Abstract
Simple Summary
As important prolific candidate genes, BMPR1B, BMP15, and GDF9 may affect the lambing performance of sheep. Therefore, regarding the three FecB genotypes of Small Tail Han (STH) sheep (FecB BB, FecB B+, and FecB ++), this study explored the gene expression characteristics of different tissues using reverse transcription PCR (RT-PCR) and real-time quantitative PCR (qPCR). The results showed that BMPR1B, BMP15, and GDF9 expression differed between the selected tissues, with all being highly expressed in the ovaries. Further analysis indicated that there was no significant difference in BMPR1B expression among the three FecB genotypes, but both GDF9 and BMP15 had the highest expression in FecB B+. As for other non-ovarian tissues, expression also varied. This study is relevant to understanding the high prolificacy of the STH breed.
Abstract
The expression characteristics of the prolific candidate genes, BMPR1B, BMP15, and GDF9, in the major visceral organs and hypothalamic–pituitary–gonadal (HPG) axis tissues of three FecB genotypes (FecB BB, FecB B+, and FecB ++) were explored in STH ewes using RT-PCR and qPCR. The results were as follows, BMPR1B was expressed in all FecB BB genotype (Han BB) tissues, and GDF9 was expressed in all selected tissues, but BMP15 was specifically expressed in the ovaries. Further study of ovarian expression indicated that there was no difference in BMPR1B expression between genotypes, but the FecB B+ genotype (Han B+) had greater expression of GDF9 and BMP15 than Han BB and FecB ++ genotype (Han ++) (p < 0.05, p < 0.01). BMP15 expression was lower in the ovaries of Han BB than in Han ++ sheep, but the reverse was shown for GDF9. The gene expression in non-ovarian tissues was also different between genotypes. Therefore, we consider that the three genes have an important function in ovine follicular development and maturation. This is the first systematic analysis of the tissue expression pattern of BMPR1B, BMP15, and GDF9 genes in STH sheep of the three FecB genotypes. These results contribute to the understanding of the molecular regulatory mechanism for ovine reproduction.
Keywords: candidate gene, FecB genotype, tissue expression, sheep
1. Introduction
Understanding of the genetics of female fertility is of particular importance for economic production of meat sheep. Earlier work [1] found that between 74% and 96% of the economic value of genetic progress under selection was attributable to increases in litter size, which is ultimately influenced by ovulation rate. However, the vast majority of sheep breeds around the world are non-prolific breeds. Effectively increasing the litter size of sheep has been an urgent scientific challenge for a long time. Since the beginning of the 1980s, researchers have searched for the major genes related to the prolificacy of sheep [2]. Until now, three kinds of major candidate genes have been studied, including those with known mutations, those with known genetic modes, as well as those that need to be further validated [3]. Among them, the bone morphogenetic protein receptor 1B (BMPR1B), bone morphogenetic protein 15 (BMP15), and growth differentiation factor 9 (GDF9) are genes with known target mutations that have been investigated in relation to litter size [4,5,6,7,8,9]. BMPR1B, also known as activin receptor-like kinase 6 (ALK6), is an important transmembrane receptor protein that is involved in the signal transduction of bone morphogenetic protein 2 (BMP2), bone morphogenetic protein 4 (BMP4), bone morphogenetic protein 6 (BMP6)/Vg-related protein 1 (Vgr1), bone morphogenetic protein 7 (BMP7)/osteogenic protein 1 (OP1), growth differentiation factor 5 (GDF5), and bone morphogenetic protein 15 (BMP15) [10]. BMPR1B has a great influence on cumulus cell expansion, ovulation cycle, and skeletal system development. Early studies found that there is a mutation locus (A746G), named FecB by the International Committee on Sheep and Goat Genetics, in the coding region of the BMPR1B, which results in the amino acid change from glutamine (Glu) to arginine (Arg) at position 249 (Q249R) [11,12]. According to previously published literature, the FecB mutation has an additive effect on enhancement of the ovine ovulation number and litter size, such that one copy of the FecB mutation may increase the ovulation number by 1.5 and the litter size by 1, and two copies by 3 and 1.5, respectively [11,13].
BMP15 and GDF9, which both belong to the transforming growth factor β (TGFβ) superfamily, are oocyte-derived growth factors and can affect the ovulation rate of sheep in a dose-sensitive manner [14,15]. Recently, in animal models with autosomal mutations and knockout genes, BMP15 has been reported as a critical gene that has great influence on the ovulation rate and litter size of mammals [14,16]. Many studies have revealed that BMP15 and GDF9 are mainly expressed in oocytes. BMP15 binds to specific receptors of the granulosa/sheath cell membrane surrounding oocytes [10,17], while GDF9 regulates follicular growth and differentiation, facilitating granulosa cell (GC) proliferation and maintaining the stability of the follicular microenvironment [18]. Recently, researchers found that the type I receptors located on granulosa cells (GC), such as BMPR1B, interact with BMP15 and GDF9, which results in the phosphorylation of downstream Smad signaling molecules, which, in turn, activates Smad-dependent and -independent signaling pathways, and regulates the transcription of downstream target genes.
Research has also found that the mutated BMPR1B (FecB) gene is widely distributed in Asian sheep breeds, including Booroola Merino sheep [11], Indian Garole sheep [19], Indonesian Javanese Thin Tail sheep [19], Kendrapada sheep [20], Chinese Hu sheep [21], and STH sheep [22]. The STH sheep, an important source of both fibre and meat in China, is an endemic polytocous breed with year-round estrus and precocious puberty. In addition, the STH sheep also has comparatively excellent characters, such as crude feed tolerance, rapid growth, and good meat quality, etc. Therefore, STH sheep provide an ideal model breed to explore the molecular genetic mechanisms related to hyperprolificacy in certain breeds [23]. Recently published research showed that the STH sheep population can be divided into three FecB genotypes, by applying the TaqMan probe method. Frequencies of genotype BB, genotype B+, and genotype ++ were identified as 0.154–0.667, 0.273–0.692, and 0–0.333, respectively [24]. To date, many studies on the expression of these three genes in the tissues of different animals have been reported [16,24,25,26,27]. However, there have been few studies on the tissue expression profiles or quantitative analyses in FecB carrier and noncarrier STH sheep. In particular, expression during the follicular phase is of interest. Herein, we report the BMPR1B, BMP15, and GDF9 expression levels of the three genotypes based on FecB in the major visceral organs, the HPG axis, and other tissues, including the oviduct and uterus in STH ewes, thus elucidating the genetic mechanism controlling high fecundity in certain breeds of sheep.
2. Material and Methods
2.1. Selection and Treatment of Experimental Sheep
Multiparous healthy ewes (approximately 2.5 years old and average weight of 60 kg) were selected from the nucleus herd of STH sheep in Shandong, China and fostered at the Sheep & Goat Breeding Farm of Tianjin Institute of Animal Sciences (Tianjin, China). All ewes were provided with high quality hay and clean water available ad libitum. The experimental ewes were divided into the three FecB genotypes using the TaqMan probe technique based on the FecB mutation including a homozygous mutant (FecB BB genotype, named Han BB), heterozygote mutant (FecB B+ genotype, named Han B+), and wild-type (FecB ++ genotype, named Han ++). All ewes were subjected to estrus synchronization administration of progesterone (CIDR device, InterAg Co., Ltd., Hamilton, New Zealand) for 12 days. Then, three healthy STH sheep from each of the three FecB genotypes were chosen for further analyses.
All experimental procedures mentioned in the present study were approved by the Science Research Department (in charge of animal welfare issue) of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (IAS-CAAS) (Beijing, China). Ethical approval was given by the animal ethics committee of IAS-CAAS (No. IASCAAS-AE-03, 12 December 2016).
2.2. Sample Collection
The tissue samples were collected 45–48 h after CIDR removal, based on preliminary test observations. During this period, the developing follicles were at their largest, but have not yet ovulated. Fourteen tissue samples from the heart, liver, spleen, lung, kidney, brain, cerebellum, hypothalamus, pituitary, ovary, oviduct, uterus, adrenal gland, and duodenum were obtained from sheep of the three FecB genotypes. The tissue samples were collected within 30 min of euthanasia. Fresh samples were frozen in liquid nitrogen in 2 mL RNase-Free tubes (Thermo Fisher Scientific, Waltham, MA, USA) immediately and stored at −80 °C in the laboratory.
2.3. Total RNA Extraction and cDNA Synthesis
Tissue RNA was extracted from the 14 tissues using a total RNA extraction kit for animal tissue (Tiangen, Beijing, China) and Trizol (Invitrogen Inc., Carlsbad, CA, USA) was used to dissolve the tissues. The quantity and quality of total RNA were monitored by 1.5% agarose gel electrophoresis and ultraviolet spectrophotometry (UV-1201, Shimadzu, Kyoto, Japan), respectively. Then, the RNAs were stored at −80 °C until use.
The first strand of cDNA was prepared following the instructions of the PrimeScriptTM RT Reagent Kit (TaKaRa Bio Inc., Dalian, China). The reaction program was as follows. 37 °C for 15 min, followed by 85 °C for 5 s, with a total volume of 20 μL which contained 1 μg of total RNA, 1 μL of PrimeScript RT Enzyme Mix I, 1 μL of Oligo dT Primer, 1 μL of random 6-mers, 4 μL of 5× PrimeScript Buffer (for Real Time), and 12 μL of RNase-free water. Prior to storage at −80 °C, the cDNA quality was evaluated by housekeeping gene (β-actin) amplification, and then the reverse products were stored at −20 °C until use.
2.4. Primer Design
Primers were designed with Premier 3.0 (version 4.1.0) online software (http://primer3.ut.ee/). Primer sequences of BMPR1B, BMP15, GDF9, and β-actin were selected from GenBank (http://www.ncbi.nlm.nih.gov/) for RT-PCR and qPCR analyses. Previously published literature also provided a reference for the primer sequence of BMP15 for PCR [28]. All primers were synthesized by the Beijing Tianyi Biotechnology Co., Ltd. (Beijing, China). The housekeeping gene (β-actin) was used as an internal control to normalize the threshold cycle (Ct) values. Primers are detailed in Table 1.
Table 1.
Primers of studied genes.
| Method | Gene Name | Primer Sequence (5′→3′) | Length (bp) | Tm (°C) | Accession No. |
|---|---|---|---|---|---|
| qPCR primer | BMPR1B | F: 5′-TGACGGACCTATACACCACA-3′ R: 5′-GTACCGAGGTCTGGCTTCTT-3′ |
121 | 60 | NM_001142888.2 |
| BMP15 | F: 5′-TGTTGGGCAAAAGCTCTGGA-3′ R: 5′-GCCATGCCACCAGAACTCAA-3′ |
106 | 60 | NM_001114767.1 | |
| GDF9 | F: 5′-AACAGACGCCACCTCTACAA-3′ R: 5′-CACGATCCAGGTTAAACAGCA-3′ |
124 | 60 | NM_001009431.1 | |
| β-actin | F: 5′-ACCCAGCACGATGAAGATCA-3′ R: 5′-GTAACGCAGCTAACAGTCCG-3 |
97 | 60 | NM_001009784.1 | |
| RT-PCR primer | BMPR1B | F: 5′-GGGTTCTACGACTCCGCTTC-3′ R: 5′-GGTTACTTTCAGGCCCATCAT-3 |
237 | 60 | NM_001142888.2 |
| BMP15 | F: 5′-GGGTTCTACGACTCCGCTTC-3′ R: 5′-GGTTACTTTCAGGCCCATCAT-3′ |
273 | 62 | NM_001114767.1 | |
| GDF9 | F: 5′-TAGTCAGCTGAAGTGGGACA-3′ R: 5′-AGCCATCAGGCTCGATGGCC-3′ |
224 | 61 | NM_001009431.1 | |
| β-actin | F: 5′-ACCCAGCACGATGAAGATCA-3′ R: 5′-GTAACGCAGCTAACAGTCCG-3′ |
187 | 61 | NM_001009784.1 |
2.5. Exploration of Gene Amplification Parameters
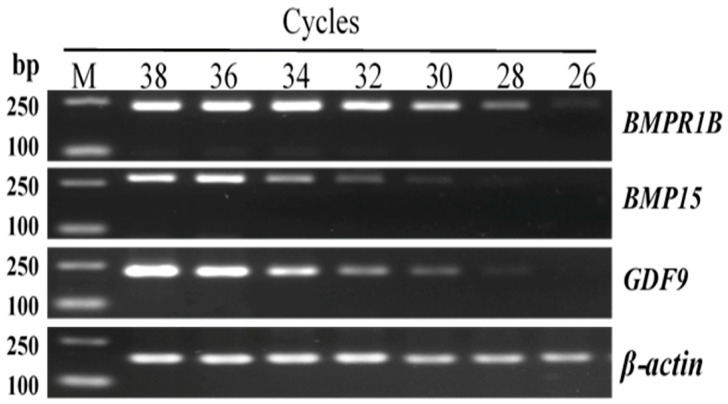
To explore the optimal amplification cycles for BMPR1B, BMP15, and GDF9 in RT-PCR, seven cycle parameters, 26, 28, 30, 32, 34, 36, and 38, were set to amplify the ovarian tissue cDNA by PCR.
2.6. RT-PCR Program and Amplification System
The reverse transcribed cDNA was used for the RT-PCR analysis. The volume of the amplification system of RT-PCR was 20 μL, including 10 μL of 2 × PCR Master Mix (Biomed, Beijing, China), 0.5 μL of 10 mmol/L primer (forward and reverse), 1.0 μL of cDNA, and 8 μL of ddH2O. The amplification program was as follows: initial denaturation at 95 °C for 5 min; followed by the optimal cycles (34, 34, and 36 cycles each for BMPR1B, BMP15, and GDF9) of denaturation at 95 °C for 30 s (see Figure 1); annealing for 30 s; and extension at 72 °C for 60 s, with a final extension at 72 °C for 5 min, and then the PCR product was stored at 4 °C.
Figure 1.
Amplification results of BMPR1B, BMP15, and GDF9 in different reaction cycles. M: DL2000 DNA marker.
2.7. qPCR
2.7.1. System and Program for Real-Time qPCR Analyses
Tissue qPCR was performed in triplicate along with the negative controls (H2O used as template) using the Roche Light Cycler®480 II. The qPCR amplification mixture was 20 μL, containing 10 μL of SYBR® Premix ExTaq II (TaKaRa Bio Inc., Dalian, China), 0.8 μL of primers (forward and reverse), 2 μL of cDNA, and the rest being ddH2O. The qPCR program is as follows, with the initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 5 s and 60 °C for 30 s. The gene expression data were normalized to the β-actin gene. After amplification, the melting curve was analyzed.
2.7.2. Establishment of the Standard Curve
One microliter of cDNA was pipetted from each sample. Then, eight gradient concentrations of cDNA were mixed and diluted: 1-, 2-, 4-, 8-, 16-, 32-, 64-, and 128-fold. BMPR1B, BMP15, GDF9, and β-actin were quantified by qPCR using this diluted cDNA as templates. The standard curves (Supplementary File 1) of the target and housekeeping genes were drawn as the Ct value (0–40) against the cDNA concentration (natural logarithm).
2.8. Data Statistics
The relative gene expression levels were calculated by the 2−ΔΔCt method [29,30]. Statistical analyses were carried out using SPSS 22.0 software (IBM Armonk, NY, USA). The levels of gene expression were analyzed for significant differences with one-way analysis of variance (ANOVA), followed by the Fisher’s least significant difference (LSD) test as a multiple comparison test [31]. All experimental data are shown as mean ± SEM. Statistical significance was taken as p < 0.05.
3. Results
3.1. RNA Extraction and cDNA Synthesis
Total RNA samples were analyzed using 1.5% agarose gel electrophoresis (U = 150 V; I = 240 mA). Three bands (representing 28S, 18S, and 5S) were detected—the 28S band was brighter than the 18S band, and the 5S band was unclear. The OD260 nm/OD280 nm ratios (1.8–2.0) of the samples RNA were all 1.9 to 2.0, which showed that the extracted total RNA was of sufficient purity with no contamination or degradation. Therefore, these tissue RNAs could be used in the follow-up experiment.
3.2. Exploration of the Amplification Parameters
In principle, to explore the optimal amplification parameters, it is important that the luminance of the gene amplification band remains unchanged with the increase in reaction cycles. As seen in Figure 1, the selective cycles of BMPR1B, BMP15, and GDF9 were 34, 36, and 36, respectively.
3.3. Tissue-Specific Expression Analysis of BMPR1B, BMP15, and GDF9
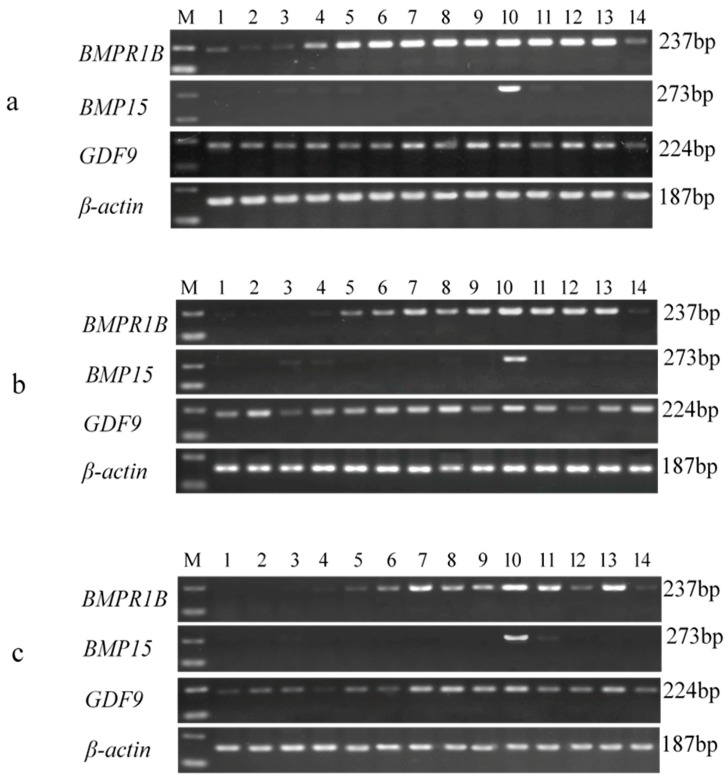
The results of the RT-PCR amplification showed that the fragment lengths of the amplification products of BMPR1B, BMP15, GDF9, and β-actin were consistent with their theoretical lengths, identified by agarose gel electrophoresis (1.5%). As seen in Figure 2, the β-actin gene, as the reference gene, was successfully expressed in all 14 tissues of STH sheep of the three FecB genotypes. BMPR1B was expressed in all selected tissues of the Han BB sheep, and was highly expressed in seven tissues (brain, cerebellum, hypothalamus, pituitary, ovary, uterus, and adrenal gland) of the Han B+ and Han ++ sheep. BMP15 was highly expressed in the ovaries of sheep of all three FecB genotypes. Additionally, GDF9 was expressed in all 14 tissues of all sheep.
Figure 2.
Tissue-specific expression analysis of BMPR1B, BMP15, GDF9, and β-actin genes in three genotypes of STH sheep. Han BB (a), Han B+ (b), and Han ++(c). 1–14: heart, liver, spleen, lung, kidney, brain, cerebellum, hypothalamus, pituitary, ovary, uterus, oviduct, adrenal gland, and duodenum, respectively. M: DL2000 DNA marker.
3.4. Expression Levels of BMPR1B, BMP15, and GDF9 in the HPG Axis
It is very difficult to quantitatively analyze gene expression using RT-PCR. Therefore, the expression levels of BMPR1B, BMP15, and GDF9 in the HPG axis (hypothalamus, pituitary, and ovary), uterus, and oviduct tissues of the STH sheep were measured by qPCR.
As shown in Figure 3, BMPR1B was expressed in five tissues with the three FecB genotypes, with the highest level being in the ovaries (p < 0.01), followed by the hypothalamus, with no significant difference between the two. However, the expression level of BMPR1B in the hypothalamus was significantly higher than those of the pituitary, oviduct, and uterus (p < 0.01), and expression levels in the pituitary and oviduct were significantly higher than those of the uterus (p < 0.01, p < 0.05). In Han B+ and Han ++ sheep, the expression level of BMPR1B was significantly higher in the oviduct than in the pituitary (p < 0.01). There was no significant difference in the BMPR1B ovarian expression levels between genotypes, but its expression in the hypothalamus of Han BB and Han B+ was higher than that in Han ++ sheep (p < 0.01, p < 0.05). BMPR1B expression levels in the pituitary and uterus were significantly higher in Han BB than in Han B+ and Han ++ sheep (p < 0.01, p < 0.05). However, BMPR1B expression in the oviduct was significantly higher in Han B+ than in Han BB and Han ++ sheep (p < 0.05).
Figure 3.
Comparison of expression of BMPR1B among three FecB genotypes (A1) and among tissues (A2). Means with different superscripts are significantly different. The significant results with a p-value lower than 0.01, 0.05 are giving two asterisks (**) and one asterisk (*), respectively. Nonsignificant results are not giving any asterisks.
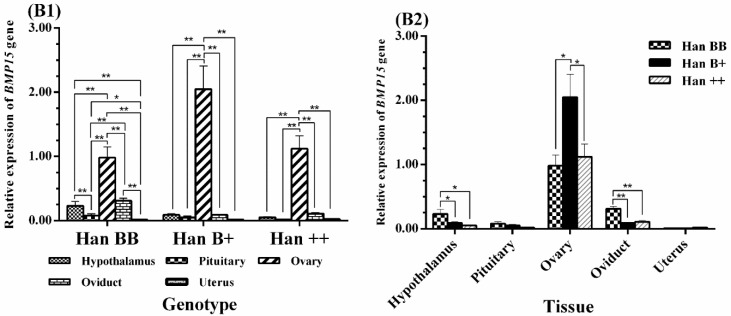
The results of the analysis are shown in Figure 4, where among the five tissues examined, BMP15 was highly significantly expressed in the ovaries (p < 0.01) and presented in trace levels in non-ovarian tissues. Additionally, the expression levels of BMP15 in the hypothalamus and oviduct of Han BB sheep were all much higher than those of the pituitary and uterus (p < 0.01), and expression in the pituitary was higher than in the uterus (p < 0.05). The tissue expression profiles of the three genotypes of sheep were analyzed, and the expression level of BMP15 in the ovaries of Han B+ sheep was significantly higher than in Han BB and Han ++ sheep (p < 0.05), and its expression in the ovaries of Han BB was lower than in Han ++ sheep; however, there was no significant difference between the two. Furthermore, the expression levels of BMP15 in the hypothalamus and oviduct tissues were much higher in Han BB than in Han B+ and Han ++ sheep (p < 0.05, p < 0.01).
Figure 4.
Comparison of expression of BMP15 among three FecB genotypes (B1) and among tissues (B2). Means with different superscripts are significantly different. The significant results with a p-value lower than 0.01, 0.05 are giving two asterisks (**) and one asterisk (*), respectively. Nonsignificant results are not giving any asterisks.
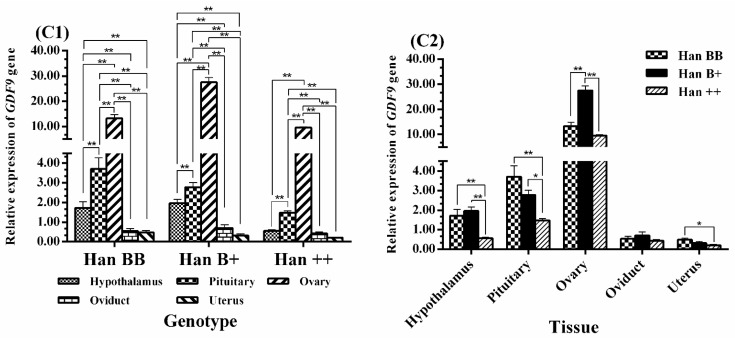
Figure 5 clearly shows that the expression levels of GDF9 in the ovaries of sheep of the three FecB genotypes showed a similar pattern to BMP15, with its expression level being significantly higher in the pituitary than in the hypothalamus, oviduct, and uterus (p < 0.01). In addition, the expression level of GDF9 was significantly higher in the hypothalamus of Han BB and Han B+ sheep than in other tissues including the oviduct and uterus (p < 0.01). In the comparison between genotypes, the expression level of GDF9 in the ovaries was much higher in Han B+ than in Han BB and Han ++ sheep (p < 0.01 or p < 0.05), and its expression in the ovaries was higher in Han BB than in Han ++ shepp, but there was no significant difference between the two (p >0.05). In addition, GDF9 expression in the hypothalamus and pituitary was significantly higher in Han BB and Han B+ than in Han ++ sheep (p < 0.01, p< 0.05), and its expression in the uterus was much higher in Han BB than in Han ++ sheep (p < 0.05).
Figure 5.
Comparison of expression of GDF9 among three FecB genotypes (C1) and among tissues (C2). Means with different superscripts are significantly different. The significant results with a p-value lower than 0.01, 0.05 are giving two asterisks (**) and one asterisk (*), respectively. Nonsignificant results are not giving any asterisks.
4. Discussion
4.1. BMPR1B Expression
Previous reports have found that BMPR1B plays the same role as the protein kinase activity of serine or threonine, which has an important influence on ovarian biological function [32]. BMPR1B expression has previously been detected in the reproductive tissues, brain, skeletal muscle, and kidney tissues of sheep [11]. In this study, BMPR1B was shown to be highly and widely expressed in tissues, including the ovary and hypothalamus, in the three FecB genotypes of STH sheep. In Chinese Merino sheep, during the estrus period BMPR1B was shown to be expressed in the ovary, ear, spinal cord, pituitary, bone, uterus, hypothalamus, kidney, skeletal muscle, and oviduct tissues, which indicates that its function is diverse and that it plays a critical role in the ovaries in addition to bone formation [33]. However, the expression levels of BMPR1B in the ovarian tissues of the three FecB genotypes in Chinese Merino sheep ewes showed no significant difference [33]. Subsequently, BMPR1B expression in the follicles of multiparous Hu sheep was found to be significantly higher than that of uniparous Hu sheep, which indicated that it may influence the ovulation rate by regulating the BMP/Smad signal pathway and some related cytokines [34]. In addition, some researchers have found that BMPR1B has no tissue-specific expression in whole tissues, and it was also shown to have a high expression level in the ovaries of the Jining Grey goat and Liaoning Cashmere goat, implying that it not only exerts a great influence on the process of bone formation, but also has a potential role in ovarian development and even in the process of reproduction. However, no significant difference in expression was shown between these two goat breeds, which suggested that the BMPR1B may not contribute to the high fecundity of goats [35]. Similarly, it has also been speculated that differences in reproductive performance may be ascribed to the structural differences in BMPR1B between Lezhi black and Tibetan goats [36].
On the basis of the similarity of expression levels of ovarian BMPR1B in the three FecB genotypes, we propose that BMPR1B plays an important biological role in the lambing performance of STH sheep through mutation rather than expression level. Early studies found that granulosa cells (GC) may be inhibited by BMPR1B, but the inhibition effect of the mutated BMPR1B was weaker for GC. Sheep carrying the FecB mutation exhibit higher ovulation numbers [11], which may be the cause of the different litter sizes of sheep in the three FecB genotypes. Moreover, the expression differences in BMPR1B in non-ovarian tissues of the HPG axis of sheep of the three FecB genotypes were not consistent with the results from goat breeds [35]. We infer that the expression profile described above may have a species discrepancy or the different timings of the sampling may have influenced the results. Regarding BMPR1B expression in non-ovarian tissues, especially in the oviduct, previous research has suggested that its expression in the ampullary region of the bovine oviduct is significantly higher than in the isthmus area and may affect the normal physiological function of oviduct epithelial cells [37]. Therefore, we needed to explore whether the difference in expression of BMPR1B in non-ovarian tissues influences the ovulation rate and litter sizes of STH sheep of the three FecB genotypes. According to the analysis of function annotation and tissue-specific expression, especially regarding the high level of expression in the ovaries, the biological function of BMPR1B appears to be diverse. It can be speculated that BMPR1B exerts a pivotal influence on ovarian function, follicular development, and maturity.
4.2. BMP15 Expression
BMP15, which is secreted by oocytes, is an indispensable signaling molecule for normal follicular development [38] and can play a critical regulatory role in the growth and differentiation of early oocytes [39,40]. Studies have found that it can activate the mRNA transcription of PFKP (phosphofructokinase, platelet type) and lactate dehydrogenase A (LDHA), which are required for granulosa cell glycolysis [41]. Likewise, it can promote the expression of kit ligand mRNA in granulosa cells [42] and antagonize the apoptosis of cumulus cells [43]. Expression of BMP15 was first detected in mouse oocytes [44]. Subsequently, other researchers have found it to have high specific expression in human oocytes [17], as well as in rats [14], pigs [25], and sheep [45]. In this study, BMP15 was shown to be highly expressed in the ovaries, with trace expression in five other tissues (spleen, lung, kidney, hypothalamus, and oviduct), which is consistent with the results in Hu sheep [46]. Previous research indicated that BMP15 can downregulate the follicle stimulating hormone (FSH) effect, promote cell proliferation, and ensure the growth and maturity of oocytes by inhibiting the expression of the FSH receptor [35]. Therefore, BMP15, based on its high expression in the ovary, may be an important factor in maintaining numerous follicular developments.
In this study, BMP15 expression was lower in the ovaries of Han BB sheep than in Han B+ and Han ++ sheep. From previous studies, it is well-known that the expression level of BMP15 in the ovaries of FecB mutant homozygous (FecB BB genotype) Booroola Romney sheep is significantly lower than that in the wide-type (FecB ++ genotype), which suggests that the higher ovulation rate of BB genotype sheep is due to a decrease in the BMP15 mRNA level and an earlier onset of luteinizing hormone (LH)-responsiveness in granulosa cells (GC) [45]. The receptor concentration of synthetic estrogen, inhibin, and follicle stimulating hormone (FSH) in follicular granulosa cells decreases due to whole or partial deletion of BMP15 function, which further leads to lower levels of FSH that maintain the follicular growth. Therefore, it can be inferred that ewes with the FecB mutant have a high ovulation rate, which may be responsible for the low expression of BMP15 in the ovaries of Han BB sheep. However, some studies have also concluded that BMP15 is a key gene in the high fecundity of goats based on results where BMP15 expression in the hircine ovary was shown to be significantly higher in a polytocous breed than in a monotocous breed [35,47]. These studies suggest that BMP15 has a substantial regulatory effect on the ovulation rates of sheep and goat [48,49]. The high level expression of BMP15 in the ovaries of Han B+ sheep, implies that BMP15 may in addition to its demonstrated function in the ovary of FecB B+ genotype STH sheep, have other influences on reproduction. For BMP15, in regard to the different expression levels in non-ovarian tissues, we hypothesize that unclear effects related to production or interrelationships with other genes could lead to this expression profile. Generally speaking, the high expression of BMP15 in the ovaries indicates that it plays a key role in the growth and maturation of oocytes. However, its expression level may be negatively correlated with the litter sizes of STH sheep.
4.3. GDF9 Expression
GDF9 is secreted by oocytes and performs pivotal regulation in follicular growth and differentiation by means of the paracrine pathway [50]. A previous study reported that GDF9 promotes the expression of hyaluronan synthase 2 and urokinase plasminogen activator (UPA) in the cumulus oocyte complex (COC) and activates the cumulus expansion [51]. GDF9 and its protein have been expressed in many mammal oocytes via in situ hybridization and immunohistochemical technologies [52,53,54]. GDF9 was also detected in GC [18] and non-ovarian tissues, including the hypothalamus pituitary and testis [26]. Particularly in Hu sheep, it is expressed in 10 tissues (hypothalamic, pituitary, ovary, oviduct, uterus, heart, liver, spleen, lung, and kidney tissues) [55], which implies that GDF9 has a wide range of biological effects. In our study, GDF9 was expressed in all tissues, of which the ovaries showed the highest expression, which indicates that it plays an important part not only in reproductive organs, but also in other organs and tissues.
Additionally, previous studies revealed that GDF9 expression in the ovaries is significantly higher in prolific Hu sheep than in non-prolific Hu sheep, indicating that it is a differentially expressed gene and could also affect the BMP/Smad metabolic pathway to regulate the litter sizes of sheep [34]. Previous research has revealed that GDF9 can exert a synergistic effect with FSH, BMP15, and other hormones or growth factors during follicular development [18]. In this study, the GDF9 expression levels in the ovaries were higher in STH sheep with Han BB and Han B+ genotypes than in those with Han ++ genotype, which indicates that GDF9 expression may affect the lambing performance of different FecB genotypes of STH sheep. However, this is an inconsistent conclusion as the GDF9 expression level in the ovaries was not significantly different between FecB carrier and noncarrier Indian sheep [56]. It was also found that the expression levels of GDF9 in the HPG axis tissues of the Jining Grey goat (multiparous breed) and Liaoning Cashmere goat (uniparious breed) are not significantly different [35]. These different findings may be the result of differences between species or breeds and the timeliness of sampling. As a consequence, GDF9 may implement a similar function in these tissues to promote oocyte maturity and further affect the ovulation rate in STH sheep. Therefore, we concluded that GDF9 function is complex and plays a positive regulatory role in the differentiation and development of antral follicles, eventually possibly increasing the ovulation rate and litter size of STH sheep with the FecB mutation.
5. Conclusions
This study found that BMPR1B, BMP15, and GDF9 are highly expressed in the ovaries of three FecB genotypes of STH sheep, which indicates that they may play pivotal functions in the ovine ovaries and promote follicular growth and maturation. The BMPR1B expression level may not differ significantly in the ovaries of STH ewes of the three genotypes, while BMP15 and GDF9 genes have an important biological function in the ovary and influence the reproduction performance of FecB B+ genotype STH sheep. This is the first systematic analysis of tissue expression patterns of the three genes tissue expression pattern in FecB genotypes of STH sheep.
Acknowledgments
We thank the help for English language editing by MDPI (www.mdpi.com/authors/english).
Supplementary Materials
The following are available online at http://www.mdpi.com/2076-2615/8/10/166/s1. Supplementary File 1: Standard Curve of target gene.
Author Contributions
This study was conceived and designed by J.T., W.H., and M.C.; J.T., W.H., X.Z. and J.Z. conducted the experiments and analyses including all the figures and tables and drafted the manuscript. R.D., Q.L., X.W., and M.C. contributed to revisions of the manuscript. M.C. assisted in explaining the results and revised the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31472078 and 31772580), Genetically Modified Organisms Breeding Major Program of China (2016ZX08009-003-006 and 2016ZX08010-005-003), Earmarked Fund for China Agriculture Research System (CARS-38), Central Public-interest Scientific Institution Basal Research Fund (Y2017JC24, 2017ywf-zd-13), Agricultural Science, and Technology Innovation Program of China (ASTIP-IAS13, CAAS-XTCX2016010-01-03, CAAS-XTCX2016010-03-03, CAAS-XTCX2016011-02-02), China Agricultural Scientific Research Outstanding Talents and Their Innovative Teams Program, China High-level Talents Special Support Plan Scientific and Technological Innovation Leading Talents Program (W02020274), and the Tianjin Agricultural Science and Technology Achievements Transformation and Popularization Program (201704020). And the APC was funded by National Natural Science Foundation of China (No. 31472078).
Conflicts of Interest
All authors declare no conflicts of interest.
References
- 1.Gabina D. Improvement of the reproductive performance of Rasa Aragonesa flocks in frequent lambing systems. II. Repeatability and heritability of sexual precocity, fertility and litter size: Selection strategies. Livest. Prod. Sci. 1989;22:87–98. doi: 10.1016/0301-6226(89)90126-7. [DOI] [Google Scholar]
- 2.Davis G.H., Montgomery G.W., Allison A.J., Kelly R.W., Bray A.R. Segregation of a major gene influencing fecundity in progeny of Booroola sheep. N. Z. J. Agric. Res. 1982;25:525–529. doi: 10.1080/00288233.1982.10425216. [DOI] [Google Scholar]
- 3.Wang J.Q., Cao W.G. Progress in exploring genes for high fertility in ewes. Hereditas. 2011;33:953–961. doi: 10.3724/SP.J.1005.2011.00953. [DOI] [PubMed] [Google Scholar]
- 4.Chu M.X., Sang L.H., Wang J.Y., Fang L., Ye S.C. Study on BMP15 and GDF9 as candidate genes for prolificacy of Small Tail Han sheep. Acta Genet. Sin. 2005;32:38–45. [PubMed] [Google Scholar]
- 5.Bodin L., Di P.E., Fabre S., Bontoux M., Monget P., Persani L., Mulsant P. A novel mutation in the bone morphogenetic protein 15 gene causing defective protein secretion is associated with both increased ovulation rate and sterility in Lacaune sheep. Endocrinology. 2007;148:393–400. doi: 10.1210/en.2006-0764. [DOI] [PubMed] [Google Scholar]
- 6.Polley S., De S., Brahma B., Mukherjee A., Vinesh P.V., Batabyal S., Arora J.S., Pan S., Samanta A.K., Datta T.K. Polymorphism of BMPR1B, BMP15 and GDF9 fecundity genes in prolific Garole sheep. Trop. Anim. Health Prod. 2010;42:985–993. doi: 10.1007/s11250-009-9518-1. [DOI] [PubMed] [Google Scholar]
- 7.Souza C.J., McNeilly A.S., Benavides M.V., Melo E.O., Moraes J.C. Mutation in the protease cleavage site of GDF9 increases ovulation rate and litter size in heterozygous ewes and causes infertility in homozygous ewes. Anim. Genet. 2014;45:732–739. doi: 10.1111/age.12190. [DOI] [PubMed] [Google Scholar]
- 8.EI Fiky Z.A., Hassan G.M., Nassar M.I. Genetic polymorphism of growth differentiation factor 9 (GDF9) gene related to fecundity in two Egyptian sheep breeds. J. Assist. Reprod. Genet. 2017;34:1683–1690. doi: 10.1007/s10815-017-1007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paz E., Quinones J., Bravo S., Montaldo H.H., Sepulveda N. Genotyping of BMPR1B, BMP15 and GDF9 genes in chilean sheep breeds and association with prolificacy. Anim. Genet. 2015;46:98–99. doi: 10.1111/age.12254. [DOI] [PubMed] [Google Scholar]
- 10.Shimasaki S., Moore R.K., Otsuka F., Erickson G.F. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 11.Wilson T., Wu X.Y., Juengel J.L., Ross I.K., Lumsden J.M., Lord E.A., Dodds K.G., Walling G.A., Mcewan J.C., O’Connell A.R. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein ib receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol. Reprod. 2001;64:1225–1235. doi: 10.1095/biolreprod64.4.1225. [DOI] [PubMed] [Google Scholar]
- 12.Souza C.J., Macdougall C., Macdougall C., Campbell B.K., McNeilly A.S., Baird D.T. The Booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1b (bmpr1b) gene. J. Endocr. 2001;169:1–6. doi: 10.1677/joe.0.169R001. [DOI] [PubMed] [Google Scholar]
- 13.Mulsant P., Lecerf F., Fabre S., Schibler L., Monget P., Lanneluc I., Pisselet C., Riquet J., Monniaux D., Callebaut I., et al. Mutation in bone morphogenetic protein receptor-ib is associated with increased ovulation rate in booroola merino ewes. Proc. Natl. Acad. Sci. USA. 2001;98:5104–5109. doi: 10.1073/pnas.091577598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galloway S.M., McNatty K.P., Cambridge L.M., Laitinen M.P.E., Juengel J.L., Jokiranta T.S., Mclaren R.J., Luiro K., Dodds K.G., Montgomery G.W. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat. Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 15.Hanrahan J.P., Gregan S.M., Mulsant P., Mullen M., Davis G.H., Powell R., Galloway S.M. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol. Reprod. 2004;70:900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- 16.Pramod R.K., Sharma S.K., Singhi A., Pan S., Mitra A. Differential ovarian morphometry and follicular expression of BMP15, GDF9 and BMPR1B influence the prolificacy in goat. Reprod. Domest. Anim. 2013;48:803–809. doi: 10.1111/rda.12165. [DOI] [PubMed] [Google Scholar]
- 17.Otsuka F., Yao Z., Lee T., Yamamoto S., Erickson G.F., Shimasaki S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J. Biol. Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- 18.Vitt U.A., Hayashi M., Klein C., Hsueh A.J.W. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Bio. Reprod. 2000;62:370–377. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- 19.Davis G.H., Galloway S.M., Ross I.K., Gregan S.M., Ward J., Nimbkar B.V., Ghalsasi P.M., Nimbkar C., Gray G.D., Subandriyo, et al. DNA tests in prolific sheep from eight countries provide new evidence on origin of the Booroola (FecB) mutation1. Biol. Reprod. 2002;66:1869–1874. doi: 10.1095/biolreprod66.6.1869. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S., Mishra A.K., Kolte A.P., Dash S.K., Karim S.A. Screening for Booroola (FecB) and Galway (FecXG) mutations in Indian sheep. Small Rumin. Res. 2008;80:57–61. doi: 10.1016/j.smallrumres.2008.09.007. [DOI] [Google Scholar]
- 21.Guan F., Liu S.R., Shi G.Q., Jun-Tao A.I., Mao D.G., Yang L.G. Polymorphism of gene in nine sheep breeds or strains and its effects on litter size, lamb growth and development. Acta Genet. Sin. 2006;33:117–124. doi: 10.1016/S0379-4172(06)60030-9. [DOI] [PubMed] [Google Scholar]
- 22.Chu M.X., Liu Z.H., Jiao C.L., He Y.Q., Fang L., Ye S.C., Chen G.H., Wang J.Y. Mutations in BMPR-IB and BMPp-15 genes are associated with litter size in Small Tailed Han sheep (Ovis aries) J. Anim. Sci. 2007;85:598–603. doi: 10.2527/jas.2006-324. [DOI] [PubMed] [Google Scholar]
- 23.Miao X.Y., Luo Q.M., Zhao H.J., Qin X.Y. Co-expression analysis and identification of fecundity-related long non-coding rnas in sheep ovaries. Sci. Rep. 2016;6:39398–39407. doi: 10.1038/srep39398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q.Y., Hu W.P., He X.Y., Pan Z.Y., Guo X.F., Feng T., Cao G.L., Huang D.W., He J.N., Di R. Establishment of high-throughput molecular detection methods for ovine high fecundity major gene FecB and their application. Acta Veterinaria et Zootechnica Sinica. 2017;48:39–51. [Google Scholar]
- 25.Li H.K., Kuo T.Y., Yang H.S., Chen L.R., Li S.L., Huang H.W. Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos. Anim. Reprod. Sci. 2008;103:312–322. doi: 10.1016/j.anireprosci.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Kathirvel M., Soundian E., Kumanan V. Differential expression dynamics of growth differentiation factor 9 (GDF9) and bone morphogenetic factor15 (BMP15) mRNA transcripts during in vitro maturation of buffalo (Bubalus bubalis) cumulus–oocyte complexes. Springerplus. 2013;2:206. doi: 10.1186/2193-1801-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain T., Haldkar M., Sarkhel B.C. Expression profile of BMPR1B gene in goat reproductive tract. Indian J. Anim. Res. 2014;48:329–335. doi: 10.5958/0976-0555.2014.00451.8. [DOI] [Google Scholar]
- 28.Mandon-Pepin B., Oustry-Vaiman A., Vigier B., Piumi F., Cribiu E., Cotinot C. Expression profiles and chromosomal localization of genes controlling meiosis and follicular development in the sheep ovary. Biol. Reprod. 2003;68:985–995. doi: 10.1095/biolreprod.102.008557. [DOI] [PubMed] [Google Scholar]
- 29.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative c(t) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 31.Meier U. A note on the power of fisher’s least significant difference procedure. Pharm. Stat. 2006;5:253–263. doi: 10.1002/pst.210. [DOI] [PubMed] [Google Scholar]
- 32.Foroughinia G., Fazileh A., Eghbalsaied S. Expression of genes involved in BMP and estrogen signaling and AMPK production can be important factors affecting total number of antral follicles in ewes. Theriogenology. 2017;91:36–43. doi: 10.1016/j.theriogenology.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Yang H., Liu S.R., ZHong F.G., Yang Y.L., Zhang Y.S. Different expression of BMPR-IB in tissues of sheep. Chin. J. Anim. Sci. 2009;45:6–8. [Google Scholar]
- 34.Xu Y.F., Li E.L., Han Y.D., Chen L., Xie Z.A. Differential expression of mRNAs encoding BMP/SMAD pathway molecules in antral follicles of high- and low-fecundity Hu sheep. Anim. Reprod. Sci. 2010;120:47–55. doi: 10.1016/j.anireprosci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Pan Z.Y., Di R., Tang Q.Q., Jin H.H., Chu M.X., Huang D.W., He J.N., Liu Q.Y., Hu W.P., Wang X.Y. Tissue-specific mRNA expression profiles of GDF9, BMP15, and BMPR1B genes in prolific and non-prolific goat breeds. Czech J. Anim. Sci. 2015;60:452–458. doi: 10.17221/8525-CJAS. [DOI] [Google Scholar]
- 36.Yang C.X., Zi X.D., Wang Y., Yang D.Q., Ma L., Lu J.Y., Niu H.R., Xiao X.L. Cloning and mRNA expression levels of GDF9, BMP15, and BMPR1B genes in prolific and non-prolific goat breeds. Mol. Reprod. Dev. 2012;79:2. doi: 10.1002/mrd.21386. [DOI] [PubMed] [Google Scholar]
- 37.Valdecantos P., Bravo M.R., Garcia E., Garcia D., Roldan-Olarte M., Miceli D. Expression of bone morphogenetic protein receptors in bovine oviductal epithelial cells: Evidence of autocrine BMP signaling. Anim. Reprod. Sci. 2017;185:89–96. doi: 10.1016/j.anireprosci.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Galloway S.M., Gregan S.M., Wilson T., Mcnatty K.P., Juengel J.L., Ritvos O., Davis G.H. BMP15 mutations and ovarian function. Mol. Cell. Endocr. 2002;191:15–18. doi: 10.1016/S0303-7207(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 39.Hosoe M., Kaneyama K., Ushizawa K., Hayashi K.G., Takahashi T. Quantitative analysis of bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDF9) gene expression in calf and adult bovine ovaries. Reprod. Biol. Endocr. 2011;9:33. doi: 10.1186/1477-7827-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S., Pandey S., Parmar M.S., Somal A., Paul A., Bsk P., Bhat I.A., Baiju I., Bharti M.K., Saikumar G. Impact of oocyte-secreted factors on its developmental competence in buffalo. Zygote. 2017;25:313–320. doi: 10.1017/S0967199417000156. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura K., Su Y.Q., Diaz F.J., Pangas S.A., Sharma S., Wigglesworth K., O’Brien M.J., Matzuk M.M., Shimasaki S., Eppig J.J. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 42.Otsuka F., Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: Its role in regulating granulosa cell mitosis. Proc. Natl. Acad. Sci. USA. 2002;99:8060–8065. doi: 10.1073/pnas.122066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussein T.S., Froiland D.A., Amato F., Thompson J.G., Gilchrist R.B. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J. Cell Sci. 2005;118:5257–5268. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- 44.Dube J.L., Wang P., Elvin J., Lyons K.M., Celeste A.J., Matzuk M.M. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol. Endocr. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 45.Crawford J.L., Heath D.A., Reader K.L., Quirke L.D., Hudson N.L., Juengel J.L., McNatty K.P. Oocytes in sheep homozygous for a mutation in bone morphogenetic protein receptor 1B express lower mRNA levels of bone morphogenetic protein 15 but not growth differentiation factor 9. Reproduction. 2011;142:53–61. doi: 10.1530/REP-10-0485. [DOI] [PubMed] [Google Scholar]
- 46.Liu S.J., Pan X.Y., LI F.D., Wang W.M., Li T.F. Characteristics and expression of BMP15 gene and its association with litter size in sheep. J. Gansu Agric. Univ. 2015;50:40–48. [Google Scholar]
- 47.Cui H.X., Zhao S.M., Cheng M.L., Guo L., Ye R.Q., Liu W.Q., Gao S.Z. Cloning and expression levels of genes relating to the ovulation rate of the Yunling black goat. Biol. Reprod. 2009;80:219–226. doi: 10.1095/biolreprod.108.069021. [DOI] [PubMed] [Google Scholar]
- 48.Almusawi S.L., Walton K.L., Heath D., Simpson C.M., Harrison C.A. Species differences in the expression and activity of bone morphogenetic protein 15. Endocrinology. 2013;154:888–899. doi: 10.1210/en.2012-2015. [DOI] [PubMed] [Google Scholar]
- 49.Zamani P., Nadri S., Saffaripour R., Ahmadi A., Dashti F., Abdoli R. A new mutation in exon 2 of the bone morphogenetic protein 15 gene is associated with increase in prolificacy of Mehraban and Lori sheep. Trop. Anim. Health Prod. 2015;47:855–860. doi: 10.1007/s11250-015-0799-2. [DOI] [PubMed] [Google Scholar]
- 50.Kaivo-Oja N., Bondestam J., Kamarainen M., Koskimies J., Vitt U., Cranfield M., Vuojolainen K., Kallio J.P., Olkkonen V.M., Hayashi M. Growth differentiation factor-9 induces Smad2 activation and inhibin B production in cultured human granulosa-luteal cells. J. Clin. Endocr. Metab. 2003;88:755–762. doi: 10.1210/jc.2002-021317. [DOI] [PubMed] [Google Scholar]
- 51.Elvin J.A., Clark A.T., Wang P., Wolfman N.M., Matzuk M.M. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocr. 1999;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 52.Hreinsson J.G., Scott J.E., Rasmussen C., Swahn M.L., Hsueh A.J.W., Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J. Clin. Endocr. Metab. 2002;87:316–321. doi: 10.1210/jcem.87.1.8185. [DOI] [PubMed] [Google Scholar]
- 53.Hsueh A.J., Mcgee E.A., Hayashi M., Hsu S.Y. Hormonal regulation of early follicle development in the rat ovary. Mol. Cell. Endocr. 2000;163:95–100. doi: 10.1016/S0303-7207(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 54.Paradis F., Novak S., Murdoch G.K., Dyck M.K., Dixon W.T., Foxcroft G.R. Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction. 2009;138:115–129. doi: 10.1530/REP-08-0538. [DOI] [PubMed] [Google Scholar]
- 55.Hu D.L., Li Q.F., Xu Y.F., Li E.L., Han Y.D., Tu F., Xie Z. The tissue expression profile, mRNA expression level and SNPs analysis on GDF9 gene in Hu sheep. J. Agric. Biotechnol. 2010;18:533–538. [Google Scholar]
- 56.Goyal S., Aggarwal J., Dubey P.K., Mishra B.P., Ghalsasi P., Nimbkar C., Joshi B.K., Kataria R.S. Expression analysis of genes associated with prolificacy in fecB carrier and noncarrier Indian sheep. Anim. Biotechnol. 2017;28:220–227. doi: 10.1080/10495398.2016.1262869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.