Abstract
Recent research suggests that exercise may help facilitate abstinence from cocaine addiction, though the mechanisms are not well understood. In mice, wheel running accelerates the extinction of conditioned place preference (CPP) for cocaine, providing an animal model for evaluating potential neurological mechanisms. The objective of this study was to quantify dynamic changes in endogenous peptides in the amygdala and dentate gyrus of the hippocampus in mice exposed to a context paired with the effects of cocaine, and in response to exercise. Male C57BL/6J mice conditioned to cocaine were housed with or without running wheels for 30 days. Following a CPP test and final exposure to either a cocaine- or saline-associated context, peptides were measured in brain tissue extracts using label-free matrix-assisted laser desorption/ionization mass spectrometry (MS) and stable isotopic labeling with liquid chromatography and electrospray ionization MS. CPP in mice was significantly reduced with running, which correlated to decreased myelin basic protein derivatives in the dentate gyrus extracts, possibly reflecting increased unmyelinated granule neuron density. Exposure to a cocaine-paired context increased hemoglobin-derived peptides in runners and decreased an actin-derived peptide in sedentary animals. These results allowed us to characterize a novel set of biomarkers that are responsive to exercise in the hippocampus and in a cocaine-paired context in the amygdala.
Introduction
Relapse presents a major obstacle to successful recovery from drug addiction. One factor that can lead to relapse, even in recovered drug abusers, is re-exposure to drug-related cues (e.g., drug paraphernalia, places where drugs were taken or people drugs were taken with), which can trigger powerful feelings of craving.1−4 Finding interventions that help extinguish the cravings induced by drug-paired cues is a critical step for designing more effective rehabilitation treatments. There is evidence that incorporating aerobic exercise can improve addiction rehabilitation outcomes,5−7 but the mechanisms are not well understood.
Exercise-induced changes in the brain have been hypothesized to alter learned associations between contextual cues and drug reward. In the conditioned place preference (CPP) paradigm, a model of drug-to-context association and reward,8−10 an animal is repeatedly exposed to a drug of abuse in one context and to a neutral substance in an alternate context so that the animal learns to associate the drug with the one context. The animal is then tested for its preference for the drug-associated context relative to the neutral context. In the animal literature, exercise has been shown to reduce cocaine-induced CPP.8−10 One mechanism by which running may accelerate the extinction of drug-to-context associations may be by modifying the levels of endogenous peptides. On the basis of previous mRNA expression studies, running is thought to induce robust peptide changes in the brain. For instance, running increases the expression of dynorphin mRNA in brain reward pathways.11,12 Considering that mRNA levels of neuropeptide precursor proteins do not always correlate to the endogenously processed peptide levels, measuring actual peptide dynamics in the brain reward regions under cocaine-induced CPP and exercise conditions may provide new insights into the chemistry of associative learning.
The objective of this study was to evaluate endogenous peptide levels in the amygdala and dentate gyrus of the hippocampus in response to running and cocaine-context re-exposure via mass spectrometry (MS), which measures the endogenous peptides directly at the sites of their action. To achieve this goal, tissue extracts were compared for detectable peptide changes induced by running and by exposure to a cocaine-associated context with two MS-based neuropeptidomics approaches, matrix-assisted laser desorption/ionization (MALDI) MS, and stable isotopic labeling using succinic anhydride (SA) followed by liquid chromatography-mass spectrometry (LC–MS).13−15 The hippocampus, and the dentate gyrus in particular, undergoes unique changes in response to exercise, including but not limited to neurogenesis,16,17 which changes the structural and molecular composition and in turn might alter the biochemical profile in this region. Thus, we hypothesized that we would observe differences in endogenous peptide levels resulting from exercise in the dentate gyrus. Because the amygdala is critical for context-induced behavior and plays a key role in CPP for cocaine,18−20 we expected the amygdala to show changes in peptide levels from exposure to a cocaine-associated context. We also expected the peptides up or down regulated in the dentate gyrus in response to exercise to prevent modulation of context-induced amygdala peptides for the following reasons. First, the hippocampus is known to project to the amygdala and modify eventual amygdala output21 and second exercise is protective against cocaine-primed and stress-induced reinstatement of cocaine seeking,22 which may be achieved by cue-induced peptide modulation.
Results and Discussion
CPP Results
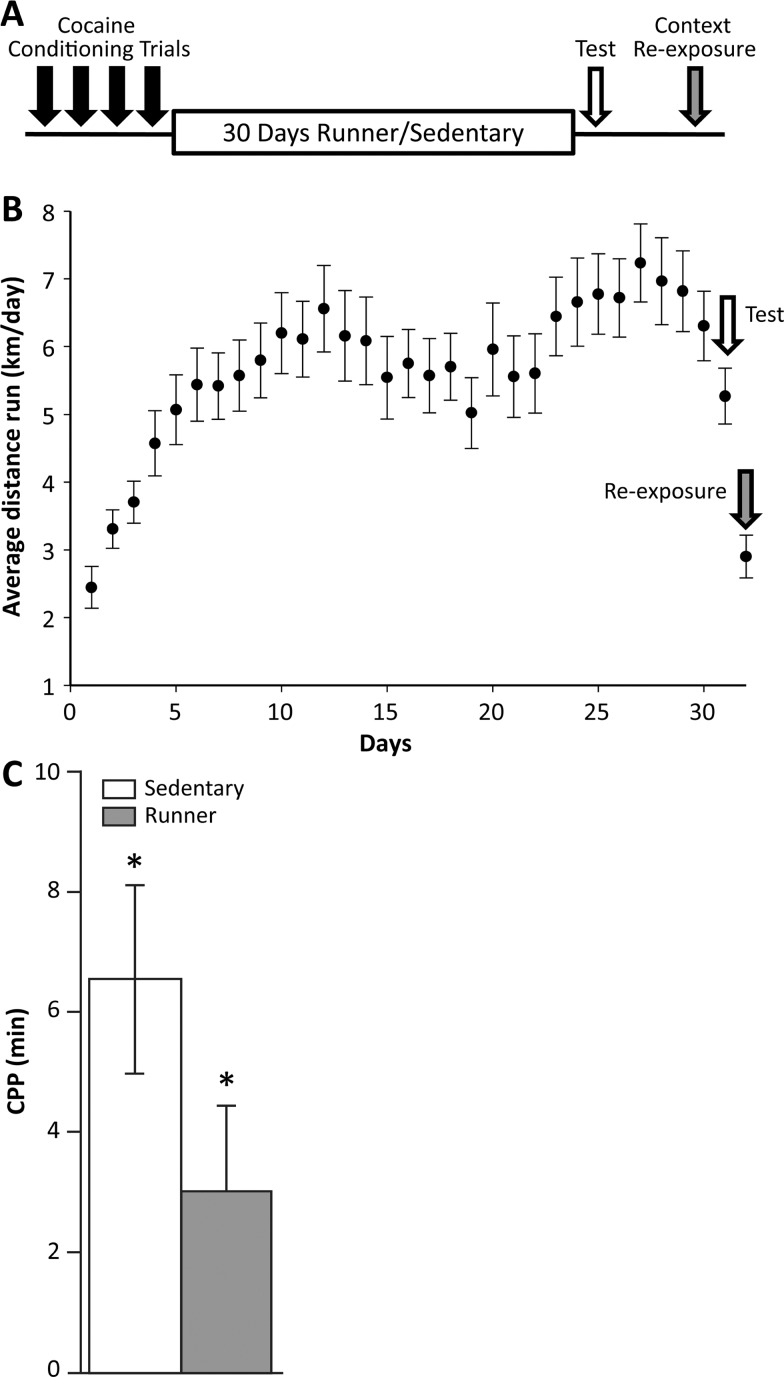
The experimental design for the CPP training, testing, and re-exposure is presented graphically in Figure 1A. The behavioral results correspond well to what was reported in previous work.9,23 For mice housed with running wheels, running increased from days 1–12 and maintained a plateau for the remaining days (e.g., day was significant, F31,1267 = 15.46, p < 0.0001) (Figure 1B). The average level of running across all mice was 5.6 ± 0.2 km/day. There was no significant effect of cohort, indicating that animals in cohort 1 and cohort 2 ran comparable distances on the running wheels.
Figure 1.
(A) Schematic diagram of the animal conditioning and testing for CPP. Mice underwent 4 days of conditioning followed by 30 days housed in cages with or without running wheels. Following that 1 day of CPP testing was performed to assess the effects of exercise on conditioning. The context re-exposure was performed with one of the two contexts (cocaine or vehicle) and sacrifice occurred immediately after context re-exposure. (B) Behavioral results in terms of wheel running. Average distance run (km/day) (±SE) for the mice housed with running wheels. The arrows indicate the testing day and the re-exposure day, both of which show reduced wheel running activity, since animals were removed from wheels for portions of these days. (C) Behavioral results for CPP testing reported as mean difference in duration (min) ±SE spent on the HOLE texture between animals receiving cocaine on a HOLE texture (conditioned stimulus (CS) + HOLE) and animals receiving cocaine on a GRID texture (CS + GRID), plotted separately for runner and sedentary mice. The white bar represents data for 45 mice (n = 22 runner CS+ HOLE; n = 23 runner CS + GRID); the gray bar represents data for 43 mice (n = 21 sedentary CS + HOLE; n = 22 sedentary CS + GRID). The asterisks indicate significant place preference at p < 0.05.
In the CPP test, a significant main effect of the cocaine-paired context (F1,82 = 25.95, p < 0.0001) was observed in both sedentary and runner mice. There was no significant effect of cohort, indicating that mice in cohort 1 and cohort 2 displayed similar magnitudes of CPP. The interaction between the cocaine-paired context and exercise was not significant F1,82 = 1.66, p = 0.20; (Figure 1C), which is consistent to previous work9 showing that the difference in CPP between sedentary and runners occurs on day 2 of CPP, not day 1.
MALDI MS Profiling
Following the CPP testing, peptides were extracted from the dentate gyrus and amygdala, and peptide profiles were investigated by MS. Initially, small portions of each extract were assayed via MALDI MS for individual animals (n = 48 total; n = 11, sedentary control group (saline-context re-exposed); n = 12, sedentary experimental group (cocaine-context re-exposed); n = 13, runner control group (saline-context re-exposed); and n = 12 runner experimental group (cocaine-context re-exposed)). Peptide signal intensities were normalized to an internal standard for statistical analysis. By optimizing the acquisition parameters, the collected spectra displayed a high degree of reproducibility (Figure S1). For dentate gyrus extracts, there were 50 ions with a signal-to-noise ratio (S/N) >3 in the mean spectrum for each of the 4 treatment groups. The 2 × 2 between-within analysis of variance (ANOVA) tests were run using the normalized intensities for each detected ion signal, where treatment (runner or sedentary) and context at re-exposure (experimental or control) were between-groups factors and the iteration (i.e., sample spot) was a within-subjects variable (five technical replicates per animal). Peptides (15) were found to differ significantly (p < 0.05) with wheel running in the dentate gyrus, whereas no peptides were found to differ significantly with exercise or with context re-exposure in the amygdala. The remaining individual peptide extracts were used to identify peptides via mass spectrometric sequencing, followed by quantification using stable isotopic labeling, as described in the following sections.
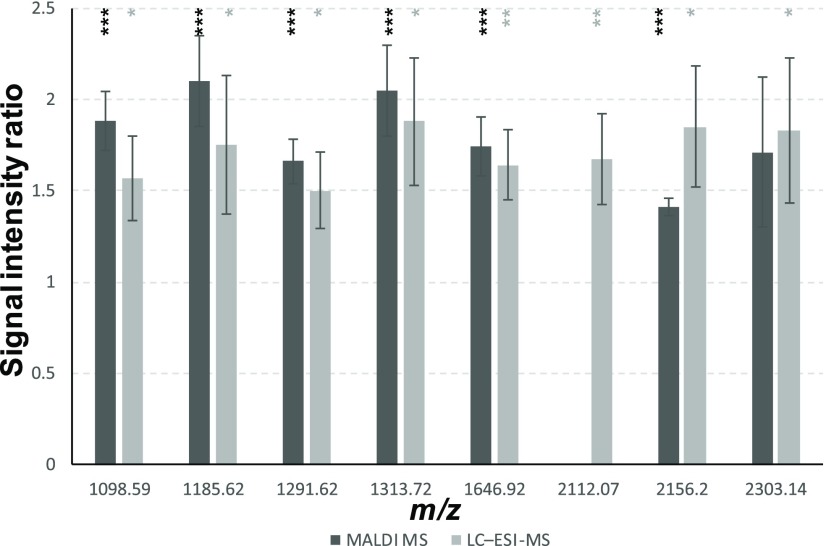
About 50% of the significantly changed peptides between runners and sedentary mice in the dentate gyrus were subsequently identified (Table S1) as shortened forms of different isoforms of myelin basic protein (MBP), a major constituent of the myelin sheath, which covers the axons of neurons and enhances transmission of action potentials along the length of the axons in white matter tracts. The measured ratios of these peptide signals indicated higher levels in the sedentary mice (Figure 2 and Table 1). This finding is consistent to neuroanatomical changes that are observed in response to exercise.24
Figure 2.
Levels of select MBP peptides in the dentate gyrus exhibit significant difference due to exercise. Level changes are presented as signal intensity ratios, sedentary/runner groups. Peptide identifications are provided in Table 1. Peptide masses reported as monoisotopic mass (after subtraction of the isotopic label). Ratios are reported as average ± SE. *p-Value <0.1, **p-value <0.05, ***p-value <0.01; p-values for MALDI MS data are based on 2 × 2 ANOVA; p-values for LC–ESI-MS data are based on Student’s t-test.
Table 1. Differences in Endogenous Peptide Signal Intensity in Response To Exercise in the Dentate Gyrus as Measured by Label-Free MALDI-TOF MS and LC–Electrospray Ionization (ESI)-MS with Stable Isotopic Labelinga.
| protein precursor | peptide sequence | Obs. mass | MALDI MS z | MALDI MS Sed/Run ratio | LC–ESI-MS z | #T | LC–ESI-MS Sed/Run ratio |
|---|---|---|---|---|---|---|---|
| unknown | 1015.59 | 1 | 1.58 ± 0.13*** | ||||
| unknown | 1049.57 | 1 | 1.35 ± 0.11** | ||||
| MBP | M.A(+42.01)SQKRPSQR.S | 1098.59 | 1 | 1.88 ± 0.16*** | 2 | 1 | 1.57 ± 0.23* |
| actin | L.RVAPEEHPVL.L | 1145.62 | 1 | 1.44 ± 0.08** | |||
| MBP | M.A(+42.01)SQKRPSQRS.K | 1185.62 | 1 | 2.10 ± 0.25*** | 2 | 1 | 1.75 ± 0.38* |
| unknown | 1226.58 | 2 | 1 | 1.09 ± 0.02** | |||
| unknown | 1237.72 | 1 | 1.42 ± 0.09** | ||||
| MBP | R.HGFLPRHRDTG.I | 1291.62 | 1 | 1.66 ± 0.12*** | 4 | 1 | 1.50 ± 0.21* |
| unknown | 1299.60 | 1 | 1.78 ± 0.15*** | ||||
| MBP | M.A(+42.01)SQKRPSQRSK.Y | 1313.72 | 1 | 2.05 ± 0.25*** | 2 | 2 | 1.88 ± 0.35* |
| unknown | 1375.68 | 1 | 1.67 ± 0.22** | ||||
| unknown | 1476.79 | 1 | 1.79 ± 0.23*** | ||||
| MBP isoform 1 | N.IVTPRTPPPSQGKGGR.D | 1646.92 | 1 | 1.74 ± 0.16*** | 2 | 2 | 1.64 ± 0.19** |
| unknown | 1660.94 | 1 | 1.68 ± 0.12*** | 2 | 2 | 1.66 ± 0.20** | |
| unknown | 1691.87 | 2 | 2 | 0.85 ± 0.04** | |||
| unknown | 1802.23 | 2 | 1 | 1.29 ± 0.06** | |||
| unknown | 2105.93 | 4 | 1 | 1.19 ± 0.04** | |||
| MBP | M.DHARHGFLPRHRDTGILD.S | 2112.07 | 4 | 1 | 1.67 ± 0.25** | ||
| unknown | 2119.94 | 4 | 1 | 1.85 ± 0.09*** | |||
| MBP | G.SLPQKSQHGRTQDENPVVH.F | 2156.20 | 1 | 1.41 ± 0.05*** | 3 | 2 | 1.85 ± 0.33* |
| unknown | 2176.97 | 4 | 1 | 1.38 ± 0.04*** | |||
| unknown | 2278.01 | 4 | 1 | 1.57 ± 0.14** | |||
| unknown | 2292.03 | 4 | 1 | 1.48 ± 0.17** | |||
| MBP | G.SLPQKSQHGRTQDENPVV | ||||||
| HF.F | 2303.14 | 1 | 1.71 ± 0.41** | 3 | 2 | 1.83 ± 0.40* | |
| unknown | 2315.04 | 5 | 1 | 1.32 ± 0.08** | |||
| unknown | 2331.04 | 5 | 1 | 1.33 ± 0.10** | |||
| unknown | 2349.05 | 4 | 1 | 1.30 ± 0.09** |
Peptide identifications are based on MS/MS of labeled samples. (.) indicates cleavage site; (_) indicates the site of isotopic labeling. Obs. mass: observed monoisotopic mass (after subtraction of the isotopic label); MALDI MS z: the charge state of the peptide signal used for relative quantitation in MALDI MS measurements; LC–ESI-MS z: the charge state of the peptide signal used for relative quantitation in LC–ESI-MS measurements; #T: the number of succinic anhydride labels covalently bonded to the peptide after labeling. Sed/Run ratio: ratio of peptide intensity in sedentary mice compared to runner mice. Ratios are reported as average ± SE. *p-Value <0.1, **p-value <0.05, ***p-value <0.01; p-values for MALDI MS data are based on 2 × 2 ANOVA; p-values for LC–ESI-MS data are based on Student’s t-test.
Relative Quantitation with Stable Isotopic Labeling and LC–MS Detection in the Dentate Gyrus
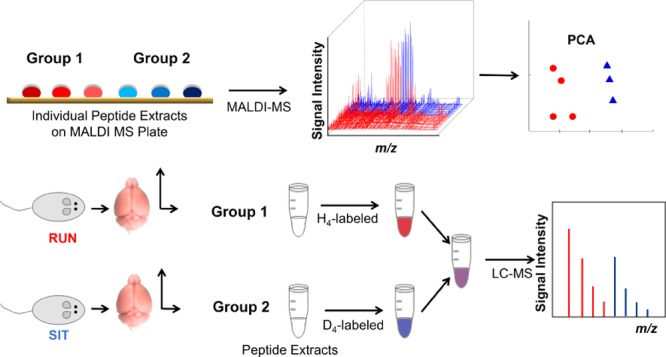
Our goal is to characterize a broad level of peptides. Thus, an additional quantitation approach was used to account for peptides in the extract potentially not detected by MALDI-TOF MS. We used LC–ESI-MS because this ionization approach creates distinct peptide lists compared to MALDI, especially true with the lack of front-end separation in our high throughput low-volume MALDI MS experiment.25−27 Because the statistical analysis of the MALDI MS peptide profiles from the dentate gyrus indicated significant peptide changes in response to exercise, stable isotopic labeling and LC–MS detection were used to measure the relative abundance of peptides in sedentary and runner mice. However, individual extracts were combined into fewer samples (three per biological replicate, three biological replicates per group, four groups, n = 36 total) to increase the amount of material for LC–MS analysis, which lowered the statistical power for stable isotopic labeling analysis compared to the MALDI MS measurements of individual samples but provided the ability to characterize lower-level peptides. Extracts from select animals from each experimental group were combined into a pooled sample for peptide identification and run separately from the quantitative measurement samples (n = 12). To isolate the effects of wheel running, context re-exposure was controlled by making the comparisons between the sedentary and runner groups for the cocaine-context re-exposed animals and the saline-context re-exposed animals, separately.
As expected, the chromatographic separation prior to MS analysis yielded a higher number of detected peptides than did the direct MALDI MS measurements. In total, 129 labeled peptide pairs were detected among all of the biological replicates (n = 3 per group), and these were used for statistical analysis. Applying the false discovery rate (FDR) correction and the criterion of FDR < 0.1, only two peptides were found to differ significantly in the dentate gyrus between the runner and sedentary groups: calculated unlabeled masses 2119.94 and 2176.97 Da. Twelve other peptides showed significant differences at an unadjusted p < 0.05; these candidate peptides are listed in Table 1. The labeled peptide pairs that were found not to be significantly different between the runner and sedentary groups are listed in Table S2.
Of the 15 peptides that were found to differ between the runner and sedentary groups in the dentate gyrus in the MALDI MS experiment, 8 were also detected by stable isotopic labeling and showed similar ratios between groups; 7 of those 8 are derivates of MBP and 1 is an unidentified peptide. Therefore, both quantitative methods reflect intrinsic chemical characteristics of the sample set. Several potential roles of MBP in cell-to-cell signaling have been proposed, including acting as a membrane actin-binding protein in which MBP might participate in the transmission of extracellular signals to the cytoskeleton in oligodendrocytes and tight junctions in myelin, and as a binder of polynucleotides in the cell nucleus, where it may affect gene expression.28 The difference in the levels of MBP between sedentary and runner animals may reflect changes in myelination due to exercise or signaling differences in runners or there may be less present structurally due to remodeling from exercise.
On the other hand, exercise has been shown to increase the volume of gray matter, which is mostly unmyelinated, in the hippocampus.29 This fits with our observation of more MBP-related peptides, which comprise the myelin in white matter, in sedentary mice. A study in mice (ages 11–13 months) performed by Latimer et al.24 found that with exercise, the levels of MBP decreased, also fitting with our results. Myelination is one form of plasticity and appears to play a role in how animals behave in learning and memory tasks.30 Rigid structuring of the white matter in the hippocampus by increased myelination in sedentary animals may lay down pathways. It may also mean that runners, with less myelin, have more cognitive flexibility in the hippocampus, which would fit with our behavioral observation that the CPP of runners starts at the same level as that of sedentary animals, but decays more rapidly.8 Furthermore, exercise-induced changes in the brain have demonstrated strong correlations with improvements in cognitive function.31,32 If extinction of CPP is considered new learning, specifically, that the intratest learning that the previously drug-paired context is no longer predictive of drug reward, these data on the procognitive effects of exercise fit with our behavioral observation of reduced CPP in runner animals. More rigid and myelinated pathways may occur in the dentate gyrus of sedentary animals, as evidenced by increased MBP-related peptides in the dentate gyrus of tested sedentary mice.
Relative Quantitation with Stable Isotopic Labeling and LC–MS Detection in the Amygdala
No significant changes were detected between treatment groups in the amygdala peptide extracts by MALDI MS, however, differential isotopic labeling and LC–MS analysis were able to measure peptide changes with context re-exposure. This discrepancy can be attributed to increased peptide detectability of the LC–MS method used with isotopic labeling. To further increase the statistical power of the LC–MS analysis, a second cohort of mice (n = 39) was added specifically for investigating the link between peptide changes and context re-exposure in the amygdala. Individual samples (n = 22, sedentary control group (saline-context re-exposed); n = 21, sedentary experimental group (cocaine-context re-exposed); n = 23, runner control group (saline-context re-exposed); and n = 21 runner experimental group (cocaine-context re-exposed, 87 mice total)) were combined in triplicates into fewer biological replicates (n = 6 per group) to increase the amount of material available for the analysis; the rest of the mice (n = 15) were used for peptide identification. Although many labeled peptide pairs (>100) were detected in each individual analysis, only 39 labeled peptide pairs were consistently detected in samples from both cohorts of the runner mice, and 36 labeled peptide pairs consistently detected in samples from both cohorts of the sedentary mice. Attempts were made to keep all conditions between cohorts consistent, however, the animals in the two cohorts were acquired and sampled at different times of the year. This discrepancy in timing could have led to some peptides being unique to one cohort of animals. Of the peptides identified by tandem MS (MS/MS) from the amygdala extracts, many were derived from structural proteins, such as MBP and actin (see Tables S1 and S3).
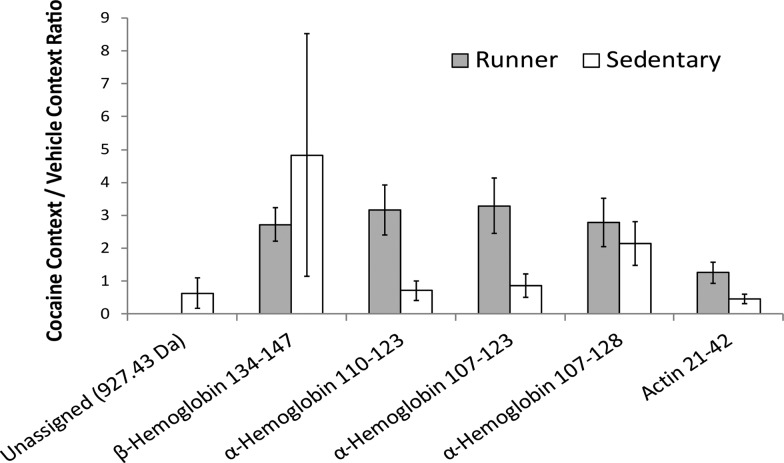
In the analysis of peptide extracts from the amygdala of runner mice, only one peptide was found to have a significant change in abundance (FDR < 0.1) between animals re-exposed to the cocaine-paired context and animals re-exposed to the control context. As determined by LC–MS/MS, the peptide is derived from β-hemoglobin and comes from the C-terminus of the protein. This may occur if a highly salient stimulus, such as a context previously paired with cocaine, activates the amygdala. Increased amygdala activation may result in increases in blood flow to the area because this would allow more hemoglobin into the amygdala, and this would affect the hemoglobin levels later detected in this region. It has been shown that cue-induced cocaine craving activates cerebral blood flow in the limbic system, particularly in the amygdala.33,34 Three other peptides, all derived from α-hemoglobin, showed differences at an unadjusted p < 0.05. All four peptides showed a higher abundance in the animals re-exposed to the cocaine-paired context compared to the control context and showed significant differences based on context re-exposure in the runner group, but not the sedentary group (Figure 3 and Table S1).
Figure 3.
Graphical representation of the fold changes in peptide levels, as measured by LC–ESI-MS with stable isotopic labeling in amygdala, from exposure to a cocaine-associated context. Level changes are presented as signal intensity ratios, cocaine context group/vehicle context group. Peptide identifications are provided in Table 1. Ratios are reported for n = 6 biological samples, with the exception of α-hemoglobin 110−123, where n = 4, and error bars represent standard error. * Unadjusted p-values <0.05, indicating fold changes are significantly different from zero based on Student’s t-test. **FDR < 0.1.
In runner mice, the peptides that showed a higher relative abundance in animals re-exposed to the cocaine-paired context compared to the control context are derived from α- and β-hemoglobin; these are nonclassical signaling peptides that have been reported to play a signaling role.35−37 The α- and β-subunits of hemoglobin are expressed in neuronal cells,38,39 and several hemoglobin-derived peptides have been found to be uniquely expressed in the brain.40 To date, there are two classes of hemoglobin-derived peptides whose roles as cell–cell signaling peptides have been extensively explored. The first class, called hemopressins, is derived from α- and β-hemoglobin and interacts with cannabinoid receptors.36,37,40 The second class, named hemorphins, consists of several β-hemoglobin-derived peptides that interact with opioid receptors.36,41 MS-based peptidomics studies have identified hemopressin and hemorphin peptides, as well as other peptides, derived from α- and β-hemoglobin.35,42−44 The hemoglobin-derived peptides identified in this study are not hemopressin or hemorphin peptides. Therefore, their peptide–receptor actions are not known. The β-hemoglobin-derived peptide found to have a higher abundance in runner mice re-exposed to a cocaine-paired context than in runner mice re-exposed to a neutral context before sacrifice comes from the C-terminal of the sequence, and has been detected in other MS-based peptidomic studies of the mouse brain.35,42 The α-hemoglobin-derived peptides identified in this study as having higher abundance in runner mice re-exposed to a cocaine-paired context than in runner mice re-exposed to a neutral context before sacrifice correlate to residues 107–123, 110–123, and 107–128. The largest of these peptides (residues 107–128) has been observed in previous peptidomic studies.35 The peptides we identified may reflect a change in amygdala signaling in response to a drug context cue in the amygdala of the runner animals or an increased blood flow in response to running and a cocaine-associated cue.
When making the same comparison of peptide extracts from the amygdala of animals re-exposed to the different contexts in the sedentary group, no peptides had a statistically significant (FDR < 0.1) change. Two peptides showed differences between cocaine- and vehicle-paired contexts at an unadjusted p < 0.05. One of these peptides is actin [21–42], and the other could not be identified (calculated unlabeled molecular weight (MW): 927.43 Da). Both peptides were decreased in the animals re-exposed to the cocaine-paired context compared to the animals re-exposed to the vehicle-paired context. The observed log2-based fold change for the actin-derived peptide between the context re-exposures (cocaine-paired context–vehicle-paired context) was −1.33 ± 0.80 (mean ± SE). There is possibility that the unidentified peptide is produced from a cytosolic protein that functions in cell–cell signaling.35
Although only one actin-derived peptide showed a significant change in the cocaine-paired context, other actin-derived peptides were detected, albeit with a high degree of variability between samples. The presence of multiple different actin-derived peptides suggests that these peptides in particular are reactive to drug cue exposure, perhaps due to cue-elicited structural remodeling of this brain area.45 Actin is involved in the remodeling of neurons, and changes in actin dynamics have been observed in tests of learning and memory.46 In addition, actin cycling has been shown to increase in response to cocaine, potentially modulating cocaine-induced reinstatement of drug seeking.47 The implication for our study is that a mere drug context re-exposure may lead to structural changes in the region of the brain that evaluates cue salience, and those changes are measurable by chemical approaches.
A graph depicting the average fold changes in signal intensity for peptides of interest in the amygdala of sedentary mice in response to context re-exposure is presented in Figure 3. A list of the identified peptides that were not found to be significantly different with context re-exposure in the amygdala is presented in Table S3. In the amygdala samples, most of the identified peptides were from ubiquitous proteins, but two peptides were derived from two prohormone precursors, somatostatin and proSAAS.
Postmortem analyses of amygdala from human drug abusers have shown cocaine-induced protein alterations relative to controls.48 A few animal studies have identified neuropeptides differentially modulated in response to a drug in distinct brain regions by MS techniques,49,50 but these subjects were pretreated with an acute dose of cocaine49 or amphetamine.50 In contrast, our context manipulation did not involve a pretreatment with acute cocaine. Rather, our results show that mere re-exposure to an environment previously associated to cocaine alters the amygdala peptide profile. We acknowledge that our results represent a correlation between context re-exposure and peptide levels and not causation. The implication may be that these amygdala peptides are associated to subjective feelings related to expecting cocaine when mice are placed in a cocaine-associated context. The peptides may not cause the subjective feelings, but they may reflect the dynamic neurochemical environment during the process of context re-exposure and the expectation of drug reward.
In summary, we identified peptide changes in the dentate gyrus in an exercise-dependent manner and in the amygdala in a context-dependent manner. Running decreased MBP derivatives in the dentate gyrus, possibly reflecting increased unmyelinated neuron density. Exposure to a cocaine-context increased several hemoglobin-derived peptides in the amygdala in runners and an actin-derived peptide in sedentary animals. This is, to the best of our knowledge, the first demonstration of peptide changes in the amygdala in response to context re-exposure alone, as opposed to drug pretreatment. Our findings identify novel molecular correlates of both drug cue exposure and intervention to extinguish the learned associations in two specific brain regions critical to the drug dependence and relapse.
Methods
Chemicals
All chemicals were obtained from Sigma-Aldrich (St Louis, MO) unless otherwise stated. The peptide standards for MALDI MS calibrations were supplied by Bruker (Billerica, MA). Heavy (D4) succinic anhydride (SA) was purchased from C/D/N Isotopes (Pointe-Claire, Quebec, Canada).
Animals
Procedures were performed with two cohorts of male C57BL/6J mice, which arrived at our facility at 5 weeks of age, from the Jackson Laboratory (Bar Harbor, ME). Because the resources available for housing, training, and testing for CPP limited the number of animals per training set, the first cohort consisted of 48 mice, the second cohort of 39.
Animals were housed four per cage in a climate-controlled environment on a reverse 12 h light/dark cycle (lights off at 1000 h) with food and water available ad libitum for 10 days. The cage dimensions were 29 × 19 × 13 cm3 (L × W × H). Mice were individually housed for 9 days before starting the experimental procedures. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines; measures were taken to minimize the number of animals used and their pain and suffering.
CPP Testing and Context Re-exposure
The experimental design for the CPP training, testing, and re-exposure is presented graphically in Figure 1A. CPP training and testing were adapted from Cunningham et al.9,51,52 and performed using chambers with interchangeable floor textures, as described in previous studies.9,51,52 Cocaine hydrochloride was dissolved in 0.9% saline and administered at a dose of 10 mg/kg via intraperitoneal (ip) injections in a volume of 10 mL/kg. The dose was chosen based on the literature and prepared according to the salt (not the base) form.53 Prior to conditioning, mice underwent habituation to familiarize them with the place conditioning chambers, and pretesting to determine individual biases in texture preference prior to drug pairing.
In the conditioning phase, conditioned stimulus (CS) trials were administered over 4 days, as follows: four CS+ trials (i.e., cocaine paired with one floor texture: HOLE or GRID) and four CS− trials (i.e., vehicle (saline), paired with the alternate floor texture). Experimental animals were weighed, received an injection of 10 mg/kg ip cocaine (CS+ trial) or vehicle (CS– trial), and were immediately placed on the appropriate floor texture. Each day, one CS+ trial and one CS– trial were administered in the morning and afternoon. The order of exposure to CS+ and CS– was counterbalanced within each group.
Following conditioning, the mice were placed individually in cages, either with or without running wheels, to create two groups: runner mice (housed with a running wheel, n = 24 in cohort 1, n = 19 in cohort 2) and sedentary mice (housed without a running wheel, n = 24 in cohort 1, n = 20 in cohort 2). The dimensions of the running wheel cages were 36 × 20 × 14 cm3 (L × W × H), with a 23 cm diameter wheel mounted in the cage top. Running wheel rotations were monitored continuously in 1 min increments via magnetic switches interfaced to a computer. Mice assigned to the sedentary group were housed in cages that did not contain locked wheels because mice will climb them,54−56 and the objective was to keep random physical activity in the sedentary group to a minimum.
After 30 days of either running or sedentary conditions, all of the mice were tested for CPP in the morning (10:00 h) for 30 min. Prior to the testing session, each mouse was weighed, injected ip with 10 mL/kg saline, and placed in the center of a dual floor texture (HOLE/GRID) conditioning chamber. The distance traveled and locations of mice within the conditioning chamber were recorded with TOPSCAN video tracking software (Clever Sys, Vienna, VA).
The day after CPP testing, animals underwent one re-exposure to context session in the morning (10:00 h; for 30 min). Mice were either re-exposed to the CPP texture they had been conditioned to associate to cocaine (n = 24 in cohort 1, n = 18 in cohort 2) or the CPP texture they had been conditioned to associate to vehicle (n = 24 in cohort 1, n = 21 in cohort 2). All animals were weighed, injected ip with 10 mL/kg saline, and placed into the center of either a HOLE or GRID conditioning chamber. All mice had, up to this point, received identical treatment, including number of total cocaine-context exposures. Mice were deliberately not placed in the dual-texture chamber at context re-exposure to control the amount of time spent on the drug-associated texture. Animals underwent context re-exposure on day 2 of CPP testing because this was the time point where the two treatment groups were most divergent in CPP in previous results.9 With the different context re-exposures, there were four behavioral groups created. For the first cohort of mice, n = 11, sedentary control group (saline-context re-exposed); n = 12, sedentary experimental group (cocaine-context re-exposed); n = 13, runner control group (saline-context re-exposed); and n = 12 runner experimental group (cocaine-context re-exposed).
A second group of mice (n = 40) was used to increase the number of replicates for investigating the link between peptide changes and the conditioned stimulus in the amygdala. The behavioral groups for the second cohort of mice were n = 11, sedentary control group (saline-context re-exposed); n = 9, sedentary experimental group (cocaine-context re-exposed); n = 9, runner control group (saline-context re-exposed); and n = 10 runner experimental group (cocaine-context re-exposed).
Tissue Sampling and Peptide Extraction
Immediately following the 30 min context re-exposure session, mice were anesthetized with 150 mg/kg sodium pentobarbital (ip) and then perfused transcardially with ice-cold saline for neuroprotection.57,58 The brain was carefully removed and immediately flash-frozen in liquid nitrogen for preservation and stored at −80 °C. For brain region sampling, frozen brains were ice-mounted on a cryostat (Leica CM3050 S, Leica Biosystems Inc) platform and cut at −40 °C in 40 μm increments until relevant morphological landmarks were observed. Frozen tissues from the bilateral basolateral amygdala and the bilateral dentate gyrus of the hippocampus were isolated via biopsy punches of 1.25 mm diameter (World Precision Instruments, Sarasota, FL) with a customized plunger. Coordinates of the punches taken were determined according to the mouse brain atlas59 and were from 0.94 to −1.94 bregma for the amygdala and −1.94 to −2.94 bregma for the hippocampus. Tissue samples from both hemispheres of the amygdala and dentate gyrus of the hippocampus were collected and immediately placed into 0.25% acetic acid for peptide extraction on ice. Acidic extractions have been shown as an effective method for peptide analysis.60
MALDI MS Peptide Profiling
Only the first cohort of animals (n = 48) was used for the MALDI MS analysis. Samples contained 5 μL of peptide extract mixed with 2 μL of a 10 μM solution of an internal standard (acidic peptide from Aplysia californica, MW 2960). Next, 0.7 μL of the spiked sample was co-crystallized with 0.7 μL of α-cyano-4-hydroxycinnamic acid matrix (13 mg/mL in 60% acetonitrile (ACN)) on a gold-coated MALDI target (Bruker). Samples were spotted as five technical replicates for each animal. Positive ion mass spectra for each sample spot were acquired in the m/z 600–5000 range on an MALDI-time-of-flight mass spectrometer (ultrafleXtreme, Bruker) in reflectron mode with high-precision calibration. Spectra were acquired in an automated fashion, sampling from the spot in a random walk manner, and utilized dynamic termination, where data collection for a spot concluded when two individual peptide signals reached a summed intensity of 1 × 104, which was determined optimal according to the S/N, signal intensity, and resolution for this sample type in pilot measurements.
Relative Quantitation with Stable Isotopic Labeling and LC–MS Detection
For the dentate gyrus, one cohort described in the behavior section was used (n = 48). Two cohorts of animals were used for relative quantitation with stable isotopic labeling and LC–MS of amygdala samples (n = 48 first cohort plus n = 39 second cohort, n = 87 total) because individual samples needed to be pooled to produce sufficient material for analysis. Pooled samples were created by combining the unused portions (from MALDI MS) of the individual extracts from three animals that had undergone identical treatment (e.g., runner mice re-exposed to the cocaine-paired context). For measurements from the dentate gyrus, pooled samples (n = 3) were created for each of the behavioral groups: sedentary mice re-exposed to the cocaine-paired context, sedentary mice re-exposed to the vehicle-paired context, runner mice re-exposed to the cocaine-paired context, and runner mice re-exposed to the control context. For the amygdala measurements, six pooled samples per treatment group (sedentary or runner) and context re-exposure (cocaine-paired context or vehicle-paired context) were created. Peptides in the pooled samples were covalently modified with H4- or D4-SA for relative quantitation, following the procedure described in Hou et al.61
For the dentate gyrus analysis, the samples were labeled and combined as follows: extracts from sedentary animals re-exposed to the cocaine-paired context (light) with extracts from runner animals re-exposed to the cocaine-paired context (heavy), and extracts from runner animals re-exposed to the control context (light) with extracts from sedentary animals re-exposed to the control context (heavy). For measurements for the amygdala extracts, the comparisons of context re-exposure were performed with both the sedentary and runner groups. The samples were labeled and combined as follows: sedentary mice re-exposed to the cocaine-paired context (H4-SA) with sedentary mice re-exposed to the vehicle-paired context (D4-SA), and runner mice re-exposed to the cocaine-paired context (H4-SA) with runner mice re-exposed to the control context (D4-SA). The samples set aside for peptide identification were also labeled with H4-SA.
Each of the labeled samples was loaded as a 1 μL sample onto a trap column (200 μm i.d. × 20 mm length, Acclaim Pepmap C18 100, 5 μm, 100 Å; Thermo Scientific, Rockwood, TN) at 20 μL/min with an Ultimate 3000 nanoHPLC system (Dionex, Sunnyvale, CA). After switching the trap in-line with a reversed phase C18 column (100 μm i.d. × 15 cm length, Magic C18, 3 μm, 200 Å; Michrom Bioresources, Auburn, CA), analytical separation was performed at 300 nL/min. The solvents used were H2O with 0.1% formic acid (FA) for solvent A and 20/80 H2O/ACN with 0.1% FA for solvent B. The multistep gradient used was 0–10 min, 4–25% B; 10–20 min, 25–30% B; 20–50 min, 30–60% B; 50–55 min, 60–75% B; 55–59 min, 75–90% B; 59–69 min, 90% B; 69 min 90–20% B; 69–73 min 20–4% B; 73–115 min 4% B. Eluting peptides were analyzed in MS mode on a quadrupole time-of-flight mass spectrometer (Impact HD, Bruker) with the CaptiveSpray source (Bruker). MS spectra were collected for an m/z range of 290–3000. Each sample was analyzed twice in MS mode for two technical replicates per sample.
Peptide Identification with LC–MS
Subsets of peptide extracts from each cohort (n = 12 and 3) were combined (without regard to treatment group) into samples for peptide sequencing by MS/MS. For peptide identification, the platform and chromatographic parameters utilized were the same as the peptide quantitation measurements. The MS/MS precursor ion selection was set for the three ions of highest intensity per MS scan, and active exclusion of previously fragmented ions after 1 min was enabled. MS and MS/MS spectra were collected for an m/z range of 290–3000. After data collection, the MS/MS spectra were exported as an .mzXML file and searched against an in-house mouse neuropeptide database and the whole mouse proteome downloaded from UniProtKB62 using PEAKS Studio 7 (Bioinformatics Solutions, Inc. Waterloo, ON, Canada). The search parameters used in the software were as follows: no enzyme, variable post-translational modifications (N-terminal pyro-Glu, acetylation, C-terminal amidation, methylation, oxidation of methionine, and the addition of light or heavy SA), mass tolerance of 0.1 Da for the precursor ion, and 0.1 Da for fragment ions. Peptide assignments were judged on the −10 log P score (>20) and mass error <100 ppm.
Statistical Analysis
The average level of running across all mice housed with running wheels (n = 46) was analyzed as a repeated measures experiment, accounting for the effects of cohort, day, and the interaction between cohort and day. For the CPP test, the time spent on the HOLE texture was modeled with cohort, exercise (runner or sedentary), context (cocaine-paired context or vehicle-paired context), the interaction between cohort and exercise, the interaction between cohort, and context and the interaction between exercise and context. Both analyses were conducted with SAS (SAS Institute Inc., Cary, NC).
With the MALDI MS peptide profiles, the raw spectra were imported into ClinProTools 2.2 software (Bruker), normalized to the total ion count, smoothed and deisotoped using the Savitzky–Golay algorithm at a width of 1.0 m/z over four cycles. The Quick Classifier algorithm in ClinProTools was then used to select putative candidate ion signals likely to differ significantly between treatment groups. Ion signals considered for statistical analysis were restricted to a S/N of 3:1. Intensity values of putative candidate peptide signals were normalized relative to the intensity of the internal standard observed in the individual sample. If the internal standard was not detected in a sample, it was omitted from the analysis. The normalized intensities for each putative candidate peptide were modeled using ClinProTools for the effects of exercise (runner or sedentary), context at re-exposure (cocaine-paired context or vehicle-paired context), and the iteration (i.e., sample spot) was a within-subject variable (five per mouse).
With the isotopically labeled samples analyzed by LC–MS, labeled peptide pairs were selected based on their charge, retention time, and mass match (Figure 4A). After detecting a labeled pair at a particular time point in the total ion chromatogram, extracted ion chromatograms (EIC) were created for the monoisotopic ion of the light and heavy species (Figure 4B). The signals in the EIC were integrated to create a combined mass spectrum that was used for quantification. For each peptide, quantitative information was obtained by summing the intensity of the first four isotopes in the spectrum for the light and heavy forms. The summed intensities were then log2 transformed, and the difference between light and heavy forms was used to calculate fold changes. Fold changes were averaged between technical replicates and were compared across the biological samples accounting for context at re-exposure (cocaine-paired context or vehicle-paired context) using SAS. Student’s t-tests were used to determine which ion signals showed fold changes significantly different from zero. Adjustment for multiple hypothesis testing was controlled by applying the Benjamini–Hochberg FDR correction.63 FDRs were computed for the set of peptides that were observed in all analyses.
Figure 4.
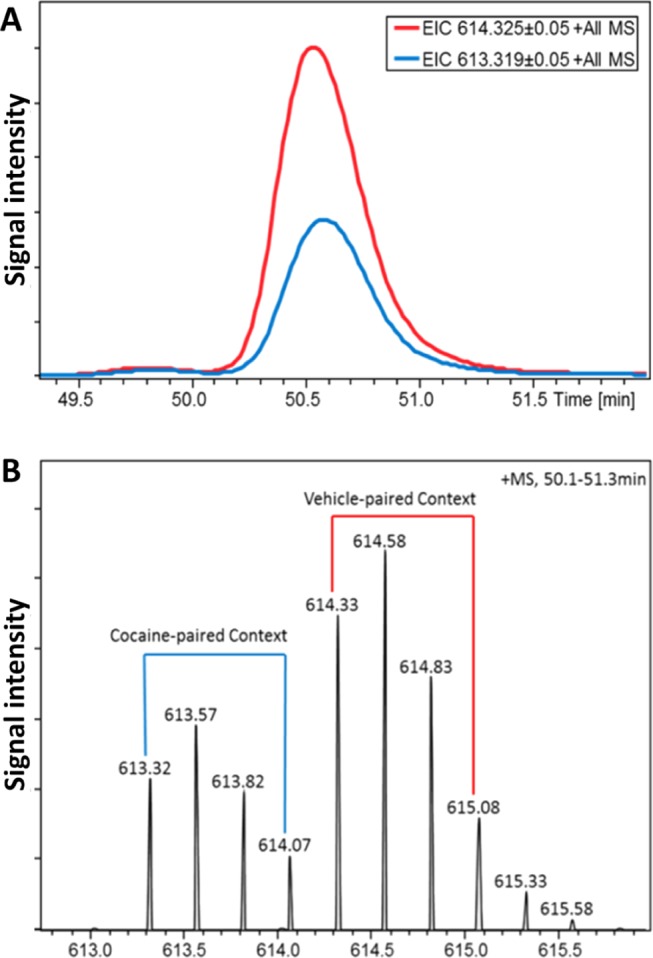
Representative chromatogram and mass spectrum of a succinic anhydride (SA)-labeled peptide from the basolateral amygdala of sedentary mice. (A) Extracted ion chromatograms (EIC) of the light (m/z 613.32, blue trace) and heavy (m/z 614.33, red trace) species in the labeled peptide pair (MW 2349.13 plus one label, charge state +4). (B) Representative, isotopically resolved mass spectrum of the labeled peptide pair. Peptide extracts from animals re-exposed to the cocaine-paired context were labeled with H4-SA (light label, blue bracket), and peptide extracts from animals re-exposed to the vehicle context were labeled with D4-SA (heavy label, red bracket).
Acknowledgments
We wish to thank the Beckman Institute Animal Facility for expert animal care.
Glossary
Abbreviations
- CPP
conditioned place preference
- EIC
extracted ion chromatogram
- ESI
electrospray ionization
- FDR
false discovery rate
- MALDI
matrix-assisted laser desorption/ionization
- MBP
myelin basic protein
- MS
mass spectrometry
- LC
liquid chromatography
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01713.
Author Present Address
∇ Waters Corporation, Beverly, Massachusetts 01915, United States (S.E.D.).
Author Contributions
# S.E.D. and M.L.M. contributed equally to this work.
Author Contributions
J.S.R. and J.V.S. conceived the project; J.S.R., E.V.R., and J.V.S. designed the study; M.L.M., S.E.D., and H.P. carried out the experiments and analyzed the data; S.E.D. carried out the MS measurements and analysis; S.E.D. and M.L.M. carried out the MS data analysis; E.V.R. provided training and advice on sampling, MS, and data analysis; B.R.S. advised on bioinformatics and statistical analysis; M.L.M. conducted CPP training, behavioral testing, and data analysis; H.P. conducted behavioral testing, and provided assistance in sample collection; S.E.D. and M.L.M. wrote the manuscript with input from E.V.R., B.R.S., J.S.R., and J.V.S.
This work was supported by the National Institute on Drug Abuse under Award Nos. R01 DA027847 to J.S.R., P30 DA018310 to J.V.S., and a Predoctoral NRSA for M.D./Ph.D. Fellowship, F30 DA 034480-01A1, awarded to M.L.M.
The authors declare no competing financial interest.
Supplementary Material
References
- Back S. E.; Gros D. F.; McCauley J. L.; Flanagan J. C.; Cox E.; Barth K. S.; Brady K. T. Laboratory-induced cue reactivity among individuals with prescription opioid dependence. Addict. Behav. 2014, 39, 1217–1223. 10.1016/j.addbeh.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond D. C.; Tiffany S. T.; Glautier S.; Remington B.. Addictive Behavior: Cue Exposure Theory and Practice; Wiley: Chichester, U.K., 1995. [Google Scholar]
- Grant S.; London E. D.; Newlin D. B.; Villemagne V. L.; Liu X.; Contoreggi C.; Phillips R. L.; Kimes A. S.; Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 12040–12045. 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. J.; Zbukvic I.; Kim J. H.; Lawrence A. J. Role of cues and contexts on drug-seeking behaviour. Br. J. Pharmacol. 2014, 171, 4636–4672. 10.1111/bph.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. A.; Abrantes A. M.; Read J. P.; Marcus B. H.; Jakicic J.; Strong D. R.; Oakley J. R.; Ramsey S. E.; Kahler C. W.; Stuart G. G.; Dubreuil M. E.; Gordon A. A. A pilot study of aerobic exercise as an adjunctive treatment for drug dependence. Ment. Health Phys. Act. 2010, 3, 27–34. 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W. J.; Peterson A. B.; Sanchez V.; Abel J.; Smith M. A. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci. Biobehav. Rev. 2013, 37, 1622–1644. 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A.; Pitts E. G. Wheel running decreases the positive reinforcing effects of heroin. Pharmacol. Rep. 2012, 64, 960–964. 10.1016/S1734-1140(12)70891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph M. L.; Merritt J. R.; Holloway A. L.; Pinardo H.; Miller D. S.; Kilby C. N.; Bucko P.; Wyer A.; Rhodes J. S. Increased adult hippocampal neurogenesis is not necessary for wheel running to abolish conditioned place preference for cocaine in mice. Eur. J. Neurosci. 2015, 41, 216–226. 10.1111/ejn.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph M. L.; Stobaugh D. J.; Miller D. S.; DeYoung E. K.; Rhodes J. S. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male c57bl/6j mice, depending on timing of wheel access. Eur. J. Neurosci. 2011, 34, 1161–1169. 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M.; Chauvet C.; Thiriet N.; El Rawas R.; Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 17145–17150. 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brené S.; Bjornebekk A.; Aberg E.; Mathe A. A.; Olson L.; Werme M. Running is rewarding and antidepressive. Physiol. Behav. 2007, 92, 136–140. 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M.; Thoren P.; Olson L.; Brene S. Running and cocaine both upregulate dynorphin mrna in medial caudate putamen. Eur. J. Neurosci. 2000, 12, 2967–2974. 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Romanova E. V.; Dowd S. E.; Sweedler J. V. Quantitation of endogenous peptides using mass spectrometry based methods. Curr. Opin. Chem. Biol. 2013, 17, 801–808. 10.1016/j.cbpa.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D.; Lim J.; Pan H.; Che F. Y. Peptidomics: identification and quantification of endogenous peptides in neuroendocrine tissues. Mass Spectrom. Rev. 2006, 25, 327–344. 10.1002/mas.20079. [DOI] [PubMed] [Google Scholar]
- Hook V.; Bandeira N. Neuropeptidomics mass spectrometry reveals signaling networks generated by distinct protease pathways in human systems. J. Am. Soc. Mass Spectrom. 2015, 26, 1970–1980. 10.1007/s13361-015-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsova O.; Tan Y. F.; Merkley C. M.; Winocur G.; Wojtowicz J. M. Early-age running enhances activity of adult-born dentate granule neurons following learning in rats. eNeuro 2017, 4, 1–9. 10.1523/ENEURO.0237-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguemeni C.; McDonald M. W.; Jeffers M. S.; Livingston-Thomas J.; Lagace D.; Corbett D. Short- and long-term exposure to low and high dose running produce differential effects on hippocampal neurogenesis. Neuroscience 2018, 369, 202–211. 10.1016/j.neuroscience.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Ladrón de Guevara-Miranda D.; Pavon F. J.; Serrano A.; Rivera P.; Estivill-Torrus G.; Suarez J.; Rodriguez de Fonseca F.; Santin L. J.; Castilla-Ortega E. Cocaine-conditioned place preference is predicted by previous anxiety-like behavior and is related to an increased number of neurons in the basolateral amygdala. Behav. Brain Res. 2016, 298, 35–43. 10.1016/j.bbr.2015.10.048. [DOI] [PubMed] [Google Scholar]
- Fuchs R. A.; Weber S. M.; Rice H. J.; Neisewander J. L. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002, 929, 15–25. 10.1016/S0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Bernardi R. E.; Ryabinin A. E.; Berger S. P.; Lattal K. M. Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learn. Mem. 2009, 16, 777–789. 10.1101/lm.1648509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner C.; Bosch D.; Gall A.; Luthi A.; Ehrlich I. Ex vivo dissection of optogenetically activated mpfc and hippocampal inputs to neurons in the basolateral amygdala: Implications for fear and emotional memory. Front. Behav. Neurosci. 2014, 8, 64 10.3389/fnbeh.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonmwan Y. E.; Schroeder J. P.; Holmes P. V.; Weinshenker D. The effects of post-extinction exercise on cocaine-primed and stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology 2015, 232, 1395–1403. 10.1007/s00213-014-3778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph M. L.; Pinardo H.; Merritt J. R.; Rhodes J. S. Parameters for abolishing conditioned place preference for cocaine from running and environmental enrichment in male C57BL/6J mice. Behav. Brain Res. 2016, 312, 366–373. 10.1016/j.bbr.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer C. S.; Searcy J. L.; Bridges M. T.; Brewer L. D.; Popovic J.; Blalock E. M.; Landfield P. W.; Thibault O.; Porter N. M. Reversal of glial and neurovascular markers of unhealthy brain aging by exercise in middle-aged female mice. PLoS One 2011, 6, e26812 10.1371/journal.pone.0026812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzyk M. A.; Ohlund L. B.; Elliott M. H.; Smith D.; Qian H.; Delaney A.; Hunter C. L.; Borchers C. H. A comparison of MS/MS-based, stable-isotope-labeled, quantitation performance on ESI-quadrupole TOF and MALDI-TOF/TOF mass spectrometers. Proteomics 2009, 9, 3328–3340. 10.1002/pmic.200800412. [DOI] [PubMed] [Google Scholar]
- Krutchinsky A. N.; Zhang W.; Chait B. T. Rapidly switchable matrix-assisted laser desorption/ionization and electrospray quadrupole-time-of-flight mass spectrometry for protein identification. J. Am. Soc. Mass Spectrom. 2000, 11, 493–504. 10.1016/S1044-0305(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Bodnar W. M.; Blackburn R. K.; Krise J. M.; Moseley M. A. Exploiting the complementary nature of LC/MALDI/MS/MS and LC/ESI/MS/MS for increased proteome coverage. J. Am. Soc. Mass Spectrom. 2003, 14, 971–979. 10.1016/S1044-0305(03)00209-5. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. 2006, 63, 1945–1961. 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W. D. S.; Olson E. A.; Weber M. Physical exercise habits correlate with gray matter volume of the hippocampus in healthy adult humans. Sci. Rep. 2013, 3, 3457 10.1038/srep03457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke M.; Gasperini R.; Young K. M. Adult myelination: Wrapping up neuronal plasticity. Neural Regener. Res. 2014, 9, 1261–1264. 10.4103/1673-5374.137571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin E. W.; Bechara R. G.; Birch A. M.; Kelly A. M. Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus 2009, 19, 973–980. 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Creer D. J.; Romberg C.; Saksida L. M.; van Praag H.; Bussey T. J. Running enhances spatial pattern separation in mice. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 2367–2372. 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts C. D.; Schweitzer J. B.; Quinn C. K.; Gross R. E.; Faber T. L.; Muhammad F.; Ely T. D.; Hoffman J. M.; Drexler K. P. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry 2001, 58, 334–341. 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Childress A. R.; Mozley P. D.; McElgin W.; Fitzgerald J.; Reivich M.; O’Brien C. P. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry 1999, 156, 11–18. 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D. Analysis of mouse brain peptides using mass spectrometry-based peptidomics: Implications for novel functions ranging from non-classical neuropeptides to microproteins. Mol. BioSyst. 2010, 6, 1355–1365. 10.1039/c003317k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I.; Dale C.; Casten K.; Geigner M.; Gozzo F.; Ferro E.; Heimann A.; Devi L. Hemoglobin-derived peptides as novel type of bioactive signaling molecules. AAPS J. 2010, 12, 658–669. 10.1208/s12248-010-9217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I.; Grushko J. S.; Golebiewska U.; Hoogendoorn S.; Gupta A.; Heimann A. S.; Ferro E. S.; Scarlata S.; Fricker L. D.; Devi L. A. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009, 23, 3020–3029. 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioli M.; Pinto M.; Cesselli D.; Zaninello M.; Lazarevic D.; Roncaglia P.; Simone R.; Vlachouli C.; Plessy C.; Bertin N.; Beltrami A.; Kobayashi K.; Gallo V.; Santoro C.; Ferrer I.; Rivella S.; Beltrami C. A.; Carninci P.; Raviola E.; Gustincich S. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 15454–15459. 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter F.; Meurers B. H.; Zhu C.; Medvedeva V. P.; Chesselet M.-F. Neurons express hemoglobin α- and β-chains in rat and human brains. J. Comp. Neurol. 2009, 515, 538–547. 10.1002/cne.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman J. S.; Sironi J.; Castro L. M.; Ferro E. S.; Fricker L. D. Hemopressins and other hemoglobin-derived peptides in mouse brain: Comparison between brain, blood, and heart peptidome and regulation in cpe(fat/fat) mice. J. Neurochem. 2010, 113, 871–880. 10.1111/j.1471-4159.2010.06653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg F.; Sanderson K.; Glämsta E.-L. The hemorphins: A new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers 1997, 43, 147–156. . [DOI] [PubMed] [Google Scholar]
- Gelman J. S.; Fricker L. Hemopressin and other bioactive peptides from cytosolic proteins: Are these non-classical neuropeptides?. AAPS J. 2010, 12, 279–289. 10.1208/s12248-010-9186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman J. S.; Dasgupta S.; Berezniuk I.; Fricker L. D. Analysis of peptides secreted from cultured mouse brain tissue. Biochim. Biophys. Acta 2013, 1834, 2408–2417. 10.1016/j.bbapap.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E.; Zamdborg L.; Southey B. R.; Atkins N.; Mitchell J. W.; Li M.; Gillette M. U.; Kelleher N. L.; Sweedler J. V. Quantitative peptidomics for discovery of circadian-related peptides from the rat suprachiasmatic nucleus. J. Proteome Res. 2013, 12, 585–593. 10.1021/pr300605p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffino L.; Giannotti G.; Racagni G.; Fumagalli F. A single cocaine exposure disrupts actin dynamics in the cortico-accumbal pathway of adolescent rats: modulation by a second cocaine injection. Psychopharmacology 2017, 234, 1217–1222. 10.1007/s00213-017-4559-z. [DOI] [PubMed] [Google Scholar]
- Hyman S. E.; Malenka R. C.; Nestler E. J. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu. Rev. Neurosci. 2006, 29, 565–598. 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Toda S.; Shen H. W.; Peters J.; Cagle S.; Kalivas P. W. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J. Neurosci. 2006, 26, 1579–1587. 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okvist A.; Fagergren P.; Whittard J.; Garcia-Osta A.; Drakenberg K.; Horvath M. C.; Schmidt C. J.; Keller E.; Bannon M. J.; Hurd Y. L. Dysregulated postsynaptic density and endocytic zone in the amygdala of human heroin and cocaine abusers. Biol. Psychiatry 2011, 69, 245–252. 10.1016/j.biopsych.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova E. V.; Lee J. E.; Kelleher N. L.; Sweedler J. V.; Gulley J. M. Mass spectrometry screening reveals peptides modulated differentially in the medial prefrontal cortex of rats with disparate initial sensitivity to cocaine. AAPS J. 2010, 12, 443–454. 10.1208/s12248-010-9204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova E. V.; Lee J. E.; Kelleher N. L.; Sweedler J. V.; Gulley J. M. Comparative peptidomics analysis of neural adaptations in rats repeatedly exposed to amphetamine. J. Neurochem. 2012, 123, 276–287. 10.1111/j.1471-4159.2012.07912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. L.; Gremel C. M.; Groblewski P. A. Drug-induced conditioned place preference and aversion in mice. Nat. Protoc. 2006, 1, 1662–1670. 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Johnson Z. V.; Revis A. A.; Burdick M. A.; Rhodes J. S. A similar pattern of neuronal fos activation in 10 brain regions following exposure to reward- or aversion-associated contextual cues in mice. Physiol. Behav. 2010, 99, 412–418. 10.1016/j.physbeh.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck J. A.; Chen G. T.; Johnson Z. V.; Rosenberg D. M.; Craig A. B.; Rhodes J. S. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol. Behav. 2008, 93, 637–650. 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Koteja P.; Garland T. Jr.; Sax J. K.; Swallow J. G.; Carter P. A. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim. Behav. 1999, 58, 1307–1318. 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Rhodes J. S.; Garland T. Jr.; Gammie S. C. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav. Neurosci. 2003, 117, 1243–1256. 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes J. S.; Koteja P.; Swallow J. G.; Carter P. A.; Garland T. Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: Repeatability and effect of genetic selection. J. Therm. Biol. 2000, 25, 391–400. 10.1016/S0306-4565(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Büki A.; Koizumi H.; Povlishock J. T. Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp. Neurol. 1999, 159, 319–328. 10.1006/exnr.1999.7139. [DOI] [PubMed] [Google Scholar]
- Haranishi Y.; Kawata R.; Fukuda S.; Kiyoshima T.; Morimoto Y.; Matsumoto M.; Sakabe T. Moderate hypothermia, but not calpain inhibitor 2, attenuates the proteolysis of microtubule-associated protein 2 in the hippocampus following traumatic brain injury in rats. Eur. J. Anaesthesiol. 2005, 22, 140–147. 10.1017/S0265021505000268. [DOI] [PubMed] [Google Scholar]
- Franklin K. B. J.; Paxinos G.. The Mouse Brain Atlas in Stereotaxic Coordinates, 3rd ed.; Academic Press: New York, NY, 2008. [Google Scholar]
- Van Dijck A.; Hayakawa E.; Landuyt B.; Baggerman G.; Van Dam D.; Luyten W.; Schoofs L.; De Deyn P. P. Comparison of extraction methods for peptidomics analysis of mouse brain tissue. J. Neurosci. Methods 2011, 197, 231–237. 10.1016/j.jneumeth.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Hou X.; Xie F.; Sweedler J. V. Relative quantitation of neuropeptides over a thousand-fold concentration range. J. Am. Soc. Mass Spectrom. 2012, 23, 2083–2093. 10.1007/s13361-012-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniprot: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y.; Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995, 57, 289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.