Abstract
Aims
Pathologic evidence supports unique sex-specific mechanisms as precursors for acute cardiovascular (CV) events. Current evidence on long-term CV risk among women when compared with men based on measures of coronary artery calcium (CAC) remains incomplete.
Methods and results
A total of 63 215 asymptomatic women and men were enrolled in the multicentre, CAC Consortium with median follow-up of 12.6 years. Pooled cohort equation (PCE) risk scores and risk factor data were collected with the Agatston score and other CAC measures (number of lesions and vessels, lesion size, volume, and plaque density). Cox proportional hazard models were employed to estimate CV mortality (n = 919). Sex interactions were calculated. Women and men had average PCE risk scores of 5.8% and 9.1% (P < 0.001). Within CAC subgroups, women had fewer calcified lesions (P < 0.0001) and vessels (P = 0.017), greater lesion size (P < 0.0001), and higher plaque density (P = 0.013) when compared with men. For women and men without CAC, long-term CV mortality was similar (P = 0.67), whereas detectable CAC was associated with 1.3-higher hazard for CV death among women when compared with men (P < 0001). Cardiovascular mortality was higher among women with more extensive, numerous, or larger CAC lesions. The relative hazard for cardiovascular disease (CVD) mortality for women and men was 8.2 vs. 5.1 for multivessel CAC, 8.6 vs. 5.9 for ≥5 CAC lesions, and 8.5 vs. 4.4 for a lesion size ≥15 mm3, respectively. Additional explorations revealed that women with larger sized and more numerous CAC lesions had 2.2-fold higher CVD mortality (P < 0.0001) as compared to men. Moreover, CAC density was not predictive of CV mortality in women (P = 0.51) but was for men (P < 0.001), when controlling for CAC volume and cardiac risk factors.
Conclusion
Our overall findings support that measures beyond the Agatston score provide important clues to sex differences in atherosclerotic plaque and may further refine risk detection and focus preventive strategies of care.
Keywords: Prognosis , Cardiovascular disease , Coronary calcium , Sex differences
Introduction
For decades, epidemiologic data documented a higher case fatality rate for cardiovascular disease (CVD) among women when compared with men.1 The higher CVD mortality for women led to many discussions regarding the underlying aetiology and whether varying precursor atherosclerotic plaque features contributed to more deaths among women. Pathobiologic and invasive angiographic evidence suggests that variable patterns of atherosclerotic plaque may be more common in women (i.e. plaque erosion vs. rupture).2–4 Although this research is far from robust, a compilation of this evidence supports a sex-specific atherosclerotic plaque profile among women that includes a smaller plaque burden, less calcified plaque, and less obstructive coronary artery disease (CAD).2,5–8 The data consistently report that despite less prevalent obstructive CAD, women have a higher acute and long-term mortality than men.9
With the advent of advanced cardiac imaging techniques, such as computed tomography, detailed visualization of composition within atherosclerotic plaque has been the focus of recent research endeavours. For the evaluation of CVD risk among asymptomatic individuals, there is abundant data that a non-contrast, computed tomography measurement of coronary artery calcium (CAC) is highly predictive of major cardiac events and total mortality.10,11 We undertook an analysis to compare sex-differences in long-term CVD mortality based on detailed measurements of CAC atherosclerotic plaque from a large multicentre study of asymptomatic women and men.
Study Methods
Enrolment criteria
The CAC Consortium is a four-centre cohort which consecutively enrolled asymptomatic women and men undergoing CAC scans between 1991 and 2010. All patients were referred by a physician for CAC scanning to further refine CVD risk screening. Patients presenting with shortness of breath or other symptoms were excluded (n = 3421), leaving 63 215 patients for the current analysis. Site enrolment is detailed in our design manuscript.12 In contrast to prior series focusing on all-cause mortality,11,13,14 the CAC Consortium includes classification of CVD mortality.
Coronary risk factor and global risk score data
Each site provided patient-level data on traditional cardiac risk factors such as age, dyslipidaemia, hypertension, diabetes, smoking, and a family history of coronary heart disease (CHD). Family history was defined as the occurrence of a CHD event prior to age 45 and 55 years of age, respectively, for a male and female first degree relatives. The PCE and Framingham risk scores were calculated for each participant.15 The National Institutes of Health (NIH)-National Heart, Lung, and Blood Institute (NHLBI)-sponsored Multi-Ethnic Study of Atherosclerosis (MESA) risk score with and without CAC was calculated for all individuals.16
Coronary artery calcium measures
Standardized methods for calculation of the CAC Agatston score were employed by multiplying the area (mm2) of CAC plaque by the categorical density of plaque in Hounsfield Units (HU) which ranges from 1 to 4 for 130–199 HU to ≥400 HU.17 The number of calcified coronary artery lesions and vessels were summed as well as the CAC volume score, measured in mm3, and average lesion size was calculated. Among a subgroup of 10 374 consecutively enrolled individuals, mean CAC density was available. Coronary artery calcium density and volume scores were divided into quartiles.18
Follow-up methods
Details of the follow-up methods are available in our design manuscript.12 In brief, each enrolling centre identified patients who died during long-term follow-up via matching with the Social Security Administration Death Master File. Death certificates were catalogued to allow for additional classification as to underlying cause of death including CVD, cancer, or from all-causes (e.g. pulmonary disease, gastrointestinal disease, or injury) from the National Death Index based on ICD-9 and ICD-10 codes.12
The median follow-up duration was 12.6 years (interquartile range = 10.9–14.2 years). A total of 919 CVD deaths were documented. Deaths attributable to all-causes (n = 3004), CHD (n = 496), and cancer (n = 1077) were also available.
Statistical methods
The primary comparison for this analysis was differences in long-term mortality between women and men. Categorical data were compared using a χ2 test while continuous data were compared using a t-test.
Time to event analysis was undertaken using univariable and multivariable Cox proportional hazards models. Survival analyses were calculated through long-term follow-up. The number at risk was reported at 5 year intervals for survival curves. Univariable and multivariable Cox proportional hazard models were devised including calculation of the hazard ratio (HR) and 95% confidence intervals (CI). Coronary artery calcium measures were highly correlated and collinear (r > 0.90), thus separate Cox regression models were undertaken. With exception, we explored a model including categorized variables including CAC lesion size ≥15 mm3 and ≥5 CAC lesions (spearman’s rho correlation = 0.70). Sex differences were examined using a first order test for interaction. We assessed the proportional hazards assumption by examining the constancy of the parallel plotted lines in the log-log graph and examining Schoenfeld’s residuals.11 Adjusted models included the PCE risk score; however, Cox models that included plaque density added covariates of CAC volume, hypertension, and dyslipidaemia, consistent with the analysis of Criqui et al.18 The proportion of excess risk explained was calculated using this equation: [HRU − HRA]/[HRU − 1] where HR is the hazard ratio and HRU is an unadjusted HR and HRA is a risk-adjusted HR.19 Receiver operating characteristics (ROC) curve analysis was used to compare mortality discrimination between the MESA risk score with and without CAC. From the ROC analysis, the area under the curve (AUC) and 95% CI were calculated.
Results
Cardiac risk factor prevalence and global risk scores
A total of 20 508 and 42 707 asymptomatic women and men were enrolled in the CAC Consortium (Table 1). Women were generally older, more often hypertensive, and had a family history of CHD; whereas men were more often smokers and obese. Nearly 21% of women as compared with 42% of men had a PCE score of >7.5%.
Table 1.
Sex differences in the prevalencea of traditional cardiac risk factors and risk scores among 63 215 asymptomatic women and men enrolled in the CAC Consortium
| Women (n = 20 508) | Men (n = 42 707) | P value | |
|---|---|---|---|
| Age (years) | 56.2 ± 10 | 53.5 ± 10 | <0.0001 |
| Hypertension (n = 63 215) | 31.2% | 29.4% | <0.0001 |
| ACE inhibitor use (n = 27 813) | 6.4% | 9.1% | <0.0001 |
| Beta blocker use (n = 28 048) | 11.5% | 9.0% | <0.0001 |
| Dyslipidaemia (n = 63 215) | 56.1% | 56.1% | 0.889 |
| Statin therapy use (n = 27 950) | 16.2% | 23.1% | <0.0001 |
| Niacin use (n = 27 650) | 3.2% | 4.3% | <0.0001 |
| Diabetes (n = 63 215) | 6.2% | 6.6% | 0.041 |
| Obesity (body mass index ≥30 kg/m2) (n = 33 515) | 23.5% | 26.7% | <0.0001 |
| Current smoking (n = 63 215) | 9.0% | 9.5% | 0.002 |
| Any family history of CVD (n = 63 215) | 53.3% | 42.9% | <0.0001 |
| Aspirin use (n = 28 204) | 38.3% | 45.2% | <0.0001 |
| PCE risk score (n = 63 215) | <0.0001 | ||
| <5% | 66.6% | 42.3% | |
| 5–7.5% | 12.4% | 16.0% | |
| >7.5% | 21.0% | 41.7% | |
| Framingham risk score (n = 63 215) | <0.0001 | ||
| <6% | 42.9% | 30.2% | |
| 6–19% | 54.3% | 52.2% | |
| ≥20% | 2.8% | 17.6% |
All risk factors are presented as proportions, except age which is presented as mean ± standard deviation values.
Coronary artery calcium measures
For women, 60%, 25%, 9%, and 5%, respectively, had a CAC score of 0, 1–100, 101–399, and ≥400 (Table 2 and Figures 1 and 2). For men, 37%, 34%, 16%, and 14%, respectively, had a CAC score of 0, 1–100, 101–399, and ≥400. Of younger women <55 years of age, 77.2% and 18.5% had CAC scores of 0 and 1–100; rates higher than that of men (P < 0.0001). Across age deciles, women had a lower prevalence of detectable CAC (Figure 1). An increase in the proportion of women with detectable CAC occurred at ∼46 years of age, nearly 10 years older than men.
Table 2.
Comparative differencesa in calcified plaque measurements among 35 146 asymptomatic women and men with detectable coronary artery calcium (score >0) enrolled in the CAC Consortium
| CAC measures | CAC 1–100 (n = 19 684) |
CAC 101–399 (n = 8612) |
CAC ≥400 (n = 6850) |
||||
|---|---|---|---|---|---|---|---|
| Women (n = 5236) | Men (n = 14448) | Women (n = 1840) | Men (n = 6772) | Women (n = 1039) | Men (n = 5811) | Sex interaction P value | |
| CAC Score | 28 ± 28 | 28 ± 37 | 204 ± 81 | 213 ± 85 | 1025 ± 826 | 1161 ± 964 | <0.0001 |
| Number of calcified vessels | 1.5 ± 0.7 | 1.6 ± 0.8 | 2.4 ± 0.9 | 2.6 ± 0.9 | 3.2 ± 0.7 | 3.3 ± 0.6 | 0.008 |
| Number of calcified lesions | 2.6 ± 2.4 | 3.5 ± 3.3 | 6.9 ± 4.2 | 9.5 ± 5.9 | 15.9 ± 9.2 | 20.2 ± 11.1 | <0.0001 |
| CAC volume (mm3) | 24 ± 22 | 25 ± 23 | 161 ± 66 | 172 ± 69 | 790 ± 578 | 932 ± 772 | <0.0001 |
| CAC density (HU) | 192 ± 43 | 184 ± 37 | 248 ± 43 | 234 ± 37 | 268 ± 39 | 258 ± 36 | 0.013 |
| Mean lesion size (mm3) | 12 ± 11 | 10 ± 10 | 36 ± 30 | 28 ± 20 | 65 ± 48 | 57 ± 43 | <0.0001 |
The mean and standard deviation (±) values are reported in each of the cell subgroups. The sex interaction P value is for each of the CAC measures listed in each row of this table.
Comparative analysis using general linear model with subsets by sex and Agatston score subgroups. P value is a test for interaction of the Agatston score subgroups by sex. Explanatory variance (or r2) was moderate and ranged from 0.38 to 0.53 for all measures. Non-parametric analysis comparing independent samples provided similar results.
Figure 1.
Comparison of the proportion of women and men with detectable coronary artery calcium (score >0) across ages 30 to ≥80 years. This line is a moving average of the proportion across the ages for women and men. An inflection in the moving average line occurred at about age 46 years for women as compared with age 37 years for men.
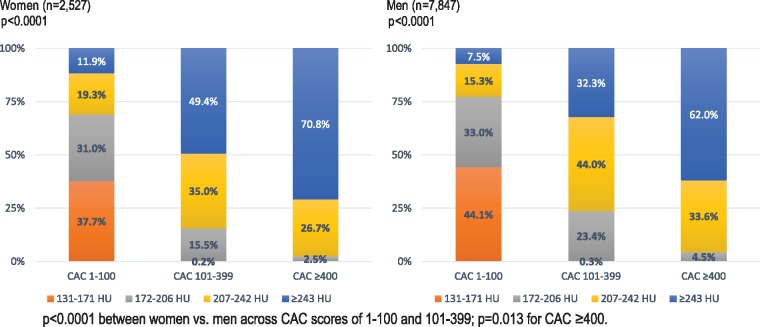
Figure 2.
Frequency of coronary artery calcium density quartiles (in Hounsfield Units) across coronary artery calcium score subgroups of 1–100, 101–399, and ≥400 within a subgroup of 10 374 asymptomatic women and men enrolled in the CAC consortium. This subgroup was limited to enrolees with a coronary artery calcium score >0.
Across the CAC subgroups in Table 2, women had fewer calcified lesions (P < 0.0001), fewer calcified vessels (P = 0.017), and a lower CAC volume (P < 0.0001). Among a subgroup of 10 374 enrolees with detectable CAC, women had higher mean plaque density values. The lowest quartile of CAC plaque density (131–171 HU) largely occurred within CAC scores of 1–100 (Figure 2). Coronary artery calcium scores ≥400 had higher CAC plaque density without any cases of plaque density from 131–171 HU.
Cox models estimating mortality among women vs. men
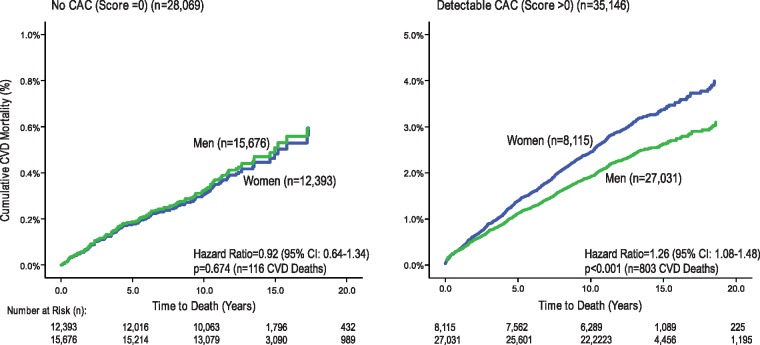
Among women and men with a 0 CAC score, similar CVD mortality was reported (Figure 3, P = 0.67) (Table 3 and Figures 3 and 4). By comparison, among those with detectable CAC, the relative hazard for CVD mortality was 1.3-fold higher for women as compared to men (Figure 3). Higher CVD mortality was reported for women as compared to men with two or more calcified vessels (Figure 4).
Figure 3.
Cardiovascular disease mortality rates among women and men with a 0 Agatston score and with detectable coronary artery calcium (score >0). Note that the y axis range varies between the survival curves for those without vs. with detectable coronary artery calcium.
Table 3.
Relative hazard (and 95% confidence intervals)a for estimating cardiovascular disease mortality by coronary artery calcium score measurements among asymptomatic women and men enrolled in the CAC Consortium
| N | Women | P value | n | Men | P value | Interaction P value | |
|---|---|---|---|---|---|---|---|
| CAC score subgroups | (n = 20 508) | (n = 42 707) | |||||
| 1–100 | 5236 | 2.93 (2.00–4.29) | <0.0001 | 14 448 | 2.13 (1.59–2.85) | <0.0001 | 0.887 |
| 101–399 | 1840 | 9.72 (6.70–14.09) | <0.0001 | 6772 | 4.98 (3.73–6.65) | <0.0001 | <0.0001 |
| ≥400 | 1039 | 26.65 (18.07–36.41) | <0.0001 | 5822 | 13.32 (10.25–17.32) | <0.0001 | <0.0001 |
| Number of calcified vessels | (n = 20 508) | (n = 42 707) | |||||
| 1 vessel | 2840 | 2.40 (1.49–3.87) | <0.0001 | 6203 | 1.91 (1.32–2.78) | 0.001 | 0.589 |
| 2 vessels | 1801 | 6.57 (4.89–10.66) | <0.0001 | 5413 | 3.29 (2.35–4.61) | <0.0001 | <0.0001 |
| 3 vessels | 1286 | 11.41 (7.69–16.94) | <0.0001 | 5667 | 7.88 (5.91–10.52) | <0.0001 | <0.0001 |
| 4 vessels | 457 | 28.22 (18.61–42.80) | <0.0001 | 2747 | 10.85 (7.96–14.78) | <0.0001 | <0.0001 |
| CAC volume (mm3) | (n = 10 394) | (n = 20 209) | |||||
| 0.2–15.0 | 1111 | 3.09 (1.54–6.22) | 0.002 | 2876 | 1.83 (1.11–3.00) | 0.025 | 0.750 |
| 15.1–61.5 | 1159 | 4.02 (2.13–7.62) | <0.0001 | 2872 | 2.06 (1.28–3.33) | 0.004 | 0.792 |
| 61.6–230.3 | 931 | 8.40 (4.79–14.72) | <0.0001 | 3081 | 3.77 (2.51–5.68) | <0.0001 | <0.0001 |
| ≥230.4 | 657 | 28.81 (17.73–46.82) | <0.0001 | 3353 | 9.90 (6.96–14.08) | <0.0001 | <0.0001 |
For this analysis, separate Cox models were calculated for women and men; with the right column including the interaction P value of women vs. men in a combined predictive model.
Adjusted by the PCE risk score. No CAC was scored as 0 and is the comparator for this analysis. Coronary artery calcium volume was categorized into quartiles.
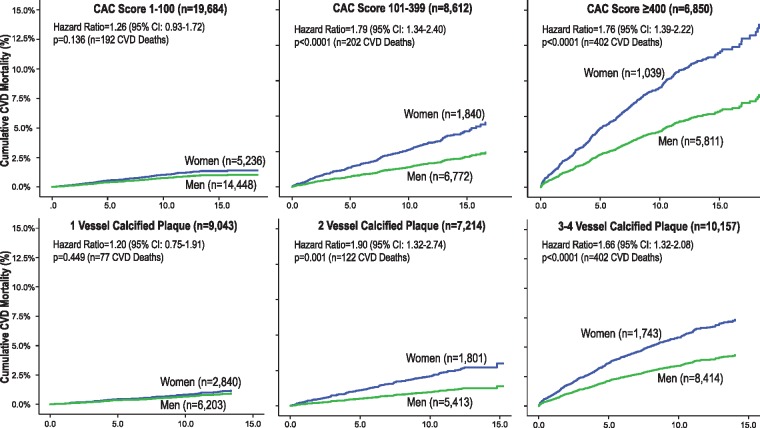
Figure 4.
Cardiovascular disease mortality among women and men by coronary artery calcium score subgroups and the number of calcified vessels.
The relative CVD mortality hazard for CAC score subgroups of 1–100, 101–399, and ≥400, respectively, ranged from 2.9 to 26.7 for women and 2.1 to 13.3 for men, respectively (Table 3). The sex interaction revealed that CVD mortality was higher for women with CAC scores >100. Supplementary material online, Table S1 reports Cox models estimating all-cause, CHD, and cancer mortality.
The AUC was greater for the integrated MESA risk score (including CAC scores) as compared to the MESA clinical risk score (without CAC scores) for women (0.824 vs. 0.812, P < 0.0001) and men (0.781 vs. 0.761, P < 0.0001).
Cardiovascular disease mortality models among women vs. men
Sex interactions were reported across the varying CAC measures. Women as compared to men with CAC in ≥2 or more vessels had a higher CVD mortality (Table 3, P < 0.004). Also, when compared with men, higher CVD mortality was documented among women with CAC volumes >61.5 mm3 (Table 3). A sex interaction was also reported for greater sized CAC lesions (P < 0.0001) (Supplementary material online, Table S2). Women with five or more calcified lesions also had a higher CVD mortality (Supplementary material online, Table S2).
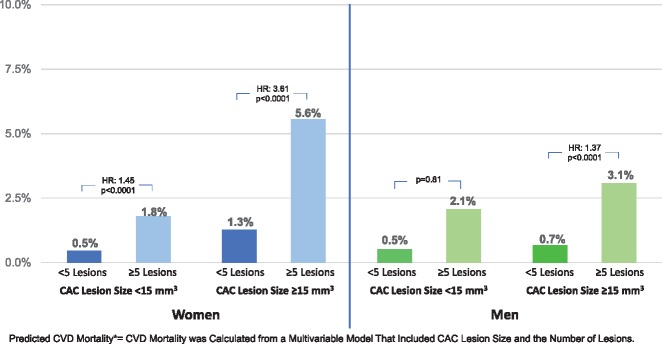
In an exploratory analysis, we examined sex differences in CVD mortality among several CAC variables. For both women and men, the number of lesions was a greater predictor of CVD mortality (P < 0.0001) as compared to lesion size. However, for women, the proportion of excess risk attributable to the size of the CAC lesion was 28% as compared to 14% for men. Based on a multivariable model including ≥5 CAC lesions and CAC lesions ≥15 mm3, the adjusted mortality was calculated (Take home figure). Take home figure plots the significantly higher CVD mortality among women with ≥5 CAC lesions and larger lesions ≥15 mm3; significantly higher than men (interaction P < 0.0001).
Take home figure.
Predicted cardiovascular disease mortality (cardiovascular disease mortality was calculated from a multivariable model that included coronary artery calcium lesion size and the number of lesions.) among women and men based on the number and size of calcified lesions. Among those with ≥5 coronary artery calcium lesions, women with larger coronary artery calcium lesions ≥15 mm3 had 2.2-fold higher cardiovascular disease mortality (P < 0.0001) when compared with men.
Exploratory analysis estimating cardiovascular disease mortality by mean coronary artery calcium density
In unadjusted models, CVD mortality increased with higher mean CAC density quartiles as compared to women and men with 0 CAC scores (P < 0.0001) (Supplementary material online, Table S2). Stepwise Cox models revealed that the volume of CAC, however, was a greater estimator of CVD mortality than CAC density for women and men. When limiting the analysis to women with detectable CAC, only five CVD deaths occurred among women with mean CAC density values of 131–171 HU and, in adjusted models, was not predictive of CVD mortality (P = 0.51). However, in a similar adjusted model, men with mean CAC density values of 131–171 HU (1st quartile) had a hazard for CVD mortality of 3.9 (P < 0.0001) as compared to higher quartile values ≥172 HU.
Discussion
From the CAC Consortium, we report for the first time on differences in long-term CVD prognosis for women and men across varied CAC measures beyond the Agatston score. Prior findings, including from our group, documented prognosis associated with the site of CAC plaque as well as the number of calcified lesions and epicardial vessels.20,21 The current analysis supports a variable profile of calcified plaque and differential prognosis among CAC measures, with women often having higher CVD mortality when compared with similar subgroups of men. Our findings support that higher risk status among women may by imparted by a more extensive burden of CAC, with numerous or multivessel CAC elevating CVD mortality risk higher than that of their male counterparts. We report on sex-specific mortality differences based on lesion size and further identify a high-risk subgroup of women. These findings from our large CAC Consortium support a sex-specific plaque phenotype impacting long-term risk in women as compared to men.
Coronary artery calcium plaque patterns among women and men
Current pathologic and invasive evidence supports a sex-specific atherosclerotic plaque profile among women that includes a smaller plaque burden, less calcified plaque, and less obstructive CAD.2,5–8,22,23 It is hypothesized that the uniqueness of atherosclerosis among women may be aetiologic for sex differences in clinical outcomes. Few reports have examined components of the Agatston score or other measures of CAC extent.18,20,21,24 Several reports have examined long-term prognosis associated with the number of calcified vessels as additive to risk estimation.21,24
In the CAC Consortium, women had a smaller volume of calcified plaque and fewer calcified lesions when compared with men. In one example, women with an Agatston score of 101–399 had on average 7 calcified lesions as compared to nearly 10 lesions for men (P < 0.0001). But, interestingly, women within a given CAC score subgroup had fewer but larger sized calcified lesions (on average) when compared with men. It would be expected that the larger calcified lesions among women represent a more expansive plaque burden, given a smaller epicardial artery and that these larger CAC lesions may differentially impact prognosis.13,14,25 Particularly, CAC encumbering multiple epicardial coronary arteries can provide data on diffuse atherosclerosis which is not be appreciated with the CAC score alone.21,24 In our analysis, women with two or more calcified vessels had a higher CVD mortality throughout more than 10 years of follow-up. Hypothetically, these larger lesions or more expansive plaque burden represent more advanced atherosclerosis but reflect plaque within a preserved but positively remodelled epicardial coronary artery and non-obstructive CAD that is commonly observed among women; more so than men. The greater prevalence of more dense plaque among women lends credence that more advanced atherosclerotic states are present in these larger sized CAC lesions. Variability across CAC measures may reflect a profile of atherosclerotic plaque unique to women.
Uniqueness of risk prediction among women
Recent consensus documents have focused on the importance of sex-specific evidence to improve CVD risk detection.26 Findings from this large, multicentre cohort reveal a consistent pattern of elevated rates of CVD mortality among women with more extensive calcified plaque. We revealed that more extensive calcification across multiple epicardial vessels or involving larger volumes of calcified plaque was associated with higher CVD mortality among women when compared with men. This consistent pattern that the same extent of coronary calcification increased risk more for women than men has been similarly observed in coronary angiography whereby multivessel CAD often has a higher risk among women than men.27,28 However, CVD risk was uniquely imparted among women with larger sized CAC lesions. Two hypotheses may be proposed including the potential for a focal lesion within a less severe burden of CAD, with women commonly having less obstructive disease. Alternatively, the deposition of CAC plaque of a larger size may be the result of sex-specific alterations in epicardial flow or blood biomarkers (e.g. higher levels of inflammation) previously reported as differential by sex. These larger CAC lesions may occur with absent, minimal, or high grade epicardial coronary stenosis; and thusly explain higher CVD mortality among women when compared with men. It also remains likely that the older ages of women provide a longer duration of exposure to risk markers and facilitation of plaque development.
For women, higher risk findings have often been attributed to greater degrees of comorbidity and advanced age as aetiologic for increasing the relative hazard for CVD events in females when compared with males.13,25 However, a recent report from the MESA revealed that a CAC score >300 was significantly predictive of incident heart failure with preserved ejection fraction among women but not men.29 Our findings and that of others support a working hypothesis that atherosclerotic plaque impairing vascular function and diastolic function may uniquely impact risk in women, even among those without obstructive CAD.29,30
Explorations regarding plaque density and cardiovascular disease mortality
The Agatston score is calculated by multiplying the area of calcified plaque by mean CAC density.17 Recent evidence, from the MESA, revealed an inverse relationship between calcified plaque density and CVD and CHD events. Thus, the combining of plaque extent with density may mask certain at-risk patient subgroups. From the MESA, the lowest quartile of plaque density (80–223 HU) was associated with higher relative event hazard and, with more dense plaque, event rates declined.18 From the CAC Consortium, the lower CAC density subgroup occurred mostly within lower CAC scores from 1–100. Only five CVD deaths occurred among women having lower plaque density scores (lowest quartile: 131–171 HU) and, in adjusted prognostic models, plaque density was not predictive of CVD mortality for women (P > 0.5) but was so for men. Several reasons may be postulated for the lack of predictive ability of CAC density among the subgroup of 2527 women, as less dense CAC plaque (131–171 HU) occurred more among younger women (<55 years of age), many of whom would be within the pre- or peri-menopausal state. Hypothetically, for younger women, preserved oestrogen status would impact plaque development and progression and, ultimately, CVD event risk.
Limitations of the evidence
Despite decades of evidence of higher case fatality rates and pathobiologic data supporting variable precursor plaque lesions for acute coronary events, our understanding of sex differences in atherosclerosis remains suboptimal. We noted a variable plaque profile with a greater size and extent of calcified lesions associated with worsening clinical outcomes for women; consistent with prior findings of elevated risk among females. However, the lack of detail on morbid events, follow-up imaging, and medical treatment would further contribute to our understanding of sex differences in atherosclerotic plaque. Importantly, the assessment of non-calcified plaque was not performed but may have improved CVD risk prediction. Menopausal history, pre-eclampsia, and gestational diabetes data were also not available. The defining of additional CAC measures, beyond the Agatston score, added a depth of detail to our report. However, as is consistent with many registries, uniformity in data collection was not possible and several CAC measures were not available in all enrolees.
Conclusions
The CAC Consortium represents the largest prognostic series reporting on detailed measures of calcified plaque and long-term follow-up,15 allowing for evaluation of sex differences impacting long-term CVD mortality. Our reports are consistent with prior angiographic series27,28 that more diffuse and extensive atherosclerosis across multiple epicardial coronary arteries or encumbering larger plaque volume accelerates risk among women. Contributing features of larger CAC lesion sizes have yet to be previously reported and likely promote higher risk status for women. Prognostication based on atherosclerotic plaque density remains exploratory and its significance as a risk marker appears to be less so for women as compared to men. Findings, such as presented herein, provide critical evidence to support future lines of inquiry on sex differences in atherosclerotic plaque and its impact on long-term clinical outcomes.
Funding
This project was supported in part by a research grant from the National Institutes of Health (NIH)-National Heart, Lung, and Blood Institute (NHLBI) [L30 HL110027].
Conflict of interest: L.J.S. received unrestricted grant from the Antinori Foundation. J.K.M. received grant support from GE Healthcare and has ownership in MDDX and AutoPlaq and served on the medical advisory board of Arineta. M.J.B. received grant support from GE Healthcare. And all other authors have none to declare.
Supplementary Material
Footnotes
See page 3736 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy630)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park S-J, Jang Y-S, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi S-Y, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang I-K.. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol 2013;62:1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virmani R, Burke AP, Farb A, Kolodgie FD.. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13–C18. [DOI] [PubMed] [Google Scholar]
- 4. Hong M-K, Mintz GS, Lee CW, Lee B-K, Yang T-H, Kim Y-H, Song J-M, Han K-H, Kang D-H, Cheong S-S, Song J-K, Kim J-J, Park S-W, Park S-J.. The site of plaque rupture in native coronary arteries: a three-vessel intravascular ultrasound analysis. J Am Coll Cardiol 2005;46:261–265. [DOI] [PubMed] [Google Scholar]
- 5. Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, Vergallo R, Minami Y, Ong DS, Lee H, Okumura K, Jang I-K.. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary. JACC Cardiovasc Interv 2015;8:1166–1176. [DOI] [PubMed] [Google Scholar]
- 6. Sheifer SE, Canos MR, Weinfurt KP, Arora UK, Mendelsohn FO, Gersh BJ, Weissman NJ.. Sex differences in coronary artery size assessed by intravascular ultrasound. Am Heart J 2000;139:649–653. [DOI] [PubMed] [Google Scholar]
- 7. Han SH, Bae JH, Holmes DR, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A Jr.. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J 2008;29:1359–1369. [DOI] [PubMed] [Google Scholar]
- 8. Vaccarino V. Ischemic heart disease in women: many questions, few facts. Circ Cardiovasc Qual Outcomes 2010;3:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK.. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 10. Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, Szklo M, Blumenthal RS, Nasir K.. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shaw LJ, Giambrone AE, Blaha MJ, Knapper JT, Berman DS, Bellam N, Quyyumi A, Budoff MJ, Callister TQ, Min JK.. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med 2015;163:14–21. [DOI] [PubMed] [Google Scholar]
- 12. Blaha MJ, Whelton SP, Al Rifai M, Dardari ZA, Shaw LJ, Al-Mallah MH, Matsushita K, Rumberger JA, Berman DS, Budoff MJ, Miedema MD, Nasir K.. Rationale and design of the coronary artery calcium consortium: a multicenter cohort study. J Cardiovasc Comput Tomogr 2017;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ.. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol 2008;52:17–23. [DOI] [PubMed] [Google Scholar]
- 14. Raggi P, Shaw LJ, Berman DS, Callister TQ.. Gender-based differences in the prognostic value of coronary calcification. J Womens Health (Larchmt) 2004;13:273–283. [DOI] [PubMed] [Google Scholar]
- 15. Blaha MJ, Whelton SP, Al Rifai M, Dardari ZA, Shaw LJ, Al-Mallah MH, Matsushita K, Rumberger JA, Berman DS, Budoff MJ, Miedema MD, Nasir K.. Rationale and design of the coronary artery calcium consortium: a multicenter cohort study. J Cardiovasc Comput Tomogr 2016;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE, Folsom AR, Khera A, Ayers C, Mahabadi A-A, Lehmann N, Jöckel K-H, Moebus S, Carr JJ, Erbel R, Burke GL.. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R.. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 18. Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA.. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014;311:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shishehbor MH, Litaker D, Pothier CE, Lauer MS.. Association of socioeconomic status with functional capacity, heart rate recovery, and all-cause mortality. JAMA 2006;295:784–792. [DOI] [PubMed] [Google Scholar]
- 20. Williams M, Shaw LJ, Raggi P, Morris D, Vaccarino V, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Nasir K, Budoff M.. Prognostic value of number and site of calcified coronary lesions compared with the total score. JACC Cardiovasc Imaging 2008;1:61–69. [DOI] [PubMed] [Google Scholar]
- 21. Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS.. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–1870. [DOI] [PubMed] [Google Scholar]
- 22. Lafont A. Basic aspects of plaque vulnerability. Heart 2003;89:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN.. Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol 2015;66:1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blaha MJ, Budoff MJ, Tota-Maharaj R, Dardari ZA, Wong ND, Kronmal RA, Eng J, Post WS, Blumenthal RS, Nasir K.. Improving the CAC score by addition of regional measures of calcium distribution: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging 2016;9:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelkar AA, Schultz WM, Khosa F, Schulman-Marcus J, O’Hartaigh BWJ, Gransar H, Blaha MJ, Knapper JT, Berman DS, Quyyumi A, Budoff MJ, Callister TQ, Min JK, Shaw LJ.. Long-term prognosis after coronary artery calcium scoring among low-intermediate risk women and men. Circ Cardiovasc Imaging 2016;9:e003742.. [DOI] [PubMed] [Google Scholar]
- 26. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shaw LJ, Bugiardini R, Merz CN.. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol 2009;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. WISE Investigators. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47:S21–S29. [DOI] [PubMed] [Google Scholar]
- 29. Sharma K, Al Rifai M, Ahmed HM, Dardari Z, Silverman MG, Yeboah J, Nasir K, Sklo M, Yancy C, Russell SD, Blumenthal RS, Blaha MJ.. Usefulness of coronary artery calcium to predict heart failure with preserved ejection fraction in men versus women (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2017;120:1847–1853. [DOI] [PubMed] [Google Scholar]
- 30. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF.. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.