Abstract
Background
In rodent models, chronic exposure to cannabis’ psychoactive ingredient, Δ9-tetrahydrocannabinol, during adolescence leads to abnormal behavior in adulthood. In female rats, this maladaptive behavior is characterized by endophenotypes for depressive-like and psychotic-like disorders as well as cognitive deficits. We recently reported that most depressive-like behaviors triggered by adolescent Δ9-tetrahydrocannabinol exposure can be rescued by manipulating endocannabinoid signaling in adulthood with the anandamide-inactivating enzyme FAAH inhibitor, URB597. However, the molecular mechanisms underlying URB597’s antidepressant-like properties remain to be established.
Methods
Here we examined the impact of adult URB597 treatment on the cellular and functional neuroadaptations that occurred in the prefrontal cortex and dentate gyrus of the hippocampus upon Δ9-tetrahydrocannabinol during adolescence through biochemical, morphofunctional, and electrophysiological studies.
Results
We found that the positive action of URB597 is associated with the rescue of Δ9-tetrahydrocannabinol-induced deficits in endocannabinoid-mediated signaling and synaptic plasticity in the prefrontal cortex and the recovery of functional neurogenesis in the dentate gyrus of the hippocampus. Moreover, the rescue property of URB597 on depressive-like behavior requires the activity of the CB1 cannabinoid receptor.
Conclusions
By providing novel insights into the cellular and molecular mechanisms of URB597 at defined cortical and hippocampal circuits, our results highlight that positive modulation of endocannabinoid-signaling could be a strategy for treating mood alterations secondary to adolescent cannabis use.
Keywords: adolescent THC, depressive-like behavior, endocannabinoid-mediated LTD, newborn neuron, URB597
Significance Statement
Adolescent THC exposure causes the development of depressive-like behaviors in adulthood in female rats, which can be rescued by the FAAH inhibitor URB597. We here provide novel insights into the cellular modifications that are associated with URB597 treatment, which may contribute to the ameliorative effects of this drug on the emotional impairments caused by adolescent THC exposure. These findings might pave the way to positive anandamide modulation as a treatment option for people suffering from depression secondary to previous cannabis abuse.
Introduction
Growing evidence from both epidemiological and experimental studies suggests that exposure to cannabis or its psychoactive ingredient, Δ9-tetrahydrocannabinol (THC), during the adolescent developmental window may be a risk factor for mental illness later in life (for reviews, see: Hall and Degenhardt 2009; Bossong and Niesink 2010; Malone et al. 2010; Rubino and Parolaro 2016). Consistent with this, we recently reported that chronic THC treatment during adolescence (i.e., from postnatal day 35 to 45) in female rats leads to abnormal behavior in adulthood, characterized by endophenotypes for depressive-like and psychotic-like disorders, as well as cognitive deficits (Rubino et al., 2008, 2009a; Realini et al., 2011; Zamberletti et al., 2014). It is worth noting that the same THC treatment in adolescent male rats triggers the development in adulthood of a less complex phenotype limited to psychotic-like signs and cognitive deficits (Rubino et al., 2008, 2009b; Zamberletti et al., 2016). This sex difference prompted us to deepen the investigation in female rats where the behavioral phenotype is associated with a decrease in CB1 receptor binding, diminished production of the endocannabinoid anandamide (AEA), deficits in endocannabinoid-mediated long-term synaptic depression (LTD) in the prefrontal cortex (PFC), and decreased cell proliferation in the dentate gyrus (DG) of the hippocampus (Realini et al., 2011; Rubino et al., 2015).
Potentiation of AEA signaling has been suggested as a potential antidepressant strategy (Bambico and Gobbi, 2008; Micale et al., 2013; Fowler, 2015). Consistent with this idea, both pharmacological blockade and genetic deletion of the enzyme responsible for AEA degradation, fatty acid amide hydrolase (FAAH), improve performance in the forced swim and tail suspension tests, the most widely used rodent behavioral assays in which immobility is used to assess the effectiveness of antidepressant drugs (Gobbi et al. 2005; Naidu et al. 2007; Adamczyk et al. 2008; Bambico et al. 2010). The potential antidepressant properties of FAAH inhibition have also been confirmed in animal models of depressive-like behaviors. Chronic treatment with the potent and selective FAAH inhibitor URB597 normalizes body weight and sucrose intake in rats exposed to chronic mild stress (Bortolato et al. 2007) and increases sucrose consumption and decreases immobility in the forced swim test in Wistar Kyoto rats (Vinod et al. 2012). Similarly, we reported that chronic URB597 treatment rescues most depressive-like behaviors triggered by adolescent THC exposure in female rats (Realini et al. 2011). Specifically, chronic URB597 treatment leads to recovery of the passive coping strategy in the forced swim test, anhedonia (measured as sucrose and palatable food consumption), and reduced social activity. Despite the potential implication for emotional control, the molecular mechanisms through which URB597 rescues behavioral dysfunctions induced by adolescent THC exposure remain to be established.
Preclinical and clinical evidence links major depression with structural and/or cellular changes in the PFC and hippocampus (for reviews, see Duman and Aghajanan, 2012; Godsil et al., 2013). Disturbances in emotional, cognitive, and autonomic regulation in mood disorders have been associated with dysfunctional connectivity within both PFC microcircuits and microcircuits formed by the PFC and its target limbic regions, including the hippocampus (Price and Drevets, 2010). Moreover, the hippocampal-PFC projection, which represents the major monosynaptic input to the PFC, plays a key role in cognitive processes and contextually dependent emotional regulation (Godsil et al., 2013). This evidence points at the PFC and hippocampal circuits as critical players for emotional control and as key substrates for some of the behavioral effects of chronic exposure to THC, which include depressive-like symptoms (Rubino et al., 2008; Realini et al., 2011).
In this study, for the first time we examined the impact of adult URB597 treatment on the cellular and functional neuroadaptations that occurred in the PFC and DG of the hippocampus upon THC during adolescence (Realini et al., 2011; Rubino et al., 2015). Specifically, we addressed the effects of URB597 on deficits of CB1 receptor-mediated signaling and plasticity in the PFC. We also surveyed URB597’s effect on morphofunctional and synaptic properties of adult hippocampal newborn neurons that, if dysfunctional, may contribute to some of the behavioral deficits associated with major depression (Perera et al., 2008; Bortolotto et al., 2014). Finally, we assessed the contribution of CB1 receptor activation to the amelioriative effects of adult URB597 treatment on depressive-like behavior caused by exposure to THC during adolescence.
METHODS
Animals
Female Sprague Dawley rats aged 28 days at arrival were obtained from Charles River Laboratories (Calco, LC, Italy) and were housed in groups of 4 in clear plastic cages on a 12-hour-light/-dark cycle (lights on 8:00 am) and in a temperature- (22±2°C) and humidity-controlled environment (50±10%). All animals were allowed free access to food and water. All experiments took place during the light phase and were performed in accordance with the guidelines released by the Italian Ministry of Health (D.L.2014/26) and the European Community directives regulating animal research (2010/63/EU). All efforts were made to minimize the number of animals used and their suffering.
Drugs
THC, a generous gift from GW Pharma Ltd, UK, was further purified to reach THC concentrations as high as 90% and dissolved in ethanol, cremophor, and saline (1:1:18). URB597 (Alexis Biochemicals, San Diego, CA) was dissolved in DMSO, Tween 80, and saline (1:1:8). AM251 (Tocris, UK) was dissolved in DMSO, Tween 80, and saline (1:1:8). All drugs were administered i.p. with an injection volume of 5 mL/kg.
Treatments
Adolescent THC Treatment
Female rats were injected with increasing doses of THC or vehicle twice a day from postnatal day (PND) 35 to PND 45 (2.5 mg/kg, PND 35–37; 5 mg/kg, PND 38–41; 10 mg/kg, PND 42–45) as previously described (Figure 1A; Rubino et al., 2008). This protocol recapitulates the effects of heavy marijuana use; according to the transformation in human equivalent dose proposed by the FDA and the average THC content of a joint, our low dose roughly corresponds to one joint, medium dose to 2 joints, and the high dose to 4 joints (Zamberletti et al., 2012; see for cannabis potency ElSohly et al., 2016).
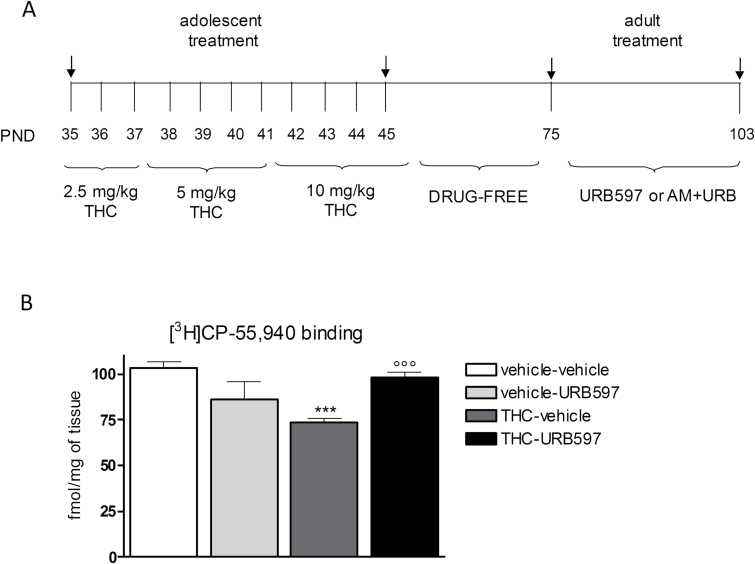
Figure 1.
(A) Treatment schedule: see Materials and Methods. AM: AM251; PND: postnatal day. (B) In the prefrontal cortex (PFC), adolescent Δ9-tetrahydrocannabinol (THC) exposure significantly reduced CB1 receptor density compared with controls. Chronic URB597 treatment completely restored CB1 receptor expression in THC-pretreated animals. Data are expressed as mean±SEM of n=4 rats/group (for each rat we considered the mean of 3 sections) and were analyzed by 2-way ANOVA followed by Tukey’s posthoc test. ***P<.001 vs vehicle; °P<.05 vs THC.
Chronic URB597 Administration
URB597 treatment was started as animals reached adulthood (PND 75) and lasted 4 weeks. During this period, rats received a daily URB597 injection at the dose of 0.3 mg/kg, as this dose was effective in rescuing behavioral impairments induced by adolescent THC exposure (Realini et al., 2011).
Another group of animals was given AM251 at the dose of 0.5 mg/kg i.p. concomitantly with URB597 administration to determine whether URB597 behavioral effects were mediated by CB1 receptors (Figure 1A). This AM251 dose was chosen because it is low enough to avoid the induction of behavioral effects per se but effective in preventing the stimulation of CB1 receptors (Fidelman et al., 2018).
Forced Swim Test
Behavioral testing was performed during the last day of URB597 treatment (PND 103). On the day of testing, animals were habituated in a quiet laboratory for 30 minutes before the experimental procedure began. Animals were tested in a modified version of the forced swim test with only the first session of swimming as previously described (Realini et al., 2011).
[3H]CP-55,940 Receptor Binding in Autoradiography
Twenty-four hours after the last URB597 injection, rats were decapitated and brains were rapidly removed, frozen in liquid nitrogen, and stored at -80°C until processing. Coronal sections (20 μm thick) were cut on a cryostat and mounted on gelatin-coated slides. The sections were stored at -80°C until processing.
[3H]CP-55,940 receptor binding was performed as previously described (Zamberletti et al., 2012).
Evaluation of Newly Generated Hippocampal Doublecortin (DCX)+ Cells
To assess the effect of drug/vehicle treatment on newly generated hippocampal neurons, at 24 hours after the last URB597 injection rats were transcardially perfused. Their brains were removed from the skull, post-fixed overnight at 4°C, and transferred first into a 15% sucrose solution, then 24 hours later into a 30% sucrose solution in phosphate-buffered saline. Coronal brain sections (40 μm thick) were then cut and prepared for immunofluorescence analysis with an anti-DCX antibody (1:200; Cell Signaling Technology Inc., Danvers, MA). As previously described (Meneghini et al., 2014), 15 DCX+ immunopositive cells (5 each in 3 different brain coronal sections) located in the DG blades and bifurcation were randomly chosen and their apical dendrite traced throughout its entire extension at 40x magnification using the Zeiss LSM Zen software. The number of DCX+ neuroblasts/mm3 in the section samples was evaluated using the same software.
Slice Preparation for Electrophysiology Studies
Coronal brain slices (350 μm thick) containing the medial PFC (mPFC) and transverse hippocampal brain slices (350 μm thick) were prepared as described (Bischofberger et al., 2006; Nazzaro et al., 2012). Electrophysiological recordings were performed 24 hours after the last injection of URB597 or vehicle. During experiments, slices were continuously superfused with aCFS (115 mM NaCl, 3.5 mM KCl, 1.2 mM NaH2PO4, 1.3 mM MgCl26H2O, 1.8 mM CaCl2, 25 mM NaHCO3, 25 mM D-glucose) at a rate of 2 mL/min at 28°C (mPFC) or 32°C (DG).
Electrophysiology
Extracellular recordings of field postsynaptic potentials (fPSP) were obtained in layer 5 (L5) of the mPFC or medial molecular layer of the DG using glass micropipettes filled with 3 M NaCl (mPFC recordings) or aCSF (DG recordings). Stimuli were delivered via a constant current isolated stimulator (Digitimer) through a bipolar twisted tungsten electrode placed in layer 2 (L2) of the mPFC (50–160 μA, 60 μs) or along the fibers of the medial perforant path (MPP) of the hippocampus (100–250 μA, 40 μs). In the DG, paired-pulse ratio was analyzed by calculating the ratio between the fPSP slope in response to the second and the first of twin stimuli (50 ms). The occurrence of paired-pulse depression confirmed the specificity of MPP stimulation (Colino and Malenka, 1993). mPFC LTD was induced using the following low-frequency stimulation (LFS) protocol: 10 Hz train for 10 minutes (Lafourcade et al., 2007; Rubino et al., 2015). DG long-term potentiation (LTP) was induced by a tetanic stimulation that consisted of 4×100 Hz trains of 0.5 seconds each repeated every 30 seconds (high-frequency stimulation [HFS]) (Snyder et al., 2001). In some DG experiments, 100 mM picrotoxin (PTX) was applied 15 minutes before LTP induction. Data were amplified and filtered (10 Hz to 3 kHz) by a differential amplifier (DAM 80 AC, World Precision Instruments) and digitized at 10 kHz (Digidata 1322, Molecular Devices). fPSP were acquired every 30 seconds. Synaptic plasticity plots were generated by averaging the peak amplitude (mPFC) or the slope (DG) of individual fPSP in 2-minute bins.
Neurogenesis-dependent LTP requires activation of glutamate receptors containing the NR2B subunit and can be induced in the DG in the presence of intact GABAergic inhibition (Snyder et al., 2001). Conversely, the LTP of mature DGCs can be induced only when local GABAergic inhibition is blocked (Wang et al., 2000; Garthe et al., 2009; Sahay et al., 2011). Thus, to specifically induce newborn neuron LTP, experiments were performed in the presence of the GABA blocker picrotoxin. To confirm that LTP obtained under these experimental conditions is mediated by newborn neurons, we applied the NR2B antagonist Ro25-6981.
Statistical Analysis
Data were reported as mean±SEM and analyzed by 1-, 2-, or 3-way ANOVA with repeated measures where necessary, followed by Tukey’s posthoc test. Statistical significance was set at P<.05.
RESULTS
URB597 Rescues Biochemical and Synaptic Alterations Induced by Adolescent THC Exposure at PFC Circuits
The PFC is one of the main brain regions affected by adolescent THC exposure in female rats (Rubino et al., 2009a; 2015). THC-pretreated rats are characterized by a persistent reduction in CB1 receptor binding and AEA levels in the PFC. These cellular alterations are associated with deficits in endocannabinoid (eCB)-mediated LTD at mPFC L2→L5 microcircuits (eCB-LTD; Rubino et al., 2015). Therefore, we asked whether URB597 treatment was able to rescue these specific neuroadaptations.
CB1 Receptor Density
To determine the effect of adult URB597 treatment on CB1 receptor density within the PFC, we performed an autoradiographic-binding assay (Figure 1B). Two-way ANOVA revealed significant main effects of THC (F1,12=5.641; P=.0220) and THC x URB597 interaction (F1,12=31.92; P<.0001) on CB1 receptor density in the PFC. Adolescent THC exposure significantly reduced CB1 receptor density by about 30% compared with controls (P<.001). Chronic URB597 treatment completely reinstates proper CB1 expression in the PFC of THC-exposed female rats (Figure 1B).
eCB-LTD
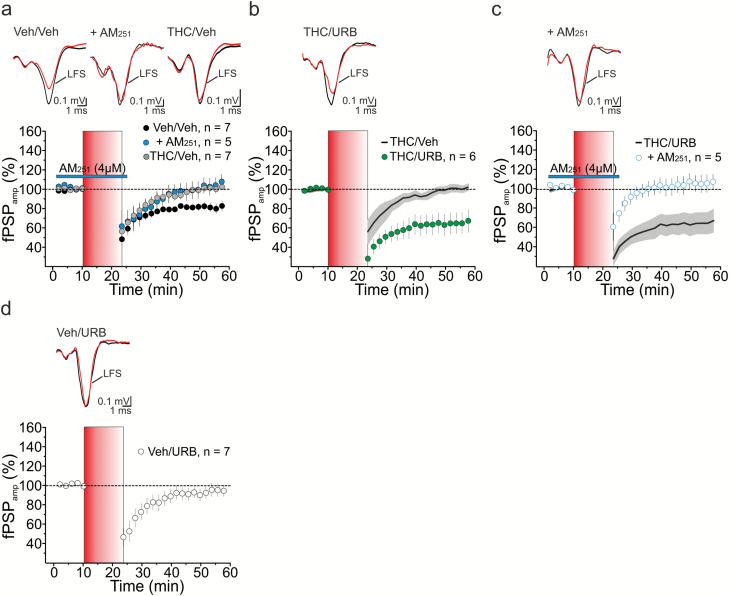
Next, we examined the effect of URB597 treatment on CB1-mediated plasticity in the mPFC of adult female rats exposed to THC or vehicle during adolescence. In vehicle-treated rats (Veh/Veh), low-frequency stimulation (LFS, 10 Hz, 10 minutes) of cortical layer 2/3 afferents to layer 5 (L2/3→L5) resulted in a long-lasting (>30 minutes) depression of extracellular fPSPs (81±4% of baseline, n=7, P<.05; Figure 2a). Consistent with previous observations (Lafourcade et al., 2007; Rubino et al., 2015), eCB-LTD was abolished by the extracellular application of the CB1 antagonist AM251 (4 µM; 102±8% of baseline, n=5, P<.05; Figure 2a) and impaired upon THC exposure during adolescence (THC/Veh, 100±3% of baseline, n=7, P>.05; Figure 2a). Adult treatment with URB597 (0.3 mg/kg) fully rescued LTD in THC-pretreated animals (THC/URB, 65±11% of baseline, n=6, P<.05; Figure 2b). This form of synaptic depression still depended on CB1 activation, as AM251 prevented LTD induction (106±9% of baseline, n=5, P>.05; Figure 2c). LFS failed to induce significant eCB-LTD in vehicle preexposed female rats when treated with URB597 in adulthood (Veh/URB, 100±3% of baseline, n=7, P>.05; Figure 2d). This may be consistent with the slight, albeit not significant, decrease in CB1 expression measured upon administration of URB597 alone (P>.05; Figure 1B).
Figure 2.
Low-frequency stimulation (LFS)-induced endocannabinoid (eCB)-mediated long-term synaptic depression (eCB-LTD) at medial prefrontal cortex (mPFC) layer 2 (L2)→L5 microcircuit. (A) LFS stimulation of cortical L2 triggered a depression of field postsynaptic potentials (fPSP) in layer 5 female rats subjected to pretreatment with vehicle (Veh/Veh) during adolescence. This form of LTD was abolished by acute application of the CB1R antagonist AM251 (4 μM) and impaired in female rats upon adolescent Δ9-tetrahydrocannabinol (THC) exposure (THC/Veh); (RM2W, F2,16=6, P=.014; Veh/Veh vs Veh/Veh+AM, P<.05; Veh/Veh vs THC/Veh, P<.05) (B, C) In vivo treatment with the fatty acid amide hydrolase (FAAH) inhibitor URB597 (0.3 mg/kg) reinstated a form of LTD at L2→L5 connections of THC-exposed rats (B, RM2W, F1,11=11, P=0.008; THC/Veh vs THC/URB, P<.01), which was sensitive to AM251 (C, RM2W, F1,9=8, P=.02; THC/URB vs THC/URB + AM, P<.05). (D) In female rats preexposed to vehicle and then treated with URB (Veh/URB), LFS failed to trigger eCB-LTD (P>.05). (A-D) Time courses of normalized fPSP amplitudes (mean±SEM). Insets, averaged recordings (5 traces) before (black line) and after (red line) the delivery of the LFS protocol. LFS is indicated by the red vertical bar (B, C) Black lines (average fPSP amplitude) and gray areas (SEM) in (B) and (C) show time courses from (A) and (B), respectively, and are reported here for comparison.
Overall, these data indicate that adult URB597 treatment rescues THC-induced deficits in eCB-mediated plasticity at mPFC L2/3→L5 microcircuits.
URB597 Reverts Morphological and Synaptic Alterations Induced in Hippocampal Newborn Neurons by Adolescent THC Exposure
In several mammalian models of neuropsychiatric disorders, including major depression, newborn neurons in the hippocampal DG have lower differentiation capabilities and proliferation (Kempermann et al., 2008; Hanson et al., 2011). Newborn neurons have unique electrophysiological properties, including increased intrinsic excitability and a lower threshold for the induction of LTP compared with mature DG cells (Snyder et al., 2001). Given these peculiar properties, it has been proposed that maturing newborn neurons are crucial for hippocampal-dependent behaviors (Saxe et al., 2006; Winocur et al., 2006). We therefore assessed the effect of adolescent THC exposure on adult newborn neuron maturation and the impact of URB597 treatment at adulthood.
Morphological and Quantitative Study of Newborn DCX+ Neuroblasts
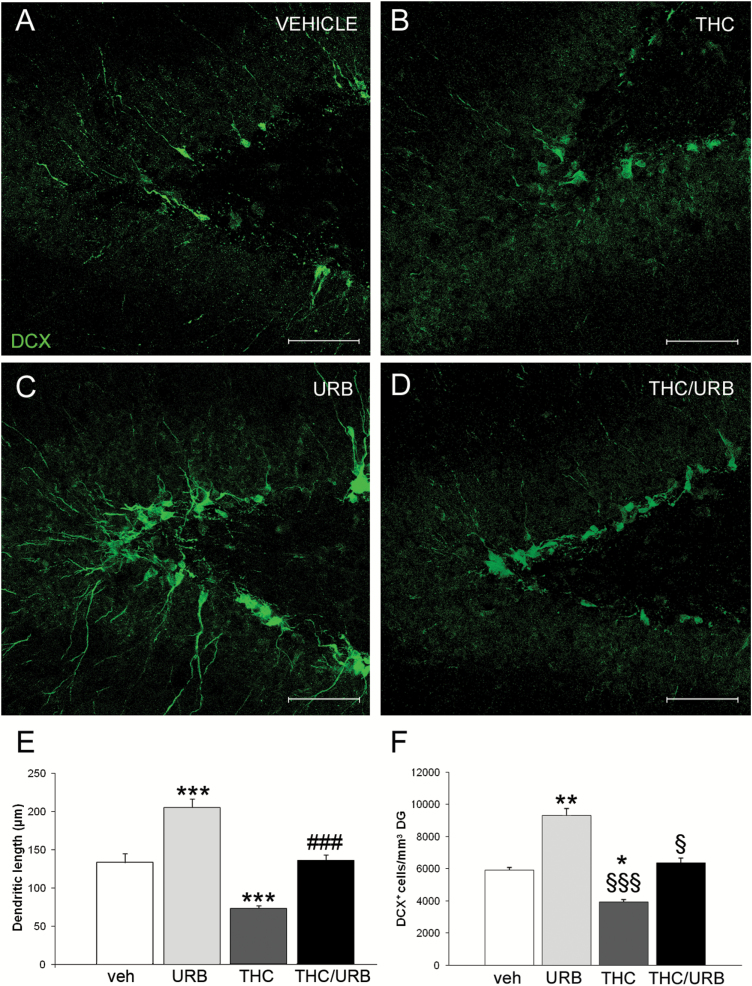
We evaluated both the morphology and number of immature newborn hippocampal neurons, identified by the expression of the marker doublecortin (DCX). We first assessed whether THC exposure during adolescence and subsequent URB597 treatment in adulthood affected the mean apical dendrite length of DCX+ cells. Two-way ANOVA analysis showed a significant effect of both THC (F1,176=141.8, P<.0001) and URB597 (F1,176=155, P<.0001; Figure 3A, C, D, E). Untreated THC-exposed rats showed a decreased dendritic length in DCX+ cells compared with the other groups (P<.001; Figure 3B, E). In contrast, dendritic length was increased in the URB597-treated rats (P<.001; Figure 3C, E). In THC-exposed animals, treatment with URB597 restored dendritic arborization to levels comparable with those of vehicle-treated rats (P<.001; Figure 3D, E).
Figure 3.
(A-D) Representative confocal microscopy images of immunofluorescent doublecortin (DCX) labeling (green) in dentate gyrus (DG) of vehicle-, Δ9-tetrahydrocannabinol (THC)-, URB-, and THC/URB-treated rats. Scale bar: 75 µm. (E) Quantification of apical dendrite length in DG DCX+ neuroblasts. Compared with the vehicle-treated group, DCX+ cells had significantly longer apical dendrites in URB-treated rats. Conversely, mean dendritic length of DCX+ cells was significantly decreased in THC- vs vehicle-treated rats. No difference was observed between vehicle- and THC/URB-treated groups. Data are expressed as mean±SEM of n=4 rats/group and were analyzed by 2-way ANOVA followed by Tukey’s posthoc test. ***P<.001 vs vehicle; ###P<.001 vs THC. (F) Quantification of DCX+ cells per mm3 of DG. URB-treated rats had significantly more DCX+ cells compared with vehicle-, THC-, and THC/URB-treated rats. Data are expressed as mean±SEM cells/mm3 of n=4 rats/group and were analyzed by 2-way ANOVA, followed by Tukey’s posthoc test. *P<.05 and **P<.01 vs vehicle-treated rats; §§§P<.001 and §P<.05 vs URB-treated rats.
Next, we evaluated the number of DCX+ cells/mm3 in the DG. We found a significant main effect of THC (F1,16=15.65, P<.001) and URB597 (F1,16=21.97; P<.0002; Figure 3A, C, D, F). Posthoc analysis showed a significant reduction of DCX+ cells in THC-treated compared with vehicle-treated rats (P<.05). Treatment with URB597 significantly increased the number of DCX+ cells/mm3 in control animals compared with the vehicle-treated (P<.01), THC-treated (P<.001), and THC/URB-treated groups (P<.05). Notably, URB597 treatment in THC-treated rats also increased the number of DCX+ cells, thus restoring the physiological density of newborn neurons in these animals. Taken together, our data indicate that in animals exposed to THC during adolescence a significant decrease in dendritic length and number of immature neurons is present, and URB597 treatment in adulthood is able to restore both density and morphological complexity of newborn neurons.
Newborn Neuron- and DG Cells-Mediated Synaptic Plasticity
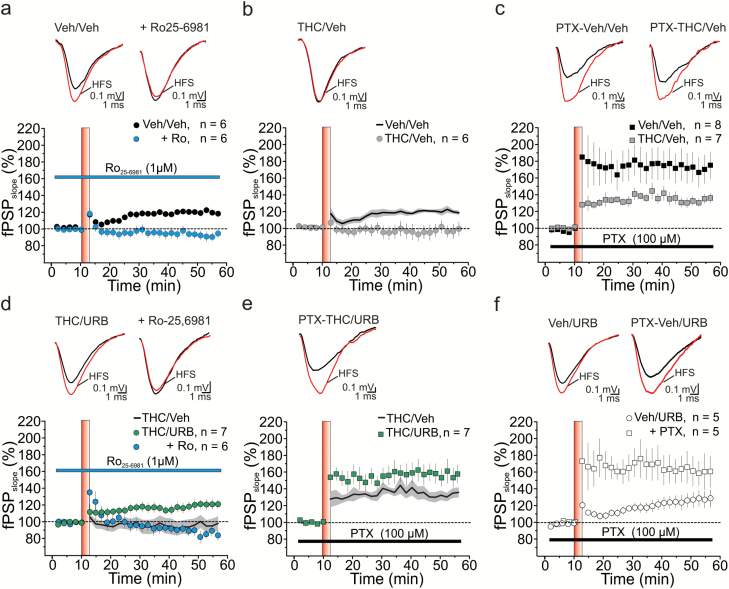
We then tested whether alterations of DCX+ cells in THC-treated rats were associated with impaired DG synaptic plasticity and whether URB597-rescued newborn neurons were physiologically functional. In the DG, newborn neuron-dependent LTP (NBN-LTP) can be induced by HFS of the medial perforant path under intact local GABAergic inhibition (120±3% of baseline, n=6, P<.05; Figure 4A). This form of LTP requires the activation of glutamate NMDA receptors containing the NR2B subunit (Snyder et al., 2001). Conversely, LTP of the synaptic response in mature DG cells occurs only upon GABAergic inhibition (Snyder et al., 2001). In female rats exposed to vehicle during adolescence, HFS elicited a long-lasting potentiation of synaptic responses in the absence of GABAA receptor blockers. Acute application of the NR2B antagonist Ro25-6981 (1 μM) abolished LTP (93±5% of baseline, n=6, P>.05; Figure 5A), thus confirming the specific induction of NBN-LTP. Conversely, in slices from female rats preexposed to THC during adolescence (THC/Veh), HFS failed to induce NBN-LTP (97±8% of baseline, n=6, P>.05; Figure 4B). In the presence of the GABAA receptor blocker PTX (100 μM), HFS triggered robust dentate granule cell LTP in both vehicle- and THC-treated rats (Veh/Veh, 174±15% of baseline, n=8, P<.05; THC/Veh, 133±5% of baseline, n=7, P<.05; Figure 4C). Nevertheless, this LTP had a smaller magnitude in THC-treated than in vehicle-treated rats (P<.05), indicating that synaptic plasticity of mature dentate granule cells is also partially affected by THC exposure during adolescence. In rats exposed to THC, URB597 treatment in adulthood fully restored NBN-LTP (THC/URB, 121±6 % of baseline, n=7, P<.05; Figure 4D). The LTP rescued upon URB597 administration was sensitive to Ro25-6981 (90±6% of baseline, n=6, P>.05; Figure 4D), further supporting that restored plasticity depends on functional newborn neurons. URB597 treatment also increased the magnitude of LTP of mature DG cells from rats preexposed to THC (THC/URB, 158±26% of baseline, n=7, P<.05; Figure 4E). In the vehicle-treated group (Veh/URB), URB597 alone had no significant effects on either NBN-LTP (128±9% of baseline, n=5, P<.05; Figure 4F) or mature dentate granule cell LTP (159±13% of baseline, n=5, P<.05; Figure 4F). In summary, URB597 administration in adulthood reverts DG synaptic dysfunctions caused by adolescent THC exposure.
Figure 4.
Synaptic plasticity at MMP→DG connections. (A) Consistent with the expression of a newborn neuron-mediated form of LTP in the dentate gyrus (DG) of vehicle-treated (Veh/Veh) rats, high-frequency stimulation (HFS) of the medial perforant path (MPP) triggered a long-lasting potentiation of field post-synaptic potentials (fPSPs), which was abolished by the specific NR2B antagonist Ro26-6981 (1 μM); (RM2W, F1,10=21, P=.001; Veh/Veh vs Veh/Veh + Ro, P<.01). (B, C) Both newborn neuron-LTP (B, RM2W, F1,10=7, P=.02; Veh/Veh vs THC/Veh, P<.05) and LTP obtained in the presence of the GABAA blocker picrotoxin (PTX, 100 μM) (C, RM2W, F1,13=5, P=.04; Veh/Veh vs THC/Veh, P<.05) were impaired in THC-treated rats during adolescence. (D, E) URB597 administration fully rescued both Ro26-6981-sensitive LTP (D) and DGC LTP (E) in THC-treated rats (D, RM2W, F2,16=7, P=.008; THC/Veh vs THC/URB, P<.05; THC/URB vs THC/URB + Ro, P<.01; E, RM2W, F1,12=5, P=.04; THC/Veh vs THC/URB, P<.05). In contrast, URB597 treatment had no effect on these 2 forms of LTP recorded in the DG of Veh/Veh rats (Veh/URB, P<.05; Veh/URB+PTX, P<.05). (A–F) Time courses of normalized fPSP slopes (mean±SEM). Insets, averaged recordings (5 traces) before (black line) and after (red line) the delivery of high-frequency stimulation (HFS) protocol. HFS is indicated by the red vertical bar (B,D,E). Black lines (average fPSP slope) and gray areas (SEM) in B, D, and E show time courses from A, B, and C, respectively, and are reported here for comparison.
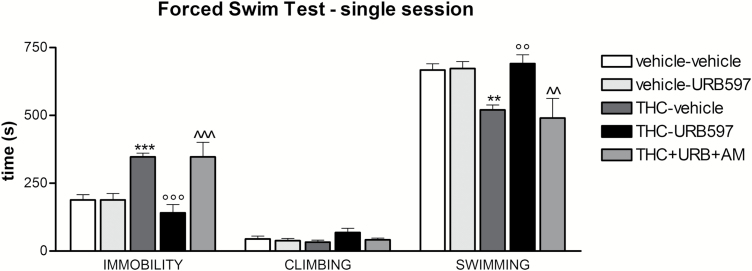
Figure 5.
Effect of co-administration of the CB1 receptor antagonist AM251 on URB597’s positive modulation of behavior in the forced swim test. Adolescent Δ9-tetrahydrocannabinol (THC) administration significantly increased immobility and decreased swimming time during the 15-minute test session. Chronic URB597 normalized the time spent immobile and swimming when administered to THC-pretreated animals, however, co-administration of AM251 completely blocked the beneficial effect of URB597. Data are expressed as mean±SEM of n=5/10 rats/group and were analyzed by 1-way ANOVA followed by Tukey’s posthoc test. **P<.01 and ***P<.001 vs vehicle; °°P<.01 and °°°P<.001 vs THC; ^^P<.01 and ^^^P<.001 vs THC + URB597.
URB597 Rescue of Passive Coping Strategy in THC-Treated Rats Requires CB1 Receptor Activation
We previously demonstrated that adult chronic URB597 treatment rescues depressive-like symptoms caused by adolescent THC exposure in female rats, namely, passive coping strategy in the FST, social withdrawal, and anhedonia (Realini et al., 2011). To investigate the relevance of CB1 receptor activation in URB597 recovery of THC-induced depressive-like effects, we treated adult female rats, preexposed to THC during their adolescence, with URB597 or with URB597 and the CB1 receptor antagonist AM251. We then examined one of the behaviors recovered by URB597, that is, the passive coping strategy in the FST (Figure 5). Statistical analysis revealed a significant effect of treatment on immobility time (F4,39=13.31; P<.0001). Adolescent THC administration approximately doubled immobility time during the 15-minute test session compared with vehicle-treated animals (P<.001). Chronic URB597 had no behavioral impact on its own but normalized the time rats spent immobile when administered to THC-pretreated animals (P<.001 vs THC). Treatment also had a significant effect on swimming activity (F4,39=8.274; P<.0001). Indeed, adolescent THC treatment decreased the time spent swimming by about 22% with respect to controls (P<.01), which URB597 administration completely normalized (P<.01 vs THC). No changes were observed in climbing behavior in any of the groups. Co-administration of AM251 completely blocked the beneficial effect of URB597 both on immobility (P<.001) and swimming behavior (P<.01). This effect was obtained at a dose of AM251 that alone did not modify behavior in the FST (veh+veh 176.2±35.85 n=5 and veh+AM251 231.0±21.94 n=4 for immobility, P>.05; veh+veh 654.2±44.54 n=5 and veh+AM251 573.2±30.32 n=4 for swimming activity, P>.05). No changes in basal locomotor activity were observed in the different cohorts of rats (data not shown). In summary, these data indicate that the beneficial effect of URB597 on coping behavior in the FST requires CB1 receptor activation.
Discussion
In this study, we provide evidence that cellular and synaptic alterations at PFC and hippocampal DG circuits induced by adolescent THC exposure in female rats are rescued in adults by enhancing AEA signaling through in vivo administration of the FAAH inhibitor URB597. At PFC circuits, we previously reported that CB1R expression and eCB-mediated LTD are impaired upon adolescent THC exposure (Rubino et al., 2015). Here for the first time we demonstrate that both are reinstated by URB597 treatment. Moreover, we also report that animals exposed to THC during adolescence show decreased density and morphological complexity of newborn neurons as well as deficits in DG synaptic plasticity; these neurobiological alterations are restored by URB597 treatment in adulthood. Furthermore we here demonstrate that URB597-mediated improvement in depressive-like behavior present in adult female rats requires CB1 receptor activation.
In the PFC, the eCB system undergoes maturational events during adolescence (Lee et al., 2013; Rubino et al., 2015). The cellular and functional consequences in adulthood triggered by adolescent THC exposure include the loss of PFC eCB-LTD at L2/3→L5 synapses and a significant reduction in the expression of CB1 receptors, which in L5 are expressed at presynaptic glutamatergic inputs (Lafourcade et al., 2007). Here, we demonstrate that URB597 administration to adult female rats is sufficient to restore normal expression levels of CB1 receptor and eCB-LTD in the PFC. This supports the hypothesis that deficits in eCB-mediated plasticity triggered by chronic THC administration are associated with the disruption in the eCB signaling in this brain region.
Together with the PFC, the hippocampus is anatomically critical for the emotional disturbances induced by adolescent THC exposure (Realini et al., 2011). We previously found that adult female rats preexposed to THC during adolescence show decreased cell proliferation in the DG of the hippocampus (Realini et al., 2011). Here we add new pieces of information reporting that the same treatment also decreased number and morphological complexity in DG newborn neurons. Moreover, enhancing AEA signaling in adulthood rescues these deficits in DG newborn neuron maturation caused by adolescent THC exposure. Recent evidence indicates that dendritic maturation of newborn hippocampal neurons may be a key component in the regulation of emotional behavior (Po et al., 2015). Stressful experiences in rodents induce dendritic atrophy in hippocampal DG (see Duman and Duman, 2015 for review). Additionally, chronic treatment with corticosterone increases depression-like behavior, and, in parallel, decreases the number and dendritic complexity of immature DCX+ neurons in the subgranular zone and granule cell layer of the hippocampus (Lussier et al., 2013). Conversely, antidepressant treatments, besides promoting neural stem/progenitor cell proliferation, also promote dendritic arborization and development of immature granule cells in parallel with the improvement in emotional behavior (Wang et al. 2008; Schloesser et al., 2015). Together, these observations raise the possibility that the ability of URB597 to rescue dendritic morphology in immature DG newborn neurons of rats exposed to THC during adolescence may contribute to the antidepressant-like action of this drug. Nevertheless, further experiments are needed to establish a clear causality between these two events.
The behavioral effects of URB597 are prevented by coadministration of low doses of the CB1 antagonist AM251. This indicates that CB1 receptor activation is crucial for URB597’s antidepressant-like properties and is consistent with the role of CB1R-mediated signaling in emotional control. Indeed, polymorphisms in the CB1R coding gene CNR1 have been associated with the etiology of major depression and the clinical response to antidepressant drugs (Barrero et al., 2005; Juhasz et al., 2009; Monteleone et al., 2010; Agrawal et al., 2012; Mitjans et al., 2013). In preclinical settings, pharmacological inhibition or genetic disruption of CB1 receptors produces symptoms that recapitulate human depression (see for review Hillard and Liu, 2014). Additionally, CB1 receptor activation seems to play an important role in adult hippocampal neurogenesis. Indeed, in mice, chronic CB1 receptor blockade inhibits cell proliferation in the DG, and CB1 receptor deficiency impairs increases in hippocampal neuroprogenitor cell turnover mediated by glutamate (Aguado et al., 2007) as well as the neurogenic effects of environmental enrichment and wheel running (Hill et al., 2010; Wolf et al., 2010).
Our data indicate not only that URB597 treatment rescues dendritic abnormalities and the number of maturing DG newborn neurons in THC-exposed rats, but that these neurons are functionally active. Indeed, we observed that NBN-LTP is deficient in THC-treated rats and restored upon URB597 administration. Thus, these neurophysiological results further support the evidence that chronic adolescent THC exposure affects hippocampal neurogenesis. Our work shows that enhancing AEA signaling through URB597 rescues these morphological and functional deficits of maturing DG newborn neurons. However, we cannot rule out a possible contribution of 2-AG to these events, as under different experimental conditions (chronic unpredictable stress and a preventive treatment) enhancement of 2-AG tone potentiated hippocampal neurogenesis and LTP in the DG (Zhang et al., 2015).
In conclusion, these data provide novel insights into the cellular modifications that are associated with in vivo URB597 treatment and which may contribute to the ameliorative effects of this drug on the emotional impairments caused by adolescent THC exposure. These mechanisms include rescue of proper CB1 expression and eCB-mediated plasticity at PFC microcircuits as well as recovery of morphological and synaptic properties of newborn hippocampal neurons. URB597 exposure per se triggers functional alterations in the deep PFC layers, together with increased number of proliferating neuroblasts in the DG. Nevertheless, whilst URB597 administration rescues depressive-like behavior in THC-treated rats, it has no behavioral effect per se on vehicle-exposed animals. This raises the possibility that the same pharmacological manipulation may have different impacts in the context of pathological neuroadaptations secondary to repeated THC exposure compared with normal physiological conditions.
In light of this evidence, it is tempting to speculate that positive AEA modulation may represent a treatment option for people suffering from depression secondary to previous cannabis abuse. It is worth noting that higher rates of comorbid mood and anxiety disorders are reported in women (see for review Rubino and Parolaro, 2015) and that depressed patients who are also cannabis users experience significantly worse emotional, affective, and social withdrawal than nonusers (Bersani et al., 2016). Future studies are therefore needed to validate the clinical effectiveness of positive AEA modulation in mood alterations triggered by adolescent cannabis exposure.
Acknowledgments
This study was supported by Dipartimento delle Politiche Antidroga, grant “Adocannabis” and by the Fondazione Istituto Italiano di Tecnologia.
Statement of Interest
None.
References
- Adamczyk P, Gołda A, McCreary AC, Filip M, Przegaliński E(2008)Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol 59:217–228. [PubMed] [Google Scholar]
- Agrawal A, Nelson EC, Littlefield AK, Bucholz KK, Degenhardt L, Henders AK, Madden PA, Martin NG, Montgomery GW, Pergadia ML, Sher KJ, Heath AC, Lynskey MT(2012)Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Arch Gen Psychiatry 69:732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, Lutz B, Guzmán M, Galve-Roperh I(2007)The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J Biol Chem 282:23892–23898. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D, Gobbi G(2010)Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 35:2083–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Gobbi G(2008)The cannabinoid CB1 receptor and the endocannabinoid anandamide: possible antidepressant targets. Expert Opin Ther Targets 12:1347–1366. [DOI] [PubMed] [Google Scholar]
- Barrero FJ, Ampuero I, Morales B, Vives F, de Dios Luna Del Castillo J, Hoenicka J, García Yébenes J(2005)Depression in parkinson’s disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1). Pharmacogenomics J 5:135–141. [DOI] [PubMed] [Google Scholar]
- Bersani G, Bersani FS, Caroti E, Russo P, Albano G, Valeriani G, Imperatori C, Minichino A, Manuali G, Corazza O(2016)Negative symptoms as key features of depression among cannabis users: a preliminary report. Eur Rev Med Pharmacol Sci 20:547–552. [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Li L, Geiger JR, Jonas P(2006)Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc 1:2075–2081. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D(2007)Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry 62:1103–1110. [DOI] [PubMed] [Google Scholar]
- Bortolotto V, Cuccurazzu B, Canonico PL, Grilli M(2014)NF-κb mediated regulation of adult hippocampal neurogenesis: relevance to mood disorders and antidepressant activity. Biomed Res Int 2014:612798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJ(2010)Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol 92:370–385. [DOI] [PubMed] [Google Scholar]
- Colino A, Malenka RC(1993)Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro. J Neurophysiol 69:1150–1159. [DOI] [PubMed] [Google Scholar]
- Duman CH, Duman RS(2015)Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett 601:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK(2012)Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC(2016)Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the united states. Biol Psychiatry 79:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidelman S, Mizrachi Zer-Aviv T, Lange R, Hillard CJ, Akirav I(2018)Chronic treatment with URB597 ameliorates post-stress symptoms in a rat model of PTSD. Eur Neuropsychopharmacol 28:630–642. [DOI] [PubMed] [Google Scholar]
- Fowler CJ.(2015)The potential of inhibitors of endocannabinoid metabolism for drug development: a critical review. Handb Exp Pharmacol 231:95–128. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G(2009)Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. Plos One 4:e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D(2005)Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A 102:18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM(2013)The hippocampal-prefrontal pathway: the weak link in psychiatric disorders?Eur Neuropsychopharmacol 23:1165–1181. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L(2009)Adverse health effects of non-medical cannabis use. Lancet 374:1383–1391. [DOI] [PubMed] [Google Scholar]
- Hanson ND, Owens MJ, Nemeroff CB(2011)Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology 36:2589–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, Gorzalka BB, Hillard CJ, Christie BR(2010)Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 20:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Liu QS(2014)Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr Pharm Des 20:3795–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G, Chase D, Pegg E, Downey D, Toth ZG, Stones K, Platt H, Mekli K, Payton A, Elliott R, Anderson IM, Deakin JF(2009)CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology 34:2019–2027. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K(2008)The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry 21:290–295. [DOI] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ(2007)Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. Plos One 2:e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT, Hill MN, Hillard CJ, Gorzalka BB(2013)Temporal changes in N-acylethanolamine content and metabolism throughout the peri-adolescent period. Synapse 67:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier AL, Lebedeva K, Fenton EY, Guskjolen A, Caruncho HJ, Kalynchuk LE(2013)The progressive development of depression-like behavior in corticosterone-treated rats is paralleled by slowed granule cell maturation and decreased reelin expression in the adult dentate gyrus. Neuropharmacology 71:174–183. [DOI] [PubMed] [Google Scholar]
- Malone DT, Hill MN, Rubino T(2010)Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol 160:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini V, Cuccurazzu B, Bortolotto V, Ramazzotti V, Ubezio F, Tzschentke TM, Canonico PL, Grilli M(2014)The noradrenergic component in tapentadol action counteracts μ-opioid receptor-mediated adverse effects on adult neurogenesis. Mol Pharmacol 85:658–670. [DOI] [PubMed] [Google Scholar]
- Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F(2013)Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol Ther 138:18–37. [DOI] [PubMed] [Google Scholar]
- Mitjans M, Serretti A, Fabbri C, Gastó C, Catalán R, Fañanás L, Arias B(2013)Screening genetic variability at the CNR1 gene in both major depression etiology and clinical response to citalopram treatment. Psychopharmacology (Berl) 227:509–519. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Bifulco M, Maina G, Tortorella A, Gazzerro P, Proto MC, Di Filippo C, Monteleone F, Canestrelli B, Buonerba G, Bogetto F, Maj M(2010)Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol Res 61:400–404. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH(2007)Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 192:61–70. [DOI] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, Parolaro D, Tkatch T, Benfenati F, Pedarzani P, Tonini R(2012)SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat Neurosci 15:284–293. [DOI] [PubMed] [Google Scholar]
- Perera TD, Park S, Nemirovskaya Y(2008)Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist 14:326–338. [DOI] [PubMed] [Google Scholar]
- Po KT, Siu AM, Lau BW, Chan JN, So KF, Chan CC(2015)Repeated, high-dose dextromethorphan treatment decreases neurogenesis and results in depression-like behavior in rats. Exp Brain Res 233:2205–2214. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC(2010)Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Vigano’ D, Guidali C, Zamberletti E, Rubino T, Parolaro D(2011)Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology 60:235–243. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D(2015)Sex-dependent vulnerability to cannabis abuse in adolescence. Front Psychiatry 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D(2016)The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry 79:578–585. [DOI] [PubMed] [Google Scholar]
- Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, Sagheddu C, Ligresti A, Tonini R, Di Marzo V, Parolaro D(2015)Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis 73:60–69. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Alberio T, Capurro V, Viganò D, Guidali C, Sala M, Fasano M, Parolaro D(2009a)The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox Res 15:291–302. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D(2009b)Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 19:763–772. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D(2008)Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33:2760–2771. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R(2011)Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR(2006)Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A 103:17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Orvoen S, Jimenez DV, Hardy NF, Maynard KR, Sukumar M, Manji HK, Gardier AM, David DJ, Martinowich K(2015)Antidepressant-like effects of electroconvulsive seizures require adult neurogenesis in a neuroendocrine model of depression. Brain Stimul 8:862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM(2001)Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol 85:2423–2431. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Xie S, Psychoyos D, Hungund BL, Cooper TB, Tejani-Butt SM(2012)Dysfunction in fatty acid amide hydrolase is associated with depressive-like behavior in wistar kyoto rats. Plos One 7:e36743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R(2008)Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM(2000)Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol 42:248–257. [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S(2006)Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16:296–304. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Bick-Sander A, Fabel K, Leal-Galicia P, Tauber S, Ramirez-Rodriguez G, Müller A, Melnik A, Waltinger TP, Ullrich O, Kempermann G(2010)Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun Signal 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamberletti E, Prini P, Speziali S, Gabaglio M, Solinas M, Parolaro D, Rubino T(2012)Gender-dependent behavioral and biochemical effects of adolescent delta-9-tetrahydrocannabinol in adult maternally deprived rats. Neuroscience 204:245–257. [DOI] [PubMed] [Google Scholar]
- Zamberletti E, Beggiato S, Steardo L Jr, Prini P, Antonelli T, Ferraro L, Rubino T, Parolaro D(2014)Alterations of prefrontal cortex gabaergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis 63:35–47. [DOI] [PubMed] [Google Scholar]
- Zamberletti E, Gabaglio M, Grilli M, Prini P, Catanese A, Pittaluga A, Marchi M, Rubino T, Parolaro D(2016)Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharmacol Res 111:459–470. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang W, Zhong P, Liu SJ, Long JZ, Zhao L, Gao HQ, Cravatt BF, Liu QS(2015)Blockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticity. Hippocampus 25:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]