Abstract
Background
Early-life stress increases the risk for posttraumatic stress disorder. However, the epigenetic mechanism of early-life stress-induced susceptibility to posttraumatic stress disorder in adulthood remains unclear.
Methods
Rat pups were exposed to maternal deprivation during postnatal days 1 to 14 for 3 hours daily and treated with the DNA methyltransferase inhibitor zebularine, L-methionine, or vehicle 7 days before contextual fear conditioning, which was used as a second stress and to mimic the reexperiencing symptom of posttraumatic stress disorder in adulthood. Long-term potentiation, dendritic spine density, DNA methyltransferase mRNA, Reelin gene methylation, and Reelin protein expression in the hippocampal CA1 were measured.
Results
Maternal deprivation enhanced contextual fear memory in adulthood. Meanwhile, maternal deprivation decreased DNA methyltransferase mRNA and Reelin gene methylation in the hippocampal CA1 on postnatal days 22 and 90. Reelin protein expression was increased in the hippocampal CA1 following contextual fear conditioning in adulthood. Furthermore, compared with rats that experienced maternal deprivation alone, rats also exposed to contextual fear conditioning showed an enhanced induction of hippocampal long-term potentiation and increased dendritic spine density in the hippocampal CA1 following contextual fear conditioning in adulthood. Zebularine pretreatment led to an enhancement of contextual fear memory, hypomethylation of the Reelin gene, and increased Reelin protein expression in adult rats, while L-methionine had the opposite effects.
Conclusions
Maternal deprivation can epigenetically program second-hit stress-induced Reelin expression and enhance the susceptibility to contextual fear memory in adulthood. These findings provide a new framework for understanding the cumulative stress hypothesis.
Keywords: early life stress, susceptibility, posttraumatic stress disorder, Reelin, methylation
Significance Statement
Early-life stress (ELS) increases the risk of posttraumatic stress disorder (PTSD) in adulthood. However, few studies have been conducted that explore the epigenetic mechanism underlying ELS-generated programming effects on a second stressor in adulthood to account for PTSD susceptibility. In the present study, we provide evidence that ELS epigenetically programs Reelin gene expression and synaptic plasticity induced by a second stress and enhances the vulnerability to contextual fear memory in adulthood. By revealing the lifelong impact of ELS, these findings help to clarify the mechanism by which adverse childhood experiences lead to the development of PTSD when individuals are coping with second or multiple stressors experienced in adulthood and provide a new framework for understanding the cumulative stress hypothesis.
Introduction
Posttraumatic stress disorder (PTSD) is a common stress-related psychiatric disorder that may occur in individuals after exposure to some severe life-threatening traumatic events. Previous studies have shown that 20% of people who experience traumatic events develop PTSD, whereas some people recover from the traumatic experiences (Block and Liberzon, 2016), suggesting that the vulnerability to PTSD differs within the population. Clinical and animal studies have indicated that early-life stress (ELS) results in enhanced susceptibility to PTSD following a second trauma exposure in adulthood (Widom, 1999).
As with studies of PTSD susceptibility in the context of ELS, abundant evidence has indicated that early adversities alter the brain both structurally and functionally during development (Cowan et al., 2016). However, recent studies have proposed that neuropsychiatric diseases not only are affected by ELS but also result from 2 (or more) stressors experienced over the lifespan based on the cumulative or diathesis stress hypothesis (Nederhof and Schmidt, 2012; Mc Elroy and Hevey, 2014). As stated, this classical theory hypothesizes that ELS elevates the risk for individuals to develop stress-related diseases in response to second or multiple stressors experienced during later life by programming effects on the nervous system (Lesse et al., 2017; Peña et al., 2017). However, the exact molecular mechanisms underlying ELS-generated vulnerability to adulthood second stressors are still unclear.
Epigenetics has been increasingly recognized as an important factor in various psychiatric disorders (Ju et al., 2016). One well-studied form of epigenetic processes is DNA methylation, a covalent modification of DNA catalyzed by DNA methyltransferases (DNMTs) (Goll and Bestor, 2005). ELS has been well established to potentially cause DNA methylation alterations in several neurotransmitter genes (Alelu-Paz et al., 2015). In addition, a previous study suggested that maternal care alters DNA methylation and has long-term effects on gene expression following a second stress in adulthood (Weaver et al., 2004). Interestingly, our previous works and other studies have suggested that ELS alters DNA methylation and Reelin gene transcription in the hippocampus (Qin et al., 2011; Gross et al., 2012; Zhang et al., 2013).
Reelin is a large extracellular matrix glycoprotein that is involved in the etiopathology of several psychiatric disorders (Fatemi et al., 2000). Recently, a series of studies showed that Reelin is also crucial for synaptic plasticity, the formation of fear memory, and long-term potentiation (LTP) in the postnatal hippocampus (Weeber et al., 2002; Qiu et al., 2006; Levenson et al., 2008). Numerous sites of the Reelin gene appear to be susceptible to methylation, potentially leading to inhibition of Reelin gene expression, and are associated with memory formation and cognition (Levenson et al., 2008). The hippocampus is involved in fear memory formation and has been highlighted to play a key role in the etiology of PTSD (Alvarez et al., 2008; Acheson et al., 2012; Maren et al., 2013). The several different subfields of the hippocampus might play distinct roles in contextual fear conditioning (CFC). For example, the CA1 area has been shown to be involved in the rapid acquisition of CFC and the retrieval of contextual memory after a long time (i.e., 24 hours) as well as the consolidation process, which is specifically important for long-term contextual fear memory (Lee and Kesner, 2004; Daumas et al., 2005; Ji and Maren, 2008; Langston et al., 2010). In contrast, the dentate gyrus and CA3 areas have primarily been associated with the rapid acquisition and retrieval of contextual memory (Lee and Kesner, 2004; Daumas et al., 2005; Langston et al., 2010). Meanwhile, the CA1 area was found to be an important output structure of the hippocampal network (Lee and Kesner, 2004). Previous studies generally focused on the effects of Reelin gene methylation on resilience to PTSD after a single ELS at a young age but ignored the influence of a second stress in adulthood (Qin et al., 2011; Palacios-García et al., 2015; Ju et al., 2016). Therefore, we hypothesized that Reelin gene methylation in the adult hippocampal CA1 might be altered by ELS and involved in PTSD susceptibility after a second stress in adulthood.
The aim of this study was to clarify whether Reelin promoter methylation in the adult hippocampal CA1 induced by ELS is involved in the development of PTSD vulnerability after a second stress in adulthood. Maternal deprivation (MD), one of the most common animal models for inducing ELS (Qin et al., 2011), was used as a potential influencing factor of PTSD, while the CFC paradigm mimicked the second stress and reexperiencing symptom of PTSD in adulthood in this study.
Methods
Animals
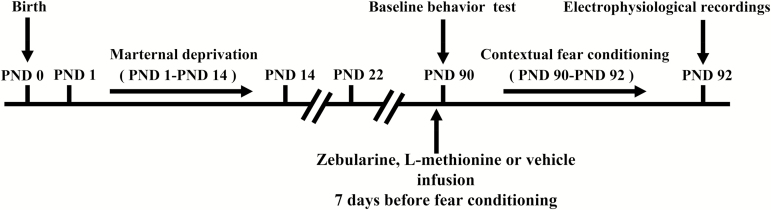
Primiparous pregnant Sprague-Dawley rats were housed individually in standard polypropylene cages with wood-chip bedding and standard rat chow and water available ad libitum. The rats (see Figure 1 for experimental setup) were kept in a temperature-controlled room (23°C±1°C) on a 12-hour-light/-dark cycle (lights on at 8:00 am). All procedures (see Figure 1 for experimental setup) were approved by the Institutional Animal Care and Use Committee of the Zhongshan School of Medicine, Sun Yat-sen University, and complied with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Figure 1.
Schematic representation of a timeline for the experimental methods.
MD Procedure (First Stress)
The birth day was designated as postnatal day 0 (PND 0). The MD procedure was conducted as previously described (Barreau et al., 2004). After PND 1, each pup was randomly assigned to either the MD group or mother-reared control (MRC) group. During MD (PND 1–14), all pups were removed from their home cages and placed into an individual clean cage where the ambient temperature was controlled at 28°C ± 1°C. Then, the new cages were moved to another room for 3 hours daily (from 9:00 am to 12:00 pm). The rat pups of the MRC group were not removed from the home cage until weaning. All rat pups were weaned on PND 22, at which time 6 same-sex rats were housed in each cage until PND 90. Only male pups were used for the experiments. Rats were weighed on PND 22 and 90.
CFC Procedure (Second Stress)
CFC was conducted on PND 90 and served as the second stressor. All rats were allowed to habituate for 5 minutes to the conditioning chamber on day 1. On day 2, the animals were placed in the conditioning chamber (conditioned stimulus) and received 3 footshocks (0.6 mA, 2 seconds) at 1-minute intervals after a 2-minute acclimation period in the chamber. All rats were retained for another 1 minute in the chamber after the last footshook. After conditioning, rats were placed in their home cages and returned to the rearing room. Twenty-four hours after fear conditioning, all the conditioned rats were returned to the chamber for 5 minutes to test for long-term contextual fear.
Cannula Implantation
For stereotaxic surgery, rats were anesthetized with 2% pentobarbital sodium. Anesthetized rats were secured on a stereotaxic frame (68001; RWD Life Science, Shenzhen, China). Cannulas (62001; RWD Life Science) were bilaterally implanted in the CA1 region of the dorsal hippocampus (AP: -3.8 mm relative to bregma; ML:±2.5 mm; DV: -3.5 mm from skull) (Miller and Sweatt, 2007). Rats were housed individually for 7 days for postoperative recovery before drug infusion. Accurate cannula placement was confirmed by postmortem histological analysis.
Drugs
Zebularine (Sigma) was dissolved in 10% DMSO and diluted to a concentration of 600 ng/mL in 0.9% sterile saline (Roth et al., 2009). L-methionine (Sigma) was dissolved and diluted to a concentration of 100 μg/mL in 0.9% sterile saline (Weaver et al., 2005).
Intra-CA1 Infusion
After 7 days of postoperative recovery as described above, MRC rats received a single infusion every day for 7 consecutive days before CFC. A total 2 μL of each drug was bilaterally infused at a rate of 0.5 μL/min for 2 minutes. Infusion cannulas were left in place for 3 minutes after the infusion to allow for diffusion of the drug.
Isolation of the CA1 Area
On PND 22, PND 90, and after completion of CFC, rats were killed and brains were rapidly removed. The CA1 was dissected from the other hippocampal subfields under a dissecting microscope, immediately frozen in liquid nitrogen, and stored at -80°C (Miller and Sweatt, 2007).
Real-Time Reverse Transcription Quantitative PCR
RNA extraction was conducted on CA1 tissue from rats that were killed on PND 22. RNA was extracted using Trizol (Invitrogen) and further purified with CHCl3. The concentrations of the RNA samples were determined spectrophotometrically, and reverse transcription (RT)-PCR was performed with commercially available reagents (AORT-0060, GeneCopoeia) following the manufacturer’s protocol. DNMT mRNA levels were measured by quantitative PCR (qPCR) using commercially available super SYBR Green PCR Mix (QP002, GeneCopoeia) and primers for DNMT1, DNMT3A, DNMT3B, and β-actin (RQP051580, RQP044961, RQP044963, RQP051050, GeneCopoeia). All primers were designed to span exon boundaries, ensuring amplification of only mRNA. Relative gene expression comparisons were carried out using β-actin as an invariant endogenous control. qPCRs and comparative Ct method were conducted according to previous protocols (Livak and Schmittgen, 2001; Pfaffl, 2001; Miller and Sweatt, 2007).
DNA Methylation Assay
DNA was extracted from the CA1 of the brain tissue from rats killed on PND 22 and 90. Genomic DNA was extracted and purified by using the standardized phenol-chloroform extraction method. Purified DNA was processed for bisulfite modification enabling high cytosine conversion rates of >99% (EpTect Bisulfite Kit, QIAGEN). The DNA methylation of the Reelin gene was determined using quantitative real-time PCR. The unmethylated Reelin DNA was detected using the following primers: forward (5’-TGTTAAATTTTTGTAGTATTGGGGATGT-3’) and reverse (5’-TCCTTAAAATAATCCAACAACACACC-3’). Methylated Reelin DNA was detected using the following primers: forward (5’-GGTGTTAAATTTTTGTAGTATTGGGGAC-3’) and reverse (5’-TCCTTAAAATAAT CCAACAACACGC-3’) (Miller and Sweatt, 2007). qPCRs and methylation index (%) equation were conducted according to previous protocols (Miller and Sweatt, 2007; Toda et al., 2014).
Western Blotting
Total protein extracted from the CA1 area of rat brain tissue was subjected to SDS-PAGE on 8% separating gels and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore). The membranes were blocked with 5% nonfat milk and then separately incubated with a 1:500 anti-Reelin monoclonal antibody (MAB5364, Millipore) and 1:1000 anti-β-actin antibody at 4°C overnight. Then the membranes were washed and incubated with a corresponding secondary antibody for 2 hours at room temperature. Reelin blots were normalized to actin blots for each individual lane. Optical densities of the individual bands were visualized using ImageJ for Windows.
Golgi-Cox Impregnation
Animals were anesthetized with 2% pentobarbital sodium and transcardially infused with 0.9% saline solution. Brains were rapidly removed. The whole-brain impregnation procedure was conducted using a kit (GMS80020.2. vA, Genmed Scientifics Inc.) as described in the manufacturer’s protocol, and the brain was then sectioned at a thickness of 180 μm on a vibratome (VT 1000S; Leica). The sections were stained, dehydrated, and coverslipped with mounting medium. The dendritic spines in the CA1 of the hippocampus were analyzed by counting the tertiary apical dendrites using a 100× oil microscope (BX51). Spine density was normalized as counted spines per 10 μm of dendrite length. Image acquisition and quantitative analysis method were conducted according to the previous protocols (Li et al., 2004; Chen et al., 2018).
Electrophysiological Recordings
Adult male animals were anesthetized with pentobarbital sodium and decapitated on PND 90 or after CFC. Brains were immediately removed and placed in oxygenated (95% O2/5% CO2) ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 120 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2.0 CaCl2, 2.0 MgSO4, 26 NaHCO3, and 10 glucose. Coronal hippocampal slices (360 μm) were prepared using a vibratome (VT 1000S, Leica). After the tissue was sectioned, hippocampal slices were allowed to recover in oxygenated ACSF at 30°C ±1°C for at least 90 minutes. The slices were gently placed into a recording chamber that was superfused (a flow rate of 4–5 mL/min) with oxygenated ACSF and maintained at 30°C. Field excitatory synaptic potentials (fEPSPs) were evoked with a 2-concentric bipolar stimulating electrode and recorded through glass pipettes (3–8 M, filled with ACSF) using Axon Multiclamp 1500A (Molecular Devices). A stimulating electrode evoked fEPSPs from the Schaffer collateral pathway at 0.05 Hz, and fEPSP responses at half of the maximum response were recorded in the stratum radiatum of the CA1. LTP was induced with high-frequency stimulation (HFS; 100 pulses at 100 Hz, 4 trains with 20-second intertrain intervals), and the average slope of 6 recording sweeps (across 2 minutes) was plotted. The magnitude of LTP was measured by the average slope of the recordings over the last 30 minutes (90 sweeps). Each group consisted of 4 to 5 slices obtained from 4 rats.
Statistical Analysis
SPSS Statistics version 19 was used for statistical analysis. Numerical data were analyzed by an unpaired t test, multiple t tests, ANOVA for repeated measures, 1-way ANOVA, or 2-way ANOVA followed by posthoc LSD test or posthoc Tukey test. In all cases, the significance level was set at P<.05. All data are shown as the means±SEM.
Results
Contextual Fear Memory Enhancement in Adulthood Was Induced by MD
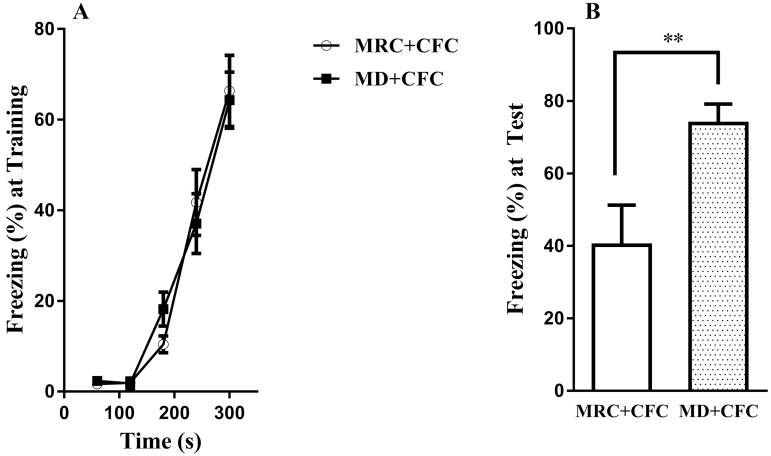
To determine whether MD affected the formation of fear memory in adulthood, a CFC paradigm, which is related to the functions of the hippocampal CA1, was performed. We found no significant difference in freezing level between the MRC+CFC and MD+CFC groups during fear conditioning training (F(1, 84)=0.02, P=.88) (Figure 2A), but the rats in the MD+CFC group showed a significantly higher level of freezing than those in the MRC+CFC group 24 hours posttraining (t(15)=3.00, P<.05) (Figure 2B), suggesting that MD enhanced contextual fear memory.
Figure 2.
Effects of maternal deprivation (MD) on contextual fear memory in adulthood. (A) The MD+contextual fear conditioning (CFC) and mother-reared control (MRC)+CFC groups acquired similar levels of freezing during fear conditioning training (n=7–10 per group). (B) A higher freezing level was exhibited in the MD+CFC group in the test performed 24 hours posttraining (n=7–10 per group). Data presented as the mean±SEM. **P<.01 compared with the MRC+CFC group.
Locomotor activity, anxiety-like behavior in a novel environment, and nociception were examined to ascertain whether CFC was influenced by baseline behaviors and perception of a footshock, which might be affected by MD. We found no significant difference between MRC and MD rat pups in body weight (t(20)=0.98, P=.34) (supplementary Figure 1A) or forelimb grasp reflex on PND 90 (t(10)=0.64, P=.54) (supplementary Figure 1B). In the open field test, the MD and MRC rats showed no significant differences in total distance (t(10)=0.22, P=.83) (supplementary Figure 1C) or percentage of the distance in the center zone (t(10)=0.04, P=.98) (supplementary Figure 1D). Furthermore, in the elevated plus maze, no significant effect of MD was found on the percent of entries in the open arm (t(16)=1.13, P=.28) or closed arm (t(16)=0.81, P=.43) (supplementary Figure 1E) or the percent of time spent in open arm (t(16)=0.67, P=.51) or closed arm (t(16)=0.46, P=.65) (supplementary Figure 1F). Finally, there was no significant difference found in the withdrawal thresholds between groups (F(1, 32)=0.05, P=.83) (supplementary Figure 1G). These results suggested that MD treatment had no obvious effect on somatic development, locomotion, habituation, thigmotaxis, anxiety-like behavior, or nociception in adulthood.
Hypomethylation of the Reelin Gene in the Hippocampal CA1 in Adulthood Was Induced by MD
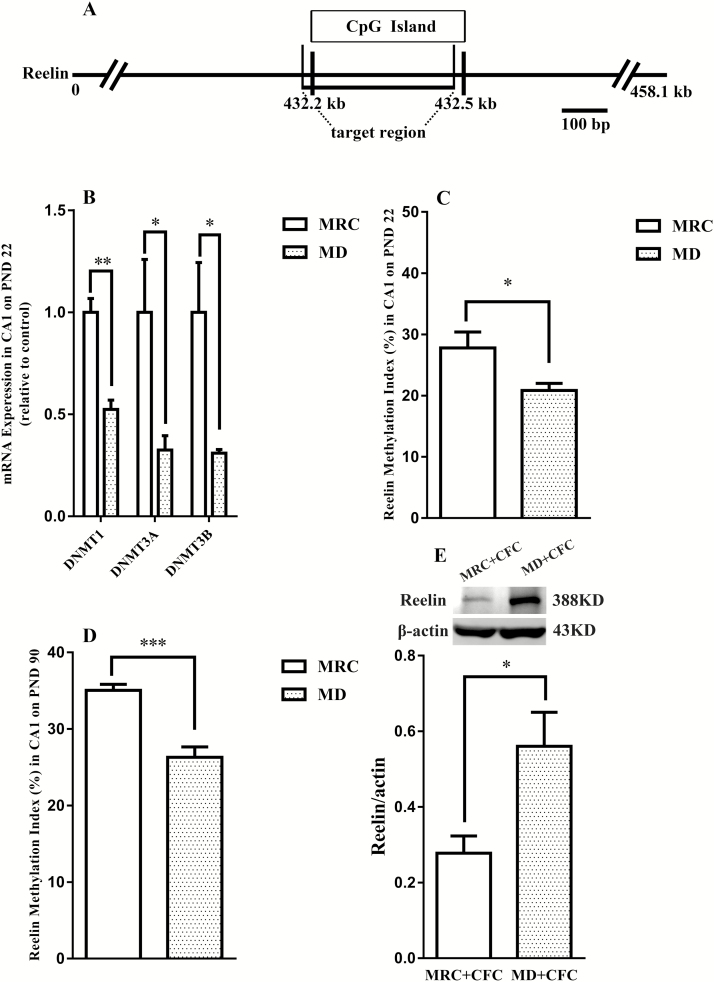
Our previous works have shown that ELS alters DNA methylation of the Reelin gene in the hippocampus on PND 22 (Qin et al., 2011). Therefore, we first investigated whether the expression of 3 DNMT isoforms, which catalyze DNA methylation (Levenson et al., 2008), in the CA1 area were altered by MD on PND 22. MD rats showed lower DNMT1, DNMT3A, and DNMT3B mRNA expression in the CA1 area than MRC rats (DNMT1: t(6)=5.82, P<.05; DNMT3A: t(6)=2.54, P<.05; DNMT3B: t(6)=2.93, P<.05) (Figure 3B).
Figure 3.
Effects of maternal deprivation (MD) on Reelin gene methylation in the hippocampal CA1. (A) Schematic representation of the location of the methylation changes. Primer sets, as described in Experimental Procedures, were designed to amplify the target region within a CpG island located in the Reelin gene. All base pair (bp) annotations are relative to the start of the promoter. The scale bar represents 100 bp (Miller and Sweatt, 2007). (B) DNA methyltransferase (DNMT)1, DNMT3A, and DNMT3B mRNA expression in the CA1 on postnatal day (PND) 22 was downregulated by MD (n=4 per group). MD decreased methylation of the Reelin gene on PND 22 (C) (n=6 per group) and PND 90 (D) (n=5–7 per group). (E) Protein expression of Reelin was increased by MD following contextual fear conditioning (CFC) (n=5 per group). Data presented as the mean±SEM. *P<.05, **P<.01, ***P<.001 compared with the mother-reared control (MRC) group or MRC+CFC group.
To evaluate whether the MD-induced hypomethylation of the Reelin gene was a long-lasting epigenetic mark in adulthood, its expression in the CA1 was examined on PND 22 and PND 90. We found that MD rats showed an obvious reduction in Reelin gene methylation on PND 22 (t(10)=2.44, P<.05) (Figure 3C) and PND 90 (t(10)=5.01, P<.001) (Figure 3D) compared with MRC rats. As the epigenetic changes are potentially associated with gene expression, we further examined whether the decreased Reelin gene methylation affected gene expression following CFC. We found a significantly greater level of Reelin protein in the MD+CFC group than in the MRC+CFC group 24 hours posttraining (t(8)=2.80, P<.05) (Figure 3E). These results showed that MD promoted the hypomethylation of the Reelin gene, which subsequently upregulated the expression of Reelin protein in the hippocampal CA1 area following CFC in adulthood.
Hypomethylation and Hypermethylation of the Reelin Gene Showed Opposite Effects on Contextual Fear Memory in Adulthood
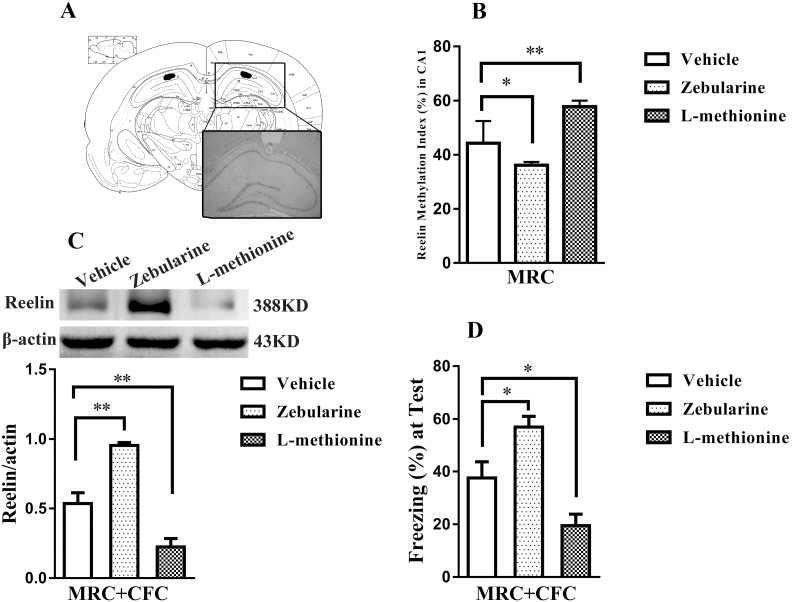
To determine whether Reelin gene DNA methylation was critically involved in contextual fear memory formation in adulthood, we examined the effects of Reelin DNA methylation on contextual fear memory and Reelin protein expression in the hippocampal CA1 area following CFC through performing intra-CA1 microinjections of zebularine or L-methionine in adult MRC rats. Zebularine is a DNA methyltransferase inhibitor that has been validated to cause DNA hypomethylation (Lubin et al., 2008). L-methionine, a methyl group donor for DNA methylation, has been proposed to cause DNA hypermethylation (Detich et al., 2003). The effects of drug treatment on Reelin DNA methylation were evident in our results (F(2, 13)=17.69, P<.001) (Figure 4B) and were in agreement with previous studies (Tremolizzo et al., 2002; Miller and Sweatt, 2007). Compared with the vehicle group, the zebularine group showed a significant reduction in Reelin gene methylation (LSD-t(13)=2.21, P<.05) (Figure 4B), while the L-methionine group revealed the opposite effect (LSD-t(13)=3.83, P<.05) (Figure 4B).
Figure 4.
Effects of Reelin gene hypomethylation or hypermethylation in the hippocampal CA1 on contextual fear memory. (A) Representative microphotograph depicting the injection site in the hippocampal CA1 (4.16 mm posterior to bregma). (B) The zebularine group showed a significant reduction in Reelin gene methylation, and the L-methionine group exhibited the opposite effects (n=5–6 per group). (C) The zebularine group showed higher Reelin protein expression than the vehicle group, whereas the L-methionine group showed the opposite effect (n=3–4 per group). (D) The zebularine group displayed a higher level of freezing, and the L-methionine group showed a lower level of freezing at 24 hours posttraining (n=6–7 per group). Data presented as the mean±SEM. *P<.05, **P<.01 compared with the vehicle group.
The expression of Reelin protein influenced by Reelin gene hypomethylation or hypermethylation in adulthood showed remarkable changes 24 h post training (F(2, 8)=31.26, P<.001) (Figure 4C). We found a significantly higher level of Reelin protein expression in the zebularine group (LSD-t(8)=4.54, P<.05) (Figure 4C) and an evidently lower level in the L-methionine group (LSD-t(8)=3.64, P<.05) (Figure 4C) compared to that in vehicle controls. These results suggested that hypomethylation and hypermethylation of the Reelin gene caused opposite alterations in Reelin gene expression in the hippocampal CA1.
The effects of DNA hypomethylation or hypermethylation on contextual fear memory were also evaluated by intra-CA1 microinjections of zebularine or L-methionine 7 days before training. Significant effects of drug treatments were observed on contextual fear memory 24 h posttraining (F(2, 17)=16.11, P<.05) (Figure 4D). Compared with the vehicle group, the zebularine group showed a significantly higher level of freezing (LSD-t(17)=2.82, P<.05) (Figure 4D), while the L-methionine group exhibited a significantly lower freezing level (LSD-t(17)=2.63, P<.05) (Figure 4D). Thus, our results confirmed that modifications of Reelin gene methylation play a crucial role in the formation of contextual fear memory.
Hippocampal LTP Induction was Facilitated by the Second Stress Compared with That in Adult Rats Who Experienced MD Alone
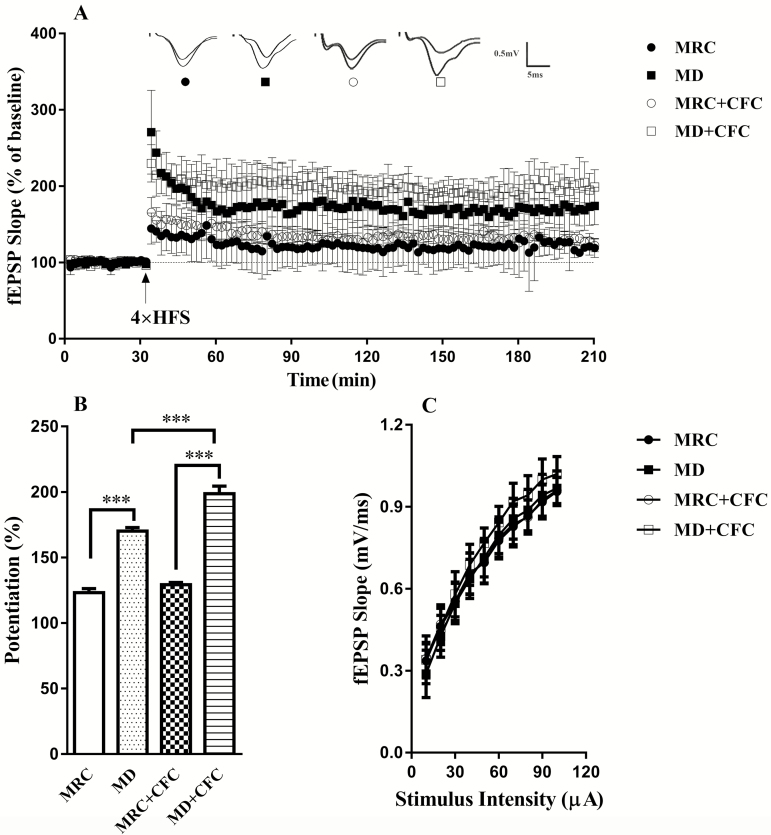
LTP is considered to be associated with learning and memory in the hippocampus (Bliss and Collingridge, 1993) at the cellular level, and lasting synaptic plasticity facilitates memory consolidation. To determine whether the fear memory-augmenting effect of MD was correlated with enhanced synaptic plasticity following a second stress, LTP in the hippocampal CA1 was measured in rats exposed to MD alone and in those exposed to MD+CFC. Two-way ANOVA revealed a significant interaction between MD and the second stress (F(1,280)=9.64, P<.05). Compared with slices from the MRC group, slices from the MD group exhibited a greater induction of LTP (MRC: 123.18±3.14%, MD: 170.22±2.59%, Tukey-t(280)=12.64, P<.001) (Figure 5B). CFC further facilitated hippocampal LTP in MD rats but showed no significant effects on LTP in MRC rats (MRC+CFC: 129.20±1.70%, MD+CFC: 198.68±5.81%, Tukey-t(280)=19.86, P<.001; MD: 170.22±2.59%, MD+CFC: 198.68±5.81%, Tukey-t(280)=7.82, P<.001; MRC: 123.18±3.14%, MRC+CFC:129.20±1.70%, Tukey-t(280)=1.68, P=.63) (Figure 5B). There was no significant difference in input-output curves or basal synaptic transmission at a series of stimulation intensities across groups (F(3,567)=0.12, P=.946, n=4–5 slices from 4 rats for each group) (Figure 5C). These results suggested that MD facilitated LTP induction in the hippocampal CA1 following CFC in adulthood.
Figure 5.
Effects of maternal deprivation (MD) on hippocampal long-term potentiation (LTP) after a second stress. (A) The field excitatory synaptic potential (fEPSP) slope was plotted from mother-reared control (MRC) slices (close circles), MD slices (close square), MRC+CFC slices (open circles), and MD+CFC slices (open square). Arrow indicates 4× high-frequency stimulation (HFS) (100 Hz, 1 second with 20-second intervals). (B) Histogram shows the average percentage of potentiation (150–180 min after HFS vs baseline). LTP was more easily induced in slices obtained from the MD group. Furthermore, CFC further facilitated the hippocampal LTP of MD rats but had no effect on MRC rats (n=4–5 slices from 4 rats for each group). (C) MD, CFC, and MD+CFC slices did not show a shift in the input-output (I-O) curve (n=4–5 slices from 4 rats for each group). Data presented as the mean±SEM. ***P<.001 compared with the MRC, MD, or MRC+CFC group.
Dendritic Spine Density in the Hippocampal CA1 Was Reversed by the Second Stress in Adulthood
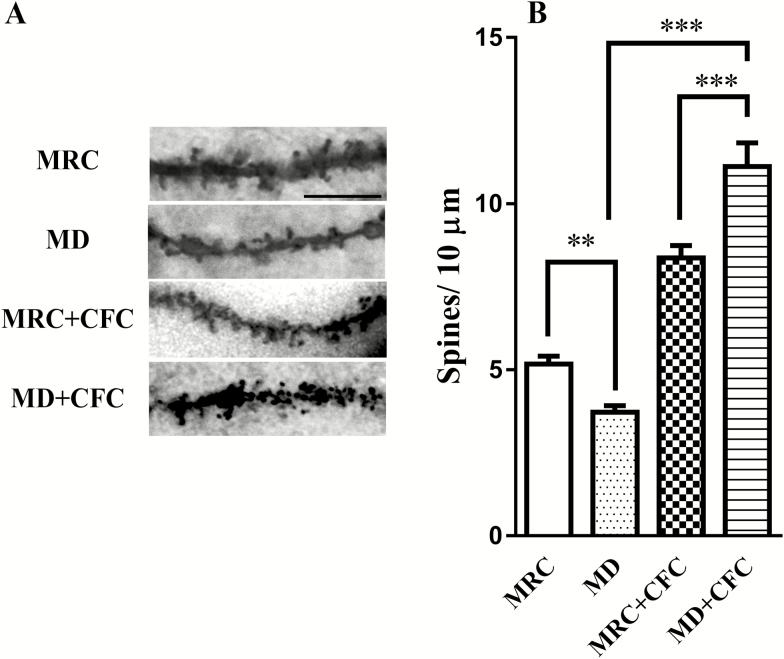
Furthermore, we examined whether the MD exposure-induced enhancement of fear memory cooccurred with structural synaptic plasticity following CFC through examining changes in dendritic spines pre- vs post-second-hit stress. Two-way ANOVA revealed a significant interaction between MD and the second stress (F(1,60)=38.58, P<.001). Furthermore, CFC reversed the reduction in dendritic spine density in the hippocampal CA1 caused by MD (MRC: 5.17±0.23, MD: 3.72±0.20, Tukey-t(60)=5.39, P<.01; MRC+CFC: 8.37±0.38, MD+CFC: 11.11±0.71, Tukey-t(60)=6.96, P<.001) (Figure 6B). The results showed that the dendritic spine density in the hippocampal CA1 was reversed by the second stress in adulthood.
Figure 6.
Effects of maternal deprivation (MD) on dendritic spines density in the hippocampal CA1 following the second stress. (A) Representative photomicrographs (×100) of tertiary apical dendrites of hippocampal CA1 pyramidal neurons. Scale bar: 5 μm. (B) The average spine density per oblique dendritic length of hippocampal CA1 pyramidal neurons was reversed in the MD+ contextual fear conditioning (CFC) group (n=7–19 slices from 4 rats for each group). Data presented as the mean±SEM. **P<.01, ***P<.001 compared with the MRC, MD, or MRC+CFC group.
Discussion
The major finding of the present study was that MD, a stressor applied in the early postnatal period, promoted the lifelong hypomethylation of the Reelin gene in the hippocampal CA1, which subsequently led to the upregulation of Reelin protein expression and the facilitation of LTP in the hippocampal CA1 area, further strengthening fear memory induced by a second stress in adulthood and potentially increasing the susceptibility of individuals to PTSD.
ELS has been demonstrated to increase the risk of developing PTSD in adulthood (Widom, 1999). The present study showed that MD did not affect normal baseline behavior or sensitivity to nociception that could affect CFC (Sultan et al., 2012). MD has the potential to alter the anxiety levels of rats, but there was no significant difference in baseline anxiety level among the groups in the present study. Meanwhile, the freezing level of the MD+CFC group was higher than that of the MRC+CFC group 24 hours posttraining. Several lines of evidence also support the observed MD-enhanced fear memory in adulthood (Oomen et al., 2010; Diehl et al., 2014; Toda et al., 2014). Our previous studies and other independent studies also reported altered Reelin mRNA and protein levels in the total hippocampus of ELS-exposed male rats at PND 90 (Gross et al., 2012; Zhang et al., 2013). Therefore, our present and previous study showed that destabilization of Reelin expression might disturb the response to a second stress in adulthood and alter the susceptibility to fear memory in MD rats. In contrast to our results, our previous study and some independent studies found that ELS hampered contextual fear memory and exerted anxiety-like behavior (Chocyk et al., 2014; Sun et al., 2014; Rana et al., 2015). However, we speculate that these discrepancies are dependent on different experimental conditions such as animal strain, sex, genetic background (Pryce and Feldon, 2003; Sun et al., 2014; Rana et al., 2015), experimental model, timing of ELS, and second stress procedure, which may cause differences in the stressful environment and the effects on the development of the brain (Lehmann et al., 1999; Meerlo et al., 1999; Chocyk et al., 2014; Pierce et al., 2014; Sun et al., 2014; Banqueri et al., 2017).
Gene-environment interactions, such as those occurring with ELS exposure, often lead to epigenetic changes in genes associated with the stress response and synaptic plasticity (Roth et al., 2009; Jawahar et al., 2015). Interestingly, in the current study, we found that MD decreased the expression of 3 DNMT subtypes and Reelin gene methylation in the CA1 area of the hippocampus on PND 22. Our results were in agreement with a previous study that reported that DNMT1 was the primary methyltransferase for the Reelin promoter (Noh et al., 2005). In addition, DNMT3A and DNMT3B may also work together to affect the methylation of the Reelin gene. Our previous study suggested that MD results in upregulation of Reelin gene methylation in the total hippocampus on postnatal day 22 (Qin et al., 2011), which seems to be inconsistent with the results of our present study. In fact, the alteration of Reelin gene methylation may differ across the subfields of the hippocampus in MD rats. Meanwhile, Reelin gene methylation level also changes dynamically from infancy to adulthood. An additional intriguing implication of the current study is that there might be a DNA demethylase regulated by MD in the hippocampal CA1. No specific DNA demethylase has been identified so far; however, current research suggests that there might be at least 2 candidates. Detich et al. have reported that overexpression of MBD2 in cell culture induced demethylation at a number of sites within the promoter region of 2 reporter genes (Detich et al., 2002). In addition, a recent study found that knockdown of Gadd45b, a regulator of active DNA demethylation, reduced expression of Reelin (Kigar et al., 2015). Our study implied the existence of a signaling cascade controlling the demethylation of the Reelin gene induced by MD and suggested that the demethylase might be identifiable in the hippocampal CA1 region. Future studies are needed to investigate the upstream molecules that may regulate DNA demethylase and DNMT activities (Levenson et al., 2004; Chwang et al., 2006). One upstream candidate molecule is extracellular signal-regulated kinase, an upstream kinase of a signaling cascade resulting in 2 chromatin modifications, phosphorylation and histone acetylation, which play an integral role in contextual fear memory (Levenson et al., 2004; Miller and Sweatt, 2007). Thus, DNMTs or demethylases might be a target for extracellular signal-regulated kinase as a means of translating general signals during MD. Furthermore, the MD-induced Reelin gene hypomethylation not only was found in early life but also had profound effects in adulthood. The relationship between the downregulation of Reelin gene methylation in adulthood and increased contextual fear memory was causative. Hypomethylation of the Reelin gene and increased Reelin protein levels caused by zebularine pretreatment in adult rats resulted in enhanced contextual fear memory, while L-methionine had the opposite effect. Furthermore, numerous studies have also suggested that contextual fear memory could be affected by the level of Reelin protein in the hippocampus (Weeber et al., 2002; Qiu et al., 2006; Reichelt et al., 2015). However, a previous study reported that infusion of zebularine immediately after CFC impaired fear memory (Miller and Sweatt, 2007). The observed inconsistencies might be due to the differences in drug dose, the time of injection, and the CFC procedures. Furthermore, zebularine infusion at different times of fear conditioning has been shown to have different effects on fear memory (Miller and Sweatt, 2007). Consistent with our findings, another study found that zebularine increased brain-derived neurotrophic factor mRNA expression and enhanced fear memory (Lubin et al., 2008). Together, our results provide compelling evidence that MD promoted the persistent hypomethylation of the Reelin gene, which was associated with downregulated DNMT expression, and finally facilitated the formation of fear memory induced by a second stress in adulthood. Furthermore, a previous study found that Reelin mRNA expression and the density of Reelin-synthesizing cells in the hippocampus during adulthood were not altered by MD alone (Gross et al., 2012), which suggested that MD-induced hypomethylation of the Reelin gene might be insufficient to alter Reelin expression. However, MD might prime a “stress-like” state in the hippocampal CA1 area, which could be activated by a second stress in adulthood, leading to upregulation of Reelin protein expression and elevated sensitivity to the second stress. Together, we concluded that MD rats might be less capable of dealing with a second stress in adulthood, predisposing them to the development of enhanced contextual fear memory.
LTP is the most widely used model to study learning and memory underlying neuronal plasticity in the hippocampus (Bliss and Collingridge, 1993). Dendritic spines are principal loci of activity-dependent synaptic rearrangements and often correlate with the functional changes associated with synaptic plasticity (Briner et al., 2011; Penzes et al., 2011). We found that LTP was more easily induced in MD rat slices on PND 90, in accordance with the results of previous studies (Zhang et al., 2014; Derks et al., 2016). Our study also showed that the second stress could further facilitate the induction of hippocampal LTP in rats that experienced MD, while the second stress had no effect on LTP in the MRC group. These results are in line with a previous study showing that CFC did not affect hippocampal fEPSP LTP (Abrari et al., 2009; Simoes et al., 2016) but do not mean that there was no behavioral effect on hippocampal potentiation. Some studies have indicated that different behavior interventions induce different forms of hippocampal potentiation (e.g., population spike and long-term depression) (Abrari et al., 2009; Aarse et al., 2016). Taken together, our results further suggested that MD might enhance contextual fear memory via hippocampal fEPSP LTP facilitated by a second stress. In addition, the change in dendritic spine density in the hippocampal CA1 was reversed by the second stress in adulthood. In line with our study, previous studies have shown that hippocampal spine formation is attenuated by ELS alone (Monroy et al., 2010; Ohta et al., 2017), while an acute stressful event could greatly increase the presence of dendritic spines (Dalla et al., 2009). These observations suggest that the reversal of the change in dendritic spine density might result from an MD-induced enhanced vulnerability to a second stress in adulthood. The Reelin protein, the expression of which was upregulated by MD-induced hypomethylation of the gene following the second stress, and the relevant receptor might be involved, as they have been shown to affect neuroplasticity and neuronal structure in the hippocampal CA1 (Stranahan et al., 2013). Reelin increased Disabled-1 and cAMP-response element binding protein activation and enhanced dendritic spine density in the hippocampal CA1 in the context of improved CA1 LTP (Rogers et al., 2011). Two major forms of N-methyl-D-aspartate receptors (NR) 2 subunits in the hippocampus, NR2A and NR2B, were developmentally regulated, subjected to activity- as well as behavior-induced changes, and determined the polarity of synaptic plasticity under certain circumstances (Levenson et al., 2008). Reelin was required for the normal developmental switch from NR2B to NR2A in the hippocampus (Sinagra et al., 2005). NR2A has been proposed to be more likely to favor the induction of LTP and control the direction of LTP than NR2B (Iafrati et al., 2014). Moreover, both NR2A and NR2B could be tyrosine phosphorylated in response to synaptic activity by Reelin (Beffert et al., 2005). Thus, the activation of Disabled-1 and cAMP-response element binding protein, the alteration of NMDAR activity by changes in subunit composition, the modulation of NMDAR conductivity via phosphorylation by Reelin, which is regulated by the MD-induced alteration of Reelin gene methylation following a second stress, might impact LTP induction and contextual fear memory in adulthood.
The major limitation of our study was that we only investigated one synaptic plasticity-related gene regulated by DNA methylation. Whether other genes were involved in the MD-induced enhanced fear memory remains unclear, and the roles of other genes and epigenetic programming should be investigated in the future.
In conclusion, our results suggest that MD promoted the lifelong hypomethylation of the Reelin gene in the hippocampal CA1, which subsequently upregulated the expression of the Reelin protein and further facilitated the induction of LTP, finally strengthening the vulnerability to fear memory following a second stress in adulthood. Importantly, these findings could be explained within the framework of the cumulative stress model, which is based on the hypothesis that individual genes interact with environmental factors (Nederhof and Schmidt, 2012; Daskalakis et al., 2013; Mc Elroy and Hevey, 2014). A great number of studies have revealed that ELS results in significant epigenetic changes of genes in or out of the hypothalamic–pituitary–adrenal axis such as glucocorticoid receptor, arginine vasopressin, and brain-derived neurotrophic factor (Gray et al., 2014; Jawahar et al., 2015). Recent studies have suggested that compared with a combination of different stressors, a single stressor results in different gene expression activation patterns, further indicating that a history of stress indeed permanently alters stress susceptibility and gene expression patterns in the hippocampus following a novel stressor, which might be regulated by epigenetic variation-induced programming effects (Lesse et al., 2017). Altogether, our findings provide further evidence for long-term epigenetic alterations induced by ELS that alter the susceptibility for contextual fear memory and the mechanism for the programming effects of ELS on gene expression in response to a second stressor in later life.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Funding
This work was supported by the Natural Science Foundation of China (Grant numbers 81273350, 81471829, 81530061).
Supplementary Material
Acknowledgments
We thank Muhammad Asim for English editing assistance and declare that the authors are entirely responsible for the scientific content of the paper.
Statement of Interest
None.
References
- Aarse J, Herlitze S, Manahan-Vaughan D(2016)The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent. Hippocampus 26:739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y, Jadid M(2009)Post-training administration of corticosterone enhances consolidation of contextual fear memory and hippocampal long-term potentiation in rats. Neurobiol Learn Mem 91:260–265. [DOI] [PubMed] [Google Scholar]
- Acheson DT, Gresack JE, Risbrough VB(2012)Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62:674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alelú-Paz R, González-Corpas A, Ashour N, Escanilla A, Monje A, Guerrero Márquez C, Algora Weber M, Ropero S(2015)DNA methylation pattern of gene promoters of major neurotransmitter systems in older patients with schizophrenia with severe and mild cognitive impairment. Int J Geriatr Psychiatry 30:558–565. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C(2008)Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci 28:6211–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banqueri M, Méndez M, Arias JL(2017)Behavioral effects in adolescence and early adulthood in two length models of maternal separation in male rats. Behav Brain Res 324:77–86. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L(2004)Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut 53:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J(2005)Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 47:567–579. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL(1993)A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39. [DOI] [PubMed] [Google Scholar]
- Block SR, Liberzon I(2016)Attentional processes in posttraumatic stress disorder and the associated changes in neural functioning. Exp Neurol 284:153–167. [DOI] [PubMed] [Google Scholar]
- Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L(2011)Developmental stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology 115:282–293. [DOI] [PubMed] [Google Scholar]
- Chen YF, Chen ZX, Wang RH, Shi YW, Xue L, Wang XG, Zhao H(2018)Knockdown of CLC-3 in the hippocampal CA1 impairs contextual fear memory. Prog Neuropsychopharmacol Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Chocyk A, Przyborowska A, Makuch W, Majcher-Maślanka I, Dudys D, Wędzony K(2014)The effects of early-life adversity on fear memories in adolescent rats and their persistence into adulthood. Behav Brain Res 264:161–172. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD(2006)ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem 13:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CS, Callaghan BL, Kan JM, Richardson R(2016)The lasting impact of early-life adversity on individuals and their descendants: potential mechanisms and hope for intervention. Genes Brain Behav 15:155–168. [DOI] [PubMed] [Google Scholar]
- Dalla C, Whetstone AS, Hodes GE, Shors TJ(2009)Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling vs masculinized females. Neurosci Lett 449:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER(2013)The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38:1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S, Halley H, Francés B, Lassalle JM(2005)Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn Mem 12:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks NA, Krugers HJ, Hoogenraad CC, Joëls M, Sarabdjitsingh RA(2016)Effects of early life stress on synaptic plasticity in the developing hippocampus of male and female rats. Plos One 11:e0164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detich N, Hamm S, Just G, Knox JD, Szyf M(2003)The methyl donor S-Adenosylmethionine inhibits active demethylation of DNA: a candidate novel mechanism for the pharmacological effects of S-Adenosylmethionine. J Biol Chem 278:20812–20820. [DOI] [PubMed] [Google Scholar]
- Detich N, Theberge J, Szyf M(2002)Promoter-specific activation and demethylation by MBD2/demethylase. J Biol Chem 277:35791–35794. [DOI] [PubMed] [Google Scholar]
- Diehl LA, Pereira Nde S, Laureano DP, Benitz AN, Noschang C, Ferreira AG, Scherer EB, Machado FR, Henriques TP, Wyse AT, Molina V, Dalmaz C(2014)Contextual fear conditioning in maternal separated rats: the amygdala as a site for alterations. Neurochem Res 39:384–393. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T(2000)Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry 5:654–663, 571. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH(2005)Eukaryotic cytosine methyltransferases. Annu Rev Biochem 74:481–514. [DOI] [PubMed] [Google Scholar]
- Gray JD, Rubin TG, Hunter RG, McEwen BS(2014)Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry 19:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CM, Flubacher A, Tinnes S, Heyer A, Scheller M, Herpfer I, Berger M, Frotscher M, Lieb K, Haas CA(2012)Early life stress stimulates hippocampal reelin gene expression in a sex-specific manner: evidence for corticosterone-mediated action. Hippocampus 22:409–420. [DOI] [PubMed] [Google Scholar]
- Iafrati J, Orejarena MJ, Lassalle O, Bouamrane L, Gonzalez-Campo C, Chavis P(2014)Reelin, an extracellular matrix protein linked to early onset psychiatric diseases, drives postnatal development of the prefrontal cortex via GluN2B-NMDARs and the mTOR pathway. Mol Psychiatry 19:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawahar MC, Murgatroyd C, Harrison EL, Baune BT(2015)Epigenetic alterations following early postnatal stress: a review on novel aetiological mechanisms of common psychiatric disorders. Clin Epigenetics 7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S(2008)Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem 15:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju LS, Jia M, Sun J, Sun XR, Zhang H, Ji MH, Yang JJ, Wang ZY(2016)Hypermethylation of hippocampal synaptic plasticity-related genes is involved in neonatal sevoflurane exposure-induced cognitive impairments in rats. Neurotox Res 29:243–255. [DOI] [PubMed] [Google Scholar]
- Kigar SL, Chang L, Auger AP(2015)Gadd45b is an epigenetic regulator of juvenile social behavior and alters local pro-inflammatory cytokine production in the rodent amygdala. Brain Behav Immun 46:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, Stevenson CH, Wilson CL, Saunders I, Wood ER(2010)The role of hippocampal subregions in memory for stimulus associations. Behav Brain Res 215:275–291. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP(2004)Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus 14:301–310. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Bettschen D, Feldon J(1999)The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav 64:705–715. [DOI] [PubMed] [Google Scholar]
- Lesse A, Rether K, Gröger N, Braun K, Bock J(2017)Chronic postnatal stress induces depressive-like behavior in male mice and programs second-hit stress-induced gene expression patterns of OxtR and AvpR1a in adulthood. Mol Neurobiol 54:4813–4819. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD (2004) Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279:40545–40559. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Qiu S, Weeber EJ(2008)The role of reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene. Biochim Biophys Acta 1779:422–431. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS(2004)Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A 101:2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD(2001)Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD(2008)Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci 28:10576–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I(2013)The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Elroy S, Hevey D(2014)Relationship between adverse early experiences, stressors, psychosocial resources and wellbeing. Child Abuse Negl 38:65–75. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Horvath KM, Nagy GM, Bohus B, Koolhaas JM(1999)The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J Neuroendocrinol 11:925–933. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD(2007)Covalent modification of DNA regulates memory formation. Neuron 53:857–869. [DOI] [PubMed] [Google Scholar]
- Monroy E, Hernández-Torres E, Flores G(2010)Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat 40:93–101. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Schmidt MV(2012)Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav 106:691–700. [DOI] [PubMed] [Google Scholar]
- Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y, Costa E, Guidotti A, Grayson DR(2005)DNA methyltransferase 1 regulates reelin mrna expression in mouse primary cortical cultures. Proc Natl Acad Sci U S A 102:1749–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta KI, Suzuki S, Warita K, Kaji T, Kusaka T, Miki T(2017)Prolonged maternal separation attenuates BDNF-ERK signaling correlated with spine formation in the hippocampus during early brain development. J Neurochem 141:179–194. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joëls M, Lucassen PJ, Krugers H(2010)Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci 30:6635–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-García I, Lara-Vásquez A, Montiel JF, Díaz-Véliz GF, Sepúlveda H, Utreras E, Montecino M, González-Billault C, Aboitiz F(2015)Prenatal stress down-regulates Reelin expression by methylation of its promoter and induces adult behavioral impairments in rats. Plos One 10:e0117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, Issler O, Loh YE, Leong T, Kiraly DD, Goodman E, Neve RL, Shen L, Nestler EJ(2017)Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356:1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM(2011)Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW.(2001)A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AN, Ryals JM, Wang R, Christianson JA(2014)Vaginal hypersensitivity and hypothalamic-pituitary-adrenal axis dysfunction as a result of neonatal maternal separation in female mice. Neuroscience 263:216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Feldon J(2003)Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev 27:57–71. [DOI] [PubMed] [Google Scholar]
- Qin L, Tu W, Sun X, Zhang J, Chen Y, Zhao H(2011)Retardation of neurobehavioral development and reelin down-regulation regulated by further DNA methylation in the hippocampus of the rat pups are associated with maternal deprivation. Behav Brain Res 217:142–147. [DOI] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ(2006)Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem 85:228–242. [DOI] [PubMed] [Google Scholar]
- Rana S, Pugh PC, Jackson N, Clinton SM, Kerman IA(2015)Inborn stress reactivity shapes adult behavioral consequences of early-life maternal separation stress. Neurosci Lett 584:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC, Maniam J, Westbrook RF, Morris MJ(2015)Dietary-induced obesity disrupts trace fear conditioning and decreases hippocampal reelin expression. Brain Behav Immun 43:68–75. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Rusiana I, Trotter J, Zhao L, Donaldson E, Pak DT, Babus LW, Peters M, Banko JL, Chavis P, Rebeck GW, Hoe HS, Weeber EJ(2011)Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem 18:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD(2009)Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões AP, Machado NJ, Gonçalves N, Kaster MP, Simões AT, Nunes A, Pereira de Almeida L, Goosens KA, Rial D, Cunha RA(2016)Adenosine A2a receptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology 41:2862–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, Weeber EJ, Manzoni OJ, Chavis P(2005)Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro. J Neurosci 25:6127–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Erion JR, Wosiski-Kuhn M(2013)Reelin signaling in development, maintenance, and plasticity of neural networks. Ageing Res Rev 12:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan FA, Wang J, Tront J, Liebermann DA, Sweatt JD(2012)Genetic deletion of Gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity. J Neurosci 32:17059–17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XM, Tu WQ, Shi YW, Xue L, Zhao H(2014)Female-dependent impaired fear memory of adult rats induced by maternal separation, and screening of possible related genes in the hippocampal CA1. Behav Brain Res 267:111–118. [DOI] [PubMed] [Google Scholar]
- Toda H, Boku S, Nakagawa S, Inoue T, Kato A, Takamura N, Song N, Nibuya M, Koyama T, Kusumi I(2014)Maternal separation enhances conditioned fear and decreases the mRNA levels of the neurotensin receptor 1 gene with hypermethylation of this gene in the rat amygdala. Plos One 9:e97421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, Guidotti A(2002)An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci U S A 99:17095–17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ(2004)Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M(2005)Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25:11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J(2002)Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem 277:39944–39952. [DOI] [PubMed] [Google Scholar]
- Widom CS.(1999)Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry 156:1223–1229. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qin L, Zhao H(2013)Early repeated maternal separation induces alterations of hippocampus reelin expression in rats. J Biosci 38:27–33. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang B, Jin J, An S, Zeng Q, Duan Y, Yang L, Ma J, Cao X(2014)Early deprivation reduced anxiety and enhanced memory in adult male rats. Brain Res Bull 108:44–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.