Abstract
This meta-analysis was conducted with the aims to summarize all available evidence on (1) prevalence of pre-existing atrial fibrillation (AF) and/or incidence of AF following kidney transplantation; (2) the outcomes of kidney transplant recipients with AF; and (3) the trends of estimated incidence of AF following kidney transplantation over time. A literature search was conducted utilizing MEDLINE, EMBASE, and the Cochrane Database from inception through March 2018. We included studies that reported (1) prevalence of pre-existing AF or incidence of AF following kidney transplantation or (2) outcomes of kidney transplant recipients with AF. Effect estimates from the individual study were extracted and combined utilizing random-effect, generic inverse variance method of DerSimonian and Laird. The protocol for this meta-analysis is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42018086192). Eight cohort studies with 137,709 kidney transplant recipients were enrolled. Overall, the pooled estimated prevalence of pre-existing AF in patients undergoing kidney transplantation was 7.0% (95% CI: 5.6–8.8%) and pooled estimated incidence of AF following kidney transplantation was 4.9% (95% CI: 1.7–13.0%). Meta-regression analyses were performed and showed no significant correlations between year of study and either prevalence of pre-existing AF (p = 0.93) or post-operative AF after kidney transplantation (p = 0.16). The pooled odds ratios (OR) of mortality among kidney transplant recipients with AF was 1.86 (3 studies; 95% CI: 1.03–3.35). In addition, AF is also associated with death-censored allograft loss (2 studies; OR: 1.55, 95% CI: 1.02–2.35) and stroke (3 studies; OR: 2.54, 95% CI: 1.11–5.78) among kidney transplant recipients. Despite advances in medicine, incidence of AF following kidney transplant does not seem to decrease over time. In addition, there is a significant association of AF with increased mortality, allograft loss, and stroke after kidney transplantation.
Keywords: atrial fibrillation, kidney transplantation, renal transplantation, transplantation, systematic reviews, meta-analysis
1. Introduction
Atrial fibrillation (AF) is one of the most frequent diagnoses, affecting 3 to 6 million people in the United States, and almost 30 million people worldwide [1,2,3,4]. Global prevalence of AF has continued to rise and is expected to reach 50 million people by 2050 [1,2,3,4,5,6]. Patients with AF carry a higher risk of mortality and adverse cardiovascular events including stroke [7,8]. Among end-stage renal disease (ESRD) patients, given hypercoagulable state [9,10] and hemodynamic changes during dialysis [11], the prevalence of AF is exceptionally high, approximately 12% [12,13], when compared to the prevalence in the general patient population of 2.5% [14]. One-year mortality risk of ESRD patients with AF is twice higher than those without AF [12,15].
Kidney transplantation is the treatment of choice for ESRD and improves the survival and quality of life for the majority of ESRD patients when compared to dialysis [16,17,18,19,20]. While advances in immunosuppression and surgical techniques have led to significant improvement in short-term survival of the renal allograft [21], long-term renal allograft survival is still an ongoing concern [22,23]. While reduced kidney function is an important risk factor for AF development [24], the improvement of renal function after successful kidney transplantation may affect the incidence of AF and potential consequences of AF [25,26,27,28,29,30,31,32]. Conversely, immunosuppressive agents, insulin resistance, and metabolic syndrome after kidney transplantation may also impact on the potential consequences of AF [29,33,34,35]. In spite of progress in transplant medicine, the trends of incidence of AF following kidney transplantation over time remain unclear [5,25,26,27,28,29,30,31,32,36,37].
Thus, this meta-analysis was conducted with the aim to summarize all available data on (1) prevalence of pre-existing AF and/or incidence of AF following kidney transplantation; (2) the outcomes of kidney transplant recipients with AF; and (3) the trends of estimated incidence of AF following kidney transplantation over time.
2. Methods
2.1. Search Strategy and Literature Review
The protocol for this meta-analysis is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42018086192). A systematic literature search of MEDLINE (1946 to March 2018), EMBASE (1988 to March 2018), and the Cochrane Database of Systematic Reviews (database inception to March 2018) was conducted (1) to assess prevalence of pre-existing AF and/or incidence of AF following kidney transplantation and (2) to evaluate the outcomes of kidney transplant recipients with AF. The systematic literature review was undertaken independently by two investigators (C.T. and R.C.) using the search strategy that combined the terms of “kidney” or “renal” AND “transplant” OR “transplantation” AND “atrial fibrillation”, which is provided in Supplementary materials. No language limitation was applied. A manual search for conceivably relevant studies using references of the included articles was also performed. This study was conducted by the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) [38] and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement [39].
2.2. Selection Criteria
Eligible studies must be clinical trials or observational studies (cohort, case-control, or cross-sectional studies) that reported prevalence of pre-existing AF or incidence of AF following kidney transplantation or outcomes of kidney transplant recipients with AF. They must provide the data on prevalence or incidence or effect estimates relative risks (RR), odds ratios (OR), or hazard ratios (HR) with 95% confidence intervals (CI). Retrieved articles were individually reviewed for eligibility by the two investigators (C.T. and R.C.). Discrepancies were addressed and solved by a third investigator (W.C.) and joint consensus. Inclusion was not limited by the size of study. The Newcastle-Ottawa quality assessment scale was applied to evaluate the quality of study for case-control study and outcome of interest for cohort study [40], as shown in Table 1.
Table 1.
(a) Main characteristic of studies included in meta-analysis of atrial fibrillation (AF) in patients undergoing kidney transplantation. (b) Main characteristic of studies included in meta-analysis of AF in patients undergoing kidney transplantation.
(a).
| Study | Aull-Watschinger et al. [26] | La Manna et al. [25] | Lenihan et al. [5] | Findlay et al. [28] |
|---|---|---|---|---|
| Country | Austria | Italy | USA | UK |
| Study design | Cohort | Cohort | Cohort | Cohort |
| Study year | 2008 | 2013 | 2015 | 2016 |
| Total number | 1633 | 304 | 62706 | 956 |
| Patients | Kidney or kidney-pancreas transplant patients in a single center | Kidney or kidney/liver transplant patients in a single center | Kidney transplant patients in the US renal Data System | Functioning kidney transplant patients in a single hospital |
| Living donor | 174/1633 (11%) | N/A | 10409/62706 (17%) | N/A |
| Anticoagulation | Antiplatelet or anticoagulation 454/1633 (28%) | N/A | N/A | Warfarin 137/956 (14%) |
| AF ascertainment | History of AF before kidney transplant; identified by medical record review | Postoperative AF until hospital discharge; identified by medical record review | History of AF before kidney transplant; identified by identified by ICD-9 code 427.3x in Medicare claims | History of AF before kidney transplant; identified by medical record review |
| Pre-operative AF | 122/1633 (7.5%) | 16/304 (5.3%) | 3794/62706 (6.1%) | 88/956 (9.2%) |
| Estimated prevalence | ||||
| Post-operative AF | N/A | POAF 25/304 (8.2%) | N/A | N/A |
| Estimated prevalence | De novo POAF 21/304 (6.9%) | |||
| Follow-up | Median 4 (IQR 1.5–6.7) years | Until hospital discharge | Mean 4.9 years | Median 5.4 years |
| Outcomes | TIA/stroke 3.30 (1.63–6.67) | POAF and myocardial ischemia 11.58 (0.70–191.06) | Death 1.46 (1.38–1.54) | Stroke 4.59 (1.92–10.94) |
| All-cause graft loss 1.41 (1.34–1.48) | Ischemic stroke in AF 1.72% at 1 year and 4.07% at 3 years | |||
| Death-censored graft loss 1.26 (1.15–1.37) | ||||
| Death-censored ischemic stroke 1.36 (1.10–1.68) | Ischemic stroke risk in non-AF 0.72% at 1 year and 2.07% at 3 years | |||
| Confounder adjustment | DM, ejection fraction, C-reactive protein, hyperlipidemia, polycystic kidney disease, duration of dialysis, sex, age, degree of carotid stenosis | None | Age, sex, race, BMI, cause of ESRD, dialysis vintage and modality, SNF utilization, number of hospital days and non-nephrology clinic visits, previous transplants, comorbidities, blood type, PRA, donor age and sex, transplant type, HLA mismatches, cold ischemia time | None |
| Newcastle-Ottawa Scale | S 3 | S 3 | S 4 | S 3 |
| C 2 | C 2 | C 2 | C 2 | |
| O 3 | O 3 | O 3 | O 3 |
AF, Atrial Fibrillation; BMI, body mass index; DM, diabetes mellitus; ESRD, end-stage renal disease; HLA, human leukocyte antigen; ICD-9, international classification of diseases, ninth; IQR, interquartile range; N/A, not available; POAF, postoperative atrial fibrillation; PRA, panel reactive antibody; S, C, O, selection, comparability, and outcome; SNF, skilled nursing facility; TIA, transient ischemic attack.
(b).
| Study | Abbott et al. [29] | Lentine et al. [30] | Lentine et al. [31] | Delville et al. [32] |
|---|---|---|---|---|
| Country | USA | USA | USA | France |
| Study design | Cohort | Cohort | Cohort | Cohort |
| Study year | 2003 | 2006 | 2008 | 2015 |
| Total number | 39628 | 31136 | 1102 | 244 |
| Patients | Kidney transplant patients in the US Renal Data System | Kidney transplant patients in the US Renal Data System | Kidney transplant patients in a single center | Kidney transplant patients aged >50 years in a single center |
| Living donor | 12259/39628 (31%) | 6993/31136 (22%) | 344/1102 (31%) | N/A |
| Anticoagulation | N/A | N/A | N/A | N/A |
| AF ascertainment | Hospitalizations for a primary diagnosis of AF; identified by ICD-9 code 427.31 | AF after kidney transplant; identified by ICD-9 code 427.3x | New-onset atrial fibrillation after kidney transplant; identified by ECG | New-onset atrial fibrillation after kidney transplant; identified by medical record review and ECG |
| Pre-operative AF | N/A | N/A | N/A | N/A |
| Estimated prevalence | ||||
| Post-operative AF | 432/39628 (1.1%) | New-onset AF | 5-year 50/1102 (4.5%) | 13/244 (5.3%) |
| At 6 months 810/31136 (2.6%) | ||||
| At 12 months 1121/31136 (3.6%) | ||||
| Estimated prevalence | ||||
| At 36 months 2273/31136 (7.3%) | ||||
| Follow-up | Mean 1.89 ± 1.15 years | Up to 36 months | 5 year | 1 year |
| Outcomes | Mortality 1.34 (1.06–1.69) | Mortality 3.25 (2.92–3.63) | N/A | N/A |
| Death-censored graft loss 1.93 (1.63–2.29) | ||||
| All-cause graft loss 2.88 (2.60–3.12) | ||||
| Confounder adjustment | Adjusted but not specified | Age, sex, race, education, employment, BMI, causes of ESRD, dialysis duration, sensitization, comorbid conditions, smoking, alcohol abuse. donor age and source, donor CMV status, degree of HLA matching, induction and maintenance immunosuppression, DGF, post-transplantation complications | N/A | N/A |
| Newcastle-Ottawa Scale | S 4 | S 4 | S 3 | S 3 |
| C 1 | C 2 | C 2 | C 2 | |
| O 3 | O 3 | O 3 | O 3 |
AF, Atrial Fibrillation; CMV, Cytomegalovirus; DGF, delayed graft function; ECG, electrocardiogram; HLA, human leukocyte antigen; ICD-9, international classification of diseases, ninth; N/A, not available; SNF, skilled nursing facility; S, C, O, selection, comparability, and outcome.
2.3. Data Abstraction
A structured data collecting form was used to obtain the following information from each study including title, name of the first author, publication year, year of the study, country where the study was conducted, demographic data of kidney transplant patients, methods used to identify AF, prevalence of pre-existing AF, incidence of postoperative AF, patient outcomes following kidney transplantation, adjusted effect estimates with 95% CI and covariates that were adjusted for in the multivariable analysis.
2.4. Statistical Analysis
Analyses were performed utilizing the Comprehensive Meta-Analysis 3.3 software (version 3; Biostat Inc, Englewood, NJ, USA). Adjusted point estimates from each study were consolidated by the generic inverse variance approach of DerSimonian and Laird, which designated the weight of each study based on its variance [41]. Given the possibility of between-study variance, we used a random-effect model rather than a fixed-effect model. Cochran’s Q test and I2 statistic were applied to determine the between-study heterogeneity. A value of I2 of 0% to 25% represents insignificant heterogeneity, 26% to 50% low heterogeneity, 51% to 75% moderate heterogeneity and 76–100% high heterogeneity [42]. The presence of publication bias was assessed by the Egger test [43].
3. Results
A total of 399 potentially eligible articles were identified using our search strategy. After the exclusion of 382 articles based on title and abstract for clearly not fulfilling inclusion criteria on the basis of type of article, study design, population, or outcome of interest, and due to some being duplicates, 17 articles were left for full-length review. Six of them were excluded from the full-length review as they did not report the outcome of interest while three articles were excluded because they were not observational studies. Thus, we included 8 cohort studies [25,26,27,28,29,30,31,32] into the final analysis with 137,709 kidney transplant recipients that were enrolled. Kappa coefficient of agreement for the investigators was 0.87. Disagreements were resolved by a third researcher (W.C.) and joint consensus. The literature retrieval, review, and selection process are demonstrated in Figure 1. The characteristics and quality assessment of the included studies are presented in Table 1 [25,26,27,28,29,30,31,32].
Figure 1.
Outline of our search methodology.
3.1. Prevalence of Pre-Existing AF and Incidence of AF after Kidney Transplantation
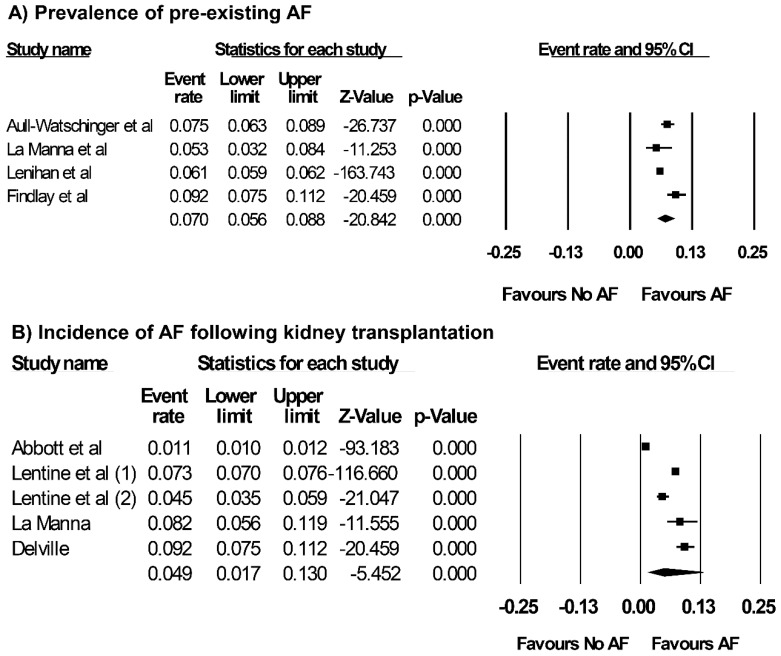
Overall, the pooled estimated prevalence of pre-existing AF in patients undergoing kidney transplantation was 7.0% (95% CI: 5.6–8.8%, I2 = 86%, Figure 2) and the pooled estimated incidence of AF following kidney transplantation was 4.9% (95% CI: 1.7–13.0%, I2 = 99%, Figure 2). When the data were limited only to new-onset AF after kidney transplant recipients, pooled estimated incidence of new-onset AF was 4.2% (95% CI: 1.6–10.6%, I2 = 94%).
Figure 2.
Forest plots of the included studies [5,25,26,28,29,30,31,32] assessing (A) prevalence of pre-existing AF in patients undergoing kidney transplantation, and (B) incidence of AF following kidney transplantation. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval.
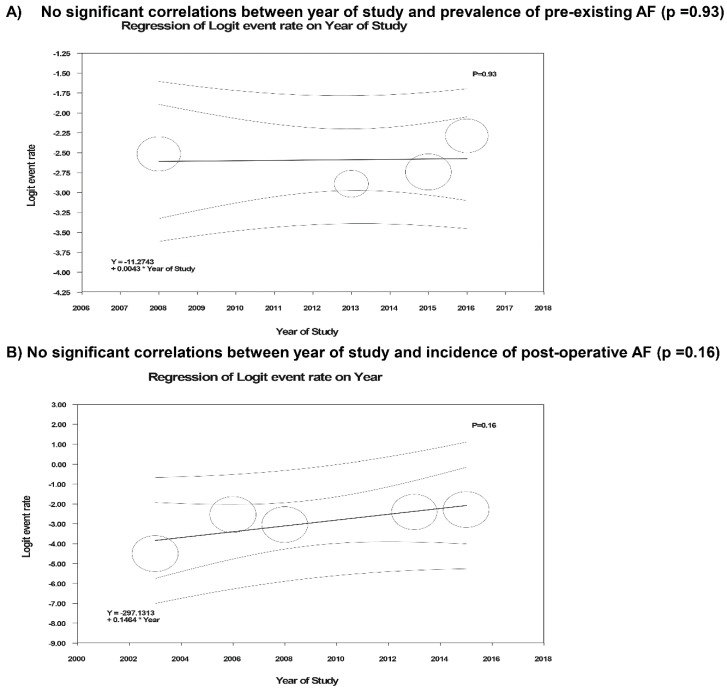
Meta-regression analyses were performed and showed no significant correlations between year of study and either prevalence of pre-existing AF (p = 0.93) or post-operative AF after kidney transplantation (p = 0.16), as shown in Figure 3.
Figure 3.
Meta-regression analyses showed (A) no significant correlations between year of study and either prevalence of pre-existing AF (p = 0.93) or (B) post-operative AF after kidney transplantation (p = 0.16). The solid black line represents the weighted regression line based on variance-weighted least squares. The inner and outer broken lines show the 95% confidence interval and prediction interval around the regression line. The circles indicate log event rates in each study.
3.2. Risk Factors of AF and Outcomes of Kidney Transplant Recipients with AF
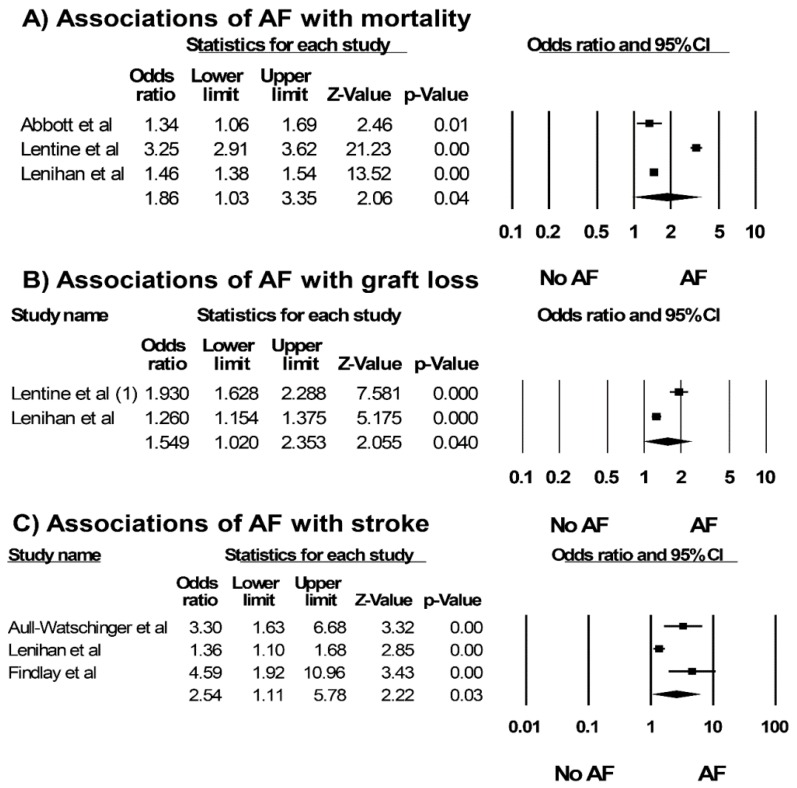
Reported risk factors associated with AF after kidney transplantation are demonstrated in Table 2 [25,29,30,31]. Older recipient age [25,29,30], higher BMI, and history of coronary artery disease/acute myocardial infarction have been demonstrated as important risk factors for AF after kidney transplantation. The pooled OR of mortality among kidney transplant recipients with AF was 1.86 (3 studies; 95% CI: 1.03–3.35, I2 = 98%, Figure 4). In addition, AF is associated with death-censored allograft loss (2 studies; OR: 1.55, 95% CI: 1.02–2.35, I2 = 94%, Figure 4) and stroke (3 studies; OR: 2.54, 95% CI: 1.11–5.78, I2 = 83%, Figure 4) among kidney transplant recipients.
Table 2.
Risk factor associated with AF after kidney transplantation.
| Studies | Follow-up Time | Risk Factor Associated with AF after Kidney Transplantation |
|---|---|---|
| Abbott et al. [29] | Mean 1.89 ± 1.15 years | Older recipient age, higher BMI, DGF, rejection, ESRD due to hypertension, cyclosporine use, Graft loss |
| Lentine et al. [30] | Up to 36 months | Older recipient age, male sex, Caucasian, non-Hispanic, ESRD due to hypertension, longer dialysis duration before transplant, CAD, DGF, older donor age, post-transplantation complications (hypertension, anemia, new-onset diabetes, MI, graft failure) |
| La Manna et al. [25] | Until hospital discharge | Older age, kidney/liver transplant, history of acute myocardial infarction |
| Lentine et al. [31] | 5 year | BMI |
AF, Atrial Fibrillation; BMI, body mass index; CAD, coronary artery disease; DGF, delayed graft function; ESRD, end-stage renal disease; MI, myocardial infarction.
Figure 4.
Associations of AF with (A) mortality, (B) death-censored allograft loss and (C) stroke among kidney transplant recipients from included studies [5,26,28,29,30,31]. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval.
3.3. Evaluation for Publication Bias
Funnel plots (Supplementary Figures S1 and S2) and Egger’s regression asymmetry tests were performed to evaluate for publication bias in analyses evaluating prevalence of pre-existing AF and incidence of postoperative AF in kidney transplant patients, respectively. There was no significant publication bias in both analyses evaluating prevalence of pre-existing AF and incidence of postoperative AF in kidney transplant patients, p = 0.33 and p = 0.68, respectively.
4. Discussion
In this meta-analysis, we demonstrated that ESRD patients who underwent kidney transplantation had a prevalence of AF of 7.0%. In addition, our study showed the pooled incidence of AF after kidney transplantation of 4.9%. Our findings showed a statistically significant association of AF after kidney transplantation with 1.9-fold increased risk of mortality, 1.6-fold increased risk of renal allograft loss, and 2.5-fold increased risk of stroke after kidney transplantation.
Based on the findings from our meta-analysis, the prevalence of pre-existing of AF among ESRD patients undergoing kidney transplantation is higher than the 2.5% prevalence in the general patient population of, [14] although it is lower than the 12% prevalence in overall ESRD patients [12,13], and the 6% prevalence in patients with end-stage liver disease undergoing liver transplantation [44]. Since not all ESRD patients are candidates for kidney transplantation due to their significant comorbidities, it is not surprising that the prevalence of AF among ESRD patients undergoing kidney transplantation from our study is lower than the prevalence among ESRD population in general. Following kidney transplantation, we demonstrated that approximately 4% of kidney transplant recipients developed new-onset AF. There are several explanations as to why kidney transplantation promotes the occurrence of AF during postoperative period. First, although hemodynamic instability during kidney transplantation is not as common as liver transplantation [45,46], conventional postoperative stress could provoke AF through hemodynamic instability [47,48,49]. In addition, hypertension and obesity, known risk factors for AF, are also common among kidney transplant recipients [31,50]. Furthermore, immunosuppressive agents are known to be associated with insulin resistance and metabolic syndrome after kidney transplantation (such as calcineurin inhibitors-induced diabetes mellitus [33] and mammalian target of rapamycin (mTOR) inhibitors-associated dyslipidemia [34]), which are important risk factors for AF [29,35]. Consistently, the majority of the included studies in our systematic reviews identified older recipient age [25,29,30], higher BMI, and a history of coronary artery disease/acute myocardial as predictors for AF development after kidney transplantation.
Leading causes of long-term mortality in kidney transplant recipients are cardiovascular complications, which, other than AF, include heart failure and myocardial infarction [51,52,53]. These cardiovascular complications were also considered as potential risk modification strategy that should not be overlooked. In general population, AF can put the patients at higher mortality risk compared to those without AF [54]. In addition to mortality risk, our study also revealed the associations of AF with renal allograft loss and stroke among kidney transplant recipients. There are several mechanisms that put the kidney transplant patients with AF at higher risk of postoperative morbidity and mortality compared to those without AF. Although high mortality in kidney transplant recipients with AF may have been contributed by other cardiovascular risks associated with AF (such as congestive heart failure and coronary artery disease) at the time even before kidney transplantation, studies have also demonstrated that AF after kidney transplantation itself is independently associated with increased mortality, morbidity, number of hospitalizations, and high healthcare cost [26,27,29,30]. In addition, amiodarone-tacrolimus interaction leading to QT prolongation and fatal arrhythmias in kidney transplantations have been reported [55,56]. Thus, this combination should be cautiously used with careful monitoring.
There are several limitations in our systematic review and meta-analysis. First, statistical heterogeneities were present in our study. Possible explanations for this heterogeneity include the differences in the methodology of diagnosis of AF and patient characteristics in each study. Despite these heterogeneities, meta-regression demonstrated no significant correlation between year of study and incidence of AF after kidney transplantation, representing no potential improvement in incidence of AF after kidney transplantation over time. Second, duration of follow up during the postoperative period by several studies assessing AF was just until hospital discharge or up to one-year post-transplantation [25,32]. Although the majority of cases of AF following kidney transplantation developed within the first year after kidney transplantation [25,26,27,28,29,30,31,32], the true incidence of AF might have been slightly higher. Third, while we demonstrated high mortality and stroke risks in kidney transplant recipients with AF, it remains unclear if anticoagulation use (warfarin and novel agents) in kidney transplant patients would improve patient outcomes; future clinical trials of anticoagulation use in the kidney transplant population with AF are needed [27,28,57]. Last, this is a meta-analysis of observational studies, and as such, it could only reveal association, not a causal-effect relationship, between kidney transplantation and AF.
5. Conclusions
In spite of progress in transplant medicine, incidence of AF following kidney transplants does not seem to decrease over time. When compared to those without AF, this meta-analysis shows that kidney transplant recipients with AF may carry higher risks of mortality, renal allograft loss, and stroke.
Acknowledgments
None. All authors had access to the data and played essential roles in writing of the manuscript.
Supplementary Materials
The following are available online at http://www.mdpi.com/2077-0383/7/10/370/s1, Figure S1: Funnel plot evaluating prevalence of pre-existing AF in kidney transplant patients, Figure S2: Funnel plot evaluating incidence of postoperative AF in kidney transplant patients, online Data S1: Search terms for systematic review.
Author Contributions
Conceptualization, T.B., N.J.K., K.S., P.U., N.P., N.R.A., W.K. and W.C.; Data curation, C.T. and R.C.; Formal analysis, W.C.; Funding acquisition, C.T. and R.C.; Investigation, C.T., R.C. and W.C.; Methodology, P.U., N.P., W.K. and W.C.; Project administration, T.B.; Resources, K.W.; Software, K.W.; Supervision, W.C.; Validation, C.T., R.C. and W.C.; Writing—original draft, C.T.; Writing—review & editing, R.C., T.B., N.J.K., K.S., P.U., N.P., N.R.A., K.W., S.A.S., F.L.K., W.K. and W.C.
Funding
This research received no external funding.
Conflicts of Interest
The authors deny any conflict of interest.
References
- 1.Lip G.Y.H., Brechin C.M., Lane D.A. The global burden of atrial fibrillation and stroke: A systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 2.Schnabel R.B., Yin X., Gona P., Larson M.G., Beiser A.S., McManus D.D., Newton-Cheh C., Lubitz S.A., Magnani J.W., Ellinor P.T., et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoni-Berisso M., Lercari F., Carazza T., Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade J., Khairy P., Dobrev D., Nattel S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 5.Lenihan C.R., Montez-Rath M.E., Scandling J.D., Turakhia M.P., Winkelmayer W.C. Outcomes after kidney transplantation of patients previously diagnosed with atrial fibrillation. Am. J. Transplant. 2013;13:1566–1575. doi: 10.1111/ajt.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyasaka Y., Barnes M.E., Gersh B.J., Cha S.S., Bailey K.R., Abhayaratna W.P., Seward J.B., Tsang T.S. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 7.January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C., Jr., Conti J.B., Ellinor P.T., Ezekowitz M.D., Field M.E., et al. American College of Cardiology/American Heart Association Task Force on Practice G. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Odutayo A., Wong C.X., Hsiao A.J., Hopewell S., Altman D.G., Emdin C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ. 2016;354 doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 9.Abbott K.C., Trespalacios F.C., Taylor A.J., Agodoa L.Y. Atrial fibrillation in chronic dialysis patients in the United States: Risk factors for hospitalization and mortality. BMC Nephrol. 2003;4 doi: 10.1186/1471-2369-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer A., Limperger V., Nowak-Gottl U. End-stage renal disease and thrombophilia. Hamostaseologie. 2016;36:103–107. doi: 10.5482/HAMO-14-11-0063. [DOI] [PubMed] [Google Scholar]
- 11.Mavrakanas T.A., Charytan D.M. Cardiovascular complications in chronic dialysis patients. Curr. Opin. Nephrol. Hypertens. 2016;25:536–544. doi: 10.1097/MNH.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman D., Sood M.M., Rigatto C., Holden R.M., Hiremath S., Clase C.M. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol. Dial. Transplant. 2012;27:3816–3822. doi: 10.1093/ndt/gfs416. [DOI] [PubMed] [Google Scholar]
- 13.Bansal N., Hsu C.Y., Go A.S. Intersection of cardiovascular disease and kidney disease: Atrial fibrillation. Curr. Opin. Nephrol. Hypertens. 2014;23:275–282. doi: 10.1097/01.mnh.0000444820.80249.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J., Gillum R.F., Kim Y.H., McAnulty J.H., Jr., Zheng Z.J., et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkelmayer W.C., Patrick A.R., Liu J., Brookhart M.A., Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J. Am. Soc. Nephrol. 2011;22:349–357. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schold J.D., Buccini L.D., Goldfarb D.A., Flechner S.M., Poggio E.D., Sehgal A.R. Association between kidney transplant center performance and the survival benefit of transplantation versus dialysis. Clin. J. Am. Soc. Nephrol. 2014;9:1773–1780. doi: 10.2215/CJN.02380314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaballo M.A., Canney M., O’Kelly P., Williams Y., O’Seaghdha C.M., Conlon P.J. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin. Kidney J. 2018;11:389–393. doi: 10.1093/ckj/sfx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe R.A., Ashby V.B., Milford E.L., Ojo A.O., Ettenger R.E., Agodoa L.Y., Held P.J., Port F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 19.Laupacis A., Keown P., Pus N., Krueger H., Ferguson B., Wong C., Muirhead N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235–242. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 20.Gill J.S., Tonelli M., Johnson N., Kiberd B., Landsberg D., Pereira B.J. The impact of waiting time and comorbid conditions on the survival benefit of kidney transplantation. Kidney Int. 2005;68:2345–2351. doi: 10.1111/j.1523-1755.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 21.Hariharan S., Johnson C.P., Bresnahan B.A., Taranto S.E., McIntosh M.J., Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N. Engl. J. Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 22.Meier-Kriesche H.U., Schold J.D., Kaplan B. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am. J. Transplant. 2004;4:1289–1295. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 23.Wazna E., Pazik J., Perkowska-Ptasinska A., Durlik M. Does histopathology of implanted kidney according to Banff 07 help predict long-term transplantation outcome? Transplant. Proc. 2018;50:1765–1768. doi: 10.1016/j.transproceed.2018.02.150. [DOI] [PubMed] [Google Scholar]
- 24.Bansal N., Zelnick L.R., Alonso A., Benjamin E.J., de Boer I.H., Deo R., Katz R., Kestenbaum B., Mathew J., Robinson-Cohen C., et al. eGFR and Albuminuria in Relation to Risk of Incident Atrial Fibrillation: A Meta-Analysis of the Jackson Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study. Clin. J. Am. Soc. Nephrol. 2017;12:1386–1398. doi: 10.2215/CJN.01860217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Manna G., Boriani G., Capelli I., Marchetti A., Grandinetti V., Spazzoli A., Dalmastri V., Todeschini P., Rucci P., Stefoni S. Incidence and predictors of postoperative atrial fibrillation in kidney transplant recipients. Transplantation. 2013;96:981–986. doi: 10.1097/TP.0b013e3182a2b492. [DOI] [PubMed] [Google Scholar]
- 26.Aull-Watschinger S., Konstantin H., Demetriou D., Schillinger M., Habicht A., Horl W.H., Watschinger B. Pre-transplant predictors of cerebrovascular events after kidney transplantation. Nephrol. Dial. Transplant. 2008;23:1429–1435. doi: 10.1093/ndt/gfm766. [DOI] [PubMed] [Google Scholar]
- 27.Lenihan C.R., Montez-Rath M.E., Shen J.I., Scandling J.D., Turakhia M.P., Chang T.I., Winkelmayer W.C. Correlates and outcomes of warfarin initiation in kidney transplant recipients newly diagnosed with atrial fibrillation. Nephrol. Dial. Transplant. 2015;30:321–329. doi: 10.1093/ndt/gfu323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Findlay M.D., Thomson P.C., MacIsaac R., Jardine A.G., Patel R.K., Stevens K.K., Rutherford E., Clancy M., Geddes C.C., Dawson J., et al. Risk factors and outcome of stroke in renal transplant recipients. Clin. Transplant. 2016;30:918–924. doi: 10.1111/ctr.12765. [DOI] [PubMed] [Google Scholar]
- 29.Abbott K.C., Reynolds J.C., Taylor A.J., Agodoa L.Y. Hospitalized atrial fibrillation after renal transplantation in the United States. Am. J. Transplant. 2003;3:471–476. doi: 10.1034/j.1600-6143.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 30.Lentine K.L., Schnitzler M.A., Abbott K.C., Li L., Xiao H., Burroughs T.E., Takemoto S.K., Willoughby L.M., Gavard J.A., Brennan D.C. Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2006;1:288–296. doi: 10.2215/CJN.00920805. [DOI] [PubMed] [Google Scholar]
- 31.Lentine K.L., Rocca-Rey L.A., Bacchi G., Wasi N., Schmitz L., Salvalaggio P.R., Abbott K.C., Schnitzler M.A., Neri L., Brennan D.C. Obesity and cardiac risk after kidney transplantation: Experience at one center and comprehensive literature review. Transplantation. 2008;86:303–312. doi: 10.1097/TP.0b013e31817ef0f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delville M., Sabbah L., Girard D., Elie C., Manceau S., Piketty M., Martinez F., Mejean A., Legendre C., Sberro-Soussan R. Prevalence and predictors of early cardiovascular events after kidney transplantation: Evaluation of pre-transplant cardiovascular work-up. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0131237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Rodriguez A.E., Trinanes J., Porrini E., Velazquez-Garcia S., Fumero C., Vega-Prieto M.J., Diez-Fuentes M.L., Luis Lima S., Salido E., Torres A. Glucose homeostasis changes and pancreatic beta-cell proliferation after switching to cyclosporin in tacrolimus-induced diabetes mellitus. Nefrologia. 2015;35:264–272. doi: 10.1016/j.nefro.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Holdaas H., Potena L., Saliba F. mTOR inhibitors and dyslipidemia in transplant recipients: a cause for concern? Transplant. Rev. (Orlando) 2015;29:93–102. doi: 10.1016/j.trre.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Pisano G., Fracanzani A.L., Caccamo L., Donato M.F., Fargion S. Cardiovascular risk after orthotopic liver transplantation, a review of the literature and preliminary results of a prospective study. World J. Gastroenterol. 2016;22:8869–8882. doi: 10.3748/wjg.v22.i40.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller-Deile J., Schwarz A., Menne J. Thromboembolism in renal transplant artery due to atrial fibrillation. Clin. Nephrol. Case Stud. 2013;1:10–13. doi: 10.5414/CNCS108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hristova M., van Beek C., Schurgers L.J., Lanske B., Danziger J. Rapidly progressive severe vascular calcification sparing the kidney allograft following warfarin initiation. Am. J. Kidney Dis. 2010;56:1158–1162. doi: 10.1053/j.ajkd.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 41.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 42.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Easterbrook P.J., Berlin J.A., Gopalan R., Matthews D.R. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 44.VanWagner L.B., Serper M., Kang R., Levitsky J., Hohmann S., Abecassis M., Skaro A., Lloyd-Jones D.M. Factors associated with major adverse cardiovascular events after liver transplantation among a national sample. Am. J. Transplant. 2016;16:2684–2694. doi: 10.1111/ajt.13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bezinover D., McQuillan P., Rossignol J., Uemura T., Kadry Z., Janicki P. Vasoplegic shock during liver transplantation: Is the preoperative cGMP plasma level a potential predictor of hemodynamic instability? Med. Sci. Monit. 2010;16:CS114–CS117. [PubMed] [Google Scholar]
- 46.Rudnick M.R., Marchi L.D., Plotkin J.S. Hemodynamic monitoring during liver transplantation: A state of the art review. World J. Hepatol. 2015;7:1302–1311. doi: 10.4254/wjh.v7.i10.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallegos-Orozco J.F., Charlton M.R. Predictors of cardiovascular events after liver transplantation. Clin. Liver Dis. 2017;21:367–379. doi: 10.1016/j.cld.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Bruhl S.R., Vetteth S., Rees M., Grubb B.P., Khouri S.J. Post-reperfusion syndrome during renal transplantation: A retrospective study. Int. J. Med. Sci. 2012;9:391–396. doi: 10.7150/ijms.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun W.Z., Huang F.Y., Fan S.Z., Chen T.L., Lee B.H., Lee T.S., Chen K.M. Evaluation of systemic hemodynamic effects on post-transplant perfusion to renal allograft. Ma Zui Xue Za Zhi. 1990;28:35–42. [PubMed] [Google Scholar]
- 50.Schwenger V., Zeier M., Ritz E. Hypertension after renal transplantation. Ann. Transplant. 2001;6:25–30. doi: 10.1007/s11906-001-0063-1. [DOI] [PubMed] [Google Scholar]
- 51.Lentine K.L., Costa S.P., Weir M.R., Robb J.F., Fleisher L.A., Kasiske B.L., Carithers R.L., Ragosta M., Bolton K., Auerbach A.D., et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 2012;60:434–480. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 52.Lentine K.L., Rocca Rey L.A., Kolli S., Bacchi G., Schnitzler M.A., Abbott K.C., Xiao H., Brennan D.C. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin. J. Am. Soc. Nephrol. 2008;3:1090–1101. doi: 10.2215/CJN.03080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glicklich D., Vohra P. Cardiovascular risk assessment before and after kidney transplantation. Cardiol. Rev. 2014;22:153–162. doi: 10.1097/CRD.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 54.January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C., Jr., Conti J.B., Ellinor P.T., Ezekowitz M.D., Field M.E., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 55.Kisters K., Cziborra M., Funke C., Brylak S., Hausberg M. Amiodarone-tacrolimus interaction in kidney transplantation. Clin. Nephrol. 2008;70:563. doi: 10.5414/CNP70563. [DOI] [PubMed] [Google Scholar]
- 56.Burger C.I., Clase C.M., Gangji A.S. Case report: Drug interaction between tacrolimus and amiodarone with QT prolongation. Transplantation. 2010;89:1166–1167. doi: 10.1097/TP.0b013e3181d2fed7. [DOI] [PubMed] [Google Scholar]
- 57.Malyszko J., Lopatowska P., Mlodawska E., Musialowska D., Malyszko J.S., Tomaszuk-Kazberuk A. Atrial fibrillation in kidney transplant recipients: Is there a place for the novel drugs? Nephrol. Dial. Transplant. 2017;33:1304–1309. doi: 10.1093/ndt/gfx265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.