Abstract
Background
The efficacy of fronto-temporal transcranial direct current stimulation in treating auditory verbal hallucinations and other psychopathological symptoms of schizophrenia patients has been examined in a small number of clinical trials with limited sample sizes, but the results are mixed. Fronto-temporal transcranial direct current stimulation has also been demonstrated to enhance patients’ insight into their mental illness in an open-label pilot study. The current investigation aimed to investigate the therapeutic effects of fronto-temporal transcranial direct current stimulation on the severity of auditory verbal hallucinations, other schizophrenia symptoms, and insight in a large double blind, randomized, sham-controlled trial.
Methods
Sixty patients with medication-refractory auditory verbal hallucinations were randomized over 2 conditions: transcranial direct current stimulation with 2-mA, twice-daily sessions for 5 consecutive days, with anodal stimulation to the left prefrontal cortex and cathodal stimulation to the left temporo-parietal junction, and sham treatment.
Results
Fronto-temporal transcranial direct current stimulation failed to cause significant changes in the severity of auditory verbal hallucinations and other schizophrenia symptoms. The levels of insight into illness (effect size=0.511, P<.001) and positive symptoms (effect size=0.781, P<.001) were largely promoted by 5 days of transcranial direct current stimulation relative to sham treatment. The beneficial effects on the 2 insight dimensions remained 1 month after transcranial direct current stimulation.
Conclusions
Fronto-temporal transcranial direct current stimulation is not more effective for auditory verbal hallucinations and other schizophrenia symptoms than sham treatment. But the results of transcranial direct current stimulation-associated improvement in awareness of illness and positive symptoms show promise and provide a new direction for future research into insight promotion interventions in schizophrenia.
Keywords: schizophrenia, transcranial direct current stimulation, fronto-temporal montage, auditory verbal hallucination, insight
Significance Statement
The efficacy of fronto-temporal transcranial direct current stimulation (tDCS) in treating auditory verbal hallucinations (AVHs) and other psychopathological symptoms of schizophrenia patients has been examined in a small number of clinical trials with limited sample sizes. Fronto-temporal tDCS has also been demonstrated to enhance patients’ insight into their mental illness in an open-label pilot study. We hypothesized that fronto-temporal tDCS may not only improve AVHs and other psychotic symptoms but also intensify insight in schizophrenia. Our main findings indicated that tDCS did not reduce AVHs and other psychopathological symptoms. After treatment with tDCS, however, patients’ insight into illness and positive symptoms rapidly heightened. The beneficial effect of tDCS on insight lasted for at least 1 month. These observations suggest that fronto-temporal tDCS is not effective for AVHs and other schizophrenia symptoms, but its effect on insight levels provides a new direction for future research into insight promotion interventions in schizophrenia.
Introduction
A large proportion of patients with schizophrenia experience auditory verbal hallucinations (AVHs), one of the core symptoms of schizophrenia (Waters, 2012). Antipsychotic medication is currently the mainstay of treatment for AVHs. However, nearly 30% patients with AVHs are nonresponsive to traditional antipsychotics (Shergill et al., 1998), and these patients do not always agree with or tolerate treatment with adequate doses of clozapine or electroconvulsive therapy to treat their medication-refractory AVHs. Researchers keep trying to develop well-tolerated alternative or add-on therapies to treat medication-refractory AVHs in schizophrenia patients. Transcranial direct current stimulation (tDCS) is one of the add-on neurostimulation therapies that has raised great interest in recent years. This noninvasive technique applies a weak direct current on the scalp and through the brain and rapidly leads to changes in cortical excitability by shifting membrane resting potentials, which facilitates either depolarization or hyperpolarization of the brain neurons (Nitsche et al., 2003). Repetitive stimulation during specific time intervals further enhances efficacy and prolongs after-effects of tDCS by modifying the efficacy of N-methyl-D-aspartate receptor (Nitsche et al., 2008).
Functional neuroanatomical studies have described a common observation of hypoactivity in the prefrontal cortex among patients with schizophrenia (Lawrie et al., 2002) and during their AVHs, the observations of hyperactivity in the left temporo-parietal brain areas (Jardri et al., 2011) and a fronto-temporal functional dysconnectivity (Alderson-Day et al., 2015). A pilot trial based on the prior observations has examined the potential treatment effects of fronto-temporal tDCS (twice daily stimulation for total 10 sessions, with the anode placed over the left prefrontal cortex and the cathode over the left temporo-parietal junction) for treating medication-refractory AVHs in schizophrenia and the results are very promising, giving hope for future clinical application (Brunelin et al., 2012). Some studies that successfully replicated the original pilot study have further confirmed a therapeutic benefit of tDCS, manifested by reductions in frequency and/or severity of AVHs in schizophrenic patients (Mondino et al., 2015, 2016; Bose et al., 2017). However, other studies that applied the stimulation once daily for 15 (Fitzgerald et al., 2014) or 5 sessions (Smith et al., 2015; Fröhlich et al., 2016) failed to replicate previous positive findings. A recent systematic review analyzed all these clinical trials with limited sample sizes (numbers ranging from 23 to 33) and pointed out that the information is incongruent and insufficient for determining the clinical use of tDCS to reduce the severity of AVHs in schizophrenia (Pondé et al., 2017). A large randomized controlled trial with higher statistical power is necessary to draw a firm conclusion.
Lack of insight into illness is common in patients with schizophrenia and has a negative impact on treatment adherence, long-term illness outcome, and prognosis (Lincoln et al., 2007). Insight in schizophrenia interacts with psychopathology symptoms in a complex way. For example, poor insight seems to be particularly associated with increased severity of positive symptoms (Vaz et al., 2002; Xavier et al., 2018). The presence of reduced insight has been considered a state characteristic that might contribute to the phenomenology of AVHs, for example, determining the meaning of AVHs (Waters et al., 2012). Therapeutic interventions do improve insight in schizophrenia (Pijnenborg et al., 2013), but 50% to 80% of schizophrenia patients have impaired insight into the presence of their mental disorder and therefore poorly comply with the recommended treatment (Lincoln et al., 2007). Researchers have been seeking specific brain regions implicated in impaired insight in schizophrenia and for interventions to promote insight among patients with schizophrenia. A recent open-label study stands on the premise that structural and functional brain deficits in prefrontal (Sapara et al., 2007; Buchy et al., 2015) and temporo-parietal cortical regions (Buchy et al., 2011; Emami et al., 2016) is highly correlated with poor insight in schizophrenia as reported in earlier studies and successfully demonstrated the insight facilitation effects of add-on fronto-temporal tDCS in schizophrenia (Bose et al., 2014). The positive effect needs to be confirmed in randomized controlled trials with adequate statistical power.
The present study aimed to examine the acute effect of 5 consecutive days of fronto-temporal tDCS and its maintenance effects at 1 month and 3 months follow-up on refractory AVHs in schizophrenia patients. We anticipated that fronto-temporal tDCS attenuates the severity of AVHs in schizophrenia. We also assessed the effects of fronto-temporal tDCS on other psychopathological symptoms and the levels of insight across the same study period.
Methods
Participants
Patients who met DSM-IV-TR criteria for schizophrenia or schizoaffective disorder were enrolled in the study. None of them had any current psychiatric comorbidity or active substance use disorder. All participants aged 20 to 65 years were provided written informed consent for the study as approved by the Institutional Review Board of Tri-Service General Hospital (no. of IRB approval: TSGHIRB-2-103-03-002; ClinicalTrials.gov ID: NCT03388554). All participants had medication-refractory auditory verbal hallucinations, which are defined as the persistence of daily hallucinations without remission under adequate treatments with antipsychotic medications at clinical efficacious tolerated dose for more than 3 months. Throughout the duration of the study, their antipsychotic treatment was unchanged. The daily dosage of antipsychotic drugs equivalent to 100 mg chlorpromazine was calculated. A randomized, double-blind, parallel arm, stimulation protocol was used in our study. An Eldith DC stimulator (Neuroconn DC Stimulator Plus, GmbH, Ilmenau, Germany) was used for stimulation, with two 7×5 cm sponge electrodes soaked in a 0.9% NaCl saline solution. In line with previous studies and based on the international 10 to 20 electrode placement system, the middle of the anode was located over a point midway between F3 and FP1, presumably corresponding to left prefrontal cortex and dorsolateral prefrontal cortex. The cathode was centered at a point midway between T3 and P3, corresponding to left temporo-parietal junction. Stimulation was applied at an intensity of 2 mA for 20 minutes, twice daily, on 5 consecutive weekdays. In sham stimulation, the 2-mA current was turned on for 30 seconds and then ramped down to 0 mA through the remainder of the 20-minute time.
Outcome Measurement
The primary outcome measure was the variation during the study period in score of the Auditory Hallucination Rating Scale (AHRS), which is used to assess multiple characteristics of AVHs and thereby quantify the severity of AVHs (Hoffman et al., 2003). Secondary outcome measures comprised the changes over time in the severity of other schizophrenia symptoms and the level of patient insight. Other schizophrenia symptoms were measured by the Positive and Negative Syndrome Scale (PANSS) and 5 main symptom dimensions of PANSS: positive, negative, grandiosity/excitement, disorganization, and depression (Lindenmayer et al., 1995). The level of patient insight was assessed by using the abbreviated version of the Scale to Assess Unawareness in Mental Disorder in schizophrenia (SUMD), which is an expert-rating scale based on a patient interview (Michel et al., 2013). The abbreviated version of SUMD comprises 9 items measuring current states of awareness as follows: (1) a mental disorder, (2) consequences of a mental disorder, (3) effects of drugs, (4) hallucinatory experiences, (5) delusional ideas, (6) disorganized thoughts, (7) blunted affect, (8) anhedonia, and (9) lack of sociability. Scores on each item range from 0 to 3. A score of 0 indicates not applicable; 1, aware; 2, somewhat aware/unaware; and 3, severely unaware. Based on the 3-dimensions approach of the abbreviated version of SUMD, the scores on the items 1 to 3, 4 to 6, and 7 to 9 were averaged to obtain the dimension score of awareness of the disease, awareness of positive symptoms, and awareness of negative symptoms, respectively. All dimension scores were linearized on a 0 to 100 scale, with 0 and 100 indicating the lowest and highest level of unawareness, respectively. A rater (H.A.C.) who was blinded to the group assignment administered the AHRS, PANSS, and the abbreviated version of SUMD at baseline, immediately after the 5 days of tDCS and 1 and 3 months after tDCS. All participants were reimbursed for their transportation costs and time spent at each appointment attended.
Statistical Analyses
IBM SPSS Statistics 21.0 software (IBM SPSS Inc., Chicago, IL) was used for analyses. For the comparisons of continuous variables between the 2 groups, Student’s t tests were used for parametric variables and the Mann-Whitney tests for nonparametric variables. The χ2 and Fisher’s tests were used to examine between-group differences in discrete variables. To compare the effects of tDCS on primary and secondary outcomes over time in the 2 groups, a repeated-measures ANOVA (RMANOVA) was used to analyze the data from the full intent-to-treat sample, with time as the within-group factor and treatment as the between-group factor. RMANCOVA analysis was used to control for baseline significant differences that might have potential confounding effects. When a significant treatment group-by-time interaction effect was found, posthoc analyses were performed by using Student’s t tests for between-group comparisons. All results are 2-tailed, statistical significance was defined as P<.05, and Bonferroni adjustment was used to correct for multiple tests in which only P<.005 (0.05/10) were considered significant. For the percent changes in primary and secondary outcomes before and after 5 days of tDCS sessions, between-groups comparisons were undertaken and Cohen’s d effect sizes were calculated by using G*power Version 3.1.9.2. Cohen’s guidelines (Cohen, 1988, 1992) identify 0.2, 0.5, and 0.8 as small, medium, and large effects, respectively. Power analysis was performed with the use of G*power Version 3.1.9.2.
Results
Sample Characteristics
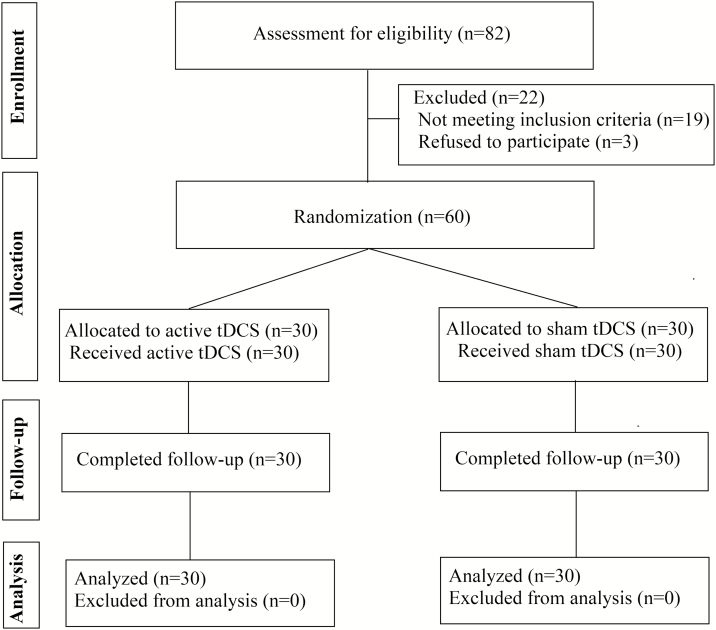
The CONSORT flow chart was shown in Figure 1. Sixty patients, all right-handed, were included in our study. Fifty-one of them had a diagnosis of schizophrenia and 9 had a diagnosis of schizoaffective disorder. Thirty patients were randomly assigned to the active tDCS group and 30 to the sham group. There was no significant between-group difference in demographic data. Patients in the active tDCS group had longer illness duration, a higher depression score of PANSS, and a higher domain score in awareness of negative symptoms of the abbreviated SUMD (Table 1).
Figure 1.
Flow diagram of the participant progress through the phases of the present randomized trial.
Table 1.
Baseline Demographic and Clinical Characteristics of the Participants
| Characteristics | Active tDCS | Sham tDCS | t/U or χ2/Fisher’s | P value |
|---|---|---|---|---|
| Number of participants | 30 | 30 | ||
| Females (%) | 16 (53.30) | 17 (56.70) | 0.07 | .80 |
| Age, mean±SD (range), years | 46.40±10.29 (22–65) | 42.17±10.29 (23–60) | 1.46 | .15 |
| Education level, mean±SD, years | 13.17±2.57 | 13.03±2.53 | 0.20 | .84 |
| BMI, mean±SD, kg/m2 | 25.77±3.75 | 25.29±4.86 | 0.43 | .67 |
| Smokers (%) | 10 (33.33) | 4 (13.30) | 3.35 | .13 |
| Onset age, mean±SD, years | 26.50±8.88 | 28.27±9.85 | -0.73 | .47 |
| Length of illness, mean±SD, years | 19.73±10.36 | 13.90±7.50 | 2.50 | .02 |
| Antipsychotic dosage, mean±SD, mg/d (chlorpromazine equivalents) | 493.81±306.76 | 493.32±284.93 | 0.01 | 1.00 |
| AHRS score, mean±SD | 29.13±5.31 | 29.07±4.83 | 0.05 | .96 |
| Positive and Negative Syndrome Scale | ||||
| Total score, mean±SD | 72.33±13.19 | 66.73±12.49 | 1.69 | .10 |
| Positive score, mean±SD | 13.53±4.64 | 12.97±3.41 | 0.54 | .59 |
| Negative score, mean±SD | 22.83±4.98 | 21.40±4.67 | 1.15 | .26 |
| Grandiosity/excitement score, mean±SD | 5.73±2.27 | 5.30±1.73 | 0.83 | .41 |
| Disorganization score, mean±SD | 11.43±2.30 | 10.60±2.31 | 1.40 | .17 |
| Depression score, mean±SD | 6.43±1.99 | 5.43±1.72 | 2.08 | .04 |
| The abbreviated version of the SUMD | ||||
| Awareness of disease, mean±SD | 90.00±13.79 | 84.07±15.90 | 363.00 | .16 |
| Awareness of positive symptoms, mean±SD | 62.59±19.89 | 55.93±15.30 | 363.50 | .18 |
| Awareness of negative symptoms, mean±SD | 86.67±16.09 | 75.19±15.35 | 290.50 | .009 |
Abbreviations: AHRS, Auditory Hallucination Rating Scale; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); SUMD, Scale to Assess Unawareness in Mental Disorder.
Statistical Power
The present total sample had a power of 0.966 to detect a small effect (Cohen’s d=0.2) for the primary and secondary outcomes.
Primary Outcome
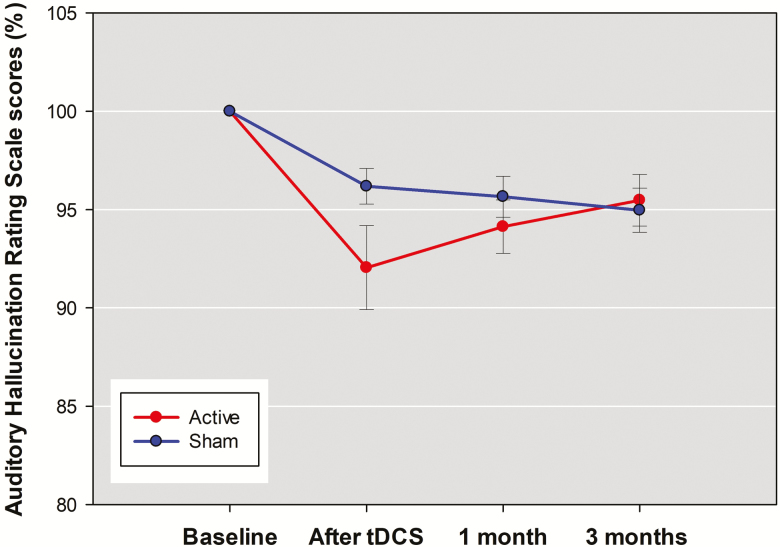
In the active tDCS group, AHRS score was decreased 7.95±11.73% immediately after tDCS, 5.86±7.43% at 1 month, and 4.52±7.17% at 3 months (Figure 1). In the sham group, AHRS score was decreased 3.81±4.98% immediately after tDCS, 4.34±5.71% at 1 month, and 5.03±6.11% at 3 months. The RMANOVA for AHRS score showed no significant interaction between group and time [F(3,56)=2.59, P=.062]. The negative result remained after controlling for confounding effects of illness duration, baseline depression, and awareness of negative symptoms by covarying them in RMANCOVA [F(3,53)=1.56, P=.211]. As seen in Table 2, the acute effect of 5 days of tDCS on auditory verbal hallucination (from 29.13±5.31 to 26.73±5.64) showed a small effect size compared with the sham group (from 29.07±4.83 to 27.97±4.91), but the effect did not reach statistical significance.
Table 2.
Percent Decrease in the Severity of Auditory Verbal Hallucinations and Other Schizophrenia Symptoms and Levels of Impaired Insight after 5 Days of tDCS or Sham Treatment in the Participants
| Outcome measures | Active tDCS (n=30) Mean±SEa |
Sham tDCS (n=30) Mean±SEa |
F | P value | Partial η2 | Effect size |
|---|---|---|---|---|---|---|
| Auditory Hallucination Rating Scale score | 7.83±1.79 | 3.94±1.79 | 2.11 | .15 | 0.037 | 0.196 |
| Positive and Negative Syndrome Scale | ||||||
| Total score | 2.88±0.66 | 0.81±0.66 | 4.37 | .041 | 0.074 | 0.283 |
| Positive score | 4.05±1.10 | 1.83±1.10 | 1.81 | .18 | 0.032 | 0.182 |
| Negative score | 1.24±0.90 | 0.38±0.90 | 0.40 | .53 | 0.007 | 0.084 |
| Grandiosity/excitement score | -4.42±2.31 | -0.07±2.31 | 1.57 | .21 | 0.028 | 0.170 |
| Disorganization score | 0.87±0.57 | 0.07±0.57 | 0.89 | .35 | 0.016 | 0.128 |
| Depression score | 2.29±1.06 | 1.16±1.06 | 0.51 | .48 | 0.009 | 0.095 |
| The abbreviated version of the SUMD | ||||||
| Awareness of disease | 31.49±3.80 | 9.92±3.80 | 14.34 | <.001 | 0.207 | 0.511 |
| Awareness of positive symptoms | 36.57±3.91 | 2.58±3.91 | 33.54 | <.001 | 0.379 | 0.781 |
| Awareness of negative symptoms | 13.77±2.72 | 0.43±2.72 | 10.71 | .002 | 0.163 | 0.441 |
Abbreviations: SUMD, Scale to Assess Unawareness in Mental Disorder; tDCS, Transcranial Direct Current Stimulation.
aValues were adjusted for covariates (illness duration, baseline depression, and awareness of negative symptoms).
Secondary Outcomes
The RMANOVA showed no significant group-by-time interaction for total PANSS score [F(3,56)=4.35, P=.008] and for the scores of symptoms dimensions of positive [F(3,56)=1.63, P=.19], negative [F(3,56)=1.84, P=.15], grandiosity/excitement [F(3,56)=0.24, P=.87], disorganization [F(3,56)=2.42, P=.08], or depression [F(3,56)=2.09, P=.11].These negative results were unchanged after controlling for confounding effects. The acute effects of tDCS on total PANSS score and the scores of symptoms dimensions showed small or even trivial effects compared with the sham group, and the effects were not statistically significant (Table 2).
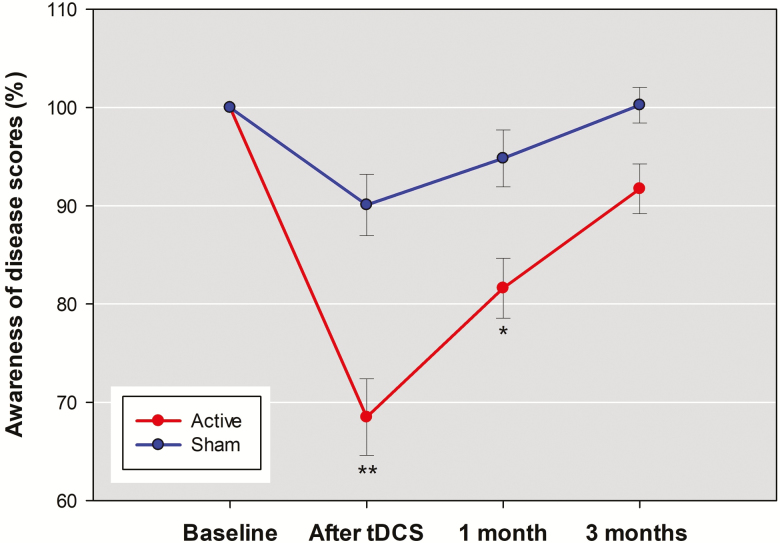
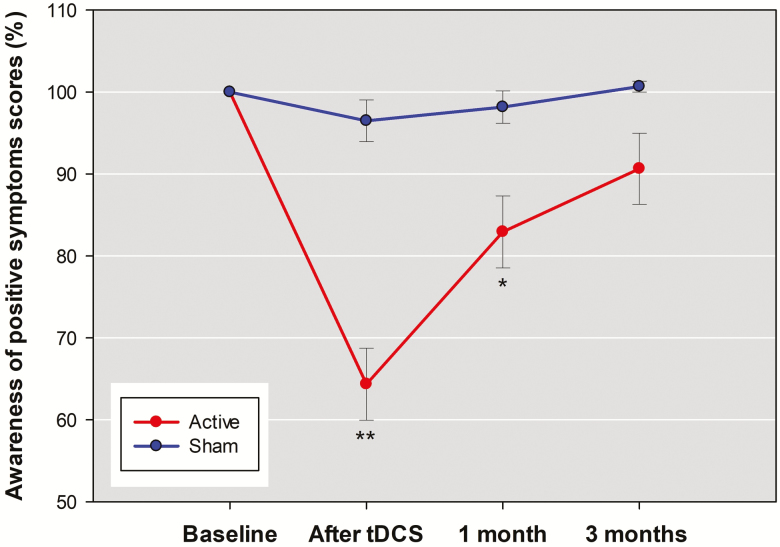
There were significant group-by-time interaction effects for the SUMD dimension scores of awareness of the disease [F(3,56)=7.97, P<.001], awareness of positive symptoms [F(3,56)=13.27, P<.001], and awareness of negative symptoms [F(3,56)=6.13, P=.001]. The results remained unchanged after adjusting for the covariates of illness duration and baseline depression in the RMANCOVA model. Additional correction for the potential confounder of awareness of negative symptoms did not change significant group-by-time interaction for the SUMD dimension scores of awareness of the disease [F(3,53)=6.06, P=.001] and awareness of positive symptoms [F(3,53)=9.89, P<.001] but reduced the interaction for awareness of negative symptoms to nonsignificant [F(3,53)=4.48, P=.007]. Posthoc analyses showed that the significant interaction effects for the SUMD dimension scores of awareness of the disease and awareness of positive symptoms were mainly due to the acute effect of 5 days of active tDCS to reduce the 2-dimension scores, with moderate effect sizes compared with sham treatment (Table 2). However, the maintenance effects (from end of treatment to 3 months) of tDCS on awareness of the disease (Figure 3) and awareness of positive symptoms (Figure 4) faded with time. Between-group differences in percent decrease of the 2 SUMD dimension scores were significant after tDCS and at 1 month but were no longer significant at 3 months.
Figure 3.
The mean percentage changes in the awareness of the disease dimension scores of the abbreviated version of the Scale to Assess Unawareness in Mental Disorder in schizophrenia (SUMD) between active stimulation group and sham group across the 4 assessments. Other descriptions are as in Figure 2.
Figure 4.
The mean percentage changes in the awareness of positive symptoms dimension scores of the abbreviated version of the Scale to Assess Unawareness in Mental Disorder in schizophrenia (SUMD) between active stimulation group and sham group across the 4 assessments. Other descriptions are as in Figure 2.
Figure 2.
The mean percentage changes in scores of Auditory hallucination Rating Scale (AHRS) between active stimulation group and sham group across the 4 assessments. Error bars indicated the SE. Posthoc analyses were undertaken to examine between-group difference at each post-baseline assessment with P <.005 (0.05/10) considered significant. *P<.005; **P<.001.
Side Effects
The study did not observe any major adverse events. Patients in the active and sham groups reported pricking [9 (30.0%) vs 5 (16.7%)], itchiness [4 (13.3%) vs 3 (10.0%)], and burning sensation [2 (6.7%) vs 2 (6.7%)] under the electrodes and daytime sedation [1 (3.3%) vs 1 (3.3%)].
Discussion
To our knowledge, this is the largest randomized controlled trial thus far examining the therapeutic effects of fronto-temporal tDCS on AVHs in schizophrenia. The pilot study by Brunelin et al. (2012) demonstrated therapeutic effects of fronto-temporal tDCS on AVHs severity in schizophrenia. Fronto-temporal tDCS was also expected to improve negative symptoms and depression symptoms through anodal tDCS acting on left dorsolateral prefrontal cortex (DLPFC) to increase its cortical excitability and thereby correct its hypoactivity on the premise that both negative symptoms (Sanfilipo et al., 2000) and depression (Grimm et al., 2008) have been linked to hypofrontality. However, we did not find any significant effects of tDCS on AVHs severity in the primary outcome measure and other schizophrenia symptoms in the secondary outcome measures. Our negative results provide an observation that the tDCS treatment for medication-refractory AVHs is not as effective as previously reported. Our study has quite strong statistical power for the primary outcome. Thus, a false-negative result due to type II statistical error coming from insufficient statistical power is presently unlikely. In fact, the clinical value of fronto-temporal tDCS for treating AVHs in schizophrenia is being called into question (Pondé et al., 2017). Evidence in magnet treatment for AVHs in schizophrenia also suggests a similar scenario, in which transcranial magnetic stimulation directed at the left temporo-parietal area showed promising impact on AVHs symptoms in earlier trials, but effect sizes of this modality have a tendency to decrease over time along with publication of larger trials (Slotema et al., 2014; Kubera et al., 2015).
On the other hand, our negative results on the effects of tDCS on other schizophrenia symptoms are also unlikely to be false-negative because the statistical power for the secondary outcomes is equally strong as that for the primary outcome. On the contrary, the positive results of tDCS reducing negative and depression symptoms reported in the pilot study were at increased risk for change findings or false-positive errors because the power of statistical tests for the secondary outcomes was greatly reduced (a power of 0.826 and 0.561 for independent t test and for RMANOVA, respectively, to detect a small effect) and Bonferroni correction was not used neither. In line with our study, several other studies reported no significant effects of fronto-temporal tDCS on schizophrenia symptoms as measured by the PANSS (Fitzgerald et al., 2014; Smith et al., 2015).
Several possible reasons might have contributed to our negative results. For one, we did not take into account the possibility that the receptor binding profiles of antipsychotics taken by the patients may impact the treatment effects of tDCS. Recent research indicated antipsychotic drug type (as defined by high vs low dopamine D2 receptor affinity) may influence the therapeutic effects of add-on tDCS on AVHs in schizophrenia (Agarwal et al., 2016). It may help clarify the issue by comparing the drug type of concomitant use of antipsychotics in our study with that in previous studies showing positive findings. Another possible reason was our heterogeneous sample comprising patients with schizophrenia and schizoaffective disorder. Some patients in our study had a wide range of symptom profiles, that is, a complex mixture of delusions, disorganized speech and behaviors, negative symptoms, depression, and AVHs. Now that fronto-temporal tDCS was scheduled to target the specific symptoms of AVHs, its therapeutic effects on AVHs might be obscured if the investigation was carried on the patient population with mixed symptoms.
The present study used conventional tDCS, which stimulates cortical areas via large sponge electrodes and with low precision spatial localization. A growing body of research suggests the use of high definition tDCS, which targets cortical areas using arrays of electrodes on the scalp and thereby focalizes the delivery of electrical current to the discrete brain regions (Borckardt et al., 2012) and emphasizes the value of electroencephalography-based approach in guiding stimulation, individualizing, and optimizing tDCS protocols (Dmochowski et al., 2017; Thut et al., 2017). The effects of tDCS on AVHs in schizophrenia should be validated by future studies using the combination of these new tools.
Research has indicated that precise identification of potential responders for the noninvasive brain stimulation is a key to success in AVHs treatment (Kubera et al., 2015). Of note, the average daily chlorpromazine equivalent dose given in our patient population is much less than that reported in the pilot study. A possible reason for our negative results is that patients with refractory AVHs may be candidate responders particularly benefiting from the prevailing stimulation protocol of fronto-temporal tDCS if they could tolerate higher dosage of antipsychotic medications. For those who are unable to tolerate high-dose antipsychotics, further studies should verify whether a more intensive stimulation protocol can improve the success rate of tDCS without jeopardizing their safety, for example, 3 times daily for 5 days (15 sessions).
In secondary outcome analyses, fronto-temporal tDCS led to improvement in insight into illness and positive symptoms. Our study is the first and largest randomized controlled trial to approve the beneficial effects of tDCS on impaired insight in schizophrenia. The positive results are unlikely due to chance alone given that the strong statistical power along with corrections for the multiple comparisons have protected these secondary outcome measures against the risk of type I error and thus have other underlying causes for their occurrence.
Insight of schizophrenia patients is a multidimensional concept that includes elements of different domains of awareness and ability to attribute symptoms to mental illness (Amador et al., 1993). In the interhemispheric imbalance theory of impaired insight in schizophrenia, insight deficits are thought to stem from left dominant brain hemisphere activity (Shad et al., 2007). The theory has gained the support of recent functional imaging research that attributes impaired insight to aberrant functional connectivity in neural networks of left hemisphere (Gerretsen et al., 2014) and suggests several left-hemispheric regions representing putative targets for noninvasive neuromodulation treatment to intensify insight in schizophrenia (Gerretsen et al., 2015). In addition, evidence has suggested that unawareness of symptoms in schizophrenia is associated with anatomical deficits in prefrontal cortex (Parellada et al., 2011) as well as increased activations in this brain region, which may be a compensatory mechanism of prefrontal impairment (Shad and Keshavan, 2015). It has also been suggested that unawareness of illness correlates with anatomical deficits (Bergé et al., 2011) and compensatory hyperactivity (Sapara et al., 2014) of prefrontal cortex, particularly in the left hemisphere (Buchy et al., 2011; Gerretsen et al., 2015). One might intuitively wonder that our positive effects of tDCS on insight are a result of the anodal tDCS acting on left prefrontal cortex to augment the endogenous effort to compensate for prefrontal deficits. However, previous research indicated the linkage between unawareness of symptoms and white matter disruption in fronto-temporal brain regions (Antonius et al., 2011). More recent research provided further evidence for the ability of tDCS to modify white matter connectivity (Lindenberg et al., 2013) and in particular fronto-temporal tDCS to increase the resting-state functional connectivity between the left TPJ and the left DLPFC in schizophrenia patients (Mondino et al., 2016). Thus, the fronto-temporal montage used in the present study is also a possible factor contributing to the positive effects on insight into positive symptoms.
In our study, the effect of tDCS on insight into negative symptoms is not as evident as that for insight into positive symptoms. Contrary to the interhemispheric imbalance theory of impaired insight in schizophrenia, some researchers reported that illness unawareness and symptoms unawareness were associated with reduced gray matter volume in right-hemispheric fronto-temporo-parietal brain regions (Gerretsen et al., 2013) and DLPFC (Shad et al., 2006), respectively. Future studies are needed to examine if bilateral bicephalic tDCS (Klein et al., 2013) with fronto-temporal montage placement could improve insight into both positive and negative symptoms. Notwithstanding some promising results on insight enhancement, it should be noted that these positive findings need to be taken with great caution because volunteer bias, a subtype of selection bias, likely occurred in our study in which psychotic patients who volunteered for such an interventional trial already had a certain awareness of their own situations and thus may not be a full representative of the whole population.
It is clear that the completion rate of our clinical trial was high and fronto-temporal tDCS was well tolerated in our participants. No significant adverse events were observed, and patients in both active and sham groups reported no significant difference in mild side effects of stimulation. All these findings further strengthen the safety profile of tDCS in schizophrenia patients (Pondé et al., 2017) and approve tDCS and sham stimulation for double-blind, sham-controlled study designs (Gandiga et al., 2006).
Our study has several limitations. First, the abbreviated version of the SUMD has better acceptability in clinical practices but narrower definition of insight than the longer version does. Lack of assessment of attribution dimension in this short-form scale limits the interpretations of our results. Second, the interpretation of tDCS-associated positive effects on insight in schizophrenia is highly speculative because our study lacks the support from functional neuroanatomical or electrophysiological evidence. The underlying mechanism of our observations should be confirmed in future research with simultaneous electroencephalography recording during tDCS in these patients. Finally, there is debate about the role of insight in the treatment of schizophrenia. Interventions to promote insight in schizophrenia have been viewed by some researchers as a double-edged sword because heightened insight has been linked not only to better clinical outcomes, but also to worse psychological outcomes (depression, low self-esteem, increased levels of self-stigma, and suicidality) (Vrbova et al., 2017; Chio et al., 2018). Researchers applying noninvasive neuromodulation treatment in an attempt to heighten insight of schizophrenia patients should monitor the risk factors so as to maximize the beneficial effects of treatment-associated insight enhancement.
CONCLUSION
In summary, we observed no therapeutic effects of fronto-temporal tDCS on AVHs and other schizophrenia symptoms. Further studies should focus on more intensive and individualized protocols and the use of more focalized stimulation device to maximize the efficacy of fronto-temporal tDCS for these symptoms. Further, our results suggest that add-on tDCS in schizophrenia patients is an effective and safe intervention to facilitate patients’ insight into illness and symptoms. The promising results should be confirmed in future replication studies.
Acknowledgments
This study was supported in part by grants from the Ministry of Science and Technology of the Taiwanese Government (MOST 106-2314-B-016-021-MY3), the Tri-Service General Hospital (TSGH-C106-110), and the National Defense Medical Research (MAB-106-023).
Statement of Interest
None.
References
- Agarwal SM, Bose A, Shivakumar V, Narayanaswamy JC, Chhabra H, Kalmady SV, Varambally S, Nitsche MA, Venkatasubramanian G, Gangadhar BN(2016)Impact of antipsychotic medication on transcranial direct current stimulation (tdcs) effects in schizophrenia patients. Psychiatry Res 235:97–103. [DOI] [PubMed] [Google Scholar]
- Alderson-Day B, McCarthy-Jones S, Fernyhough C(2015)Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci Biobehav Rev 55:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador XF, Strauss DH, Yale SA, Flaum MM, Endicott J, Gorman JM(1993)Assessment of insight in psychosis. Am J Psychiatry 150:873–879. [DOI] [PubMed] [Google Scholar]
- Antonius D, Prudent V, Rebani Y, D’Angelo D, Ardekani BA, Malaspina D, Hoptman MJ(2011)White matter integrity and lack of insight in schizophrenia and schizoaffective disorder. Schizophr Res 128:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O(2011)Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand 123:431–439. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, Madan A, Barth K, George MS(2012)A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tdcs) on pain perception. J Pain 13:112–120. [DOI] [PubMed] [Google Scholar]
- Bose A, Shivakumar V, Narayanaswamy JC, Nawani H, Subramaniam A, Agarwal SM, Chhabra H, Kalmady SV, Venkatasubramanian G(2014)Insight facilitation with add-on tdcs in schizophrenia. Schizophr Res 156:63–65. [DOI] [PubMed] [Google Scholar]
- Bose A, Shivakumar V, Agarwal SM, Kalmady SV, Shenoy S, Sreeraj VS, Narayanaswamy JC, Venkatasubramanian G(2017)Efficacy of fronto-temporal transcranial direct current stimulation for refractory auditory verbal hallucinations in schizophrenia: a randomized, double-blind, sham-controlled study. Schizophr Res. 195:475–480. [DOI] [PubMed] [Google Scholar]
- Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, Saoud M, Mechri A, Poulet E(2012)Examining transcranial direct-current stimulation (tdcs) as a treatment for hallucinations in schizophrenia. Am J Psychiatry 169:719–724. [DOI] [PubMed] [Google Scholar]
- Buchy L, Ad-Dab’bagh Y, Malla A, Lepage C, Bodnar M, Joober R, Sergerie K, Evans A, Lepage M(2011)Cortical thickness is associated with poor insight in first-episode psychosis. J Psychiatr Res 45:781–787. [DOI] [PubMed] [Google Scholar]
- Buchy L, Hawco C, Joober R, Malla A, Lepage M(2015)Cognitive insight in first-episode schizophrenia: further evidence for a role of the ventrolateral prefrontal cortex. Schizophr Res 166:65–68. [DOI] [PubMed] [Google Scholar]
- Chio FHN, Mak WWS, Chan RCH, Tong ACY(2018)Unraveling the insight paradox: One-year longitudinal study on the relationships between insight, self-stigma, and life satisfaction among people with schizophrenia spectrum disorders. Schizophr Res doi: 10.1016/j.schres.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Cohen L.(1988)Statistical power analysis for the behavioral sciencies. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Cohen J.(1992)Statistical power analysis. Curr Dir Psychol Sci 1:98–101. [Google Scholar]
- Dmochowski JP, Koessler L, Norcia AM, Bikson M, Parra LC(2017)Optimal use of EEG recordings to target active brain areas with transcranial electrical stimulation. Neuroimage 157:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami S, Guimond S, Mallar Chakravarty M, Lepage M(2016)Cortical thickness and low insight into symptoms in enduring schizophrenia. Schizophr Res 170:66–72. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, McQueen S, Daskalakis ZJ, Hoy KE(2014)A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul 7:813–816. [DOI] [PubMed] [Google Scholar]
- Fröhlich F, Burrello TN, Mellin JM, Cordle AL, Lustenberger CM, Gilmore JH, Jarskog LF(2016)Exploratory study of once-daily transcranial direct current stimulation (tdcs) as a treatment for auditory hallucinations in schizophrenia. Eur Psychiatry 33:54–60. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG(2006)Transcranial DC stimulation (tdcs): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117:845–850. [DOI] [PubMed] [Google Scholar]
- Gerretsen P, Chakravarty MM, Mamo D, Menon M, Pollock BG, Rajji TK, Graff-Guerrero A(2013)Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum Brain Mapp 34:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Mamo DC, Fervaha G, Remington G, Pollock BG, Graff-Guerrero A(2014)Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: resting state functional connectivity. Schizophr Res 160:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Chakravarty MM, Lerch JP, Mamo DC, Remington G, Pollock BG, Graff-Guerrero A(2015)Illness denial in schizophrenia spectrum disorders: a function of left hemisphere dominance. Hum Brain Mapp 36:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G(2008)Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fmri study in severe major depressive disorder. Biol Psychiatry 63:369–376. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, Krystal JH(2003)Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry 60:49–56. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pouchet A, Pins D, Thomas P(2011)Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry 168:73–81. [DOI] [PubMed] [Google Scholar]
- Klein E, Mann A, Huber S, Bloechle J, Willmes K, Karim AA, Nuerk HC, Moeller K(2013)Bilateral bi-cephalic tdcs with two active electrodes of the same polarity modulates bilateral cognitive processes differentially [corrected]. Plos One 8:e71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera KM, Barth A, Hirjak D, Thomann PA, Wolf RC(2015)Noninvasive brain stimulation for the treatment of auditory verbal hallucinations in schizophrenia: methods, effects and challenges. Front Syst Neurosci 9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC(2002)Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51:1008–1011. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Lüllmann E, Rief W(2007)Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr Bull 33:1324–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Nachtigall L, Meinzer M, Sieg MM, Flöel A(2013)Differential effects of dual and unihemispheric motor cortex stimulation in older adults. J Neurosci 33:9176–9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JP, Grochowski S, Hyman RB(1995)Five factor model of schizophrenia: replication across samples. Schizophr Res 14:229–234. [DOI] [PubMed] [Google Scholar]
- Michel P, Baumstarck K, Auquier P, Amador X, Dumas R, Fernandez J, Lancon C, Boyer L(2013)Psychometric properties of the abbreviated version of the scale to assess unawareness in mental disorder in schizophrenia. BMC Psychiatry 13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondino M, Haesebaert F, Poulet E, Suaud-Chagny MF, Brunelin J(2015)Fronto-temporal transcranial direct current stimulation (tdcs) reduces source-monitoring deficits and auditory hallucinations in patients with schizophrenia. Schizophr Res 161:515–516. [DOI] [PubMed] [Google Scholar]
- Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J(2016)Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull 42:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W(2003)Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A(2008)Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1:206–223. [DOI] [PubMed] [Google Scholar]
- Parellada M, Boada L, Fraguas D, Reig S, Castro-Fornieles J, Moreno D, Gonzalez-Pinto A, Otero S, Rapado-Castro M, Graell M, Baeza I, Arango C(2011)Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: a 2-year longitudinal study. Schizophr Bull 37:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg GH, van Donkersgoed RJ, David AS, Aleman A(2013)Changes in insight during treatment for psychotic disorders: a meta-analysis. Schizophr Res 144:109–117. [DOI] [PubMed] [Google Scholar]
- Pondé PH, de Sena EP, Camprodon JA, de Araújo AN, Neto MF, DiBiasi M, Baptista AF, Moura LM, Cosmo C(2017)Use of transcranial direct current stimulation for the treatment of auditory hallucinations of schizophrenia - a systematic review. Neuropsychiatr Dis Treat 13:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A(2000)Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry 57:471–480. [DOI] [PubMed] [Google Scholar]
- Sapara A, Ffytche DH, Birchwood M, Cooke MA, Fannon D, Williams SC, Kuipers E, Kumari V(2014)Preservation and compensation: the functional neuroanatomy of insight and working memory in schizophrenia. Schizophr Res 152:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapara A, Cooke M, Fannon D, Francis A, Buchanan RW, Anilkumar AP, Barkataki I, Aasen I, Kuipers E, Kumari V(2007)Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr Res 89:22–34. [DOI] [PubMed] [Google Scholar]
- Shad MU, Keshavan MS(2015)Neurobiology of insight deficits in schizophrenia: an fmri study. Schizophr Res 165:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Keshavan MS(2006)Prefrontal subregions and dimensions of insight in first-episode schizophrenia–a pilot study. Psychiatry Res 146:35–42. [DOI] [PubMed] [Google Scholar]
- Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A(2007)Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry 19:437–446. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Murray RM, McGuire PK(1998)Auditory hallucinations: a review of psychological treatments. Schizophr Res 32:137–150. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, van Lutterveld R, Hoek HW, Sommer IE(2014)Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol Psychiatry 76:101–110. [DOI] [PubMed] [Google Scholar]
- Smith RC, Boules S, Mattiuz S, Youssef M, Tobe RH, Sershen H, Lajtha A, Nolan K, Amiaz R, Davis JM(2015)Effects of transcranial direct current stimulation (tdcs) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr Res 168:260–266. [DOI] [PubMed] [Google Scholar]
- Thut G, Bergmann TO, Fröhlich F, Soekadar SR, Brittain JS, Valero-Cabré A, Sack AT, Miniussi C, Antal A, Siebner HR, Ziemann U, Herrmann CS(2017)Guiding transcranial brain stimulation by EEG/MEG to interact with ongoing brain activity and associated functions: a position paper. Clin Neurophysiol 128:843–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz FJ, Béjar A, Casado M(2002)Insight, psychopathology, and interpersonal relationships in schizophrenia. Schizophr Bull 28:311–317. [DOI] [PubMed] [Google Scholar]
- Vrbova K, Prasko J, Ociskova M, Latalova K, Holubova M, Grambal A, Slepecky M(2017)Insight in schizophrenia - a double-edged sword?Neuro Endocrinol Lett 38:457–464. [PubMed] [Google Scholar]
- Waters F.(2012)Multidisciplinary approaches to understanding auditory hallucinations in schizophrenia and nonschizophrenia populations: the International Consortium on Hallucination Research. Schizophr Bull 38:693–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F, Allen P, Aleman A, Fernyhough C, Woodward TS, Badcock JC, Barkus E, Johns L, Varese F, Menon M, Vercammen A, Larøi F(2012)Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull 38:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier RM, Pan W, Dungan JR, Keefe RSE, Vorderstrasse A(2018)Unraveling interrelationships among psychopathology symptoms, cognitive domains and insight dimensions in chronic schizophrenia. Schizophr Res 193:83–90. [DOI] [PubMed] [Google Scholar]