Abstract
Background
Before tyrosine kinase inhibitor (TKI) therapy can be administered in patients with advanced non‐small cell lung cancer (NSCLC), EGFR mutation testing is required. However, few studies have evaluated the extent of EGFR testing in real‐world practice in China.
Methods
A multicenter, observational study of EGFR testing in NSCLC patients in North China was conducted. Treatment‐naïve patients or those with postoperative recurrent stage IIIB/IV NSCLC were enrolled. The primary objective was EGFR testing rate. Secondary objectives included EGFR mutation status, EGFR testing methods and specimens, factors associated with EGFR testing, and overall survival with or without EGFR testing.
Results
Overall, 2809 patients with stage IIIB/IV NSCLC were enrolled; 90.78% had adenocarcinoma. The EGFR screening rate was 42.54%. EGFR testing rates were higher in tumor samples obtained by lymph node puncture, and in patients with urban medical insurance, adenocarcinoma, non‐smokers, or those located in developed cities (all P < 0.001). The EGFR mutation rate was 46.44%. The most commonly used specimens for EGFR testing were biopsy tumor samples (67.53%). PCR‐based methods (72.05%), Sanger sequencing (5.36%), and Luminex liquid chip (5.10%) were the most frequently used testing platforms. Similar positive EGFR mutation rates were achieved with different platforms. TKI therapy was the first‐line treatment administered to most EGFR‐positive patients (56.22%), and chemotherapy in EGFR‐negative patients (84.88%). Overall survival was higher in EGFR‐tested than in untested patients (27.50 vs. 19.73 months; P = 0.007).
Conclusion
Real‐world EGFR testing rates for NSCLC in North China were relatively low because of clinical and social factors, including medical insurance coverage.
Keywords: Clinical practice, epidermal growth factor receptor, mutation, non‐small‐cell lung cancer, tyrosine kinase inhibitor
Introduction
Lung cancer is a leading cause of cancer‐related death worldwide. It is estimated that 154 000 patients will die from lung cancer in the United States in 2018.1 Lung cancer is also the leading cause of cancer‐related death in China, with approximately 610 000 deaths reported in 2015.2, 3 Non‐small‐cell lung cancer (NSCLC) constitutes over 80% of all new lung cancer diagnoses.4 As with most other cancer treatments, the therapy for NSCLC depends on tumor stage. Platinum‐based chemotherapy is the standard treatment for NSCLC, but the five‐year survival rate for advanced NSCLC is only 2%.5
Tyrosine kinase inhibitors (TKIs) have shown profound clinical efficacy in NSCLC patients harboring EGFR mutations, and are now the preferred first‐line treatment for advanced NSCLC with EGFR mutations.6 Given the benefit of TKI therapy for EGFR mutations, international guidelines were updated in 2011 to recommend EGFR molecular testing for NSCLC patients to select candidates for TKI therapy.5, 7, 8, 9 EGFR mutations more frequently occur in women, non‐smokers, and NSCLC patients with adenocarcinomas, and vary between gender and different pathological tumor types.10 Furthermore, EGFR mutation rates are much higher in Asian than in Caucasian patients (approximately 50% vs. 10%).5, 11
The benefits of TKI therapy in patients with EGFR‐mutant NSCLC are well recognized, but the real‐world practice of EGFR testing for NSCLC patients varies between countries. In 2010, the EGFR testing rate for NSCLC patients was 16.8% in the United States,12 compared to 63.5% in Korea.13 In 2012, a Swedish study reported that 49% of advanced NSCLC patients were tested for the EGFR gene,11 while a 2014 study conducted in New Zealand revealed the EGFR testing rate to be 67%.14 As EGFR mutations are more common in Asian compared to Western patients, EGFR molecular testing in Chinese patients is important. However, the EGFR testing rate in China was 9.6% in 2010,15 and although it increased to 18.3% in 2011, it was much lower than the testing rate in Japan in the same year at 64.8%.16
As indicated, the EGFR testing rate is relatively low in China, and limited updated data are available on the real‐world practice of EGFR testing post‐2011, when several international and national guidelines were revised to recommend EGFR molecular testing for advanced NSCLC. Thus, this study investigated the real‐world practice of EGFR testing in North China, with secondary objectives of the clinical and social factors associated with EGFR testing rates, the positive rates yielded from different testing platforms, and clinical outcomes in patients with or without EGFR testing (NCT02620657).
Methods
Study design
This was a non‐interventional, observational study of EGFR gene testing status in advanced NSCLC patients in North China (NCT02620657). Treatment‐naïve patients or those with postoperative recurrent stage IIIB/IV NSCLC across 28 research centers in 11 cities in North China were included. These cities were divided into three tiers according to their level of economy, education, and industry. Beijing, the capital of China, is a Tier‐1 city; Tier‐2 cities included Harbin, Changchun, Shenyang, and Dalian, which are either provincial capital cities or bigger developed cities; Tier‐3 included six developing cities, Anshan, Daqing, Jilin, Jinzhou, Siping, and Yanji.
The study was conducted in accordance with the Declaration of Helsinki, and in line with the principles of Good Clinical Practice, as well as applicable regulatory requirements. The institutional review boards at each site approved the study protocol before patient enrollment. The requirement of informed consent was waived because of the observational nature of this study.
Patients and inclusion/exclusion criteria
The inclusion criteria were as follows: (i) patients with histologically or pathologically confirmed stage IIIB/IV NSCLC, (ii) treatment‐naïve patients or those with postoperative recurrent NSCLC between 1 January and 31 December 2014 in North China, and (iii) NSCLC patients with complete medical records.
Patients’ medical records were reviewed by experienced physicians or trained nurses. Baseline clinical characteristics included: age at diagnosis, gender, smoking history, tumor histology, tumor node metastasis (TNM) stage (according to the 7th edition of the American Joint Committee on Cancer TNM staging system), EGFR test result, EGFR detection method, therapy regimen, and survival outcome. This study mainly assessed the EGFR testing rates in patients with adenocarcinoma, and the proportions of non‐adenocarcinoma patients in each center were kept below 10%.
Study objectives
The primary objective of this study was the real‐world practice of EGFR gene testing in advanced NSCLC patients in North China. The secondary objectives included EGFR mutation status, platforms and sample types used for EGFR detection, potential factors affecting EGFR testing, and overall survival (OS) of patients who did or did not undergo EGFR testing and their first‐line treatment regimens.
Statistical analysis
The estimated EGFR gene testing rate was 30.04% in accordance with a previously published study.17 Overall, an enrollment target of 3000 individuals with NSCLC was set for this study using Wilson's estimate calculations. Analysis was based on a full analysis set for all patients. Categorical data were presented as frequencies and percentages; continuous variables were expressed as mean, standard deviation, median, minimum, and maximum. Logistic regression analysis was performed to identify factors associated with EGFR gene testing. Independent variables associated with EGFR testing based on bivariate chi‐square tests were then analyzed by multivariate logistic regression. The OS was defined from the date of NSCLC diagnosis to the date of death, 30 March 2017 (censored observation), disease progression after third‐line therapy, or the end of third‐line therapy, whichever came first. All statistical analyses were performed using SAS version 7.1 (SAS Institute Inc., Cary, NC, USA). All tests were two‐sided and statistical significance was defined as P < 0.05.
Results
Patient characteristics
A total of 26 187 NSCLC patients from 28 research centers in North China were screened; 2809 patients met the inclusion/exclusion criteria and were enrolled in the study. The patients’ clinical characteristics are summarized in Table 1. The majority of patients were newly diagnosed with NSCLC (93.52%), stage IV (79.49%), with adenocarcinomas (90.78%). The proportions of women and non‐smokers (never and former) were 43.57% and 70.02%, respectively. More than 60% of patients had urban medical insurance, and 87.75% (2465/2809) of patients were treated in Tier‐1 and Tier‐2 cities in North China.
Table 1.
Clinical characteristics and EGFR mutation testing rates
| Characteristic | Patients, n (%) | EGFR testing rate (%) |
|---|---|---|
| Total | 2809 (100) | 1195/2809 (42.54) |
| Age at diagnosis (years) | ||
| < 65 | 2112 (75.19) | 940/2112 (42.54) |
| ≥ 65 | 697 (24.81) | 255/697 (36.59) |
| Gender | ||
| Male | 1585 (56.43) | 624/1585 (39.37) |
| Female | 1224 (43.57) | 571/1224 (46.65) |
| Tumor stage | ||
| IIIB | 576 (20.51) | 187/576 (32.47) |
| IV | 2233 (79.49) | 1008/2233 (45.14) |
| Histology | ||
| Adenocarcinoma | 2550 (90.78) | 1146/2550 (44.94) |
| Non‐squamous carcinoma | 259 (9.22) | 49/259 (18.92) |
| Smoking | ||
| Never | 1605 (57.14) | 729/1605 (45.42) |
| Former | 362 (12.89) | 185/362 (51.10) |
| Current | 842 (29.98) | 281/842 (33.37) |
| Treatment history† | ||
| Naïve | 2627 (93.52) | 1129/2627 (42.98) |
| Recurrence | 180 (6.41) | 65/180 (36.11) |
| Medical insurance‡ | ||
| Urban medical insurance | 1877 (66.82) | 837/1877 (44.59) |
| Rural cooperative medical insurance | 707 (25.17) | 258/707 (36.49) |
| Self‐funded | 224 (7.97) | 99/224 (44.20) |
| City level | ||
| Tier‐1 city | 717 (25.53) | 495/717 (69.04) |
| Tier‐2 city | 1748 (62.23) | 652/1728 (37.30) |
| Tier‐3 city | 344 (12.25) | 48/344 (13.95) |
| Hospital level | ||
| Grade‐1 level A General Hospital | 1491 (53.08) | 585/1491 (39.24) |
| Grade‐1 level A Specialized Hospital | 1211 (43.11) | 580/1211 (47.89) |
| Grade‐1 level B Specialized Hospital | 75 (2.67) | 15/75 (20.00) |
| Grade‐2 level A Specialized Hospital | 32 (1.14) | 15/32 (46.88) |
Data was missing for two patients.
Includes one patient with unknown medical insurance status who underwent EGFR testing.
EGFR mutation testing
In this study, 42.54% (1195/2809) of NSCLC and 44.94% of adenocarcinoma patients underwent EGFR testing (Table 1). EGFR testing rates were higher in patients aged < 65 years, women, patients with stage IV NSCLC, and non‐smokers. When stratified by city level, hospital level, and medical insurance type, testing rates ranged from 13.95% to 69.04% (patients treated in a Tier‐1 city).
The highest EGFR testing rate of 73.51% (111/151) was found in patients whose tumor samples were obtained by lymph node puncture (Fig 1), followed by those obtained by lung puncture.
Figure 1.

EGFR testing rates of study patients. ECOG, Eastern Cooperative Oncology Group.
A total of 2498 (88.93%) patients were referred for EGFR gene testing by their physician. However, more than half (1317/2498, 52.72%) of the patients refused to undergo EGFR testing even when recommended by their doctors, and this accounted for 81.60% of all non‐tested patients (Fig 2a). Reasons that EGFR testing was not conducted included the high cost of testing (46.77%), expensive TKI therapy (37.66%), and time constraints as the patient required immediate therapy (Fig 2b).
Figure 2.

(a) Proportion of patients who did not undergo EGFR testing. ( ) No testing although physician recommended test, (
) No testing although physician recommended test, ( ) No testing because physician did not recommend test, (
) No testing because physician did not recommend test, ( ) other reasons, and (
) other reasons, and ( ) unknown reasons. (b) Patient reasons for declining EGFR testing although a physician recommended the test. TKI, tyrosine kinase inhibitor. (
) unknown reasons. (b) Patient reasons for declining EGFR testing although a physician recommended the test. TKI, tyrosine kinase inhibitor. ( ) High detection fee, (
) High detection fee, ( ) expensive TKI, (
) expensive TKI, ( ) unknown, and (
) unknown, and ( ) time constraint.
) time constraint.
Clinical and social features associated with EGFR testing
Associations between clinical and social variables with EGFR testing were assessed by multivariate logistic regression analysis. As shown in Table 2, EGFR testing was associated not only with a patient's clinical characteristics, such as tumor pathology, tumor stage, and smoking status, but also with social features, including medical insurance type and city level, as well as willingness to follow their doctor's recommendation for EGFR testing.
Table 2.
Independent factors associated with EGFR testing†
| Factors | Odds ratio | 95% CI | P |
|---|---|---|---|
| Stage | |||
| IIIB | Reference | 0.005 | |
| IV | 0.715 | 0.566–0.902 | |
| Histological type | |||
| Adenocarcinoma | Reference | < 0.001 | |
| Non‐adenocarcinoma | 0.437 | 0.300–0.637 | |
| Smoking status | |||
| Never | Reference | < 0.001 | |
| Former | 1.102 | 0.828–1.467 | 0.506 |
| Current | 0.660 | 0.537–0.812 | < 0.001 |
| ECOG score | |||
| 0 | Reference | < 0.001 | |
| 1 | 1.079 | 0.810–1.437 | 0.603 |
| 2 | 0.967 | 0.680–1.375 | 0.853 |
| ≥ 3 | 0.320 | 0.178–0.575 | < 0.001 |
| Procedure to obtain samples | |||
| Lung puncture | Reference | < 0.001 | |
| Lymph node puncture | 3.358 | 2.123–5.311 | < 0.0001 |
| Hydrothorax | 0.979 | 0.727–1.317 | 0.887 |
| Endobronchial ultrasound | 0.612 | 0.338–1.106 | 0.104 |
| Bronchoscopy | 0.578 | 0.457–0.732 | < 0.001 |
| Patient willingness to undergo EGFR testing | |||
| Testing without physician referral | Reference | < 0.001 | |
| Testing with physician referral | 50.025 | 27.824–89.942 | |
| Medical insurance type | |||
| Rural cooperative medical insurance | Reference | < 0.001 | |
| Urban medical insurance | 1.556 | 1.255–1.928 | < 0.001 |
| Self‐funded | 1.531 | 1.044–2.244 | 0.029 |
| City level | |||
| Tier‐1 city | Reference | < 0.001 | |
| Tier‐2 city | 0.127 | 0.097–0.167 | < 0.001 |
| Tier‐3 city | 0.014 | 0.008–0.025 | < 0.001 |
Multivariate analyses were performed for variables of P < 0.05 during univariate analysis by logistic regression model.
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group.
EGFR testing was more likely to be performed in patients with adenocarcinoma, and with urban medical insurance, and less likely in smokers, those with Eastern Cooperative Oncology Group (ECOG) performance status ≥ 3, and in patients who were treated in Tier‐3 cities. A strong independent positive predictor for EGFR mutation testing was that the tumor sample was obtained by lymph node puncture (odds ratio [OR] 3.358, 95% confidence interval [CI] 2.123–5.311; P < 0.0001).
EGFR mutation status
Among 1195 patients who underwent EGFR mutation testing, 555 had EGFR gene mutations. Most (493/555, 88.82%) of these were single mutations, including 480 patients with TKI‐sensitive‐EGFR mutations and 19 with resistant‐EGFR mutations.
Exon 19 deletions and exon 21 L858R mutations were the two most common sensitive‐EGFR mutations (Table 3). Exon 20 insertions were the most common TKI‐resistant EGFR mutations (9/13, 69.23%). Complex mutations harboring both TKI‐sensitive and TKI‐resistant EGFR mutations, such as T790M+L858R, T790M+L861Q, and G719X+S768I were found in 15 patients.
Table 3.
EGFR gene mutation status in EGFR‐mutant patients
| EGFR gene status | Patients (n), N = 555 | Percentage (%) |
|---|---|---|
| Single mutation | 493 | 88.82 |
| TKI‐sensitizing mutation | ||
| 19Del | 235 | 42.34 |
| L858R | 222 | 40.00 |
| G719X | 15 | 2.70 |
| L861Q | 5 | 0.90 |
| S768I | 3 | 0.54 |
| Total | 480 | 86.49 |
| TKI‐resistant mutation | ||
| Exon 20 insertion | 9 | 1.62 |
| T790M | 4 | 0.72 |
| Total | 13 | 2.34 |
| Complex mutation | ||
| T790M + L858R | 2 | 0.36 |
| T790M + L861Q | 1 | 0.18 |
| G719X + S768I | 1 | 0.18 |
| 19Del + L858R | 8 | 1.44 |
| L858R + L861Q | 1 | 0.18 |
| G719X + L861Q | 1 | 0.18 |
| 19Del + L858R + L861Q | 1 | 0.18 |
| Total | 15 | 2.70 |
| Unknown mutation | 47 | 8.47 |
19Del, Exon 19 deletion; TKI, tyrosine kinase inhibitor.
Platforms used for EGFR mutation detection
Multiple molecular platforms are available to detect EGFR gene mutations. In this study, EGFR mutations were detected by real‐time PCR‐based methods, Sanger sequencing, and Luminex liquid chip, among others (Fig 3a). The most common was the PCR‐based method (861/1195, 72.05%).
Figure 3.
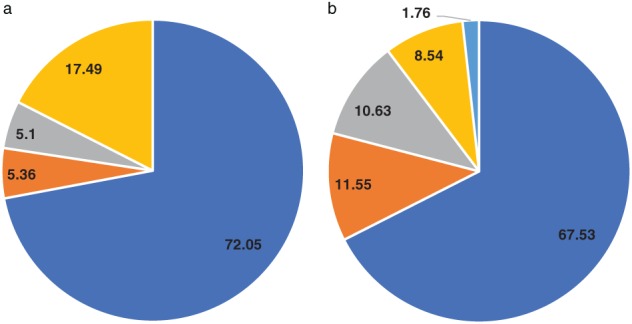
Different (a) platforms ( ) PCR‐based method, (
) PCR‐based method, ( ) sanger sequencing, (
) sanger sequencing, ( ) luminex liquid chip, and (
) luminex liquid chip, and ( ) others and (b) specimens used for EGFR detection (%). (
) others and (b) specimens used for EGFR detection (%). ( ) Biopsy tumor sample, (
) Biopsy tumor sample, ( ) cytological sample, (
) cytological sample, ( ) surgically resected sample, (
) surgically resected sample, ( ) blood sample, and (
) blood sample, and ( ) others.
) others.
The EGFR‐positive rate from PCR‐based platforms was 45.99% (396/861), and 44.80% (56/125) for Sanger sequencing and Luminex liquid chip methods. Thus, different EGFR detection methods yielded similar EGFR‐positive rates.
Specimens used for EGFR mutation detection
Tumor specimens used to detect EGFR gene mutations in this study were mainly biopsy tumor samples (807/1195, 67.53%), followed by cytological samples and surgically resected specimens. Less than 10% of patients had their blood samples tested for EGFR mutations (Fig 3b).
Considering the different types of tumor specimens used to detect the EGFR gene, the positive rates in biopsy tumor tissues, cytology specimens, and resected tumor specimens were 48.82%, 45.65%, and 51.97%, respectively. These specimens had similar positive rates for EGFR detection, and were higher than those gained from blood samples (20.59%).
First‐line treatment for patients with and without EGFR testing, and survival outcomes
Consistent with current guidelines, in this survey the preferred first‐line treatment for patients with EGFR mutations (312/555, 56.22%) was TKI therapy, followed by chemotherapy for those without EGFR mutations (522/615, 84.88%). However, 5.85% (36/615) of patients without EGFR mutations and 5.27% (85/1614) of untested patients received TKI therapy.
In addition, the survival details of 1261 patients were further analyzed. The median OS in these patients was 22.67 months (range: 20.47–25.03). Patients in the EGFR testing group had greater OS compared to those in the untested group (27.50 vs. 19.73 months; hazard ratio [HR] 0.767, 95% CI 0.658–0.894; P = 0.007). The one and two‐year OS rates were also higher than those of the untested group (74.10% and 52.60% vs. 64.50% and 43.85%, respectively).
Discussion
This was a large observational study of the real‐world practice of EGFR testing in NSCLC patients from 28 centers covering different levels of cities and hospitals in North China in 2014. We found that 42.54% of stage IIIB/IV NSCLC patients were tested for EGFR mutations. This rate was significantly higher than those obtained during 2010–2011 in China,15, 16 and is consistent with the professional guidelines for EGFR testing released after 2011.8 These findings indicate that physicians are becoming more aware of the role that EGFR mutations play when making decisions over personalized TKI therapy for NSCLC patients. However, given that 90% of patients in this study were selected with advanced adenocarcinoma and were more likely to have undergone EGFR status testing,18, 19 the true EGFR testing rate in North China is still low. The 42.54% test rate in our study sample was lower than the rates found in the CTONG1506 study for 2015–2016 in China (71.4%),20 as well as in Taiwan (54.3%), and Japan (64.8%) in 2014.13, 14, 16
The reason why more than half of the patients in this study did not undergo EGFR testing is likely multifactorial. In the real‐world setting, multiple clinical and social factors affect EGFR testing rates in North China, including patient characteristics, tumor specimen types, EGFR detection platforms, and doctors’ awareness of EGFR testing, as well as the costs associated with EGFR testing and TKI therapy.
EGFR testing is also more likely to be performed in patients with adenocarcinomas and in non‐smokers. Tumor specimens obtained by lymph node puncture had the highest EGFR testing rates (73.51%), followed by those obtained by lung puncture. However, EGFR tests are less likely to be conducted in patients treated in Tier‐3 cities. The EGFR testing rate in a Tier‐1 city was 69.04% in our study, similar to the 71.4% found in the CTONG1506 study that focused on larger Chinese cities, such as Beijing, Shanghai, and Nanjing.20 In China, patients covered by rural medical insurance have relatively low incomes. The high costs of EGFR testing (46.77%) and TKI therapy (37.66%) discourage a large proportion of patients from undergoing EGFR testing, even if recommended by treating doctors. Previous studies have reported that reimbursement of EGFR testing costs by medical insurance companies contributes to high EGFR testing rates in Japan.16 Thus, governmental policies that enable reimbursement of EGFR testing costs are important to the implementation of EGFR testing and TKI therapy regimens for NSCLC patients. The influence of economic burden on EGFR testing was further revealed by a study carried out in Canada, which showed that EGFR testing rates dropped substantially once related funding was discontinued.21
EGFR mutation testing can be performed on tumor specimens obtained via many techniques: surgical resection, endoscopy, transthoracic needle biopsy, or lung puncture. In this study, biopsy lymph node punctate samples were the most frequently used specimen for EGFR gene testing. Tumor tissue is the gold standard for clinical genetic analyses, but adequate tissue samples are not always available for all patients. Data have shown that cytology samples can be used for EGFR mutation testing.22 Because of the observational nature of our study, the overall agreement between different sample types was not compared. The positive rates of biopsy tumor tissues, cytology specimens, and resected tumor specimens were 48.82%, 45.65%, and 51.97%, respectively. These results are consistent with the findings of a recent study that demonstrated EGFR positive rates in histological and cytological samples of 42.5% and 37.5%, respectively.23 Compared to tissue and cytology samples, blood samples are easily obtained and can also be used for EGFR mutation detection,24 but highly sensitive platforms, such as digital PCR, are required.25 Although blood samples generate a lower positive rate of EGFR testing (20.59%), this method has advantages, especially when adequate tissue samples are not available.26
The EGFR mutation rate in our study was 46.44% (555/1195), and 86.49% of these were TKI‐sensitive mutations, such as 19 deletions and L858R mutations; these mutations comprised up to 90% of all sensitive mutations, consistent with the results of previous reports.27 A variety of technology platforms are available to detect EGFR mutations, including DNA sequencing, real‐time PCR‐based methods, restriction fragment length polymorphism, mass spectrometry‐based genotyping, peptide nucleic acid clamp, and high‐performance liquid chromatography. DNA sequencing is the initial method used for EGFR gene detection, but has low sensitivity; more sensitive methods are required for samples with a low tumor content. Real‐time PCR‐based methods, Sanger DNA sequencing, and Luminex liquid chip were used in our study, and these platforms generated similar positive rates for EGFR testing, indicating that physicians can choose appropriate methods in accordance with patient characteristics and techniques available in hospitals.
EGFR mutation testing in lung cancer is used to guide patient selection for TKI therapy. In this study, 56.22% of NSCLC patients with EGFR mutation were administered TKI therapy. Most international guidelines recommend that TKI therapy should not be prescribed as first‐line therapy for patients without sensitive mutations; however, 5.85% of patients without EGFR mutations and 5.27% of EGFR‐unknown patients in our sample received TKI therapy. Efforts should be enhanced to further improve the clinical education of the benefits of personalized TKI therapy for doctors and patients.
We also investigated the impact of EGFR testing and subsequent first‐line therapy regimens on patient outcomes. Patients in the EGFR testing group had better OS compared to those in the untested group (27.50 vs. 19.73 months; P = 0.007). This could be associated with TKI therapy because the majority of untested patients did not receive TKI therapy, and EGFR testing results are essential in selecting the candidate patients who will benefit most from such therapy. A recent study demonstrated that EGFR testing was associated with prolonged OS (HR 0.76; P < 0.0001) because EGFR‐mutant patients benefited more from first‐line TKI therapy.14
There are several limitations to this study. The major limitation is its retrospective real‐world nature, which might explain why 75.19% of patients were aged < 65 years. However, several strengths, including the large number of patients, the assessment of EGFR testing in clinical settings, and the different platforms and specimens used for EGFR testing add to the importance of our findings. Another limitation is that the patients were enrolled in North China; however, the screened patient samples were adequate, and the patient characteristics and EGFR mutation rates were similar to previous studies.15, 16 We believe the key findings in our study, including EGFR testing rates and the clinical and social features affecting EGFR testing, can be generalized to China or Asian countries.
In conclusion, the real‐world EGFR testing rate is relatively low in North China. Close cooperation between doctors, patients, and government is required to improve the clinical practice of EGFR testing in NSCLC patients. These include strengthening education on personalized therapy, increasing doctor and patient awareness of EGFR gene detection, and improving social medical insurance coverage in order to further implement EGFR testing to benefit NSCLC patients. A variety of EGFR testing platforms and techniques are available and generate similar positive rates; therefore doctors are in a position to choose appropriate methods to better support personalized TKI therapy.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by the Health and Family Planning Commission of Jilin Province (No: 2015Z094) and AstraZeneca, China. We would like to thank all patients and their families, as well as the investigators, researchers, nurses, study coordinators, and operation staff involved in this study. Medical writing was performed by Nucleus Global and funded by AstraZeneca, China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Sun K, Zheng R et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018; 30: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gridelli C, Rossi A, Carbone DP et al Non‐small‐cell lung cancer. Nat Rev Dis Primers 2015; 1: 15009. [DOI] [PubMed] [Google Scholar]
- 5. Ettinger DS, Wood DE, Akerley W et al Non‐small cell lung cancer, Version 6.2015. J Natl Compr Cancer Netw 2015; 13: 515–24. [DOI] [PubMed] [Google Scholar]
- 6. Masters GA, Temin S, Azzoli CG et al Systemic therapy for stage IV non‐small‐cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2015; 33: 3488–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindeman NI, Cagle PT, Beasley MB et al Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. (Published erratum appears in J Thorac Oncol 2013;8:1343) J Thorac Oncol 2013; 8: 823–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keedy VL, Temin S, Somerfield MR et al American Society of Clinical Oncology provisional clinical opinion: Epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non‐small‐cell lung cancer considering first‐line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 2011; 29: 2121–7. [DOI] [PubMed] [Google Scholar]
- 9. Reck M, Popat S, Reinmuth N et al Metastatic non‐small‐cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014; 25 ((Suppl 3)): iii27–39. [DOI] [PubMed] [Google Scholar]
- 10. Rosell R., Moran T., Queralt C., et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361 (2009) 958–67. [DOI] [PubMed] [Google Scholar]
- 11. Sandelin M, Berglund A, Sundström M et al Patients with non‐small cell lung cancer analyzed for EGFR: Adherence to guidelines, prevalence and outcome. Anticancer Res 2015; 35: 3979–85. [PubMed] [Google Scholar]
- 12. Enewold L, Thomas A. Real‐world patterns of EGFR testing and treatment with erlotinib for non‐small cell lung cancer in the United States. PLoS One 2016; 11: e0156728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi YL, Sun JM, Cho J et al EGFR mutation testing in patients with advanced non‐small cell lung cancer: A comprehensive evaluation of real‐world practice in an East Asian tertiary hospital. PLoS One 2013; 8: e56011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKeage M, Elwood M, Tin Tin S et al EGFR mutation testing of non‐squamous NSCLC: Impact and uptake during implementation of testing guidelines in a population‐based registry cohort from Northern New Zealand. Target Oncol 2017; 12: 663–75. [DOI] [PubMed] [Google Scholar]
- 15. Xue C, Hu Z, Jiang W et al National survey of the medical treatment status for non‐small cell lung cancer (NSCLC) in China. Lung Cancer 2012; 77: 371–5. [DOI] [PubMed] [Google Scholar]
- 16. Yatabe Y, Kerr KM, Utomo A et al EGFR mutation testing practices within the Asia Pacific region: Results of a multicenter diagnostic survey. J Thorac Oncol 2015; 10: 438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu YL, Zhong WZ, Li LY et al Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non‐small cell lung cancer: A meta‐analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007; 2: 430–9. [DOI] [PubMed] [Google Scholar]
- 18. Shigematsu H, Lin L, Takahashi T et al Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005; 97: 339–46. [DOI] [PubMed] [Google Scholar]
- 19. Shi Y, Au JS, Thongprasert S et al A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou Q, Song Y, Zhang X et al A multicenter survey of first‐line treatment patterns and gene aberration test status of patients with unresectable Stage IIIB/IV nonsquamous non‐small cell lung cancer in China (CTONG 1506). BMC Cancer 2017; 17: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellis PM, Verma S, Sehdev S, Younus J, Leighl NB. Challenges to implementation of an epidermal growth factor receptor testing strategy for non‐small‐cell lung cancer in a publicly funded health care system. J Thorac Oncol 2013; 8: 1136–41. [DOI] [PubMed] [Google Scholar]
- 22. Brandao G.D., Brega E.F., Spatz A.. The role of molecular pathology in non‐small‐cell lung carcinoma‐now and in the future. Curr Oncol 19 (Suppl 1) (2012) S24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Satouchi M, Tanaka H, Yoshioka H et al Detection of epidermal growth factor receptor gene T790M mutation in cytology samples using the cobas® EGFR mutation test. Lung Cancer 2017; 111: 190–4. [DOI] [PubMed] [Google Scholar]
- 24. Maheswaran S, Sequist LV, Nagrath S et al Detection of mutations in EGFR in circulating lung‐cancer cells. N Engl J Med 2008; 359: 366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buder A, Tomuta C, Filipits M. The potential of liquid biopsies. Curr Opin Oncol 2016; 28: 130–4. [DOI] [PubMed] [Google Scholar]
- 26. Li X, Ren R, Ren S et al Peripheral blood for epidermal growth factor receptor mutation detection in non‐small cell lung cancer patients. Transl Oncol 2014; 7: 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–81. [DOI] [PubMed] [Google Scholar]


