Abstract
Purpose:
To describe the patterns of regression of choroidal melanoma after treatment with plaque brachytherapy.
Methods:
Retrospective interventional case series including 170 consecutive patients treated with 103Pd eye plaque radiation for choroidal melanoma. Outcome measures were changes in tumor thickness, surface characteristics, tumor vascularity, ultrasonography, fluorescein angiography, optical coherence tomography, and histopathology.
Results:
The mean initial tumor thickness was 3.9 mm (median 2.8 mm; range 2–11.3 mm) that decreased to 1.7 mm (median 1.2 mm; range 0–7.1 mm) after plaque brachytherapy. On imaging, tumors were pigmented in 51% (n = 86/170), amelanotic in 10% (n = 17/170), and variably pigmented in 39% (n = 67/170). Tumor pigmentation increased in 64% (n = 106/166), decreased in 18% (n = 30/166), and was unchanged in 18% (n = 30/166). Of the 120 that demonstrated intrinsic vascularity, 10% (n = 12/120) had decreased tumor-related vascularity and 90% (n = 108/120) showed complete resolution. Subretinal fluid was present in 34% (n = 58/170) of eyes at presentation. Of them, 15% (9; n = 9/58) had persistent SRF at last follow-up. On ultrasound imaging, 88% (n = 149/170) tumors presented with low to moderate internal reflectivity of which 61% (n = 91/149) showed increased reflectivity on regression. We noted a crescendo–decrescendo fluctuation in the presence of orange pigment lipofuscin along with complete resolution of drusenoid retinal pigment epithelial detachments. In the entire series of 170 patients, there was 0.5% (1) failure of local control, 2% (4) secondary enucleations, and 6% (10) patients developing metastasis.
Conclusion:
Findings related to choroidal melanoma regression after 103Pd plaque brachytherapy included decreased intrinsic tumor vascularity, decreased tumor-related subretinal fluid, increased pigmentation, specific changes in orange pigment lipofuscin and resolution of drusenoid retinal pigment epithelial detachments, as well as decreased tumor thickness with an increase in internal reflectivity on ultrasound.
Keywords: Choroidal melanoma, regression, pigment, exudative, ultrasound
Introduction
Radiation has increasingly replaced enucleation as an eye and vision-sparing alternative treatment for uveal melanoma. Five-year mean local control rates of up to 97% have been reported.1 Though tumor size is the most well validated predictor of metastatic disease, the importance of initial local control cannot be understated.2,3 In 2016, a multicenter, international data-sharing study involving 3217 patients reported significantly increased risk (6.3 hazard ratio) of metastasis if their initial uveal melanoma treatment fails.3,4
Death typically follows metastasis, because there exists no curative treatment for metastatic uveal melanoma. In that local tumor recurrence has been proven to be associated with a significantly higher risk of systemic metastasis,4 definitive local control (to prevent recurrence-related added metastatic risk) is currently the best way to “treat” metastatic uveal melanoma.3,4
That said, the best way to make plaque radiation therapy effective is to follow consensus guidelines for medical physics measurements and clinical application.5,6 Both the American Association of Physicists in Medicine (AAPM) and the American Brachytherapy Society (ABS) have sponsored multicenter, international efforts to define quality assurance, medical physics practices, and consensus guidelines. The ABS standards were created by 47 eye cancer specialists from 10 countries.6
In 1990, P.T.F. initiated the first preclinical and clinical studies using 103Pd ophthalmic plaques for treatment of choroidal melanomas.7,8 Since that time, multiple comparative dosimetry studies (including those of the AAPM TG-129) have demonstrated that in comparison to iodine-125 (125I), 103Pd photons are more rapidly absorbed within uveal melanomas, while less radiation reaches most normal ocular structures.5,9,10 These attributes, coupled with careful 103Pd plaque-size selection (2- to 3-mm safety margins) and precise episcleral placement, has yielded some of the highest reported rates of local control associated with low rates of metastatic melanoma.11,12
Once irradiated, eye cancer specialists are tasked with monitoring the tumor for regression and recurrence. Prior descriptions of uveal melanoma regression have largely concentrated on tumor-size (typically height) following brachytherapy with cobalt (60Co), iodine (125I) and ruthenium (106Ru) plaques, charged particles, and gamma knife.13–24 However, in 1982, Wilkes and Gragoudas25 examined choroidal melanoma regression after proton beam irradiation, where they considered tumor thickness, pigmentation, vascularity, and subretinal fluid (SRF). Most recently, our group described regression patterns of iris melanoma following 103Pd plaque.26 Therein, we noted that tumor vascularity, pigmentation, and thickness were the most important parameters when looking for regression.
In this era of a shifting paradigm toward eye and vision-sparing radiation, it is important to know which tumor characteristics are associated with local control. Herein, we describe the clinical features of regressing choroidal melanomas seen after 103Pd plaque brachytherapy.
Methods
This study adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act of 1996. We obtained approval from The New York Eye Cancer Center Internal Review Board to perform a retrospective chart review of 170 consecutive patients who met our inclusion criteria and had undergone ophthalmic plaque brachytherapy for choroidal melanoma between 2001 and 2012.
To provide a long-term perspective, no patients treated after 2012 were included in this study. Specific inclusion criteria were patients with at least 1-year follow-up, tumors not involving ciliary body, tumors in which location allowed observation, eyes in which media permitted adequate fundus photography, optical coherence tomography (OCT), fundus auto fluorescence (FAF), ultrasonography, and fluorescein angiography (FA).
Ophthalmic examinations were inclusive of but not limited to history, Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity, pupillary reactions, ocular motility, intraocular pressure, anterior segment, and fundus examination. Features recorded at presentation included tumor pigmentation (melanotic, amelanotic, or variable), tumor epicenter quadrant, and tumor location (posterior, equatorial, or anterior).
Fundus photography was used to determine the presence of orange pigment lipofuscin (OP) and drusenoid retinal pigment epithelial detachments. FA was used to evaluate tumor intrinsic vascularity as well as SRF. OCT was most sensitive for the presence of SRF as was FAF for OP. Ultrasonography was primarily used to determine tumor configuration (dome or mushroom-shaped), internal tumor reflectivity (low, moderate, or high), tumor basal dimensions, and tumor thickness.
Initial American Joint Committee on Cancer (AJCC) staging involved whole-body positron emission tomography-computed tomography (PET-CT).27 Post-treatment surveillance involved abdominal radiographic imaging every 6 months for the first 5 years and then every year thereafter. Based on the American College of Radiology Appropriateness Criteria for hepatic metastasis, the order of sensitivity was contrast-enhanced magnetic resonance imaging, computed tomography, and lastly ultrasound imaging.28
For this study, local recurrence was defined as ⩾0.5 mm of apical tumor growth (by ultrasonography) or ⩾1 mm of vascularized marginal growth (by comparative fundus photography and FA). Hemorrhagic or exudative tumor enlargement was observed for regression prior to being considered growth.
Palladium-103 plaque radiation therapy
Comparative preoperative dosimetry (125I vs103Pd seeds in gold ophthalmic plaques) were performed for each patient in this series. Plaque parameters included plaque size, type of plaque, apical tumor dose, and hours of irradiation. Critical ocular structures were defined as the tumor, subjacent sclera, lens, fovea, and central optic disc. Calculations of dose to opposite eye wall most closely represented organ dose. This analysis resulted in the use of 103Pd in every case.5,10
In this series, plaque treatments were consistent with the 2014 multicenter international ABS consensus guidelines for plaque brachytherapy of uveal melanoma. Therefore, surgery comprised tumor localization and plaque insertion, including scleral trans-illumination and intraoperative ultrasound to define tumor margins.29 Each plaque was constructed and placed to cover the entire tumor plus a 2- to 3-mm tumor-free safety margin.5,6,30 All patients received continuous radiation for 5–7 days.6
The mean tumor apex palladium-103 radiation dose was a mean 79.5 Gray (Gy) (median 80 Gy; range 5.9–99.8 Gy). The mean plaque size was 15 mm (median 16 mm; range 10–22 mm; Table 1).
Table 1.
Dosimetry and plaque treatment parameters.
| Variable | Value | |
|---|---|---|
| Plaque size (mm), no. (%) | 10 | 4 (2) |
| 12 | 17 (10) | |
| 14 | 57 (34) | |
| 16 | 48 (28) | |
| 18 | 25 (15) | |
| 20 | 12 (7) | |
| 22 | 7 (4) | |
| 103Pd seeds, no. | Median | 13 |
| Mean | 15 | |
| Range | 5–30 | |
| Radiation dose to tumor apex (Gy) | Median | 80 |
| Mean | 79.5 | |
| Range | 5.9–99.8 | |
| Dose to lens (Gy) | Median | 6.5 |
| Mean | 11.2 | |
| Range | 0.9–100.3 | |
| Dose to fovea (Gy) | Median | 33.8 |
| Mean | 53.9 | |
| Range | 2.6–291.9 | |
| Dose to optic nerve (Gy) | Median | 32.4 |
| Mean | 47.2 | |
| Range | 3.6–257.3 | |
| Duration of treatment (hours) | Median | 166 |
| Mean | 164.1 | |
| Range | 118–172 |
Gray (Gy) = 100 cGy.
Follow-up
As possible, posttreatment fundus photography, ultrasonography, OCT, and FAF were obtained every 3–4 months after brachytherapy. FA was typically performed every 6 months to monitor changes in tumor vascularity, radiation retinopathy, and optic neuropathy. Ultrasound-imaging was used to measure the tumor and monitor for extra scleral extension. At each visit, these test results were compared with the patient’s prior test examinations.
Results
The mean age of the patients was 61.3 years (median 63 years; range 22–86 years). The mean follow-up duration was 71 months (median 69.5 months; range 12–201 months). The mean initial largest basal tumor dimension was 11 mm (median 10.2 mm; range 4–19.4 mm). According to the 8th edition of the American Joint Committee on Cancer staging system for uveal melanoma, there were 86-T1, 59-T2, 21-T3, and 4-T4 sized tumors (Table 2).31
Table 2.
Patient demographic data and pretreatment tumor classifications.
| Features | Categories | No. | % |
|---|---|---|---|
| Race | White | 161 | 95 |
| Hispanic | 5 | 3 | |
| Asian | 3 | 2 | |
| Gender | Male | 91 | 54 |
| Female | 79 | 46 | |
| Eye | Right | 75 | 44 |
| Left | 95 | 56 | |
| Associated comorbidities | Hypertension | 64 | 38 |
| Diabetes | 19 | 11 | |
| Other cancers | 24 | 14 | |
| Tumor epicenter quadrant | Superotemporal | 57 | 34 |
| Inferotemporal | 52 | 30.5 | |
| Superonasal | 24 | 14 | |
| Inferonasal | 32 | 19 | |
| Foveal | 4 | 2 | |
| Disc | 1 | 0.5 | |
| Tumor location | Posterior | 129 | 76 |
| Equatorial | 24 | 14 | |
| Anterior | 17 | 10 | |
| AJCC staginga | T1 | 86 | 51 |
| T2 | 59 | 35 | |
| T3 | 21 | 12 | |
| T4 | 4 | 2 |
According to American Joint Committee on Cancer (AJCC) cancer staging manual, eighth edition.31
Data were available from all 170 patients at 1 year, 136 patients at 3 years, 101 patients at 5 years, and 22 patients at 10 years of follow-up. Data were analyzed for regression patterns with respect to changes in tumor thickness, pigmentation, and vascularity changes in ultrasonography and OCT features at 1-, 3-, 5-, and 10-year follow-up (Table 3).
Table 3.
Regression pattern analysis at 1, 3, 5, and 10 years.
| Feature | Before surgery (n = 170) | 1 year (n = 170) | 3 years (n = 136) | 5 years (n = 101) | 10 years (n = 22) | Last follow-up (mean 6 years; n = 166) |
|---|---|---|---|---|---|---|
| Mean tumor thickness (mm) | 3.9 | 2.4 | 1.5 | 1.4 | 0.7 | 1.7 |
| Pigmentation, no. (%) | 170 (100) | n = 170 | n = 136 | n = 101 | n = 22 | n = 166 |
| Increased | 123 (73) | 84 (62) | 62 (61) | 11 (50) | 106 (64) | |
| Decreased | 16 (9) | 28 (20) | 20 (20) | 6 (27) | 30 (18) | |
| Persistent | 31 (18) | 24 (18) | 19 (19) | 5 (23) | 30 (18) | |
| Orange pigment, no. (%) | 136 (80) | n = 170 | n = 136 | n = 101 | n = 22 | n = 166 |
| Present | 144 (85) | 39 (29) | 14 (14) | 2 (9) | 37 (22) | |
| Absent | 26 (15) | 97 (71) | 87 (86) | 20 (91) | 129 (78) | |
| Subretinal fluid, no. (%) | 58 (34) | n = 170 | n = 136 | n = 101 | n = 22 | n = 166 |
| Absent | 116 (68) | 112 (82) | 83 (82) | 21 (95) | 117 (70) | |
| Persistent | 54 (32) | 24 (18) | 18 (18) | 1 (5) | 49 (30) | |
| Intrinsic vascularity, no. (%) | 120 (70) | n = 120 | n = 86 | n = 34 | n = 10 | n = 120 |
| Resolved | 20 (17) | 29 (34) | 25 (74) | 10 (100) | 108 (90) | |
| Persistent | 100 (83) | 57 (66) | 9 (26) | 0 (0) | 12 (10) | |
| Internal reflectivity, no. (%) | 170 (100) | n = 170 | n = 136 | n = 101 | n = 22 | n = 166 |
| Low | 21 (12) | 7 (5) | 0 (0) | 0 (0) | 6 (4) | |
| Moderate | 63 (37) | 39 (29) | 30 (30) | 0 (0) | 48 (29) | |
| High | 86 (51) | 90 (66) | 71 (70) | 22 (100) | 112 (67) |
Comparison of regression characteristics before and after treatment
Change in tumor size on ultrasound
The mean initial tumor thickness was 3.9 mm (median 2.8 mm; range 2–11.3 mm) which reduced to 1.7 mm (median 1.2 mm; range 0–7.1 mm) at last follow-up. Radiation induced a mean 44% reduction in thickness in this series. All tumors showed reduction in tumor thickness except the one with recurrence.
Ultrasonographic internal reflectivity prior to radiation revealed that 13% (n = 22/170) tumors with low, 75% (n = 127/170) moderate, and 12% (n = 21/170) were highly reflective. Of the tumors with low to moderate internal reflectivity, 61% (n = 91/149) demonstrated an increase in internal reflectivity (Table 4). Tumors that started with high internal reflectivity remained highly reflective.
Table 4.
Comparison of regression characteristics before and after treatment.
| Features | Characteristics | Before Rx (n = 170) |
After Rx (n = 166) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Clinically | Pigmentation | Pigmented | 86 | 51 | 106 | 64 |
| Amelanotic | 17 | 10 | 30 | 18 | ||
| Variable | 67 | 39 | 30 | 18 | ||
| Orange pigment | Granular | 85 | 50 | 12 | 7 | |
| Gross | 51 | 30 | 25 | 15 | ||
| Absent | 34 | 20 | 129 | 78 | ||
| FAF | Orange pigment | Granular | 42 | 25 | 8 | 5 |
| Gross | 20 | 12 | 17 | 10 | ||
| Absent | 29 | 17 | 47 | 28 | ||
| N/A | 79 | 46 | 94 | 57 | ||
| OCT | Drusenoid retinal pigment epithelial detachments | Present | 30 | 18 | 0 | 0 |
| Absent | 140 | 82 | 166 | 100 | ||
| Subretinal fluid | Apical | 26 | 16 | 9 | 6 | |
| Dependent | 18 | 10 | 0 | 0 | ||
| Foveal | 14 | 8 | 40 | 24 | ||
| Absent | 112 | 66 | 117 | 70 | ||
| FFA | Intrinsic vascularity | Microaneurysms | 100 | 59 | 12 | 7 |
| Vessels | 20 | 12 | 0 | 0 | ||
| Absent | 50 | 29 | 154 | 93 | ||
| USG | Tumor configuration | Dome | 148 | 87 | 86 | 52 |
| Mushroom-shaped | 22 | 13 | 19 | 11 | ||
| Flat | 0 | 0 | 61 | 37 | ||
| Internal reflectivity | Low | 22 | 13 | 6 | 4 | |
| Moderate | 127 | 75 | 48 | 29 | ||
| High | 21 | 12 | 112 | 67 | ||
FAF: fundus auto fluorescence; OCT: optical coherence tomography; FFA: fundus fluorescein angiography; USG: ultrasonography.
Ultrasonographic shape prior to radiation revealed 87% (n = 148/170) to be dome-shaped and 13% (n = 22/170) to form a mushroom shape. Of the dome-shaped tumors, 58% (n = 86/148) remained dome-shaped and 41% (n = 61/148) became flat. Of the mushroom shaped tumors, 86% (n = 19/22) maintained their mushroom shape with reduced thickness. No patient had evidence of extra scleral extension.
Change in tumor color
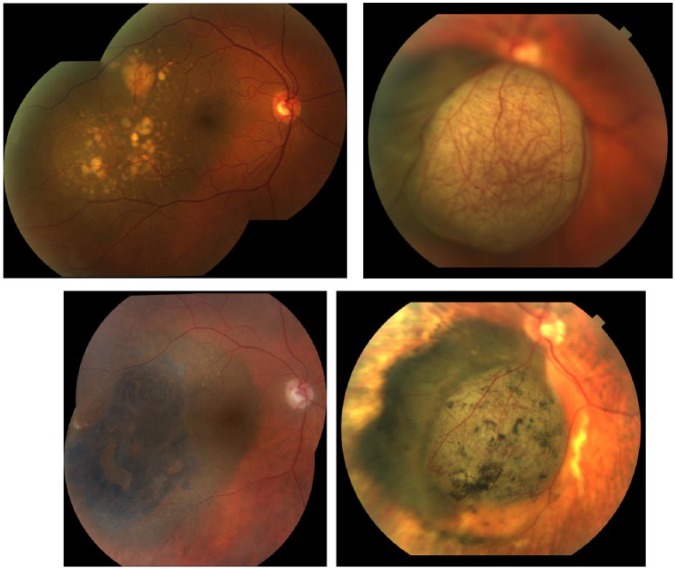
Fundus photography revealed that 51% (n = 86/170) of tumors were pigmented, 39% (n = 67/170) were variably pigmented, and 10% (n = 17/170) were amelanotic. At the last follow-up, tumor pigmentation was found to be increased in 64% (n = 106/166), decreased in 18% (n = 30/166), and unchanged in 18% (n = 30/166; Figure 1).
Figure 1.
Fundus photographs of a T2-sized choroidal melanoma (top left) before surgery and (bottom left) 8 years after brachytherapy. Note the change in pigmentation to bluish black, resolution of DRPEDs, and increase in orange pigment lipofuscin. Fundus photographs of a mushroom-shaped choroidal melanoma (top right) before surgery and (bottom right) 3 years after brachytherapy. Note the resolution of intrinsic tumor vascularity.
At last follow-up, subgroup analysis of the 129 tumors located posterior to the equator, 61% (n = 79/129) became more pigmented, 22% (n = 29/129) variably pigmented, and 13% (n = 17/129) became atrophic scars. Of tumors centered at the equator, 91% (n = 22/24) became more pigmented. Of interest, 76% (n = 13/18) of the anteriorly located tumors became atrophic scars.
Change in OP
Clinically: at presentation, 50% (n = 85/170) tumors had granular and 30% (n = 51/170) had gross OP. After 1 year of treatment, OP was present in 85% (n = 144/170). After 3 years of treatment, OP diminished to 29% (n = 39/136) and almost disappeared to 9% (n = 2/22) after 10 years. It is important to note that 14% (n = 12/85) had persistent granular and 49% (n = 25/51) had persistent gross OP at the last follow-up (Figure 1).
Imaging observations: on fundus autofluorescence, 25% (n = 42/170) tumors had granular and 12% (n = 20/170) had gross orange pigmentation. At the last follow-up, 19% (n = 8/42) had persistent granular and 85% (n = 17/20) had persistent gross OP. It is important to note that the patient who developed recurrence showed increase in orange pigmentation on autoflorescence at the site of recurrence.
Changes in OCT
Subretinal fluid (SRF) was present in 34% (n = 58/170) eyes, out of which, 45% (n = 26/58) had apical, 31% (n = 18/58) had dependent, and 24% (n = 14/58) had subfoveal fluid. After 18 months of treatment, resolution of SRF began and only 15% (n = 9/58) patients had persistent SRF due to tumor at last follow-up. The dependent SRF resolved in all patients and 24% (n = 40/166) patients developing radiation maculopathy/retinopathy ended up with persistent intraretinal fluid in the macular retina.
Drusenoid retinal pigment epithelial detachments (DRPED) were present on 18% (n = 30/170) of tumors prior to radiation. All (n = 30/30) of these tumors showed resolution of DRPED at last follow-up (Figure 1).
Changes on FA
On FA, dot micro-aneurysms were noted in 59% (n = 100/170) and intrinsic tumor vessels in 12% (n = 20/170) tumors prior to radiation. At last follow-up, 88% (n = 88/100) of micro-aneurysms had resolved. Similarly, 100% (n = 20/20) of intrinsic tumor blood vessels had resolved.
Visual acuity
Pretreatment visual acuities were a median of 20/25 (mean, 20/32; range, 20/16 to counting fingers). They decreased to a median of 20/32 (mean, 20/63; range, 20/16 to no light perception) at a mean 71 months follow-up (Table 5).
Table 5.
Complications and visual, ocular, and systemic outcomes at last follow-up.
| Variable | No. (n = 170) | % | ||
|---|---|---|---|---|
| Complications | Dry eye | 0 | 0 | |
| Scleromalacia | 0 | 0 | ||
| Cataracta | 15 | 9 | ||
| Glaucoma (including NVG) | 10 | 6 | ||
| Radiation retinopathy | 82 | 48 | ||
| Radiation maculopathy | 26 | 15 | ||
| Radiation optic neuropathy | 24 | 14 | ||
| Outcomes | Vision outcomesb | Good (20/16–20/40) | 95 | 56 |
| Intermediate (20/50–20/200) | 39 | 23 | ||
| Poor (<20/200) | 36 | 21 | ||
| Ocular outcomes | Tumor recurrence | 1 | 0.5 | |
| Enucleation | 4 | 2 | ||
| Eye salvage | 166 | 98 | ||
| Systemic outcomes | Distant metastases | 10 | 6 | |
| No distant metastases | 160 | 94 |
NVG: neovascular glaucoma.
New onset and worsening of preexisting disease.
Final best-corrected visual acuity.
Subgroup analysis revealed that out of 80% (n = 136/170) who had a baseline acuity of 20/16–20/40, 66% (n = 89/136) retained 20/16–20/40 at last follow-up. Reductions in visual acuity in 18 patients was attributed to radiation induced retinopathy and maculopathy.
Subgroup analysis of the 14% (n = 24/170) who had a baseline acuity of 20/50–20/200 showed that 25% (n = 6/24) improved to 20/16–20/40, and 42% (n = 10/24) retained 20/50–20/200 at last follow-up.
Subgroup analysis of the 6% (n = 10/170) who had a baseline acuity of <20/200, 10% (n = 1/10) improved to 20/50–20/200, and 90% (n = 9/10) continued with <20/200 at last follow-up This finding was mainly attributed to tumor location beneath the fovea or involving the optic disc.
Cataract and radiation vasculopathy
Of the patients who were phakic at the time of treatment 10% (n = 15/151) developed radiation-induced cataract. These cataracts were considered as acceptable and safely treatable consequences related to plaque brachytherapy.
Radiation retinopathy alone was the most common cause of treatment-related vision loss present in 48% (n = 82/170) patients. All patients were examined for the presence of radiation maculopathy and radiation optic neuropathy (RON) and were staged according to the Ophthalmic Radiation Oculopathy for Vision Prognosis Classification.32 No radiation oculopathy (Stage 0) was noted in 41% (n = 69/170) eyes at last follow-up. Stages 1–3 retinopathy was noted in 44% (n = 75/170). Stage 4 retinopathy primarily characterized by vitreous hemorrhage and RON was noted in 15% (n = 26/170). Initiated at the first clinical sign of neuropathy or maculopathy, 96 patients have been treated with intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy to suppress radiation-induced retinopathy or/and optic neuropathy.33–35
Unusual regression patterns
Seven irradiated melanomas were found to evolve into peculiar donut configuration (atrophy surrounded by pigmentation surrounded by atrophy) at last follow-up. All these tumors shared the common features of posterior location and an overlying fibrotic component prior to radiation therapy.
Also, melanomas with a mushroom-shape regressed such that the main body of the tumor shrunk, the mushroom configuration persisted, though to a darker color.
Histopathology
On histopathological evaluation of the four enucleated eyes, 75% (n = 3/4) had 1–2 mitotic figures/40 high-power fields showing that cells can enter mitosis even after irradiation. As radiation induces programmed DNA-damage-mediated cell death, the cytotoxic effect becomes histopathologically apparent only when cells enter mitosis over subsequent years.36 In addition, no radiation-treated choroidal melanoma was reported to have vascular loops. Thus, these four cases offer histopathologic corroboration of our angiographic observations that resolution of vascularity was a consistent finding associated with tumor regression.
Tumor resolution and metastasis
In this series, local tumor control (no recurrence) was achieved in 99.5% (n = 169/170) patients. Eye retention was seen in 98% (n = 166/170) patients. Four (2%) patients (n = 4/170) required secondary enucleation for neovascular glaucoma. Ten (6%) patients (n = 10/170) developed metastasis.
Discussion
In this study, side-by-side comparison of fundus photographs, OCT, FA, and ultrasonography from each follow-up visit was useful for detecting changes in pigmentation, SRF, vascularity, tumor size, and reflectivity after treatment.
Ultrasonography
Radiation induced a mean 44% reduction in tumor thickness in this series. We observed that the signs of tumor regression are decreased tumor thickness along with increased internal reflectivity.
Tumor color
At last follow-up, tumor pigmentation was found to be increased in 64% (n = 106/166) eyes. The tumors that were pigmented at presentation typically developed a dark bluish hue. Tumors located in the posterior and equatorial fundus typically regressed with increased pigmentation, whereas the tumors located in the anterior position more commonly regressed to chorioretinal atrophy. This may be due a vaso-occlusive radiation effect on the thinner peripheral choroid.
Orange Pigment
OP has been found after damage to various other human tissues like heart, liver, colon, and testis and is considered to be a nonspecific sign of cell death.37 In this study, we have noticed that OP gradually increased over the first 3 years after radiation. The clumps of gross OP consolidated and became more prominent, as if the OP revealed itself or more likely increased after treatment. Tumors that started with granular OP evolved to reveal gross OP. Then, 3 years after treatment, OP declined and almost disappeared after 5–10 years of treatment. This latter finding, and as we observed on our solitary recurrence, suggests that the presence of new OP should be considered along with other signs of recurrence.
Radiation vasculopathy
Leakage from the tumor decreased within first 3 years as the tumor regressed. However, radiation-related exudative retinal changes started to appear between 18 and 24 months after treatment.35 The most common cause of SRF at the last follow-up was radiation-related retinal vascular changes (including intraretinal fluid). intraretinal fluid. Radiation maculopathy was largely supressed with intravitreal anti-VEGF therapy.33–35
Tumor vascularity
Tumor vascularity reduced and typically disappeared over time. This is an “in-vivo” demonstration of radiation’s vaso-occlusive effect on uveal melanoma and choroidal vasculature.38 Partial reduction or complete elimination of intrinsic tumor vascularity was found to be the most consistent finding related to tumor regression. This finding suggests that the presence of new tumor vascularity suggests tumor recurrence.
In the literature review, descriptions of regression of choroidal melanoma after treatment have largely been limited to ultrasonographic tumor thickness reduction.13–24 Rashid et al.39 reported 43% decrease in thickness and 42% decrease in cross-sectional area of tumor after 106Ru and 125I plaque brachytherapy. A recent study by Rashid et al.40 also reported that initial tumor thickness was the predominant clinical predictor of regression rate of choroidal melanomas after 106Ru and 125I brachytherapy. Abramson et al.15 also reported that the amount of reduction in tumor thickness after 60Co and 125I therapy was the same, irrespective of location of tumor. Salvi et al.41 noted that the regression of tumor height at 3, 6, and 12 months with 125I did not statistically correlate with tumor location. In our study, all lesions, irrespective of location and shape, show similar amounts of reduction in tumor thickness. Using proton therapy, Wilkes and Gragoudas25 noted resolution of the secondary serous retinal detachments in 100% (our study 85%) and destruction of the tumor’s vasculature with elimination of leakage on FA in 66% (our study 90%). In addition, the 1982 Wilkes study could not report on newly available OCT and FAF findings and nor did they analyze radiation-related changes or vision outcomes.
With respect to local control: in a review of 100 patients treated with 60Co plaque, Cruess et al.13 reported that there was no difference in the rate and extent of tumor regression between the patients who developed metastasis and the ones who did not. Kaiserman et al.42 found that the initial tumor thickness regression rate was significantly higher in patients who developed metastasis (6% per month) compared to patients who did not (4% per month), with 106Ru plaque. Glynn et al.43 following proton beam radiotherapy, found that tumors regressing rapidly were significantly more likely to develop metastasis within 2 years of treatment, while tumors with slow regression developed metastasis after 2 years of treatment. In our study, we found no difference in the rate of tumor regression between patients who developed metastasis and those who remained well systemically.
In terms of visual acuity: Marconi et al.44 noted that after 106Ru plaque brachytherapy, 56% of patients had visual acuity of 20/25 initially, out of which more than half stayed with 20/40. Patel et al.45 compared 103Pd with 125I and found that >20/40 visual acuity was noted in 65% with 103Pd versus 30% with 125I. Papakostas et al.46 noted visual acuity of ⩾20/200 in 8.7% patients and counting fingers in 22% patients after proton beam radiotherapy. In our study, we noted excellent median visual acuity of 20/32 with 103Pd plaque brachytherapy.
In that, choroidal melanomas have been reported to appear in various configurations: dome, mushroom-shaped, and irregular. They can be melanotic, amelanotic, or demonstrate variable pigmentation. They may be associated with orange pigment, vascularity, or SRF. Clearly, a single parameter of change (as measured thickness) does not comprehensively define regression of choroidal melanomas.
Advantages of our study
This study benefits from its long duration of follow-up, the use of a single treatment modality, and that all surgeries were performed by one, experienced surgeon (P.T.F.) who has chaired or participated in recent ABS and AAPM guideline initiatives.5,6,30 With such high (99.5%) local control (at a mean duration of 71 months after 103Pd plaque brachytherapy), our outcomes should be considered reliable characteristics of choroidal melanoma regression.
Limitations of this study
This study was retrospective. Although reasonably sized compared to published reports in the literature, a large multicenter cooperative “big data” registry study would improve validation and allow for observations after alternative methods of radiation therapy.
In conclusion
Findings related to choroidal melanoma regression (after 103Pd plaque brachytherapy) include increased tumor pigmentation of posterior tumors, an evolution from increased to decreased OP and complete resolution of DRPED. FA and histopathology revealed resolution of intrinsic tumor vascularity, while OCT showed decreased tumor-related, exudative SRF. Finally, ultrasound imaging revealed decreased tumor thickness with a trend toward synchronous increased internal reflectivity. We hope this study serves to aid to the clinical judgment of those who treat and follow patients with choroidal melanoma.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by The Eye Cancer Foundation, Inc., Dr Maheshwari received a fellowship Grant from The Eye Cancer Foundation (http://eyecancercure.com).
References
- 1. Finger PT, Chin KJ, Duvall G, et al. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology 2009; 116(4): 790–796. [DOI] [PubMed] [Google Scholar]
- 2. Egger E, Schalenbourg A, Zografos L, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys 2001; 51(1): 138–147. [DOI] [PubMed] [Google Scholar]
- 3. American Joint Committee on Cancer Ophthalmic Oncology Task Force. International validation of the American Joint Committee on Cancer’s 7th edition classification of uveal melanoma. JAMA Ophthalmol 2015; 133(4): 376–383. [DOI] [PubMed] [Google Scholar]
- 4. Ophthalmic Oncology Task Force. Local recurrence significantly increases the risk of metastatic uveal melanoma. Ophthalmology 2016; 123(1): 86–91. [DOI] [PubMed] [Google Scholar]
- 5. Chiu-Tsao S-T, Astrahan MA, Finger PT, et al. Dosimetry of (125)I and (103)Pd COMS eye plaques for intraocular tumors: report of Task Group 129 by the AAPM and ABS. Med Phys 2012; 39(10): 6161–6184. [DOI] [PubMed] [Google Scholar]
- 6. American Brachytherapy Society—Ophthalmic Oncology Task Force. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy 2014; 13(1): 1–14. [DOI] [PubMed] [Google Scholar]
- 7. Finger PT, Berson A, Szechter A. Palladium-103 plaque radiotherapy for choroidal melanoma: results of a 7-year study. Ophthalmology 1999; 106(3): 606–613. [DOI] [PubMed] [Google Scholar]
- 8. Finger PT, Berson A, Ng T, et al. Palladium-103 plaque radiotherapy for choroidal melanoma: an 11-year study. Int J Radiat Oncol Biol Phys 2002; 54(5): 1438–1445. [DOI] [PubMed] [Google Scholar]
- 9. Finger PT, Lu D, Buffa A, et al. Palladium-103 versus iodine-125 for ophthalmic plaque radiotherapy. Int J Radiat Oncol Biol Phys 1993; 27(4): 849–854. [DOI] [PubMed] [Google Scholar]
- 10. Finger PT, Zhou D, Kalach N, et al. 103Pd versus 125I ophthalmic plaque brachytherapy: preoperative comparative radiation dosimetry for 319 uveal melanomas. J Radiat Oncol 2014; 3(4): 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagendran ST, Finger PT, Campolattaro BN. Extraocular muscle repositioning and diplopia: associated with ophthalmic plaque radiation therapy for choroidal melanoma. Ophthalmology 2014; 121(11): 2268–2274. [DOI] [PubMed] [Google Scholar]
- 12. Maheshwari A, Finger PT. A 12-year study of slotted palladium-103 plaque radiation therapy for choroidal melanoma: near, touching, or surrounding the optic nerve. Am J Ophthalmol 2018; 188: 60–69. [DOI] [PubMed] [Google Scholar]
- 13. Cruess AF, Augsburger JJ, Shields JA, et al. Regression of posterior uveal melanomas following cobalt-60 plaque radiotherapy. Ophthalmology 1984; 91(12): 1716–1719. [DOI] [PubMed] [Google Scholar]
- 14. Augsburger JJ, McNeary BT, von Below H, et al. Regression of posterior uveal malignant melanomas after cobalt plaque radiotherapy. Graefes Arch Clin Exp Ophthalmol 1986; 224(5): 397–400. [DOI] [PubMed] [Google Scholar]
- 15. Abramson DH, Servodidio CA, McCormick B, et al. Changes in height of choroidal melanomas after plaque therapy. Br J Ophthalmol 1990; 74(6): 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demirci H, Saponara F, Khan A, et al. Regression rate of posterior uveal melanomas following iodine-125 plaque radiotherapy. Middle East Afr J Ophthalmol 2015; 22(1): 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaiserman I, Anteby I, Chowers I, et al. Changes in ultrasound findings in posterior uveal melanoma after ruthenium-106 brachytherapy. Ophthalmology 2002; 109(6): 1137–1141. [DOI] [PubMed] [Google Scholar]
- 18. Georgopoulos M, Zehetmayer M, Ruhswurm I, et al. Tumour regression of uveal melanoma after ruthenium-106 brachytherapy or stereotactic radiotherapy with gamma knife or linear accelerator. Ophthalmologica 2003; 217(5): 315–319. [DOI] [PubMed] [Google Scholar]
- 19. Novak-Andrejcic K, Jancar B, Hawlina M. Echographic follow-up of malignant melanoma of the choroid after brachytherapy with 106Ru. Klin Monbl Augenheilkd 2003; 220(12): 853–860. [DOI] [PubMed] [Google Scholar]
- 20. Bartlema YM, Oosterhuis JA, Journée-De Korver JG, et al. Combined plaque radiotherapy and transpupillary thermotherapy in choroidal melanoma: 5 years’ experience. Br J Ophthalmol 2003; 87(11): 1370–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee CS, Lee SC, Lee K, et al. Regression of uveal melanoma after Ru-106 brachytherapy and thermotherapy based on metabolic activity measured by positron emission tomography/computed tomography. Retina 2014; 34(1): 182–187. [DOI] [PubMed] [Google Scholar]
- 22. Gragoudas ES, Egan KM, Saornil MA, et al. The time course of irradiation changes in proton beam-treated uveal melanomas. Ophthalmology 1993; 100(10): 1555–1559. [DOI] [PubMed] [Google Scholar]
- 23. Mosci C, Lanza FB, Mosci S, et al. Quantitative echography in primary uveal melanoma treated by proton beam therapy. Can J Ophthalmol 2014; 49(1): 60–65. [DOI] [PubMed] [Google Scholar]
- 24. Müllner K, Langmann G, Pendl G, et al. Echographic findings in uveal melanomas treated with the Leksell gamma knife. Br J Ophthalmol 1998; 82(2): 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkes SR, Gragoudas ES. Regression patterns of uveal melanomas after proton beam irradiation. Ophthalmology 1982; 89(7): 840–844. [DOI] [PubMed] [Google Scholar]
- 26. Chaugule SS, Finger PT. Regression patterns of iris melanoma after palladium-103 (103Pd) plaque brachytherapy. Ophthalmology 2017; 124(7): 1023–1030. [DOI] [PubMed] [Google Scholar]
- 27. Freton A, Chin KJ, Raut R, et al. Initial PET/CT staging for choroidal melanoma: AJCC correlation and second nonocular primaries in 333 patients. Eur J Ophthalmol 2012; 22(2): 236–243. [DOI] [PubMed] [Google Scholar]
- 28. Expert Panel on Gastrointestinal Imaging:, Kaur H, Hindman NM, et al. ACR appropriateness criteria® suspected liver metastases. J Am Coll Radiol 2017; 14(5S): S314–S325. [DOI] [PubMed] [Google Scholar]
- 29. Finger PT, Tran HV, Turbin RE, et al. High-frequency ultrasonographic evaluation of conjunctival intraepithelial neoplasia and squamous cell carcinoma. Arch Ophthalmol 2003; 121(2): 168–172. [DOI] [PubMed] [Google Scholar]
- 30. Finger PT. Radiation therapy for choroidal melanoma. Surv Ophthalmol 1997; 42(3): 215–232. [DOI] [PubMed] [Google Scholar]
- 31. Kivela T Simpson ER Grossniklaus HE et al.. Uveal melanoma. In:Amin MB, Edge SB, Greene FL, et al. (eds) AJCC cancer staging manual. New York: Springer International Publishing, 2016, pp. 805–813. [Google Scholar]
- 32. Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol 2005; 89(6): 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Finger PT, Chin K. Anti-vascular endothelial growth factor bevacizumab (Avastin) for radiation retinopathy. Arch Ophthalmol 2007; 125(6): 751–756. [DOI] [PubMed] [Google Scholar]
- 34. Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys 2008; 70(4): 974–977. [DOI] [PubMed] [Google Scholar]
- 35. Finger PT, Chin KJ. Intravitreous ranibizumab (lucentis) for radiation maculopathy. Arch Ophthalmol 2010; 128(2): 249–252. [DOI] [PubMed] [Google Scholar]
- 36. Char DH, Saunders W, Castro JR, et al. Helium ion therapy for choroidal melanoma. Ophthalmology 1983; 90(10): 1219–1225. [DOI] [PubMed] [Google Scholar]
- 37. Piña-Oviedo S, Ortiz-Hidalgo C, Ayala AG. Human colors-the rainbow garden of pathology: what gives normal and pathologic tissues their color? Arch Pathol Lab Med 2017; 141(3): 445–462. [DOI] [PubMed] [Google Scholar]
- 38. Nag S, Quivey JM, Earle JD, et al. The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys 2003; 56(2): 544–555. [DOI] [PubMed] [Google Scholar]
- 39. Rashid M, Heikkonen J, Kivelä T. Tumor regression after brachytherapy for choroidal melanoma: reduction of thickness and cross-sectional area by shape and regression pattern. Invest Ophthalmol Vis Sci 2015; 56(4): 2612–2623. [DOI] [PubMed] [Google Scholar]
- 40. Rashid M, Heikkonen J, Singh AD, et al. Clinical predictors of regression of choroidal melanomas after brachytherapy: a growth curve model. Ophthalmology 2018; 125: 747–754. [DOI] [PubMed] [Google Scholar]
- 41. Salvi SM, Aziz HA, Dar S, et al. Uveal melanoma regression after brachytherapy: relationship with chromosome 3 monosomy status. Ocul Oncol Pathol 2017; 3(2): 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaiserman I, Anteby I, Chowers I, et al. Post-brachytherapy initial tumour regression rate correlates with metastatic spread in posterior uveal melanoma. Br J Ophthalmol 2004; 88(7): 892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Glynn RJ, Seddon JM, Gragoudas ES, et al. Evaluation of tumor regression and other prognostic factors for early and late metastasis after proton irradiation of uveal melanoma. Ophthalmology 1989; 96(10): 1566–1573. [DOI] [PubMed] [Google Scholar]
- 44. Marconi DG, de Castro DG, Rebouças LM, et al. Tumor control, eye preservation, and visual outcomes of ruthenium plaque brachytherapy for choroidal melanoma. Brachytherapy 2013; 12(3): 235–239. [DOI] [PubMed] [Google Scholar]
- 45. Patel KR, Prabhu RS, Switchenko JM, et al. Visual acuity, oncologic, and toxicity outcomes with 103Pd vs. 125I plaque treatment for choroidal melanoma. Brachytherapy 2017; 16(3): 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Papakostas TD, Lane AM, Morrison M, et al. Long-term outcomes after proton beam irradiation in patients with large choroidal melanomas. JAMA Ophthalmol 2017; 135(11): 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]