Wood is one of the largest pools of carbon on Earth, and its decomposition is dominated in most systems by fungi. Wood-degrading fungi specialize in extracting sugars bound within lignin, either by removing lignin first (white rot) or by using Fenton-generated reactive oxygen species (ROS) to “loosen” wood cell walls, enabling selective sugar extraction (brown rot). Although white rot lignin-degrading pathways are well characterized, there are many uncertainties in brown rot fungal mechanisms. Our study addressed a key uncertainty in how brown rot fungi deploy ROS without damaging themselves or the enzymes they secrete. In addition to revealing differentially expressed genes to promote ROS generation only in early decay, our study revealed three spatial control mechanisms to avoid/tolerate ROS: (i) constraining Fenton reactant concentrations (H2O2, Fe2+), (ii) quenching ROS via antioxidants, and (iii) secreting ROS-tolerant enzymes. These results not only offer insight into natural decomposition pathways but also generate targets for biotechnological development.
KEYWORDS: antioxidant capacity, Fenton reaction, glycosyl hydrolases, hydroxyl radicals, oxidative stress tolerance, wood decay
ABSTRACT
Brown rot wood-degrading fungi deploy reactive oxygen species (ROS) to loosen plant cell walls and enable selective polysaccharide extraction. These ROS, including Fenton-generated hydroxyl radicals (HO˙), react with little specificity and risk damaging hyphae and secreted enzymes. Recently, it was shown that brown rot fungi reduce this risk, in part, by differentially expressing genes involved in HO˙ generation ahead of those coding carbohydrate-active enzymes (CAZYs). However, there are notable exceptions to this pattern, and we hypothesized that brown rot fungi would require additional extracellular mechanisms to limit ROS damage. To assess this, we grew Postia placenta directionally on wood wafers to spatially segregate early from later decay stages. Extracellular HO˙ production (avoidance) and quenching (suppression) capacities among the stages were analyzed, along with the ability of secreted CAZYs to maintain activity postoxidation (tolerance). First, we found that H2O2 and Fe2+ concentrations in the extracellular environment were conducive to HO˙ production in early (H2O2:Fe2+ ratio 2:1) but not later (ratio 1:131) stages of decay. Second, we found that ABTS radical cation quenching (antioxidant capacity) was higher in later decay stages, coincident with higher fungal phenolic concentrations. Third, by surveying enzyme activities before/after exposure to Fenton-generated HO˙, we found that CAZYs secreted early, amid HO˙, were more tolerant of oxidative stress than those expressed later and were more tolerant than homologs in the model CAZY producer Trichoderma reesei. Collectively, this indicates that P. placenta uses avoidance, suppression, and tolerance mechanisms, extracellularly, to complement intracellular differential expression, enabling this brown rot fungus to use ROS to degrade wood.
IMPORTANCE Wood is one of the largest pools of carbon on Earth, and its decomposition is dominated in most systems by fungi. Wood-degrading fungi specialize in extracting sugars bound within lignin, either by removing lignin first (white rot) or by using Fenton-generated reactive oxygen species (ROS) to “loosen” wood cell walls, enabling selective sugar extraction (brown rot). Although white rot lignin-degrading pathways are well characterized, there are many uncertainties in brown rot fungal mechanisms. Our study addressed a key uncertainty in how brown rot fungi deploy ROS without damaging themselves or the enzymes they secrete. In addition to revealing differentially expressed genes to promote ROS generation only in early decay, our study revealed three spatial control mechanisms to avoid/tolerate ROS: (i) constraining Fenton reactant concentrations (H2O2, Fe2+), (ii) quenching ROS via antioxidants, and (iii) secreting ROS-tolerant enzymes. These results not only offer insight into natural decomposition pathways but also generate targets for biotechnological development.
INTRODUCTION
Brown rot fungi are ubiquitous decomposers of woody plant material, particularly in coniferous forests, and they are well-known pests in lumber (1–3). The mechanistic strategies of brown rot fungi are different than those of lignin-degrading white rot fungi. Brown rot fungi rapidly depolymerize and metabolize cellulose and hemicellulose, leaving a large fraction of lignin behind in brownish residues (4, 5). Interestingly, brown rot fungi reliably degrade wood at a higher rate than white rot fungi in single-strain wood decay microcosms (6), yet they are characterized by the loss of >60% of the genes known to be involved in white rot (e.g., ligninolytic peroxidases) (7–9).
This “more-with-less” paradox has most often been explained by a novel oxidative step deployed by brown rot fungi at the earliest stages of decay. Instead of removing lignin as a requisite pretreatment to access carbohydrates, brown rot fungi initiate the wood attack using reactive oxygen species (ROS), particularly, hydroxyl radicals produced by the Fenton reaction (Fe2+ + H2O2 → Fe3+ + HO− + HO˙) (10, 11). To maintain a viable concentration of Fe2+ to drive the Fenton reaction near the hyphal front, many have proposed that brown rot fungi employ hydroquinones (e.g., 2,5-dimethoxyhydroquinone and 4,5-dimethoxycatechol) that act as Fe2+-reducing agents (12–14). Likewise, H2O2 production is considered to be regulated by extracellular oxidases such as alcohol oxidase and glyoxal oxidase (15). Although measuring these Fenton reagents presents challenges in solid wood (16), fungus-facilitated HO˙ has been implicated in depolymerizing and carbonylating carbohydrates (17), removing side chain hemicelluloses that reduce fiber strength (18), and modifying lignin via demethylation, oxidation, and cleavage (19–21). These alterations made in early brown rot enable fungal hydrolytic enzymes to gain access, secondarily, during a saccharification step.
Given the nonspecific nature of these early oxidative reactions, brown rot fungi need mechanisms to avoid or tolerate self-inflicted damage from ROS (11). This was long thought to involve microenvironmental gradients such as pH differentials across the fungus-wood interface (22). These gradients have not been supported as a plausible mechanism, however, and transcriptomics for Postia placenta recently revealed that the differential expression of genes involved in H2O2 production (i.e., glucose oxidase) and Fe2+ regulation (i.e., ferric reductase) is upregulated at early wood decay stages and then downregulated (23). Also, most carbohydrate-active enzymes (CAZYs), particularly glycoside hydrolases (GHs) such as endoglucanases, have been shown to be inducible (24) after an initial delay, presumably to avoid being secreted into an oxidatively damaging extracellular environment. This collectively indicates that differential expression helps focus ROS away from most CAZYs toward an active oxidative zone at the hyphal front, as brown rot fungi invade wood.
There are several indications that the differential control of intracellular gene regulation is not the only mechanism enabling brown rot fungi to partition ROS from CAZYs extracellularly. First, there are exceptions to this on/off oxidative hydrolytic shift among differentially expressed genes. Some CAZY genes, such as those encoding pectinases, are upregulated significantly in early decay stages, and the proteins produced by these genes presumably diffuse into a harsh oxidative environment (23). Early-secreted enzymes, including these pectinases and others targeting side-chain hemicellulose, have been implicated in loosening plant cell walls (25, 26) but would presumably be at risk without an additional means of protection. Second, brown rot fungi can regulate genes using induction/repression feedback and transcription factors (24, 27). Once products are secreted, however, they require additional means to spatially direct and control (on/off) oxidative reactions that cause collateral damage.
For these reasons, we hypothesized that brown rot fungi harness additional oxidative damage control mechanisms that complement, extracellularly, the differential gene expression controlled within hyphal cells. Specifically, we tested early “exceptional” enzymes for their oxidative tolerance and assessed the oxidative potential in the environment into which they are secreted as a potential mechanism of avoidance. To do this, we grew P. placenta along wood strips and separated, spatially, areas of early and late decay. Within these decayed sections, we used two standard measures of Fenton reactant concentrations to gauge HO˙ production capacity, and we used a cation quenching assay to gauge antioxidant capacity. Additionally, we compared the oxidative tolerance (HO˙ stress, by measuring activity loss) of P. placenta GHs implicated in early brown rot (pectinase) with that of enzymes secreted after incipient decay (α-d-galactosidase, α-l-arabinofuranosidase, β-glucosidase, mannanase, xylanase, and endoglucanase). Finally, for reference, we compared the tolerances of these enzymes in the brown rot fungus P. placenta against those of enzymes with equivalent function in a non-brown-rot fungus, Trichoderma reesei. Collectively, our results indicate that brown rot fungi likely use avoidance (controlling ROS potential), suppression (antioxidants), and tolerance (equipping early-secreted CAZYs for oxidative stress) to enable their unique ROS-based wood decay strategy.
RESULTS AND DISCUSSION
Fungal ROS damage control. (i) Avoidance.
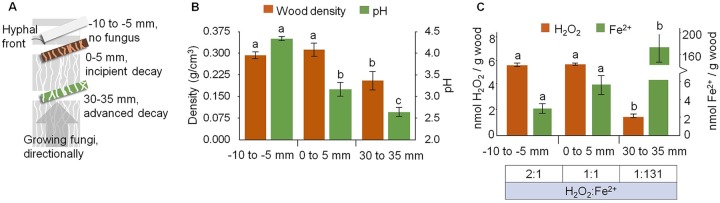
Postia placenta decayed wood and reduced the pH significantly as it colonized along wood wafers, using a directional growth design (Fig. 1A and B). Along this decay gradient, molar ratios of H2O2:Fe2+ were strikingly different in early versus later decay stages (Fig. 1C), likely to restrict HO˙ to early decay stages. The ∼4× decrease in H2O2 content in wood is in line with a sequential “two-step” expression of ROS-generating genes and GH genes, including genes involved with H2O2 production and degradation (23, 27, 28). Notably, some H2O2 either had diffused into the noncolonized section ahead of the hyphal front (−10 to −5 mm) or may have been generated there by diffusible enzymes or low-molecular-weight hydroquinones (13). These hydroquinones, in association with iron-mobilizing oxalate secreted by brown rot fungi, may help control the Fenton “potential” by chelating, reducing, and altering the solubility of Fe near the hyphal front (10). In contrast with the H2O2 pattern, Fe2+ content increased ∼40× in the later decay stage (Fig. 1C). A low pH (<3.0) plus the antioxidant environment (as discussed below) in later decay stages might help stabilize Fe2+ (29, 30). Moreover, given that the iron homeostasis implies the reduction of iron after its uptake (31, 32), the abundance of Fe2+ might be important to support the propagation of hyphal cells at later decay.
FIG 1.
H2O2 and Fe2+ contents are staggered as P. placenta decays wood wafers. (A) Schematic of the directional wood colonization system that spatially segregates decay stages. (B) A decrease in wood density and pH at later decay. (C) Contents of H2O2 and Fe2+ per wood mass (means ± standard deviations; n = 3). Different lowercase letters denote significant differences per two-way randomized ANOVA (α = 0.05). The ratio of H2O2 to Fe2+ for each wood wafer section is shown at the bottom (higher Fe2+ content corresponds to a lower Fenton potential).
These ratios would promote HO˙ near the hyphal front to enable the ROS-based brown rot pretreatment step. A ratio of H2O2:Fe2+ of near 1:1 will favor the Fenton reaction (33, 34), and the Fe2+ present in uncolonized wood (−10 to −5 mm) would also react with H2O2 there to drive the Fenton reaction ahead of the hyphal front, eliminating damage risk completely. In later stages, the very low H2O2:Fe2+ (1:131) would limit ROS risk, as Fe2+ acts as a radical scavenger to quench HO˙ (35, 36). This would create a “safe zone” for GHs that lack tolerance to oxidative stress but that are critical for hydrolyzing polysaccharides released after the brown rot pretreatment step.
(ii) Suppression.
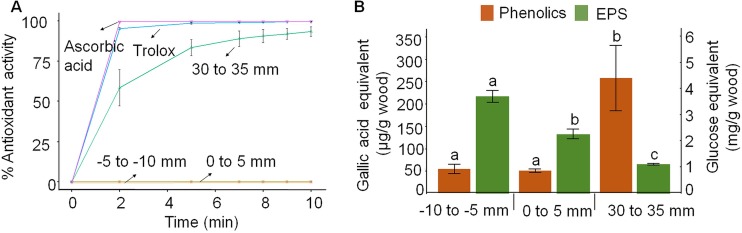
The antioxidant capacity (quenching), measured as the free radical scavenging activity with ABTS˙+ as the substrate, was clearly detected at the advanced decay stage (30- to 35-mm section), but not at the early decay stage (Fig. 2A). This is in line with the strategy of targeting ROS production “forward” near the hyphal front but actively suppressing it later. To explain this novel antioxidant pattern in P. placenta (Polyporales), we assessed phenolics and polysaccharides often associated with the same quenching role in Agaricales fungi (37, 38). We found that phenolic contents, but not fungal polysaccharides, were correlated with antioxidant capacity (Fig. 2B). Phenolic contents were dramatically increased ∼4× in the 30- to 35-mm sections (P < 0.05), which may be related to the onset of idiophasic growth during advanced decay (39) or to the increase of dihydroxyl aromatic compounds by brown rot lignin modifications (19). In either case, future metabolomic studies of P. placenta would help identify with higher specificity the compounds responsible for the antioxidant capacity observed.
FIG 2.
Antioxidant capacity increases with progressing brown rot wood decay. (A) The antioxidant capabilities of metabolites in P. placenta were measured as ABTS cation radical scavenging activity, with ascorbic acid (1.0 mg/ml) and trolox (0.12 mg/ml) as positive controls. (B) Potential antioxidant compounds such as phenolics and extracellular polysaccharides (EPS) were evaluated in the fungal extracts (means ± standard deviations; n = 3).
(iii) Tolerance.
Although ROS are targeted toward the hyphal front, ahead of most CAZYs, there remain exceptions among cell wall-loosening enzymes that are secreted early into the harsh oxidative environment of early brown rot (23). These would, hypothetically, need an additional tolerance mechanism, and so we tested brown rot GH tolerances to HO˙, generated through the addition of Fenton reagents, using bulk protein extracts to represent the natural conditions of decay. T. reesei was used as an outgroup for comparison purposes given that this organism has been characterized as a “workhorse” cellulase producer, and as a non-brown-rot-type fungus, it does not harness an ROS mechanism to degrade plant cell walls (40).
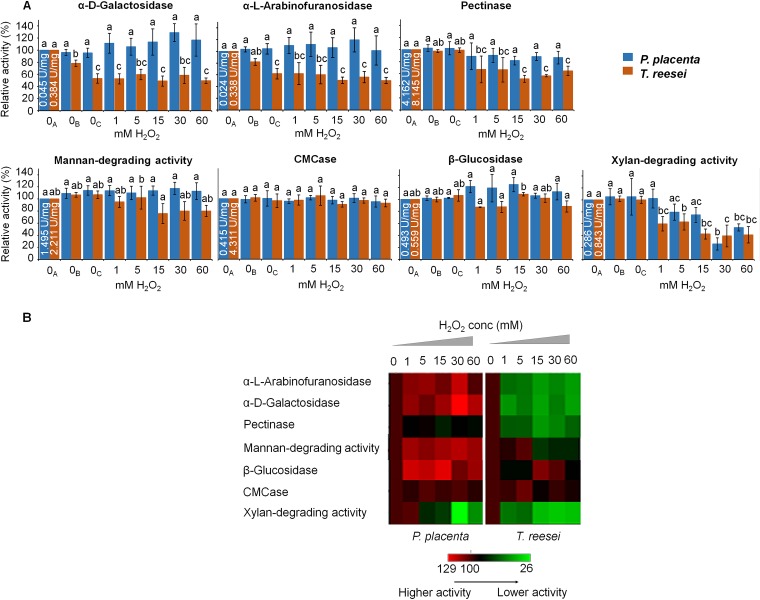
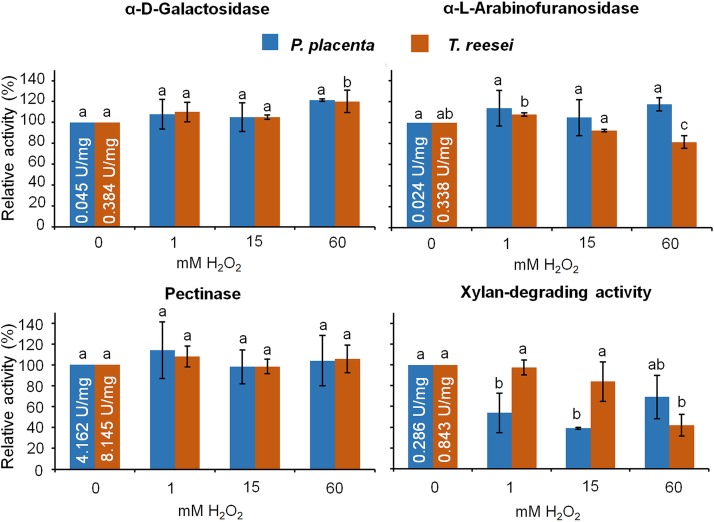
There were, as hypothesized, enzymes involved in the earliest brown rot decay stages that exhibited significant tolerance to oxidative stress relative to that of a non-brown-rot fungus, T. reesei. This included GHs pectinase, α-d-galactosidase, and α-l-arabinofuranosidase, implicated in loosening the recalcitrant wood matrix by the hydrolysis of the pit membranes (pectinases) or by cleaving the hemicellulose cross-links to cellulose microfibers and lignin (25, 26, 41) (Fig. 3A). The α-d-galactosidase and α-l-arabinofuranosidase were also rerun at equal enzyme activities rather than protein contents, with the same overall results: T. reesei samples showed an activity abatement of 39.3% ± 9.6% (1 mM) and 44.7% ± 10.8% (15 mM) for α-d-galactosidase and 31.3% ± 2.0% (1 mM) and 41.8% ± 4.5% (15 mM) for α-l-arabinofuranosidase. The oxidative tolerance of α-d-galactosidase and α-l-arabinofuranosidase in P. placenta is notable, as these enzymes have been associated with the early degradation of hemicellulose during brown rot (41) but can have complicated expression patterns (23).
FIG 3.
Enzyme oxidative tolerances as relative activities. (A) Damage of GH activities due to the treatment with the Fenton reagents. 0A, control without Fe2+ or H2O2; 0B, control with only 1 mM Fe2+ and catalase added from the beginning of the experiment; 0C, control with only 1 mM FeSO4 and catalase added after 1 h of incubation; 1, 5, 15, 30, and 60 mM values refer to the concentrations of H2O2 used in each treatment, in which the FeSO4 concentration was fixed at 1 mM. The values are shown as activities of treated samples relative to that of the untreated control (0A) (means ± standard deviations; n = 3). Bars with the same lowercase letters in the same series are not significantly different (P > 0.05). Initial specific enzyme activities are embedded as units per milligram within the controls. (B) Heat map of oxidative stress tolerance in the brown rot fungus P. placenta relative to that of T. reesei. Relative activities (%) after in vitro Fenton treatment with Fe2+ and 1 to 60 mM H2O2 are shown as coded colors relative to that of nontreated samples. Mean values of GH activities were used for the heat map analysis.
Along with the oxidative tolerance of enzymes associated with early brown rot, the degradation activities associated with main-chain hemicellulose and cellulose in later decay stages showed no “boost” in tolerance relative to that of T. reesei. This includes cellulase, β-glucosidase, mannanase, and xylanase, which, given that we used bulk protein assays, are best labeled as substrate-specific activities (CMCase, mannan-degrading activity, xylan-degrading activity). All the tolerance differences between T. reesei and P. placenta were more evident using a heat map to qualitatively summarize and group relative activities (Fig. 3B). However, a bar graph showing variability revealed that only xylan-degrading activity was reliably reduced by ROS, while P. placenta and T. reesei maintained similarly high apparent ROS tolerances for the other enzymes.
These tolerance patterns were first observed using H2O2 at various concentrations with a set Fe2+ level when H2O2 was present but without any Fe2+ present in the “0 mM” H2O2 treatments (Fig. 3A, 0A). Adding controls of Fe only (Fig. 3A, 0B and 0C) and H2O2 only (Fig. 4) revealed some oxidative potential for both, although more for Fe2+, supported by a carbonylation assessment that indicated more ROS-based oxidative damage (42) in bulk enzyme extracts in T. reesei than in P. placenta (Table 1). This might reflect some potential for Fe2+ to auto-oxidize in the presence of molecular oxygen, leading to the production of HO˙ in the absence of H2O2 (43). Collectively, these analyses imply that our treatments generated oxidative stress when Fenton reactants were present and that P. placenta enzyme activities were more robustly tolerant to this stress than those of T. reesei.
FIG 4.
Treatment controls using only H2O2. Damage of GH activities susceptible to the Fenton reaction was assessed for some of the H2O2 concentrations used in the Fenton treatment (1, 15, and 60 mM). The values are shown as activities of treated samples (1, 15, and 60 mM H2O2) relative to untreated samples (0 mM H2O2) (means ± standard deviations; n = 3). Bars with the same lowercase letters in the same series are not significantly different (P > 0.05). Initial specific enzyme activities are embedded as units per milligram within the controls.
TABLE 1.
Carbonylation content pre- and post-Fenton treatment in the bulk extracts from P. placenta and T. reesei
| Fungus | Carbonylation content (fmol of DNP/μg of total protein) after treatment witha: |
|||
|---|---|---|---|---|
| Control | 1 mM H2O2 | 5 mM H2O2 | 60 mM H2O2 | |
| P. placenta | 55.6 ± 10.2 A | 63.8 ± 15.2 A | 67.8 ± 21.5 A | 76.9 ± 34.2 A |
| T. reesei | 62.4 ± 27.3 A | 103.5 ± 11.0 AB | 121.5 ± 38.9 B | 135.7 ± 35.9 B |
Different uppercase letters indicate statistically different means within a row. DNP, dinitrophenyl-derivatized protein.
These results help shed light on how P. placenta might maximize the efficiency of lignocellulose deconstruction by producing enzymes able to resist the Fenton-based oxidative conditions during early brown rot and, in turn, minimize the energy spent on enzyme production during colonization. Thus, P. placenta may be protecting some enzymes essential for the initial deconstruction of the lignocellulosic complex, namely, pectinase, α-d-galactosidase, and α-l-arabinofuranosidase, from oxidative damage by the coexisting ROS. Additionally, this would avoid any potential losses in several late-upregulated main-chain GHs due to the diffusion or leaking of ROS.
Further discussion on ROS tolerance in GH enzymes.
It is important to note that none of the GH enzymes in P. placenta have been characterized with respect to their tolerance against oxidative stress, and in general, little is known about their stability properties. In this study, we found that some enzyme activities (e.g., mannan degrading, CMCase) that rely on enzymes in the same GH family (GH 5) fared similarly in the presence of the Fenton reagents for both fungi tested. In other cases, we found similar oxidative tolerance issues (i.e., functional similarities) among enzymes from different GH families. Xylan-degrading activity, for example, was reduced after oxidative stress in both species, but xylanases in P. placenta are encoded by four GH10 genes while those of T. reesei include three GH11 genes. Nevertheless, P. placenta xylanase showed a higher tolerance than T. reesei, especially at low concentrations of H2O2 (<15 mM) (Fig. 3A). This result might depend upon unexplored structural differences between GH10 and GH11 xylanases that, for instance, affect the efficiency at which both types of enzymes degrade arabinoxylans, with GH 10 showing a higher efficiency (44).
Interestingly, the low tolerance of P. placenta xylanase to oxidative stress contrasts with the high tolerance to denaturing agents such as urea or guanidine previously evidenced in purified samples of P. placenta decayed wood extracts (45, 46). In these studies, P. placenta xylanase showed a significant carbohydrate composition that reached a value between 50% and 60%. Although the carbohydrate presence may contribute to oxidative stability, this only happens indirectly if those moieties are blocking the access to critical oxidizable residues such as methionine, lysine, histidine, cysteine, proline, tryptophan, tyrosine, and arginine. Therefore, in this case, it can be assumed that these glycosidic moieties are likely located far from the critical residues in the protein; thus, they do not confer any oxidative tolerance to the enzyme.
It is hard to make specific assertions at this point, considering that all these activities might be affected by the presence of isoenzymes or different interactions occurring in the bulk extract. Therefore, the underlying mechanisms of the oxidative damage tolerance of specific GHs in brown rot fungi need to be addressed as a relevant functional trait in future work by the characterization of individual enzymes on the basis of protein sequences and structures.
Conclusion.
In this study, we found that P. placenta creates an extracellular environment that support ROS activity at the hyphal front and quenches it in later stages. In line with this, side-chain hemicellulases and pectinases expressed during early wood decay stages showed significant oxidative tolerance to Fenton-generated ˙OH relative to those of other CAZYs within P. placenta and to CAZYs of a non-brown-rot industrial strain of T. reesei. These results are unanimously in line with a two-step oxidative hydrolytic brown rot mechanism to spatially control ROS generation but offer a new alternative explanation (versus pH differentials) for the extracellular component of ROS damage control.
MATERIALS AND METHODS
Fungal cultures.
Postia placenta MAD 698-R (ATCC 44394) and Trichoderma reesei RUT-C30 (ATCC 56765) were maintained on malt extract agar plates (MEA; 2% malt extract, 0.2% yeast extract, and 1.5% agar) for routine subculturing. For wood wafer trials, Postia placenta MAD 698-R was inoculated on aspen wafers (Populus sp.; size, 60 mm by 25 mm by 2.5 mm) in soil block microcosms to segregate decay stages temporally and spatially, as described in Zhang et al. (23). Specifically, wafers propped in a vertical position on a predeveloped hyphal mat were colonized until the hyphal front reached 50 mm in height (i.e., equivalent to 3 to 4 weeks of growth) and then were harvested and sectioned. Three locations representing various decay stages were collected: −10 to −5 mm (no colonization), 0 to 5 mm (early decay), and 30 to 35 mm (later decay). The 0-mm mark represents the horizontal line of the hyphal front. To obtain a sufficient amount of substrate for analyses but capture biological variability, replicates were analyzed for each location by using pooled samples from 36 wafers (i.e., each representative was 12 wafers).
Trichoderma reesei RUT-C30, a non-brown-rot fungus used for out comparisons, was cultured in a liquid medium containing 2% malt extract and 0.2% yeast extract for enzyme production. Five discs (size, 3-mm diameter) taken from the edge of active growing mycelia were used for inoculations. Cultivation was at 25°C and 150 rpm for 7 days, and then the supernatant was collected by centrifuging cultures at 4°C and 15,000 × g. Three bioreplicates from independent cultures were included for the enzymatic analysis.
Wood decay parameters.
The pH of each wafer section was found by soaking the wafer section in a 5 mM calcium chloride solution for 1 h, followed by measuring the resulting pH of the solution using a calibrated pH probe (47). The soaked wafer section (“green” weight) was then used to displace 5 mM calcium chloride solution on a tared balance to determine the weight of the solution displaced, which was subsequently converted to the volume of solution displaced. The wafer section was then dried at 100°C for 2 days and weighed to obtain the oven dry weight. The volume of solution displaced and the oven dry weight were used to calculate the wafer section density (48).
Extractions.
Aspen wood sections were extracted with either buffer A (50 mM citrate-phosphate buffer, pH 3.3) or buffer B (50 mM acetate buffer, 0.5 M NaCl, 0.05% Tween 80, pH 5.0). Buffer A was used for extraction prior to analyzing H2O2, Fe2+, phenolics, and exopolysaccharides (EPS) as well as the antioxidant activity. The buffer volume (ml) to sample weight (g) ratio was 3:1. The extraction process was started by 5 min of vacuum to help the buffer penetrate the wood lumen, followed by shaking at 180 rpm for 90 min at 4°C. Then, the extracts were collected by taking the supernatant after centrifugation of the mixture at 2,576 × g for 7 min at 4°C. Buffer B was applied for protein extraction prior to analyses of glycoside hydrolase tolerance to oxidative stress. The buffer volume (ml) to sample weight (g) ratio was 1.5:1. The extraction was at 80 rpm and 4°C for 24 h, and then the mixture was centrifuged at 2,576 × g and 4°C for 7 min to collect the supernatant. Before the enzymatic analysis, crude extracts were exchanged to 50 mM citrate (pH 5.0) buffer using 10-kDa-cutoff Amicon Ultra-15 centrifugal filter units (Millipore-Sigma, USA). The protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad, USA).
Fenton reactants (H2O2, Fe2+).
H2O2 was measured by the Amplex Red10 (AR10, 10-acetyl-3,7-dihydroxyphenoxazine; Thermo Fisher Scientific, USA) method, which was evaluated as highly sensitive and selective for H2O2 quantification in decayed wood in a previous report (16). Briefly, 500 μl of extract and 500 μl of working solution (0.1 mM AR10 and 2 U/ml horseradish peroxidase in 50 mM phosphate buffer, pH 7.4) were mixed and incubated for 30 min at room temperature to allow oxidation of AR10 by H2O2. The absorbance of oxidized AR10 was measured at 570 nm, and H2O2 was quantified relative to a standard curve. As a blank control, H2O2 in the extract was degraded by incubating samples with 2,990 units of catalase at 25°C for 1 h, validated to completely remove 10 mM H2O2.
Ferrozine reagent was used to determine reduced iron (Fe2+) in decayed wood as previously described (49). Briefly, 200 μl of extracts was mixed with 1 ml of Ferrozine reagent {1.94 mM ferrozine [3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid sodium salt; Sigma, USA] in 50 mM HEPES buffer, pH 7.0}. After 20 min of color development, the absorbance of the iron(II)-ferrozine complex was measured at 562 nm and quantified relative to a standard curve from a series of sequential dilutions of FeCl2. The extract without the addition of the Ferrozine reagent was used as the blank control.
Antioxidant capacity.
The antioxidizing ability of fungal metabolites in decayed wood was determined by measuring the reduction of ABTS˙+ to ABTS, as described by Koleva et al. (50). Briefly, an ABTS solution (7 mM) was converted to its radical cation form ABTS˙+ by reacting with K2S2O8 (7 mM) at 27°C for 16 h in the dark and then was adjusted to an absorbance at 734 nm (A734) of 0.7 for subsequent use. To measure antioxidant capacity, 980 μl of ABTS˙+ was mixed with 20 μl of extracts or positive antioxidant controls (e.g., 1.0 mg/ml ascorbic acid and 0.12 mg/ml trolox [6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid]) at 27°C to measure the changes in A734. The antioxidant capacity was presented as the percentage of inhibition (% Inh) ABTS˙+ formation by the equation
| (1) |
where Absctrl and Abssample represent the absorbances of the starting oxidized solution and of the solution after the reaction with the samples to be evaluated, respectively.
Antioxidant agents.
Fungal phenolic compounds and extracellular polysaccharides (EPS) were assessed as key antioxidants implicated in quenching oxygen radicals in other Agaricales fungi (37, 38). Fungal phenolics were determined spectrophotometrically at 760 nm using Folin-Ciocalteu reagent according to the method described by Diaz et al. (51). On the other hand, extracellular polysaccharides (EPS) in decayed wood were precipitated with ethanol and determined using the phenol-sulfuric acid method for total carbohydrates as described by Wei et al. (52).
Enzyme tolerance of oxidative stress.
Crude protein extracts were subjected to Fenton-generated HO˙ stress and then assessed for posttreatment activities. For Fenton treatment (i.e., ˙OH), equal amounts of protein (1.3 μg) from P. placenta and T. reesei were incubated with FeSO4 (1 mM) plus variable amounts of H2O2 (1, 5, 15, 30, and 60 mM) in 50 mM citrate buffer (pH 5.0). After 1 h, the residual H2O2 was degraded by incubating with 2,990 units of catalase at 25°C for 1 h. The residual activities of these treated proteins were then assessed for glycoside hydrolases (GHs). Four different controls were used in these experiments. One control (0A) had neither Fe2+ nor H2O2 but had catalase added at the end of the 1-h incubation period used in the other treatments. The second control (0B) was without H2O2 but with 1 mM Fe2+ and catalase added from time zero of incubation, with the aim to address any potential Fe2+ auto-oxidation. The third control (0C) was without H2O2 but with 1 mM Fe2+ and catalase added at the end of the 1-h incubation period. Finally, the fourth set of controls had H2O2 at different concentrations (1, 15, and 60 mM) and catalase added after 1 h of incubation.
The α-d-galactosidase, α-l-arabinofuranosidase, and β-glucosidase activities were evaluated using p-nitrophenol derivatives p-nitrophenyl α-d-galactopyranoside, p-nitrophenyl-α-l-arabinofuranoside, and p-nitrophenyl β-d-glucopyranoside (Sigma, USA), respectively, as previously described (53). The released p-nitrophenol was determined by measuring the absorbance at 420 nm, using a p-nitrophenol standard curve. One unit of activity was defined as the amount of enzyme needed to release 1 μg of p-nitrophenol per minute per microgram of protein.
Mannan-degrading activity, CMCase, and pectinase activities were measured using locust bean gum (glucomannan), carboxymethylcellulose, and polygalacturonic acid as the substrates, respectively, according to the methods used by Zhang et al. (23) and by Debing et al. (54). The amount of reducing sugars was measured by reacting the samples with the dinitrosalicylic reagent (300 g/liter sodium potassium tartrate, 10 g/liter dinitrosalicylic acid, 16 g/liter NaOH). After heating in boiling water and incubating on ice, the absorbance was measured at 540 nm. Depending on the substrate employed for the enzyme activity assay, different sugars such as mannose, glucose, or galacturonic acid were used to build the standard curves. One unit of activity was defined as the amount of enzyme needed to release 1 μmol of sugar equivalent per minute per microgram of protein.
Xylan-degrading enzyme activity was measured with Remazol brilliant blue R-D-Xylan (RBB-xylan) (Sigma, USA) substrate as previously reported by Biely et al. (55). One unit of activity was reported as the increase in absorbance (595 nm) of solubilized Remazol brilliant blue-xylan per minute per microgram of protein.
Carbonylation assessment.
Bulk extracts of both P. placenta and T. reesei were subjected to the Fenton reaction under 3 different conditions (1 mM H2O2, 5 mM H2O2, and 60 mM H2O2), all including 1 mM FeSO4. A control without any of the Fenton reagents was included. The proteins in the samples were precipitated after 1 h of incubation using cold acetone (56) and redissolved in 50 mM citrate buffer, pH 5.0. Protein carbonylation was detected using the OxyBlot protein detection kit (Millipore). The carbonylated samples were dot blotted onto polyvinylidene difluoride (PVDF) membranes and developed with an Opti-4CN substrate kit (Bio-Rad). The membranes were dried, scanned on a UPV bioimaging system, and quantified by optical density using LabWorks 4.6 software (UPV, Inc., Upland, CA, USA). Three replicates were included for each sample.
Statistical analyses.
Data were analyzed using a two-way randomized analysis of variance (ANOVA) in R ×64 3.4.0, with post hoc Tukey's means comparisons to find significant differences. Significance was at an α value of 0.05.
ACKNOWLEDGMENTS
We thank the Fulbright Commission, Colciencias, for funding the lead author. The work was supported internally by the College of Biological Sciences at the University of Minnesota as well as by the McIntire Stennis Project MIN-12-087 of the Minnesota Agricultural Experiment Station.
REFERENCES
- 1.Gilbertson RL, Ryvarden L. 1986. North American polypores. Fungiflora, Oslo, Norway. [Google Scholar]
- 2.Zabel RA, Morrell JJ. 1992. Wood microbiology: decay and its prevention. Academic Press, San Diego, CA. [Google Scholar]
- 3.Hibbett DS, Donoghue MJ. 2001. Analysis of character correlations among wood decay mechanisms, mating systems, and substrate ranges in homobasidiomycetes. Syst Biol 50:215–242. doi: 10.1080/10635150121079. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson KE, Blanchette RA, Ander P. 1990. Microbial and enzymatic degradation of wood and wood components. Springer, Berlin, Germany. [Google Scholar]
- 5.Schilling JS, Kaffenberger JT, Liew FJ, Song Z. 2015. Signature wood modifications reveal decomposer community history. PLoS One 10:e0120679. doi: 10.1371/journal.pone.0120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ASTM International. 2007. Standard test method for wood preservatives by laboratory soil-block cultures. D1413-07e1 AS ASTM International, West Conshohocken, PA. [Google Scholar]
- 7.Martinez D, Challacombe J, Morgenstern I, Hibbett D, Schmoll M, Kubicek CP, Ferreira P, Ruiz-Duenas FJ, Martinez AT, Kersten P, Hammel KE, Vanden Wymelenberg A, Gaskell J, Lindquist E, Sabat G, Bondurant SS, Larrondo LF, Canessa P, Vicuna R, Yadav J, Doddapaneni H, Subramanian V, Pisabarro AG, Lavín JL, Oguiza JA, Master E, Henrissat B, Coutinho PM, Harris P, Magnuson JK, Baker SE, Bruno K, Kenealy W, Hoegger PJ, Kües U, Ramaiya P, Lucas S, Salamov A, Shapiro H, Tu H, Chee CL, Misra M, Xie G, Teter S, Yaver D, James T, Mokrejs M, Pospisek M, Grigoriev IV, Brettin T, Rokhsar D, Berka R, Cullen D. 2009. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Natl Acad Sci U S A 106:1954–1959. doi: 10.1073/pnas.0809575106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TK, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, et al. . 2012. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719. doi: 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- 9.Riley R, Salamov AA, Brown DW, Nagy LG, Floudas D, Held BW, Levasseur A, Lombard V, Morin E, Otillar R, Lindquist EA, Sun H, LaButti KM, Schmutz J, Jabbour D, Luo H, Baker SE, Pisabarro AG, Walton JD, Blanchette RA, Henrissat B, Martin F, Cullen D, Hibbett DS, Grigoriev IV. 2014. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc Natl Acad Sci U S A 111:9923–9928. doi: 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodell BS, Jellison J, Daniel G, Paszczynski A, Fekete F, Krishnamurthy S, Jun L, Xu G. 1997. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J Biotechnol 53:133–162. doi: 10.1016/S0168-1656(97)01681-7. [DOI] [Google Scholar]
- 11.Hammel KE, Kapich AN, Jensen KA, Ryan ZC. 2002. Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb Technol 30:445–453. doi: 10.1016/S0141-0229(02)00011-X. [DOI] [Google Scholar]
- 12.Jensen KA, Houtman CJ, Ryan ZC, Hammel KE. 2001. Pathway for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microbiol 67:2705–2711. doi: 10.1128/AEM.67.6.2705-2711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki MR, Hunt CG, Houtman CJ, Dalebroux ZD, Hammel KE. 2006. Fungal hydroquinones contribute to brown rot of wood. Environ Microbiol 8:2214–2223. doi: 10.1111/j.1462-2920.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- 14.Korripally P, Timokhin VI, Houtman CJ, Mozuch MD, Hammel KE. 2013. Evidence from Serpula lacrymans that 2,5-dimethoxyhydroquinone is a lignocellulolytic agent of divergent brown rot basidiomycetes. Appl Environ Microbiol 79:2377–2383. doi: 10.1128/AEM.03880-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel G, Volc J, Filonova L, Plíhal O, Kubátová E, Halada P. 2007. Characteristics of Gloeophyllum trabeum alcohol oxidase, an extracellular source of H2O2 in brown rot decay of wood. Appl Environ Microbiol 73:6241–6253. doi: 10.1128/AEM.00977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castaño JD, Zhang J, Schilling JS. 2018. Evaluation of colorimetric assays for determination of H2O2 in planta during fungal wood decomposition. J Microbiol Methods 145:10–13. doi: 10.1016/j.mimet.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Kirk TK, Ibach R, Mozuch MD, Conner AH, Highley TL. 1991. Characteristics of cotton cellulose depolymerized by a brown-rot fungus, by acid, or by chemical oxidants. Holzforschung 45:239–244. doi: 10.1515/hfsg.1991.45.4.239. [DOI] [Google Scholar]
- 18.Irbe I, Andersons B, Chirkova J, Kallavus U, Andersone I, Faix O. 2006. On the changes of pinewood (Pinus sylvestris L.). Chemical composition and ultrastructure during the attack by brown-rot fungi Postia placenta and Coniophora puteana. Int Biodeterior Biodegradation 57:99–106. doi: 10.1016/j.ibiod.2005.12.002. [DOI] [Google Scholar]
- 19.Filley TR, Cody GD, Goodell B, Jellison J, Noser C, Ostrofsky A. 2002. Lignin demethylation and polysaccharide decomposition in spruce sapwood degraded by brown-rot fungi. Org Geochem 33:111–124. doi: 10.1016/S0146-6380(01)00144-9. [DOI] [Google Scholar]
- 20.Kirk TK. 1975. Effects of a brown-rot fungus, Lenzites trabea, on lignin in spruce wood. Holzforschung 29:99–107. doi: 10.1515/hfsg.1975.29.3.99. [DOI] [Google Scholar]
- 21.Yelle DJ, Ralph J, Lu L, Hammel K. 2008. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ Microbiol 10:1844–1849. doi: 10.1111/j.1462-2920.2008.01605.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyde SM, Wood PM. 1997. A mechanism for production of hydroxyl radicals by the brown-rot fungus Coniophora puteana: Fe(III) reduction by cellobiose dehydrogenase and Fe(II) oxidation at a distance from the hyphae. Microbiology 143:259–266. doi: 10.1099/00221287-143-1-259. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Presley GN, Hammel KE, Ryu J-S, Menke JR, Figueroa M, Hu D, Orr G, Schilling JS. 2016. Localizing gene regulation reveals a staggered wood decay mechanism for the brown rot fungus Postia placenta. Proc Natl Acad Sci U S A 113:10968–10973. doi: 10.1073/pnas.1608454113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Schilling JS. 2017. Role of carbon source in the shift from oxidative to hydrolytic wood decomposition by Postia placenta. Fungal Genet Biol 106:1–8. doi: 10.1016/j.fgb.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Green F III, Tschernitz JL, Kuster TA, Highley TL. 1995. Hydrolysis of bordered pits during colonization of conifers by brown-rot decay. International Research Group for Wood Preservation IRG/WP/ 95–10103. U.S. Department of Agriculture, Forest Service, Madison, WI. [Google Scholar]
- 26.Glass NL, Schmoll M, Cate JHD, Coradetti S. 2013. Plant cell wall deconstruction by ascomycete fungi. Annu Rev Microbiol 67:477–498. doi: 10.1146/annurev-micro-092611-150044. [DOI] [PubMed] [Google Scholar]
- 27.Skyba O, Cullen D, Douglas CJ, Mansfield SD. 2016. Gene expression patterns of wood decay fungi Postia placenta and Phanerochaete chrysosporium are influenced by wood substrate composition during degradation. Appl Environ Microbiol 82:4387–4400. doi: 10.1128/AEM.00134-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Presley GN, Schilling JS. 2017. Distinct growth and secretome strategies for two taxonomically divergent brown rot fungi. Appl Environ Microbiol 83:e02987-16. doi: 10.1128/AEM.02987-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varela E, Tien M. 2003. Effect of pH and oxalate on hydroquinone-derived hydroxyl radical formation during brown rot wood degradation. Appl Environ Microbiol 69:6025–6031. doi: 10.1128/AEM.69.10.6025-6031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Presley GN, Zhang J, Schilling JS. 2016. A genomics-informed study of oxalate and cellulase regulation by brown rot wood-degrading fungi. Fungal Genet Biol 112:64–70. doi: 10.1016/j.fgb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 31.De Luca NG, Wood PM. 2000. Iron uptake by fungi: contrasted mechanisms with internal or external reduction. Adv Microb Physiol 43:39–74. doi: 10.1016/S0065-2911(00)43002-X. [DOI] [PubMed] [Google Scholar]
- 32.Kosman DJ. 2003. Molecular mechanisms of iron uptake in fungi. Mol Microbiol 47:1185–1197. doi: 10.1046/j.1365-2958.2003.03368.x. [DOI] [PubMed] [Google Scholar]
- 33.de Oliveira TD, Martini WS, Santos MDR, Matos MAC, da Rocha LL. 2015. Caffeine oxidation in water by Fenton and Fenton-like processes: effects of inorganic anions and ecotoxicological evaluation on aquatic organisms. J Braz Chem Soc 26:178–184. doi: 10.5935/0103-5053.20140237. [DOI] [Google Scholar]
- 34.Karale RS, Manu B, Shrihari S. 2014. Fenton and photo-Fenton oxidation processes for degradation of 3-aminopyridine from water. APCBEE Procedia 9:25–29. doi: 10.1016/j.apcbee.2014.01.005. [DOI] [Google Scholar]
- 35.Wang C, Zhang S, Zhang Z, Zeng M, Yuji S. 2014. Optimization and interpretation of Fenton and UV/Fenton processes for degradation of syringyl lignin. J Environ Anal Chem 2014:2–6. doi: 10.4172/2380-2391.1000115. [DOI] [Google Scholar]
- 36.Chan KH, Chu W. 2003. The dose and ratio effects of Fe(II) and H2O2 in Fenton's process on the removal of atrazine. Environ Technol 24:703–710. doi: 10.1080/09593330309385606. [DOI] [PubMed] [Google Scholar]
- 37.Carvajal AESS, Koehnlein EA, Soares AA, Eler GJ, Nakashima ATA, Bracht A, Peralta RM. 2012. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. Lebenson Wiss Technol 46:493–499. doi: 10.1016/j.lwt.2011.11.018. [DOI] [Google Scholar]
- 38.Puttaraju NG, Venkateshaiah SU, Dharmesh SM, Urs SMN, Somasundaram R. 2006. Antioxidant activity of indigenous edible mushrooms. J Agric Food Chem 54:9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- 39.Jaszek M, Osińska-Jaroszuk M, Janusz G, Matuszewska A, Stefaniuk D, Sulej J, Polak J, Ruminowicz M, Grzywnowicz K, Jarosz-Wilkołazka A. 2013. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. Biomed Res Int 2013:497492. doi: 10.1155/2013/497492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodell BS, Qian Y, Jellison J. 2008. Fungal decay of wood: soft rot-brown rot-white rot, p 9–31. In Schultz TP, Militz H, Freeman MH, Goodell B, Nicholas DD (ed), Development of commercial wood preservatives, vol 982 ACS Symposium Series. American Chemical Society, Washington, DC. [Google Scholar]
- 41.Curling SF, Clausen CA, Winandy JE. 2002. Relationships between mechanical properties, weight loss, and chemical composition of wood during incipient brown-rot decay. For Prod J 52:34–39. [Google Scholar]
- 42.Khosravifarsani M, Shabestani-Monfared A, Pouramir M, Zabihi E. 2016. Effects of Fenton reaction on human serum albumin: an in vitro study. Electron Physician 8:2970–2976. doi: 10.19082/2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiller E. 2004. Free radicals and inhalation pathology, p 310 Springer-Verlag, Berlin, Germany. [Google Scholar]
- 44.Morgan NK, Wallace A, Bedford MR, Choct M. 2017. Efficiency of xylanases from families 10 and 11 in production of xylo-oligosaccharides from wheat arabinoxylans. Carbohydr Polym 167:290–296. doi: 10.1016/j.carbpol.2017.03.063. [DOI] [PubMed] [Google Scholar]
- 45.Green F III, Clausen CA, Micales JA, Highley TL, Wolter KE. 1989. Carbohydrate-degrading complex of the brown-rot fungus Postia placenta: purification of β-1,4-xylanase. Holzforschung 43:25–31. doi: 10.1515/hfsg.1989.43.1.25. [DOI] [Google Scholar]
- 46.Micales JA, Green F, Clausen C, Highley T. 1987. Physical properties of β-1,4-xylanase produced by Postia placenta. Implications of the control of brown rot. International Research Group on Wood Preservation document no. IRG/WP/1318. U.S. Department of Agriculture, Forest Service, Madison, WI. [Google Scholar]
- 47.Shortle WC, Dudzik KR, Smith KT. 2010. Development of wood decay in wound-initiated discolored wood of eastern red cedar. Holzforschung 64:529–536. doi: 10.1515/hf.2010.051. [DOI] [Google Scholar]
- 48.Chave J. 16 February 2005. Measuring wood density for tropical forest trees. A field manual for the CTFS sites. Université Paul Sabatier, Toulouse, France. [Google Scholar]
- 49.Hynes MJ, Coinceanainn OM. 2002. Investigation of the release of iron from ferritin by naturally occurring antioxidants. J Inorg Biochem 190:8–21. [DOI] [PubMed] [Google Scholar]
- 50.Koleva II, Niederländer HAG, van Beek TA. 2001. Application of ABTS radical cation for selective on-line detection of radical scavengers in HPLC eluates. Anal Chem 73:3373–3381. doi: 10.1021/ac0013610. [DOI] [PubMed] [Google Scholar]
- 51.Diaz P, Jeong SC, Lee S, Khoo C, Koyyalamudi SR. 2012. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin Med 7:26. doi: 10.1186/1749-8546-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei ZH, Duan YY, Qian YQ, Guo XF, Li YJ, Jin SH, Zhou ZX, Shan SY, Wang CR, Chen XJ, Zheng Y, Zhong JJ. 2014. Screening of Ganoderma strains with high polysaccharides and ganoderic acid contents and optimization of the fermentation medium by statistical methods. Bioprocess Biosyst Eng 37:1789–1797. doi: 10.1007/s00449-014-1152-2. [DOI] [PubMed] [Google Scholar]
- 53.Mallek-Fakhfakh H, Belghith H. 2016. Physicochemical properties of thermotolerant extracellular β-glucosidase from Talaromyces thermophilus and enzymatic synthesis of cello-oligosaccharides. Carbohydr Res 419:41–50. doi: 10.1016/j.carres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Debing J, Peijun L, Stagnitti F, Xianzhe X, Li L. 2006. Pectinase production by solid fermentation from Aspergillus niger by a new prescription experiment. Ecotoxicol Environ Saf 64:244–250. doi: 10.1016/j.ecoenv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Biely P, Mislovičová D, Toman R. 1988. Remazol brilliant blue-xylan: a soluble chromogenic substrate for xylanases. Methods Enzymol 160:536–541. doi: 10.1016/0076-6879(88)60165-0. [DOI] [Google Scholar]
- 56.Fic E, Kedracka-Krok S, Jankowska U, Pirog A, Dziedzicka-Wasylewska M. 2010. Comparison of protein precipitation methods for various rat brain structures prior to proteomic analysis. Electrophoresis 31:3573–3579. doi: 10.1002/elps.201000197. [DOI] [PubMed] [Google Scholar]