Abstract
Aquaporins (AQPs) are transmembrane proteins essential for controlling the flow of water and other molecules required for development and stress tolerance in plants, including important crop species such as wheat (Triticum aestivum). In this study, we utilized a genomic approach for analyzing the information about AQPs available in public databases to characterize their structure and function. Furthermore, we validated the expression of a suite of AQP genes, at the transcriptional level, including accessions with contrasting responses to drought, different organs and water stress levels. We found 65 new AQP genes, from which 60% are copies expanded by polyploidization. Sequence analysis of the AQP genes showed that the purifying selection pressure acted on duplicate genes, which was related to a high conservation of the functions. This situation contrasted with the expression patterns observed for different organs, developmental stages or genotypes under water deficit conditions, which indicated functional divergence at transcription. Expression analyses on contrasting genotypes showed high gene transcription from Tonoplast Intrinsic Protein 1 (TIP1) and 2 (TIP2), and Plasma Membrane Intrinsic Protein 1 (PIP1) and 2 (PIP2) subfamilies in roots and from TIP1 and PIP1 subfamilies in leaves. Interestingly, during severe drought stress, 4 TIP genes analyzed in leaves of the tolerant accession reached up to 15-fold the level observed at the susceptible genotype, suggesting a positive relationship with drought tolerance. The obtained results extend our understanding of the structure and function of AQPs, particularly under water stress conditions.
Keywords: wheat, aquaporins, gene expression, evolution, water stress responses
1. Introduction
Triticum aestivum—bread wheat or common wheat is the world’s largest crop (~220 million ha) and it is cultivated in a wide variety of environments, many of them with severe water limitations [1,2]. The development of drought-tolerant and water-use efficient wheat genotypes is of global interest because stress by water deficit and/or drought can drastically reduce yield and grain quality [2]. In Mediterranean climate regions, wheat and other annual crops are exposed to water deficit during grain filling, leading to what has been called ‘terminal drought stress’ [3].
Tolerance to water deficit is a complex trait under polygenic control. In wheat, water uptake and hydraulic conductivity by roots are strongly influenced by aquaporins (AQPs) [4,5]. These proteins are located in the cell membranes and form channels which mediate the absorption and movement of water between the tissues and organs of the plant [6]. Recent studies, aimed at optimizing the efficient use of water and nutrients, have highlighted the functional importance of this type of channel, codified by a multigenic family [7]. Aquaporins belong to the superfamily of major intrinsic proteins (MIPs) and they are essential to facilitate the bidirectional flow of water or other uncharged small molecules, such as urea, carbon dioxide, boric acid or silicon, through biological membranes [8,9,10,11]. Structurally, they have six trans-membrane (TM) alpha helices and two short TM alpha helices that have a conserved NPA domain (Asp-Pro-Ala) and a selectivity pore ar/R (aromatic/Arg) that regulates the passage of water [12]. According to their intracellular location and phylogenetic distribution, AQPs have been divided into seven families. Within these, five are well known: intrinsic plasma membrane (PIP), intrinsic tonoplast (TIP), intrinsic nodulin 26-like (NIP), intrinsic small (SIP) and intrinsic uncharacterized (XIP) [13].
The role of AQPs in increasing membrane permeability to facilitate the mobilization of water and other small molecules essential for plant growth and development has been well documented [6]. AQPs are important in many processes for example, seed germination [14], leaf hydraulic and stomatal conductance [15] and cell elongation [16]. Their physiological role has been demonstrated in several studies on different species. In Gossypium hirsutum, GhPIP2 is relevant in the rapid formation of fibers [17]. In Arabidopsis thaliana, AtNIP5;1 is essential for cell elongation and fertility processes, allowing the mobilization of boron [10]. The ectopic expression of an antisense construct targeting Brassica napus BnPIP1 in tobacco causes morphological deformations [18]. The activity of AQP is regulated by the phosphorylation mediated by kinases or phosphatases [13,19].
Aquaporin genes are differentially expressed in response to internal signals or environmental stresses [20]. In Solanum lycopersicum, 23 of 32 AQP genes modulated their expression in different tissues and some of them did this in a specific way [21]. Different genotypes of Musa acuminata grown under water stress also showed differential expression patterns with respect to PIP genes such as MaPIP1-7, MaPIP2-6 and MaPIP2-10, among others [22]. In Hordeum vulgare, the expression levels of PIP and TIP genes were higher than that of NIP and SIP [23], similarly to that observed in A. thaliana [24]. Salinity in H. vulgare differentially modulated AQPs, especially PIP genes, with differences between tissues and even between sensitive and tolerant genotypes [24]. Other studies have shown that ectopic expression of TaAQP7, TaAQP8 and TaNIP genes in transgenic tobacco plants and A. thaliana increased tolerance to water and salt stress in both species [25,26,27]. Equally, ectopic expression of VfPIP1 [28], MaPIP1 [29], PgTIP1 [30] and OsPIP1-1 [31] increased drought tolerance in transgenic A. thaliana plants and a similar effect was observed with SlPIP2;2 in S. lycopersicum [32].
More than 1200 AQP genes have been identified in 31 plant species, including 34 in Oryza sativa, 35 in A. thaliana, 54 in Populus trichocarpa, 31 in Zea mays and 40 in H. vulgare [33]. In silico methods and databases of sequenced genomes have facilitated the characterization of these genes in several species such as S. lycopersicum, M. acuminata and A. thaliana [21,22,34]. In wheat, a comparative analysis and synteny with O. sativa AQPs revealed only 24 PIP and 11 TIP genes [35]. A subsequent in silico analysis added 6 PIP, 1 TIP, 4 NIP and 2 SIP genes to the list [36]. Currently, new genomic information resources are available, including the first version of the wheat genome sequence (http://www.wheatgenome.org/), allowing the study of the genes encoding these proteins [37]. However, additional research is required for organizing the accumulated information generated by these different sources and using it to deepen the analysis of the structure and function of these proteins.
Triticum aestivum is a hexaploid species with genomes A, B and D. Genetic analyses have established that Triticum urartu (A-genome donor) and Aegilops speltoides (B-genome donor) are the progenitors of the allotetraploid wild emmer wheat [Triticum turgidum ssp. dicoccoides (körn.) Thell] (2n = 4x = 28; AABB), which subsequently hybridized with Aegilops tauschii (D-genome donor) to form the current genome composition of bread wheat (2n = 6x = 42; AABBDD) [38]. These events have modified the number and/or levels of expression of different gene families, such as EXP expansins, SWEET sugar transporters, MAPK and MAPKK kinases, PHT1 phosphate transporters, among others, implicating a change in their biological role [39,40,41,42].
In this study, we carried out a complete genome analysis utilizing the information about AQPs available in public databases. We analyzed their amino acid sequences, physico-chemical properties, gene structure and synteny. Furthermore, we explored the transcript profiles of a suite of AQP genes using accessions with contrasting responses to drought, different organs and water stress levels, to test the relationship between differential expression of those genes and a differential response to water deficit. The evolutionary history of duplicated genes and the spatio-temporal expression patterns of the AQP family, suggests possible divergences at the functional level. We conclude that these functional differences could be involved in the drought tolerance mechanism of wheat plants.
2. Materials and Methods
2.1. Identification and Phylogenetic Analysis of Aquaporins in Wheat
Sequences of approximately 99.000 genes in the wheat genome were obtained from EnsemblPlants [43] to establish a local database and determine genes corresponding to AQPs. Aquaporins encoded in the genome were identified using the MIP aquaporin-specific domains (Pfam PF00230) [44] as a profile for hidden Markov model (HMM) chains with a cut-off value of 1× 10−10 in the HMMER v3.1 software [45]. In addition, an automatic search and sequence annotation analysis was carried out using the HAMAP-Scan software [46]. Manual validation of each sequence was done by comparative analysis with the BLASTp databases (https://blast.ncbi.nlm.nih.gov), Pfam and CDD [47], sequences lacking the conserved amino acids in the selectivity pore and those encoding truncated proteins were discarded, allowing the elimination of false positives. The complete length of each protein sequence of wheat AQPs and those of model species such as A. thaliana, O. sativa and P. trichocarpa [48], were used as templates for multiple alignment with ClustalO [49]. The phylogenetic tree was constructed using MEGA7 software [50] and the neighbor-joining method with 5000 iterations bootstrap.
2.2. Analysis of the DNA Sequence and Predicted Proteins
The exon-intron structure of the AQP genes was obtained from the GSDS software [48]. The conserved motifs in the amino acid sequence were identified using the web tool MEME [51] and analyzed with InterPro Scan 5 [52]. The isoelectric point (pI) and molecular weight (MW) were predicted with ExPaSy’s Compute pI/Mw tool [53], the transmembrane domains of each protein were predicted using the TMHMM Server v. 2.0 [54] and sub-cellular localization using the WoLF PSORT tool [55].
2.3. Expansion Pattern and Collinearity Analyses for the Aquaporin Family
The AQP genes were mapped in the genome of wheat by identifying their A, B and D sub-genomes position provided by the EnsemblPlants database. Groups of AQP genes were classified as paralogs in the wheat genome according to the same database. To establish the expansion pattern, those duplicate AQP genes that were in distinct sub-genomes of wheat according to the ENSEMBL Gene Tree tool were regarded as copies generated by polyploidization, or segmental duplication, while two or more AQP genes with the same chromosomal localization were defined as tandem duplication [56].
For the collinearity analysis, AQP genes from T. urartu were identified by alignment with BLASTn hosted in the EnsemblPlant database using the AQP gene sequences of the sub-genome A of wheat. Next, the location of the orthologue genes on the chromosome of T. urartu was obtained from the databases published by [57]. The syntenic relationships between AQP gene paralogs on each wheat chromosome, together with the collinearity map between wheat and T. urartu, were obtained using the web-based service ClicO FS [58].
2.4. Nonsynonymous Substitution Rate (Ka) and Synonymous Substitution Rate (Ks) Calculations
To determine the selection pressure that is exerted on duplicate AQP genes, we calculated the ratio Ka/Ks (purifying selection Ka/Ks < 1, neutral selection Ka/Ks = 1 and positive selection Ka/Ks > 1) [59]. To obtain the non-synonymous substitution rate and synonymous substitution rate of the AQP genes, the codon evolution rate was calculated using the duplicate genes previously found with the ClustalW codons by MEGA7 program [47] and using the neighbor-joining model [56]. The approximate date of the duplication events was estimated using T = Ks/2λ × 10−6 million years ago (Mya), based on the clock-like rates (λ) in grasses of 6.5 × 10−9 [60].
2.5. Expression Patterns of Aquaporin Genes in Several Organs and Developmental Stages and in Wheat Seedlings Growing Under Osmotic Stress
Microarrays data for wheat were obtained from IWGSC using the WheatEXP tool (https://wheat.pw.usda.gov/WheatExp/). The expression data for AQP genes were obtained in three developmental stages and five different organs (root, leaf, stem, spike and grain) according to the Zadoks scale [61,62]. For the analysis of expression patterns during osmotic stress, we used expression data of leaves of one-week-old seedlings grown in 20% PEG-6000 [15]. The magnitude of change in stress condition was expressed as the ratio compared to the initial condition (time zero). Expression profiles were grouped and presented as heatmaps [63].
2.6. Expression Analysis of Aquaporin Genes in a Drought-Tolerant and Susceptible Genotypes of Wheat Grown with and Without Water Stress during Grain Filling
Two genotypes of spring wheat (T. aestivum L.) with contrasting tolerances to water stress were selected according to the yield tolerance index (YTI) from a previous study [2]. One was advanced line with high yield potential under water stress conditions, “Fontagro 8” with a YTI of 0.60; and the other line, “QUP2569” with a lower YTI of 0.17, was considered as susceptible. The plants grew in a greenhouse, with a temperature range between 18 and 25 °C.
Five seeds of each genotype were sown in 7.5 L pots of 26 cm in diameter and 21.1 cm height, filled with a 1:1:1 mixture of organic soil mix (Anasac, Talca, Chile), perlite and river sand, representing a total weight per pot of 4.9 kg. After the emergence of the second leaf, the seedlings were thinned to one per pot. Plants were fertilized until the tillering stage using Hoagland nutrient solution (PhytoTechnology Laboratories, Lenexa, KS, USA). All pots were irrigated at field capacity (CC) to anthesis stage (Z61) [61]. From anthesis onward, two water regimes were established, 100% CC (control) and without irrigation (water stress). The stomatal conductance (gs) was evaluated on three flag leaves of each replica between 11:00 and 15:00 h using an infrared gas analyzer (IRGA) CIRAS2 (PPSystem) (Table S1). The first sampling of leaves and roots were carried out when the gs of the treatment plants without irrigation reached about 50% of the control plants in each genotype, sampling 10 plants from the control treatment and 10 plants from the water stress treatment. Then, 20 pots were kept without irrigation and a second sampling was carried out when the gs of the stressed plants reached about 30% of the control plants (two days after the first sampling). The ten remaining water stress treatment pots were watered to CC and after two days a third sampling was taken. There were 60 replicates pots of each genotype (n = 120), considering two treatments and three sampling times. The samples collected were immediately frozen in liquid nitrogen and stored at −80 °C until analysis.
RNA extraction from leaves and roots was done using the TRIzol® reagent (Life Technologies, Carlsbad, CA, USA) and the manufacturer’s protocol. RNA concentration and quality were determined spectrophotometrically using the Infinite® 200 PRO NanoQuant (Tecan, Männedorf, Switzerland). The integrity of the RNA was verified by agarose gel electrophoresis. Subsequently, the samples were treated with DNase Turbo™ Ambion® (Life Technologies) according to the manufacturer’s instructions. The RNA concentration was then standardized to 25 µg µL−1 and finally the complementary DNA synthesis was performed using the First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA) according to the protocol described by the manufacturer [64].
The gene expression of a selected suite of AQP genes was measured at the transcript level, by quantitative PCR (qPCR) using a Stratagene Mx3000p thermal cycler (Agilent Technologies, Santa Clara, CA, USA). The Maxima SYBR Green/ROX qPCR Master MIX kit (Thermo Scientific) was used for all reactions according to the protocol described by the manufacturer. For each sample (three biological replicates), qPCR was done in triplicate (three technical replicates), using 10 μL of Master MIX, 0.5 μL of 250 nM primers, 1 μL of cDNA and nuclease free water to a final volume of 20 µL. Amplification was followed by a melting curve analysis from 55 to 95 °C with continuous fluorescence measurement. Expression quantification was normalized using the Ta-α-Tubulin gene which is constitutively expressed in the organs analyzed [65,66]. The primers used for qPCR are described in Table S2. Transcript levels of each gene were evaluated using the 2−ΔΔCT method [67]. The qPCR data was analyzed using ANOVA (one-way analysis of variance) tests (with a significance of α = 0.05). Data management and standardization were performed with R version 3.2.5 [68].
3. Results
3.1. The Wheat Genome has 113 Non-Redundant Aquaporin Genes
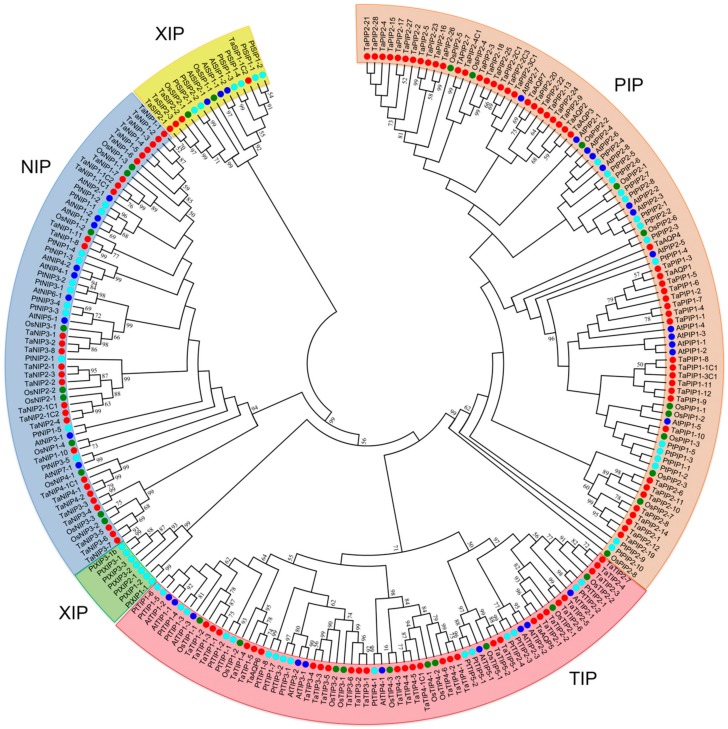
We obtained 123 sequences of non-redundant AQP gene homologs in the wheat genome. Among these, 10 pseudogenes were omitted from the subsequent analyses. Consequently, we used 113 AQPs (Table S3) to construct a phylogenetic tree together with the orthologue AQP sequences of A. thaliana, O. sativa and P. trichocarpa. The tree displayed five large families; all wheat sequences were grouped into the TIP, PIP, NIP and SIP families and no wheat AQP grouped in the clade XIP, composed only of P. trichocarpa AQPs. Altogether, 29 proteins were grouped in the TIP family, 52 in the PIP family, 4 in the SIP family and 28 in the NIP family. Similar proportions of members were established in related species, such as H. vulgare (Table S4). Then, we assigned each wheat AQP a name according to previously identified AQPs and their orthologues of O. sativa and A. thaliana (Figure 1, Table S5).
Figure 1.
Phylogenetic tree for Aquaporins (AQP) identified in Triticum aestivum, Arabidopsis thaliana, Populus trichocarpa and Oryza sativa. Five protein families (PIP, NIP, TIP, XIP and NIP) were identified. In red circles, 113 AQPs from wheat; in light-blue, 56 from P. trichocarpa; in dark-blue, 36 from A. thaliana; in green, 33 from O. sativa (Table S4).
3.2. Each Aquaporin Subfamily Presents Particular Physicochemical and Structural Characteristics
For the 113 wheat AQPs proteins, basic physicochemical characteristics were analyzed such as molecular weight, isoelectric point, subcellular localization, conserved motifs and amino acids associated with the selectivity pore, among others (Table S3). Generally, the AQP proteins had molecular weights (MWs) ranging from 19.3 to 47.2 kDa and isoelectric points (IPs) between 5.33 and 9.82. At the sequence level, 82 AQP proteins had 6 transmembrane domains and the remaining had 4, 5 or 7 domains (Table S6). Subcellular localization prediction for the AQP proteins indicated that they are located in the plasma membrane (59.3%), tonoplast (22.1%), chloroplast (11.5%), mitochondria (3.5%), endoplasmic reticulum (2.7%) and peroxisome (0.89%). Regarding the conserved NPA motifs, 106 AQP proteins maintained the sequence of the first conserved motif and 110 the second NPA motif. The ar/R selectivity filter present in the PIP family was conserved in all its members, while in the TIP family the filter was conserved at the subfamily level. On the other hand, the NIP family presented the greatest variations. For example, TaNIP1-11 and TaNIP1-10 change their amino acids in the first and second positions, respectively, while in the NIP3 subfamily only the amino acid at the fourth position was conserved among all their members (Table S3).
3.3. Gene Structure and Conserved Motifs of Wheat Aquaporin Proteins Confirm the Phylogenetic Classification
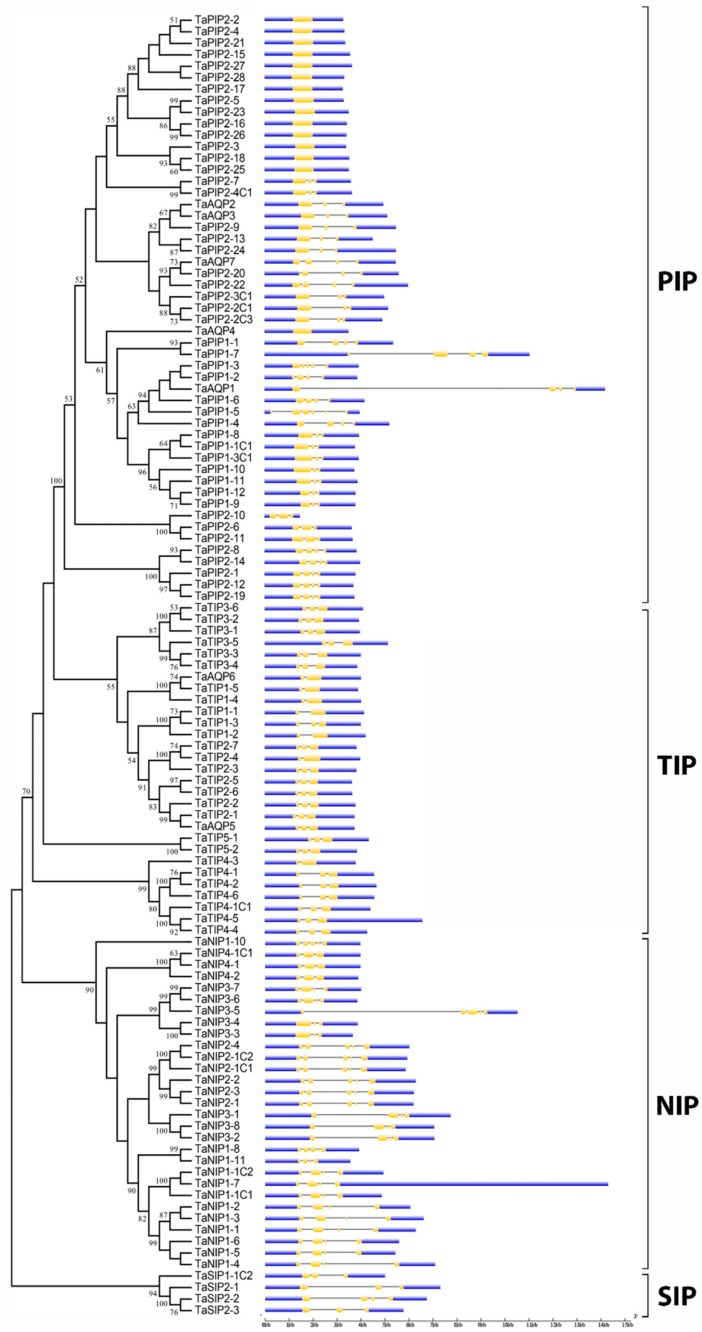
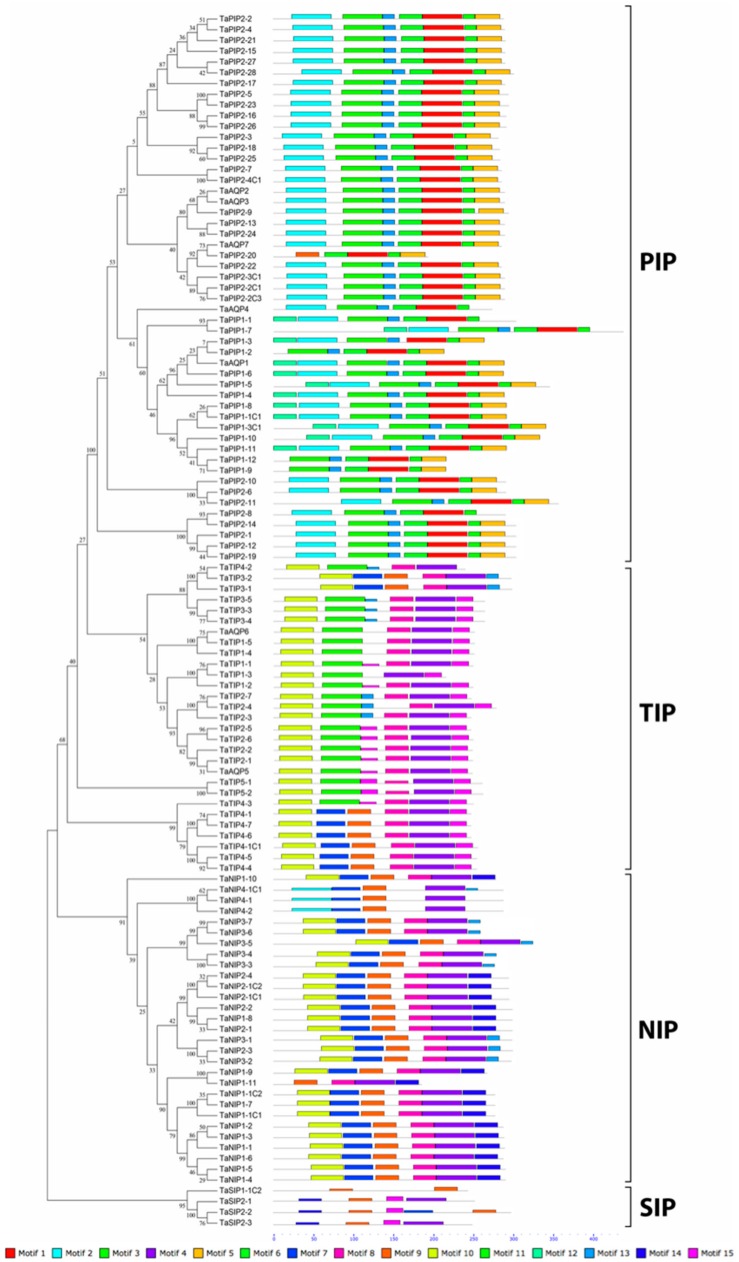
Gene exon-intron structural and motif analyses are relevant for the evolutionary study of gene families and to support the phylogenetic tree. The distribution of introns and exons in the 113 AQP genes was analyzed, revealing that the divergences in gene structures are consistent with phylogenetic classification (Figure 2, Table S7). All TIP genes had 1 to 2 introns. Within the PIP family, the PIP1 subfamily had 2 to 5 introns and the longest intron of all AQPs found, TaAQP1 10.5 kb. A significant number of PIP2 subfamily members had not introns and only some cases had 2 to 3 introns. The NIP family had 2 to 4 introns and the SIP family had 2 to 3. In general, the length and number of introns within each family or subfamily of AQP was similar to those of related species, such as H. vulgare. Then, to strengthen our phylogenetic analysis, a strategy for MEME analysis was also developed. Fifteen motifs were identified, named from 1 to 15 (Figure 3). Among these, the sequences of motifs from 1 to 10 matched with AQPs domains according to the analysis with InterPro Scan 5 (Table S8). The different motifs were grouped correspondingly with each big family or subfamily. Thus, similar domain patterns supported the relationships and phylogenetic classification of wheat AQP proteins.
Figure 2.
Exon-intron structure of wheat AQPs. In yellow, exons; in blue, the 3′ and 5′ UTR regions; and in the grey lines, introns.
Figure 3.
Identification of conserved motifs in wheat AQPs. The MEME database identified 15 motifs using the complete amino acid sequence represented proportionally by grey lines. Each color represents a different motif described in Table S7.
3.4. Segmental Duplications of Aquaporin Genes Have Contributed to High Number of Members in the Plasma Membrane Intrinsic Protein Family
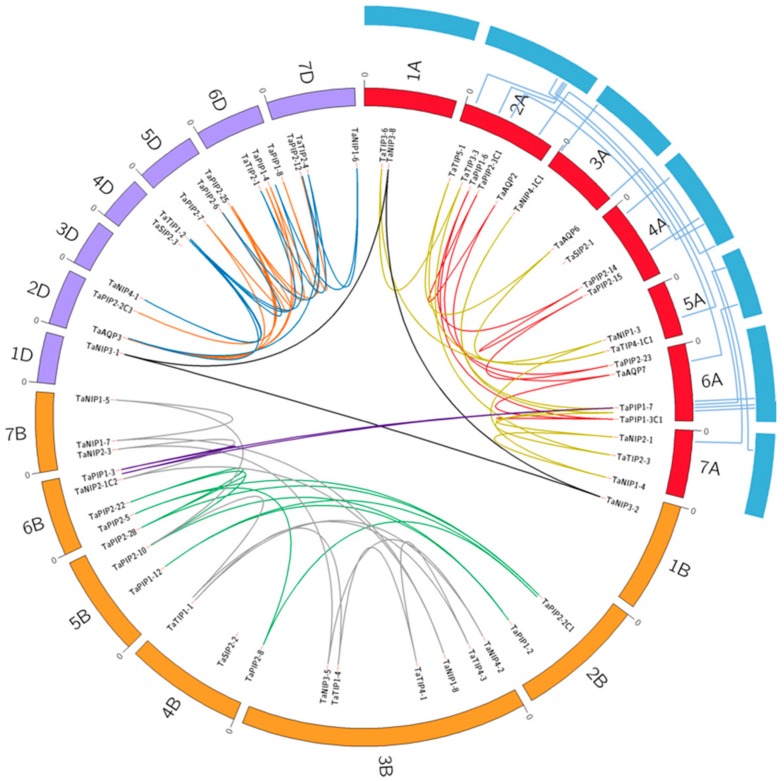
From the 113 AQP genes identified, the chromosomal location of 76 genes was determined and 8 clades of paralog genes were established (Figure 4, Table S9). Among them, 35 genes were located in sub-genome A, 42 in B and 36 in D. All chromosomes include AQP genes. The sets of chromosomes 7 and 1 were those with higher and lower abundance of genes, with 21 and 6 AQP genes, respectively. Polyploidization events play an important role in the expansion of the AQP genes in wheat, whose origins go back to the hybridization events between the species of T. urartu, A. speltoides and A. tauschii [69]. In this way, wheat had 24 groups of genes that were homolog copies on each of the A, B and D chromosomes, 15 pairs were on the A, B or D chromosomes and 11 genes were unique copies. Among them, TaAQP3 – TaPIP2-24 and TaPIP2-27 – TaPIP2-28 were tandem duplications and 21 pairs were segmental duplications concentrated mainly in the PIP family (Table 1 and Table S10). In the same context, to determine if the array of genes of the AQP family on the D sub-genome of wheat was conserved with respect to the genome of T. urartu, 24 pairs of orthologue genes were identified. Interestingly, some of these genes could be associated with the abiotic stress tolerance capacity of T. urartu. Among them, 8 genes maintained their chromosomal location in respect to wheat genome (Figure 4, Table S11), evidencing a medium collinearity level between the AQP genes of both species. This suggests that about half of the genes have changed their chromosomal location in the D sub-genome due to chromosomal inversion and crossover events.
Figure 4.
Analysis of synteny and chromosomal distribution of duplicated AQP genes of T. aestivum homolog groups and collinearity among AQP genes from T. aestivum and T. urartu. The locations of AQP genes are indicated on respective A, B and D sub-genomes of T. aestivum. Eight paralog groups are displayed in different colors. The light blue on the periphery represents chromosomes of T. urartu. The collinearity among TuAQPs and TaAQPs genes is represented by the lines between orthologue gene-pairs.
Table 1.
Evolutionary parameters for duplicated AQP genes. Table shows Ka/Ks ratios, duplication and selection types and time of divergence.
| AQP Pairs | Duplication Type | Ka 1 | Ks 2 | Ka/Ks | Selection | Time (mya 3) | ||
|---|---|---|---|---|---|---|---|---|
| TaAQP1 | vs. | TaPIP1-2 | Segmental | 0.03 | 0.08 | 0.34 | purifying | 6.08 |
| TaAQP2 | vs. | TaPIP2-13 | Segmental | 0.02 | 0.16 | 0.09 | purifying | 12.54 |
| TaPIP2-9 | vs. | TaPIP2-24 | Segmental | 0.02 | 0.14 | 0.13 | purifying | 10.85 |
| TaNIP1-3 | vs. | TaNIP1-4 | Segmental | 0.06 | 0.18 | 0.32 | purifying | 13.77 |
| TaNIP1-2 | vs. | TaNIP1-5 | Segmental | 0.04 | 0.15 | 0.25 | purifying | 11.54 |
| TaNIP1-1 | vs. | TaNIP1-6 | Segmental | 0.06 | 0.20 | 0.28 | purifying | 15.23 |
| TaPIP1-11 | vs. | TaPIP1-3C1 | Segmental | 0.01 | 0.12 | 0.05 | purifying | 9.15 |
| TaPIP1-10 | vs. | TaPIP1-12 | Segmental | 0.01 | 0.27 | 0.05 | purifying | 20.38 |
| TaPIP2-1 | vs. | TaPIP2-14 | Segmental | 0.05 | 0.13 | 0.41 | purifying | 10.23 |
| TaPIP1-12 | vs. | TaPIP1-1C1 | Segmental | 0.01 | 0.20 | 0.06 | purifying | 15.46 |
| TaPIP1-9 | vs. | TaPIP1-8 | Segmental | 0.01 | 0.22 | 0.06 | purifying | 16.62 |
| TaPIP2-11 | vs. | TaPIP2-1 | Segmental | 0.23 | 0.36 | 0.63 | purifying | 27.69 |
| TaPIP2-10 | vs. | TaPIP2-19 | Segmental | 0.27 | 0.33 | 0.82 | purifying | 25.15 |
| TaPIP2-6 | vs. | TaPIP2-12 | Segmental | 0.27 | 0.32 | 0.84 | purifying | 24.46 |
| TaPIP2-19 | vs. | TaPIP2-8 | Segmental | 0.16 | 0.22 | 0.71 | purifying | 17.00 |
| TaPIP2-17 | vs. | TaPIP2-28 | Segmental | 0.03 | 0.22 | 0.12 | purifying | 16.69 |
| TaPIP2-4 | vs. | TaPIP2-2 | Segmental | 0.04 | 0.24 | 0.15 | purifying | 18.38 |
| TaPIP2-28 | vs. | TaPIP2-27 | Tandem | 0.02 | 0.27 | 0.06 | purifying | 20.38 |
| TaPIP2-27 | vs. | TaPIP2-21 | Segmental | 0.02 | 0.18 | 0.09 | purifying | 13.77 |
| TaPIP2-26 | vs. | TaPIP2-23 | Segmental | 0.18 | 0.29 | 0.60 | purifying | 22.46 |
| TaPIP2-16 | vs. | TaPIP2-5 | Segmental | 0.17 | 0.25 | 0.69 | purifying | 18.92 |
| TaPIP2-18 | vs. | TaPIP2-3 | Segmental | 0.72 | 0.52 | 1.38 | positive | 40.31 |
| TaAQP3 | vs. | TaPIP2-24 | Tandem | 0.02 | 0.14 | 0.11 | purifying | 10.85 |
1 Ka: number of nonsynonymous substitutions per non-synonymous site; 2 Ks: number of synonymous substitutions per synonymous site. 3 Mya: million years ago.
To determine possible evolutionary forces acting on wheat AQP genes, the Ka/Ks ratio (synonymous and nonsynonymous substitutions per site) was estimated for each pair of duplicated genes (Table 1). Practically all the Ka/Ks ratios were less than 1 (between 0.05 and 0.84), suggesting that the purifying selection has an important role in maintaining the function of the AQP genes in wheat. Only on the pair TaPIP2-18 – TaPIP2-3 (Ka/Ks = 1.38) a positive selection pressure has been exerted. We also calculated the time of divergence between these duplicated genes and found that the duplications of the AQP genes were between 6,08 and 40.31 million years ago (mya), indicating that these duplications in the AQP family in wheat occurred before of the divergence of the common ancestor of the Triticum-Aegilops group (~4–5 mya) [70].
3.5. Spatio-Temporal Expression of Aquaporin Genes in Wheat
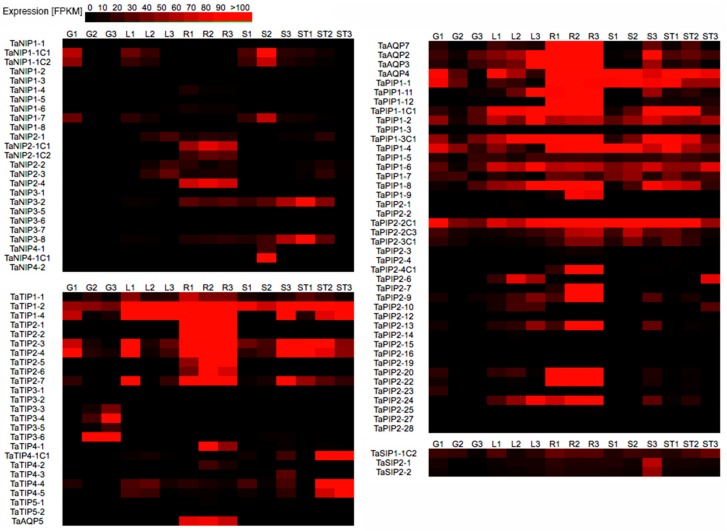
Analysis of gene expression profiles is a useful tool to understand their biological functions. Therefore, we analyzed the expression patterns of wheat AQP genes using microarray data obtained from public databases. In this approach, 94 of the 113 AQP genes (83.18%) were identified and a heatmap was designed to evaluate the abundance of transcripts in roots, leaves, stem, spikes and grains according to the Zadoks growth scale (Figure 5, Table S12). We observed that all AQP genes were expressed in at least one organ, with the exception of TaTIP5-2, indicating that they had remarkable organ and developmental specificity. For example, some NIP genes had high transcript levels in spikes, roots and stems, as TaNIP1-1C1, TaNIP1-1C2, TaNIP1-7, TaNIP4-1C1, or TaNIP2-1C1, among others. In the case of the TIP family, some TIP1 and TIP2 subfamily genes were expressed in the three stages of root, leaf, spike or stem development. Transcripts of the PIP family were accumulated in all the analyzed organs and developmental stages. Most of the members of this family were expressed in all developmental stages of roots and in one or two stages in leaf, stem, spike or grain. Within this family, TaAQP7, TaTIP1-12 and TaTIP2-22 were specifically expressed in roots, whereas TaPIP1-5 had constitutive levels in all organs and stages of development. On the other hand, TaPIP1-6 accumulated at different degrees in all organs and stages evaluated. In the SIP family only TaSIP1-1C2 transcripts accumulated in all organs, unlike TaSIP2-1 and TaSIP2-2, which were observed only in spikes.
Figure 5.
Analysis of AQP expression in different organs and developmental stages of wheat. Each heatmap corresponds to a subfamily of AQP. The intensity of the red color indicates the level of up-regulation of the gene in FPKM values. Development stages are indicated according to Zadoks scale: G1: Grain_Z71; G2: Grain_Z75; G3: Grain_Z85; L1: Leaf_Z10; L2: Leaf_Z23; L3: Leaf_Z71; R1: Root_Z10; R2: Root_Z13; R3: Root_Z39; S1: Spike_Z32; S2: Spike_Z39; S3: Spike_Z65; ST1: Stem_Z30; ST2: Stem_Z32; ST3: Stem_Z65. Microarray data were obtained from [62].
3.6. Wheat Aquaporin Gene Expression in Response to Osmotic Stress
In order to understand the role of AQP genes in stress under water deficit in wheat, we analyzed their expression patterns under osmotic stress, using microarray data obtained from WheatExp database (Figure 6, Table S13). The results showed that osmotic stress regulated differentially the accumulation of AQP transcripts. NIP genes tended to be down-regulated after 6 hours of stress. In the NIP1 subfamily, TaNIP1-3, TaNIP1-4 and TaNIP1-6 were strongly repressed, as was TaNIP2-1C1 from the NIP2 subfamily. Similarly, the expression of SIP genes was also down-regulated. In contrast, the transcripts of the majority of the TIP family increased (12 of 25 genes) at 6 hours of stress, with the exception of TaTIP1-1, TaTIP2-2 and TaTIP4-1C1 that were repressed. On the other hand, in the PIP family, 17 genes were up-regulated and 16 down-regulated at 6 hours of stress, whereas the expression levels of 13 genes were not altered. In this case, most down-regulated genes belonged to the PIP2 family (13 genes). In summary, we observed that some genes highly expressed in leaves or roots, such as: TaPIP1-1, TaPIP1-3C1, TaAQP7, TaPIP2-2C1, TaTIP2-24, TaPIP1-2, TaTIP2-3, TaTIP2-4, or TaTIP4-1, were also highly induced during stress, suggesting that they could be associated with the drought tolerance capacity of wheat.
Figure 6.
Analysis of AQP gene expression of wheat seedling leaves under osmotic stress. Green indicates a negative change or down-regulation and red indicates a positive change or up-regulation at 1 or 6 hours after treatment. The intensity of the colors indicates the fold change. Microarray data were obtained from [14].
3.7. Expression Analysis of Aquaporin Genes in Drought-Tolerant and Susceptible Wheat Genotypes Grown with and without Water Stress during Grain Filling
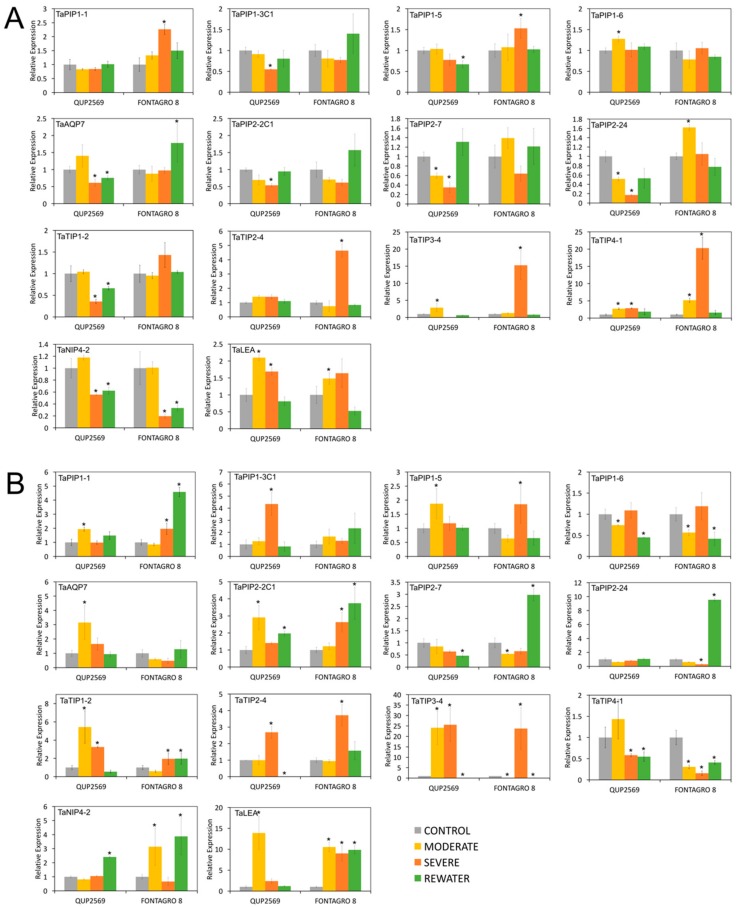
To investigate in more depth the relationship between the expression patterns of AQP genes and the tolerance capacity of wheat, we performed an analysis of relative expression of some genes previously mentioned by qPCR in leaves and roots between two wheat genotypes (accessions) with contrasting tolerance responses to water deficit (Figure 7). In this sense, we observed important differences at the transcript level between the drought-tolerant (Fontagro 8) and the drought-susceptible genotype (QUP2569). In leaves, as stress intensity increased, some AQP genes of the PIP1 and PIP2 subfamilies, like TaPIP1-1, TaPIP1-5, or TaPIP2-24, were clearly up-regulated in the tolerant genotype, whereas in the genotype susceptible the expression levels were down-regulated (Figure 7A). In the case of the TIP family, they also increased their expression levels during severe stress. On the other hand, an opposite effect was seen in the expression of AQP genes in roots, where the tolerant genotype showed a down-regulation of TaPIP1-1, TaPIP1-5, TaAQP7, TaPIP2-1C1, TaTIP2-1, TaTIP3-4 and TaTIP4-1 genes under moderate stress, while the susceptible one presented up-regulation (Figure 7B). Interestingly, during the rehydration of the tolerant genotype, several genes of the PIP2 subfamily were induced in leaves and roots. This could be associated with a better internal water balance and faster rehydration ability of Fontagro 8.
Figure 7.
Expression profiles of AQP genes for drought-tolerant (Fontagro 8) and drought-susceptible (QUP2569) genotypes with and without water stress. Quantitative PCR analyses for 13 AQP genes in leaves (A) and roots (B) of wheat genotypes under well-watered conditions (control), moderate water stress, severe water stress and re-watering. Columns and error bars represent the mean and standard deviation for three biological by three technical replicates. * p-value < 0.05.
4. Discussion
The increasing number of sequenced genomes opens new horizons in the study of functional genomics and the precision of annotating new genes [71,72]. In this sense, genome-wide identification methods allow greater accuracy in the characterization of multiple gene families, such as AQPs. These play an important role in the cellular homeostasis of different organs and tissues, increasing the permeability of the plasma membrane to water and essential nutrients [2,33]. Different studies in species like O. sativa [73], Z. mays [74], Sorghum bicolor [75], Setaria italica [76], or H. vulgare identified AQPs in cereals. In addition, previously, 48 AQP genes [35,36] were identified in wheat by EST analysis. In this study, 65 new non-redundant AQP genes were identified, a number that is consistent with the allohexaploid genome, as predicted earlier by Forrest and Bhave [77], who suggested that a higher number of AQP genes are present in the wheat genome.
Consistent with the AQP classification of O. sativa [73], H. vulgare [23], A. thaliana [78] and P. trichocarpa [79], the 113 wheat AQP genes total grouped in 4 families: NIP, TIP, PIP and SIP. At the same time, NIP separated according to the pattern of conserved motifs of 5 subfamilies: NIP1, NIP2, NIP3, NIP4 and NIP5; PIP of 2 subfamilies: PIP1 and PIP2; TIP of 5 subfamilies: TIP1, TIP2, TIP3, TIP4 and TIP5; and SIP of 2 subfamilies: SIP1 and SIP2. The structure of these groups is closely related to the genetic relationships with O. sativa, A. thaliana and in a lower degree with P. trichocarpa, which supports previous phylogenetic classifications [22,80,81]. Coincidentally, the structural exon-intron patterns of the genes of all subfamilies of wheat AQPs are specific to each subfamily and similar in number and length only to closely related species, such as H. vulgare [23]. It would reflect a high degree of conservation for this gene family, despite the processes of natural and artificial polyploidy that occurred in wheat [82].
By evolutionary analyses, polyploidy events and segmental and tandem duplications have had an important role in increasing the total number of genes [82,83]. In wheat, each sub-genome provides approximately one-third of the family of AQPs. However, 20% are genes duplicated in tandem or segmentally, indicating that both mechanisms have contributed significantly to the expansion of the members of this family, especially of the PIP family. A similar result has been reported in B. rapa [71] and Z. mays [74]. During evolutionary processes, events of crossover and inversion of important chromosome zones, even distal, can modify the location and function of hundreds of genes [84]. In this context, the collinearity levels between the orthologue AQP genes of the wheat D sub-genome and T. urartu reveal that their localization has been conserved, which is consistent with the auxin/indole-3-acetic acid family of genes in wheat [56]. However, some pairs of orthologue genes (e.g., TaAQP6 or TaAQP7) present different locations between both genomes, suggesting re-arrangements throughout the evolution of the AQP gene family in wheat, similar to that evidenced by the MADS-box family of this same species [85]. The different duplications identified in this work reached values of Ka/Ks ratio <1, indicating that the evolutionary history of the AQP genes is under a purifying selection pressure, which suggests a high conservation of the duplicated genes, similar to the AQP family in B. rapa [71]. Taken together and despite the structural redundancy, it is evident that for several pairs of duplicated genes (for example, TaPIP1-12 and TaPIP1-1C1) the patterns of organ expression and specific time of expression are different, eventually revealing divergence and functional diversity [86]. This is interesting because it suggests that functional changes are often regulated by genetic and epigenetic interactions between homoalleles, providing a sufficient amount of plasticity required for coping with new environmental scenarios [87].
The microarray data analysis revealed that a large number of AQP genes, unique or duplicates, especially PIP and TIP, are highly expressed in wheat leaves, consistently with homolog genes reports in other species [29,88,89,90] and confirmed here with the expression analysis. The comparison of two contrasting wheat genotypes growing in greenhouse conditions with and without water stress during grain filling revealed different AQP expression patterns in leaves and roots between the tolerant and the susceptible genotypes. In the tolerant genotype, the relative abundance of transcripts for different AQP genes in roots decreased under moderate water stress, while that in the susceptible one it increased. Interestingly, this inverse pattern is similar to that reported in contrasting O. sativa genotypes, a monocotyledonous species related to wheat [91]. On the other hand, we observed that in the tolerant genotype, the expression levels of several PIP1, PIP2 and TIP genes increase during the re-watering. It is probably that the flow of water mediated by PIP1 or PIP2 towards the cytoplasm and by TIPs towards the vacuole in tolerant genotype is efficiently conducted for its compartmentalization. This hypothesis agrees with studies of PIP1 from Salicornia bigelovii or AQP7 from wheat expressed in Nicotiana tabacum, PgTIP1 from Panax ginseng in Glycine max and SlPIP2-7 from S. lycopersicum or JcPIP2-7 from Jatropha curcas in A. thaliana [25,32,92,93,94]. However, there is no total consensus about the physiological mechanisms associated with the function of AQP genes and how and/or why these genes are up or down-regulated in plants under the same water stress [95]. Different studies have associated the down-regulation of AQPs with an adaptive mechanism to reduce the effects of water deficit stress [96,97] but future analyses associated with the functional characterization of these candidate genes or proteins will be necessary to confirm these results and to draw general conclusions.
5. Conclusions
The breeding of wheat cultivars with augmented tolerance to water deficit requires, among other subjects, an exhaustive characterization of the molecular mechanisms that underlie physiological processes in the plant. The characterization of proteins related to the control of water status in tissues and organs, from a structural and functional genomic point of view, could contribute to this goal. The results obtained in this study updated the classification of AQP genes and proteins recently generated by several sequencing projects. Furthermore, they showed that, in spite of the presence of a high number of AQP genes in wheat (113), an important number of them are copies coming from polyploidization events and from segmental or tandem duplications in the ancestral species (60%). Although the conservation rate is also high, there is functional diversity as revealed by the expression patterns of several copies of AQP genes in different organs and between contrasting genotypes when they are subjected to stress by water deficit. Thus, this research contributes relevant information for future studies on wheat AQPs and confirms the complexity associated with their regulation.
Acknowledgments
The authors are also thankful for the support from the Plant Breeding and Phenomic Center of the University of Talca and Simón Ruiz of the Laboratory of Functional Genomics, Institute of Biological Sciences, University of Talca, Chile. We thank the Academic Writing Center in the Programa de Idiomas at Universidad de Talca, Chile.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4425/9/10/497/s1, Table S1: Evaluation of physiological parameters of wheat contrasting genotypes under water stress, Table S2: Primers used in this study, Table S3: Characterization of genes identified in this study, Table S4: Number of proteins in each family and subfamily of aquaporins in different species, Table S5: Accession codes of AQP genes used in the phylogenetic tree, Table S6: Predicted transmembrane domains of AQP proteins, Table S7: Pattern of introns and exons in T. aestivum and H. vulgare, Table S8: Regular expression profile of the conserved motifs defined by MEME of AQPs, Table S9: Chromosomal location and paralog groups of AQP genes, Table S10: Homologs and duplications of AQP genes, Table S11: Pairs of orthologue genes identified in T. aestivum and T. Urartu, Table S12: Analysis of AQP gene expression in different organs and stages of wheat development, Table S13: Analysis of AQP gene expression in wheat seedlings responding to osmotic stress.
Author Contributions
Conceptualization, J.M.-E., N.B.-S., F.P.G. and A.d.P.; Data curation, N.B.-S.; Formal analysis, J.M.-E., N.B.-S. and A.G.; Funding acquisition, N.B.-S.; Investigation, J.M.-E., N.B.-S. and A.G.; Methodology, J.M.-E. and N.B.-S.; Resources, N.B.-S., F.G. and A.P; Software, J.M.-E.; Supervision, F.P.G. and A.d.P; Visualization, J.M.-E. Writing—original draft, J.M.-E. and N.B.-S.; Writing—review & editing, F.P.G. and A.d.P.
Funding
This research was funded by CONICYT through the projects Posdoctoral No. 3150021, Fondecyt No. 1150353 and Programa Nacional de Becas de Posgrado No. 21170702.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhou M. Barley Production and Consumption. In: Zhang G., Li C., editors. Genetics and Improvement of Barley Malt Quality. Springer; Berlin, Germany: 2009. pp. 1–17. Advanced Topics in Science and Technology in China. [Google Scholar]
- 2.Blum A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009;112:119–123. doi: 10.1016/j.fcr.2009.03.009. [DOI] [Google Scholar]
- 3.Del Pozo A., Yáñez A., Matus I.A., Tapia G., Castillo D., Sánchez-Jardón L., Araus J.L. Physiological traits associated with wheat yield potential and performance under water-stress in a Mediterranean environment. Front. Plant Sci. 2016;7:987. doi: 10.3389/fpls.2016.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramley H., Turner D.W., Tyerman S.D., Turner N.C. Water flow in the roots of Crop species: The influence of root structure, aquaporin activity and waterlogging. Adv. Agron. 2007;96:133–196. [Google Scholar]
- 5.Bramley H., Turner N.C., Turner D.W., Tyerman S.D. Roles of morphology, anatomy and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol. 2009;150:348–364. doi: 10.1104/pp.108.134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurel C., Verdoucq L., Luu D.T., Santoni V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 7.Johansson I., Karlsson M., Johanson U., Larsson C., Kjellbom P. The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta. 2000;1465:324–342. doi: 10.1016/S0005-2736(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 8.Javot H., Lauvergeat V., Santoni V., Martin-Laurent F., Güçlü J., Vinh J., Heyes J., Franck K.I., Schäffner A.R., Bouchez D., et al. Role of a single aquaporin isoform in root water uptake. Plant Cell. 2003;15:509–522. doi: 10.1105/tpc.008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uehlein N., Lovisolo C., Siefritz F., Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;25:734–737. doi: 10.1038/nature02027. [DOI] [PubMed] [Google Scholar]
- 10.Takano J., Wada M., Ludewig U., Schaaf G., Von Wirén N., Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell. 2006;18:1498–1509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J.F., Tamai K., Yamaji N., Mitani N., Konishi S., Katsuhara M., Ishiguro M., Murata Y., Yano M. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- 12.Maurel C., Boursiac Y., Luu D.T., Santoni V., Shahzad Z., Verdoucq L. Aquaporins in plants. Physiol Rev. 2015;95:1321–1358. doi: 10.1152/physrev.00008.2015. [DOI] [PubMed] [Google Scholar]
- 13.Afzal Z., Howton T.C., Sun Y., Mukhtar M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016;4:9. doi: 10.3390/jdb4010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z., Xin M., Qin J., Peng H., Ni Z., Yao Y., Sun Q. Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat (Triticum aestivum L.) BMC Plant Biol. 2015;15:152. doi: 10.1186/s12870-015-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pou A., Medrano H., Flexas J., Tyerman S.D. A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ. 2013;36:828–843. doi: 10.1111/pce.12019. [DOI] [PubMed] [Google Scholar]
- 16.Obroucheva N., Sin’kevich I. Aquaporins and cell growth. Russ. J. Plant Physiol. 2010;57:153–165. doi: 10.1134/S1021443710020019. [DOI] [Google Scholar]
- 17.Li D.D., Wu Y.J., Ruan X.M., Li B., Zhu L., Wang H., Li X.B. Expressions of three cotton genes encoding the PIP proteins are regulated in root development and in response to stresses. Plant Cell Rep. 2013;28:291–300. doi: 10.1007/s00299-008-0626-6. [DOI] [PubMed] [Google Scholar]
- 18.Yu Q.J., Hu Y.L., Li J.F., Wu Q., Lin Z.P. Sense and antisense expression of plasma membrane aquaporin BnPIP1 from Brassica napus in tobacco and its effects on plant drought resistance. Plant Sci. 2005;169:647–656. doi: 10.1016/j.plantsci.2005.04.013. [DOI] [Google Scholar]
- 19.Eto K., Noda Y., Horikawa S., Uchida S., Sasaki S. Phosphorylation of aquaporin-2 regulates its water permeability. J. Biol. Chem. 2010;285:40777–40784. doi: 10.1074/jbc.M110.151928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaldenhoff R., Fischer M. Aquaporins in plants. Acta Physiol. 2006;187:169–176. doi: 10.1111/j.1748-1716.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- 21.Reuscher S., Akiyama M., Mori C., Aoki K., Shibata D., Shiratake K. Genome-wide identification and expression analysis of aquaporins in tomato. PLoS ONE. 2013;8:e79052. doi: 10.1371/journal.pone.0079052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu W., Hou X., Huang C., Yan Y., Tie W., Ding Z., Wei Y., Liu J., Miao H., Lu Z., et al. Genome-wide identification and expression analyses of aquaporin gene family during development and abiotic stress in banana. Int. J. Mol. Sci. 2015;16:19728–19751. doi: 10.3390/ijms160819728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hove R.M., Ziemann M., Bhave M. Identification and expression analysis of the barley (Hordeum vulgare L.) aquaporin gene family. PLoS ONE. 2015;10:e0128025. doi: 10.1371/journal.pone.0128025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexandersson E., Fraysse L., Sjovall-Larsen S., Gustavsson S., Fellert M., Karlsson M., Johanson U., Kjellbom P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005;59:469–484. doi: 10.1007/s11103-005-0352-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhou S., Hu W., Deng X., Ma Z., Chen L., Huang C., Wang C., Wang J., He Y., Yang G., et al. Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS ONE. 2012;7:e52439. doi: 10.1371/journal.pone.0052439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu W., Yuan Q., Wang Y., Cai R., Deng X., Wang J., Zhou S., Chen M., Chen L., Huang C., et al. Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiol. 2012;53:2127–2141. doi: 10.1093/pcp/pcs154. [DOI] [PubMed] [Google Scholar]
- 27.Gao Z., He X., Zhao B., Zhou C., Liang Y., Ge R., Shen Y., Huang Z. Overexpressing a putative aquaporin gene from wheat, TaNIP, enhances salt tolerance in transgenic Arabidopsis. Plant Cell Physiol. 2010;51:767–775. doi: 10.1093/pcp/pcq036. [DOI] [PubMed] [Google Scholar]
- 28.Cui X.H., Hao F.S., Chen H., Chen J., Wang X.C. Expression of the Vicia faba VfPIP1 gene in Arabidopsis thaliana plants improves their drought resistance. J. Plant Res. 2008;121:207–214. doi: 10.1007/s10265-007-0130-z. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y., Hu W., Liu J., Zhang J., Jia C., Miao H., Xu B., Jin Z. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014;14:59. doi: 10.1186/1471-2229-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Y., Lin W., Cai W., Arora R. Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta. 2007;226:729–740. doi: 10.1007/s00425-007-0520-4. [DOI] [PubMed] [Google Scholar]
- 31.Guo L., Wang Z.Y., Lin H., Cui W.E., Chen J., Liu M., Chen Z.L., Qu L.J., Gu H. Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 2006;16:277–286. doi: 10.1038/sj.cr.7310035. [DOI] [PubMed] [Google Scholar]
- 32.Li R., Wang J., Li S., Zhang L., Qi C., Weeda S., Zhao B., Ren S., Guo Y.D. Plasma membrane intrinsic proteins SlPIP2;1, SlPIP2;7 and SlPIP2;5 conferring enhanced drought stress tolerance in tomato. Sci. Rep. 2016;6:31814. doi: 10.1038/srep31814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deshmukh R.K., Sonah H., Bélanger R.R. Plant aquaporins: Genome-wide identification, transcriptomics, proteomics and advanced analytical tools. Front. Plant Sci. 2016;7:1896. doi: 10.3389/fpls.2016.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quigley F., Rosenberg J.M., Shachar-Hill Y., Bohnert H.J. From genome to function: The Arabidopsis aquaporins. Genome Biol. 2002;3:research0001.0001–research0001.0017. doi: 10.1186/gb-2001-3-1-research0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forrest K., Bhave M. The PIP and TIP aquaporins in wheat form a large and diverse family with unique gene structures and functionally important features. Funct. Int. Gen. 2008;8:115–133. doi: 10.1007/s10142-007-0065-4. [DOI] [PubMed] [Google Scholar]
- 36.Pandey B., Sharma P., Pandey D.M., Sharma I., Chatrath R. Identification of new aquaporin genes and single nucleotide polymorphism in bread wheat. Evol. Bioinform. 2013;9:437–452. doi: 10.4137/EBO.S12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International Wheat Genome Sequencing Consortium A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science. 2014;345:1251788. doi: 10.1126/science.1251788. [DOI] [PubMed] [Google Scholar]
- 38.Wicker T., Mayer K.F., Gundlach H., Martis M., Steuernagel B., Scholz U., Simková H., Kubaláková M., Choulet F., Taudien S., et al. Frequent gene movement and pseudogene evolution is common to the large and complex genomes of wheat, barley and their relatives. Plant Cell. 2011;23:1706–1718. doi: 10.1105/tpc.111.086629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teng W., Zhao Y.-Y., Zhao X.-Q., He X., Ma W.-Y., Deng Y., Chen X.P., Tong Y.P. Genome-wide Identification, Characterization and Expression Analysis of PHT1 Phosphate Transporters in Wheat. Front. Plant Sci. 2017;8:543. doi: 10.3389/fpls.2017.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan H., Yue H., Zhao X., Wang M., Song W., Nie X. Genome-Wide Identification and Analysis of MAPK and MAPKK Gene Families in Bread Wheat (Triticum aestivum L.) Genes. 2017;8:284. doi: 10.3390/genes8100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y., Wang Z.Y., Kumar V., Xu X.F., Yuan P., Zhu X.F., Li T.Y., Jia B., Xuan Y.H. Genome-wide identification of the SWEET gene family in wheat. Gene. 2018;642:284–292. doi: 10.1016/j.gene.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J.F., Xu Y.Q., Dong J.M., Peng L.N., Feng X., Wang X., Li F., Miao Y., Yao S.K., Zhao Q.Q., et al. Genome-wide identification of wheat (Triticum aestivum) expansins and expansin expression analysis in cold-tolerant and cold-sensitive wheat cultivars. PLoS ONE. 2018;13:e0195138. doi: 10.1371/journal.pone.0195138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kersey P.J., Allen J.E., Armean I., Boddu S., Bolt B.J., Carvalho-Silva D., Christensen M., Davis P., Falin L.J., Grabmueller C., et al. Ensembl Genomes 2016: More genomes, more complexity. Nucleic Acids Res. 2016;44:D574–D580. doi: 10.1093/nar/gkv1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R., Eddy S.R., Heger S.R., Hethernigton K., Holm L., Mistry J., et al. Pfam: The protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finn R.D., Clements J., Eddy S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedruzzi I., Rivoire C., Auchincloss A.H., Coudert E., Keller G., de Castro E., Baratin D., Cuche B.A., Bougueleret L., Poux S., et al. HAMAP in 2015: Updates to the protein family classification and annotation system. Nucleic Acids Res. 2015;43:D1064–D1070. doi: 10.1093/nar/gku1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchler-Bauer A., Anderson J.B., Cherukuri P.F., DeWeese-Scott C., Geer L.Y., Gwadz M., He S., Hurwitz D.I., Jackson J.D., Ke Z., et al. CDD: A Conserved Domain Database for protein classification. Nucleic Acids Res. 2005;33:D192–D196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu B., Jin J., Guo A.Y., Zhang H., Luo J., Gao G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey T.L., Williams N., Misleh C., Li W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2016;34:W369–W373. doi: 10.1093/nar/gkl198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones P., Binns D., Chang H.Y., Fraser M., Li W., McAnulla C., McWilliam H., Maslen J., Mitchell A., Nuka G., et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R.D., Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Möller S., Croning M.D.R., Apweiler R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics. 2001;17:646–653. doi: 10.1093/bioinformatics/17.7.646. [DOI] [PubMed] [Google Scholar]
- 55.Horton P., Park K.J., Obayashi T., Fujita N., Harada H., Adams-Collier C.J., Nakai K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao L., Zhang X., Han X., Zhang L., Li X., Zhan H., Ma J., Luo P., Zhang W., Cui L., et al. A genome-wide analysis of the auxin/indole-3-acetic acid gene family in hexaploid bread wheat (Triticum aestivum L.) Front. Plant Sci. 2015;6:770. doi: 10.3389/fpls.2015.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ling H.Q., Zhao S., Liu D., Wang J., Sun H., Zhang C., Fan H., Li D., Dong L., Tao Y., et al. Draft genome of the wheat A-genome progenitor Triticum urartu. Nature. 2013;496:87–90. doi: 10.1038/nature11997. [DOI] [PubMed] [Google Scholar]
- 58.Cheong W.H., Tan Y.C., Yap S.J., Ng K.P. ClicO FS: An interactive web-based service of Circos. Bioinformatics. 2015;31:3685–3687. doi: 10.1093/bioinformatics/btv433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 60.Mao H., Yu L., Li Z., Yan Y., Han R., Liu H., Ma M. Genome-wide analysis of the SPL family transcription factors and their responses to abiotic stresses in maize. Plant Gene. 2016;6:1–12. doi: 10.1016/j.plgene.2016.03.003. [DOI] [Google Scholar]
- 61.Zadoks J.C., Chang T.T., Konzak C.F. A decimal code for the growth stages of cereals. Weed Res. 1974;14:415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- 62.Choulet F., Alberti A., Theil S., Glover N., Barbe V., Daron J., Pingault L., Sourdille P., Couloux A., Paux E., et al. Structural and functional partitioning of bread wheat chromosome 3B. Science. 2014;345:1249721. doi: 10.1126/science.1249721. [DOI] [PubMed] [Google Scholar]
- 63.Toufighi K., Brady S.M., Austin R., Ly E., Provart N.J. The Botany Array Resource: E-northerns, expression angling, and promoter analyses. Plant J. 2005;43:153–163. doi: 10.1111/j.1365-313X.2005.02437.x. [DOI] [PubMed] [Google Scholar]
- 64.Pérez-Díaz R., Madrid-Espinoza J., Salinas-Cornejo J., González-Villanueva E., Ruiz-Lara S. Differential Roles for VviGST1, VviGST3 and VviGST4 in Proanthocyanidin and Anthocyanin Transport in Vitis vinífera. Front. Plant Sci. 2016;7:1166. doi: 10.3389/fpls.2016.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paolacci A.R., Tanzarella O.A., Porceddu E., Ciaffi M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol. 2009;10:11. doi: 10.1186/1471-2199-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rampino P., Pataleo S., Gerardi C., Mita G., Perrotta C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2016;29:2143–2152. doi: 10.1111/j.1365-3040.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- 67.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- 68.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2∆∆C(T) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 69.Qin L., Zhao J., Li T., Hou J., Zhang X., Hao C. TaGW2, a Good Reflection of Wheat Polyploidization and Evolution. Front. Plant Sci. 2017;8:318. doi: 10.3389/fpls.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chalupska D., Lee H.Y., Faris J.D., Evrard A., Chalhoub B., Haselkorn R., Gornicki P. Acc homoeoloci and the evolution of wheat genomes. Proc. Natl. Acad. Sci. USA. 2008;105:9691–9696. doi: 10.1073/pnas.0803981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kayum M.A., Park J.I., Nath U.K., Biswas M.K., Kim H.T., Nou I.S. Genome-wide expression profiling of aquaporin genes confer responses to abiotic and biotic stresses in Brassica rapa. BMC Plant Biol. 2017;17:23. doi: 10.1186/s12870-017-0979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun H., Li L., Lou Y., Zhao H., Gao Z. Genome-wide identification and characterization of aquaporin gene family in moso bamboo (Phyllostachys edulis) Mol. Biol. Rep. 2016;43:437–450. doi: 10.1007/s11033-016-3973-3. [DOI] [PubMed] [Google Scholar]
- 73.Sakurai J., Ishikawa F., Yamaguchi T., Uemura M., Maeshima M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005;46:1568–1577. doi: 10.1093/pcp/pci172. [DOI] [PubMed] [Google Scholar]
- 74.Chaumont F., Barrieu F., Wojcik E., Chrispeels M.J., Jung R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001;125:1206–1215. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reddy P.S., Rao T., Sharma K., Vadez V. Genome-wide identification and characterization of the aquaporin gene family in Sorghum bicolor (L.) Plant Gene. 2001;1:18–28. doi: 10.1016/j.plgene.2014.12.002. [DOI] [Google Scholar]
- 76.Azad A., Ahmed J., Alum A., Hasan M., Ishikawa T., Sawa Y., Katsuhara M. Genome-wide characterization of major intrinsic proteins in four grass plants and their non-aqua transport selectivity profiles with comparative perspective. PLoS ONE. 2016;11:e0157735. doi: 10.1371/journal.pone.0157735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forrest K., Bhave M. Major intrinsic proteins (MIPs) in plants: A complex gene family with major impacts on plant phenotype. Funct. Int. Gen. 2007;7:263–289. doi: 10.1007/s10142-007-0049-4. [DOI] [PubMed] [Google Scholar]
- 78.Johanson U., Karlsson M., Johansson I., Gustavsson S., Sjovall S., Fraysse L., Weig A., Kjellbom P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001;126:1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta A.B., Sankararamakrishnan R. Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: Characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol. 2009;9:134. doi: 10.1186/1471-2229-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Devos K., Gale M. Genome Relationships. The Grass Model in Current Research. Plant Cell. 2000;12:637–646. doi: 10.1105/tpc.12.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sorrells M.E., La Rota M., Bermudez-Kandianis C.E., Greene R.A., Kantety R., Munkvold J.D., Miftahudin, Mahmoud A., Ma X., Gustafson P.J., et al. Comparative DNA Sequence Analysis of Wheat and Rice Genomes. Genome Res. 2003;13:1818–1827. doi: 10.1101/gr.1113003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glover N., Daron J., Pingault L., Vandepoele K., Paux E., Feuillet C., Choulet F. Small-scale gene duplications played a major role in the recent evolution of wheat chromosome 3B. Genome Biol. 2015;16:188. doi: 10.1186/s13059-015-0754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schoenfelder K.P., Fox D.T. The expanding implications of polyploidy. J. Cell Biol. 2015;209:485–491. doi: 10.1083/jcb.201502016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lukaszewski A.J., Kopecky D., Linc G. Inversions of chromosome arms 4AL and 2BS in wheat invert the patterns of chiasma distribution. Chromosoma. 2012;121:201–208. doi: 10.1007/s00412-011-0354-5. [DOI] [PubMed] [Google Scholar]
- 85.Ma J., Yang Y., Luo W., Yang C., Ding P., Liu Y., Qiao L., Chang Z., Geng H., Wang P., et al. Genome-wide identification and analysis of the MADS-box gene family in bread wheat (Triticum aestivum L.) PLoS ONE. 2017;12:e0181443. doi: 10.1371/journal.pone.0181443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ha M., Kim E.D., Chen Z.J. Duplicate genes increase expression diversity in closely related species and allopolyploids. Proc. Natl. Acad. Sci. USA. 2009;106:2295–2300. doi: 10.1073/pnas.0807350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feldman M., Levy A. Genome evolution due to allopolyploidization in wheat. Genetics. 2012;192:763–774. doi: 10.1534/genetics.112.146316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alexandersson E., Danielson J.A., Råde J., Moparthi V.K., Fontes M., Kjellbom P., Johanson U. Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant Gene. 2010;61:650–660. doi: 10.1111/j.1365-313X.2009.04087.x. [DOI] [PubMed] [Google Scholar]
- 89.Jang J.Y., Rhee J.Y., Chung G.C., Kang H. Aquaporin as a membrane transporter of hydrogen peroxide in plant response to stresses. Plant Signal. Behav. 2012;7:1180–1181. doi: 10.4161/psb.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lian H.L., Yu X., Lane D., Sun W.N., Tang Z.C., Su W.A. Upland rice and lowland rice exhibited different PIP expression under water deficit and ABA treatment. Cell Res. 2006;16:651–660. doi: 10.1038/sj.cr.7310068. [DOI] [PubMed] [Google Scholar]
- 91.Ai-hua X., Cui K., Wang W., Wang Z., Huang J., Nie L., Yong L., Shao-bing P. Differential responses of water uptake pathways and expression of two Aquaporin genes to water-deficit in rice seedlings of two genotypes. Rice Sci. 2017;24:187–197. doi: 10.1016/j.rsci.2017.03.001. [DOI] [Google Scholar]
- 92.Khan K., Agarwal P., Shanware A., Sane V.A. Heterologous expression of two Jatropha Aquaporins imparts drought and salt tolerance and improves seed viability in transgenic Arabidopsis thaliana. PLoS ONE. 2015;10:e0128866. doi: 10.1371/journal.pone.0128866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.An J., Cheng C., Hu Z., Chen H., Cai W., Yu B. The Panax ginseng PgTIP1 gene confers enhanced salt and drought tolerance to transgenic soybean plants by maintaining homeostasis of water, salt ions and ROS. Environ. Exper. Bot. 2018;155:45–55. doi: 10.1016/j.envexpbot.2018.06.025. [DOI] [Google Scholar]
- 94.Sun X., Deng Y., Liang L., Jia Z., Xiao Z., Su J. Overexpression of a PIP1 gene from Salicornia bigelovii in tobacco plants improves their drought tolerance. J. Am. Soc. Hortic. Sci. 2017;142:235–245. doi: 10.21273/JASHS04098-17. [DOI] [Google Scholar]
- 95.Tyerman S.D., Niemietz C.M., Bramley H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002;25:173–194. doi: 10.1046/j.0016-8025.2001.00791.x. [DOI] [PubMed] [Google Scholar]
- 96.Siemens J.A., Zwiazek J.J. Effects of water deficit stress and recovery on the root water relations of trembling aspen (Populus tremuloides) seedlings. Plant Sci. 2003;165:113–120. doi: 10.1016/S0168-9452(03)00149-3. [DOI] [Google Scholar]
- 97.Srivastava A.K., Penna S., Nguyen D.V., Tran L.S. Multifaceted roles of aquaporins as molecular conduits in plant responses to abiotic stresses. Crit. Rev. Biotechnol. 2016;36:389–398. doi: 10.3109/07388551.2014.973367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.