Abstract
Background
Immunotherapy is a new paradigm for the treatment of non‐small‐cell lung cancer (NSCLC), and targeting the PD‐1 or PD‐L1 pathway is a promising therapeutic option. Although PD‐1/PD‐L1 inhibitors are more effective than standard chemotherapy in lung cancer, clinicians are afraid to actively use them because of hyperprogression and pseudoprogression. The aim of this study was to investigate the factors associated with tumor response and serious outcomes.
Methods
We retrospectively collected the medical records of 51 patients with advanced NSCLC who received PD‐1/PD‐L1 inhibitors between January 2016 and February 2018.
Results
The mean patient age was 63.9 years, and 72.5% (37/51) were male. Most (92.2%, 47/51) had received previous systemic treatment. The overall response rate was 21.6% (11/51). The response rate was significantly lower in patients with pleural or pericardial metastasis than in patients without pleural or pericardial metastasis (4.3% vs. 35.7%; P = 0.007). Patients with pleural or pericardial metastasis had a significantly higher rate of adverse events of any grade (91.3% vs. 50.0%; P = 0.002) and grade 3–5 adverse events (52.2% vs. 25.0%; P = 0.046).
Conclusion
Pleural or pericardial metastasis is a significant factor affecting the efficacy and rate of adverse events in advanced NSCLC patients treated with PD‐1/PD‐L1 inhibitors. Clinicians should pay attention to the use of immune checkpoint inhibitors in lung cancer patients with pleural or pericardial metastasis.
Keywords: Adverse event, immune checkpoint inhibitor, non‐small cell lung cancer, pericardial metastasis, pleural metastasis
Introduction
Until recently, standard treatment for advanced non‐small cell lung cancer (NSCLC) patients without actionable mutations was platinum‐based chemotherapy. The median overall survival (OS) in such patients was approximately one year, and the prognosis was poor.1 Recently, drugs targeting the immune checkpoint pathway based on the mechanism of immune evasion of cancer have been developed. These drugs show promising effects in various cancers, particularly melanoma and NSCLC. Immunotherapy is changing the paradigm of NSCLC treatment.
One feature of cancer is immune escape, which is complicated and difficult to overcome; the immune checkpoint is considered an important step in immune escape. Immune checkpoint inhibitors bind to the PD‐1 receptor or PD‐L1 and allow activated T cells to attack tumor cells by blocking the binding of the PD‐1 ligand of tumor cells to the PD‐1 receptor of immune cells.2, 3, 4, 5 The efficacy and safety of immune checkpoint inhibitors in advanced NSCLC have been demonstrated in various clinical trials. Checkmate 017 and 057, KENOTE‐010, OAK, and POLAR trials reported superior efficacy and survival benefits of nivolumab, pembrolizumab, and atezolizumab in patients with previously treated NSCLC.6, 7, 8, 9, 10, 11 In KENOTE‐024, pembrolizumab was associated with significantly longer progression‐free survival (PFS) and OS in previously untreated advanced NSCLC patients with high PD‐L1 expression (tumor proportion score [TPS] ≥ 50%), compared to platinum‐based chemotherapy.12
Many studies have shown that PD‐1/PD‐L1 inhibitors exhibit less toxicity and greater efficacy than platinum‐based chemotherapy; however, only approximately 20% of unselected patients benefit from PD‐1/PD‐L1 inhibitors. Many studies have investigated the potential predictive biomarkers of the efficacy of immune checkpoint inhibitors, including PD‐L1 expression, tumor mutation burden, and tumor‐infiltrating lymphocytes, but this area is still unclear.13, 14 The use of PD‐1/PD‐L1 inhibitors in an unselected population is challenging considering the more frequent immune‐related adverse events (AEs), such as pneumonitis, rash, and hypothyroidism, and the low benefits and high costs.15 In addition, early negative discordant crossover of OS curves commonly occurred in randomized controlled trials of immune checkpoint inhibitors. The cause of this mortality is unclear, but it may be a result of immune checkpoint inhibitor toxicity and the tumor growth‐promoting effects of immunotherapy.16 The clinicopathologic features of patients who have serious AEs (SAEs) associated with PD‐1/PD‐L1 inhibitors are unknown, and there is no precise biomarker to predict side effects following immunotherapy.
The purpose of this study was to investigate the clinicopathologic factors associated with tumor response and serious outcomes following immunotherapy and establish a subpopulation of patients at higher risk of SAEs caused by immunotherapy.
Methods
Patients
We retrospectively collected and analyzed the medical records of 51 patients with advanced NSCLC who received PD‐1/PD‐L1 inhibitors between January 2016 and February 2018. The eligibility criteria were: histological or cytological confirmation of NSCLC; age ≥ 18 years; Eastern Cooperative Oncology Group performance score (ECOG PS) of 0–2; administered more than one dose of atezolizumab, nivolumab, or pembrolizumab; and no prior therapy using an immune checkpoint inhibitor, regardless of PD‐L1 status. PD‐L1 expression was assessed using the PD‐L1 immunohistochemical 22C3 pharmDx kit (Dako North America, Carpinteria, CA, USA). Patients with advanced or metastatic disease before immunotherapy, including recurrence or progression after surgery, even at an early stage of diagnosis, were enrolled. Patients who had EGFR mutations or ALK rearrangement were included if the disease progressed after targeted therapy.
Patients were ineligible if: they were receiving immunosuppressive treatment or systemic glucocorticoids; or if they had other malignant disease, uncontrolled autoimmune disease, active interstitial lung disease, or uncontrolled disease that might have affected survival.
Treatments
Patients were administered intravenous atezolizumab (1200 mg every 3 weeks), nivolumab (3 mg per kg of body weight every 2 weeks), or pembrolizumab (200 mg in previously untreated patients and 2 mg per kg of body weight every 3 weeks in previously treated patients). Treatment was continued until the patient had confirmed investigator‐assessed disease progression, had unacceptable SAEs, or withdrew consent. Patients whom the investigator assessed may obtain a clinical benefit could continue treatment after radiologic disease progression.
Response and adverse events
Computed tomography (CT) was performed every six to eight weeks during treatment. The response to treatment was assessed based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Toxicities were reviewed, and a complete blood count with a differential count, blood chemistry panel, and vital signs were assessed every two or three weeks during treatment. AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Dysimmune toxicities caused by immune system imbalance, which mainly involve the skin, gut, liver, endocrine glands, or lung but can affect any tissue, were categorized as immune‐related AEs.17
Statistical analysis
Fisher's exact and independent t‐tests were used to analyze differences in patients’ clinicopathological data. Multivariate analyses were performed using logistic regression analysis. Results are shown as the mean ± standard deviation, and P < 0.05 was considered statistically significant. Survival was estimated using the Kaplan–Meier method, and survival rates were compared using the log‐rank test. SPSS version 20 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
Patient baseline characteristics
Between January 2016 and February 2018, 51 patients received at least one dose of immune checkpoint inhibitors. The baseline characteristics of the included patients are shown in Table 1. The mean age was 63.9 years (range: 33–86), and 72.5% (37/51) were male. Current or former smokers accounted for 66.7% (34/51). The histologic types of tumors were squamous cell carcinoma (51.0%), adenocarcinoma (35.3%), mixed type (7.8%), and other (5.9%). Most patients had an ECOG PS score of 0 or 1. Some patients with early‐stage carcinomas at diagnosis were also included in the study, but the stage prior to immunotherapy was IIIB or higher. Immediately before immunotherapy, there were 10 patients without distant metastasis, 23 with pleural or pericardial metastasis, 2 with lung‐to‐lung metastasis, and 16 with distant metastasis. Of the 39 (76.5%) patients whose tumor samples were assessable for PD‐L1 expression, 34 (87.2%) had PD‐L1 expression on at least 1% of tumor cells, including 23 (59.0%) with PD‐L1 expression on at least 50% of tumor cells. Most patients (92.2%, 47/51) had received at least one line of previous systemic treatment: 49.0% had received pembrolizumab, 39.2% nivolumab, and 11.8% atezolizumab. The mean number of treatment cycles of immune checkpoint inhibitors was 5.69 (range: 1–21).
Table 1.
Patient baseline characteristics
| Variable | Mean (range) or number of patients (%) |
|---|---|
| Age, years | 63.9 (33–86) |
| Gender | |
| Male | 37 (72.5) |
| Female | 14 (27.5) |
| Disease stage at diagnosis | |
| IB | 1 (2.0) |
| IIA | 1 (2.0) |
| IIB | 2 (3.9) |
| IIIA | 5 (9.8) |
| IIIB | 4 (7.8) |
| IV | 38 (74.5) |
| Histology | |
| Squamous | 26 (51.0) |
| Adenocarcinoma | 18 (35.3) |
| Mixed | 4 (7.8) |
| Other | 3 (5.9) |
| EGFR | |
| Mutant | 5 (9.8) |
| Wild type | 46 (90.2) |
| PD‐L1 expression | |
| < 1% | 5 (9.8) |
| Low (1–49%) | 11 (21.6) |
| High (> 50%) | 23 (45.1) |
| Unknown | 12 (23.5) |
| Smoking status | |
| Never | 17 (33.3) |
| Former | 16 (31.4) |
| Current | 18 (35.3) |
| Number of prior regimens | |
| 0 | 4 (7.8) |
| 1 | 23 (45.1) |
| ≥ 2 | 24 (47.1) |
| ECOG | |
| 0 | 8 (15.7) |
| 1 | 34 (66.7) |
| 2 | 9 (17.6) |
| Agent | |
| Atezolizumab | 6 (11.8) |
| Nivolumab | 20 (39.2) |
| Pembrolizumab | 25 (49.0) |
| Metastatic sites before immunotherapy | |
| Pleural or pericardial metastasis | 23 (45.1) |
| Lung to lung (only) | 2 (3.9) |
| Distant metastasis | 16 (31.4) |
| No distant metastasis | 10 (19.6) |
| Number of cycles of immunotherapy | 5.69 (1–21) |
ECOG, Eastern Cooperative Oncology Group.
Efficacy
The overall response rate, assessed according to RECIST, was 21.6%. The disease control rate including partial response (PR) and stable disease (SD) was 47.1%. Response evaluation was not conducted in 6 patients (11.8%) because of treatment discontinuation as a result of unacceptable SAEs or patient refusal.
To identify the important factors affecting the response rate, we analyzed various epidemiologic and clinical factors (Table 2). There were no statistically significant differences in age, gender, smoking status, PD‐L1 expression status, or histology, excluding types of immune checkpoint inhibitors and metastatic sites. The response rate was significantly higher in patients without pleural or pericardial metastasis than in patients with pleural or pericardial metastasis (odds ratio [OR] 25.97, 95% confidence interval [CI] 2.54–265.61; P = 0.006). In addition, patients receiving pembrolizumab had a significantly higher response rate than patients receiving atezolizumab or nivolumab (OR 14.73, 95% CI 2.25–96.34; P = 0.005). Pembrolizumab should be prescribed to patients with high PD‐L1 expression (TPS ≥ 50%) and the other drugs to patients with low or no PD‐L1 expression (TPS < 50%). The efficacy of PD‐1/PD‐L1 inhibitors differs between patients with high PD‐L1 expression and those with low or no PD‐L1 expression.
Table 2.
Univariate and multivariate analyses of factors associated with the response rate to a PD‐1/PD‐L1 inhibitor
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P | OR (95% CI) | P |
| Age (years) | ||||
| < 55 | 1.0 | — | — | — |
| 55–70 | 1.74 (0.18–17.22) | 0.636 | — | — |
| ≥ 70 | 4.44 (0.42–46.55) | 0.213 | — | — |
| Gender | ||||
| Male | 1.0 | — | — | — |
| Female | 0.21 (0.02–1.80) | 0.251 | ||
| Smoking status | ||||
| Never | 1.0 | — | — | — |
| Former | 2.12 (0.41–10.88) | 0.367 | ||
| Current | 0.93 (0.16–5.42) | 0.939 | ||
| EGFR | ||||
| Wild type | 1.0 | — | — | — |
| Mutant | 0.76 (0.65–0.90) | 0.572 | ||
| PD‐L1 expression | ||||
| Unknown and < 1% | 1.0 | — | — | — |
| Low (1–49%) | 2.81 (0.39–20.46) | 0.307 | — | — |
| High (≥ 50%) | 2.65 (0.46–15.15) | 0.274 | — | — |
| Histology | ||||
| SqCC | 1.0 | — | — | — |
| Adeno | 0.54 (0.12–2.46) | 0.429 | — | — |
| Other | 0.45 (0.05–4.46) | 0.497 | — | — |
| Agent | ||||
| Atezolizumab, nivolumab | 1.0 | — | — | — |
| Pembrolizumab | 6.75 (1.29–35.42) | 0.024 | 14.73 (2.25–96.34) | 0.005 |
| Pleural or pericardial metastasis | ||||
| Yes | 1.0 | — | — | — |
| No | 12.22 (1.43–104.71) | 0.022 | 25.97 (2.54–265.61) | 0.006 |
Adeno, adenocarcinoma; CI, confidence interval; OR, odds ratio; SqCC, squamous cell carcinoma.
Pleural or pericardial metastasis
Pleural or pericardial metastasis was confirmed by cytology and/or biopsy or by imaging studies, including CT and 18F‐fluorodeoxyglucose (FDG)‐positron emission tomography (PET). In principle, pleural or pericardial metastases should be confirmed by cytology and/or biopsy; however, if pathologic diagnosis was difficult because of low effusion, the chest CT findings of a radiologist and FDG–PET findings of a nuclear medicine specialist were combined. FDG‐PET combined with CT has high specificity and accuracy for the detection of pleural malignancies.18, 19 In the 23 patients with pleural or pericardial metastases included in this study, 11 were diagnosed by cytology and/or biopsy and 12 via a combination of FDG‐PET and CT.
Patients with and without pleural or pericardial metastasis were analyzed separately (Table 3). There were no significant differences between the groups in age, gender, smoking status, histology, EGFR mutation status, PD‐L1 expression, ECOG PS, number of prior regimens, type of agent, or number of distant metastases. Among the patients with pleural or pericardial metastasis, 20 (87.0%) had pleural metastases, 2 of which also had pericardial involvement and 2 had peritoneal seeding. Three patients had pericardial invasion alone.
Table 3.
Differences in baseline characteristics and clinical outcomes between patients with and without pleural or pericardial metastasis
| Variable | Pleural or pericardial metastasis (n = 23) |
No pleural or pericardial metastasis (n = 28) |
P |
|---|---|---|---|
| Age (years) | 61.70 ± 12.60 | 65.71 ± 9.39 | 0.198 |
| Male gender | 15 (65.2) | 22 (78.6) | 0.454 |
| Smoking status | |||
| Never | 10 (43.5) | 7 (25.0) | 0.054 |
| Former | 9 (39.1) | 7 (25.0) | |
| Current | 4 (17.4) | 14 (50.0) | |
| EGFR | |||
| Mutant | 3 (13.0) | 2 (7.1) | 0.647 |
| Wild type | 20 (87.0) | 26 (92.9) | |
| PD‐L1 expression | |||
| Unknown/< 1% | 7 (30.4) | 10 (35.7) | 0.257 |
| Low (1–49%) | 3 (13.0) | 8 (28.6) | |
| High (> 50%) | 13 (56.5) | 10 (35.7) | |
| Histology | |||
| Squamous | 9 (39.1) | 17 (60.7) | 0.215 |
| Adenocarcinoma | 9 (39.1) | 9 (32.1) | |
| Other | 5 (21.7) | 2 (7.1) | |
| Number of prior regimens | |||
| 0 | 2 (8.7) | 2 (7.1) | 0.913 |
| 1 | 11 (47.8) | 12 (42.9) | |
| ≥ 2 | 10 (43.5) | 14 (50.0) | |
| ECOG | |||
| 0 | 3 (13.0) | 5 (17.9) | 0.838 |
| 1 | 15 (65.2) | 19 (67.9) | |
| 2 | 5 (21.7) | 4 (14.3) | |
| Agent | |||
| Atezolizumab | 1 (4.3) | 5 (17.9) | 0.326 |
| Nivolumab | 9 (39.1) | 11 (39.3) | |
| Pembrolizumab | 13 (56.5) | 12 (42.9) | |
| Number of distant metastases | |||
| 0 | 8 (34.8) | 10 (35.7) | 1.000 |
| 1 | 6 (26.1) | 7 (25.0) | |
| ≥ 2 | 9 (39.1) | 11 (39.3) | |
| Number of cycles of immunotherapy | 4.52 ± 3.54 | 6.64 ± 5.84 | 0.117 |
| AEs | |||
| Any grade | 21 (91.3) | 14 (50.0) | 0.002 |
| Grade 3–5 | 12 (52.2) | 7 (25.0) | 0.046 |
| Response | |||
| Cannot be evaluated | 4 (17.4) | 2 (7.1) | 0.037 |
| PR | 1 (4.3) | 10 (35.7) | |
| SD | 6 (26.1) | 7 (25.0) | |
| PD | 12 (52.2) | 9 (32.1) | |
AEs, adverse events; ECOG, Eastern Cooperative Oncology Group; PD, progressive disease; PR, partial response; SD, stable disease.
Of the patients with pleural or pericardial metastasis, 1 (4.3%) achieved a PR with an immune checkpoint inhibitor, and 6 (26.1%) had SD. In patients without pleural or pericardial metastasis, 10 (35.7%) achieved a PR, and 7 (25.0%) had SD. The response rate was significantly lower in patients with pleural or pericardial metastasis than in patients without (4.3% vs. 35.7%; P = 0.007).
The one patient who achieved a PR despite pleural metastasis had received 12 cycles of an immune checkpoint inhibitor and was still receiving the treatment. The patient had received pembrolizumab as first‐line treatment because the tumor sample had high PD‐L1 expression (TPS ≥ 50%). In addition, the patient underwent the first treatment with a chest catheter insertion because of a large amount of pleural effusion at diagnosis. All six patients with an SD response in the pleural or pericardial metastasis group had received > 5 cycles of immunotherapy, and their diseases were consistently well controlled.
The median PFS of patients without pleural or pericardial metastasis was 4.0 months (95% CI 2.0–6.0), which was significantly longer than that of patients with pleural or pericardial metastasis (1.6 months, 95% CI 1.4–1.8). The median OS of patients without pleural or pericardial metastasis was 9.3 months (95% CI 0.8–17.8), which was longer than that of patients with pleural or pericardial metastasis (6.1 months, 95% CI 0.0–12.8), although this difference was not statistically significant (Fig 1).
Figure 1.
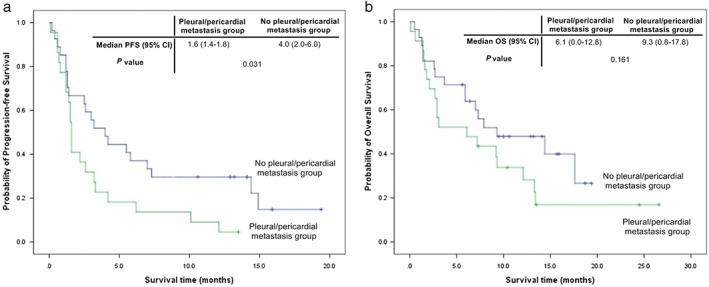
(a) Progression‐free survival (PFS) and (b) overall survival (OS) according to the presence or absence of pleural or pericardial metastasis. CI, confidence interval.
Adverse events
Adverse events of any grade occurred in 35 (68.6%) patients, and grade 3 or greater in 19 (37.3%) (Table 4). The most common any‐grade AEs were fatigue (29.4%) and dyspnea (9.8%). Stomatitis, nausea, vomiting, or back pain occurred in four patients (7.8%). Pleural effusion, ascites, rash, or constipation occurred in three patients (5.9%). Pruritus, insomnia, elevated alanine aminotransferase level, anorexia, or pericardial effusion occurred in two patients (3.9%). Of these, seven patients (13.7%) experienced immune‐related AEs (irAEs). The most common irAE was a rash (n = 3). Other irAEs included liver dysfunction (n = 2), hypothyroidism (n = 1), and pneumonitis (n = 1). Most irAEs were mild (grade 1–2), except for one case of grade 3 pneumonitis.
Table 4.
AEs that occurred in at least 3% of all treated patients
| AEs | Any grade | Grade 3–4 |
|---|---|---|
| Any event | 35 (68.6) | 19 (37.3) |
| Fatigue | 15 (29.4) | 3 (5.9) |
| Dyspnea | 5 (9.8) | 4 (7.8) |
| Stomatitis | 4 (7.8) | 0 |
| Nausea | 4 (7.8) | 0 |
| Vomiting | 4 (7.8) | 0 |
| Pain (back, extremity) | 4 (7.8) | 2 (3.9) |
| Pleural effusion | 3 (5.9) | 3 (5.9) |
| Ascites | 3 (5.9) | 2 (3.9) |
| Rash | 3 (5.9) | 0 |
| Constipation | 3 (5.9) | 0 |
| Pruritus | 2 (3.9) | 0 |
| Insomnia | 2 (3.9) | 0 |
| Elevated ALT | 2 (3.9) | 1 (2.0) |
| Pericardial effusion | 2 (3.9) | 1 (2.0) |
| Anorexia | 2 (3.9) | 0 |
AEs, adverse events; ALT, alanine aminotransferase.
Patients with pleural or pericardial metastasis had significantly higher any‐grade AEs (91.3% vs. 50.0%; P = 0.002) and grade 3–5 AEs (52.2% vs. 25.0%; P = 0.046). Grade 3–5 AEs occurred in 12 patients with pleural or pericardial metastasis and included: dyspnea (3 patients); pleural effusion (3 patients); fatigue (2 patients); and elevated alanine aminotransferase levels, ascites, pericardial effusion, and back pain (1 patient each). SAEs occurred in five patients, all of whom had pleural or pericardial metastasis.
Serious adverse events
Three patients discontinued immunotherapy as a result of SAEs and two patients died (Table 5). These were not considered clinically as treatment‐related deaths because of the patients’ unstable condition before drug administration. The changes on chest radiography before and after PD‐1/PD‐L1 inhibitor administration in patients who discontinued immunotherapy as a result of SAEs are shown in Figure 2. Within one to two weeks after immunotherapy, all three patients developed respiratory distress symptoms with a sudden increase in pleural or pericardial effusion that required intervention, such as catheter insertion or thoracentesis, and hospitalization.
Table 5.
Serious AEs that led to the discontinuation of immunotherapy or death
| Patient (No.) |
Age | Gender | Smoking status (P‐Y) | Histology | PD‐L1 expression | Site of metastatic lesions | Agent | Initial response | AEs |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | Never | Adeno | Unknown | Pleura, Peritoneum | Nivolumab | Unevaluated | Uncontrolled ascites Skin rash |
| 2 | 55 | F | 2.5 | SqCC | High | Pleura | Nivolumab | PD | Uncontrolled pleural effusion Elevated liver enzyme |
| 3 | 34 | F | Never | Adeno | High | Pericardium | Pembrolizumab | PD | Uncontrolled pleural effusion Recurrent pericardial effusion |
| 4 | 68 | M | Never | Adeno | Unknown | Pleura | Nivolumab | Unevaluated | Death |
| 5 | 55 | M | 15 | SqCC | Unknown | Pericardium | Nivolumab | Unevaluated | Death |
Adeno, adenocarcinoma; AEs, adverse events; PD, progressive disease; P‐Y, pack‐year; SqCC, squamous cell carcinoma.
Figure 2.
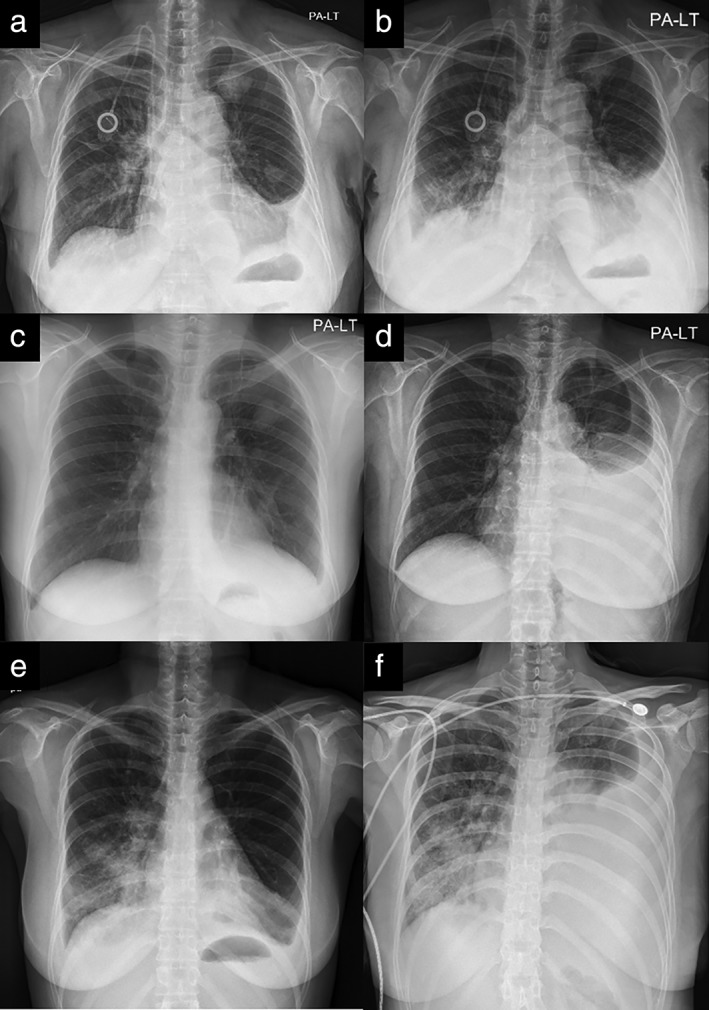
Changes on chest radiography in three patients who discontinued treatment as a result of serious adverse events. Patient #1: (a) pre‐treatment and (b) after two weeks of treatment. Patient #2: (c) pre‐treatment and (d) after two weeks of treatment. Patient #3: (e) pre‐treatment and (f) after one week of treatment.
Discussion
To our knowledge, this is the first study to identify various clinicopathologic factors in relation to AEs and SAEs in advanced NSCLC patients administered immune checkpoint inhibitors. In addition, we investigated patients who required attention prior to the administration of PD‐1/PD‐L1 inhibitors.
In our study, the response rate to immune checkpoint inhibitors was 21.6% (11/51), regardless of the status of PD‐L1 expression, similar to the 20% rate reported in real‐world settings.20 Of the 23 patients with high PD‐L1 expression (TPS ≥ 50%), 19 (82.6%) had received pembrolizumab. The response rate of these patients was 31.6% (6/19), higher than the efficacy observed among all patients in this study. In the group with high PD‐L1 expression, pembrolizumab was preferentially used, and the first‐line setting was also included, such that the response to pembrolizumab was significantly higher than those to the other immunotherapeutic agents. Any‐grade AEs after immunotherapy occurred in 35 (68.6%) patients, and grade 3 or higher AEs in 19 (37.3%) of 51 patients. This incidence is higher than previously reported grade 3–5 treatment‐related AEs of 9.5% (pembrolizumab) and 13% (nivolumab), probably because this study was conducted in a real clinical setting. Three patients (5.9%) experienced AEs that led to treatment discontinuation, similar to the 4–6% rate reported in a phase III trial of PD‐1/PD‐L1 inhibitors.9
In analysis of the factors associated with a significant difference in the efficacy and AEs of immunotherapy, patients with pleural or pericardial metastasis had a significantly lower response rate and more AEs. Grade 3–5 and any‐grade AEs were significantly more common in patients with pleural or pericardial metastasis (Table 3). Most of these patients had wet metastasis, such as malignant pleural or pericardial effusion, but three patients had pleural or pericardial nodules without effusion. Patients with pleural or pericardial metastasis may more often develop SAEs and exhibit a poor response following the use of an immune checkpoint inhibitor because of the large disease burden itself or because the immunologic reaction is more aggressively exhibited in patients with pleural or pericardial metastasis. Most patients (80.4%, 41/51) had distant metastases before immunotherapy. Of 23 patients with pleural or pericardial metastasis, 15 had metastasis to other solid organs (bone, lung, liver, or brain), as well as pleural or pericardial metastasis. Although the presence of pleural or pericardial metastasis may indicate that the disease burden was high, the number of distant metastases was not significantly different between the groups (with vs. without pleural or pericardial metastasis). Therefore, the results of this study were not simply caused by differences in tumor burden. Although the three patients who discontinued immunotherapy because of SAEs had a small amount of pleural effusion or ascites not requiring drainage before administration of the immune checkpoint inhibitor, pleural effusion or ascites rapidly increased to then require intervention, such as chest catheter insertion or paracentesis, and symptoms such as dyspnea or abdominal distension were observed within one to two weeks after administration of the PD‐1/PD‐L1 inhibitor. This phenomenon can be considered an immune‐related reaction or pseudoprogression. In a similar report of two cases, recurrent effusions may have been secondary to pseudoprogression because the patients showed disease improvement after continuous immunotherapy.21 Generally, there are many lymphocytes in malignant pleural effusion and pericardial effusion; thus, re‐activated lymphocytes could cause more frequent and severe immunologic reactions in these lymphocyte‐enriched niches.
Pseudoprogression, which is defined as initial tumor growth followed by subsequent tumor regression, has been described with immunotherapy.22, 23 However, it has not been sufficiently investigated as many studies have focused mainly on efficacy, as the number of patients using an immune checkpoint inhibitor has increased dramatically. In Checkmate 017, of the 135 patients, 28 (20.7%) were treated with nivolumab after initial progression as defined by RECIST version 1.1, with 9 patients displaying a nonconventional pattern of benefit.7 Checkmate 057 showed that 71 patients treated with nivolumab (24%) continued treatment after initial progression, of whom 16 (23%) had a nonconventional pattern of benefit.6 As reported in two studies, the incidence of pseudoprogression is uncommon, at 5–6%. The mechanism and pattern of pseudoprogression and its impact on the efficacy of immunotherapy have not been clearly established; it is based on clinicians’ judgment after combining radiologic and clinical findings. When progression is identified in a response assessment, if it is pseudoprogression, continued immunotherapy may be helpful to the patient, but if it is real progression, the patient may miss the opportunity to be treated with other medications. Therefore, it is necessary to discriminate between pseudoprogression and true progression during immunotherapy. In a case of pseudoprogression in a melanoma patient with brain metastasis, histopathologic results showed that there was a small cluster of tumor cells, rare CD4 T lymphocytes, and few CD8 T lymphocytes, but hemorrhage, reactive astrocytosis, and inflammatory cells were observed around the tumor cells.24 In patients with pleural or pericardial metastasis, body fluid analysis before and after treatment may be helpful in differentiating pseudoprogression, and further studies are needed in the future.
In most cancers, tumor‐associated macrophages (TAMs) have been identified as a major component of inflammatory infiltrated cells.25 TAMs are also a major component of malignant pleural effusion, which is associated with cancer progression.26 When analyzed for malignant pleural effusion in patients with lung cancer, the proportion of PD‐1+, Tim‐3+, and CTLA‐4+ cells in CD4 and CD8 T cells was higher than in paired peripheral blood.27 In other words, because PD‐1 expression is higher in malignant effusion than in peripheral blood, the use of PD‐1/PD‐L1 inhibitors may induce excessive immune reactions, resulting in an increased amount of effusion. This may lead to more frequent immune‐related side effects in patients with malignant pleural effusion, but few have been identified.
A limitation of this study is the small number of patients, which was not sufficient to allow us to generalize the results. In addition, it is difficult to explain the mechanism of low efficacy and greater AEs in patients with pleural or pericardial metastasis treated with an immune checkpoint inhibitor. Some patients with pleural or pericardial metastasis experienced disease improvement without side effects. Investigation into the clinicopathologic factors that differ depending on the presence or absence of response or side effects in patients with pleural or pericardial metastasis is needed, and larger patient samples to reach statistical significance. Analyzing T cells and inflammatory cytokines in fluid samples such as pleural effusion, pericardial effusion, or ascites before and after treatment with immune checkpoint inhibitors in patients with pleural or pericardial metastasis may be helpful to understand these mechanisms, and additional research in this area is needed.
In conclusion, patients with pleural or pericardial metastasis receiving immunotherapy have a significantly poorer prognosis, with low efficacy and more common and serious AEs. Clinicians should pay attention to the use of immune checkpoint inhibitors in lung cancer patients with pleural or pericardial metastasis.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF‐2018R1D1A1B07050870).
References
- 1. Leighl N. Treatment paradigms for patients with metastatic non‐small‐cell lung cancer: First‐, second‐, and third‐line. Curr Oncol 2012; 19: S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anagnostou VK, Brahmer JR. Cancer immunotherapy: A future paradigm shift in the treatment of non–small cell lung cancer. Clin Cancer Res 2015; 21: 976–84. [DOI] [PubMed] [Google Scholar]
- 4. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD‐1 signaling pathway. J Biomed Sci 2017; 24: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non–small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horn L, Spigel DR, Vokes EE et al Nivolumab versus docetaxel in previously treated patients with advanced non–small‐cell lung cancer: Two‐year outcomes from two randomized, open‐label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017; 2017: 2074–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbst RS, Baas P, Kim D‐W et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 10. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fehrenbacher L, Spira A, Ballinger M et al Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–46. [DOI] [PubMed] [Google Scholar]
- 12. Brahmer JR, Kim ES, Zhang J et al KEYNOTE‐024: Phase III trial of pembrolizumab (MK‐3475) vs platinum‐based chemotherapy as first‐line therapy for patients with metastatic non‐small cell lung cancer (NSCLC) that expresses programmed cell death ligand 1 (PD‐L1). J Clin Oncol 2015; 33 (15 Suppl 1): Abstract TPS8103. [Google Scholar]
- 13. Jiang T, Liu H, Qiao M et al Impact of clinicopathologic features on the efficacy of PD‐1/PD‐L1 inhibitors in patients with previously treated non–small‐cell lung cancer. Clin Lung Cancer 2018; 19: e177–84. [DOI] [PubMed] [Google Scholar]
- 14. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor‐based immunotherapy. Lancet Oncol 2016; 17: e542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non–small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 16. Winquist E, Kuruvilla S, Nichols AC et al Early mortality with immune checkpoint inhibitors (IOs) in solid tumors: An inconvenient truth? J Clin Oncol 2018; 36 (Suppl): Abstract 12121. [Google Scholar]
- 17. Michot J, Bigenwald C, Champiat S et al Immune‐related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 2016; 54: 139–48. [DOI] [PubMed] [Google Scholar]
- 18. Schaffler GJ, Wolf G, Schoellnast H et al Non–small cell lung cancer: Evaluation of pleural abnormalities on CT scans with 18F FDG PET. Radiology 2004; 231: 858–65. [DOI] [PubMed] [Google Scholar]
- 19. Gupta NC, Rogers JS, Graeber GM et al Clinical role of F‐18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest 2002; 122: 1918–24. [DOI] [PubMed] [Google Scholar]
- 20. Fujimoto D, Yoshioka H, Kataoka Y et al Efficacy and safety of nivolumab in previously treated patients with non‐small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer 2018; 119: 14–20. [DOI] [PubMed] [Google Scholar]
- 21. Kolla BC, Patel MR. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy‐ a report of two cases. J Immunother Cancer 2016; 4: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurra V, Sullivan RJ, Gainor JF et al Pseudoprogression in cancer immunotherapy: Rates, time course and patient outcomes. J Clin Oncol 2016; 34 (15 Suppl): Abstract 6580. [Google Scholar]
- 23. Liu G, Chen T, Li R, Zhu L, Liu D, Ding Z. Well‐controlled pleural effusion indicated pseudoprogression after immunotherapy in lung cancer: A case report. Thorac Cancer 2018; 9: 1190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen JV, Alomari AK, Vortmeyer AO et al Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res 2016; 4: 179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coffelt SB, Hughes R, Lewis CE. Tumor‐associated macrophages: Effectors of angiogenesis and tumor progression. Biochim Biophys Acta 2009; 1796: 11–8. [DOI] [PubMed] [Google Scholar]
- 26. Kaczmarek M, Nowicka A, Kozłowska M, Żurawski J, Batura‐Gabryel H, Sikora J. Evaluation of the phenotype pattern of macrophages isolated from malignant and non‐malignant pleural effusions. Tumor Biol 2011; 32: 1123–32. [DOI] [PubMed] [Google Scholar]
- 27. Li L, Yang L, Wang L et al Impaired T cell function in malignant pleural effusion is caused by TGF‐beta derived predominantly from macrophages. Int J Cancer 2016; 139: 2261–9. [DOI] [PubMed] [Google Scholar]


