Abstract
Ganoderma is a significant source of natural fungal medicines and has been used for the treatment of various diseases for many years. However, the use of Ganoderma in cancer immunotherapy is poorly elucidated. In this study, we have analyzed 2,398 English-language papers and 6,968 Chinese-language papers published between 1987 and 2017 by using bibliometrics. A steady growth in the number of publications was observed before 2004, followed by an exponential increase between 2004 and 2017. The most common category for publications about Ganoderma was “Pharmacology & Pharmacy,” in which immunomodulation (25.60%) and cancer treatment (21.40%) were the most popular subcategories. Moreover, we have provided an overview of the bioactive components and combinatorial immunomodulatory effects for the use of Ganoderma in the treatment of cancer, including the major pathways of immune cells. Immunomodulatory protein and polysaccharides are the key bioactive factors responsible for cancer immunotherapy, and the NF-κB and MAPK pathways are the most comprehensively investigated major pathways. Our results indicate that Ganoderma has a broad-spectrum application for the treatment of cancer through the regulation of the immune system. This review provides guidance for future research into the role of Ganoderma in cancer immunotherapy.
Keywords: Ganoderma, lingzhi, bibliometrics, cancer immunotherapy, mechanism
Introduction
Ganoderma, also called Lingzhi, is one of the most well-known medicinal species. Regarded as the “marvelous herb,” it is used widely in China, America, Japan, Korea, and other countries (Meng et al., 2011). According to traditional Chinese medicine (TCM) theory, Ganoderma has the ability to enhance body resistance, i.e., “Fuzheng Guben” (Yue et al., 2006). “Channel tropism” (Gui-Jing) links the functions of herbal drugs to their corresponding internal organs, channels, and various body parts to allow the interpretation of their functional mechanisms. The channel tropism of Ganoderma is the heart, lung, and liver, according to Gui-Jing theory. The main Ganoderma species are G. lucidum, G. sinensis, G. applanatum, G. tsugae, G. atrum, and G. formosanum. G. lucidum and G. sinensis are recorded in ChP2015 (Pharmacopeia of the People's Republic of China), and G. lucidum is recorded in USP40-NF35 (U.S. Pharmacopeia/National Formulary; Gao et al., 2004). The production of Ganoderma occurs mainly through artificial cultivation, which has provided an abundance of materials for the market; the yield has already surpassed that of wild Ganoderma (Chen et al., 2017). Methods used for Ganoderma identification include microscopy, TLC, spectroscopy, chromatography, chemical fingerprinting, and DNA sequencing. DNA sequencing has recently been used for classification of different Ganoderma species, with HPLC, UPLC, LC-Q-TOF-MS, HPTLC, and GC-MS have been commonly applied for quality evaluation (Toh Choon et al., 2012; Hennicke et al., 2016). Ganoderma has been used for the clinical treatment of chronic bronchitis, bronchial asthma, leukopenia, coronary heart disease, arrhythmia, and acute infectious hepatitis. However, at present, it does not have the potential to be used as first-line therapy, but only as an addition to conventional therapy in a clinical setting (Gao and Zhou, 2003; Unlu et al., 2016).
Chemical drugs for cancer treatment, such as cisplatin and cyclophosphamide, can cause side effects, such as nephrotoxicity, which are detrimental to the quality of life of patients (Aguirre-Moreno et al., 2013). In addition to this toxicity, the resistance of some cancer cells to treatment has led to the need for the evaluation of alternative approaches. Hence, chemotherapy does not completely meet the treatment need and immunotherapy is a promising alternative method as it results in fewer side effects. The use of cancer immunotherapy has gained acceptance because immune cells play notable roles in the control of cancer (Blattman and Greenberg, 2004). Immune cells can identify cancer cells as dangerous and consequently attack them; thus, the use of cancer vaccines to treat growing tumors is considered an excellent therapeutic strategy (Rosenberg et al., 2004). Herbal medicines have also been examined in clinical trials for cancer immunotherapy. Shing et al. found that a 6 months treatment using G. lucidum increased the mitogen-induced lympho-proliferative responses in immunocompromised children with tumors (Shing et al., 2008).
Bibliometrics is a method of document analysis that can count and analyze a large number of articles and monitor the trends in research (Kim and Park, 2011). Previous studies have reviewed the anticancer and/or immunomodulatory effects of G. lucidum and their potential immunological mechanisms (Lin and Zhang, 2004; Xu et al., 2011). However, the bioactive substances and corresponding immunoregulatory effects of Ganoderma in the treatment of cancer have not yet been investigated. Therefore, we have provided an overview of the research trend on Ganoderma determined from bibliometrics and reviewed its bioactive components and combinatorial immunomodulatory effects for use as a cancer treatment. We have also summarized the major diseases and pathways involved, clinical studies, and preliminary assessments of toxicity.
Literature analysis
Bibliometrics is defined as the application of statistics and mathematics to analyze bibliographical metadata linked to scholarly publications. Bibliometrics uses a literature system and literature metrology characteristics as research objects to quantitatively and qualitatively analyze the studies. Bibliometrics can be used to monitor the trends in the scientific development of a research domain; it can be used to analyze the trends and provide a comprehensive perspective on a topic. Therefore, we analyzed a specific question from the review of published literature by using current software programs (Aggarwal et al., 2016). Using professional bibliometrics software, such as CiteSpaceV (Chen et al., 2014) and RAWGraphs, we performed a bibliometric analysis of the publications on Ganoderma between 1987 and 2017 from the Web of Science (WoS), PubMed, and CNKI databases, which were the most suitable databases for this type of evaluation. We found 2,205 articles in WoS and 1,368 articles in PubMed with “Ganoderma,” “Lingzhi,” or “Reishi” as the key words. After removal of the duplicates, a total of 2,398 English-language articles (included in the Science Citation Index) were retrieved. We also found 6,968 Chinese-language articles on CNKI with the Chinese word for “Lingzhi” as the key word. We analyzed publication counts, cooperation between countries, and research categories. We found that immunomodulation and antitumor research were the most popular research subcategories; subsequently, from examination of the relevant literature, the topic of this review was determined to be cancer immunotherapy.
Publication counts
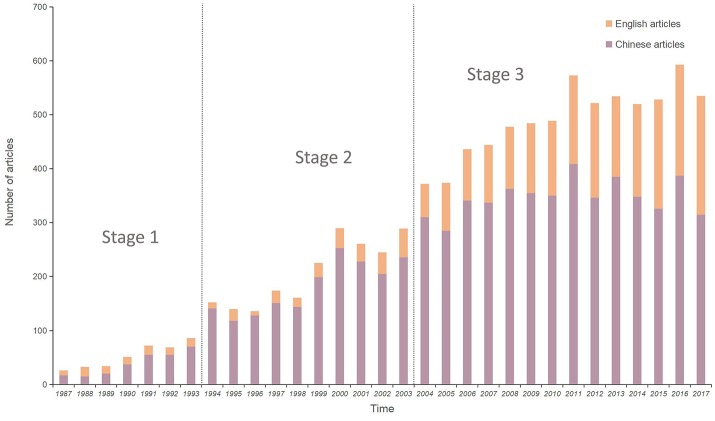
The publication counts for each year from 1987 to 2017 are shown in Figure 1. From on the number of publications, This 30 years period was preliminarily divided into three stages: Stage 1, from 1987 to 1993, was considered as the budding period, when <100 papers were published annually; Stage 2, from 1994 to 2003, was known as the development period, when the number of annual publications increased linearly from 100 to 300; Stage 3, from 2004 to 2017, was the “boom period,” when the annual number of papers increased rapidly; in particular, the number of English-language papers doubled annual. Research interest into Ganoderma widened over the years examined; moreover, the number of English-language studies has recently increased rapidly, revealing the potential research value of Ganoderma.
Figure 1.
Statistical analysis for published articles of genus Ganoderma.
Cooperation between countries
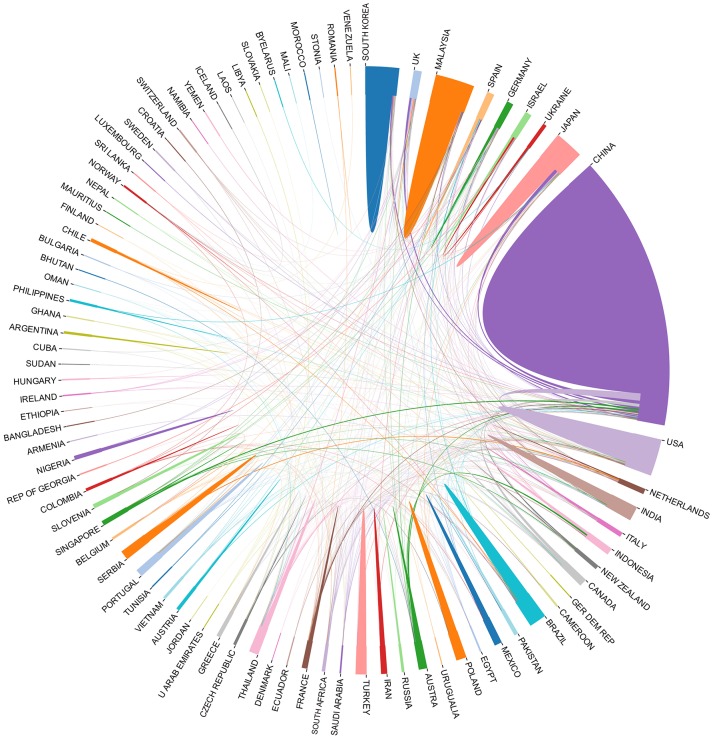
The relationships between many countries with active Ganoderma researchers, based on their publications included in the Science Citation Index, are illustrated in Figure 2. In total, 84 countries were involved in the study of Ganoderma. China, the United States, Malaysia, Japan, and South Korea have the highest output and the most extensive cooperation was found among these countries.
Figure 2.
Statistical analysis for relationship among countries for Ganoderma research. Different countries are represented by different colors, and the size represents the number of publications.
Subject categories and major historical developments
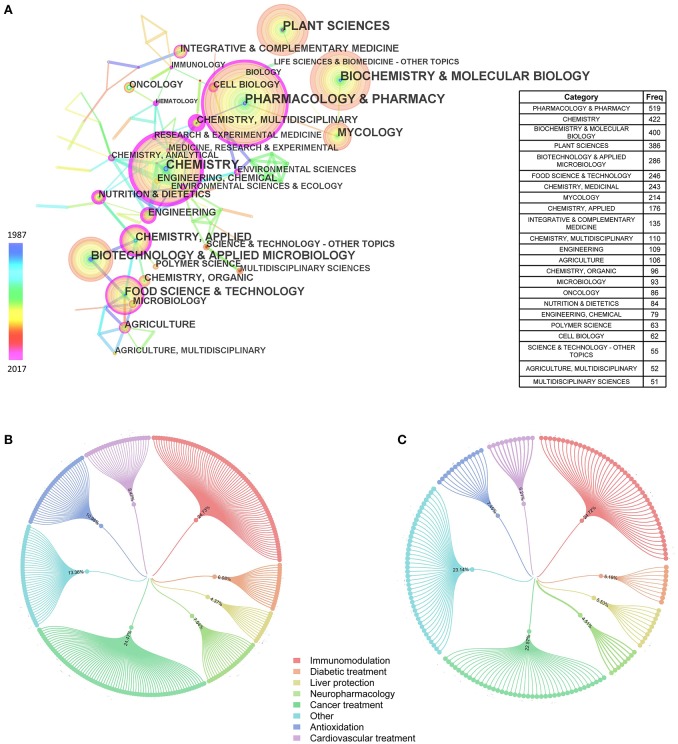
The categories of articles about Ganoderma that were included in the Science Citation Index are shown in Figure 3A. After the software analysis, we have displayed only subjects with a frequency of 50 or more. The most abundant category, “Pharmacology & Pharmacy,” had a frequency of 519, followed by the categories of “Chemistry” (422) and “Biochemistry & Molecular Biology” (400). From further reading, we found 1,512 Chinese-language articles and 880 English-language articles included in the Science Citation Index that described the pharmacological effects of Ganoderma. These pharmacological effects were subdivided into several specific effects (Figures 3B,C), such as immunomodulation, cancer treatment, antioxidation, cardiovascular treatment, diabetic treatment, liver protection, and neuropharmacology. The immunomodulation effect-related studies occupied the largest proportion of the eight areas of pharmacology, followed by cancer treatment, both in Chinese-language articles (24.73 and 24.47%, respectively) and in English-language articles (24.72 and 22.57%, respectively). Furthermore, in English-language articles, the number of citations was 17,692 and the average citation per item, which is the average number of articles cited for all items in the results set, was 20.43.
Figure 3.
Analysis for subject categories of Ganoderma. (A) Subjects of 50 frequencies or more (included in Science Citation Index). Nodes represent objects analyzed. And the larger nodes, the more frequently they occur. The connections among nodes represent the cooperative relationships. The thicker the connections, the closer they consociate. (B) Classification of pharmacological effects in Chinese articles (C) Classification of pharmacological effects in English articles.
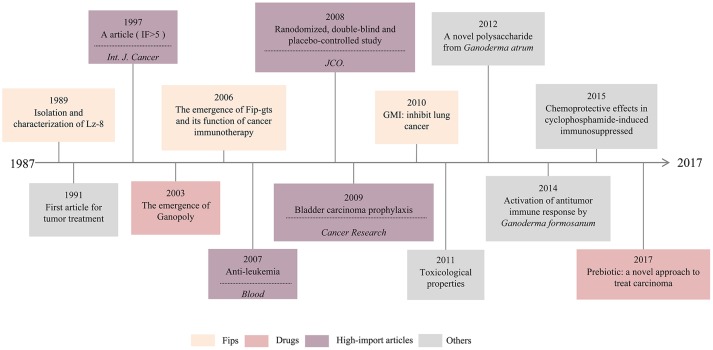
Further analysis of the English-language articles led to the identification of a total of 196 articles related to cancer immunotherapy. The timeline of major historical developments that are related to Ganoderma in cancer immunotherapy is shown in Figure 4. We found three types of fungal immunomodulatory proteins (Fips) that played important roles; Lz-8 was the first of these discovered. Moreover, the first study of the effect of Ganoderma on the inhibition of tumor growth occurred as early as 1991. In 2003, Ganopoly appeared as a new drug, and has since been used widely in clinical practice. Furthermore, the toxicology and immunology of Ganoderma were partly addressed in 2011 and its chemoprotective effects against cyclophosphamide-induced immunosuppression were studied in 2015. In addition, prebiotics were investigated as a novel approach for the treatment of carcinoma in 2017. Cancer immunotherapy has emerged one of the most popular fields of Ganoderma research. Hence, we have focused on the immunomodulatory effects of this genus and its constituent active components for use in cancer treatment.
Figure 4.
Timeline of major historical developments of Ganoderma on cancer treatment.
Immunomodulatory effects of ganoderma and its active components on cancer treatment
Many pharmacological and clinical studies have shown that Ganoderma can play an antitumor role through the regulation of the immune system (Boh et al., 2007). The therapeutic effects of Ganoderma are attributed to fungal immunomodulation proteins (FIPs), polysaccharides, and triterpenoids. Furthermore, we have specifically summarized active components of Ganoderma and their corresponding pharmacological effects.
Fungal immunomodulation proteins
FIPs are small molecular proteins purified from various fungi, such as Ganoderma. These proteins are functional families of Ganoderma components with anticancer effects (Table 1). Four types of immunoregulatory proteins, Lingzhi-8 (Lz-8), Fip-gts, GMI, and Fip-gat, have been isolated and purified from Ganoderma.
Table 1.
Pharmacological effects of immunomodulatory proteins of Ganoderma.
| Source | Protein | Cell lines/Mice | Optimum treatment concentration/dose | Duration | Pharmacological effect(s) | References |
|---|---|---|---|---|---|---|
| G. lucidum | (r)Lz-8 | A549, CL1-5, H226, LLC1 cells, C57BL/6 mice | 5 μg/ml | 12 h, 4 weeks | Induced changes in epithelial to mesenchymal transition by interfering with cell and focal adhesion kinase (FAK) functions in lung cancer cells. | Lin and Hsu, 2016 |
| SGC-7901 cell | 0.5 μg/ml | 24 h | Induced endoplasmic reticulum stress-mediated autophagic cell death. | Liang et al., 2012 | ||
| Human primary and Jurkat T cells | 1 μg/ml | 24 h | Induced IL-2 gene expression via the Src-family protein tyrosine kinase. | Hsu et al., 2008 | ||
| LLC1 cell, C57BL/6 mice | 10 μg/ml, 7.5 mg/kg | 48 h, 18 days | Inhibited growth and induced apoptosis of lung cancer cells by promoting epidermal growth factor receptor (EGFR) degradation. | Lin et al., 2017 | ||
| MBT-2 cell, C57BL/6, C3H/HeN, C3H/HeJ mice | 10 μg/ml | 90 days | Improved the therapeutic effect of DNA vaccine against MBT-2 tumor in mice. | Lin et al., 2011 | ||
| G. tsugae | (r)Fip-gts | HeLa, SiHa, and Caski cells | 0.15 μM | 24 h | Suppressed cervical cancer cell migration and enhanced the inhibition of FIP-gts upon migration. | Wang P. H. et al., 2007 |
| A549, MRC-5 cells | 8 μg/ml | 48 h | Regulated telomerase in A549 cells. | Liao et al., 2006 | ||
| A549, H1299, A549-p53, H1299-p53 stable cells | 1.2 μM | 48 h | Induced suppression of telomerase activity in lung cancer cells by post-translational modifications of hTERT protein | Liao et al., 2007 | ||
| A549, CaLu-1 cells, nude mice | 1.2 μM, 12.8 mg/kg | 48 h, 33 days | Inhibited A549 cell growth. A549 cells treated with reFIP-gts grew slower than cells treated with PBS alone in vivo. | Liao et al., 2008 | ||
| G. microsporum | GMI | A549, CaLu-1 cells, nude mice | 1.2 μM, 160 μg/mouse | 48 h, 66 days | Induced lung cancer cell death by activating autophagy, but did not induce apoptotic cell death. | Hsin et al., 2011 |
| A549, CCL-185 cells | 8 μg/mg | 24 h | Exhibited an inhibitory effect on EGF-induced migration and invasion. | Lin et al., 2010 | ||
| A549, CaLu-1 cells | 1.2 μM | 48 h | Inhibited lysosome degradation on autophagosome formation | Hsin et al., 2012 | ||
| A549, CaLu-1 cells | 1.2 μM (GMI) + 5 μM (Cisplatin) | 48 h | Induced apoptosis via autophagy and might be a potential cisplatin adjuvant against lung cancer. | Hsin et al., 2015 | ||
| G. atrum | (r)Fip-gat | MDA-MB-231 cell | 9.96 μg/ml | 48 h | Triggered significant cell cycle arrest at the G1/S transition and pronounced increase in apoptotic cell population. | Xu et al., 2016 |
Lz-8, an immunomodulatory protein from G. lucidum, was first isolated and cloned in 1989. Primarily composed of 110 amino acids, Lz-8 has an immunoglobulin-like structure that forms non-covalently linked homodimers with biological activity (Kino et al., 1989). Lz-8 exerted significant therapeutic effects on gastric cancer and specific lung cancers. Liang et al. found that recombinant Lz-8 (rLz-8) induced autophagic cell death through aggregation in the endoplasmic reticulum (ER), which triggered ER stress and the ATF4-CHOP pathway in SGC-7901 human gastric cancer cells (Liang et al., 2012). Moreover, rLz-8 might be a useful chemotherapeutic agent for the treatment of lung cancer because owing the key role of FAK targets in metastasis (Lin and Hsu, 2016). In addition, Lin et al. reported a novel anticancer effect of rLz-8 through targeting EGFR mutation or overexpression and EGFR-dependent processes in lung cancer cells (Lin et al., 2017).
Fip-gts is an immunomodulatory protein purified from G. tsugae. The DNA encoding this protein was isolated from a cDNA library by using a reverse transcriptase-polymerase chain reaction (Lin et al., 1997). The recombinant FIP-gts (rFip-gts) suppressed telomerase activity in a dose-dependent manner through the downregulation of the telomerase catalytic subunit (Liao et al., 2006). RFip-gts inhibited telomerase activity in lung cancer cells in vitro through effects on nuclear export mechanisms, which may have been mediated by the ER stress-induced intracellular calcium level (Liao et al., 2007). In vivo studies showed that the growth of A549 cells in nude mice treated with rFIP-gts was significantly slower than those treated with PBS, which confirmed that lung tumor growth could be inhibited by rFIP-gts (Liao et al., 2008). Moreover, this protein was also shown to affect cervical cancer cells.
GMI is an immunomodulatory protein cloned from G. microsporum. The amino acid sequence of this protein shared 83% homology with that of FIP-gts (Chiu et al., 2015). In vitro studies found that GMI inhibited the EGF-induced phosphorylation and activation of EGFR and AKT pathway kinases in a dose-dependent manner (Lin et al., 2010). Hsin et al. found that autophagosomal accumulation induced autophagic cell death in a model of GMI treatment, and ATP6V0A1, a subunit of vesicular H+-ATPases, regulated autophagosome lysosome fusion. Hsin et al. also revealed that GMI and cisplatin induced apoptosis via autophagy/caspase-7-dependent and survivin- and ERCC1-independent pathways (Hsin et al., 2012). In vivo studies suggested that the oral administration of GMI inhibited tumor growth and induced autophagy in nude mice that were administered a subcutaneous injection of A549 cells (Hsin et al., 2011).
Fip-gat is an immunomodulatory protein from G. atrum containing 111 amino acids. Xu et al. treated MDA-MB-231 cells with different concentrations of recombinant Fip-gat in vitro and found that this protein reduced cell viability in a dose-dependent manner (Xu et al., 2016). Treatment with FIP-gat triggered a significant degree of cell cycle arrest in the G1/S transition and a pronounced increase in the apoptotic cell population.
Polysaccharides and other active components
Polysaccharides (Meng et al., 2014) and other active components of Ganoderma also play key roles in its use for cancer treatment owing to their immunomodulatory effects (Table 2). Their effects are described below relative to different diseases.
Table 2.
Pharmacological effects of other bioactive components than proteins of Ganoderma.
| Source | Components | Cell lines/mice | Optimum treatment concentration/Dose | Duration | Pharmacological effect(s) | References |
|---|---|---|---|---|---|---|
| G. lucidum | Water extract | γ-ray-irradiated mice | 400 mg/kg | 35 days | Enhanced the recovery of cellular immuncompetence from γ-ray-irradiation. | Chen and Hau, 1995 |
| RAW 264.7 cell | 100 μg/ml | 24 h | Inhibited LPS-induced NO production in RAW 264.7 macrophages. | Song et al., 2004 | ||
| NK92, pNK, K562 cells | 5% effector/target ratio | 24 h | Induced NK cell cytotoxicity against various cancer cell lines by activating NKG2D/NCR receptors and MAPK signaling pathways. | Chang et al., 2014 | ||
| Ethanolic extract | MDA-MB 231, B16-F10 cells | 250 μg/ml | 48 h | Decreased the viability of both cancer cells in a time- and concentration-dependent manner. | Barbieri et al., 2017 | |
| Polysaccharide | HL-60 and U937 cells | 100 μg/ml | 5 days | Increased IL-1 and IL-6 and might play an indirect role in potentiating anti- tumor immunity in vitro. | Wang et al., 1997 | |
| C57BL/6j, BALB/c mice | 12.8 mg/L | 5 days | Promoted the cytotoxicity of specific cytotoxic T-lymphocytes induced by dendritic cells (DC), which were pulsed with P815 tumor antigen during the stage of antigen presentation. | Cao and Lin, 2003 | ||
| LAK cells, C57BL/6j mice | 400 or 100 mg/L | 8 days | Mediated the anti-tumor activity through complement receptor type 3. | Zhu and Lin, 2005 | ||
| L929, P815, YAC-1 cells, C57BL/6 mice | 400 or 100 mg/L | 15 days | Promoted cytokine-induced killer (CIK) cell proliferation and cytotoxicity were relevant to enhancing IL-2, TNF production. | Zhu and Lin, 2006 | ||
| S180, Heps, EAC cells, ICR species mice | 300 mg/kg | 8 days | Inhibited the growth of inoculated S180, Heps, and EAC tumor cells in mice. | Pang et al., 2007 | ||
| S180 cell, BALB/c mice | 200 mg/kg | 14 days | Activated the immune response of the host organism by the stimulation of NK cells, T cells, and macrophages. | Wang et al., 2012 | ||
| rats of Wistar strain | 2.6 mg/ml | 48 h | Enhanced the antioxidant enzyme activities, and reduced levels of IL-1b, IL-6, and TNF-α in rats with cervical cancer. | Chen et al., 2009 | ||
| B16F10 cell, C57BL/6 and BABL/c mice | 12.8 μg/ml | 72 h | Had antagonistic effects on the immunosuppression induced by B16F10 culture supernatant. | Sun et al., 2011a | ||
| B16F10 cell, BALB/c mice | 400 μg/ml | 5 days | Suppressed lymphocyte proliferation and perforin and granzyme B production in lymphocytes after induction with phytohemagglutinin. | Sun et al., 2011b | ||
| B16F10 cell | 400 μg/ml | 48 h, 21 days | Enhanced major histocompatibility complex (MHC) class I, more efficient immune cell-mediated cytotoxicity against these B16F10 cells might be induced. | Sun et al., 2012 | ||
| B16, A375 cells, C57Bl/6J mice | 400 μg/ml | 21 days | Inhibited the adhesion of fibrinogen to melanoma cells and reversed the blocking effect of the fibrin coat on NK cytotoxicity against melanoma cells. | Zheng et al., 2012 | ||
| HepG2 cell | Unknown | Unknown | Inhibited HepG2 cells directly through regulation of hepato-carcinoma genes. | Shen et al., 2014 | ||
| Lymphocytes of cancer patients | 12.8 μg/ml | 48 h | Antagonized lung cancer patient plasma-induced suppression of lymphocyte activation by phytohemagglutinin. | Sun et al., 2014 | ||
| H22 cell, Kunming, BALB/c male mice | 200 mg/kg | 4 weeks | Inhibited hepatocellular carcinoma through miR-125b inhibiting regulatory T cell (Treg) accumulation and function. | Li A. M. et al., 2015 | ||
| β-glucan | Neutrophils | 100 μg/ml | 24 h | Induced anti-apoptotic effects on neutrophils relying on activation of Akt-regulated signaling pathways. | Hsu et al., 2002 | |
| 10 μg/ml | 24 h | Promoted the activation and maturation of immature DC. | Lin et al., 2005 | |||
| THP-1, U937 cells | 100 μg/ml | 72 h | Induced selected monocytic leukemic cell differentiation into DCs with immuno-stimulatory function. | Chan et al., 2007 | ||
| A fucose-containing glycoprotein | Con A-stimulated mouse spleen cells | 0.01–0.1 μg/ml | 72 h | Stimulated the expression of cytokines, especially IL-1, IL-2, and INF-g. | Wang et al., 2002 | |
| F3 | BALB/c mice spleen cells | 100 μg/ml | 48 h | Activated the expression of IL-1, IL-6, IL-12, IFN-c, TNF-a, GM-CSF, G-CSF, and M-CSF. | Chen et al., 2004 | |
| L-fucose (FMS) | LLC1 cell, C57BL/6J mice | 240 mg/kg | 28 days | Induced antibodies against murine Lewis lung carcinoma cells, with increased antibody-mediated cytotoxicity and reduced production of tumor-associated inflammatory mediators. | Liao et al., 2013 | |
| Proteoglycan | Lymphocytes from BALB/c mice spleens | 500 μg/ml | 72 h | Activated B cells and expressed CD71 and CD25 on the cell surface. Enhanced the expression of protein kinase C α and protein kinase C γ in B cells. | Zhang et al., 2002 | |
| Triterpenes | A549 cell, C57BL/6 mice | 120 mg/kg | 14 days | Had anti-lung cancer activity in vitro and in vivo via enhancement of immunomodulation and induction of cell apoptosis. | Feng et al., 2013 | |
| Ganoderic acid Me | YAC-1, LLC cells, C57BL/6 mice | 28 mg/kg | 20 days | Up-regulated expression of Nuclear Factor-κB after the treatment of GA-Me, which might be involved in the production of IL-2. | Wang G. et al., 2007 | |
| 2LL cells, C57BL/6 mice | 10 μg/ml | 48 h | Induced the apoptosis of competent T cells and increased the proportion of Treg cells | Que et al., 2014 | ||
| G. sinensis | Lipid extract | U937, HepG2 cells | 12.8 μg/ml | 72 h | Re-establish the antitumor activity of the immunosuppressive tumor-associated macrophages. | Sun et al., 2011a |
| G. applanatum | Polysaccharide | S180 Transplanted Mice | 20 mg/kg | 10 days | Restored the NK activity and the IL-2 and IFNy production of the spleen cells, which were suppressed by the tumor. | Gao and Yang, 1991 |
| Exo-biopolymer (EXP) | S180 cell, BALB/c mice. | 80 mg/kg | 16 days | Inhibited the growth of solid tumor and increased the natural killer (NK) cell activity. | Jeong et al., 2008 | |
| unknown | Breast cancer cells | Unknown | Unknown | Stimulated macrophages in immunosuppressive breast cancer microenvironment. | Javed et al., 2016 | |
| G. tsugae | mycelium extracts | C3H/HeN mice | 50 mg/kg | 10 days | Elevated the splenic NK activity and serum IFN titers. | Won et al., 1992 |
| G. atrum | Polysaccharide | S180 cell, Kunming mice | 100 mg/kg | 18 days | Induced anti-tumor activity via the mitochondrial apoptotic pathway related to activation of host immune response. | Li et al., 2011 |
| CT26 cell, BALB/c mice | 200 mg/kg | 14 days | Activated macrophages via TLR4-dependent signaling pathways, improved immunity, and inhibited tumor growth. | Zhang et al., 2013 | ||
| RAW264.7 cell, C3H/HeN, C3H/HeJ mice | 160 μg/ml | 48 h | Induced TNF-a secretion through TLR4/ROS/PI3K/Akt/MAPKs/NF-κB pathways during macrophage activation. | Yu et al., 2014 | ||
| CT26 cell, BALB/c mice | 200 mg/kg | 15 days | Exerted antitumor activity in vivo by inducing apoptosis via mitochondria-mediated apoptotic pathway and enhanced host immune system function. | Zhang et al., 2014 | ||
| 100 mg/kg | 18 days | Activated peritoneal macrophages and spleen lymphocytes in cyclophosphamide-treated mice. | Yu et al., 2015a | |||
| G. formosanum | PS-F2 | S180, B16, C26 cells C57BL/6, BALB/c mice | 50 mg/kg | 24 days | Activated host immune responses against ongoing tumor growth. | Wang et al., 2014 |
Lung cancer
Feng et al. evaluated the inhibitory effect of triterpenes of G. lucidum on cell proliferation and tumor growth. The IC50 of triterpenes on A549 cells was 24.63 μg/mL (Feng et al., 2013). Triterpenes could significantly inhibit tumor growth in Lewis tumor-bearing mice (30, 60, and 120 mg/kg), and the indices of immune organs, including the spleen and thymus, were increased remarkably by the treatment with triterpenes. Moreover, an in vitro study by Liao et al. found that the L-fucose (Fuc)-enriched Reishi polysaccharide fraction (FMS) could inhibit the growth of cancer cells through an increase in the antibody-mediated cytotoxicity and the reduction of the production of tumor-associated inflammatory mediators, particularly monocyte chemoattractant protein-1 (MCP-1). In vivo studies showed a significant increase in the peritoneal B1 B-cell population, suggesting the FMS-mediated anti-glycan IgM production (Liao et al., 2013). Sun et al. recently showed that the plasma of patients with lung cancer suppressed the proliferation, CD69 expression, and perforin and granzyme B production in lymphocytes upon activation by PHA (Sun et al., 2014). These effects were partially or fully reversed by G. lucidum polysaccharides (GLPS). Furthermore, Que et al. suggested that Ganoderic acid Me, a pure lanostane triterpene of G. lucidum contributing to the indoleamine 2,3-dioxygenase, helped create a tolerogenic milieu in lung tumors by directly inducing T cell apoptosis, inhibiting CD8+T cell activation, and enhancing Treg-mediated immunosuppression (Que et al., 2014).
Liver cancer
Zhang et al. indicated that, in addition to its direct tumoricidal activity, the lipid extract from G. sinensis spores could exert an anticancer effect through the stimulation of the activation of human macrophages/monocytes (Zhang et al., 2009). Furthermore, Shen et al. found that the anticancer mycelia of GLPS could be used to disclose the differential expression of miRNA in human hepatocarcinoma cells through the comprehensive investigation of miRNA expression in polysaccharide-treated cancer cells (Shen et al., 2014). Li et al. elaborated that GLPS significantly suppressed the tumor growth in hepatoma-bearing mice. This effect was associated with an increase in the ratio of the effector T cells (Teffs) to regulatory T cells (Tregs) (Li A. M. et al., 2015). Moreover, GLPS eliminated the Treg-induced suppression Teff proliferation through increased IL-2 secretion.
Melanoma
Sun et al. found that GLPS promoted B16F10 melanoma cells to induce lymphocyte proliferation, CD69 and FasL expression, and IFN-γ production. The authors also indicated that GLPS improved the ability of B16F10 cells to activate lymphocytes (Sun et al., 2011b). In addition, the culture supernatant of B16F10 melanoma cells (B16F10-CS) inhibited lymphocyte proliferation and the production of perforin and granzyme B in lymphocytes after induction with phytohemagglutinin and lymphocyte proliferation in the mixed lymphocyte reaction (Sun et al., 2011a). They also found that GLPS could enhance the activity of major histocompatibility complex (MHC) class I molecules and costimulatory molecules, and improve the efficiency of immune cell-mediated cytotoxicity against B16F10 cells (Sun et al., 2012). Barbieri et al. demonstrated that the ethanolic extracts of G. lucidum significantly inhibited the release of IL-8, IL-6, MMP-2, and MMP-9 in cancer cells under pro-inflammatory conditions (Barbieri et al., 2017). Wang et al. revealed that the continuous administration of the G. formosanum polysaccharide PS-F2 activated the host immune responses against ongoing tumor growth (Wang et al., 2011, 2014).
Leukemia
Wang et al. revealed that GLPS might play an indirect role in potentiating antitumor immunity in vivo through an increase in the levels of IL-1 and IL-6 (Wang et al., 1997). Lin et al. showed that GLPS promoted the cytotoxicity of specific cytotoxic T-lymphocytes (CTL) induced by dendritic cells (DCs) (Cao and Lin, 2003). These lymphocytes were pulsed with P815 tumor antigens during the stage of antigen presentation and the reported mechanisms of cytotoxicity involved the IFNγ and granzyme B pathways. In addition, the found that GLPS (400 or 100 mg/mL), which promoted CIK cell proliferation and cytotoxicity, enhanced IL-2 and TNF production, and the protein and mRNA expression of granzyme B and perforin in CIK cells through a synergistic interaction with cytokines, decreasing doses of IL-2 and anti-CD3 by 75 and 50%, respectively, which might be irrelevant to nitric oxide (NO) (Zhu and Lin, 2006). Moreover, Chan et al. suggested that GLPS could induce selected monocytic leukemic cell differentiation into DCs with immunostimulatory function (Chan et al., 2007). Chang et al. prepared a water extract of G. lucidum and examined its effect on natural killer (NK) cells; they observed that the treatment increased cytotoxicity in NK cells through the stimulation of perforin and granulysin secretion (Chang et al., 2014).
Colon cancer
Zhang et al. found that G. atrum polysaccharides could activate macrophages via TLR4-dependent signaling pathways, improve immunity, and inhibit tumor growth (Zhang et al., 2013). Wang et al. revealed that the continuous administration of G. formosanum polysaccharide PS-F2 activated the host immune responses against ongoing tumor growth (Wang et al., 2014). In addition, Yu et al. indicated that the chemoprotective effects of G. atrum polysaccharide might be attributable to its ability to activate peritoneal macrophages and spleen lymphocytes in cyclophosphamide-treated mice (Yu et al., 2015a).
Major pathways of cancer immunotherapy of ganoderma in immune cells
Dendritic cells and T-Lymphocytes
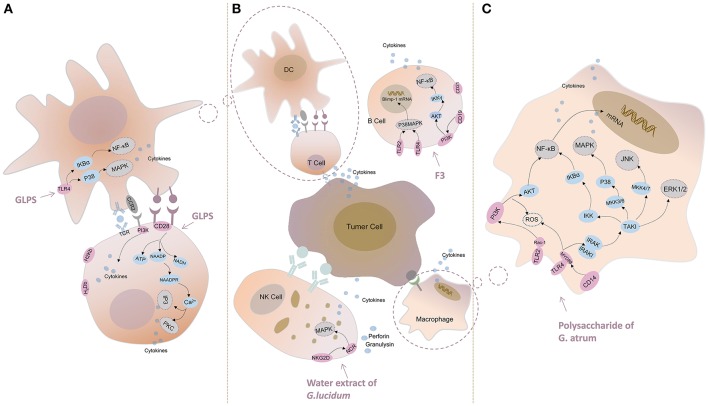
Toll-like receptor (TLR)-4 inhibited the GLPS-induced production of IL-12 and IL-10, which suggested a vital role in DC signaling after incubation with GLPS. Further studies showed that GLPS could augment the activity of κB (IκB) kinase and nuclear factor (NF)-κB inhibitors, as well as the phosphorylation of IκBα and p38 mitogen-activated protein kinase (MAPK) (Lin et al., 2005; Figure 5A).
Figure 5.
Major pathways of cancer immunotherapy of Ganoderma in immune cells. (A) GLPS induces NF-κB activation and p38 mitogen-activated protein kinase (MAPK) phosphorylation in DC. GLPS might activate T cells via inositol triphosphate/Ca2+ (IP3/Ca2+) and protein kinase C (PKC) pathways. (B) F3 induces the expression of Blimp-1mRNA through p38 MAPK pathway and mediates intracellular signal through NF-κB pathway in B cell. The water extract of G. lucidum activates NK cells by the mechanism of activating NKG2D/NCR receptors and MAPK signaling pathway. (C) The polysaccharide of G. atrum induced macrophage activation through MAPK (JNK, ERK1/2) and NF-κB signaling pathways.
Sun et al. revealed that GLPS enhanced the effect of H-2Kb and H-2Db, and B7-1 and B7-2 (two prominent MHC class I molecules in C57BL mice) on B16F10 cells and that the mRNAs of these molecules improved the efficiency of the antitumor cytotoxicity in GLPS-treated cells (Sun et al., 2012). Li et al. inferred that GLPS might activate T cells via the inositol triphosphate/Ca2+ (IP3/Ca2+) and protein kinase C (PKC) pathways, because the extracellular receptor was bound by GLPS (Li et al., 2013; Li X. L. et al., 2015; Figure 5A).
B lymphocytes and natural killer cells
Lin et al. showed that the interaction of F3 (the main polysaccharide fraction of G. lucidum) with TLR4/TLR2, followed by signaling through p38 MAPK, was involved in the induction of Blimp-1 mRNA (Figure 5B) and that the intracellular signal was mediated by the NF-κB pathway (Lin et al., 2006).
Chang et al. indicated that G. lucidum induced cytotoxicity in various cancer cell lines through the activation of the NKG2D/NCR receptors and MAPK signaling pathways, which ultimately culminated in the exocytosis of perforin and granulysin (Chang et al., 2014; Figure 5B).
Macrophage
Kuo et al. revealed that the dried mycelia of G. lucidum also induced NF-κB activation in murine RAW264.7 macrophages, which indicated that NF-κB activation was one of the most important signaling pathways (Kuo et al., 2006). Pro-inflammatory cytokines (TNF-α, IL-1β, or IFN-γ) were able to bind to their respective receptors and induce iNOS expression via the activation of NF-κB. Yu et al. indicated that the signaling mechanism might be that of G. atrum polysaccharide-induced macrophage activation through TLR4-mediated NF-κB and MAPK (p38, ERK1/2, and JNK) signaling pathways, thereby initiating the release of cytokines, such as TNF-α and IL-1β, and effector molecules, such as NO, in macrophages (Yu et al., 2015b). The results suggested that the polysaccharide of G. atrum exerted its antitumor activity through the improvement of immune system functions and acted as an antitumor agent with immunomodulatory activity (Figure 5C). Yu et al. concluded that the polysaccharide of G. atrum induced TNF-α secretion through the TLR4/ROS/PI3K/Akt/MAPKs/NF-κB pathways during macrophage activation (Yu et al., 2014). To investigate the possible signaling pathways involved in the activation of macrophages of S180 tumor-bearing mice by the polysaccharide of G. atrum, Huang et al. simulated macrophages and observed an increase in the phosphorylation of NF-κB, Akt, and MAPK family proteins, which was indicative of the activation of the NF-κB pathway (Huang et al., 2016). These findings further indicated the possible involvement of the NF-κB signaling pathway in TNF-α secretion and mRNA expression (Figure 5C).
Clinical studies
Selections of clinical studies are presented. In 2003, Gao et al. investigated the effects of Ganopoly on the immune function of 34 patients with advanced-stage cancer. They found that it enhanced the immune responses in patients with advanced-stage cancer through an increase in the number of CD3+ (and similar) cells (Gao et al., 2003). In 2008, Shing et al. found that a 6 months treatment G. lucidum increased the mitogen-induced lympho-proliferative responses in immune-compromised children with tumors (Shing et al., 2008). In 2012, a pilot study suggested that the spore powder of G. lucidum had beneficial effects on cancer-related fatigue and quality of life in 48 patients with breast cancer undergoing endocrine therapy, without any significant adverse effects. The experimental group made statistically significant improvements in the domains of physical well-being and the fatigue subscale after intervention (Zhao et al., 2012). In addition, a study of five patients with gynecological cancer showed that they achieved stability in the disease after the ingestion of Lingzhi in the form of fruit body water extract and spores (Suprasert et al., 2014). Some modest benefit was also found when the mushroom was administered with standard chemotherapy (Chen and Alpert, 2016).
Toxicology
The toxicology and immunology of Ganoderma have been partly investigated in current studies. Wanmuang et al. presented a case in which fatal fulminant hepatitis occurred after taking Lingzhi powder for 1–2 months (Wanmuang et al., 2007). In addition, a patient was diagnosed with non-Hodgkins lymphoma and presented with chronic watery diarrhea whilst taking Lingzhi (Suprasert et al., 2014). However, no abnormal clinical-symptoms or deaths and no significant difference in body weight and food intake rate was found in Wistar rats during the 30 days administration period (Cheng et al., 2008). No mutagenicity was observed, as indicated by negative results from the Ames test, micronucleus test of polychromatic erythrocyte, sperm abnormality test, and chromosome aberration test in Kunming mice (Zhang et al., 2016).
Disscusion
The present review provides the most up-to-date analysis of Ganoderma research over a 30-years period by using CiteSpaceV and RAW Graphs. We found that the number of studies have increased significantly over time, especially during Stage 3 (Figure 1). We inferred that chemical drugs may exhibit certain side effects. Hence, the medicinal capabilities of the Ganoderma fungi have been gradually elucidated. In addition, China, the United States, Malaysia, Japan, and South Korea are the world leaders in Ganoderma research, based on outputs and close cooperation among the 84 countries active in the research area (Figure 2). Remarkably, the output of China is ~20% of the total output, which gives it the highest output of these countries. Based on a large amount of data, we summarized the subject categories of the research and found that “Pharmacology & Pharmacy” is the leading category. In the subcategories within pharmacology, immunomodulatory effects and cancer treatment occupy the largest proportion of the eight areas of pharmacology in Chinese-language and English-language articles. These finding revealed a new trend, which was the use of Ganoderma in cancer immunotherapy research.
Cancer is a disease with a high death rate. Chemotherapy does not completely meet the needs for cancer treatment and immunotherapy is a promising alternative method owing to the fewer side effects observed. Ganoderma, a medicinal mushroom, could be administered as an adjunct to conventional treatment to enhance the tumor response and stimulate host immunity. At the species level, studies on G. lucidum predominate; other species are less well-studied. With regard to the effective components, FIPs and polysaccharides are dominant; of which Lz-8 and polysaccharides from G. lucidum are the most researched. Ganoderma also plays important roles in many aspects of immune regulation for cancer treatment, not only the activation of T or B lymphocytes, macrophages, NK cells, and other immune cells, but in the promotion of the in vitro proliferation of undifferentiated spleen cells, and the production of cytokines and antibodies. NF-κB and MAPK, the most comprehensively investigated major pathways, are shown to be activated and release cytokines that subsequently inhibit the growth of tumor cells. TLR-4 is an effective receptor involved in the host defense mechanism of the immune response to polysaccharides. In addition, some researchers have used Ganoderma in combination with drug treatments for cancer, such as the combination of GMI and cisplatin and the combination of G. atrum polysaccharides with cyclophosphamide to reduce the side effects of the drug. We found that the immunotherapy of lung cancer, liver cancer, melanoma, leukemia, and colon cancer were thoroughly studied in vivo and vitro, particularly lung and liver cancers. This observation was basically consistent with the channel tropism of Ganoderma in TCM theory. Moreover, this review has made a preliminary analysis of the safety of Ganoderma through the exploration of the reported toxicology. With regard to adverse effects, there were generally no serious side effects from the use of Lingzhi, but patients should be monitored while receiving Lingzhi, as liver toxicity and chronic watery diarrhea are reported side effects.
Ganoderma is one of the most widely used herbal fungi and is a promising anticancer immunotherapy agent owing to its low toxicology and efficacy as a combination therapy. However, the mechanistic pathways lack specificity and do not accurately select specific targets; in addition, most results are derived from in vitro studies. Future studies should focus on the combination therapies of Ganoderma and clinical chemotherapy drugs to alleviate the side effects of these drugs. Furthermore, the safety and toxicity should be thoroughly explored. The major bioactive components should to be investigated and corresponding in vivo pharmacokinetic studies should be performed. The mechanisms underlying immune modulation and interactions should be determined.
Author contributions
YC conducted and designed the review and wrote the MS. XX and SL contributed to the language editing. LH and JG conducted the designed the review.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding from the National Natural Science Foundation of China (No. 81473315) and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2016-12M-3-015) are gratefully acknowledged.
References
- Aggarwal A., Lewison G., Idir S., Peters M., Aldige C., Boerckel W., et al. (2016). The state of lung cancer research: a global analysis. J. Thorac. Oncol. 11, 1040–1050. 10.1016/j.jtho.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Aguirre-Moreno A., Villeda-Hernandez J., Campos-Pena V., Herrera-Ruiz M., Montiel E., Tello I., et al. (2013). Anticonvulsant and neuroprotective effects of oligosaccharides from lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Higher Basidiomycetes). Int. J. Med. Mushrooms 15, 555–568. 10.1615/IntJMedMushr.v15.i6.40 [DOI] [PubMed] [Google Scholar]
- Barbieri A., Quagliariello V., Del Vecchio V., Falco M., Luciano A., Amruthraj N. J., et al. (2017). Anticancer and anti-inflammatory properties of Ganoderma lucidum extract effects on melanoma and triple-negative breast cancer treatment. Nutrients 9:E210. 10.3390/nu9030210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman J. N., Greenberg P. D. (2004). Cancer immunotherapy: a treatment for the masses. Science 305, 200–205. 10.1126/science.1100369 [DOI] [PubMed] [Google Scholar]
- Boh B., Berovic M., Zhang J., Zhi-Bin L. (2007). Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 13, 265–301. 10.1016/S1387-2656(07)13010-6 [DOI] [PubMed] [Google Scholar]
- Cao L. Z., Lin Z. B. (2003). Regulatory effect of Ganoderma lucidum polysaccharides on cytotoxic T-lymphocytes induced by dendritic cells in vitro. Acta Pharmacol. Sin. 24, 321–326. [PubMed] [Google Scholar]
- Chan W. K., Cheung C., Law H. K. W., Lau Y. L., Chan G. C. (2007). Ganoderma lucidum polysaccharides can induce human monocytic leukemia cells into dendritic cells with immunotolerogenic function. Blood 1:9 10.1186/1756-8722-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. J., Chen Y. Y. M., Lu C. C., Lin C. S., Martel J., Tsai S. H., et al. (2014). Ganoderma lucidum stimulates NK cell cytotoxicity by inducing NKG2D/NCR activation and secretion of perforin and granulysin. Innate Immun. 20, 301–311. 10.1177/1753425913491789 [DOI] [PubMed] [Google Scholar]
- Chen B., Ke B., Ye L., Jin S., Jie F., Zhao L., et al. (2017). Isolation and varietal characterization of Ganoderma resinaceum from areas of Ganoderma lucidum production in China. Sci. Hortic. 224, 109–114. 10.1016/j.scienta.2017.06.002 [DOI] [Google Scholar]
- Chen C., Dubin R., Kim M. C. (2014). Emerging trends and new developments in regenerative medicine: a scientometric update (2000–2014). Expert Opin. Biol. Ther. 14, 1295–317. 10.1517/14712598.2014.920813 [DOI] [PubMed] [Google Scholar]
- Chen H. S., Tsai Y. F., Lin S., Lin C. C., Khoo K. H., Lin C. H., et al. (2004). Studies on the immuno-modulating and anti-tumor activities of Ganoderma lucidum (Reishi) polysaccharides. Bioorg. Med. Chem. 12, 5595–5601. 10.1016/j.bmc.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Chen Q. M., Alpert J. S. (2016). Nutraceuticals: evidence of benefit in clinical practice? Am. J. Med. 129, 897–898. 10.1016/j.amjmed.2016.03.036 [DOI] [PubMed] [Google Scholar]
- Chen W. C., Hau D. M. (1995). Effects of Ganoderma lucidum on cellular immunocompetence in gamma-irradiated mice. Phytother. Res. 9, 533–535. 10.1002/ptr.2650090716 [DOI] [PubMed] [Google Scholar]
- Chen X. P., Chen Y., Li S. B., Chen Y. G., Lan J. Y., Liu L. P. (2009). Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 77, 389–393. 10.1016/j.carbpol.2009.01.009 [DOI] [Google Scholar]
- Cheng P. C., Hsu C. Y., Chen C. C., Lee K. M. (2008). In vivo immunomodulatory effects of Antrodia camphorata polysaccharides in a T1/T2 doubly transgenic mouse model for inhibiting infection of Schistosoma mansoni. Toxicol. Appl. Pharmacol. 227, 291–298. 10.1016/j.taap.2007.10.023 [DOI] [PubMed] [Google Scholar]
- Chiu L. Y., Hu M. E., Yang T. Y., Hsin I. L., Ko J. L., Tsai K. J., et al. (2015). Immunomodulatory protein from Ganoderma microsporum induces pro-death autophagy through akt-mTOR-p70S6K pathway inhibition in multidrug resistant lung cancer cells. PLoS ONE 10:e0125774. 10.1371/journal.pone.0125774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Yuan L., Du M., Chen Y., Zhang M. H., Gu J. F., et al. (2013). Anti-lung cancer activity through enhancement of immunomodulation and induction of cell apoptosis of total triterpenes extracted from Ganoderma luncidum (Leyss. ex Fr.) Karst. Molecules 18, 9966–9981. 10.3390/molecules18089966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Yang G. Z. (1991). Effects of Ganoderma applanatum polysaccharide on cellular and humoral immunity in normal and sarcoma-180 transplanted mice. Phytother. Res. 5, 134–138. 10.1002/ptr.2650050310 [DOI] [Google Scholar]
- Gao Y., Chan E., Zhou S. (2004). Immunomodulating activities of Ganoderma, a mushroom with medicinal properties. Food Rev. Int. 20, 123–161. 10.1081/FRI-120037158 [DOI] [Google Scholar]
- Gao Y., Zhou S., Jiang W., Huang M., Dai X. (2003). Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol. Invest. 32, 201–215. 10.1081/IMM-120022979 [DOI] [PubMed] [Google Scholar]
- Gao Y. H., Zhou S. F. (2003). Cancer prevention and treatment by Ganoderma, a mushroom with medicinal properties. Food Rev. Int. 19, 275–325. 10.1081/FRI-120023480 [DOI] [Google Scholar]
- Hennicke F., Cheikh-Ali Z., Liebisch T., Macia-Vicente J. G., Bode H. B., Piepenbring M. (2016). Distinguishing commercially grown Ganoderma lucidum from Ganoderma lingzhi from Europe and East Asia on the basis of morphology, molecular phylogeny, and triterpenic acid profiles. Phytochemistry 127, 29–37. 10.1016/j.phytochem.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Hsin I. L., Ou C. C., Wu M. F., Jan M. S., Hsiao Y. M., Lin C. H., et al. (2015). GMI, an immunomodulatory protein from Ganoderma microsporum, potentiates cisplatin-induced apoptosis via autophagy in lung cancer cells. Mol. Pharm. 12, 1534–1543. 10.1021/mp500840z [DOI] [PubMed] [Google Scholar]
- Hsin I. L., Ou C. C., Wu T. C., Jan M. S., Wu M. F., Chiu L. Y., et al. (2011). GMI, an immunomodulatory protein from Ganoderma microsporum, induces autophagy in non-small cell lung cancer cells. Autophagy 7, 873–882. 10.4161/auto.7.8.15698 [DOI] [PubMed] [Google Scholar]
- Hsin I. L., Sheu G. T., Jan M. S., Sun H. L., Wu T. C., Chiu L. Y., et al. (2012). Inhibition of lysosome degradation on autophagosome formation and responses to GMI, an immunomodulatory protein from Ganoderma microsporum. Br. J. Pharmacol. 167, 1287–1300. 10.1111/j.1476-5381.2012.02073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. Y., Hua K. F., Wu W. C., Hsu J., Weng S. T., Lin T. L., et al. (2008). Reishi immuno-modulation protein induces interleukin-2 expression via protein kinase-dependent signaling pathways within human T cells. J. Cell. Physiol. 215, 15–26. 10.1002/jcp.21144 [DOI] [PubMed] [Google Scholar]
- Hsu M. J., Lee S. S., Lin W. W. (2002). Polysaccharide purified from Ganoderma lucidum inhibits spontaneous and Fas-mediated apoptosis in human neutrophils through activation of the phosphatidylinositol 3 kinase/Akt signaling pathway. J. Leukoc. Biol. 72, 207–216. 10.1189/jlb.72.1.207 [DOI] [PubMed] [Google Scholar]
- Huang J. Q., Nie Q. X., Liu X. Z., Zhang S. S., Nie S. P., Huang D. F., et al. (2016). Ganoderma atrum polysaccharide modulates TNF-alpha secretion and mRNA expression in macrophages of S-180 tumor-bearing mice. Food Hydrocoll. 53, 24–30. 10.1016/j.foodhyd.2014.12.035 [DOI] [Google Scholar]
- Javed S., Payne G. W., Lee C. H. (2016). Ganoderma applanatum–potential target for stimulating macrophages in immunosuppressive breast cancer microenvironment. Breast Cancer Res. Treat. 159, 181. [Google Scholar]
- Jeong Y. T., Yang B. K., Jeong S. C., Kim S. M., Song C. H. (2008). Ganoderma applanatum: a promising mushroom for antitumor and immunomodulating activity. Phytother. Res. 22, 614–619. 10.1002/ptr.2294 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Park J.H. (2011). Trends in Ginseng research in 2010. J. Ginseng Res. 35, 389–398. 10.5142/jgr.2011.35.4.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino K., Yamashita A., Yamaoka K., Watanabe J., Tanaka S., Ko K., et al. (1989). Isolation and characterization of a new immunomodulatorry protein, Ling Zhi-8 (LZ-8), from Ganoderma lucidum. J. Biol. Chem. 264, 472–478. [PubMed] [Google Scholar]
- Kuo M. C., Weng C. Y., Ha C. L., Wu M. J. (2006). Ganoderma lucidum mycelia enhance innate immunity by activating NF-kappa B. J. Ethnopharmacol. 103, 217–222. 10.1016/j.jep.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Li A. M., Shuai X. Y., Jia Z. J., Li H. Y., Liang X. B., Su D. M., et al. (2015). Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J. Transl. Med. 13:100. 10.1186/s12967-015-0465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Lee D. S., Kang Y., Yao N. Q., An R. B., Kim Y. C. (2013). Protective effect of ganodermanondiol isolated from the Lingzhi mushroom against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 53, 317–324. 10.1016/j.fct.2012.12.016 [DOI] [PubMed] [Google Scholar]
- Li W. J., Chen Y., Nie S. P., Xie M. Y., He M., Zhang S. S., et al. (2011). Ganoderma atrum Polysaccharide induces anti-tumor activity via the mitochondrial apoptotic pathway related to activation of host immune response. J. Cell. Biochem. 112, 860–871. 10.1002/jcb.22993 [DOI] [PubMed] [Google Scholar]
- Li X. L., He L. P., Yang Y., Liu F. J., Cao Y., Zuo J. J. (2015). Effects of extracellular polysaccharides of Ganoderma lucidum supplementation on the growth performance, blood profile, and meat quality in finisher pigs. Livest. Sci. 178, 187–194. 10.1016/j.livsci.2015.04.001 [DOI] [Google Scholar]
- Liang C., Li H., Zhou H., Zhang S., Liu Z., Zhou Q., et al. (2012). Recombinant Lz-8 from Ganoderma lucidum induces endoplasmic reticulum stress-mediated autophagic cell death in SGC-7901 human gastric cancer cells. Oncol. Rep. 27, 1079–1089. 10.3892/or.2011.1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. H., Hsiao Y. M., Hsu C. P., Lin M. Y., Wang J. C. H., Huang Y. L., et al. (2006). Transcriptionally mediated inhibition of telomerase of fungal immunomodulatory protein from Ganoderma tsugae in A549 human lung adenocarcinoma cell line. Mol. Carcinog. 45, 220–229. 10.1002/mc.20161 [DOI] [PubMed] [Google Scholar]
- Liao C. H., Hsiao Y. M., Lin C. H., Yeh C. S., Wang J. C. H., Ni C. H., et al. (2008). Induction of premature senescence in human lung cancer by fungal immunomodulatory protein from Ganoderma tsugae. Food Chem. Toxicol. 46, 1851–1859. 10.1016/j.fct.2008.01.044 [DOI] [PubMed] [Google Scholar]
- Liao C. H., Hsiao Y. M., Sheu G. T., Chang F. T. I., Wang P. H., Wu M. F., et al. (2007). Nuclear translocation of telomerase reverse transcriptase and calcium signaling in repression of telomerase activity in human lung cancer cells by fungal immunomodulatory protein from Ganoderma tsugae. Biochem. Pharmacol. 74, 1541–1554. 10.1016/j.bcp.2007.07.025 [DOI] [PubMed] [Google Scholar]
- Liao S. F., Liang C. H., Ho M. Y., Hsu T. L., Tsai T. I., Hsieh Y. S., et al. (2013). Immunization of fucose-containing polysaccharides from Reishi mushroom induces antibodies to tumor-associated Globo H-series epitopes. Proc. Natl. Acad. Sci. U.S.A. 110, 13809–13814. 10.1073/pnas.1312457110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. C., Yu Y. L., Shih C. C., Liu K. J., Ou K. L., Hong L. Z., et al. (2011). A novel adjuvant Ling Zhi-8 enhances the efficacy of DNA cancer vaccine by activating dendritic cells. Cancer Immunol. Immunother. 60, 1019–1027. 10.1007/s00262-011-1016-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H., Sheu G. T., Lin Y. W., Yeh C. S., Huang Y. H., Lai Y. C., et al. (2010). A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 45, 1537–1542. 10.1016/j.procbio.2010.06.006 [DOI] [Google Scholar]
- Lin K. I., Kao Y. Y., Kuo H. K., Yang W. B., Chou A., Lin H. H., et al. (2006). Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2-mediated induction of transcription factor Blimp-1. J. Biol. Chem. 281, 24111–24123. 10.1074/jbc.M601106200 [DOI] [PubMed] [Google Scholar]
- Lin T. Y., Hsu H. Y. (2016). Ling Zhi-8 reduces lung cancer mobility and metastasis through disruption of focal adhesion and induction of MDM2-mediated Slug degradation. Cancer Lett. 375, 340–348. 10.1016/j.canlet.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Lin T. Y., Hsu H. Y., Sun W. H., Wu T. H., Tsao S. M. (2017). Induction of Cbl-dependent epidermal growth factor receptor degradation in Ling Zhi-8 suppressed lung cancer. Int. J. Cancer 140, 2596–2607. 10.1002/ijc.30649 [DOI] [PubMed] [Google Scholar]
- Lin W. H., Hung C. H., Hsu C. I., Lin J. Y. (1997). Dimerization of the N-terminal amphipathic alpha-helix domain of the fungal immunomodulatory protein from Ganoderma tsugae (Fip-gts) defined by a yeast two-hybrid system and site-directed mutagenesis. J. Biol. Chem. 272, 20044–20048. 10.1074/jbc.272.32.20044 [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Liang Y. C., Lee S. S., Chiang B. L. (2005). Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappa B and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 78, 533–543. 10.1189/jlb.0804481 [DOI] [PubMed] [Google Scholar]
- Lin Z. B., Zhang H. N. (2004). Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol. Sin. 25, 1387–1395. [PubMed] [Google Scholar]
- Meng J., Hu X., Shan F., Hua H., Lu C., Wang E., et al. (2011). Analysis of maturation of murine dendritic cells (DCs) induced by purified Ganoderma lucidum polysaccharides (GLPs). Int. J. Biol. Macromol. 49, 693–699. 10.1016/j.ijbiomac.2011.06.029 [DOI] [PubMed] [Google Scholar]
- Meng L. Z., Xie J., Lv G. P., Hu D. J., Zhao J., Duan J. A., et al. (2014). A comparative study on immunomodulatory activity of polysaccharides from two official species of Ganoderma (lingzhi). Nutr. Cancer 66, 1124–1131. 10.1080/01635581.2014.948215 [DOI] [PubMed] [Google Scholar]
- Pang X., Chen Z., Gao X., Liu W., Slavin M., Yao W., et al. (2007). Potential of a novel polysaccharide preparation (GLPP) from Anhui-grown Ganoderma lucidum in tumor treatment and immunostimulation. J. Food Sci. 72, S435–S442. 10.1111/j.1750-3841.2007.00431.x [DOI] [PubMed] [Google Scholar]
- Que Z., Zou F., Zhang A., Zheng Y., Bi L., Zhong J., et al. (2014). Ganoderic acid Me induces the apoptosis of competent T cells and increases the proportion of Treg cells through enhancing the expression and activation of indoleamine 2,3-dioxygenase in mouse lewis lung cancer cells. Int. Immunopharmacol. 23, 192–204. 10.1016/j.intimp.2014.08.001 [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Yang J. C., Restifo N. P. (2004). Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915. 10.1038/nm1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Park H. S., Xia Y. M., Kim G. S., Cui S. W. (2014). The polysaccharides from fermented Ganoderma lucidum mycelia induced miRNAs regulation in suppressed HepG2 cells. Carbohydr. Polym. 103, 319–324. 10.1016/j.carbpol.2013.12.044 [DOI] [PubMed] [Google Scholar]
- Shing M. K., Leung T. F., Chu Y. L., Li C. Y., Chik K. W., Leung P. C., et al. (2008). Randomized, double-blind and placebo-controlled study of the immunomodulatory effects of Lingzhi in children with cancers. J. Clin. Oncol. 26(15 Suppl.), 14021–14021. 10.1200/jco.2008.26.15_suppl.14021 [DOI] [Google Scholar]
- Song Y. S., Kim S. H., Sa J. H., Jin C., Lim C. J., Park E. H. (2004). Anti-angiogenic and inhibitory activity on inducible nitric oxide production of the mushroom Ganoderma lucidum. J. Ethnopharmacol. 90, 17–20. 10.1016/j.jep.2003.09.006 [DOI] [PubMed] [Google Scholar]
- Sun L. X., Li W. D., Lin Z. B., Duan X. S., Lia X. F., Yang N., et al. (2014). Protection against lung cancer patient plasma-induced lymphocyte suppression by Ganoderma lucidum polysaccharides. Cell. Physiol. Biochem. 33, 289–299. 10.1159/000356669 [DOI] [PubMed] [Google Scholar]
- Sun L. X., Lin Z. B., Duan X. S., Lu J., Ge Z. H., Li X. F., et al. (2012). Enhanced MHC class I and costimulatory molecules on B16F10 cells by Ganoderma lucidum polysaccharides. J. Drug Target. 20, 582–592. 10.3109/1061186X.2012.697167 [DOI] [PubMed] [Google Scholar]
- Sun L. X., Lin Z. B., Duan X. S., Lu J., Ge Z. H., Li X. J., et al. (2011a). Ganoderma lucidum polysaccharides antagonize the suppression on lymphocytes induced by culture supernatants of B16F10 melanoma cells. J. Pharm. Pharmacol. 63, 725–735. 10.1111/j.2042-7158.2011.01266.x [DOI] [PubMed] [Google Scholar]
- Sun L. X., Lin Z. B., Li X. J., Li M., Lu J., Duan X. S., et al. (2011b). Promoting effects of Ganoderma lucidum polysaccharides on B16F10 cells to activate lymphocytes. Basic Clin. Pharmacol. Toxicol. 108, 149–154. 10.1111/j.1742-7843.2010.00632.x [DOI] [PubMed] [Google Scholar]
- Suprasert P., Apichartpiyakul C., Sakonwasun C., Nitisuwanraksa P., Phuackchantuck R. (2014). Clinical characteristics of gynecologic cancer patients who respond to salvage treatment with Lingzhi. Asian Pac. J. Cancer Prev. 15, 4193–4196. 10.7314/APJCP.2014.15.10.4193 [DOI] [PubMed] [Google Scholar]
- Toh Choon R. L., Sariah M., Siti Mariam M. N. (2012). Ergosterol from the soilborne fungus Ganoderma boninense. J. Basic Microbiol. 52, 608–612. 10.1002/jobm.201100308 [DOI] [PubMed] [Google Scholar]
- Unlu A., Nayir E., Kirca O., Ozdogan M. (2016). Ganoderma lucidum (reishi mushroom) and cancer. J. Buon. 21, 792–798. [PubMed] [Google Scholar]
- Wang C. L., Lu C. Y., Hsueh Y. C., Liu W. H., Chen C. J. (2014). Activation of antitumor immune responses by Ganoderma formosanum polysaccharides in tumor-bearing mice. Appl. Microbiol. Biotechnol. 98, 9389–9398. 10.1007/s00253-014-6027-6 [DOI] [PubMed] [Google Scholar]
- Wang C. L., Pi C. C., Kuo C. W., Zhuang Y. J., Khoo K. H., Liu W. H., et al. (2011). Polysaccharides purified from the submerged culture of Ganoderma formosanum stimulate macrophage activation and protect mice against Listeria monocytogenes infection. Biotechnol. Lett. 33, 2271–2278. 10.1007/s10529-011-0697-2 [DOI] [PubMed] [Google Scholar]
- Wang G., Zhao J., Liu J., Huang Y., Zhong J. J., Tang W. (2007). Enhancement of IL-2 and IFN-gamma expression and NK cells activity involved in the anti-tumor effect of ganoderic acid Me in vivo. Int. Immunopharmacol. 7, 864–870. 10.1016/j.intimp.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Wang P. H., Yang S. F., Chen G. D., Han C. P., Chen S. C., Lin L. Y., et al. (2007). Human nonmetastatic clone 23 type 1 gene suppresses migration of cervical cancer cells and enhances the migration inhibition of fungal immunomodulatory protein from Ganoderma tsugae. Reprod. Sci. 14, 475–485. 10.1177/1933719107305035 [DOI] [PubMed] [Google Scholar]
- Wang P. Y., Zhu X. L., Lin Z. B. (2012). Antitumor and immunomodulatory effects of polysaccharides from broken-spore of Ganoderma lucidum. Front. Pharmacol. 3:135. 10.3389/fphar.2012.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Hsu M. L., Hsu H. C., Tzeng C. H., Lee S. S., Shiao M. S., et al. (1997). The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int. J. Cancer 70, 699–705. [DOI] [PubMed] [Google Scholar]
- Wang Y. Y., Khoo K. H., Chen S. T., Lin C. C., Wong C. H., Lin C. H. (2002). Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorg. Med. Chem. 10, 1057–1062. 10.1016/S0968-0896(01)00377-7 [DOI] [PubMed] [Google Scholar]
- Wanmuang H., Leopairut J., Kositchaiwat C., Wananukul W., Bunyaratvej S. (2007). Fatal fulminant hepatitis associated with Ganoderma lucidum (Lingzhi) mushroom powder. J. Med. Assoc. Thai. 90, 179–181. [PubMed] [Google Scholar]
- Won S. J., Lin M. T., Wu W. L. (1992). Ganoderma tsugae mycelium enhances splenic natural-killer-cell activity and serum interferon-production in mice. Jpn. J. Pharmacol. 59, 171–176. 10.1254/jjp.59.171 [DOI] [PubMed] [Google Scholar]
- Xu H., Kong Y. Y., Chen X., Guo M. Y., Bai X. H., Lu Y. J., et al. (2016). Recombinant FIP-gat, a fungal immunomodulatory protein from Ganoderma atrum, induces growth inhibition and cell death in breast cancer cells. J. Agric. Food Chem. 64, 2690–2698. 10.1021/acs.jafc.6b00539 [DOI] [PubMed] [Google Scholar]
- Xu Z., Chen X., Zhong Z., Chen L., Wang Y. (2011). Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 39, 15–27. 10.1142/S0192415X11008610 [DOI] [PubMed] [Google Scholar]
- Yu Q., Nie S. P., Wang J. Q., Huang D. F., Li W. J., Xie M. Y. (2015a). Molecular mechanism underlying chemoprotective effects of Ganoderma atrurn polysaccharide in cyclophosphamide-induced immunosuppressed mice. J. Funct. Food. 15, 52–60. 10.1016/j.jff.2015.03.015 [DOI] [Google Scholar]
- Yu Q., Nie S. P., Wang J. Q., Huang D. F., Li W. J., Xie M. Y. (2015b). Toll-like receptor 4 mediates the antitumor host response induced by Ganoderma atrum polysaccharide. J. Agric. Food Chem. 63, 517–525. 10.1021/jf5041096 [DOI] [PubMed] [Google Scholar]
- Yu Q., Nie S. P., Wang J. Q., Yin P. F., Huang D. F., Li W. J., et al. (2014). Toll-like receptor 4-mediated ROS signaling pathway involved in Ganoderma atrum polysaccharide-induced tumor necrosis factor-alpha secretion during macrophage activation. Food Chem. Toxicol. 66, 14–22. 10.1016/j.fct.2014.01.018 [DOI] [PubMed] [Google Scholar]
- Yue G. G., Fung K. P., Tse G. M., Leung P. C., Lau C. B. (2006). Comparative studies of various Ganoderma species and their different parts with regard to their antitumor and immunomodulating activities in vitro. J. Altern. Complement. Med. 12, 777–789. 10.1089/acm.2006.12.777 [DOI] [PubMed] [Google Scholar]
- Zhang J., Gao X., Pan Y., Xu N., Jia L. (2016). Toxicology and immunology of Ganoderma lucidum polysaccharides in Kunming mice and Wistar rats. Int. J. Biol. Macromol. 85, 302–310. 10.1016/j.ijbiomac.2015.12.090 [DOI] [PubMed] [Google Scholar]
- Zhang J., Tang Q., Zimmerman-Kordmann M., Reutter W., Fan H. (2002). Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganoderma lucidum. Life Sci. 71, 623–638. 10.1016/S0024-3205(02)01690-9 [DOI] [PubMed] [Google Scholar]
- Zhang J. P., Zheng L. M., Wang J. H., Magnusson K. E., Liu X. (2009). Lipid extract from completely sporoderm-broken germinating Ganoderma sinensis spores elicits potent antitumor immune responses in human macrophages. Phytother. Res. 23, 844–850. 10.1002/ptr.2707 [DOI] [PubMed] [Google Scholar]
- Zhang S., Nie S., Huang D., Feng Y., Xie M. (2014). A novel polysaccharide from Ganoderma atrum exerts antitumor activity by activating mitochondria-mediated apoptotic pathway and boosting the immune system. J. Agric. Food Chem. 62, 1581–1589. 10.1021/jf4053012 [DOI] [PubMed] [Google Scholar]
- Zhang S. S., Nie S. P., Huang D. F., Li W. J., Xie M. Y. (2013). Immunomodulatory effect of Ganoderma atrum polysaccharide on CT26 tumor-bearing mice. Food Chem. 136, 1213–1219. 10.1016/j.foodchem.2012.08.090 [DOI] [PubMed] [Google Scholar]
- Zhao H., Zhang Q., Zhao L., Huang X., Wang J., Kang X. (2012). Spore powder of Ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: a pilot clinical trial. Evid. Based Complement. Altern. Med. 2012:809614. 10.1155/2012/809614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Jia Y., Zhao J., Wei Q., Liu Y. (2012). Ganoderma lucidum polysaccharides eradicates the blocking effect of fibrinogen on NK cytotoxicity against melanoma cells. Oncol. Lett. 3, 613–616. 10.3892/ol.2011.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Lin Z. (2006). Modulation of cytokines production, granzyme B and perforin in murine CIK cells by Ganoderma lucidum polysaccharides. Carbohyd. Polym. 63, 188–197. 10.1016/j.carbpol.2005.08.002 [DOI] [Google Scholar]
- Zhu X. L., Lin Z. B. (2005). Effects of Ganoderma lucidum polysaccharides on proliferation and cytotoxicity of cytokine-induced killer cells. Acta Pharmacol. Sin. 26, 1130–1137. 10.1111/j.1745-7254.2005.00171.x [DOI] [PubMed] [Google Scholar]