Summary
Common data elements (CDEs) are becoming more common as more areas of preclinical research have generated CDEs. Herein we provide an overview of the progress to date in generating CDEs for preclinical epilepsy research. Currently there are CDEs that have been developed for Physiology (in vivo), Behavior, Pharmacology, and Electroencephalography (EEG). Together the CDEs and methodologic considerations associated with these CDEs are laid out in consecutive manuscripts published in Epilepsia Open, each describing CDEs for their respective topic area. In addition to the overview of progress for the 4 subjects, core characteristics (Core CDEs) are described and explained. Data collection using a case report form (CRF) is described, and considerations that are involved in using the CDEs and CRFs are discussed.
Keywords: Translation, Case report form, Rigor, Reproducibility, Transparency
1.
Key Points.
Common data elements (CDEs) are fundamental bits of data to be reported for all studies of a particular type
The use of CDEs in preclinical epilepsy research will increase rigor, standardization, and transparency
The ILAE/AES Joint Translational Task Force has created CDEs in 4 main areas, Physiology, Pharmacology, Behavior, and EEG, and also a set of Core CDEs
Core CDEs contain the most common pieces of information that are useful for a preclinical epilepsy study
Organization of Preclinical Epilepsy Common Data Elements (CDEs)
In light of several concerns related to current preclinical research endeavors, common data elements (CDEs), which are “data elements that are common to multiple data sets across different studies,”1 have emerged as one approach to improve preclinical research. For example, preclinical research studies have not always described methods and presented data in ways that make results comparable from one study to the next. This has made it hard to conduct so‐called meta‐analyses for preclinical epilepsy research. Such meta‐analyses attempt to consider large data sets from multiple laboratories to discern commonalities and differences. This type of large‐scale comparison can be used to identify important directions for future studies. One of the ways that one could address the problems related to large‐scale comparisons is by defining common methods, terms, and units for data that are usually of interest for comparisons. From this idea CDEs were developed. Strictly speaking, CDEs are the fundamental elements of data reported in the results sections of publications.1, 2 They are as their name implies, the fundamental data that are common to many types of research, in this case preclinical epilepsy research.
Additional benefits of preclinical epilepsy CDEs have been described before.3, 4, 5 CDEs for clinical epilepsy research have also been discussed,2 and are available online.6 For preclinical epilepsy, the International League Against Epilepsy (ILAE) and the American Epilepsy Society (AES) as well as the National Institute for Neurological Disorders and Stroke (NINDS) partnered to bring together an international group of individuals working in the field of epilepsy research. These individuals were chosen because they represented many countries and types of expertise in epilepsy and included junior as well as senior investigators. Representation by a diversity of individuals was a goal. There were 3 chairpersons (J.F., A.P., and H.S.) who organized the general CDE effort, as well as an additional leader (A.G.) who interfaced between other translational groups and the individuals working on CDEs. To include input from NINDS, Dr. Brandy Fureman and subsequently Dr. Vicky Whittemore were also included. Dr. Lauren Harte‐Hargrove assisted, helping coordinate and generate drafts of text, tables, and forms described further below. As manuscripts were prepared and forums were organized, input was also provided by the ILAE and AES leadership.
To begin the work, 4 areas of preclinical research were selected and are presented in this supplement: Physiology (in vivo), Behavior, Pharmacology, and EEG. These topics were chosen because of the need for CDEs in these subject areas, but they are by no means considered to be the only topics that are relevant to preclinical epilepsy research. In the future, additional topics should be developed until all areas of preclinical epilepsy are addressed.
Physiology (in vivo) refers to experimental approaches such as recording body temperature or blood pressure.7 Behavior includes tests in animals that are used to understand cognitive ability and other abilities relevant to the comorbidities of epilepsy.8 Examples include memory, depression, and anxiety. Pharmacology CDEs address tests that are intended for studies of drugs on seizures, either in a normal animal, genetically modified condition (i.e., transgenic), or an animal with seizures or epilepsy. Examples include the maximal electroshock test of seizures in rodents, kindling, or induction of status epilepticus.9 EEG CDEs address the data derived from EEG recording, either in a brain before seizures occur, during seizures, or afterward.10 All of the CDEs were developed with research using laboratory rats or mice in mind, or other laboratory animals that are periodically used in preclinical research such as primates.
For each topic, 2 chairpersons led a group of individuals (Table 1). Together the work groups chose their approach to develop CDEs, meaning their rationales for selecting subtopics, and other considerations. Forms used as templates for data collection (case report forms, or CRFs) were generated. Explanations of the methods associated with the data collection were drafted also. These are called “companions” in the manuscripts that follow in this Supplement to Epilepsia Open. meant to help guide the readers on the use of the CDEs and CRFs and not necessarily to create a mandate for a particular set of methods. Instead, each work group was tasked with developing CDEs, CRFs, and explanatory material that would be of maximum utility in preclinical research given the methods and other considerations of laboratories working in epilepsy in the current era.
Table 1.
List of work group co‐chairs and group members
| Work group title | Work group co‐chairs | Work group members |
|---|---|---|
| Physiology | Jan Gorter, Astrid Nehlig | Karen Borges, Gordon Buchanan, Stefanie Dedeurwaerdere, Daniel Friedman, Heidi Grabenstatter, Katarzyna Lukasiuk, Fulvio Scorza, Erwin van Vliet |
| Behavior | Nigel Jones, Andrey Mazarati | Liset Menendez de la Prida, Lisa E. Kalynchuk, Pierre‐Pascal Lenck‐Santini, Karine Sarkisova, Jana Veliskova |
| Pharmacology | Claudia Brandt, Melissa Barker‐Haliski | Jeremy Barry, Teresa Ravizza, Michael Rogawski, Ilse Smolders, Bo Xiao |
| EEG | Tomonori Ono, Aristea Galanopoulou (TASK1 Liaison) | Joost Wagenaar, Emmanuel Raffo, Ryosuke Hanaya. Filippo Sean Giorgi, Petr Fabera, John Jefferys |
Common data elements (CDEs) are currently being developed for preclinical research for several disorders, including not only epilepsy but also traumatic brain injury (TBI).11 Of interest, some of the questions raised by the research community about these CDEs are similar, despite the fact that different methods are used in each type of preclinical research topic, and the issues involved in preclinical research can be quite different for epilepsy, TBI, and spinal cord injury (SCI). The questions that have been commonly asked among epilepsy researchers were gleaned from vetting early drafts of CDEs and CRFs in 2016 at an Open Forum immediately following the annual meeting of AES in 2016.5 In addition, questions were raised by reviewers in the fall of 2016 who are outside the field of epilepsy but have expertise in Physiology, Behavior, Pharmacology, or EEG. Additional questions were raised by individuals working on translational endeavors of the ILAE and AES. Although it is fair to say that the idea of CDEs was welcomed by the vast majority, specific concerns about CDEs were raised. A common conception was that CDEs would increase the burden on researchers from a financial perspective. Concerns were raised about how to easily understand CDE tables and CRFs and whether CDEs will hinder creativity or scientific freedom. Many researchers did not appreciate the benefits of using CDEs for their own laboratory.
None of these issues are problematic if one becomes familiar with CDEs and CRFs. For example, researchers will not have an increased financial burden of any kind because CDEs and CRFs will be freely available on the web. They will be free to download and offered in various formats that are common and flexible (e.g., Microsoft Excel). One would expect a savings of time rather than increased time to conduct research as CDEs and CRFs make the time of laboratory personnel more efficient. They also allow Principal Investigators (PIs; heads of laboratories) to collect data from many individuals and merge files easily. In contrast, currently there are many laboratories where the PIs cannot easily take data from one postdoctoral fellow or student and merge it with another because of individual differences in data collection and methods. Those who made time‐consuming records might not be able to include them in an ultimate report of the project if others did not keep the same type of records. Thus, a problem that CDEs address is that measurements are not consistently entered, or entry is misplaced or forgotten entirely by different individuals even in the same lab. CRFs provide a template for an entire laboratory or series of laboratories. Many questions have binary answers or simple check marks. If a project needs data entry by more than one individual, the data entries will be the same.
Regarding the benefit of CDEs, it is true that authors of a single study may not benefit from comparing their data to another study using similar methods. However, it is easy to see that adoption of CDEs will ultimately benefit every author. The reason is that all published studies need to establish in introductory material what research has come before their experiments, and how the published results impact the studies of others. Without standards for reporting, some studies may seem relevant but become difficult to compare when considered in detail. Careful review of CDEs should make it clearer why studies differ, if one study cannot reproduce the findings of another. If findings are more transparent, and reproducibility more likely, authors will have more compelling published findings.
There are also other benefits of CDEs. Although not immediately realized, these will be important in the long‐term and benefit all individuals conducting research. One such benefit is that researchers may not have to repeat what has been done by others, and doubt what has been done by others, because research will be more transparent and rigorous. It will be more likely that differences in outcomes will be possible to trace to variables that are different. The transparency will come from the availability of data sets in a more universal style, and greater rigor will come from the addition of types of data that might have been absent in the past. For example, some research studies in epilepsy in the past did not note sex or age.12
An immediate benefit of using CDEs can be appreciated by considering the normal burden on PIs of training. For example, it is time‐consuming to tell individuals new to epilepsy research how to conduct experiments, and there is also an additional burden to explain how to write down data or record data in notebooks or by computer. Without training, data are acquired that could be incomplete. This problem is addressed if a PI can provide CRFs for data collection.
Regarding scientific freedom, use of the CDEs and CRFs do not determine experimental design or influence methods, although they do help remind experimenters of variables that are valuable that otherwise might be forgotten or dismissed. Furthermore, if studies of behavior, for example, are not relevant to a study for any reason, the CDEs and CRFs for behavior are not used.
Core CDEs
File names: Core CRF.docx; Core CDE.xlsx; CRF for Uploaded Files.docx
Core CDEs describe the general information collected from animal subjects. They are collected for a study of preclinical epilepsy research where a subject may undergo experimentation for physiology, behavior, pharmacology and/or EEG. Core CDEs are fundamental, and common to all experiments, whereas Physiology, Behavior, Pharmacology, or EEG CDEs may not always be needed.
The Core CDE and CRF files can be downloaded as a zip folder from the Supporting Information section at the end of the article (Data S1). Additionally, the CRF for Core Animal Characteristics can be found in Table 2 and the CRF for Uploaded Files can be found in Table 3. A portion of the CDE Chart can be found in Table 4 (because of its very large size, the entire chart can be found in Supporting Information). There are many types of core information that are important for animal subjects and can be organized into the following subheadings A‐H. The divisions are not intended to influence experiments; instead the order and organization is intended to reflect how the different data elements are typically considered in a Methods section of a publication.
Table 2.
Core animal characteristics case report form
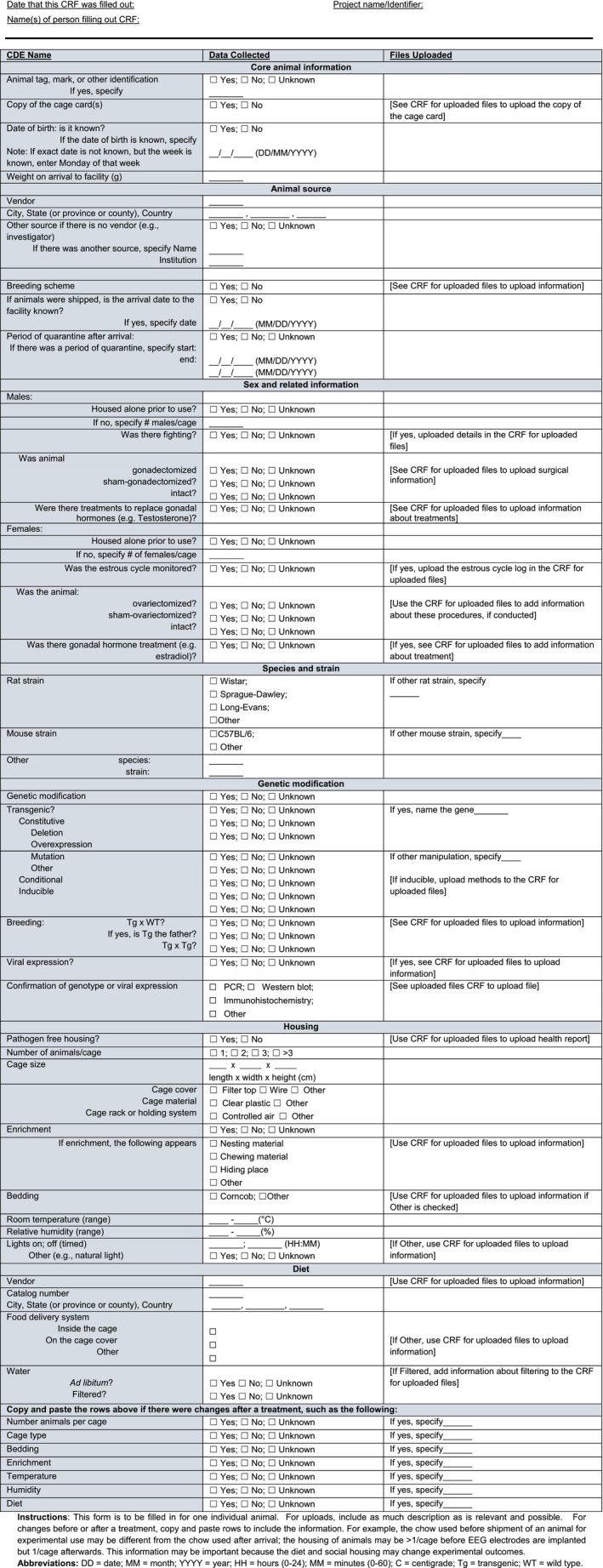
Table 3.
Case report form for uploaded files
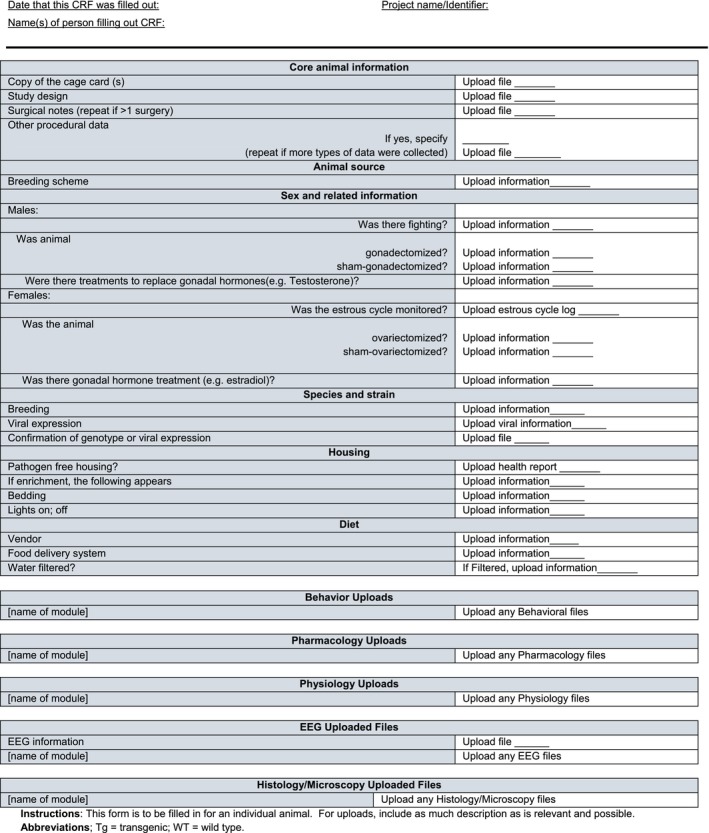
Table 4.
Sample of common data elements (CDEs) taken from the core animal characteristics CDE chart
| CDE Name | Variable name | Definition/description | Question text | Permissible value | Description of permissible value | Data type | Quantifier | Instructions |
|---|---|---|---|---|---|---|---|---|
| Core Animal Characteristics Animal tag, mark, or other identification Indicator | CoreCharTagMarkInd | Indicates whether or not there is an animal tag or mark or other identification used to identify the animal | Animal tag, mark, or other identification | Yes;no; unknown | Yes, there is an animal tag or mark or other identification; No, there is not an animal tag or mark or other identification; It is not known if there is an animal tag or mark or other identification | Alpha‐numeric | Yes/No/Unknown | Choose one: was there an animal tag or mark or other identification used to identify the animal? |
| Core Animal Characteristics If animal tag or mark, specify Text | CoreCharTagMarkTxt | Specifies the animal tag or mark used to identify the animal | If yes, specify | Alpha‐numeric | Animal tag or mark | Record the animal tag or mark used to identify the animal and upload file if available | ||
| Core Animal Characteristics Study design Indicator | CoreCharStudyDesInd | Indicates whether or not there is information regarding the study design available | Study design | Yes;no/Unknown | Yes, there is a study design available; No, there is not a study design available | Alpha‐numeric | Yes/No/Unknown | Choose one: is information regarding the study design available? Upload the file, if available |
| Core Animal Characteristics Surgical notes (repeat if >1 surgery) Indicator | CoreCharSurgNotesInd | Indicates whether or not there are surgical notes available | Surgical notes (repeat if >1 surgery) | Yes;no/Unknown | Yes, there are surgical or procedural notes available; No, there are not surgical or procedural notes available | Alpha‐numeric | Yes/No/Unknown | Choose one: are there surgical notes available? Upload the file if available |
Core animal information
This section covers general information. Using CRFs can greatly facilitate collecting information. Most animals have an identification code for a given study, which can be simple, such as animal 1, 2, 3, and so on. A separate “CRF for Uploaded Files” also has space to upload files related to the epilepsy induction procedure, such as how pilocarpine was administered, the date, dose, time of injection, and other details prior to and after the injection.
The CRF has a cell where a link to the cage card can be uploaded. This can be useful if there is a history of errors in logging information or forgetting to log information such as date of birth. “Human error” is all too common and the CRF is one way to help address it. Other files such as the study design and details related to surgery (e.g., EEG electrode implantation, cannula implantation) can be uploaded in the CRF.
Regarding date of birth, some vendors do not provide more than a general time of birth. Instead they provide animals of a range of body weights. If the specific date is not known, any information about the general time of birth should be entered. If the week is known, one way to standardize the CDE is to make the Monday of that week the birth date.
Animal source
The source of animals refers to how animals were obtained, that is, they might have been bred in the institution where the experiments are conducted or purchased from a vendor who shipped pregnant animals or postnatal animals. Animals may have been shipped from another laboratory, bred, and then experiments were conducted on the offspring. Documenting these details is valuable because the potential for stress to occur early in life can lead to later life changes in seizures,13 behavior,14, 15 or neuronal structure, in some cases.15, 16, 17 For example, litter size may differ depending upon the source of the animals—whether from an external vendor, or from an in‐house breeding source, and this may have an effect on variables such as weight gain.18 The use of a surrogate dam, and the quality of that dam, may also have an impact on the offspring. If animals are shipped shortly after birth, separation from the mother and the possibility that they will not eat or drink for a prolonged period may lead to dehydration or changes in the diet that can influence seizures.19, 20 Another variable that is helpful to document is when animals were imported into a quarantine area of the host institution and for how long. Although not in the CRF, it is helpful to ensure that the quarantine area is free of animals who have an illness or have just had surgery because it may cause stress in the exposed animals that have had no surgery but are nearby. The same is true for the other areas where animals are housed.
Sex and related information
Recent efforts in the United States and Europe are making it more important for investigators to log data regarding sex.21, 22 This raises several issues relevant to neuroendocrinology that are often not discussed in publications but are important to consider. Several examples are included in the CRF for Core CDEs.
The proper identification of an animal as male or female is essential, but not trivial. Determining the accurate sex of an animal can often be difficult at neonatal ages and requires an investigator or technician who is skilled in this area. The importance of accurate sexing becomes evident when one considers that type of housing—whether mice are isolated, housed with another of the same sex or housed with others of the opposite sex—can influence behavior and potentially experimental outcomes.23
An important issue is not only whether an animal is female or male but also its social housing. Most animals are different behaviorally and sometimes experimentally if they are housed with the same sex compared to isolation. This is particularly important in epilepsy research where animals might have been housed with others initially and then separated after an EEG electrode implant or a cannula implantation to prevent a cage mate from disrupting the implant (e.g., by grooming or gnawing on the implant of another animals in the same cage). The initial social housing might be unavoidable because housing animals in isolation is expensive. Although these changes in housing could affect experimental results, if the timing of such changes is noted at least experimenters can evaluate the potential for one study to differ from another due to the timing of social housing and isolation.
An issue for male housing specifically is that testosterone values can change depending on the numbers of animals per cage and time in a cage with additional males. Research has shown that rodents can establish social dominance and testosterone values can climb in the dominant male or fall in the subordinate male.24 Given the evidence that testosterone and its metabolites can alter behavior and seizures25, 26, 27 these are important considerations.
For females housed together compared to isolation there is no evidence that hormone values change or that there is a hierarchy that develops. However, females show elevated testosterone after status epilepticus28 and can fight other females if housed together afterward (unpublished results, H.E.S.). Therefore, isolation is important in females as well as males.
Estrous cycle monitoring has been described in detail elsewhere for rats and mice.29, 30 This is important in females because the hormones that fluctuate during the estrous cycle influence seizures, behavior, and many additional aspects of the brain and periphery.31, 32, 33, 34 The CRF for Core CDEs points users toward the Uploaded Files CRF where these details can be uploaded, and the section about females can also be expanded or contracted depending on the way females are used, that is, for a study of the estrous cycle, ovariectomy, or hormone administration on seizures.
One final issue to keep in mind pertains not only to the sex of the animals, but to the sex of the scientist or technician who is primarily handling the animals and/or conducting experiments. The sex of the scientist or technician is important because it has been shown that olfactory exposure to human males induces a physiologic stress response in male rodents compared to human females.35 It is possible that increased stress levels may affect research outcomes such as seizure susceptibility,36 and it is therefore important to make a note of the sex of the technician or experimenter in case this information is needed to help interpret experimental results.
Species and strain
In most epilepsy research today, animals are either rats or mice. There are several issues that are important to consider, however. One is the vendor and their location. This may seem overly detailed, but it has been published that the vendor and even the location of the vendor of C57BL6 mice can influence epilepsy research.37
Genetic modification
Researchers are using transgenic manipulations to conduct epilepsy research at an increasing rate. This makes it very important to clarify exactly what was done to an animal. The origin of the animal at a specific vendor or collaborating institution is important, as well as where the transgenic line was originally produced, that is, an investigator created the transgenic and then gave it to Jackson Laboratories. This information is usually available from the vendor and the Core CRF points users to the CRF for Uploaded Files where details can be uploaded as a single file. It is also valuable to note how the background strain was obtained and if the mouse was backcrossed to a new strain, the number of generations it was backcrossed.
Clarifying how expression was confirmed (e.g., deletion for a knockout and expression for an overexpressing mouse line) is valuable because sometimes the deletion is not 100% and the degree of overexpression varies. For those situations that are complex, for example, the expression varies in different tissues, a file can be uploaded to document the variability. The CRF has educational value in this context because the most junior members of a laboratory may not recognize that these are important aspects of their research.
Housing
Housing is an important variable in animal research, with numerous publications documenting surprising effects of the environment on experimental results.38, 39, 40 Housing also is highly variable from laboratory to laboratory, making it likely that differences in housing contribute to differences in results. There are also different housing standards around the world, making it difficult to create CDEs that can be used by all. One of the common variables is whether the animals are kept in an environment that is pathogen free. The definition of “pathogen‐free” may vary so the Core CRF contains a link to the CRF for Uploaded Files where users can upload detailed information. Theoretically it would be valuable to publish this information in Appendix S1 because the degree that animals are maintained as free of pathogens could be important. For example, a benign virus may be present, and some veterinarians may still consider the colony relatively free of pathogens, but another veterinarian might not. By providing a Health Report, the CRF contains exactly what was tested and the results, avoiding the issues related to interpreting what is or is not “pathogen‐free.”
Another possible consideration is whether the animal is housed in a specialized holding room, as often occurs after EEG or other types of epilepsy experiments that require invasive procedures such as surgeries to implant electrodes. Novel housing after such procedures is most often an abrupt change from the animal's original housing environment, and the population of other animals housed in the room is very different from the original population to which the animal was exposed. These sudden changes in housing environment have the potential to be a large stressor that may impact seizure susceptibility.36 Moreover, animals in neighboring cages often change more frequently, adding an additional potential stressor. Therefore, it is important to record this information and consider these factors when analyzing results.
The cages used for animals are important in several ways. Across the world the dimensions vary, so including this information is much better than simply stating that the cage is standard, or not including any information at all which is what is typical. Whether the cage is made of transparent plastic or cage walls are dark has an influence on what the animal can see; if nothing is possible to see, the environment is less stimulating that a transparent plastic cage. This affects the overall enrichment of the environment, discussed further below. Again, there are findings that have been published showing how much a variable such as enrichment can matter,38, 39, 40 supporting the inclusion of these types of information in the CRF for Core CDEs.
Enrichment of the cage is often considered to be, for example, a plastic container where the animal can “hide” or a nestlet that animals can use to make a nest. Therefore, these examples are found in the CRF for Core CDEs. The temperature, humidity, and light‐dark cycle are also common variables that are important to specify.41, 42
Diet
Several studies have shown that the composition of the diet can affect seizures.43, 44 Although the most common example is the ketogenic diet, other diets have also been shown to affect rodents.43, 44 For females, the degree that phytoestrogens are included in the diet is very important because it has been shown to exert an influence on the brain and behavior.45 Furthermore, dietary nutritional supplementation can influence aspects of breeding including litter sizes.46 In both sexes, the maternal diet and the diet during early life can have effects in adulthood.47 The diets of most animals are known and can be uploaded by importing a file from the vendor to the CRF for Uploaded Files.
Although it is often not specified, the water and its delivery may be a variable in research studies. In some laboratories, highly purified water is used, whereas in other laboratories this may not be possible or prohibitively expensive. Automatic watering systems can avoid this issue but are not always available. To the extent these details can be provided the better, but the CRF contains only the most common types of information: whether water is ad libitum or filtered.
Complex experimental designs
Increasing use of complex experimental designs makes it harder and harder to compare epilepsy research studies. For example, experimenters may use a virus to express channelrhodopsin in a specific cell type in a single brain region. Those animals may be subjected to epilepsy induction and video‐EEG at multiple times of their life, with or without pharmacology. For this reason, the last part of the CRF allows investigators to copy and paste rows from the parts A‐G to make a part H where values can be entered for each part of an experiment.
Conclusions
CDEs for preclinical epilepsy have now been generated for 4 subtopics, and the CDEs, CRFs and guidelines have received comments from experts within and outside of epilepsy research. Many of the comments have raised concerns that are readily addressable by considering exactly what CDEs are, their utility, and flexibility. The CDEs, CRFs, and companion papers have the potential to streamline data collection and make it more standardized, transparent, and cost/time‐effective. They can help correct errors and train junior staff to attend to important aspects of their experiments. It is envisioned that this effort to produce some of the first CDEs in preclinical epilepsy research will begin an interactive and growing interest in CDEs that will ultimately expand to all aspects of epilepsy research and the epilepsy research community worldwide.
Disclosure/Conflict of interest
J.A.F. A.S.G., L.C.H., A.P. and H.E.S. received travel reimbursement for meetings for the work done through the TASK3 of the ILAE/AES Joint Translational Task Force. L.C.Harte‐Hargrove is currently Associate Director of Research at Citizens United for Research in Epilepsy (CURE) but this position has created no conflict of interest for the content of this manuscript. A.S. Galanopoulou has received royalties for publications from Elsevier and is a co‐Editor‐in‐Chief of Epilepsia Open. She has also received honorarium from Mallinckrodt for participation in a scientific advisory board but has no conflicts of interest in regard to this manuscript. J.A. French receives New York University (NYU) salary support from the Epilepsy Foundation and for consulting work on behalf of the Epilepsy Study Consortium for Acorda, Adamas, Alexza, Anavex, Axcella Health, Biogen, BioPharm Solutions, Cerecor, Concert Pharmaceuticals, Engage, Eisai, GlaxoSmithKline, GW Pharma, Marinus, Nestle‐Health Science, Neurelis, Novartis, Pfizer, Pfizer‐Neusentis, Ovid, Sage, SK Life Sciences, Sunovion, Takeda, UCB Inc., Upsher Smith, Xenon Pharmaceuticals, Zogenix, and Zynerba. J.A. French has also received research grants from Acorda, Alexza, Eisai Medical Research, Lundbeck, Pfizer, SK Life Sciences, Sunovion, UCB Inc., Upsher‐Smith, and Vertex, as well as grants from the Epilepsy Research Foundation, Epilepsy Study Consortium, Epilepsy Therapy Project, and NINDS. She is on the editorial board of Lancet Neurology, Neurology Today, and Epileptic Disorders. She has received travel reimbursement related to research, advisory meetings, or presentation of results at scientific meetings from the Epilepsy Study Consortium, the Epilepsy Foundation, Eisai, GW Pharma, Marinus, Nestle Life Sciences, Pfizer, Sage, SK life Sciences, Takeda, UCB Inc., Upsher‐Smith, Zogenix, Zynerba. H.E. Scharfman is a member of the Scientific Board of Advisors for Pyramid Biosciences. The remaining authors have no further conflicts of interest to disclose. The authors have read the journal's position on issues involved in ethical publication and affirm this report is consistent with those guidelines.
Supporting information
Data S1. Core CRF and CDE files. The CDE and CRF modules linked to this article can be found and downloaded as a zip folder.
Acknowledgments
This report was written by experts selected by the International League Against Epilepsy (ILAE) and the American Epilepsy Society (AES) (TASK3 group of the ILAE/AES Joint Translational Task Force) and was approved for publication by the ILAE and the AES. Opinions expressed by the authors, however, do not necessarily represent the policy or position of the ILAE or the AES. We are also grateful to the AES, ILAE, and National Institutes of Health (NIH)/NINDS for partial sponsoring the activities of the ILAE/AES Joint Translational Task Force.
A. Galanopoulou acknowledges grant support by NINDS RO1 NS091170, U54 NS100064, the US Department of Defense (W81XWH‐13‐1‐0180), and research funding from the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. J.A. French acknowledges research support from New York University, Epilepsy Research Consortium and Epilepsy Foundation. A. Pitkänen receives grant support from the Academy of Finland (272249 and 273909), and the European Union 7th Framework Program no:o 602102 EPITARGET, U54 NS1000064. H.E. Scharfman acknowledges the support of the NY State Office of Mental Health.
Biography
Dr. Lauren C. Harte‐Hargrove is Project Manager of the ILAE/AES Joint Translational Task Force.

Contributor Information
Lauren C. Harte‐Hargrove, Email: laurenharte@gmail.com
Helen E. Scharfman, Email: hscharfman@nki.rfmh.org
References
- 1. National Institutes of Health . NIH Common Data Element (CDE) Resource Portal, 2013. Available at: https://www.nlm.nih.gov/cde/glossary.html#cdedefinition. Accessed November 21, 2017.
- 2. Loring DW, Lowenstein DH, Barbaro NM, et al. Common data elements in epilepsy research: development and implementation of the NINDS epilepsy CDE project. Epilepsia 2011;52:1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. EPITARGET . Common Data Elements CRF Modules & Guidelines, 2015. Available at: http://www.epitarget.eu/cdes/. Accessed November 21, 2017.
- 4. EPITARGET . First EPITARGET Results, n.d. Available at: http://www.epitarget.eu/results/the-projectfirst-results/. Accessed November 21, 2017.
- 5. Harte‐Hargrove LC, French JA, Pitkänen A, et al. Common data elements for preclinical epilepsy research: Standards for data collection and reporting. A TASK3 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia 2017;58(Suppl 4):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institute of Neurological Disorders and Stroke . NINDS Common Data Elements: Epilepsy, 2017. Available at: https://www.commondataelements.ninds.nih.gov/Epilepsy.aspx#tab=Data_Standards. Accessed November 21, 2017.
- 7. Gorter JA, van Vliet EA, Dedeurwaerdere S, et al. A companion to the preclinical common data elements for rodent epilepsy models. A report of the TASK3 Physiology Working Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018;3(S1):68–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mazarati A, Jones NC, Galanopoulou AS, et al. A companion to the preclinical common data elements on neurobehavioral comorbidities of epilepsy. A report of the TASK3 Behavior Working Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018;3(S1):23–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker‐Haliski M, Harte‐Hargrove LC, Ravizza T, et al. A companion to the preclinical common data elements for pharmacological studies in animal models of seizures and epilepsy. A report of the TASK3 pharmacology working group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018;3(S1):52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ono T, Wagenaar J, Giorgi FS, et al. A companion to the preclinical common data elements and case report forms for rodent EEG studies. A report of the TASK3 EEG Working Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018;3(S1):89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith DH, Hicks RR, Johnson VE, et al. Pre‐clinical traumatic brain injury common data elements: toward a common language across laboratories. J Neurotrauma 2015;32:1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis 2014;72(Pt B): 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koe AS, Jones NC, Salzberg MR. Early life stress as an influence on limbic epilepsy: an hypothesis whose time has come? Front Behav Neurosci 2009;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korosi A, Naninck EFG, Oomen CA, et al. Early‐life stress mediated modulation of adult neurogenesis and behavior. Behav Brain Res 2012;227:400–409. [DOI] [PubMed] [Google Scholar]
- 15. Brunson KL, Kramar E, Lin B, et al. Mechanisms of late‐onset cognitive decline after early‐life stress. J Neurosci 2005;25:9328–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karten YJG, Olariu A, Cameron HA. Stress in early life inhibits neurogenesis in adulthood. Trends Neurosci 2005;28:171–172. [DOI] [PubMed] [Google Scholar]
- 17. Kramar G, Jones NC, Morris J. Early life stress enhancement of limbic epileptogenesis in adult rats: mechanistic insights. PLoS ONE 2011;6:e24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Epstein HT. The effect of litter size on weight gain in mice. J Nutr 1978;108:120–123. [DOI] [PubMed] [Google Scholar]
- 19. Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ion flux, and changes in brain excitability. Epilepsy Res 1998;32:275–285. [DOI] [PubMed] [Google Scholar]
- 20. Yuen AW, Sander JW. Rationale for using intermittent calorie restriction as a dietary treatment for drug resistant epilepsy. Epilepsy Behav 2014;33:110–114. [DOI] [PubMed] [Google Scholar]
- 21. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014;509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Science Europe . Practical Guide to Improving Gender Equality in Research Organisations, 2017. Available at: https://www.scienceeurope.org/wp-content/uploads/2017/01/SE_Gender_Practical-Guide.pdf. Accessed November 21, 2017.
- 23. Meaney MJ, Stewart J. Environmental factors influencing the affiliative behavior of male and female rats (rattus norvegicus). Learn Behav 1979;7:3987–405. [Google Scholar]
- 24. Machida T, Yonezawa Y, Noumura T. Age‐associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm Behav 1981;15:238–245. [DOI] [PubMed] [Google Scholar]
- 25. Edwards HE, Burnham WM, MacLusky NJ. Testosterone and its metabolites affect afterdischarge thresholds and the development of amygdala kindled seizures. Brain Res 1999;838:151–157. [DOI] [PubMed] [Google Scholar]
- 26. Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α‐androstanediol and 17β‐estradiol. Neuroscience 2004;129:195–207. [DOI] [PubMed] [Google Scholar]
- 27. Breuer ME, McGinnis MY, Lumia AR, et al. Aggression in male rats receiving anabolic androgenic steroids: effects of social and environmental provocation. Horm Behav 2001;40:409–418. [DOI] [PubMed] [Google Scholar]
- 28. Scharfman HE, Kim M, Hintz TM, et al. Seizures and reproductive function: insights from female rats with epilepsy. Ann Neurol 2008;64:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caligioni CS. Assessing Reproductive status/stages in mice. Curr Protoc Neurosci 2009; 48:A.41.1–A.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 2007;80:84–97. [DOI] [PubMed] [Google Scholar]
- 31. Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia 2006;47:1423–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luine VN, Richards ST, Wu VY, et al. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav 1998;34:149–162. [DOI] [PubMed] [Google Scholar]
- 33. Gould E, Woolley CS, Frankfurt M, et al. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 1990;10:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MacKenzie G, Maguire J. The role of ovarian hormone‐derived neurosteroids on the regulation of GABAA receptors in affective disorders. Psychopharmacology 2014;231:3333–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorge RE, Martin LJ, Isbester KA, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 2014;11:629–6532. [DOI] [PubMed] [Google Scholar]
- 36. Maguire J, Salpekar JA. Stress, seizures and hypothalamic‐pituitary‐adrenal axis targets for the treatment of epilepsy. Epilepsy Behav 2013;26:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borges K, Gearing M, McDermott DL. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol 2003;182:21–34. [DOI] [PubMed] [Google Scholar]
- 38. Lewejohann L, Reinhard C, Schrewe A, et al. Environmental bias? Effects of housing conditions, laboratory environment and experimenter on behavioral tests. Genes Brain Behav 2006;5:64–72. [DOI] [PubMed] [Google Scholar]
- 39. Crabbe JC, Wahlsten D, Dudek BC. Genetics of Mouse behavior: interactions with laboratory environment. Science 1999;284:1670–1672. [DOI] [PubMed] [Google Scholar]
- 40. Marashi V, Barneo A, Ossendorf E, et al. Effects of different forms of environmental enrichment on behavioral, endocrinological, and immunological parameters in male mice. Horm Behav 2003;43:281–292. [DOI] [PubMed] [Google Scholar]
- 41. Van der Meer E, Van Loo PL, Baumans V. Short‐term effects of a disturbed light‐dark cycle and environmental enrichment on aggression and stress‐related parameters in male mice. Lab Anim 2004;38:376–383. [DOI] [PubMed] [Google Scholar]
- 42. Clough G. Environmental effects on animals use in biomedical research. Biol Rev Camb Philos Soc 1982;57:487–523. [DOI] [PubMed] [Google Scholar]
- 43. Lee SH, Choi BY, Kim JH, et al. Late treatment with choline alfoscerate (l‐alpha glycerylphosphorylcholine, α‐GPC) increases hippocampal neurogenesis and provides protection against seizure‐induced neuronal death and cognitive impairment. Brain Res 2017;1654:66–76. [DOI] [PubMed] [Google Scholar]
- 44. Polataeva II, Surina NM, Ashapkin VV, et al. Maternal methyl‐enriched diet in rat reduced the audiogenic seizure proneness in progeny. Pharmacol Biochem Behav 2014;127:21–26. [DOI] [PubMed] [Google Scholar]
- 45. Luine V, Attalla S, Mohan G, et al. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res 2006;1126:183–187. [DOI] [PubMed] [Google Scholar]
- 46. Lecker J, Froberg‐Fejko K. Using environmental enrichment and nutritional supplementation to improve breeding success in rodents. Lab Anim 2016;45:406–407. [DOI] [PubMed] [Google Scholar]
- 47. Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev 2003;27:385–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Core CRF and CDE files. The CDE and CRF modules linked to this article can be found and downloaded as a zip folder.


