Abstract
Irritable bowel syndrome (IBS) remains a prevalent and difficult-to-manage gastrointestinal condition. There is growing interest in the use of traditional medicine to manage IBS. In particular, curcumin, a biologically active phytochemical, has demonstrated anti-inflammatory and anti-oxidant properties and mucosal protective effects in rat models of colitis. This meta-analysis thus aimed to investigate the hypothesis that curcumin improves IBS symptoms. Using the keywords (curcumin OR turmeric OR Indian saffron OR diferuloylmethane OR curcuminoid) AND (irritable bowel syndrome OR IBS), a preliminary search on the PubMed, Medline, Embase, PsychINFO, Web of Science, and Google Scholar databases yielded 1080 papers published in English between 1 January 1988 and 1 May 2018. Five randomized, controlled trials were systematically reviewed and 3 were included in the final meta-analysis. Random-effects meta-analysis based on three studies and 326 patients found curcumin to have a beneficial albeit not statistically significant effect on IBS symptoms (pooled standardized mean difference from baseline IBS severity rating −0.466, 95% CI: −1.113 to 0.182, p = 0.158). This is the first meta-analysis to examine the use of curcumin in IBS. With its unique anti-oxidant and anti-inflammatory activities and ability to modulate gut microbiota, curcumin is a potentially useful addition to our armamentarium of agents for IBS. It also appears safe and well-tolerated, with no adverse events reported in the available trials. However, current findings are based on a considerably limited evidence base with marked heterogeneity. More robust clinical trials involving a standardized curcumin preparation and larger sample sizes should be encouraged.
Keywords: IBS, irritable bowel syndrome, functional, curcumin, turmeric, Indian saffron, natural product
1. Introduction
Irritable bowel syndrome (IBS) is an exceedingly common and debilitating gastrointestinal condition, characterized by chronic abdominal pain and a change in the frequency or form of stool [1]. It affects an estimated 10% to 15% of the global population [2] and carries a significant disease burden in terms of decreased productivity, increased healthcare costs and reduced health-related quality of life [3].
Despite the prevalence of IBS, its pathophysiology remains incompletely understood. Increasing research on the human microbiome has highlighted the role of gut microbial dysbiosis in IBS [4]. Studies on post-infectious IBS have provided etiological insights into the pathogenesis of IBS. It is well documented that following infective gastroenteritis, more than 10% of affected individuals go on to develop post-infectious IBS [5]. A consequence of infective gastroenteritis is the disruption of normal gut flora. Though the gut microbiota is known to have inter-individual variations, relative increase in Bacteroides and Prevotella bacteria have been frequently reported in patients with IBS compared to healthy controls [6]. The microbiome is thought to modulate inflammation and act either directly or indirectly through microbial metabolites. Probiotics [7], rifaximin [8], and other attempts to restore normal gut flora have been demonstrated to alleviate IBS symptoms [9]. However, repeated courses of treatment are often required, raising cost concerns. Current therapies are also limited and based on a weak evidence base [10].
Research efforts to find new and more effective gut microbiota-based therapies are ongoing. There is growing enthusiasm in the use of traditional medicine and many patients with IBS frequently turn to complementary and alternative (CAM) therapies [11]. Curcumin (diferuloylmethane), a bright yellow phytochemical, is the main curcuminoid found in turmeric (Curcuma longa), a popular spice used during food preparation in South Asia and the Middle East [12]. Curcumin has long been used in Ayurvedic medicine to treat various inflammatory conditions e.g., arthritis and ulcers [13]. It is also found in the traditional Chinese medicine Jieyu-wan and Xiaoyao-san, which are prescribed to manage stress and mood disorders [14].
Modern studies on the pharmacology and use of curcumin have reported its potent anti-oxidant [15], anti-inflammatory [16] and anti-depressant [17] effects. It also has demonstrated mucosal protective effects in rat models of colitis [18] and reported efficacy in patients with inflammatory bowel disease [19]. Curcumin also has poor bioavailability [20] and majority of it is excreted in the faeces unmetabolized [21]. This means that after ingestion, curcumin reaches the gut almost unaltered and could exert potentially beneficial effects on the gut microbiota. As no meta-analysis has been done up till now to investigate the efficacy of curcumin in patients with IBS, this meta-analysis is, thus, timely and necessary to summarise current evidence and generate hypotheses for further research.
2. Methods
A systematic literature search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Using the keywords (curcumin OR turmeric OR Indian saffron OR diferuloylmethane OR curcuminoid) AND (irritable bowel syndrome OR IBS), a preliminary search on the PubMed, Medline, Embase, PsychINFO, Web of Science and Google Scholar databases yielded 1080 papers published in English between 1 January 1988 and 1 May 2018. Grey literature was searched using Google search. Title/abstract screening were performed independently by three researchers (Q.X.N., W.Y.L., and N.V.) to identify articles of interest. For relevant abstracts, full articles were obtained, reviewed and also checked for references of interest. If necessary, the authors of the articles were contacted to provide additional data.
Full articles were obtained for all selected abstracts and reviewed by three researchers (Q.X.N., W.Y.L., and N.V) for inclusion. Any disagreement was resolved by discussion and consensus. The inclusion criteria for this review were: (1) published randomized, controlled trial, (2) curcumin administered as an active intervention, (3) study participants with IBS, and (4) available outcome measures for treatment efficacy. Trials that were not placebo-controlled were excluded as a high placebo response rate is known for clinical trials involving patients with IBS [22].
Methodological quality of the eligible clinical trials was appraised using the Cochrane Collaboration’s tool for assessing risk of bias [23]. As different scales and scoring systems, e.g., the IBS symptom-related quality of life (IBSQOL) and IBS Symptom Severity Score (IBS-SSS), were used in the various studies to assess IBS severity pre- and post-intervention, the primary outcome measure of interest was the standardized mean difference (SMD) for mean reduction in IBS symptoms from baseline with curcumin, per-protocol analysis. Estimates were pooled and where appropriate, 95% confidence intervals (95% CI) and p-values were calculated.
Heterogeneity amongst the different studies pooled was examined using the I2 statistic and Cochran’s Q test. I2 > 50% indicates substantial heterogeneity. Due to the small number of studies available, a funnel plot and sensitivity analyses were not done. All analyses were done using MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014).
3. Results
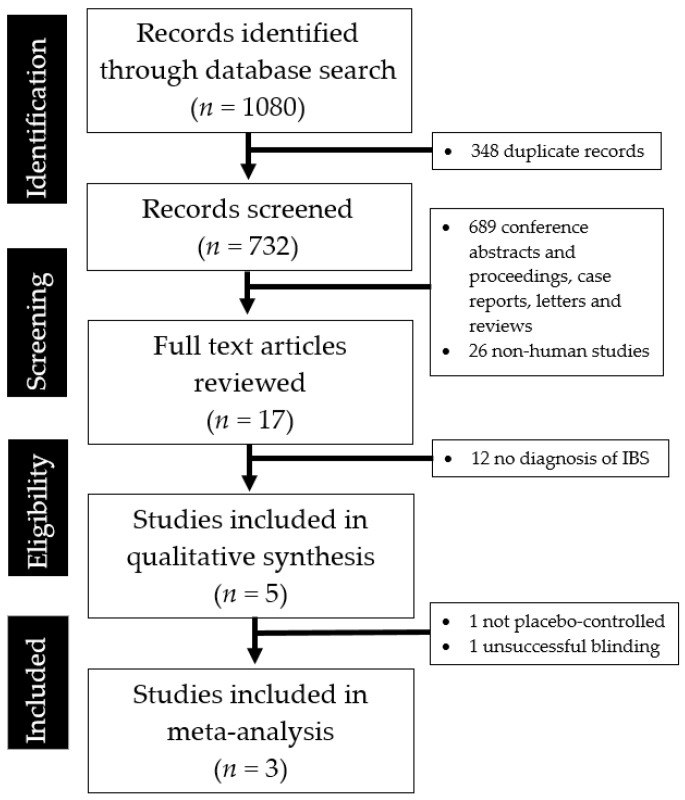
Of the 1080 citations retrieved, 17 full papers were selected for further review. Five studies were systematically reviewed and their key findings were summarised in Table 1. A total of three studies with a total of 326 individuals with IBS were included in the final meta-analysis. One study [24] was excluded as it was only partially blinded and was not placebo-controlled, while another study [25] was excluded as the sample size was limited and there was a high drop-out rate (11 out of 32 participants) and consequent loss of statistical power. Patient blinding was also unsuccessful in that study, as the majority of the patients correctly identified the active intervention or placebo during the trial. Further reason for exclusion from the meta-analysis is that the study [25] investigated a curcumin-containing Ayurvedic preparation with a proportionately small amount of turmeric compared to the other constituents (curry (Murraya koenigii), pomegranate (Punica granatum), and turmeric (Curcuma longa rhizome pulvis) in a 6:3:1 ratio). The abstraction process and reasons for exclusion were detailed in Figure 1. None of the authors had to be contacted to provide additional data.
Table 1.
Studies included in the systematic review (arranged alphabetically by first author’s last name).
| Author, Year | Study Design | Country | Sample Size | Curcumin Dose and Formulation | Study Duration | Diagnosis of IBS | Conclusions |
|---|---|---|---|---|---|---|---|
| Alt, 2017 [26] | Double-blind, placebo-controlled randomized trial | Germany | 99 |
|
8 weeks | Rome III | Significant improvement in IBS symptoms, compared to placebo (p < 0.001). Improvements seen as early as 4 weeks into treatment. No serious adverse events reported. |
| Bundy, 2004 [24] | Partially blinded, randomized, two-dose trial | United Kingdom | 207 |
|
8 weeks | Rome II | Significantly reduced IBS symptomatology in both treatment groups after 8 weeks (p < 0.001). No serious adverse events reported. |
| Brinkhaus, 2005 [25] | Double-blind, placebo-controlled, randomized trial | Germany | 106 |
|
18 weeks | Extensive clinical examination ruling out organic causes | Both herb-based monotherapy did not significantly improve IBS symptoms compared to placebo. |
| Lauche, 2016 [27] | Double-blind, placebo-controlled, randomized crossover trial | Germany | 32 |
|
4 weeks | Rome III | No significant difference between Ayurvedic preparation and placebo for IBS symptom severity (p = 0.26). |
| Portincasa, 2016 [28] | Double-blind, placebo-controlled, randomized trial | Italy | 121 |
|
30 days | Rome III | Significant improvement in IBS symptoms (p < 0.001) and quality of life. No serious adverse events reported. |
Figure 1.
PRISMA flow diagram showing the studies identified during the literature search and abstraction process.
Methodological quality of the eligible clinical trials was appraised using the Cochrane Collaboration’s tool for assessing risk of bias as shown in Table 2.
Table 2.
Results of Cochrane collaboration’s tool for assessing risk of bias.
| Study (Author, Year) | Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Alt, 2017 [26] | + | + | + | + | ? | ? |
| Bundy, 2004 [24] | + | ? | - | + | ? | - |
| Brinkhaus, 2005 [25] | ? | + | + | + | ? | ? |
| Lauche, 2016 [27] | + | - | - | + | ? | ? |
| Portincasa, 2016 [28] | - | + | + | + | ? | ? |
Key: + low risk of bias; - high risk of bias; ? unclear risk of bias.
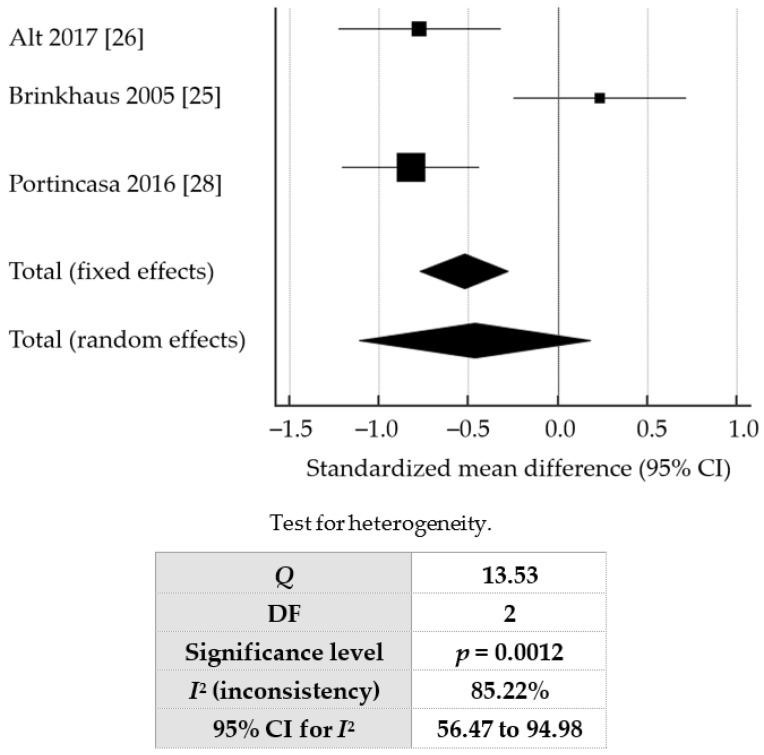
Applying per-protocol analysis and a random-effects model, the pooled SMD from baseline IBS severity rating was −0.466 (95% CI: −1.113 to 0.182, p = 0.158), which showed a beneficial, albeit not statistically significant, effect of curcumin on IBS symptoms, compared to placebo. The forest plot is shown in Figure 2. A random-effects model was applied due to the high degree of heterogeneity observed (I2 = 85.22%).
Figure 2.
Forest plot showing overall standardized mean difference (SMD) for change in baseline IBS severity rating.
4. Discussion
Overall, random-effects meta-analysis based on three studies and 326 subjects found curcumin to have beneficial but not statistically significant effects on IBS symptoms. Of the five clinical trials reviewed, three reported positive and significant effects for curcumin-containing products [24,26,28], while two [25,27] found no significant effects compared to placebo and advised against monotherapy with curcumin. It is important to note that in the study by Brinkhaus et al. [25], diagnosis of IBS was not based on the established Rome Criteria [29], but rather an intensive clinical examination ruling out other organic causes. The study also recruited particularly treatment-refractory patients with IBS; most of them had a diagnosis of IBS for an average of seven years prior to the study, have had abdominal complaints for an average of 15 years and previous treatments were unsuccessful. This could have accounted for the apparent lack of effect observed for curcumin in the abovementioned study [25]. In the other study by Lauche et al. [27], which found non-significant effects compared to placebo, the sample size was limited and there was a high drop-out rate (11 out of 32 participants) and consequent loss of statistical power. Patient blinding was also unsuccessful in that study [27], as the majority of the patients correctly identified the active intervention or placebo during the trial. It was encouraging that curcumin had demonstrated positive effects in the other two placebo-controlled trials [24,28], which recruited male and female patients with at least moderate symptom severity (IBS-SSS ranging from 250 to 300).
To the best of our knowledge, no prior meta-analysis has been done to investigate the efficacy of curcumin on IBS symptoms. Although the meta-analysis did not find an overall statistically significant effect, the potentially relevant properties of curcumin for IBS sufferers may still have clinical significance or utility. Curcumin’s beneficial effects in IBS are likely related to its unique combination of anti-oxidant [15] and anti-inflammatory [16] activities. Curcumin has been shown to attenuate circulating interleukin-6 (IL-6) levels [30] and regulate key mediators of cellular inflammation, including 5-lipoxygenase (5-LOX), cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) [31]. Current studies on patients with IBS have highlighted a pro-inflammatory phenotype in these patients, with higher circulating IL-6 levels, immune activation and chronic, low-grade, subclinical mucosal inflammation [32]. Curcumin also has demonstrated mucosal protective effects in rat models of colitis [18] and reported efficacy, even in patients with inflammatory bowel disease [19].
A recent study using a rat model of IBS also found that curcumin was able to exert beneficial effects via the “brain-gut” axis [33]. Curcumin administration increased serotonin (5-HT), brain-derived neurotrophic factor (BDNF) and phosphorylation of cAMP response element-binding protein (pCREB) expression in the hippocampus and colon [33]. Psychosocial factors have been implicated in the etiology of IBS [34]. Stress is thought to potentiate immune activation as it stimulates pro-inflammatory cytokines and nuclear factor (NF)-κB [35]. A dysregulated hypothalamic-pituitary-adrenal (HPA) axis could contribute to visceral hypersensitivity [36], which is typically seen in patients with IBS. Abnormal 5-HT functioning is associated with altered gut motility and enhanced nociceptive pain sensitivity [37], both symptoms characteristic of IBS. Interestingly, laboratory studies with chronically stressed mice have found that curcumin administration was able to reverse the effects of chronic stress on behaviour, the HPA axis and BDNF protein levels [38].
Another factor that could contribute to the therapeutic effect of curcumin is related to its poor oral bioavailability [20]. The majority of the curcumin ingested is excreted in the faeces unmetabolized [21]. This means that after ingestion, curcumin reaches the gut almost unaltered and could exert potentially beneficial effects on the gut and gut microbiota. In Sprague Dawley rats with hepatic steatosis (induced by a high-fat diet), curcumin not only restored intestinal barrier integrity (increased expression of tight junction proteins ZO-1 and occluding), it markedly altered the overall composition of the gut microbiota, towards that of lean rats maintained on a normal diet [39]. Also underlying the disease process of IBS is altered intestinal permeability, specifically, decreased expression of the tight junction proteins ZO-1 and α-cathenin [40]. Curcumin has demonstrated positive effects on intestinal permeability in animal models, improving the structure of intestinal tight junctions and upregulating the expression of occludin in the intestinal mucosa [41]. These could explain its positive effects on IBS symptomatology.
With regard to the possible side effects of curcumin use, human trials assessing its safety and toxicity have found it to be safe, with occasional reports of slight giddiness, nausea, and diarrhoea [42]. Encouragingly, no adverse events were also reported in any of the trials reviewed in this study [24,25,26,28]. However, at higher doses, curcumin may interact with some medications, such as anticoagulants [43]. Quality longitudinal studies are needed to confirm the safety of curcumin use.
Finally, the limitations of our current study should be discussed. As only three studies were available for meta-analysis, a funnel plot or sensitivity analysis was not feasible. Second, inter-study variability in terms of curcumin formulation (even combined with peppermint oil, a known antispasmodic [44], in one study [26]) and the diagnosis of IBS likely contributed to the overall heterogeneity of the meta-analysis, and could have affected the reliability of current findings. Furthermore, curcumin was mixed with at least one other therapeutic or potentially therapeutic material in two of the trials analysed [26,28], hence, it is difficult to quantify a curcumin-attributable SMD and determine whether the therapeutic benefits observed in these two studies were due to curcumin. More robust clinical trials involving a standardized curcumin preparation and larger sample sizes are warranted. Finally, as IBS is often a chronic, relapsing condition [45], follow-up studies over a longer duration should be conducted to ensure that the clinical improvement observed with curcumin use would be stable in the long-term. Current studies ranged from only 8 to 18 weeks [24,25,26,28].
5. Conclusions
Current evidence suggests that curcumin has a positive albeit not statistically significant effect (compared to placebo) on IBS symptoms, alleviating pain and improving quality-of-life scores in patients with at least moderate symptom severity. With its unique anti-oxidant and anti-inflammatory activities and ability to modulate gut microbiota, it is a potentially useful addition to our armamentarium of agents for managing IBS. Curcumin also appears to be safe and well-tolerated, with no serious adverse events reported in any of the trials reviewed. However, current findings are based on a considerably limited evidence base with marked heterogeneity. More robust clinical trials involving a standardized curcumin preparation and larger sample sizes should be encouraged.
Author Contributions
Q.X.N. conceived, designed and carried out the study and the relevant data analysis and interpretation, while A.Y.S.S. and W.-S.Y. supervised the study. W.L. and N.V. carried out the study and the relevant data analysis and interpretation. D.Y.L. contributed to the data analysis and interpretation. All authors contributed toward drafting and critically revising the paper, and agree to be accountable for all aspects of the work.
Funding
No funding received.
Conflicts of Interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Drossman D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology. 2016;150:1262–1279. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Lovell R.M., Ford A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Akehurst R.L., Brazier J.E., Mathers N., O’Keefe C., Kaltenthaler E., Morgan A., Platts M., Walters S.J. Health-related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics. 2002;20:455–462. doi: 10.2165/00019053-200220070-00003. [DOI] [PubMed] [Google Scholar]
- 4.Barbara G., Cremon C., De Giorgio R., Dothel G., Zecchi L., Bellacosa L., Carini G., Stanghellini V., Corinaldesi R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr. Gastroenterol. Rep. 2011;13:308–315. doi: 10.1007/s11894-011-0195-7. [DOI] [PubMed] [Google Scholar]
- 5.Thabane M., Kottachchi D.T., Marshall J.K. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 2007;26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- 6.Jalanka-Tuovinen J., Salojärvi J., Salonen A., Immonen O., Garsed K., Kelly F.M., Zaitoun A., Palva A., Spiller R.C., de Vos W.M. Faecal microbiota composition and host–microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–1745. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.J., Camilleri M., McKinzie S., Lempke M.B., Burton D.D., Thomforde G.M., Zinsmeister A.R. A randomized controlled trial of a probiotic, VSL# 3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharara A.I., Aoun E., Abdul-Baki H., Mounzer R., Sidani S., ElHajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am. J. Gastroenterol. 2006;101:326. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 9.Chassard C., Dapoigny M., Scott K.P., Crouzet L., Del’homme C., Marquet P., Martin J.C., Pickering G., Ardid D., Eschalier A., et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 10.Ng Q.X., Soh A.Y., Lim D.Y., Yeo W.S. Agomelatine, a novel therapeutic option for the management of irritable bowel syndrome. J. Clin. Pharm. Ther. 2018;43:752–756. doi: 10.1111/jcpt.12749. [DOI] [PubMed] [Google Scholar]
- 11.Langmead L., Chitnis M., Rampton D.S. Use of complementary therapies by patients with IBD may indicate psychosocial distress. Inflamm. Bowel Dis. 2002;8:174–179. doi: 10.1097/00054725-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Goel A., Aggarwal B.B. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 13.Singh S. From exotic spice to modern drug? Cell. 2007;130:765–768. doi: 10.1016/j.cell.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Ng Q.X., Koh S.S., Chan H.W., Ho C.Y. Clinical use of curcumin in depression: A meta-analysis. J. Am. Med. Dir. Assoc. 2017;18:503–508. doi: 10.1016/j.jamda.2016.12.071. [DOI] [PubMed] [Google Scholar]
- 15.Ak T., Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong S., Zeng Q., Mitchell E., Xiu J., Duan Y., Li C., Tiwari J.K., Hu Y., Cao X., Zhao Z. Curcumin Enhances Neurogenesis and Cognition in Aged Rats: Implications for Transcriptional Interactions Related to Growth and Synaptic Plasticity. PLoS ONE. 2012;7:e31211. doi: 10.1371/journal.pone.0031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubbad A., Oriowo M.A., Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol. Cell. Biochem. 2009;322:127–135. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- 19.Holt P.R., Katz S., Kirshoff R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 20.Ammon H., Wahl M. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 21.Sharma R.A., McLelland H.R., Hill K.A., Ireson C.R., Euden S.A., Manson M.M., Pirmohamed M., Marnett L.J., Gescher A.J., Steward W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 22.Dorn S.D., Kaptchuk T.J., Park J.B., Nguyen L.T., Canenguez K., Nam B.H., Woods K.B., Conboy L.A., Stason W.B., Lembo A.J. A meta-analysis of the placebo response in complementary and alternative medicine trials of irritable bowel syndrome. Neurogastroenterol. Motil. 2007;19:630–637. doi: 10.1111/j.1365-2982.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bundy R., Walker A.F., Middleton R.W., Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: A pilot study. J. Altern. Complement. Med. 2004;10:1015–1018. doi: 10.1089/acm.2004.10.1015. [DOI] [PubMed] [Google Scholar]
- 25.Brinkhaus B., Hentschel C., Keudell C.V., Schindler G., Lindner M., Stützer H., Kohnen R., Willich S.N., Lehmacher W., Hahn E.G. Herbal medicine with curcuma and fumitory in the treatment of irritable bowel syndrome: A randomized, placebo-controlled, double-blind clinical trial. Scand. J. Gastroenterol. 2005;40:936–943. doi: 10.1080/00365520510023134. [DOI] [PubMed] [Google Scholar]
- 26.Alt F., Chong P.W., Teng E., Uebelhack R. Evaluation of Benefit and Tolerability of IQP-CL-101 (Xanthofen) in the Symptomatic Improvement of Irritable Bowel Syndrome: A Double-Blinded, Randomised, Placebo-Controlled Clinical Trial. Phytother. Res. 2017;31:1056–1062. doi: 10.1002/ptr.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauche R., Kumar S., Hallmann J., Lüdtke R., Rampp T., Dobos G., Langhorst J. Efficacy and safety of ayurvedic herbs in diarrhoea-predominant irritable bowel syndrome: A randomised controlled crossover trial. Complement. Ther. Med. 2016;26:171–177. doi: 10.1016/j.ctim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Portincasa P., Bonfrate L., Scribano M.L., Kohn A., Caporaso N., Festi D., Campanale M.C., Di Rienzo T., Guarino M., Taddia M., et al. Curcumin and Fennel Essential Oil Improve Symptoms and Quality of Life in Patients with Irritable Bowel Syndrome. J. Gastrointestin. Liver Dis. 2016;25:151–157. doi: 10.15403/jgld.2014.1121.252.ccm. [DOI] [PubMed] [Google Scholar]
- 29.Thompson W.G., Longstreth G.F., Drossman D.A., Heaton K.W., Irvine E.J., Mueller-Lissner S.A., Talley N.J., Whitehead W.E., Corazziari E. Rome II: Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology, and Treatment: A Multinational Consensus. 2nd ed. Degnon Associates; McLean, VA, USA: 2000. pp. 351–432. [Google Scholar]
- 30.Derosa G., Maffioli P., Simental-Mendía L.E., Bo S., Sahebkar A. Effect of curcumin on circulating interleukin-6 concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016;111:394–404. doi: 10.1016/j.phrs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 32.Ng Q.X., Soh A.Y., Loke W., Lim D.Y., Yeo W.-S. The role of inflammation in irritable bowel syndrome (IBS) J. Inflamm. Res. 2018 doi: 10.2147/JIR.S174982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y., Wu S., Li J., Wang R., Xie X., Yu X., Pan J., Xu Y., Zheng L. The effect of curcumin on the brain-gut axis in rat model of irritable bowel syndrome: Involvement of 5-HT-dependent signaling. Metab. Brain Dis. 2015;30:47–55. doi: 10.1007/s11011-014-9554-z. [DOI] [PubMed] [Google Scholar]
- 34.Ng Q.X., Soh A.Y., Loke W., Venkatanarayanan N., Lim D.Y., Yeo W.-S. Systematic Review with Meta-analysis: The Association between Post-traumatic Stress Disorder (PTSD) and Irritable Bowel Syndrome (IBS) J. Gastroenterol. Hepatol. 2018 doi: 10.1111/jgh.14446. [DOI] [PubMed] [Google Scholar]
- 35.Pace T.W., Mletzko T.C., Alagbe O., Musselman D.L., Nemeroff C.B., Miller A.H., Heim C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 36.Chang L., Sundaresh S., Elliott J., Anton P.A., Baldi P., Licudine A., Mayer M., Vuong T., Hirano M., Naliboff B.D., et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol. Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grover M., Camilleri M. Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J. Gastroenterol. 2013;48:177–181. doi: 10.1007/s00535-012-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y., Ku B., Tie L., Yao H., Jiang W., Ma X., Li X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006;1122:56–64. doi: 10.1016/j.brainres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Feng W., Wang H., Zhang P., Gao C., Tao J., Ge Z., Zhu D., Bi Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017;1861:1801–1812. doi: 10.1016/j.bbagen.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Vivinus-Nebot M., Frin-Mathy G., Bzioueche H., Dainese R., Bernard G., Anty R., Filippi J., Saint-Paul M.C., Tulic M.K., Verhasselt V., et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744–752. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 41.Wang J., Ghosh S.S., Ghosh S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017;312:438–445. doi: 10.1152/ajpcell.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chainani-Wu N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa) J. Altern. Complement. Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 43.Liu A.C., Zhao L.X., Lou H.X. Curcumin alters the pharmacokinetics of warfarin and clopidogrel in Wistar rats but has no effect on anticoagulation or antiplatelet aggregation. Planta Med. 2013;79:971–977. doi: 10.1055/s-0032-1328652. [DOI] [PubMed] [Google Scholar]
- 44.Hiki N., Kurosaka H., Tatsutomi Y., Shimoyama S., Tsuji E., Kojima J., Shimizu N., Ono H., Hirooka T., Noguchi C., et al. Peppermint oil reduces gastric spasm during upper endoscopy: A randomized, double-blind, double-dummy controlled trial. Gastrointest. Endosc. 2003;57:475–482. doi: 10.1067/mge.2003.156. [DOI] [PubMed] [Google Scholar]
- 45.Chey W.D., Kurlander J., Eswaran S. Irritable bowel syndrome: A clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]