The oppa2 gene encodes an oligopeptide permease essential for the production of clavulanic acid. A transcriptomic analysis of S. clavuligerus ΔoppA2::aac in comparison to the parental strain S. clavuligerus ATCC 27064 is reported. The lack of OppA2 results in different expression of 233 genes, including genes for proteases and genes for transport systems. The expression of the clavulanic acid genes in the oppA2 mutant is not significantly affected, but the genes for holomycin biosynthesis are strongly upregulated, in agreement with the higher holomycin production by this strain. The oppA2-mutant is known to release N-acetylglycyl-clavaminic acid to the broth. Cosynthesis assays using non-clavulanic acid-producing mutants showed that the addition of pure N-acetylglycyl-clavaminic acid to mutants in which clavulanic acid formation was blocked resulted in the recovery of clavulanic acid production, but only in mutants blocked in the early steps of the pathway. This suggests that N-acetylglycyl-clavaminic acid is a previously unknown late intermediate of the clavulanic acid pathway.
KEYWORDS: oligopeptide permeases, ATP binding cassettes, Streptomyces clavuligerus, oppA2 gene, clavulanic acid, transcriptional analysis
ABSTRACT
The oppA2 gene encodes an oligopeptide-binding protein similar to the periplasmic substrate-binding proteins of the ABC transport systems. However, oppA2 is an orphan gene, not included in an ABC operon. This gene is located in the clavulanic acid (CA) gene cluster of Streptomyces clavuligerus and is essential for CA production. A transcriptomic study of the oppA2-null mutant S. clavuligerus ΔoppA2::aac showed changes in the expression levels of 233 genes from those in the parental strain. These include genes for ABC transport systems, secreted proteins, peptidases, and proteases. Expression of the clavulanic acid, clavam, and cephamycin C biosynthesis gene clusters was not significantly affected in the oppA2 deletion mutant. The genes for holomycin biosynthesis were upregulated 2-fold on average, and the level of upregulation increased to 43-fold in a double mutant lacking oppA2 and the pSCL4 plasmid. Strains in which oppA2 was mutated secreted into the culture the compound N-acetylglycyl-clavaminic acid (AGCA), a putative intermediate of CA biosynthesis. A culture broth containing AGCA, or AGCA purified by liquid chromatography-mass spectrometry (LC-MS), was added to the cultures of various non-CA-producing mutants. Mutants blocked in the early steps of the pathway restored CA production, whereas mutants altered in late steps did not, establishing that AGCA is a late intermediate of the biosynthetic pathway, which is released from the cells when the oligopeptide-binding protein OppA2 is not available.
IMPORTANCE The oppa2 gene encodes an oligopeptide permease essential for the production of clavulanic acid. A transcriptomic analysis of S. clavuligerus ΔoppA2::aac in comparison to the parental strain S. clavuligerus ATCC 27064 is reported. The lack of OppA2 results in different expression of 233 genes, including genes for proteases and genes for transport systems. The expression of the clavulanic acid genes in the oppA2 mutant is not significantly affected, but the genes for holomycin biosynthesis are strongly upregulated, in agreement with the higher holomycin production by this strain. The oppA2-mutant is known to release N-acetylglycyl-clavaminic acid to the broth. Cosynthesis assays using non-clavulanic acid-producing mutants showed that the addition of pure N-acetylglycyl-clavaminic acid to mutants in which clavulanic acid formation was blocked resulted in the recovery of clavulanic acid production, but only in mutants blocked in the early steps of the pathway. This suggests that N-acetylglycyl-clavaminic acid is a previously unknown late intermediate of the clavulanic acid pathway.
INTRODUCTION
ATP-binding transport cassettes (ABC) are ubiquitous systems typically consisting of (i) a periplasmic substrate-binding protein, OppA, with high substrate specificity in Gram-negative bacteria, (ii) two hydrophobic integral membrane proteins, OppB and OppC, containing at least six membrane-spanning domains, with their N- and C-terminal ends located in the cytoplasm, and (iii) two ATP binding cytoplasmic proteins, OppD and OppE, which provide the energy to transport the substrate by ATP hydrolysis (1). In Gram-positive bacteria, the OppA subunit is located on the cell surfaces and is anchored in the membrane by a lipophilic acyl residue (2). Some of these ABC-type systems are involved in peptide transport and play important roles in Gram-positive bacteria, such as cell wall recycling (3), signaling at the onset of Bacillus subtilis sporulation (4), and aerial mycelium formation in Streptomyces species (5, 6). In Streptomyces coelicolor, the BldK transporter imports an extracellular signaling molecule that governs aerial mycelium formation through the lantibiotic-like peptide SapB (5, 7, 8). A similar system is present in Streptomyces griseus (9). Genes for ABC transport systems are normally clustered and coordinately expressed, as occurs in Salmonella enterica serovar Typhimurium (10) or in the S. coelicolor BldKABCDE cluster (5). However, isolated genes encoding OppA subunits are occasionally found in bacteria without the cognate genes encoding the OppBCDE subunits.
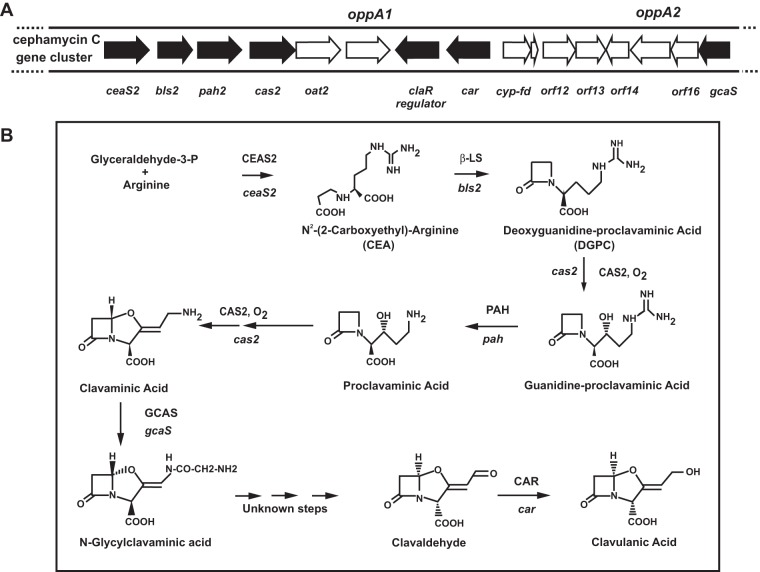
Streptomyces clavuligerus produces the β-lactamase inhibitor clavulanic acid (CA). This compound is formed by the condensation of arginine and glyceraldehyde-3-phosphate by the N2-(2-carboxyethyl)-arginine synthase (CeaS2), encoded by ceaS2 (also named pyc [11]). Cyclization of N2-(2-carboxyethyl)-arginine by the β-lactam synthase (Bls), encoded by bls2, results in deoxyguanidine-proclavaminic acid formation. This compound is transformed through several enzymatic steps into (3S,5S)-clavaminic acid, N-glycylclavaminic acid (NGCA), (3R,5R)-clavaldehyde, and finally (3R,5R)-clavulanic acid (12) (Fig. 1). Most of the genes encoding enzymes for the steps leading to NGCA (ceaS2, bls2, pah2, cas2, and gcaS) and the gene involved in the final step of clavulanic acid biosynthesis (car) have been characterized; however, the functions of several genes essential for CA formation (oppA1, cyp, orf12, orf13, orf14, oppA2, orf16) and located in the CA cluster are still unknown.
FIG 1.
Clavulanic acid gene cluster and pathway. (A) Clavulanic acid gene cluster. Filled arrows represent genes encoding enzymes of the pathway and the transcriptional cluster activator, ClaR. Open arrows represent genes with unclear functions. Note that some genes with unknown functions—oppA1, cyp-fd, orf12, and oppA2—are essential for clavulanic acid biosynthesis. The locations of genes oppa1 and oppA2 are indicated above the diagram. (B) Clavulanic acid biosynthesis pathway. The names and chemical structures of the intermediate compounds are given. The enzymes and genes involved in each step are shown.
Two genes encoding orphan oligopeptide-binding proteins, oppA1 and oppA2, are located in the S. clavuligerus clavulanic acid gene cluster (13, 14) (Fig. 1A). These orphan OppA proteins are likely to be substrate-binding proteins rather than components of a complete ABC transport system, since the oppB-to-oppE genes, forming the corresponding ABC transport system, are not located close to oppA1 or oppA2. However, genes for complete ABC transporter systems are present elsewhere in the S. clavuligerus genome. OppA1 and OppA2 are both essential for CA production but appear to have different substrate-binding specificities and functions, since they cannot replace each other when expressed in the other gene-disrupted mutant (15).
Crystal structures of the oligopeptide-binding proteins complexed with tripeptide and tetrapeptide ligands have been obtained (16). The crystal structure of S. clavuligerus OppA2 binds di- or tripeptides containing arginine, a CA precursor, and arginine-containing complex molecules (17). Since all the oppA2 mutants studied secrete the arginine-derived peptide N-acetylglycyl-clavaminic acid (AGCA) (14, 15), we hypothesize that this compound might be a ligand for OppA2 and an intermediate of the CA pathway. Therefore, it was important to know whether the lack of CA formation was due to an effect of OppA2 on the transcription of the clavulanic acid biosynthesis genes or to a role of OppA2 in the cellular localization of AGCA and its conversion to clavulanic acid.
The characterization of the OppA2 function was hampered by the recent finding that the mutant S. clavuligerus oppA2::aph (15), now renamed S. clavuligerus oppA2::aph pSCLlow, contains lower copy numbers of plasmids pSCL2 and pSCL4 in the mycelium than the wild-type S. clavuligerus strain ATCC 27064 (18). Plasmid pSCL4 (1.8 Mb) is an important reservoir of secondary metabolite gene clusters, whereas pSCL1 (11 kb) and pSCL2 (120 kb) are not so well described (19). Therefore, a new strain with oppA2 deleted, but carrying standard copy numbers of plasmids pSCL1, pSCL2 and pSCL4, was constructed (18) and is designated S. clavuligerus ΔoppA2::aac (referred to as S. clavuligerus ΔoppA2::aac pSCL4+ in reference 18). Both S. clavuligerus oppA2::aph pSCLlow and S. clavuligerus ΔoppA2::aac overproduce the yellow antibiotic holomycin, which is encoded by the holomycin gene cluster (20, 21).
This work presents a transcriptomic study of S. clavuligerus ΔoppA2::aac to elucidate the effect of the oppA2 mutation on the expression of the CA biosynthesis genes and to prove, through cosynthesis assays, that AGCA is an intermediate in the clavulanic acid pathway.
RESULTS
Characteristics of the oppA2 gene and its encoded protein.
The oppA2 gene, located in the clavulanic acid gene cluster, encodes an oligopeptide-binding orphan protein similar to those of ABC systems. OppA2 has 562 amino acids and possesses 75% and 81% identity with the homologous OppA2 proteins from Saccharomonospora viridis (WP_015787617.1, Svir_33430) and Streptomyces flavogriseus (WP_014152681.1, Sfla_0541), encoded by genes located in the clavulanic acid clusters of these actinomycetes (22). The amino acid identity between OppA2 and OppA1 (SCLAV_4192), also located in the S. clavuligerus clavulanic acid gene cluster, is 49%, whereas the amino acid identity decreases to 29 to 41% when OppA2 is compared with other oligopeptide transporters, such as those encoded by SCLAV_3976 (BldKB), SCLAV_4035, and SCLAV_4406, all of them genes located in complete ABC transporter clusters in the S. clavuligerus genome, suggesting that OppA2 is a protein specific for clavulanic acid biosynthesis.
Transcriptomic analysis of S. clavuligerus ΔoppA2::aac compared to its parental strain, S. clavuligerus ATCC 27064.
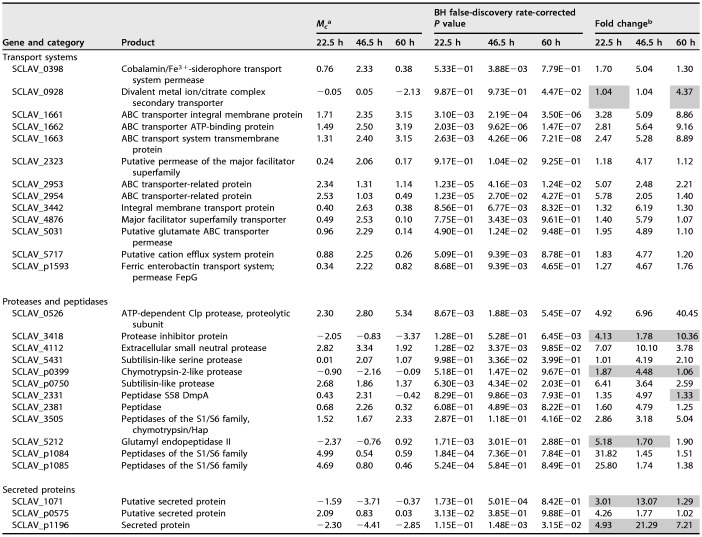
A comparative transcriptomic analysis of S. clavuligerus ΔoppA2::aac and the parental strain S. clavuligerus ATCC 27064 was carried out to determine the effect of OppA2 on gene transcription. Three percent of the genes analyzed showed statistically significant differences in their expression (233 genes of the 7,589 present in the microarray). Those genes with Mc values (binary log values of the differential transcription between the mutant and the wild-type strain) greater than 2 or less than −2 for at least one of the sampling times and with statistically significant changes in expression levels (Benjamini-Hochberg [BH] false-discovery rate-corrected P value, <0.05) were considered in the study. The differentially expressed genes were classified as follows: (i) 28 genes encoding transport component systems, secreted proteins, peptidases, or proteases, (ii) 10 regulatory genes, (iii) 45 genes located in secondary metabolite gene clusters, (iv) 79 genes with diverse functions, and (v) 71 genes encoding hypothetical proteins (see Table S1 in the supplemental material).
Genes encoding transport systems, secreted proteins, proteases, or peptidases whose expression is affected in the oppA2 mutant.
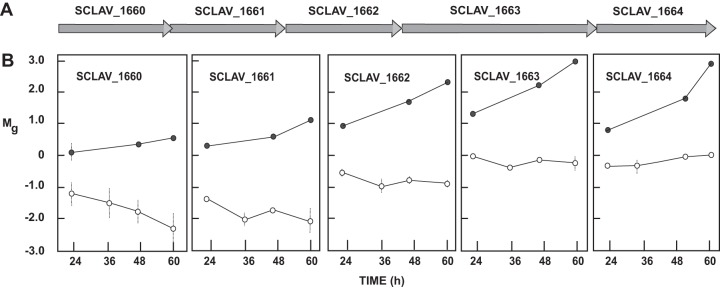
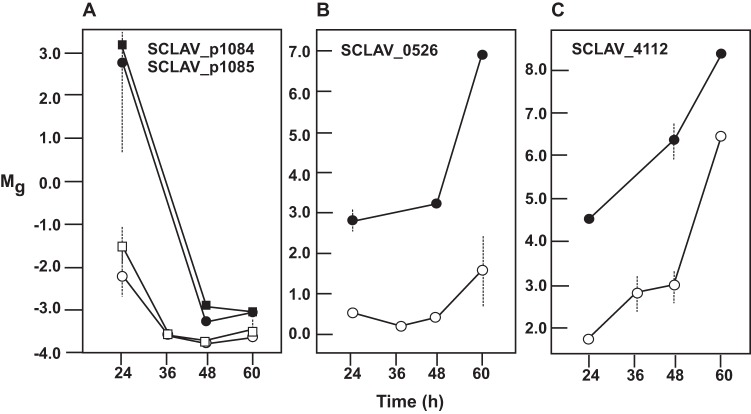
The lack of the orphan peptide-binding protein OppA2 upregulates 12 genes encoding components for transport systems (Table 1) in the S. clavuligerus ΔoppA2::aac mutant at the three sampling times used and downregulates SCLAV_0928, a divalent metal ion/citrate complex secondary transporter. Six of the upregulated genes encode members of ABC transport systems. The clustered genes SCLAV_2953 and SCLAV_2954, coding for a peptide transport system, showed the highest upregulation (5.1- and 5.8-fold, respectively). Likewise, the changes in the expression of genes SCLAV_1661 to -1663, coding for an ABC transport system, increased steadily in the oppA2 mutant strain, as high as 9-fold over expression in the control strain (Fig. 2). A group of 15 genes differentially transcribed in the oppA2 mutant encode secreted proteins, proteases, or peptidases (Table 1). All these genes were up- or downregulated in S. clavuligerus ΔoppA2::aac at least 4-fold at some sampling time. Three of these genes (SCLAV_3505, SCLAV_p1084, and SCLAV_p1085) encode peptidases of the S1/S6 family. The last two genes (as well as SCLAV_p1086 and SCLAV_p1087, encoding hypothetical proteins) are located upstream of res2, in the clavulanic acid paralogous gene cluster, and showed 32- and 26-fold-higher expression, respectively, in the oppA2 mutant than in the wild-type strain in the early-exponential phase (Fig. 3A). The role of these peptidases in the CA paralogous gene cluster is unknown, but their strong upregulation suggests a linkage between OppA2 and the CA paralogous gene cluster. A strong upregulatory transcriptional effect was observed for a gene encoding the ATP-dependent Clp protease subunit (SCLAV_0526 [Fig. 3B]) and the adjacent SCLAV_0527 gene (not shown), especially at stationary phase (40-fold increase), but no significant effect was observed for genes encoding other components of the Clp complex (SCLAV_1784 to SCLAV_1786). Also upregulated were the genes encoding the extracellular neutral protease (SCLAV_4112 [Fig. 3C]) and the subtilisin-like protease (SCLAV_p0750), which were expressed at some sampling times at levels 10.1- and 6.4-fold higher than those in the wild-type strain, respectively (Table 1). Coregulated expression of independent substrate-binding proteins and secreted proteins has been described previously (23).
TABLE 1.
Genes differentially transcribed in S. clavuligerus ΔoppA2::aac and S. clavuligerus ATCC 27064
The transcription values in S. clavuligerus ATCC 27064 are taken as 1.0.
Shaded values correspond to genes that were downregulated at the indicated times.
FIG 2.
Transcriptomic pattern of the SCLAV_1660-to-SCLAV_1664 gene cluster. (A) Organization of the genes in the cluster. (B) Transcriptomic levels (Mg values) of the genes SCLAV_1660 to SCLAV_1664 in S. clavuligerus ATCC 27064 (open circles) and S. clavuligerus ΔoppA2::aac (filled circles). Vertical lines show standard deviations.
FIG 3.
Transcriptomic patterns of genes encoding peptidases and proteases in S. clavuligerus ATCC 27064 (open symbols) and S. clavuligerus ΔoppA2::aac (filled symbols). (A) Transcriptomic levels (Mg values) of SCLAV_p1084 (circles) and SCLAV_p1085 (squares), encoding type S1/S6 peptidases. (B) Mg values of SCLAV_0526, encoding the ATP-dependent Clp protease. (C) Mg values of SCLAV_4112, encoding an extracellular neutral protease. Vertical lines show standard deviations.
Transcription of antibiotic biosynthesis gene clusters in the strain lacking OppA2.
S. clavuligerus oppA2-null mutants do not produce clavulanic acid, behave like the wild-type strain in relation to cephamycin C production, and produce large amounts of holomycin (14, 15). The expression of the genes encoding these antibiotics was analyzed in the wild-type strain and the mutant S. clavuligerus ΔoppA2::aac.
Clavulanic acid and clavam gene clusters.
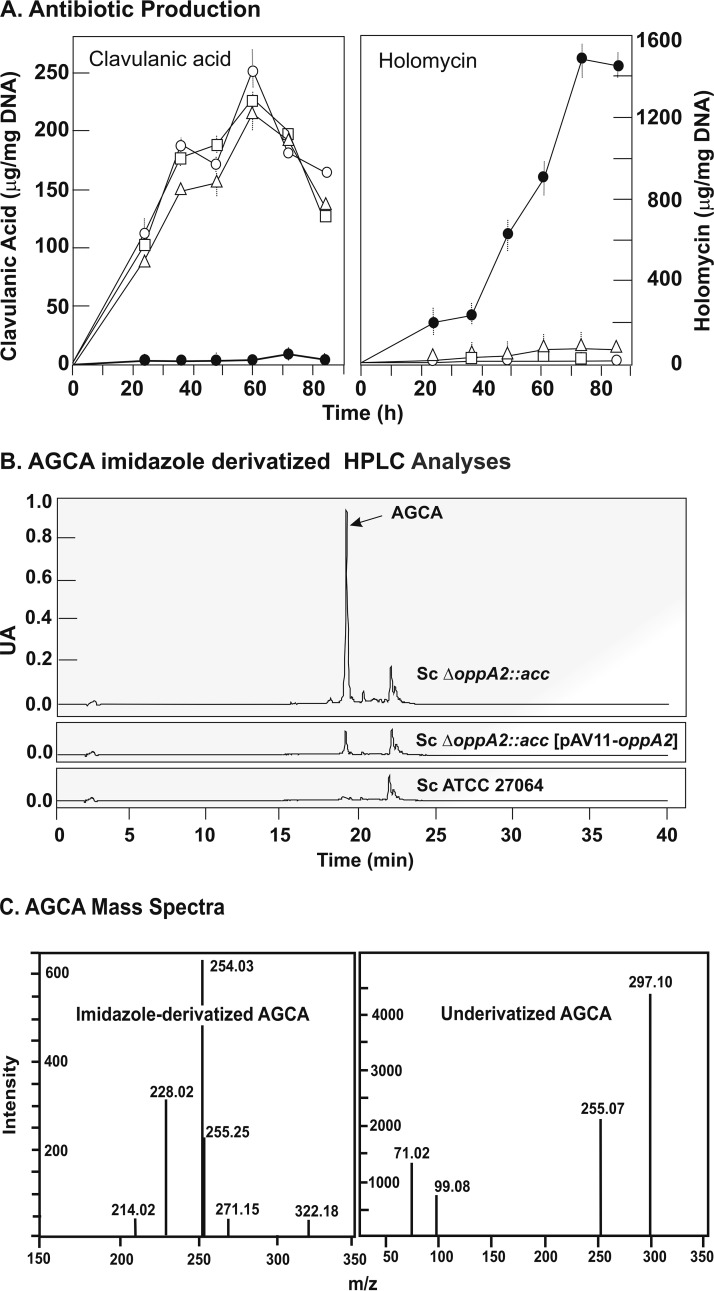
The lack of OppA2 completely blocked clavulanic acid production in the mutant S. clavuligerus ΔoppA2::aac (Fig. 4A, left). The transcription of oppA2 was null in the oppA2 deletion mutant (Table 2); however, the expression of the upstream gene (orf16) that is cotranscribed with oppA2 (24) was not significantly affected. Similar expression of orf14 in the wild-type strain and the oppA2 mutant (Table 2) was also observed, ruling out the possibility that oppA2 deletion exerted a polar effect on orf14 expression; this was also confirmed by complementation experiments of the oppA2 mutation (see below). The BH false-discovery rate-corrected P values of <0.05 for the 18 genes of the CA cluster indicate that there were no significant transcriptional differences due to the lack of OppA2 (Table 2). In summary, all the evidence indicates that the OppA2 protein does not exert transcriptional control on the CA gene cluster, although the mutant did not produce CA (Fig. 4A, left).
FIG 4.
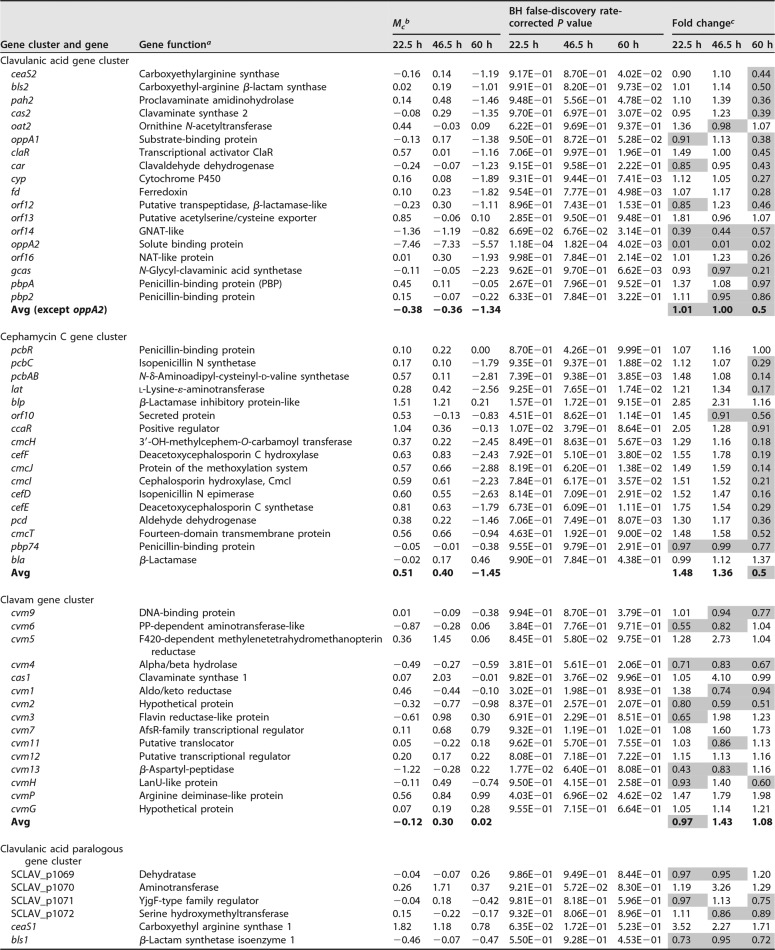
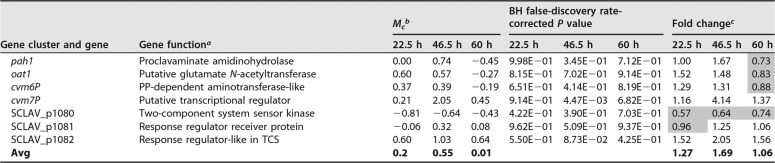
Effects of the oppA2 complementation in S. clavuligerus oppA2::acc[pAV11-oppA2]. (A) Production of antibiotics by S. clavuligerus ATCC 27064 (open circles), S. clavuligerus ΔoppA2::aac (filled circles), S. clavuligerus oppA2::acc[pAV11-oppA2] (triangles), and S. clavuligerus ΔoppA2::aac[pAV11] (squares). (Left) Production of clavulanic acid; (right) production of holomycin. (B) HPLC analysis of AGCA formation by the distinct strains. The name of each strain is given beside the HPLC pattern. The AGCA peak is indicated by an arrow. (C) Mass spectra of imidazole-derivatized AGCA (left) and of underivatized AGCA (right).
TABLE 2.
Transcriptomic data for genes of the clavulanic acid, cephamycin C, clavam, and clavulanic acid paralogous gene clusters in S. clavuligerus ΔoppA2::acc versus S. clavuligerus ATCC 27064
PP, pyridoxal phosphate; TCS, two-component systems.
The transcription values in S. clavuligerus ATCC 27064 are taken as 1.0.
Values shaded in gray correspond to downregulated genes at the indicated times.
No significant differences in the transcription level of the chromosomal clavam gene cluster (SCLAV_2922 to SCLAV_2929) or of the pSCL4-located clavulanic acid paralogous gene cluster (SCLAV_p1070 to SCLAV_p1082) were observed (Table 2), although the expression of SCLAV_p1079, encoding the transcriptional regulator Cvm7P, increased 4.1-fold at the exponential-growth phase.
Cephamycin C gene cluster.
The differences in the expression of 16 genes (pcbR to pbp74; SCLAV_4198 to SCLAV_4215) of the cephamycin gene cluster between the two strains were quite low; on average, 1.5- and 1.3-fold increases in transcription at the early-exponential and exponential phases, and 2.3-fold decreases at the stationary-growth phase, were observed (Table 2). The most affected genes were those coding for isopenicillin N epimerase, deacetylcephalosporin C hydroxylase, and C-7 cephalosporin hydroxylase (cefD, cefE, cmcI), which were downregulated 5- to 7-fold at the stationary-growth phase. This slight effect of OppA2 on the expression of the cephamycin C genes agrees with the similar levels of cephamycin C production by the oppA2 mutant and the wild-type strain that were observed in this work (average, 80% production with respect to the wild-type strain at the different sampling times), and by other authors (14, 25).
The lack of OppA2 strongly upregulates the holomycin gene cluster.
Holomycin is not detectable in cultures of the wild-type strain, S. clavuligerus ATCC 27064, whereas S. clavuligerus ΔoppA2::aac, as also reported for other oppA2-null mutants, produced large amounts of holomycin (15) (see Fig. S1 in the supplemental material). This also occurs in strains carrying oppA2 but lacking plasmid pSCL4 (18, 26, 27). In this work, the expression of the genes for holomycin biosynthesis (hlm) in S. clavuligerus ATCC 27064 and S. clavuligerus ΔoppA2::aac, with oppA2 deleted, is compared; in addition, the S. clavuligerus pSCL4− mutant, which carries a normal oppA2 gene but lacks plasmid pSCL4, and the double mutant S. clavuligerus ΔoppA2::aac pSCL4−, which lacks both oppA2 and plasmid pSCL4, were included in the study (Table 3). All the hlm genes were upregulated in the three mutant strains. The upregulation was higher at the exponential phase of growth, coinciding with the holomycin production phase, and also at the stationary phase in the double mutant (Fig. S1).
TABLE 3.
Effect of the lack of OppA2, the lack of the pSCL4 plasmid, or the lack of both pSCL4 and OppA2 on the transcription of the hlm genes for holomycin biosynthesisa
| Mutant | Gene |
Mc |
BH false-discovery rate-corrected P value |
Fold change |
Gene functionb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 22.5 h | 46.5 h | 60 h | 22.5 h | 46.5 h | 60 h | 22.5 h | 46.5 h | 60 h | |||
| S. clavuligerus ΔoppA2::aac | hlmA | 0.93 | 0.59 | 0.58 | 8.1E−01 | 8.2E−01 | 8.7E−01 | 1.90 | 1.51 | 1.50 | Acyltransferase |
| hlmB | 1.19 | 1.54 | 0.48 | 6.9E−01 | 3.5E−01 | 8.8E−01 | 2.28 | 2.92 | 1.39 | Acyl-CoA dehydrogenase | |
| hlmC | 0.72 | 0.62 | 0.39 | 8.5E−01 | 7.8E−01 | 9.1E−01 | 1.64 | 1.54 | 1.31 | Thiosterase | |
| hlmD | 0.76 | 0.25 | 0.58 | 8.3E−01 | 9.2E−01 | 8.5E−01 | 1.69 | 1.19 | 1.49 | Oxidoreductase | |
| hlmE | 0.79 | 0.41 | 063 | 8.4E−01 | 8.8E−01 | 8.5E−01 | 1.73 | 1.33 | 1.54 | NRPS | |
| hlmF | 1.34 | 0.42 | 0.45 | 7.5E−01 | 9.0E−01 | 9.2E−01 | 2.52 | 1.34 | 1.37 | Cys-decarboxylase like | |
| hlmG | 0.56 | 0.18 | 0.47 | 9.0E−01 | 9.5E−01 | 8.9E−01 | 1.48 | 1.13 | 1.39 | Globin | |
| hlmH | 1.91 | 0.98 | 0.53 | 4.4E−01 | 6.3E−01 | 8.8E−01 | 3.76 | 1.97 | 1.45 | MFS transporter | |
| hlmI | 1.44 | 0.83 | 0.68 | 6.7E−01 | 6.9E−01 | 8.3E−01 | 2.72 | 1.78 | 1.60 | Thioredoxin reductase | |
| hlmK | 0.50 | 1.93 | 3.07 | 9.1E−01 | 2.5E−01 | 1.3E−01 | 1.42 | 3.80 | 8.35 | Thiosterase domain | |
| hlmL | 1.77 | 0.61 | 0.37 | 1.8E−03 | 2.7E−01 | 6.7E−01 | 3.42 | 1.52 | 1.29 | Condensation domain | |
| hlmM | 1.67 | 0.42 | 0.15 | 6.8E−03 | 5.4E−01 | 9.1E−01 | 3.18 | 1.33 | 1.11 | LuxR-type regulator | |
| S. clavuligerus pSCL4− | hlmA | 3.60 | 4.01 | 2.78 | 1.27E−02 | 4.34E−03 | 3.95E−02 | 12.13 | 16.12 | 6.84 | Acyltransferase |
| hlmB | 2.44 | 3.01 | 0.99 | 6.47E−02 | 1.62E−02 | 4.75E−01 | 5.42 | 8.03 | 1.99 | Acyl-CoA dehydrogenase | |
| hlmC | 3.72 | 4.49 | 2.23 | 4.72E−03 | 6.43E−04 | 6.86E−02 | 13.19 | 22.44 | 4.69 | Thiosterase | |
| hlmD | 3.52 | 4.08 | 2.30 | 6.29E−03 | 1.34E−03 | 5.47E−02 | 11.45 | 16.92 | 4.92 | Oxidoreductase | |
| hlmE | 4.06 | 4.80 | 2.99 | 3.38E−03 | 5.13E−04 | 1.89E−02 | 16.62 | 27.79 | 7.94 | NRPS | |
| hlmF | 4.33 | 5.17 | 3.24 | 1.06E−02 | 1.96E−03 | 4.05E−02 | 20.15 | 36.11 | 9.42 | Cys-decarboxylase like | |
| hlmG | 4.23 | 4.87 | 3.09 | 2.63E−03 | 5.09E−04 | 1.67E−02 | 18.77 | 29.15 | 8.53 | Globin | |
| hlmH | 3.37 | 5.10 | 3.87 | 1.51E−02 | 3.10E−04 | 3.21E−03 | 10.31 | 34.35 | 14.64 | MFS transporter | |
| hlmI | 3.57 | 5.46 | 3.84 | 8.91E−03 | 1.22E−04 | 3.01E−03 | 11.84 | 44.08 | 14.28 | Thioredoxin reductase | |
| hlmK | 2.05 | 2.57 | 1.16 | 1.49E−01 | 4.82E−02 | 4.11E−01 | 4.14 | 5.93 | 2.24 | Thiosterase domain | |
| hlmL | 0.82 | 1.18 | 1.26 | 6.94E−02 | 6.63E−03 | 3.00E−03 | 1.77 | 2.26 | 2.40 | Condensation domain | |
| hlmM | 0.88 | 1.75 | 1.25 | 7.32E−02 | 3.78E−04 | 5.98E−03 | 1.84 | 3.36 | 2.38 | LuxR-type regulator | |
| S. clavuligerus ΔoppA2::aac pSCL4− | hlmA | 6.17 | 3.03 | 7.06 | 4.40E−02 | 7.10E−01 | 7.80E−02 | 72.16 | 8.16 | 133.56 | Acyltransferase |
| hlmB | 5.14 | 2.67 | 4.90 | 3.40E−02 | 9.20E−01 | 7.70E−02 | 35.30 | 6.38 | 29.88 | Acyl-CoA dehydrogenase | |
| hlmC | 5.69 | 3.25 | 6.10 | 5.10E−01 | 5.50E−01 | 7.80E−02 | 51.78 | 9.49 | 68.76 | Thiosterase | |
| hlmD | 5.73 | 2.92 | 5.79 | 4.50E−01 | 5.80E−01 | 9.80E−02 | 53.21 | 7.56 | 55.47 | Oxidoreductase | |
| hlmE | 6.24 | 3.30 | 6.75 | 4.80E−02 | 4.60E−01 | 9.60E−02 | 75.48 | 9.86 | 108.00 | NRPS | |
| hlmF | 7.12 | 3.97 | 7.53 | 4.70E−02 | 6.90E−01 | 1.30E−01 | 138.63 | 15.64 | 185.17 | Cys-decarboxylase like | |
| hlmG | 5.64 | 3.21 | 6.10 | 7.20E−03 | 4.00E−01 | 2.40E−01 | 49.88 | 9.24 | 68.39 | Globin | |
| hlmH | 4.77 | 2.89 | 6.54 | 7.20E−01 | 2.00E−01 | 3.40E−01 | 27.35 | 7.39 | 92.89 | MFS transporter | |
| hlmI | 4.49 | 3.24 | 7.13 | 8.60E−01 | 1.90E−01 | 1.60E−01 | 22.47 | 9.47 | 140.36 | Thioredoxin reductase | |
| hlmK | 3.61 | 2.23 | 4.16 | 6.70E−01 | 9.30E−03 | 2.20E−01 | 12.23 | 4.71 | 17.89 | Thiosterase domain | |
| hlmL | 1.79 | 1.29 | 2.41 | 3.20E−01 | 2.30E−03 | 1.30E−01 | 3.45 | 2.44 | 5.30 | Condensation domain | |
| hlmM | 1.89 | 1.16 | 3.00 | 3.40E−02 | 3.90E−01 | 3.30E−02 | 3.70 | 2.24 | 8.02 | LuxR-type regulator | |
The hlmA-to-hlmM genes correspond to SCLAV_5267 to SCLAV_5278 in the StrepDB database. Values for the mutants are given relative to those for S. clavuligerus ATCC 27064, taken as 1.0.
Acyl-CoA, acyl coenzyme A; NRPS, nonribosomal peptide synthetase; MFS, major facilitator superfamily.
In S. clavuligerus ΔoppA2::aac, the increase in the transcription level of the hlmM gene, encoding a LuxR-type regulator, was 3.2-fold at the early-exponential phase. In this strain, the average global transcriptional increase of all hlm genes at all the sampling times was 2-fold. The highest changes were detected for hlmK, encoding a thiosterase (8.3-fold); hlmH, encoding a major facilitator superfamily (MFS) transporter (3.7-fold); and hlmL, encoding a condensation domain (3.4-fold) (Table 3).
The lack of plasmid pSCL4 resulted in a greater effect on hlm gene expression than seen with the oppA2-deleted strain (Table 3). In S. clavuligerus pSCL4−, the average transcriptional level for the 12 hlm genes, considering all the sampling times, was 12.6-fold higher than that in the wild-type strain, and hlmF and hlmI (encoding a cysteine decarboxylase-like protein and a thioredoxin disulfide reductase) were the most affected genes (36- and 44-fold increases, respectively).
A synergistic effect on holomycin biosynthesis was observed for the lack of pSCL4 in an oppA2-null background. The expression of hlmM increased to 8.2-fold at 60 h, and the average expression of all the genes in S. clavuligerus ΔoppA2::aac pSCL4−, considering all sampling times, was 43-fold, with hlmF and hlmI transcription expression levels increasing to 185- and 140-fold at 60 h, respectively (Table 3). This additive effect of the lack of OppA2 and plasmid pSCL4 on the transcription of hlm genes fits well with the level of holomycin production by strains lacking either pSCL4 or OppA2 and by the double mutant (18) (Fig. S1 in the supplemental material).
Characterization of S. clavuligerus ΔoppA2::acc[pAV11-oppA2], a strain complemented in oppA2.
All strains lacking OppA2 release the peptide N-acetylglycyl-clavaminic acid (AGCA) (14, 15). To confirm that the release of AGCA and the differences on clavulanic acid and holomycin production were due to the lack of OppA2, and not to an indirect effect on the transcription of orf14, located downstream of oppA2, the S. clavuligerus ΔoppA2::acc mutant was complemented with plasmid pAV11-oppA2. As shown in Fig. 4A, the complemented strain S. clavuligerus ΔoppA2::acc[pAV11-oppA2] restored clavulanic acid production (left panel) to 85% of the level of S. clavuligerus ATCC 27064 and reduced holomycin production to levels similar to those of the wild-type strain (right panel). The AGCA peptide was extracted from the broth of the three cultures. High-performance liquid chromatography (HPLC) analysis of imidazole-derivatized AGCA extracts showed the presence of a single peak absorbing at 311 nm, with a retention time (Rt) of 18.3 min, in S. clavuligerus ΔoppA2::acc extracts (Fig. 4B), as described previously for AGCA (14). The level of AGCA in cultures of S. clavuligerus ΔoppA2::acc[pAV11-oppA2] was reduced to 13% of the level in the noncomplemented strain, and AGCA was almost undetectable in extracts of the wild-type culture broth.
Purification and characterization of AGCA.
To confirm that the peak with an Rt of 18.3 min was AGCA, this compound was purified from S. clavuligerus ΔoppA2::aac broth. The purified compound, when derivatized with imidazole, gave a mass spectrum identical to that published previously (14), with a major fragment of 254 m/z (Fig. 4C, left). To avoid interference by imidazole with the interpretation of the fragments, the mass spectrum of underivatized AGCA was obtained (Fig. 4C, right); AGCA showed a major peak of 297.10 m/z corresponding to the AGCA molecule, and a fragment of 255.07 m/z, which might correspond to the molecule lacking the -COCH3 group.
Clavulanic acid cosynthesis by nonproducer strains.
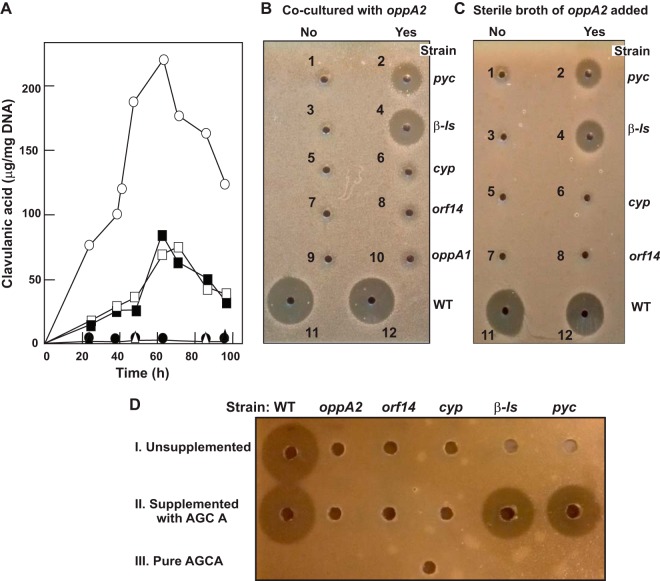
Clavulanic acid could not be detected in the broth or the mycelium of S. clavuligerus ΔoppA2::aac. Since the lack of OppA2 prevented CA biosynthesis but did not exert a significant effect on the expression of the clavulanic acid gene cluster, a study was carried out to determine whether the lack of CA production by S. clavuligerus ΔoppA2::aac was related to the AGCA release reported for oppA2 mutants (14, 15). To assess whether the AGCA could be used by other CA-negative mutants to produce clavulanic acid, cosynthesis assays were carried out with S. clavuligerus ΔoppA2::aac and the non-clavulanic acid-producing strains S. clavuligerus pyc::aph, S. clavuligerus RFL35, S. clavuligerus cyp::aph, S. clavuligerus oppA1::aac, and S. clavuligerus orf14::aac (a medium-dependent nonproducer mutant). Solid cosynthesis assays, in which the strains never came in contact, resulted in an almost undetectable inhibition halo, which was observed only in the strip carrying the plates seeded with S. clavuligerus ΔoppA2::aac and S. clavuligerus pyc::aph (not shown). Since the diffusion of intermediates in cosynthesis in solid medium might limit the complementation studies, cosynthesis assays were carried out using the liquid medium method, as summarized in Table 4. Clear clavulanic acid formation was already observed at 20 h of growth when S. clavuligerus pyc::aph or S. clavuligerus RFL35 was cocultured with S. clavuligerus ΔoppA2::aac (Fig. 5A). CA formation in mixed cultures was maximal at 60 h of growth and reached about 37% of the level produced by the wild-type strain. Conversely, mixed cultures of S. clavuligerus ΔoppA2::aac with S. clavuligerus cyp::aph, S. clavuligerus orf14::aac, or S. clavuligerus oppA1::aac did not result in clavulanic acid production (Fig. 5B, assays 6, 8, and 10). The inhibition zone produced by CA in the mixed cultures (Fig. 5B, assays 2 and 4) was clearly different from the small, diffuse inhibition zones due to holomycin (Fig. 5A, assays 6, 8, and 10), an antibiotic produced only by S. clavuligerus ΔoppA2::aac, which was very weakly active on Klebsiella pneumoniae growth. Mixed cultures of S. clavuligerus ΔoppA2::aac with S. clavuligerus cyp::aph, S. clavuligerus orf14::aac, or S. clavuligerus oppA1::aac did not produce clavulanic acid (Fig. 5B, assays 6, 8, and 10).
TABLE 4.
Cosynthesis assays in liquid SA mediuma
| Cosynthesis assaya | Strain (gene mutated)b |
Clavulanic acid bioassay resultc | |
|---|---|---|---|
| Donor | Receptor | ||
| 1 | S. clavuligerus ΔoppA2::acc (oppA2) | S. clavuligerus pyc::aph (ceaS) | ++++ |
| 2 | S. clavuligerus ΔoppA2::acc (oppA2) | S. clavuligerus RFL35 (bls2) | ++++ |
| 3 | S. clavuligerus ΔoppA2::acc (oppA2) | S. clavuligerus orf14::aph | −−−−− |
| 4 | S. clavuligerus ΔoppA2::acc (oppA2) | S. clavuligerus cyp::aph (cyp) | −−−−− |
| 5 | S. clavuligerus ΔoppA2::acc (oppA2) | S. clavuligerus oppA1::aph (oppA1) | −−−−− |
| 6 | S. clavuligerus cyp::aph (cyp) | S. clavuligerus pyc::aph (ceaS) | −−−−− |
| 7 | S. clavuligerus cyp::aph (cyp) | S. clavuligerus RFL35 (bls2) | −−−−− |
| 8 | S. clavuligerus pyc::aph (ceaS) | S. clavuligerus ΔoppA2::acc (oppA2) | −−−−− |
| 9 | S. clavuligerus RFL35 (bls2) | S. clavuligerus ΔoppA2::acc (oppA2) | −−−−− |
Assays 1 to 5 were also carried out using mixed cultures of both strains. The complementation results in the bioassay were identical.
Donor strains were from spent sterile supernatants; receptor strains were from growing cultures.
++++, positive cosynthesis; −−−−−, negative cosynthesis. CA bioassays of each separated strain were always negative. None of the strains shown in this table produces clavulanic acid by itself. All the strains produced cephamycin C.
FIG 5.
Clavulanic acid cosynthesis by nonproducer strains. (A) Liquid culture cosynthesis. Shown is clavulanic acid production by S. clavuligerus ATCC 27064 (open circles), S. clavuligerus ΔoppA2::aac (filled circles), S. clavuligerus pyc::aph (filled triangles), and S. clavuligerus RFL35 (open triangles), as well as by cocultures of S. clavuligerus pyc::aph and S. clavuligerus ΔoppA2::aac (filled squares) and cocultures of S. clavuligerus RFL35 and S. clavuligerus ΔoppA2::aac (open squares). (B) Bioassays of the 60-h samples of the pure liquid-medium cultures of the strains used in the assays for which results are shown in panel A (left side) and cocultures of the same strains with S. clavuligerus ΔoppA2::acc (right side). The strains used in the culture are S. clavuligerus pyc::aph (assays 1 and 2), S. clavuligerus RFL35 (assays 3 and 4), S. clavuligerus cyp::aph (assays 5 and 6), S. clavuligerus orf14::aph (assays 7 and 8), S. clavuligerus oppA1::aac (assays 9 and 10), and S. clavuligerus ATCC 27064 (assays 11 and 12). Notice the large inhibition zone due to CA formation (assays 2 and 4) and the small diffuse inhibition zones (assays 6, 8, and 10) due to the effect of holomycin on the Klebsiella-seeded plates. (C) Cosynthesis by addition of filtered sterile broth of S. clavuligerus ΔoppA2::aac. Shown are results of bioassays of the 60-h samples of the pure liquid-medium cultures of strains (left side) and the effects of adding 50 ml of the filtered sterile spent broth of an S. clavuligerus ΔoppA2::acc culture (right side). The strains used are S. clavuligerus pyc::aph (assays 1 and 2), S. clavuligerus RFL35 (assays 3 and 4), S. clavuligerus cyp::aph (assays 5 and 6), S. clavuligerus orf14::aph (assays 7 and 8), and S. clavuligerus ATCC 27064 (assays 11 and 12). (D) Clavulanic acid production by cultures supplemented with pure AGCA. (I) Unsupplemented cultures; (II) cultures supplemented with AGCA (1 mM); (III) pure AGCA (1 mM). The strains used were S. clavuligerus ATCC 27064 (WT), S. clavuligerus ΔoppA2::aac (oppA2), S. clavuligerus orf14::aph (orf14), S. clavuligerus cyp::aph (cyp), S. clavuligerus RFL35 (β-ls), and S. clavuligerus pyc::aph (pyc).
The bioactivity shown in Fig. 5B (assays 2 and 4) suggested that a metabolite was transferred from a donor strain and converted to clavulanic acid by an acceptor. The donor and acceptor strains in the cosynthesis assays were characterized by interchanging the culture broth between pairs of strains. Spent filter-sterilized broth from S. clavuligerus ΔoppA2::aac cultures was added to the mycelium of each of the non-CA-producing strains, and the cultures were kept growing for 24 h. No bioactivity was detected in unsupplemented cultures of any of the strains (Fig. 5C, assays 1, 3, 5, and 7). A clear inhibition zone was observed in supplemented cultures of S. clavuligerus pyc::aph and S. clavuligerus RFL35 (Fig. 5C, assays 2 and 4) but not in supplemented cultures of S. clavuligerus cyp::aph and S. clavuligerus orf14::aac (Fig. 5C, assays 6 and 8). The reciprocal assay in which filter-sterilized broth of either S. clavuligerus pyc::aph or S. clavuligerus RFL35 was added to S. clavuligerus ΔoppA2::aac mycelium (not shown) did not result in CA formation, thus confirming that S. clavuligerus ΔoppA2::aac was the donor strain and S. clavuligerus pyc::aph or S. clavuligerus RFL35 was the converter strain.
The intermediate released by the donor strain S. clavuligerus ΔoppA2::aac and taken by the converter strains might be AGCA, which is released into the broth by the former strain. Indeed, cultures of S. clavuligerus pyc::aph or S. clavuligerus RFL35 did not form clavulanic acid when supplemented with the broth of a strain that did not accumulate AGCA, such as S. clavuligerus cyp::aph (not shown).
Production of clavulanic acid by cultures supplemented with pure AGCA.
To confirm that AGCA was responsible for the CA production, pure AGCA was added at 1 mM to cultures of the different non -CA-producing strains used in this work. AGCA itself did not show bioactivity in the clavulanic acid assay (Fig. 5DIII), nor was the typical yellow color due to holomycin production formed in the cultures (not shown). It can be observed that the wild-type strain produced similar levels of clavulanic acid in the absence of AGCA and after AGCA supplementation (Fig. 5DI and II), indicating that the endogenous supply of this intermediate is not limiting for CA production under the conditions used in this work. CA was not produced by cultures of S. clavuligerus ΔoppA2::aac, S. clavuligerus cyp::aph, or S. clavuligerus orf14::aac (Fig. 5DII). However, S. clavuligerus pyc::aph and S. clavuligerus RFL35 cultures recovered the ability to produce clavulanic acid after AGCA supplementation (5DII) and produced about 40% of the CA produced by the parental strain, confirming that these two early-blocked mutants still have the enzymes for the conversion of AGCA into clavulanic acid.
DISCUSSION
OppA periplasmic proteins are important in chemotaxis and virulence due to their substrate-binding and transport abilities as part of ABC systems (23, 28). In addition, ecto-ATPase activity (29) or chaperone-like functions in protein folding (30) have been described for these proteins.
Hitherto, the role of OppA2, essential for clavulanic acid biosynthesis, has been unclear. In this study, additional information about this oligopeptide permease has been obtained through transcriptomic analysis, cosynthesis assays, and complementation studies.
The expression of 233 genes was significantly affected by the lack of OppA2. The holomycin gene cluster (hlm) was overexpressed, whereas the expression levels of clavulanic acid, cephamycin C, and clavam gene clusters were not affected. The high expression levels of the hlm genes in S. clavuligerus ΔoppA2::aac, which lacks OppA2 but has standard copy numbers of plasmids pSCL1, pSCL2 and pSCL4, are independent of the effect of the lack of the megaplasmid pSCL4, as shown in S. clavuligerus pSCL4− and described previously (18). This suggests two different mechanisms of hlm gene cluster activation. The upregulation of the hlmC, hlmE, and hlmF genes, encoding biosynthetic enzymes, and of hlmH, coding for an MFS transporter, probably responds to the overexpression of the LuxR-type regulator encoded by hlmM. But the expression of hlmM itself might be controlled by other negative regulators. The overproduction of proteases and peptidases in the oppA2 mutant (Table 1) might trigger a proteolysis activity which inactivates negative regulators controlling the holomycin gene cluster, thus releasing holomycin formation. A similar phenomenon generates morphological and physiological differentiation in S. coelicolor in response to alterations in the synthesis of the ATP-dependent protease Clp (31).
The oppA2 transcript dropped to background levels in the oppA2 mutant, but the expression of other clavulanic acid biosynthesis genes remained unaffected; therefore, the lack of the OppA2 protein itself must be responsible for the CA-null production. The CA pathway in the oppA2 mutant must be functional up to the formation of N-glycyl-clavaminic acid (NGCA), by condensation of glycine and clavaminic acid (Fig. 1), since all the genes involved (ceaS2 to gcaS) are transcribed. The N-glycyl-clavaminic acid synthetase (GcaS) does not use N-acetyl-glycine as a cosubstrate (32) to form N-acetyl-glycylclavaminic acid (AGCA); therefore, the AGCA molecule must be generated by acetylation of NGCA (32). The protein encoded by orf14 (SCLAV_4185) is a member of the GCN5-related N-acetyltransferases (GNAT) and carries NAT domains; however, it should act downstream of AGCA formation, since the addition of pure AGCA to the orf14 mutant does not result in clavulanic acid cosynthesis.
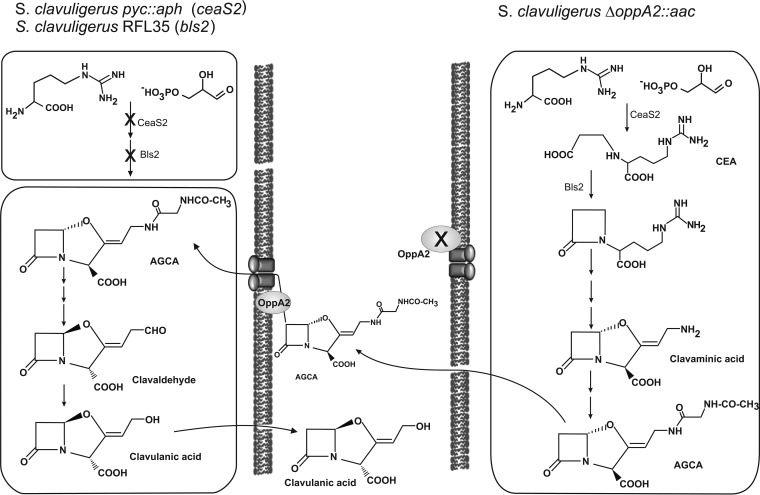
AGCA is structurally similar to N-acetyl-glycyl-arginine, one of the arginine-derived peptides that has been reported to be bound by OppA2 (17). Thus, OppA2 might bind AGCA, carry it to the correct position in the cell, and translocate it to the next enzyme of the pathway, which is determining for the proper conversion of AGCA to clavulanic acid. In the absence of OppA2, the AGCA peptide is released to the medium (14, 15). The positive cosynthesis by S. clavuligerus ΔoppA2::aac and either S. clavuligerus pyc::aph or S. clavuligerus RFL35, in which these mutants blocked in the early steps of the pathway act as converters (receptors), indicates that the AGCA released by S. clavuligerus ΔoppA2::aac (the donor strain) can be bound and correctly located by the OppA2 protein of the receptor strain and then is processed by the enzymes of the late steps of the pathway, which are functional in the two converting receptor strains, to form clavulanic acid (Fig. 6). The CA-null mutants S. clavuligerus cyp::aph, S. clavuligerus orf14::aac, and S. clavuligerus oppA1::aac are unable to form clavulanic acid from AGCA, suggesting that the steps involving the formation of CA from AGCA are affected in these mutants.
FIG 6.
Scheme for clavulanic acid biosynthesis. The model explains the performance of the donor strain, S. clavuligerus ΔoppA2::aac, on the right and the performance of the receptor strains, S. clavuligerus pyc::aph (with ceaS2 disrupted) and S. clavuligerus RFL35 (with bls2 deleted), on the left. The compound transferred in the cosynthesis and the pathway for clavulanic acid formation are shown. Notice the presence of a functional OppA2 protein in each of the receptor strains.
To proceed to form CA, the N-acetyl-glycyl group from AGCA must be released by an oxidative deamination in enzymatic steps that are still obscure. The final step of the pathway is the reduction of the C-5 aldehyde group of clavaldehyde by the clavaldehyde reductase (33) to produce clavulanic acid.
In summary, the existing evidence suggests that clavulanic acid is formed through a modified peptide intermediate, AGCA. This peptide is bound by the OppA2 protein and is probably delivered to a membrane-bound complex formed by the late enzymes of the biosynthetic pathway with the final formation and secretion of clavulanic acid. When pure AGCA, or AGCA present in the broth of the oppA2 mutant, is added to cultures of a receptor strain, the functional OppA2 protein of this converter strain binds AGCA from the medium and proceeds with the formation of clavulanic acid.
MATERIALS AND METHODS
Strains and culture conditions.
The wild-type strain S. clavuligerus ATCC 27064 was used as the control and parental strain. S. clavuligerus ΔoppA2::aac is an oppA2 deletion strain in which the oppA2 gene is replaced by an apramycin resistance cassette; S. clavuligerus pSCL4− carries the wild-type oppA2 gene but lacks the pSCL4 plasmid; S. clavuligerus ΔoppA2::aac pSCL4− is a double mutant in which the oppA2 gene is replaced by an apramycin resistance cassette and the pSCL4 plasmid is lacking. The origin and characteristics of the Streptomyces strains used in this work are shown in Table 5.
TABLE 5.
Characteristics of the Streptomyces strains used in this work
| Strain | Characteristic(s) | Plasmid content | Origin or reference(s) |
|---|---|---|---|
| S. clavuligerus ATCC 27064 | Control strain | Standard copy no. of pSCL1, pSCL2, pSCL4 | ATCC |
| S. clavuligerus ΔoppA2::aac | oppA2 deleted; apramycin resistant; previously named S. clavuligerus ΔoppA2::aac pSCL4+ | Standard copy no. of pSCL1, pSCL2, pSCL4 | 18 |
| S. clavuligerus pSCL4− | Lacking plasmid pSCL4 | Lacking plasmid pSCL4; standard copy no. of pSCL1 and pSCL2 | 18 |
| S. clavuligerus ΔoppA2::aac pSCL4− | oppA2 deleted; apramycin resistant; lacking plasmid pSCL4 | Lacking plasmid pSCL4; standard copy no. of pSCL1 and pSCL2 | 18 |
| S. clavuligerus ΔoppA2::acc[pAV11-oppA2] | oppA2 inserted in plasmid pAV11 to complement the oppA2-deletion in the parental strain; apramycin and hygromycin resistant | Standard copy no. of pSCL1, pSCL2, pSCL4 | This work |
| S. clavuligerus ΔoppA2::acc[pAV11] | oppA2 deleted; apramycin, thiostrepton resistant | Standard copy no. of pSCL1, pSCL2, pSCL4 | This work |
| S. clavuligerus pyc::aph | ceaS2 disrupted; kanamycin resistant | Standard copy no. of pSCL1, pSCL2, pSCL4 | 11, 36 |
| S. clavuligerus RFL35 | blS2 deleted; thiostrepton resistant | Standard copy no. of pSCL1, pSCL2, pSCL4 | 43 |
| S. clavuligerus cyp::aph | cyp disrupted; kanamycin resistant | Standard copy no. of pSCL1, pSCL2, pSCL4 | 25 |
| S. clavuligerus orf14::aph | orf14 disrupted; kanamycin resistant | Standard copy no. of pSCL1, pSCL2, pSCL4 | 25 |
| S. clavuligerus oppA1::aac | oppA1 disrupted; apramycin resistant | Standard copy no. of pSCL1, pSCL2, pSCL4 | 15 |
The strains were grown at 28°C and 220 rpm in 100 ml of tryptic soy broth (TSB) medium to an optical density at 600 nm (OD600) of 5 to 8. A volume of 5 ml was taken, and the mycelium was washed with a 0.9% NaCl solution and was used to inoculate 500-ml baffled flasks containing 100 ml of starch-asparagine (SA) medium (34); the cultures were incubated during growth at the same temperature with shaking. Growth was estimated by measuring the DNA concentration using the diphenylamine assay (35).
In AGCA-feeding experiments, the strains were grown in TSB medium for 24 h. These cultures were used to inoculate 25-ml flasks containing 5 ml of SA medium with an initial OD600 of 0.25. After 36 h, AGCA was added to a final concentration of 1 mM. Samples for clavulanic acid bioassays were taken at 60 h of culture.
Complementation of the oppA2 deletion.
A DNA fragment (2,063 bp) containing the oppA2 gene with its promoter was amplified by PCR using primers CP-oppA2D (ACCGACACCGATCAGAAGAG) and CP-oppA2R (CACCACAGCAGTGTCCAGTC), sequenced, and cloned into the EcoRV site of the conjugative-integrative plasmid pAV11 to produce pAV11-oppA2. The EcoRV site is located downstream of the anhydrotetracycline-inducible promoter tcp-830. Plasmid pAV11-oppA2 was introduced into S. clavuligerus ΔoppA2::acc by Escherichia coli conjugation, and the complemented strain S. clavuligerus ΔoppA2::acc[pAV11-oppA2] was selected by apramycin and hygromycin resistance. As a control, the S. clavuligerus ΔoppA2::acc[pAV11] strain, lacking the oppA2 gene, was constructed. The presence of oppA2 in the complemented strain was confirmed by PCR and sequencing of the amplicon. When required, the complemented strains were grown in the presence of anhydrotetracycline (10 μg/ml).
Antibiotic production.
Antibiotics were assayed from culture supernatants. The clavulanic acid concentration was quantified using Klebsiella pneumoniae ATCC 29665 as described previously (11, 36). Controls without penicillin G in the bioassay were always carried out to confirm that the inhibition zone was due to clavulanic acid. Holomycin was quantified by bioassays using Micrococcus luteus ATCC 9341 or by HPLC as described by de la Fuente et al. (37). Cephamycin C was tested by bioassays using Escherichia coli Ess22-31, which is supersensitive to β-lactam antibiotics (38).
Purification and characterization of AGCA.
Streptomyces clavuligerus ΔoppA2::aac was grown in SA medium for 48 h as described above. The collected broth (total volume, 1,000 ml) was filtered and concentrated to a final volume of 5 ml. The concentrated broth (100 μl) was analyzed by preparative HPLC using a SunFire C18 column (10 μm; 10 by 150 mm; Waters). As the mobile phase, acetonitrile (A) and water containing 0.05% trifluoroacetic acid (TFA) (B) were used under isocratic conditions, 35% A and 65% B, with a flow rate of 5 ml/min. The location of the sample containing AGCA (Rt, 4.5 min) was determined by collecting samples every 0.5 min, derivatizing them with imidazole (39), and then performing HPLC analysis (Rt of imidazole-derivatized AGCA, 18.3 min) and mass spectrometry as described previously (14). Samples were collected from 50 HPLC runs and were lyophilized to obtain 11.8 mg of pure AGCA.
HPLC-MS analyses were done in an Alliance chromatographic system coupled to a ZQ4000 mass spectrometer using an Atlantis T3 column (3 μm; 2.1 by 150 mm; Waters, Milford, MA). Samples (10 μl) were injected and were eluted with 0.1% formic acid in water for 4 min, followed by a linear gradient from 0 to 40% acetonitrile over 16 min at 0.2 ml/min. MS analyses of the underivatized AGCA were carried out by electrospray ionization in negative mode, with a capillary voltage of 3 kV and a cone voltage of 10 V. AGCA was detected by selected ion recording at m/z 297.
Cosynthesis studies.
To study clavulanic acid formation by cosynthesis between S. clavuligerus ΔoppA2::aac and other non-clavulanic acid-producing mutants, two types of experiments were done. First, for solid-medium cosynthesis, the tested strains were grown in close physical proximity, but not in contact, on SA plates for 36 and 48 h. Clavulanic acid formation was detected by a bioassay of a 5-cm strip of the solid culture carrying the ends of each strain culture and the empty central space. Control strips carrying each of the tested strains separately were included in the bioassay. Second, for liquid-medium cosynthesis, S. clavuligerus ΔoppA2::aac (A) and the strain to be studied for cosynthesis (B) were grown separately in 100 ml of TSB medium as described above. The mycelia were washed with 0.9% NaCl, and 2.5% (vol/vol) of each strain mycelium was used to jointly inoculate 500-ml baffled flasks containing 100 ml of SA medium. The mixed cultures were grown for 60 h.
To determine the donor and converter strains in clavulanic acid cosynthesis experiments, S. clavuligerus ΔoppA2::aac (A) and the strain to be studied (B) were grown separately in 100 ml of SA medium for 36 h as described above. From each culture, 50 ml was filtered under sterile conditions through a 0.45-μm-pore-diameter filter; then the filtered spent broth of strain A was added to the culture of strain B, and vice versa, keeping a final 100-ml volume for each culture. The strains were allowed to grow for an additional 24 h. As a control, separated cultures (strains A and B) were used, in which no interchange of the supernatant was performed.
RNA extraction and purification.
RNA was extracted from samples taken at the early-exponential, exponential, and stationary phases of growth (22.5, 46.5, and 60 h). An additional sample of the wild-type strain was taken at 36 h. For RNA isolation, samples from S. clavuligerus ATCC 27064, S. clavuligerus ΔoppA2::aac, S. clavuligerus ΔoppA2::aac pSCL4−, and S. clavuligerus pSCL4− cultures in SA medium were taken at 22.5 h, 46.5 h, and 60 h and were stabilized with 2 volumes of RNAprotect Bacteria reagent (Qiagen) for 5 min, and finally 150 μl lysozyme (30 mg/ml) in Tris-EDTA (TE) buffer was added to the stabilized mycelium pellet of 1 ml of culture samples. After 10 min, 600 μl buffer RLT (Qiagen) with β-mercaptoethanol was added to the samples, which were then mixed by vortexing, transferred to Lysing Matrix B (MP Biomedicals) microtubes, and processed in a FastPrep instrument (MP Biomedicals) with the following program: 30 s at 6.5 m/s; 1 min at 0°C; 30 s at 6.5 m/s; 1 min at 0°C. One volume of phenol-chloroform-isoamyl alcohol was added to the extracts, and the aqueous phase was applied to RNeasy minikit columns (Qiagen) according to the manufacturer's instructions. RNA preparations were incubated with DNase I (Qiagen) to eliminate DNA contamination. The RNA was spectrophotometrically quantified in a NanoDrop ND-1000 UV-Vis spectrophotometer (Thermo Scientific), and its integrity was determined using a Bioanalyzer 2100 instrument and the RNA 6000 Nano LabChip kit (Agilent Technologies). Only RNAs with RNA integrity values above 7.0 were used.
Microarray procedures.
Agilent microarrays with a format of 8×15K were designed previously (40) to target a total of 7,589 S. clavuligerus loci, including 5,710 chromosomal and 1,581 pSCL4-coding sequences. Nucleic acid labeling, hybridization and washing conditions, image scanning, and statistical analysis were performed as described previously (40). Briefly, RNA preparations were labeled with Cy3-dCTP and were hybridized altogether with Cy5-labeled genomic DNA (gDNA), used as the common reference; fluorescence signals were detected with an Agilent G2565BA scanner and were quantified with Feature Extraction software (Agilent), and the signal values normalized and processed with the limma package (41) in the R environment (42). The Mg value, the normalized binary log of Cy3/Cy5 net intensities, is proportional to the abundance of the transcript for a particular gene (40). The Mg transcription values (GSE data sets) of the six experimental conditions were compared using three contrasts, mutant versus wild type, corresponding to the three growth times studied. For each gene and comparison, P values and Mc values were calculated from biological duplicates. Mc values are binary log values of the differential transcription between the mutant and the wild-type strain (positive and negative values indicate upregulation and downregulation, respectively). The Benjamini-Hochberg (BH) false-discovery rate adjustment was applied to the P values, so a result was considered statistically significant when the BH-corrected P value was < 0.05.
Accession number(s).
The microarray data used in this work have been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus database under accession numbers GSE66683, GSE92399, and GSE99917.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant BIO2013-34723 from the Spanish Ministry of Economy and Competitiveness. R. Álvarez-Álvarez and Y. Martínez-Burgo received a PFU fellowship from the Spanish Ministry of Science and Innovation.
We appreciate the gift of the S. clavuligerus RFL35 strain from C. A. Townsend. The advice of R. Pérez-Redondo in initial experiments is appreciated.
P.L., J.F.M., and R.Á.-Á. conceived and designed the research. R.Á.-Á. and Y.M.-B. performed the research. A.R.-G. performed bioinformatic studies. P.L. wrote the manuscript.
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01701-18.
REFERENCES
- 1.Pearce SR, Mimmack ML, Gallagher MP, Gileadi U, Hyde SC, Higgins CF. 1992. Membrane topology of the integral membrane components, OppB and OppC, of the oligopeptide permease of Salmonella typhimurium. Mol Microbiol 6:47–57. doi: 10.1111/j.1365-2958.1992.tb00836.x. [DOI] [PubMed] [Google Scholar]
- 2.Monnet V. 2003. Bacterial oligopeptide-binding proteins. Cell Mol Life Sci 60:2100–2114. doi: 10.1007/s00018-003-3054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodell EW, Higgins CF. 1987. Uptake of cell wall peptides by Salmonella typhimurium and Escherichia coli. J Bacteriol 169:3861–3865. doi: 10.1128/jb.169.8.3861-3865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perego M, Higgins CF, Pearce SR, Gallagher MP, Hoch JA. 1991. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol Microbiol 5:173–185. doi: 10.1111/j.1365-2958.1991.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 5.Nodwell JR, McGovern K, Losick R. 1996. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol Microbiol 22:881–893. doi: 10.1046/j.1365-2958.1996.01540.x. [DOI] [PubMed] [Google Scholar]
- 6.Nodwell JR, Losick R. 1998. Purification of an extracellular signaling molecule involved in production of aerial mycelium by Streptomyces coelicolor. J Bacteriol 180:1334–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keijser BJ, van Wezel GP, Canters GW, Vijgenboom EJ. 2002. Developmental regulation of the Streptomyces lividans ram genes: involvement of RamR in regulation of the ramCSAB operon. J Bacteriol 184:4420–4429. doi: 10.1128/JB.184.16.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connor TJ, Nodwell JR. 2005. Pivotal roles for the receiver domain in the mechanism of action of the response regulator RamR of Streptomyces coelicolor. J Mol Biol 351:1030–1047. doi: 10.1016/j.jmb.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Akanuma G, Ueki M, Ishizuka M, Ohnishi Y, Horinouchi S. 2011. Control of aerial mycelium formation by the BldK oligopeptide ABC transporter in Streptomyces griseus. FEMS Microbiol Lett 315:54–62. doi: 10.1111/j.1574-6968.2010.02177.x. [DOI] [PubMed] [Google Scholar]
- 10.Hiles ID, Gallagher MP, Jamieson DJ, Higgins CF. 1987. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol 195:125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Redondo R, Rodríguez-García A, Martín JF, Liras P. 1999. Deletion of the pyc gene blocks clavulanic acid biosynthesis except in glycerol-containing medium: evidence for two different genes in formation of the C3 unit. J Bacteriol 181:6922–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liras P, Santamarta I, Pérez-Redondo R. 2010. Clavulanic acid and clavams biosynthesis and regulation, p 167–178. In Dyson P. (ed), Streptomyces: molecular biology and biotechnology. Horizon Press, Norwich, United Kingdom. [Google Scholar]
- 13.Mellado E, Lorenzana LM, Rodríguez-Sáiz M, Díez B, Liras P, Barredo JL. 2002. The clavulanic acid biosynthetic cluster of Streptomyces clavuligerus: genetic organization of the region upstream of the car gene. Microbiology 148:1427–1438. doi: 10.1099/00221287-148-5-1427. [DOI] [PubMed] [Google Scholar]
- 14.Jensen SE, Paradkar AS, Mosher RH, Anders C, Beatty PH, Brumlik MJ, Griffin A, Barton B. 2004. Five additional genes are involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. Antimicrob Agents Chemother 48:192–202. doi: 10.1128/AAC.48.1.192-202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenzana LM, Pérez-Redondo R, Santamarta I, Martín JF, Liras P. 2004. Two oligopeptide-permease-encoding genes in the clavulanic acid cluster of Streptomyces clavuligerus are essential for production of the β-lactamase inhibitor. J Bacteriol 186:3431–3438. doi: 10.1128/JB.186.11.3431-3438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tame JR, Dodson EJ, Murshudov G, Higgins CF, Wilkinson AJ. 1995. The crystal structures of the oligopeptide-binding protein OppA complexed with tripeptide and tetrapeptide ligands. Structure 3:1395–1406. doi: 10.1016/S0969-2126(01)00276-3. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie AK, Valegård K, Iqbal A, Caines MEC, Kershaw NJ, Jensen SE, Schofield CJ, Andersson I. 2010. Crystal structures of an oligopeptide-binding protein from the biosynthetic pathway of the β-lactamase inhibitor clavulanic acid. J Mol Biol 396:332–344. doi: 10.1016/j.jmb.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Álvarez-Álvarez R, Rodríguez-García A, Martínez-Burgo Y, Robles-Reglero V, Santamarta I, Pérez-Redondo R, Martín JF, Liras P. 2014. A 1.8-Mb-reduced Streptomyces clavuligerus genome: relevance for secondary metabolism and differentiation. Appl Microbiol Biotechnol 98:2183–2195. doi: 10.1007/s00253-013-5382-z. [DOI] [PubMed] [Google Scholar]
- 19.Medema MH, Trefzer A, Kovalchuk A, van den Berg M, Muller U, Heijne W, Wu L, Alam MT, Ronning CM, Nierman WC, Bovenberg RA, Breitling R, Takano E. 2010. The sequence of a 1.8-Mb bacterial linear plasmid reveals a rich evolutionary reservoir of secondary metabolic pathways. Genome Biol Evol 2:212–224. doi: 10.1093/gbe/evq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Walsh CT. 2010. Identification of the gene cluster for the dithiolopyrrolone antibiotic holomycin in Streptomyces clavuligerus. Proc Natl Acad Sci U S A 107:19731–19735. doi: 10.1073/pnas.1014140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liras P. 2014. Holomycin, a dithiolopyrrolone compound produced by Streptomyces clavuligerus. Appl Microbiol Biotechnol 98:1023–1030. doi: 10.1007/s00253-013-5410-z. [DOI] [PubMed] [Google Scholar]
- 22.Álvarez-Álvarez R, Martínez-Burgo Y, Pérez-Redondo R, Braña AF, Martín JF, Liras P. 2013. Expression of the endogenous and heterologous clavulanic acid cluster in Streptomyces flavogriseus: why a silent cluster is sleeping. Appl Microbiol Biotechnol 97:9451–9463. doi: 10.1007/s00253-013-5148-7. [DOI] [PubMed] [Google Scholar]
- 23.Sievers S, Lund A, Menéndez-Gil P, Nielsen A, Mollerup MS, Nielsen SL, Larsson PB, Borch-Jensen J, Johansson J, Kallipolitis BH. 2015. The multicopy sRNA LhrC controls expression of the oligopeptide-binding protein OppA in Listeria monocytogenes. RNA Biol 12:985–997. doi: 10.1080/15476286.2015.1071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santamarta I, López-García MT, Kurt A, Nárdiz N, Álvarez-Álvarez R, Pérez-Redondo R, Martín JF, Liras P. 2011. Characterization of DNA-binding sequences for CcaR in the cephamycin-clavulanic acid supercluster of Streptomyces clavuligerus. Mol Microbiol 81:968–981. doi: 10.1111/j.1365-2958.2011.07743.x. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzana LM. 2003. Agrupación de genes de biosíntesis de ácido clavulánico en Streptomyces clavuligerus. Análisis de la región corriente arriba del gen car. PhD thesis, Universidad de León, León, Spain. [Google Scholar]
- 26.Charusanti P, Fong NI, Nagarajan H, Pereira AR, Li HJ, Abate EA, Su Y, Gerwick WH, Palsson BO. 2012. Exploiting adaptive laboratory evolution of Streptomyces clavuligerus for antibiotic discovery and overproduction. PLoS One 7:e33727. doi: 10.1371/journal.pone.0033727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robles-Reglero V, Santamarta I, Álvarez-Álvarez R, Martín JF, Liras P. 2013. Transcriptional analysis and proteomics of the holomycin gene cluster in overproducer mutants of Streptomyces clavuligerus. J Biotechnol 163:69–76. doi: 10.1016/j.jbiotec.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta A, Sureka K, Mitra D, Saha B, Sanyal S, Das AK, Chakrabarti P, Jackson M, Gicquel B, Kundu M, Basu J. 2010. An oligopeptide transporter of Mycobacterium tuberculosis regulates cytokine release and apoptosis of infected macrophages. PLoS One 58:e12225. doi: 10.1371/journal.pone.0012225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopfe M, Henrich B. 2004. OppA, the substrate-binding subunit of the oligopeptide permease, is the major ecto-ATPase of Mycoplasma hominis. J Bacteriol 186:1021–1028. doi: 10.1128/JB.186.4.1021-1028.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richarme G, Caldas TD. 1997. Chaperone properties of the bacterial periplasmic substrate-binding proteins. J Biol Chem 272:15607–15612. doi: 10.1074/jbc.272.25.15607. [DOI] [PubMed] [Google Scholar]
- 31.de Crécy-Lagard V, Servant-Moisson P, Viala J, Grandvalet C, Mazodier P. 1999. Alteration of the synthesis of the Clp ATP-dependent protease affects morphological and physiological differentiation in Streptomyces. Mol Microbiol 32:505–517. doi: 10.1046/j.1365-2958.1999.01364.x. [DOI] [PubMed] [Google Scholar]
- 32.Arulanantham H, Kershaw NJ, Hewitson KS, Hughes CE, Thirkettle JE. 2006. ORF17 from the clavulanic acid biosynthesis gene cluster catalyzes the ATP-dependent formation of N-glycyl-clavaminic acid. J Biol Chem 281:279–287. doi: 10.1074/jbc.M507711200. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson NH, Baggaley KH, Cassels R, Davison M, Elson SW, Fulston M, Tyler JW, Woroniecki SR. 1994. Evidence that the immediate biosynthetic precursor of clavulanic acid is its N-aldehyde analogue. J Chem Soc Chem Commun (Camb) 1994:1281–1282. doi: 10.1039/c39940001281. [DOI] [Google Scholar]
- 34.Aidoo KA, Wong A, Alexander DC, Rittammer RA, Jensen SE. 1994. Cloning, sequencing and disruption of a gene from Streptomyces clavuligerus involved in clavulanic acid biosynthesis. Gene 147:41–46. doi: 10.1016/0378-1119(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 35.Burton K. 1956. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 62:315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-Redondo R. 2000. Genética de la producción de ácido clavulánico en Streptomyces clavuligerus. PhD thesis, Universidad de León, León, Spain. [Google Scholar]
- 37.de la Fuente A, Lorenzana LM, Martín JF, Liras P. 2002. Mutants of Streptomyces clavuligerus with disruptions in different genes for clavulanic acid biosynthesis produce large amounts of holomycin: possible cross-regulation of two unrelated secondary metabolic pathways. J Bacteriol 184:6559–6565. doi: 10.1128/JB.184.23.6559-6565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu WS, Braña AF, Demain AL. 1984. Carbon source regulation of cephem antibiotic production by resting cells of Streptomyces clavuligerus and its reversal by protein synthesis inhibitors. Enzyme Microb Technol 6:155–160. doi: 10.1016/0141-0229(84)90023-1. [DOI] [Google Scholar]
- 39.Foulstone M, Reading C. 1982. Assay of amoxicillin and clavulanic acid, the components of augmentin, in biological fluids with high-performance liquid chromatography. Antimicrob Agents Chemother 22:753–762. doi: 10.1128/AAC.22.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Burgo Y, Álvarez-Álvarez R, Rodríguez-García A, Liras P. 2015. The pathway-specific regulator ClaR of Streptomyces clavuligerus has a global effect on the expression of genes for secondary metabolism and differentiation. Appl Environ Microbiol 81:6637–6648. doi: 10.1128/AEM.00916-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 437:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Core Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.r-project.org/. [Google Scholar]
- 43.Bachmann BO, Rongfeng L, Townsend CA. 1998. β-Lactam synthetase: a new biosynthetic enzyme. Proc Natl Acad Sci U S A 95:9082–9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.