Abstract
Peripheral nerves have the capacity to regenerate due to the presence of neuroprotective glia of the peripheral nervous system, Schwann cells. Upon peripheral nerve injury, Schwann cells create a permissive microenvironment for neuronal regrowth by taking up cytotoxic glutamate and secreting neurotrophic signaling molecules. Impaired peripheral nerve repair is often caused by a defective Schwann cell response after injury, and there is a critical need to develop new strategies to enhance nerve regeneration, especially in organisms with restricted regenerative potential, such as humans. One approach is to explore mechanisms in lower organisms, in which nerve repair is much more efficient. A recent study demonstrated that the antiparasitic drug, ivermectin, caused hyperinnervation of primordial eye tissue in Xenopus laevis tadpoles. Our study aimed to examine the role of ivermectin in mammalian nerve repair. We performed in vitro assays utilizing human induced neural stem cells (hiNSCs) in co-culture with human dermal fibroblasts (hDFs) and found that ivermectin-treated hDFs promote hiNSC proliferation and migration. We also characterized the effects of ivermectin on hDFs and found that ivermectin causes hDFs to uptake extracellular glutamate, secrete glial cell-derived neurotrophic factor, develop an elongated bipolar morphology, and express glial fibrillary acidic protein. Finally, in a corresponding in vivo model, we found that localized ivermectin treatment in a dermal wound site induced the upregulation of both glial and neuronal markers upon healing. Taken together, we demonstrate that ivermectin promotes peripheral nerve regeneration by inducing fibroblasts to adopt a glia-like phenotype.
1. Introduction
Unlike the central nervous system, the peripheral nervous system (PNS) has a substantial capacity for repair upon injury. Although peripheral nerve repair is relatively efficient, there are instances in which this process is impaired or even prevented. Approximately 20 million Americans sustain peripheral nerve damage resulting from medical disorders and/or trauma.1 For example, neuropathy, a condition that occurs upon peripheral nerve damage or disruption, often produces pain and/or loss of sensation and movement, and can result from diabetes, certain autoimmune diseases, human immunodeficiency virus infection, and chemotherapy treatment. Furthermore, 3–10% of all traumatic injuries result in acute peripheral nerve damage requiring surgical intervention.2 Fewer than half of traumatic injury patients who undergo surgical nerve repair regain good to excellent motor function and sensation. This type of irreparable peripheral nerve damage can negatively affect a patient’s quality of life and may cause severe and permanent sensory and motor function defects that can result in complete paralysis or development of chronic neuropathic pain.3 Axonal loss and defective axonal regrowth are responsible for these critical deficits in peripheral nerve repair, while it has been suggested that the microenvironment surrounding axons plays an essential role in this process.
Innate peripheral nerve repair capacity can be attributed at least in part to the highly regenerative glial cells of the PNS, the Schwann cells. Several animal studies have indicated that poor axon regeneration after peripheral nerve injury is due at least in part to a defective Schwann cell response.4 Schwann cells myelinate peripheral nerves and play an essential role in axon guidance and regeneration. Upon peripheral nerve injury, distal axons degenerate, whereas resident Schwann cells dedifferentiate, become proliferative, and help to create a permissive microenvironment for subsequent neuronal regrowth.5 For example, Schwann cells have been implicated in the uptake of cytotoxic glutamate upon neuronal injury.6 Furthermore, Schwann cells also secrete extracellular signaling molecules to enhance nerve regeneration. One of the extrinsic factors upregulated by Schwann cells in response to peripheral nerve injury is glial cell-derived neurotrophic factor (GDNF).7 GDNF has been shown to promote neuronal growth and survival and has been widely used in the development of various therapeutic strategies for experimental nerve repair, such as diabetic neuropathy8 and sciatic nerve transection.9 Differentiated Schwann cells typically exhibit an elongated, bipolar morphology, and begin to upregulate certain cytoskeletal proteins, including glial fibrillary acidic protein (GFAP). GFAP appears during the formation of immature Schwann cells and is downregulated upon myelination.10 Interestingly, GFAP plays a prominent role in peripheral nerve regeneration specifically, as it was shown that neuronal regrowth after injury was delayed in GFAP-null mice, which is likely due to deficits in Schwann cell regulation.11
Strategies to treat peripheral nerve damage have been developed, but have a number of limitations. For patients requiring repair of larger nerve defects and/or traumatic injuries, the primary treatment is an autologous nerve graft, which has multiple drawbacks, such as limited availability of sacrificial nerve tissue, donor site morbidity, and potential neuroma formation.3 Current treatment for diabetic neuropathy, which is caused by distal nerve death in the extremities as a result of poor vascularization and high blood glucose levels, is pain management, which does not treat the actual nerve damage. There have also been several experimental models that attempt to improve outcomes of diabetic neuropathy. For example, a recent study utilized herpes simplex virus vector-mediated gene transfer of vascular endothelial growth factor for subcutaneous inoculation in the skin in a mouse model of diabetic neuropathy.12 Although this localized treatment was able to significantly increase the presence of nerve fibers in the skin, this method of virally induced gene transfer is not suitable for certain clinical applications.
Given the various shortcomings of current treatment options, there is a critical need to identify novel targets and to develop new strategies to combat the issue of impaired nerve regeneration. One approach is to examine mechanisms in lower organisms, in which peripheral nerve repair is much more robust and efficient. Certain species of salamander, such as the axolotl, can regenerate entire amputated limbs, including completely functional neural components with tactile sensation and motion.13 Many studies have focused on understanding what makes these amphibians retain such a high regenerative capacity into adulthood, whereas so many other vertebrate species do not. Interestingly, denervating the axolotl limb upon amputation inhibits limb regeneration, suggesting that proper innervation is crucial not only for restoring nerve function, but also for overall limb tissue repair.14
Although this organism has the innate capacity for complete regeneration, similar species are not as regenerative, but show a much higher capacity for repair than mammalian systems. For example, the South African clawed frog Xenopus laevis, can partly regenerate the tail, spinal cord, and limbs, but not to the same extent as salamanders, as its regenerative capacity steadily decreases as the animal matures.15 Understanding mechanisms by which to enhance the regenerative capacity as these animals transition from regenerative to nonregenerative can be helpful in devising strategies for enhancing mammalian repair, especially given the well-established reduction in regenerative capacity during aging in humans.16 For example, a recent study in a Xenopus host engraftment model demonstrated that ivermectin could enhance innervation of primordial eye tissue engrafted onto the flanks of early stage tadpoles.17 Ivermectin is an established antiparasitic drug that is used widely in both clinical and veterinary medicines. In humans, ivermectin is used in the treatment of onchocerciasis, but is also effective against other worm infestations, such as strongyloidiasis, as well as some parasitic skin diseases, including scabies.18 At higher doses, ivermectin has been shown to act as a positive regulator of a variety of ion channels and receptors, such as glycine19 and purinergic receptors,20 many of which are also present in mammalian cells. Locally stimulating these ion channels at the site of nerve damage has the potential to initiate nerve growth and repair. We hypothesized that this newly identified method of increasing innervation in nonregenerative stage frogs may also be useful for inducing innervation in organisms with restricted regenerative potential, like humans.
The goal of this work was to assay the effects of ivermectin on mammalian peripheral nerve repair. To this end, we first developed a series of in vitro assays utilizing human induced neural stem cells (hiNSCs).21 Using a three-dimensional (3D) bilayer collagen gel co-culture system incorporating human dermal fibroblasts (hDFs) and hiNSCs as a simple in vitro tool for understanding the complex interactions between these two different cell types, we found that pretreating hDFs with ivermectin caused adjacent hiNSCs to significantly increase proliferation. Similarly, in migration assays using predifferentiated hiNSCs, we found that ivermectin-treated hDFs caused a significant increase in neuronal migration. We also further characterized the effects of ivermectin on hDFs in vitro, and found that ivermectin caused hDFs to uptake extracellular glutamate, secrete GDNF, develop an elongated bipolar morphology, and express GFAP, suggesting that ivermectin causes hDFs to adopt a glia-like phenotype. Finally, we translated our in vitro findings to a relevant in vivo model and found that localized ivermectin treatment in a dermal wound site induced the upregulation of both glial and neuronal markers upon healing. Taken together, our data reveal a novel role for ivermectin in promoting peripheral nerve regeneration during mammalian wound healing.
2. Results
2.1. Treating Dermal Fibroblasts with Ivermectin Induces Proliferation in Co-cultured hiNSCs
We developed a 3D bilayer collagen gel co-culture system consisting of human induced neural stem cells (hiNSCs) fluorescently labeled with DiD dye and human dermal fibroblasts (Figure 1A). These hiNSCs are highly proliferative and express Sox1, Sox2, and Nestin (Figure S1). Prior to embedding in separate collagen gels, both cell types were transiently treated with dimethyl sulfoxide (DMSO) or 1 μM ivermectin and subsequently washed repeatedly to remove residual drug. hDFs were seeded into the bottom gel, labeled hiNSCs seeded into the top gel, and the bilayer constructs were subsequently cultured for 5 days.
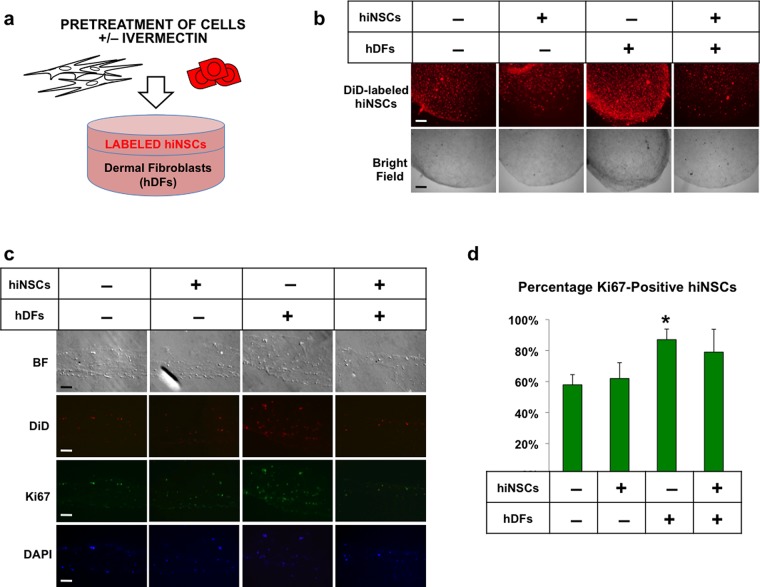
Figure 1.
Treatment of dermal fibroblasts with ivermectin induces proliferation in adjacent neural stem cells in 3D co-cultures. (a) Schematic diagram of experimental design. Human dermal fibroblasts (hDFs) and human induced neural stem cells (hiNSCs) fluorescently labeled with DiD dye were separately treated with or without 1 μM ivermectin (as indicated by “+” or “–”, respectively) and subsequently washed repeatedly to remove the drug, seeded into 3D bilayer collagen gel constructs, and cultured for 5 days. (b) Low-magnification view of 3D collagen gel constructs, scale bar: 500 μM. (c) Cryosections of collagen gels immunostained for proliferation marker, Ki67, scale bar: 100 μM. (d) Quantification of Ki67-positive DiD-labeled neural stem cells. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; as determined by one-way analysis of variance (ANOVA) with post-hoc Tukey test. Error bars show mean ± SD.
Similar to findings from previous Xenopus experiments that indicated noncell-autonomous effects on neural growth,17 we observed increased neural growth only in those constructs, in which hDFs were pretreated with ivermectin (Figure 1B). Upon cryosectioning these bilayer gels, we found that hiNSCs in these constructs expressed significantly higher levels of proliferation marker Ki67 (Figure 1C,D), suggesting that ivermectin-treated hDFs induced proliferation in adjacent hiNSCs in 3D bilayer co-cultures.
2.2. Ivermectin-Treated Fibroblasts Induce Migration of Differentiated Neurons
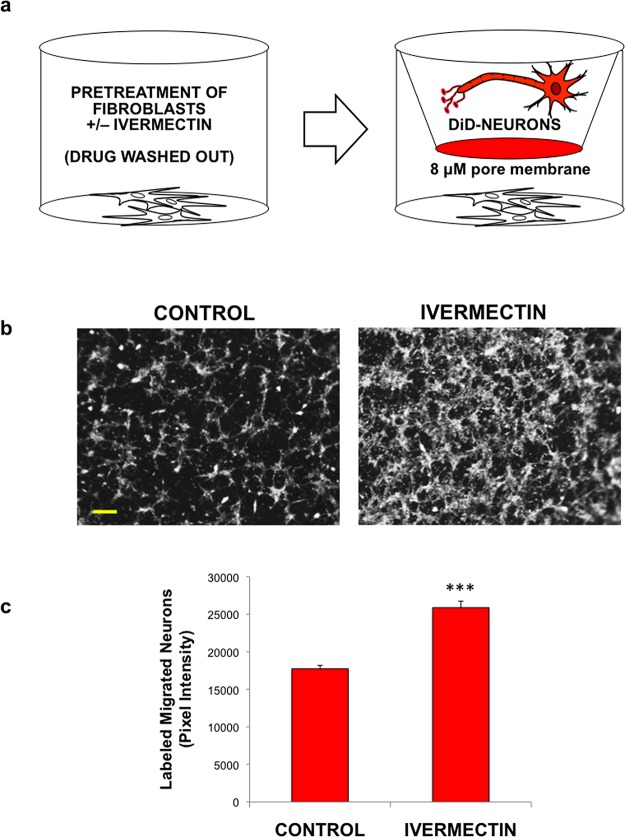
Having established that ivermectin-treated hDFs induced proliferation of neural stem cells, we also aimed to study their potential effects on neuronal migration. To test this, we seeded hDFs into the bottom transwell of cell culture plates, treated with DMSO or 1 μM ivermectin, then subsequently washed repeatedly to remove residual drug (Figure 2A). In the top of the transwell on the coated 8 μM pore membrane, we seeded DiD-labeled human neurons to minimize the effect of proliferation in the assay. We utilized hiNSCs that had been predifferentiated for 1 week that were no longer proliferative and expressed high levels of pan-neuronal marker β III tubulin (Tuj1) (Figure S2A). These transwell systems were cultured in low serum media overnight (to further minimize potential cell proliferation), and the relative number of cells migrating to the bottom of transwells was quantified (Figure 2B,C). Interestingly, ivermectin-treated hDFs demonstrated a significant increase in neuronal migration relative to control-treated cells.
Figure 2.
Treatment of dermal fibroblasts with ivermectin induces migration of differentiated neurons. (a) Schematic diagram of experimental design. Human dermal fibroblasts were seeded into the bottom of cell culture plates, subsequently treated with or without ivermectin, and washed repeatedly to remove the drug. Differentiated DiD-labeled neurons were seeded onto coated transwells (8 μM pore size), which were placed into the wells containing fibroblasts. Cells were cultured in low serum media (to minimize potential cell proliferation) overnight, and the relative number of cells migrating to the bottom of transwells was quantified. (b) Images of fluorescently labeled neurons that migrated to the bottom of transwells upon co-culture with dermal fibroblasts pretreated with or without ivermectin, scale bar: 200 μM. (c) Quantification of migrated cells. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; as determined by two-tailed t-test. Error bars show mean ± SD.
Taken together, we demonstrate that ivermectin treatment of stromal hDFs, but not hiNSCs, had a profound effect on neural growth. Importantly, because the drug was washed out extensively prior to establishing both co-culture models, this suggests that the related effects were the result of the initial pretreatment of separate cell types and not the effect of the drug itself on the entire co-culture constructs.
2.3. Treatment with Ivermectin Causes Dermal Fibroblasts to Adopt Functional Characteristics of Glial Cells
We first hypothesized that ivermectin-treated fibroblasts were removing potentially cytotoxic glutamate from the extracellular microenvironment. To test this, we treated hDFs with various concentrations of ivermectin overnight, then assayed the cell culture media to determine extracellular glutamate concentration. It was previously shown that fibroblast cell culture media contains a large quantity of glutamate as a result of the addition of l-glutamine, a precursor of glutamate, to many types of media formulations.22 Interestingly, we found that treatment of hDFs with ivermectin resulted in a significant dose-dependent decrease of extracellular glutamate concentration (Figure 3A), suggesting that ivermectin-treated fibroblasts might also be able to uptake extracellular glutamate released by neurons upon injury.
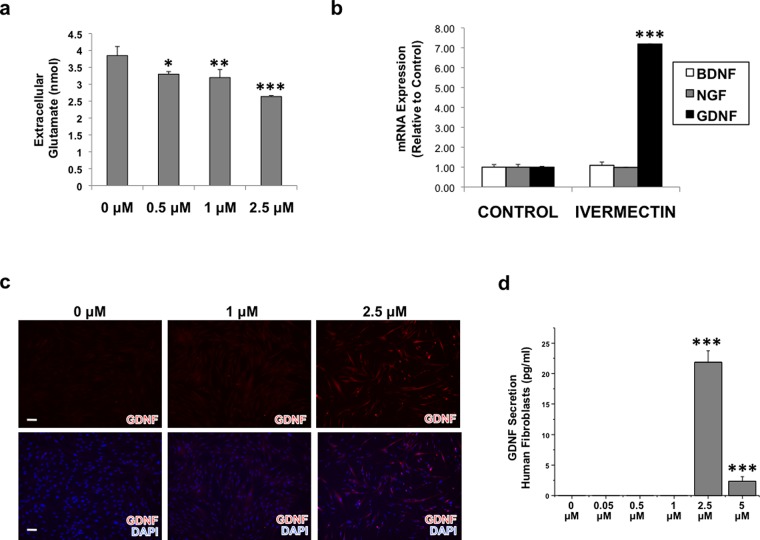
Figure 3.
Treatment with ivermectin causes dermal fibroblasts to uptake extracellular glutamate and to express glial cell line-derived neurotrophic growth factor (GDNF). (a) Dermal fibroblasts were treated with various concentrations of ivermectin overnight, and cell culture media was assayed to determine extracellular glutamate concentration. (b) Dermal fibroblasts were treated with or without 1 μM ivermectin for 4 days, then subjected to quantitative real-time polymerase chain reaction (qRT-PCR) analysis for various neurotrophic growth factors. (c) Immunostaining results of dermal fibroblasts treated with ivermectin show an increase in GDNF expression with increasing ivermectin concentration, scale bar: 100 μM. (d) Enzyme-linked immunosorbent assay (ELISA) of cell culture media harvested from dermal fibroblasts treated with ivermectin for 4 days indicates that GDNF is secreted from ivermectin-treated fibroblasts. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; as determined by one-way ANOVA with post-hoc Tukey test. Error bars show mean ± SD.
We hypothesized that ivermectin treatment of hDFs was eliciting some type of paracrine effect on neighboring hiNSCs. As such, we also aimed to determine whether ivermectin caused fibroblasts to express any neurotrophic factors. We treated hDFs with vehicle or 1 μM ivermectin for 4 days, then subjected them to qRT-PCR analysis to assay for expression of various growth factors. We found that although there was no significant effect of ivermectin on brain-derived neurotrophic factor or nerve growth factor after 4 days, ivermectin-treated hDFs dramatically upregulated expression of glial cell line-derived neurotrophic factor (GDNF) (Figure 3B), a factor shown to be highly expressed by astrocytes23 and Schwann cells.7 To confirm if GDNF was also upregulated at the protein level, we treated hDFs with increasing amounts of ivermectin and immunostained against GDNF, and found that exposure to ≥1 μM ivermectin increased GDNF expression (Figure 3C). Upon determining that GDNF was upregulated at both the mRNA and protein level, we also aimed to understand if GDNF was also being secreted. hDFs were treated with increasing concentrations of ivermectin for 4 days, and hDF-conditioned media was harvested, filtered, and subjected to GDNF ELISA. Interestingly, we found that at concentrations ≥2.5 μM, ivermectin caused hDFs to secrete significant levels of GDNF protein into the media (Figure 3D). Ivermectin concentrations ≥5 μM appeared to be cytotoxic in vitro (Figure S3).
To further elucidate the role of ivermectin-induced GDNF expression in hDFs on co-cultured hiNSCs, we performed neuron migration experiments (as in Figure 2) in the presence of an antibody that functionally blocks GDNF, and found that this antibody was able to diminish the effects of ivermectin-treated hDFs on hiNSC migration (Figure S4).
2.4. Ivermectin-Treated Fibroblasts Demonstrate Glia-like Morphology and Upregulate GFAP
Having established that ivermectin causes hDFs to adopt certain characteristics of glial cells, including glutamate uptake and release of GDNF, we also aimed to understand if ivermectin-treated hDFs also become more phenotypically similar to glia. Similar to previous experiments, hDFs were treated with increasing concentrations of ivermectin for up to 8 days, then subjected to qRT-PCR, and immunostaining. We also subjected samples to GeneQuery Human Schwann Cell Biology qPCR Array Kit and found that ivermectin-treated hDFs highly express multiple genes implicated in various aspects of Schwann cell biology, including differentiation and maintenance, peripheral nerve regeneration, and extracellular matrix synthesis, including (but not limited to) SRY-box 10 (Sox10), S100 calcium-binding protein B (S100B), myelin basic protein, growth associated protein 43 (GAP43), and neural cell adhesion molecule 1 (NCAM1) (Figure S5).
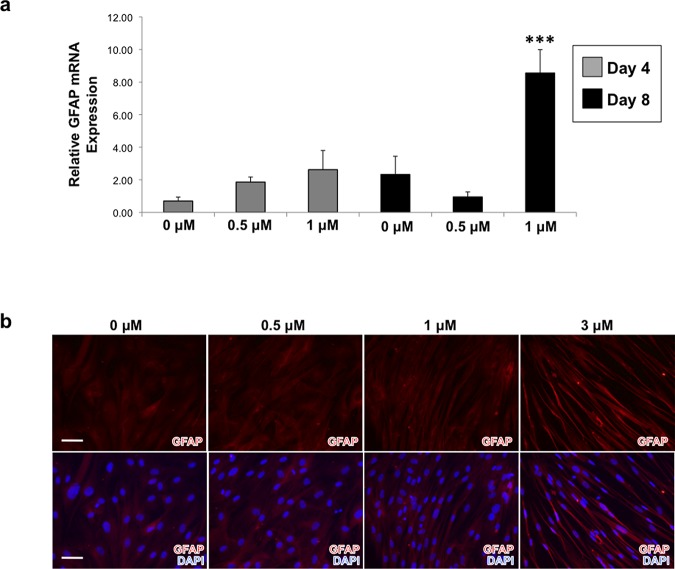
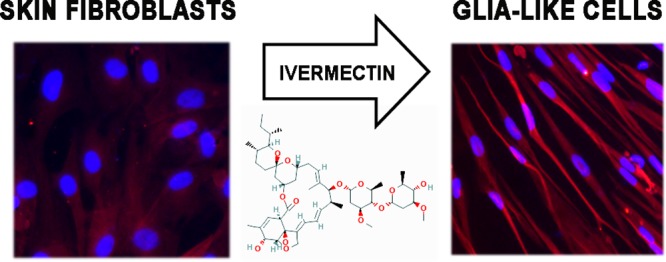
Furthermore, qRT-PCR analysis revealed that hDFs treated with 1 μM ivermectin expressed significantly higher levels of glial fibrillary acidic protein (GFAP) at both D4 and D8 (Figure 4A). Similarly, we demonstrated that increasing concentrations of ivermectin resulted in higher protein expression of GFAP, as well as morphological changes reminiscent of a Schwann cell-like phenotype, becoming very thin and elongated relative to control fibroblasts (Figure 4B). Finally, we repeated several experiments using different formulations of ivermectin to confirm that the observed effects were the result of the drug and not a nonspecific artifact of drug synthesis. We found that for all formulations of ivermectin tested, GFAP was upregulated and the phenotypic elongated morphology was also observed (Figure S6), suggesting that our findings regarding the effects of ivermectin on hDFs were likely caused by the principal component of the drug itself and not by potentially contaminating byproducts of the manufacturing process.
Figure 4.
Treatment of dermal fibroblasts with increasing concentrations of ivermectin results in the upregulation of GFAP as well as the development of an elongated morphology reminiscent of Schwann cells. (a) Dermal fibroblasts were treated with varying concentrations of ivermectin for 4 and 8 days, then subjected to qRT-PCR analysis of GFAP expression. (b) GFAP immunostaining demonstrates that dermal fibroblasts treated with relatively higher concentrations of ivermectin for 8 days results in an increase of GFAP expression as well as a change in morphology, which resembles a Schwann cell-like phenotype, scale bar: 100 μM. ***P ≤ 0.001; as determined by one-way ANOVA with post-hoc Tukey test. Error bars show mean ± SD.
2.5. Effect of Ivermectin in an in Vivo Model of Wound Healing
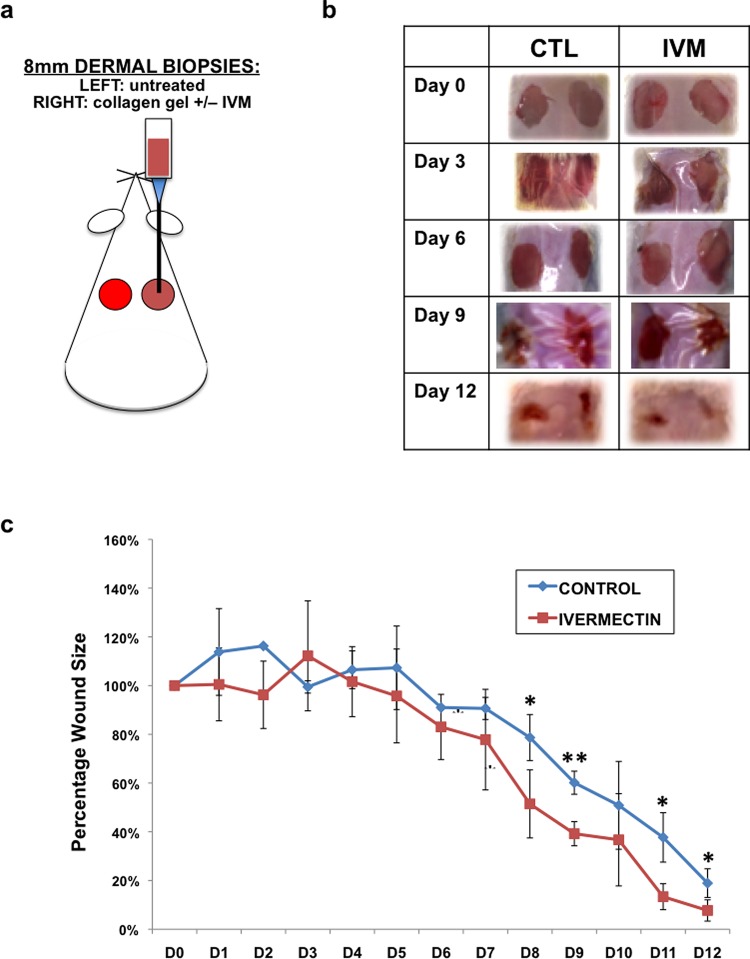
Although our in vitro results clearly demonstrated a role of ivermectin in promoting nerve regeneration through the transformation of dermal fibroblasts into a more glial-like phenotype, it was important to understand if these effects could also be seen in an in vivo model. After generating compelling data, which suggested that ivermectin-treated dermal fibroblasts promote the growth of co-cultured neurons, we selected a physiologically relevant in vivo system that would include both relevant cell types (dermal fibroblasts and peripheral nerves) and provide a simplified system for localized drug delivery of ivermectin. For this purpose, we selected a dermal wound healing model. Two 8 mm diameter full-thickness biopsies were removed from the dorsal skin of adult male BALB/c mice. In the wound on the right side, 30 μL collagen gels containing 10 μM ivermectin or DMSO (control) were pipetted onto the wound and allowed to solidify (Figure 5A). The left side wounds remained untreated and served as additional controls. Both wounds were sealed using Tegaderm, and wound progression was followed over the course of 12 days. We determined that ivermectin partially aided in wound healing as determined by quantification of wound size over time (Figure 5B). At days 8–9 as well as 11–12, ivermectin-treated wounds were significantly smaller than DMSO-treated controls.
Figure 5.
Ivermectin promotes wound healing of dermal biopsies in vivo. (a) Schematic diagram of experimental design. Biopsies (2 × 8 mm2) were taken from the dorsal dermal layer of each mouse. In the right side wound, 30 μL collagen gels containing 10 μM ivermectin or DMSO (control) were pipetted onto the wound and allowed to solidify. The left side wounds remained untreated, and served as additional controls. Both wounds were sealed using Tegaderm, and wound progression was followed over the course of 12 days. (b) Images of gross morphology of wound healing over time. (c) Quantification of wound size over time. *P ≤ 0.05, **P ≤ 0.01; as determined by two-tailed t-test. Error bars show mean ± SD.
2.6. Healed Ivermectin-Treated Wounds Have Increased Neuronal and Glial Marker Expression
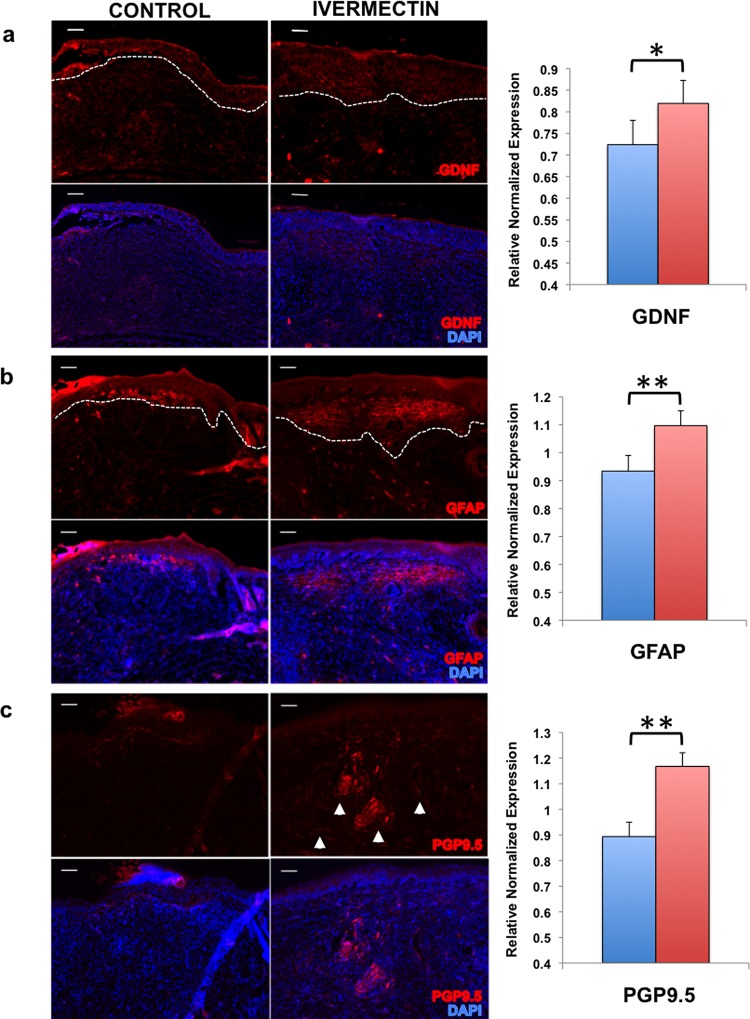
We next assayed for the presence of both neuronal and glial markers in the healed dermal tissue. This is especially important as impaired wound healing often produces scar tissue, in which nerves fail to regenerate properly, resulting in loss of sensation at the wound site. Upon sacrifice at D12, we excised and fixed the wound tissue, subjected tissue sections to immunostaining, and found significantly higher expression of secreted factor GDNF (Figure 6A), glial marker GFAP (Figure 6B), and peripheral nerve marker (PGP9.5) (Figure 6C) in those mice treated with ivermectin-loaded collagen gels as compared to vehicle-control collagen gels. These findings correspond to our in vitro data to suggest that ivermectin also promotes nerve growth by inducing the generation of glia-like cells in an in vivo model of wound healing.
Figure 6.
Ivermectin facilitates wound healing by inducing the differentiation of glia-like cells that promote nerve growth. Cryosections of the wound sites were immunostained and quantified to assay for the presence of (a) glial-derived growth factor (GDNF), (b) glial fibrillary acidic protein (GFAP), and (c) peripheral nerve marker (PGP9.5), scale bar: 100 μM. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; as determined by two-tailed t-test. Error bars show mean ± SD.
3. Discussion
Although the peripheral nervous system has an innate repair capacity, there are various cases, in which this process is impaired, especially in organisms with restricted regenerative potential, such as humans. Given the limitations of current treatment options, there is a critical need to develop new strategies to enhance nerve regeneration. One approach is to examine mechanisms in lower vertebrates, in which peripheral nerve repair is quite efficient. The anthelminic drug ivermectin was recently shown to enhance innervation in regenerative stage frogs. Previous experiments performed in X. laevis tadpoles demonstrated a role for ivermectin in promoting increased nerve growth from ectopic eye tissue.17 Fluorescently labeled donor eye primordia or unlabeled host tadpoles were treated with or without ivermectin before ectopic engraftment on the host animal flank. Hyperinnervation of the engrafted eye was only seen in those tadpoles, in which the donor tadpole received ivermectin pretreatment (and not vice versa).17 These experiments revealed that ivermectin treatment of non-neuronal stromal tissue can be exploited to induce the expansion of neurons from the adjacent engrafted neuronal tissue. Although these results were striking, it is important to understand whether this phenomenon was specific to this amphibian model or could be recapitulated in mammalian systems.
In this work, we provide a role for ivermectin in promoting peripheral nerve growth in mammals. We discovered that co-culturing hiNSCs with ivermectin-treated fibroblasts resulted in increased hiNSC proliferation and migration. In addition, we found that ivermectin causes fibroblasts to adopt a glial-like phenotype; increasing uptake of extracellular glutamate, expressing neurotrophic factor GDNF, and displaying characteristics of Schwann cells, including elongated morphology and GFAP expression. These transformed glial-like cells allow for the expansion of resident neurons, providing a supportive environment for nerve regeneration. Furthermore, we demonstrate a physiologically relevant in vivo role for ivermectin in promoting nerve regeneration using a murine model of wound healing. Importantly, our results are in accordance with current findings implicating a critical role for peripheral glia during mammalian tissue regeneration. It was recently shown that dermal injury activates peripheral glia in an in vivo model of full-thickness skin repair. Further, it was demonstrated that depletion of these activated glia functionally impairs the wound healing process.24 Similarly, transplantation of Schwann cell precursors promoted digit tip regeneration via localized secretion of paracrine factors in a murine amputation model25
Given that FDA-approved Ivermectin is already currently used to treat a variety of infestations, including scabies, lice, and onchocerciasis,26 its use could be further adapted for clinical applications in peripheral nerve repair. It is known that ivermectin eradicates parasitic invertebrates by binding and activating glutamate-gated chloride channels present only in neurons and muscle cells of these organisms, ultimately leading to muscle paralysis and death.26 In mammals, these types of glutamate-gated chloride channels were only thought to be expressed in the brain, and were thereby protected by the blood–brain barrier: rationale that has lead to its deemed safety for human use. Indeed, at low levels comparable to what is used in both clinical and veterinary medicines, there is essentially no discernible effect on these types of mammalian brain-specific glutamate-gated chloride channels. It has been shown, however, that ivermectin at higher concentrations (i.e., micromolar range) can act as an allosteric modulator of multiple channels, including the human glycine receptor;19 γ-aminobutyric acid A (GABAA) receptors from chicken,27 rodents,28 and humans;29 chicken and human α7 nicotinic receptors;30 as well as human purinergic receptors P2X420 and P2X7.31 Many of these receptors are found in multiple cell types in mammals and more specifically humans, suggesting that the effects of ivermectin may be more widespread than initially realized.
Within the microenvironment of a healing wound, there are multiple cell types involved in the healing process, many of which are known to express a number of relevant ion channels. For example, human fibroblasts have been shown to express many of the aforementioned receptors, such as glycine,32 GABA,33 purinergic,34 and nicotinic.35 This endogenous expression combined with the relatively nonspecific binding and functioning of ivermectin on a variety of different ion channels and receptors makes it somewhat challenging to identify which specific receptor or receptors ivermectin is acting upon in our in vitro and in vivo systems. This complexity of potential interactions of ivermectin with multiple channels and receptors limits insight into mode of action. However, the findings here provide compelling evidence for a broader impact of ivermectin, in both downstream efficacy and potential clinical utility.
Although the effects of ivermectin on neuronal and glial growth in vivo were quite striking, it is important to acknowledge that the drug’s effect on wound closure was not as profound. It is accepted that wound healing in healthy mice is particularly difficult to improve experimentally,36 and more specifically, the BALB/c wildtype mice used in this study are known to heal relatively quickly.37 It will be important to assess whether this drug can also play a role in improving nerve regeneration in other models, in which the nerve defect is more profound and/or impaired. For example, similar experiments could be repeated in various in vivo models of neuropathy, which is often associated with other co-morbidities, such as diabetes, autoimmune disorders, and chemotherapy treatment.38 Ivermectin could also be explored for its potential use in promoting repair of larger nerve defect models, such as sciatic nerve resection or spinal cord injury. Furthermore, because we demonstrate that ivermectin causes fibroblasts to secrete GDNF, this technique can also be adapted as a method to induce endogenous localized delivery of GDNF as a potential analgesic39 to promote innervation in ischemic tissue40 or perhaps modified further to develop strategies of GDNF delivery for treating Parkinson’s disease.41
4. Materials and Methods
4.1. Generation of hiNSCs
hiNSC lines were generated as previously described.21 Briefly, human neonatal foreskin fibroblasts (a gift from Dr. Jonathan Garlick, Tufts University) were infected with a lentivirus expressing reprogramming factors OCT4, KLF4, SOX2, and cMYC in a polycistronic vector (Addgene #24603, a gift from Jose Cibelli). Concentrated virus was used in combination with polybrene (Millipore) in fibroblast media at an MOI of 1–2. Media were ultimately changed to hiNSC media: knockout (KO) Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% KO xeno-free SR, 20 ng/mL recombinant bFGF, 1% Glutamax, 1% antibiotic-antimycotic, and 0.1 mM b-mercaptoethanol, which also contained 1% KO growth-factor cocktail (GFC) (Invitrogen). Cells were later trypsinized and plated onto mouse embryonic fibroblast (MEF) feeder layers previously inactivated by mitomycin C. hiNSC medium (without KO-GFC) was subsequently changed every 1–3 days. At day 30 or later, colonies were mechanically picked and passaged onto freshly mitotically inactivated MEFs. Colonies were expanded by enzymatically passaging using TrypLE (Invitrogen) on MEF feeders. Each picked colony represented one hiNSC line.
4.2. Ivermectin Preparation
Various formulations of ivermectin were purchased from multiple sources, including Sigma-Aldrich (Natick, MA), Tocris (Minneapolis, MN) or Cayman Chemical (Ann Arbor, MI). For all preparations of the drug, DMSO was used for reconstitution from its lyophilized form.
4.3. Three-Dimensional Collagen Gel hiNSC Co-cultures
Human dermal fibroblasts (hDFs) and hiNSCs were treated with 1 μM ivermectin or 1 μM DMSO (control) for 6 h. hiNSCs were dissociated and labeled with lipophilic fluorescent dye DiD (Invitrogen) according to manufacturer’s protocol to monitor their growth in the collagen gels. Collagen gel mix was made with 68% 1.5× DMEM (Invitrogen), 30% Rat Tail Collagen I (Corning), and 2.5% 0.8 M NaHCO3. The bottom layer of the collagen gel was seeded with 104 hDFs/gel in 20 μL gels, pipetted into four well plates (Nunc), and allowed to partially solidify for 20 min at 37 °C. The top layer was seeded with 104 labeled hiNSCs/gel in 30 μL gels, pipetted on top of the bottom layer, and allowed to fully set at 37 °C. Once completely solidified, hiNSC media were added to wells, and 3D constructs were cultured for 5 days in hiNSC media without FGF.
4.4. Neuron Migration Assay
hDFs were seeded into the bottom of cell culture plates, subsequently with 1 μM ivermectin or 1 μM DMSO (control) for 6 h, and washed repeatedly with 1× phosphate-buffered saline (PBS) to remove residual drug. hiNSCs were predifferentiated on gelatin in low-FGF media for 1 week prior to seeding. Differentiated neurons labeled with lipophilic fluorescent dye DiD (Invitrogen) were seeded onto CELLstart-coated (Invitrogen) transwells (8 μM pore size), which were placed into the wells containing hDFs. Cells were cultured in low serum media (to minimize potential cell proliferation) overnight in the presence or absence of functional blocking antibody goat anti-GDNF (AF-212-NA, R&D Systems, Minneapolis, MN) at relatively low concentration of 10 μL/10 mL or high concentration of 100 μL/10 mL, and the relative number of DiD-labeled neurons migrating to the bottom of transwells was quantified.
4.5. Glutamate Uptake Assay
hDFs were treated with various concentrations of ivermectin or DMSO overnight, cell culture media was harvested and filtered, then subjected to a glutamate assay (Sigma) according to manufacturer’s instructions.
4.6. qRT-PCR
Total RNA was isolated using the RNeasy Mini kit (Qiagen), and cDNA was generated using MLV-reverse transcriptase (Invitrogen, CA) according to the manufacturers’ instructions. Quantitative RT-PCR was performed using the iQ5 real-time PCR detection system (BioRad) and normalized based on housekeeping gene GAPDH. All primer sequences are listed in Table S1. We also performed GeneQuery Human Schwann Cell Biology qPCR Array Kit (ScienCell Research Laboratories, Carlsbad, CA) according to the manufacturers’ instructions.
4.7. GDNF ELISA
A GDNF ELISA (Promega) was performed according to manufacturer’s instructions using cell culture media harvested and filtered from hDFs treated with various concentrations of ivermectin or DMSO for 4 days.
4.8. Immunostaining
All in vitro cultures as well as in vivo tissue samples were fixed in 4% paraformaldehyde, then washed with 1× phosphate-buffered saline (PBS). Three-dimensional collagen gels and excised tissues were cryosectioned prior to immunostaining. Samples were incubated with blocking buffer: 1× PBS containing 10% goat serum and 0.1% triton X-100. Primary antibodies were added to blocking buffer and incubated with samples overnight at 4 °C. The next day, samples were washed several times with 1× PBS, then incubated with a corresponding fluorescently conjugated secondary antibody in blocking buffer for 1 h at room temperature (away from light). Nuclei were counterstained with DAPI (Invitrogen). All antibodies used in this study are listed in Table S2.
4.9. Microscopy
Brightfield and fluorescent images were obtained using a Keyence BZ-X700 microscope and associated software. Images of whole mount 3D samples were taken using an Olympus MVX10 macroscope and associated software.
4.10. In Vivo Wound Assay
Animal studies were conducted under approved protocol #M2013–142 at Tufts University. Eight weeks old male BALB/c mice (Charles River Laboratories) weighing 20–25 g were first shaved on the back and depilated with Nair (Carter-Wallace Inc., New York, NY). Mice were then anesthetized with isoflurane, and an 8 mm biopsy punch was utilized to make two round, full-thickness excisional wounds. In the right side wound, 30 μL collagen gels containing 10 μM ivermectin or DMSO (control) were pipetted onto the wound and allowed to solidify. The left side wounds remained untreated and served as additional controls. Both wounds were sealed using Tegaderm, and wound progression was followed over the course of 12 days. Three animals were used per treatment per experiment. At the conclusion of each study, animals were euthanized and wound site tissue excised for further analysis.
4.11. Statistics
All data are expressed as mean ± SD, including at least three independent samples analyzed per experiment. Statistically significant differences were determined by two-tailed t-test or one-factor ANOVA with post-hoc Tukey test using the statistics software SYSTAT12 (Systat). A P-value less than 0.05 was considered significant.
Acknowledgments
The authors thank Jonathan M. Grasman for helpful discussion.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01451.
Characterization of proliferating hiNSCs; characterization of predifferentiated hiNSCs; toxicity of ivermectin in vitro; functionally blocking ivermectin-induced GDNF upregulation by hDFs prevents migration of differentiated neurons; treatment of dermal fibroblasts with ivermectin causes an upregulation in multiple genes involved in Schwann cell differentiation and maintenance, peripheral nerve regeneration and extracellular matrix (ECM) synthesis; different formulations of ivermectin produce similar results in hDFs in vitro; list of primer sequences used for qRT-PCR analysis; list of antibodies used for immunofluorescence analysis (PDF)
Author Contributions
D.M.C. conceived the idea, performed experiments and data analysis, and wrote the paper. J.E.G. and S.C. performed experiments and data analysis. D.L.K. and M.L. supervised the project. All authors edited the final manuscript.
This research was supported by the Allen Discovery Center program through The Paul G. Allen Frontiers Group (12171) as well as the National Institutes of Health (NIH) (R01NS092847 and P41EB002520).
The authors declare no competing financial interest.
Notes
The authors declare that data supporting the findings of this study are available within the manuscript and its Supporting Information files.
Supplementary Material
References
- Lundborg G.; Richard P. Nerve injury and repair--a challenge to the plastic brain. J. Peripher. Nerv. Syst. 2003, 8, 209–226. 10.1111/j.1085-9489.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- Martins R. S.; Bastos D.; Siqueira M. G.; Heise C. O.; Teixeira M. J. Traumatic injuries of peripheral nerves: a review with emphasis on surgical indication. Arq. Neuro-Psiquiatr. 2013, 71, 811–814. 10.1590/0004-282X20130127. [DOI] [PubMed] [Google Scholar]
- Grinsell D.; Keating C. P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fu S. Y.; Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J. Neurosci. 1995, 15, 3886–3895. 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Painter M. W.; Brosius Lutz A.; Cheng Y. C.; Latremoliere A.; Duong K.; Miller C. M.; Posada S.; Cobos E. J.; Zhang A. X.; Wagers A. J.; Havton L. A.; Barres B.; Omura T.; Woolf C. J. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 2014, 83, 331–343. 10.1016/j.neuron.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. M.; Babetto E.; Beirowski B. Axon degeneration: make the Schwann cell great again. Neural Regener. Res. 2017, 12, 518–524. 10.4103/1673-5374.205000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Saitoh F.; Wakatsuki S.; Tokunaga S.; Fujieda H.; Araki T. Glutamate signals through mGluR2 to control Schwann cell differentiation and proliferation. Sci. Rep. 2016, 6, 29856 10.1038/srep29856. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Campana W. M.; Mantuano E.; Azmoon P.; Henry K.; Banki M. A.; Kim J. H.; Pizzo D. P.; Gonias S. L. Ionotropic glutamate receptors activate cell signaling in response to glutamate in Schwann cells. FASEB J. 2017, 31, 1744–1755. 10.1096/fj.201601121R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P.; Rosen K. M.; Hedstrom K.; Rey O.; Guha S.; Hart C.; Corfas G. Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in Schwann cells through purinergic signaling and the PKC-PKD pathway. Glia 2013, 61, 1029–40. 10.1002/glia.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Liu G. S.; Shi J. Y.; Lai C. L.; Hong Y. R.; Shin S. J.; Huang H. T.; Lam H. C.; Wen Z. H.; Hsu K. S.; Chen C. H.; Howng S. L.; Tai M. H. Peripheral gene transfer of glial cell-derived neurotrophic factor ameliorates neuropathic deficits in diabetic rats. Hum. Gene Ther. 2009, 20, 715–27. 10.1089/hum.2009.002. [DOI] [PubMed] [Google Scholar]; b Hedstrom K. L.; Murtie J. C.; Albers K.; Calcutt N. A.; Corfas G. Treating small fiber neuropathy by topical application of a small molecule modulator of ligand-induced GFRalpha/RET receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 2325–30. 10.1073/pnas.1308889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Chen Z. Y.; Chai Y. F.; Cao L.; Lu C. L.; He C. Glial cell line-derived neurotrophic factor enhances axonal regeneration following sciatic nerve transection in adult rats. Brain Res. 2001, 902, 272–276. 10.1016/S0006-8993(01)02395-2. [DOI] [PubMed] [Google Scholar]; b Li Q.; Ping P.; Jiang H.; Liu K. Nerve conduit filled with GDNF gene-modified Schwann cells enhances regeneration of the peripheral nerve. Microsurgery 2006, 26, 116–121. 10.1002/micr.20192. [DOI] [PubMed] [Google Scholar]
- Jessen K. R.; Mirsky R.; Lloyd A. C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harbor Perspect. Biol. 2015, 7, a020487 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo D.; Dina G.; Lorenzetti I.; Malaguti M.; Morana P.; Del Carro U.; Comi G.; Messing A.; Quattrini A.; Previtali S. C. Loss of glial fibrillary acidic protein (GFAP) impairs Schwann cell proliferation and delays nerve regeneration after damage. J. Cell Sci. 2006, 119, 3981–3993. 10.1242/jcs.03168. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M.; Krisky D.; Wolfe D.; Glorioso J. C.; Mata M.; Fink D. J. HSV-mediated gene transfer of vascular endothelial growth factor to dorsal root ganglia prevents diabetic neuropathy. Gene Ther. 2005, 12, 1377–1384. 10.1038/sj.gt.3302533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Haas B. J.; Whited J. L. Advances in Decoding Axolotl Limb Regeneration. Trends Genet. 2017, 553–565. 10.1016/j.tig.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Maden M. Axolotl/newt. Methods Mol. Biol. 2008, 461, 467–480. 10.1007/978-1-60327-483-8_32. [DOI] [PubMed] [Google Scholar]
- a Kumar A.; Brockes J. P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012, 35, 691–699. 10.1016/j.tins.2012.08.003. [DOI] [PubMed] [Google Scholar]; b Singer M. The influence of the nerve in regeneration of the amphibian extremity. Q. Rev. Biol. 1952, 27, 169–200. 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- a Beck C. W.; Izpisua Belmonte J. C.; Christen B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev. Dyn. 2009, 238, 1226–48. 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]; b Tseng A. S.; Levin M. Tail regeneration in Xenopus laevis as a model for understanding tissue repair. J. Dent. Res. 2008, 87, 806–16. 10.1177/154405910808700909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth C. M. Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 1974, 9, 853–858. 10.1016/S0022-3468(74)80220-4. [DOI] [PubMed] [Google Scholar]
- a Blackiston D. J.; Anderson G. M.; Rahman N.; Bieck C.; Levin M. A novel method for inducing nerve growth via modulation of host resting potential: gap junction-mediated and serotonergic signaling mechanisms. Neurotherapeutics 2015, 12, 170–184. 10.1007/s13311-014-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Blackiston D. J.; Vien K.; Levin M. Serotonergic stimulation induces nerve growth and promotes visual learning via posterior eye grafts in a vertebrate model of induced sensory plasticity. npj Regener. Med. 2017, 2, 8 10.1038/s41536-017-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S.; Crump A. The life and times of ivermectin - a success story. Nat. Rev. Microbiol. 2004, 2, 984–9. 10.1038/nrmicro1048. [DOI] [PubMed] [Google Scholar]
- Shan Q.; Haddrill J. L.; Lynch J. W. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J. Biol. Chem. 2001, 276, 12556–64. 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Priel A.; Silberberg S. D. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J. Gen. Physiol. 2004, 123, 281–293. 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns D. M.; Chwalek K.; Moore Y. E.; Kelley M. R.; Abbott R. D.; Moss S.; Kaplan D. L. Expandable and Rapidly Differentiating Human Induced Neural Stem Cell Lines for Multiple Tissue Engineering Applications. Stem Cell Rep. 2016, 7, 557–70. 10.1016/j.stemcr.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S.; Ishii T. A novel function of glutamine in cell culture: utilization of glutamine for the uptake of cystine in human fibroblasts. J. Cell. Physiol. 1988, 137, 360–6. 10.1002/jcp.1041370221. [DOI] [PubMed] [Google Scholar]
- Moretto G.; Walker D. G.; Lanteri P.; Taioli F.; Zaffagnini S.; Xu R. Y.; Rizzuto N. Expression and regulation of glial-cell-line-derived neurotrophic factor (GDNF) mRNA in human astrocytes in vitro. Cell Tissue Res. 1996, 286, 257–262. 10.1007/s004410050695. [DOI] [PubMed] [Google Scholar]
- Parfejevs V.; Debbache J.; Shakhova O.; Schaefer S. M.; Glausch M.; Wegner M.; Suter U.; Riekstina U.; Werner S.; Sommer L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat. Commun. 2018, 9, 236 10.1038/s41467-017-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. P.; Yuzwa S. A.; Carr M. J.; Mahmud N.; Storer M. A.; Krause M. P.; Jones K.; Paul S.; Kaplan D. R.; Miller F. D. Dedifferentiated Schwann Cell Precursors Secreting Paracrine Factors Are Required for Regeneration of the Mammalian Digit Tip. Cell Stem Cell 2016, 19, 433–448. 10.1016/j.stem.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Crump A.; Omura S. Ivermectin, ‘wonder drug’ from Japan: the human use perspective. Proc. Jpn. Acad., Ser. B 2011, 87, 13–28. 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E.; Baur R. Effect of avermectin B1a on chick neuronal gamma-aminobutyrate receptor channels expressed in Xenopus oocytes. Mol. Pharmacol. 1987, 32, 749–752. [PubMed] [Google Scholar]
- a Adelsberger H.; Lepier A.; Dudel J. Activation of rat recombinant alpha(1)beta(2)gamma(2S) GABA(A) receptor by the insecticide ivermectin. Eur. J. Pharmacol. 2000, 394, 163–70. 10.1016/S0014-2999(00)00164-3. [DOI] [PubMed] [Google Scholar]; b Krůsek J.; Zemkova H. Effect of ivermectin on gamma-aminobutyric acid-induced chloride currents in mouse hippocampal embryonic neurones. Eur. J. Pharmacol. 1994, 259, 121–8. 10.1016/0014-2999(94)90500-2. [DOI] [PubMed] [Google Scholar]
- a Estrada-Mondragon A.; Lynch J. W. Functional characterization of ivermectin binding sites in alpha1beta2gamma2L GABA(A) receptors. Front. Mol. Neurosci. 2015, 8, 55. 10.3389/fnmol.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dawson G. R.; Wafford K. A.; Smith A.; Marshall G. R.; Bayley P. J.; Schaeffer J. M.; Meinke P. T.; McKernan R. M. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid(A) receptor. J. Pharmacol. Exp. Ther. 2000, 295, 1051–60. [PubMed] [Google Scholar]
- Krause R. M.; Buisson B.; Bertrand S.; Corringer P. J.; Galzi J. L.; Changeux J. P.; Bertrand D. Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 1998, 53, 283–94. 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Nörenberg W.; Sobottka H.; Hempel C.; Plotz T.; Fischer W.; Schmalzing G.; Schaefer M. Positive allosteric modulation by ivermectin of human but not murine P2X7 receptors. Br. J. Pharmacol. 2012, 167, 48–66. 10.1111/j.1476-5381.2012.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booken D.; Henrich-Kellner C.; Klein D.; Goerdt S.; Kurzen H. Glycine Receptors are Present in Human Epidermis. Open Dermatol. J. 2008, 2, 51–56. 10.2174/1874372200802010051. [DOI] [Google Scholar]
- Ito K.; Tanaka K.; Nishibe Y.; Hasegawa J.; Ueno H. GABA-synthesizing enzyme, GAD67, from dermal fibroblasts: evidence for a new skin function. Biochim. Biophys. Acta 2007, 1770, 291–6. 10.1016/j.bbagen.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Solini A.; Chiozzi P.; Morelli A.; Fellin R.; Di Virgilio F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J. Cell Sci. 1999, 112, 297–305. [DOI] [PubMed] [Google Scholar]
- Arredondo J.; Hall L. L.; Ndoye A.; Nguyen V. T.; Chernyavsky A. I.; Bercovich D.; Orr-Urtreger A.; Beaudet A. L.; Grando S. A. Central role of fibroblast alpha3 nicotinic acetylcholine receptor in mediating cutaneous effects of nicotine. Lab. Invest. 2003, 83, 207–25. 10.1097/01.LAB.0000053917.46614.12. [DOI] [PubMed] [Google Scholar]
- Werner S. A novel enhancer of the wound healing process: the fibroblast growth factor-binding protein. Am. J. Pathol. 2011, 179, 2144–2147. 10.1016/j.ajpath.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. A.; Amare M.; Naik S.; Kovalchuk A. L.; Tadaki D. Differential cutaneous wound healing in thermally injured MRL/MPJ mice. Wound Repair Regener. 2007, 15, 577–88. 10.1111/j.1524-475X.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- Vinik A.; Ullal J.; Parson H. K.; Casellini C. M. Diabetic neuropathies: clinical manifestations and current treatment options. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 269–81. 10.1038/ncpendmet0142. [DOI] [PubMed] [Google Scholar]
- Boucher T. J.; Okuse K.; Bennett D. L.; Munson J. B.; Wood J. N.; McMahon S. B. Potent analgesic effects of GDNF in neuropathic pain states. Science 2000, 290, 124–7. 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Shvartsman D.; Storrie-White H.; Lee K.; Kearney C.; Brudno Y.; Ho N.; Cezar C.; McCann C.; Anderson E.; Koullias J.; Tapia J. C.; Vandenburgh H.; Lichtman J. W.; Mooney D. J. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol. Ther. 2014, 22, 1243–53. 10.1038/mt.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. K.; Gill S. S. GDNF delivery for Parkinson’s disease. Acta Neurochir., Suppl. 2007, 97, 135–54. 10.1007/978-3-211-33081-4_16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.