Abstract
Background:
Acute bacterial skin and skin structure infections (ABSSSI) are a leading cause of hospitalization, but are often treated inappropriately in the inpatient setting. A multifaceted stewardship intervention was implemented to encourage prescribing of guideline-concordant therapy (GCT).
Objective:
To examine the impact of this initiative on antimicrobial prescribing practices and patient outcomes.
Methods:
This was a single-center, retrospective study of adult inpatients admitted with a primary or secondary diagnosis of ABSSSI, classified by type and severity based on signs of systemic infection. Patients treated during the pre-intervention period (pre-IP) were compared with patients treated during the post-intervention period (post-IP). The primary endpoint was receipt of GCT. Secondary endpoints included receipt of anti-anaerobic antibiotic (AAA) or broad-spectrum antibiotics (BSA).
Results:
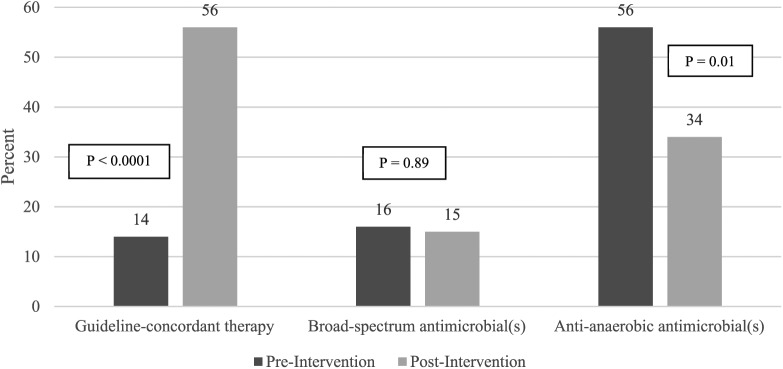
A total of 125 patients were included, 64 in the pre-IP and 61 in the post-IP. There was a statistically significant increase in prescribing of GCT during the post-IP compared with the pre-IP (14% versus 56%, p < 0.0001) and a decrease in use of AAA (56% versus 34%, p = 0.01). No difference was observed with use of BSA (16% versus 15%, p = 0.89). Use of the computerized order set during the post-IP was low (18%). There was a numerical, but non-significant reduction in 30-day readmission (14.1% versus 6.6%, p = 0.17).
Conclusion:
The multifaceted intervention was effective for improving prescribing of GCT for ABSSSI. Given low use of the computerized order set, improved prescribing seemed to be driven by provider education. Strategies around ongoing education may be key to sustain positive results of stewardship interventions.
Keywords: antibiotics, bacterial infections, clinical practice guidelines, evidence-based practice, infectious diseases
Introduction
Acute bacterial skin and skin structure infections (ABSSSI) are a significant burden on the United States healthcare system.1 Rates of ABSSSI have substantially increased since 2000, becoming one of the most common infections seen in clinical practice, estimated to cause 870,000 hospital admissions.2 Although ABSSSI represent an increasing burden on the healthcare system, relatively little data have been published regarding its treatment in the inpatient setting.
In 2014, the Infectious Diseases Society of America (IDSA) released updated evidence-based guidelines for the treatment of ABSSSI. These guidelines provide recommendations for treatment of a spectrum of ABSSSI, including cellulitis, wound infections, uncomplicated abscesses, and necrotizing fasciitis.3 Adherence to the IDSA guidelines in clinical practice has been highlighted as an opportunity for focus of antimicrobial stewardship programs in a study by Jenkins and colleagues, who retrospectively evaluated a cohort of patients hospitalized for ABSSSI at a large teaching hospital. Use of inappropriate antibiotics, such as those with broad Gram-negative activity or anaerobic activity was frequent, occurring in up to 83% of all patients. In addition, the average duration of therapy was 13 days, exceeding the guideline-recommended duration of 5–10 days for most patients with ABSSSI.1,3
The use of technology, such as computerized order sets, is an important tool for optimizing stewardship efforts.4 Implementation of computerized order sets, which can be used to provide recommendations for antimicrobial treatment at the time of prescribing, has been shown to reduce use of broad-spectrum antibiotics (BSA) and reduce length of stay.5–7
As such, a clinical pathway, computerized order set, and provider education for treatment of ABSSSI were implemented at nine hospitals within Atrium Health as a major stewardship initiative to encourage appropriate antibiotic prescribing. The purpose of this study was to examine the rate of prescribing of guideline-concordant therapy (GCT) for treatment of ABSSSI between the pre-intervention period (pre-IP) and post-intervention period (post-IP).
Methods
Study design and participants
We conducted a retrospective, single-center, pre- and post-intervention quasi-experimental study at an 874-bed, academic medical center from April 2015 to November 2015 to determine the effect of a multifaceted stewardship intervention on treatment of ABSSSI. The multidisciplinary intervention was launched in August of 2015, approximately halfway through the study period. A robust antimicrobial stewardship program with multidisciplinary support was in place during the entirety of the study period. This study was approved by the Institutional Review Board, and the need for written informed consent was waived. Patients with a primary or secondary diagnosis of ABSSSI based on ICD-9 discharge codes were identified.
Patients over the age of 18 admitted to a medical service were included. Excluded patients were those with necrotizing fasciitis, sepsis, diabetic foot infections with ulcers, deep wounds, animal or human bites, water-associated infections, oral abscesses, cellulitis of the perineum or perirectal area, immunocompromised patients, positive bacterial cultures from another site during the index admission, microbiologic history of infection caused by multidrug-resistant organisms in the last year, and admission to the hematology/oncology floor or intensive care unit. Immunocompromised patients included solid organ or bone marrow transplant recipients, neutropenic patients with absolute neutrophil count (ANC) <0.5 cells/mm3 or expected to decline below 0.5 cells/mm3 in the next 48 h, and those receiving chemotherapy at the time of infection.
Two independent investigators collected data from the medical record to determine the type (purulent or non-purulent) and severity of infection (mild, moderate, or severe). In the event of a discrepancy between the first two reviewers, a third investigator conducted an independent chart review to determine presence of purulence and severity. An infection was classified as purulent if fluctuance, purulence, pus, or a drainable abscess was mentioned in the provider notes or elsewhere in the electronic medical record. Consistent with the IDSA guidelines, severity was determined based on the number of signs of systemic infection (temperature greater than 38°C or less than 36°C, heart rate greater than 90, respiratory rate greater than 24, white blood cell count greater than 12,000/mm3 or less than 4000/mm3, or acute hypotension). Patients without signs of systemic infection were classified as having a mild ABSSSI, those with one sign of systemic infection but who were hemodynamically stable were classified as having a moderate ABSSSI, and those with two or more signs of systemic infection plus acute hypotension or organ dysfunction were classified as having a severe ABSSSI.
Intervention
Patients in the pre-IP (April 2015 until August 2015) were treated at the discretion of the medical team, without the assistance of any computerized order set, clinical pathway, or education. For the patients in the post-IP (mid-August until November 2015), providers were educated on GCT recommendations (Appendices 1 and 2) and were given the option to treat patients using the guideline-based computerized order set for ABSSSI. Education entailed an in-person, didactic presentation at a department meeting by a stewardship pharmacist and physician, as well as email communication distributed to all clinicians. The clinical pathway (published on the hospital intranet) and computerized order set included options for both purulent and non-purulent cellulitis, and preferred antibiotics were listed based on severity of illness. The service lines targeted for the intervention included hospitalists, adult medicine attendings, and medical residents. All providers within these groups were encouraged to attend the in-person education presentations and utilize the clinical pathway with computerized order set.
Outcomes
The primary outcome was the percent of patients who received GCT. An antibiotic regimen that was recommended by the IDSA clinical guideline was considered preferred, while any regimen that was not recommended by the guideline was considered non-preferred. Secondary outcomes included the rate of use of BSA (piperacillin/tazobactam, cefepime, ceftazidime, carbapenems, aztreonam, fluoroquinolones, tigecycline, and aminoglycosides) for treatment of ABSSSI, the use of anti-anaerobic antibiotics (AAA) (ampicillin/sulbactam, piperacillin/tazobactam, carbapenems, metronidazole, and clindamycin) when not indicated, total duration of antibiotic therapy, length of stay, 30-day infection-related readmission rate, and the percent of patients presenting with Clostridium difficile within 1 month of completing antibiotic therapy.
Statistics
Descriptive statistics, including means and standard deviations (SDs) for continuous variables, or counts and percentages for categorical variables, were calculated. Chi-square test comparing the rate of BSA utilization between pre- and post-IP along with the corresponding 95% confidence intervals on the difference in the proportions were used. Secondary outcomes such as BSA use, AAA use when not indicated, frequency of use of the ABSSSI order set, 30-day infection-related readmission rate, and the percent of patients presenting with C. difficile within 1 month of completing antibiotic therapy were analyzed using the chi-square or Fisher’s exact test. The duration of antimicrobial therapy and length of stay were analyzed using the t-test or the non-parametric Wilcoxon rank-sum test. SAS Enterprise Guide®, version 6.1, was used for all analyses. Two-tailed p-values of less than 0.05 were considered statistically significant.
Results
Of the 781 patients screened, a total of 125 patients met eligibility criteria for the study, with 64 patients in the pre-IP and 61 patients in the post-IP. Baseline demographics (Table 1) were similar between groups except for a higher percentage of patients in the pre-IP receiving antibiotics prior to admission compared to the post-IP (44% versus 23%, respectively). In addition, a diagnosis of moderate severity, purulent cellulitis was most commonly observed in 76% of the pre-IP and a diagnosis of moderate severity, non-purulent cellulitis was most commonly observed in 60% of the post-IP. In general, patients were middle-aged with a moderate severity, most commonly non-purulent ABSSSI.
Table 1.
Baseline demographics.
| Characteristic | Pre-intervention N = 64 n (%) |
Post-intervention N = 61 n (%) |
|---|---|---|
| Age (mean) | 53 (SD 18.3) | 49 (SD 18.6) |
| Sex (male) | 29 (45) | 28 (46) |
| Race | ||
| Caucasian | 39 (61) | 32 (52) |
| African American | 19 (30) | 25 (41) |
| Asian | 1 (2) | 0 |
| Other | 5 (8) | 4 (7) |
| Received antibiotics prior to admission | 28 (44) | 14 (23) |
| Recurrent ABSSSI in last 30 days | 19 (30) | 15 (25) |
| ABSSSI type | ||
| Purulent | 25 (39) | 26 (43) |
| Non-purulent | 39 (61) | 35 (57) |
| Severity | ||
| Mild | 9 (14) | 11 (18) |
| Moderate | 37 (58) | 31 (51) |
| Severe | 18 (28) | 19 (31) |
| Received I&D or drainage | 13 (20) | 15 (25) |
ABSSSI, acute bacterial skin and skin structure infections; SD, standard deviation; I&D, incision and debridement.
Primary and secondary outcomes
Results for the primary and secondary outcomes are detailed in Figure 1. There was a statistically significant improvement in the primary outcome with utilization rates of GCT increasing from 14% to 56% (p < 0.0001) following the intervention. No patients received GCT in the pre-intervention groups with mild and severe classification of severity. GCT rates improved in mild and severe purulent (50% and 83%, respectively) and mild and severe non-purulent (71% and 57%, respectively). Rates in moderate severity, purulent ABSSSI improved from 5% to 40% and remained relatively unchanged in moderate severity, non-purulent ABSSSI. More patients in the post-IP with mild, purulent received no antibiotics (0% in the pre-IP and 50% in the post-IP), where the IDSA guidelines recommend incision and debridement alone. In the post-IP, use of the computerized order set did not result in increases in prescribing of GCT or decreases in use of BSA (Table 2).
Figure 1.
Primary and secondary outcomes.
Table 2.
Effect of computerized order set use on prescribing (post-intervention period only).
| Outcome | Order set used N = 11 n (%) |
Order set not used N = 50 n (%) |
|---|---|---|
| Received preferred therapy | 5 (45) | 29 (58) |
| Received broad-spectrum antibiotic(s) | 3 (27) | 6 (12) |
In terms of the secondary outcomes, there was a statistically significant improvement in use of AAA. Although use of AAA decreased in the post-IP (56% versus 34%, respectively), inappropriate use of clindamycin remained a significant opportunity for improvement. There was no difference in BSA utilization between the groups.
Other outcomes
Results for the other outcomes are detailed in Table 3. There were no statistically significant differences observed in the duration of therapy, length of stay, 30-day readmission, or Clostridium difficile rates. Duration of antimicrobial therapy and length of stay were generally low in both the pre- and post-IP. Despite non-statistical significance, approximately half the number of 30-day readmissions was observed in the post-IP.
Table 3.
Other clinical outcomes.
| Outcome | Pre-intervention N = 64 |
Post-intervention N = 61 |
p-value |
|---|---|---|---|
| Mean duration of therapy with all antimicrobials (days) | 3.6 | 3.8 | 0.9078 |
| Mean length of stay (days) | 4.1 | 4.4 | 0.9319 |
| 30-day readmission | 9 (14.1%) | 4 (6.6%) | 0.1694 |
| Clostridium difficile infection within 30 days of completion of antibiotics | 0 | 0 | N/A |
Discussion
ABSSSI is a common cause for hospital admission and is often not treated according to national guidelines.1 Inappropriate use of antibiotics is associated with consequences such as prolonged hospitalization, increased resistance, risk of medication-related adverse events such as Clostridium difficile infection, and excess healthcare cost.4 In addition, selection of non-preferred antibiotics may increase treatment failure rates. We implemented a multifaceted antimicrobial stewardship intervention that included design of a clinical pathway, embedding of the clinical pathway into a computerized order set, and provider education that was directed at improving GCT of ABSSSI in the inpatient setting. Our findings demonstrate an improvement in the primary outcome, receipt of GCT, in the post-IP patient cohort. This seemed to be driven mostly by decreases in methicillin-resistant Staphylococcus aureus (MRSA)-directed agents for non-purulent cellulitis, decreases in inappropriate use of clindamycin (not recommended first-line in the institutional guideline due to low empiric susceptibility rates for Staphylococcus aureus) and less use of any antibiotics for mild, purulent cellulitis, where incision and debridement are sufficient therapy.3 There was no difference in the use of BSA agents between the two periods. This was not surprising given the unexpectedly low rate of BSA utilization in the pre-IP. There was also a statistically significant improvement in the use of AAA, likely driven by the decreased use of clindamycin in the post-IP.
Two previous studies have similarly examined the impact of a stewardship intervention on antimicrobial prescribing for ABSSSI in inpatients.8,9 In both studies, a clinical practice guideline, educational campaign, and computerized order set were implemented. However, our study was unique in that it included a primary endpoint of GCT based on type and severity of infection in accordance with the 2014 IDSA guidelines for treatment of ABSSSI, which was not directly assessed in prior studies. Jenkins and colleagues demonstrated a decrease in duration of therapy, BSA use, microbiological cultures, and requests for inpatient consultations after implementation of their multifaceted intervention.8 In a more recent study, Housman and colleagues demonstrated a decrease in BSA utilization; however, they did not observe a difference in broader clinical outcomes such as duration of therapy, length of stay, and hospital readmissions.9 We observed similar results, likely due to the short duration of therapy and length of stay at baseline. While readmissions were low, we did note an approximately 50% decrease in the post-IP, although this did not reach statistical significance.
Use of the computerized order set was low, and its use did not have an impact on prescribing of GCT. An effect may have been seen if utilization of the order set was higher, but the sample size in each of the groups was so low that non-preferred prescribing in one patient had a large effect on the overall percentage. This indicates that provider education and availability of the guideline on the hospital intranet were likely the interventions that had the largest impact on prescribing. Use of computerized order sets alone is likely not the best strategy to drive preferred antimicrobial use, and other mechanisms are needed to improve use of GCT.
To promote provider uptake of these interventions, other methods such as academic detailing could be considered, where trained pharmacists or physicians visit with other providers in real-time to discuss appropriate therapeutic choices based on available evidence. Other institutions have implemented stewardship and infectious diseases physician review of patients admitted through the emergency department with ABSSSI to provide feedback and recommendations to the attending physicians. This strategy resulted in decreases in length of stay and all-cause readmission.10
This study has several limitations. First, the stringent exclusion criteria may decrease the applicability of the study to more severely ill patients including those with underlying immunocompromise, associated bacteremia, or if admitted to an intensive care unit. Also, it was a retrospective chart review that relied upon provider documentation in the electronic medical record; there may have been factors that were not recorded in the vitals and labs section of the chart that influenced a provider’s perception of severity. Despite our best efforts to objectify classification of disease severity by two to three independent reviewers, there was still some inherent subjectivity in these classifications and may have varied from the provider evaluating the patient at the time of diagnosis. Since provider education was found to be such an integral driver of utilization of preferred therapy, it is likely that the effects observed in our study period may not be sustained over time. This highlights the importance of continuous rather than one-time education, and also the need for other mechanisms to improve preferred antibiotic use.
Conclusion
The multifaceted intervention was effective for improving prescribing of GCT for ABSSSI. Although improved in the post-IP, inappropriate use of clindamycin remained an opportunity for improvement. Given low use of the computerized order set, the effects seemed to be driven by provider education. Strategies around ongoing education may be key to sustain positive results of stewardship interventions related to ABSSSI and other infections.
Supplemental Material
Supplemental material, supplemental_appendices for Effect of a multifaceted stewardship intervention on antibiotic prescribing and outcomes for acute bacterial skin and skin structure infections by Danya Roshdy, Rupal Jaffa, Kelly E. Pillinger, Josh Guffey, Nigel Rozario, Lisa Davidson and Lewis McCurdy in Therapeutic Advances in Infectious Disease
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Danya Roshdy, Pharmacy Department, Atrium Health, Charlotte, NC, USA.
Rupal Jaffa, Pharmacy Department, Atrium Health, Charlotte, NC, USA.
Kelly E. Pillinger, Pharmacy Department, Atrium Health, Charlotte, NC, USA
Josh Guffey, Pharmacy Department, Atrium Health, Charlotte, NC, USA UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC, USA.
Nigel Rozario, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, NC, USA.
Lisa Davidson, Division of Infectious Diseases, Atrium Health, Charlotte, NC, USA.
Lewis McCurdy, Division of Infectious Diseases, Atrium Health, Charlotte, NC, USA.
References
- 1. Jenkins T, Sabel A, Sarcone E, et al. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis 2010; 51: 895–903. [DOI] [PubMed] [Google Scholar]
- 2. Moran G, Abrahamian F, Lovecchio F, et al. Acute bacterial skin infections: developments since the 2005 IDSA guidelines. J Emerg Med 2013; 44: e397–e412. [DOI] [PubMed] [Google Scholar]
- 3. Stevens D, Bisno A, Chambers H, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59: e10–e52. [DOI] [PubMed] [Google Scholar]
- 4. Barlam T, Cosgrove S, Abbo L, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paul M, Andreassen S, Tacconelli E, et al. Improving empirical antibiotic treatment using TREAT, a computerized decision support system: cluster randomized trial. J Antimicrob Chemother 2006; 58: 1238–1245. [DOI] [PubMed] [Google Scholar]
- 6. Yong M, Buising K, Cheng A, et al. Improved susceptibility of gram-negative bacteria in an intensive care unit following implementation of a computerized antibiotic decision support system. J Antimicrob Chemother 2010; 65: 1062–1069. [DOI] [PubMed] [Google Scholar]
- 7. Evans R, Pestotnik S, Classen D, et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998; 338: 232–238. [DOI] [PubMed] [Google Scholar]
- 8. Jenkins T, Knepper B, Sabel A, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med 2011; 171: 1072–1079. [DOI] [PubMed] [Google Scholar]
- 9. Housman E, Livings S, Knee A, et al. Improving management of hospitalized adults with uncomplicated cellulitis or cutaneous abscess. Open Forum Infect Dis 2017; 4: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasquale T, Trienski T, Olexia D. Impact of an antimicrobial stewardship program on patients with acute bacterial skin and skin structure infections. Am J Health Syst Pharm 2014; 71: 1136–1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplemental_appendices for Effect of a multifaceted stewardship intervention on antibiotic prescribing and outcomes for acute bacterial skin and skin structure infections by Danya Roshdy, Rupal Jaffa, Kelly E. Pillinger, Josh Guffey, Nigel Rozario, Lisa Davidson and Lewis McCurdy in Therapeutic Advances in Infectious Disease