Abstract
Background:
There has been a limited literature with regards to comparison between the pre-operative hormonal/Her-2 neu assessment by immunostaining on fine-needle aspiration (FNA) versus core needle biopsies (CNBs) and their correlation with grading of breast carcinoma.
Materials and Methods:
Two hundred fifty FNAs and 201 CNBs from 252 patients with breast carcinoma were subjected immunocytochemical/histochemical (ICC/IHC) staining along with the grading by the Robinson cytologic and modified Scarff-Bloom-Richardson scoring systems, respectively. Depending on the material adequacy, IHC was also performed on cell blocks. Sensitivity, specificity, and predictive values of ICC were calculated. The kappa statistics was performed to see the power of the study. Cytologic versus histologic gradings were compared and analysed by percentage analysis.
Results:
Sensitivity of ICC on FNAs for ER, PR, and Her-2neu was 49%, 28.8%, and 46%, respectively, while specificity was 84.5%, 90.6%, and 86.6%, respectively, with a fair agreement on kappa statistics. Her-2neu positivity on CNB versus FNA had a moderate agreement. Her-2neu staining of 3+ was seen in most of the Grade-2 tumours on FNA.
Conclusions:
Fairly reliable results on grading and hormonal/Her-2neu status are possible when ICC is performed on qualitatively superior FNA material. This is particularly useful in the management of patients in certain settings like inoperable cases.
Keywords: ER, grading, HER-2neu, immunocytochemistry, immunohistochemistry, PR
INTRODUCTION
The role of fine-needle aspiration cytology (FNAC) in various palpable or deep-seated lesions is well established. In patients with breast carcinoma, it is particularly useful as a part of the triple test in confirming the diagnosis of malignancy. It is also helpful in assessing the lymph node status (staging) and in diagnosing the metastatic disease.[1] In the recent years, there also has been emphasis on its role in assessing the predictive markers of breast cancer. Estrogen receptor (ER), progesterone receptor (PR), and Her2-neu expression status, and histologic grading serve as potential prognostic indicators in breast carcinoma.[2] Although, a core needle biopsy (CNB) is considered the ‘gold standard’ for assessing the receptor status by immunohistochemistry (IHC); quite often it remains non-representative. Also, performing IHC on CNB is more time consuming than immunocytochemistry (ICC) on fine-needle aspirates (FNAs).[3] Thus, CNB remains an alternative rather than a substitute for FNAC.[1] Moreover, FNA is minimally invasive in comparison to CNB. In addition, FNAC grading of breast carcinoma has shown to be an useful parameter in assessing the aggressiveness of breast cancer and deciding neoadjuvant chemotherapy.[4,5]
There has been a paucity of literature with regards to a comparison between immunostaining on FNA versus CNB samples in assessing the hormonal/Her2-neu receptor status.[5,6,7,8,9,10] The present study has made an effort to compare the efficacy of both these techniques in conjunction with cytologic and histologic grading using FNAs, cell blocks (CBs), and CNBs.
MATERIALS AND METHODS
The study was conducted in the Department of Pathology for a period of 3 years with the prior approval from the institute's Scientific Advisory and Ethics Committee. The study material comprised of FNAs from 252 patients with the primary breast lesions that were interpreted on FNAC as ‘carcinoma breast’, ‘suspicious of malignancy’, or ‘atypical changes’. After obtaining an informed consent from the patients, FNAC procedure was done (as part of the routine diagnostic work up) using the standard protocol. Five-to-seven smears were made in each case, one of which was fixed in 95% ethanol for Papanicolaou (Pap) staining, while two were air dried for May-Grünwald-Giemsa (MGG) staining; the remaining air-dried smears were wrapped in an aluminum foil and stored at 4°C for performing ICC to assess the ER, PR, and Her-2neu status. Whenever possible, the residual material in the needle hub was rinsed in 10% neutral-buffered formalin for CB preparation; the standard plasma thrombin method was adopted for making CBs and sections were cut at 5 μ. A sufficient material was available for CBs in 175/252 cases. We kept the fact in mind that invasive and in-situ ductal carcinomas are not always distinguishable on FNAC alone.
ICC procedure
The air-dried smears stored at 4°C were treated with ammonium chloride for 2 min for lysing red blood cells and then were fixed in methanol for 2 min, followed by formalin vapour fixation for 10 min to block the endogenous peroxidase. They were then kept in running tap water for 5 min, followed by distilled water for 5 min; subsequently, heated in citrate buffer (pH = 6) for epitope retrieval in a microwave for ER, and pressure cooker for PR and Her-2. Primary antibody was added and incubated for 30 min. Biotinylated linked antibodies were added using the labelled streptavidin-biotin kit. The chromogen used was diaminobenzidine (DAB) and the monoclonal antibodies used were ER (Biogenex), PR (pre-diluted), and Her-2 (DAKO), (1:200 dilution). Of the 252 cases, ICC was performed for ER in 227, PR in 234, and Her-2neu in 225 cases. Due to availability of sufficient material, Her-2neu immunostaining could be performed on 102 CBs as well. Tissue sections were available for IHC correlation in 205/252 cases. The histologic grading was done using the modified Scarff-Bloom-Richardson scoring system.[11] The ER, PR, and Her-2 status by IHC were compared with FNAC and ICC. Routine (Pap and MGG) FNAC smears were examined and reviewed by two pathologists (Pampa Ch Toi and Siddaraju Neelaiah); the Robinson's cytologic grading[4] was done on Pap-stained smears.
The ICC results of ER and PR were expressed as positive (>10% cells with positive nuclear staining) or negative.[12] ICC with Her-2neu was interpreted as negative, 1+, 2+, or 3+ based on the intensity of membrane staining,[13] and the percentage positivity.[14] The same criteria were applied for Her-2neu assessment on CBs.
Finally, cytologic grading and hormonal/Her-2neu status on cytology specimens (ICC) was compared with histologic grading and hormonal/Her-2neu status on histologic sections (IHC). Histologic grading and IHC findings were considered the ‘gold standard’. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. The kappa statistics was performed to see the power of the study, while a simple percentage analysis for the comparison of cytologic versus histologic grading and ICC versus IHC.
RESULTS
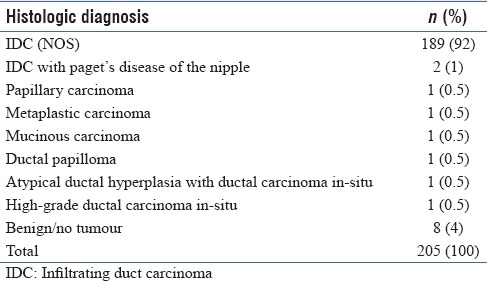
There were 252 FNAs and 205 tissue sections [which included 201 CNBs, 3 modified radical mastectomies (MRMs), and 1 lymphadenectomy specimens]. The disproportionate numbers of FNAs and CNBs is due to variability in the adequacy of material in these specimens. Cytologic and histologic diagnoses are shown in Tables 1 and 2, respectively; 96% cases had 100% cytohistologic concordance.
Table 1.
Cytologic diagnosis on breast FNA
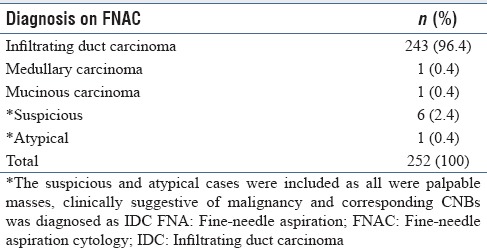
Table 2.
Histologic diagnosis
ICC versus IHC interpretation of ER, PR, and Her-2neu
ICC for ER was done in 227 cases and IHC in 187 CNBs of which 168 cases were comparable with a cytohistologic concordance of 69.6%. Sensitivity, specificity, PPV, and NPV were 49%, 84.5%, 70%, and 69.5%, respectively, with a kappa value of 0.352 (fair agreement) and 95% confidence interval (CI) of 0.212–0.493 [Table 3]. ICC for PR was done on 234 FNAs and 187 CNBs of which 169 cases were comparable with a cytohistologic concordance of 71.5%. Sensitivity, specificity, PPV, and NPV for PR were 28.8%, 90.6%, 57.7%, and 74.1%, respectively; the kappa value was 0.226 (fair agreement) with a 95% CI of 0.074–0.377 [Table 3]. Immunostaining for Her-2neu was done in 225 FNAs (ICC) and 187 CNBs (IHC), which was comparable in 127 cases with a cytohistologic concordance of 70% cases. Cases with 2 + Her-2neu score were considered negative and thus sensitivity, specificity, PPV, and NPV of ICC for Her-2neu were 46.0%, 86.6%, 70.5%, and 69.8%, respectively. There was a fair agreement with a kappa value of 0.347 and 95% CI of 0.185–0.508. Comparison between IHC and ICC for ER, PR, and Her-2neu are shown in Figure 1a–c.
Table 3.
Comparison of IHC with ICC for ER, PR and Her2neu
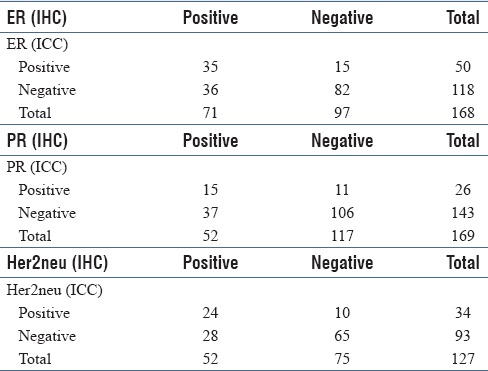
Figure 1.
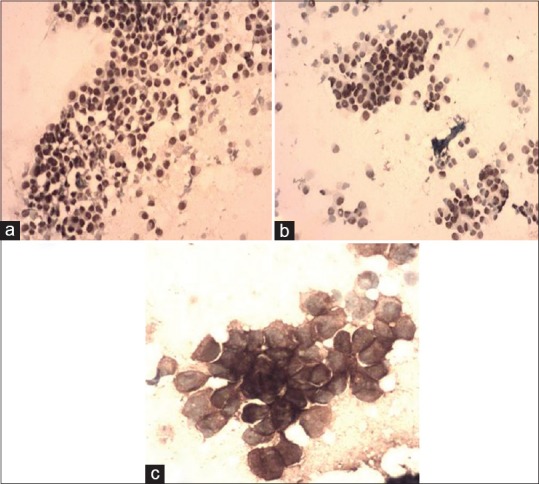
(a-c) (same case), IDC Grade-1 on FNAC, (a) ER positive, (ICC × 100); (b) PR positive, (ICC × 100); and (c) Her-2neu 3+, (ICC × 200)
Immunostaining on CBs versus histologic sections
Comparison of Her-2neu expression between CB (IHC), FNAC (ICC), and tissue section (IHC) are shown in Figure 2a–c. CBs were made in 175 cases of which 114 had adequate cellular elements. Her-2neu was done on 102/114 CBs which was comparable with FNA in 74 cases with a sensitivity, specificity, PPV, and NPV of 58.8%, 90%, 83%, and 72%, respectively. The kappa value was 0.499 (moderate agreement) with a 95% CI of 0.307–0.692 and a concordance of 75.67%. Her-2neu IHC results on tissue sections were available in 53 cases for comparison Her-2neu status on CBs; thus CBs had a sensitivity, specificity, PPV, and NPV of 72%, 74%, 66%, and 79.3% with a kappa value of 0.463 (moderate agreement), and 95% CI of 0.223–0.703. Concordance was 88.67%.
Figure 2.
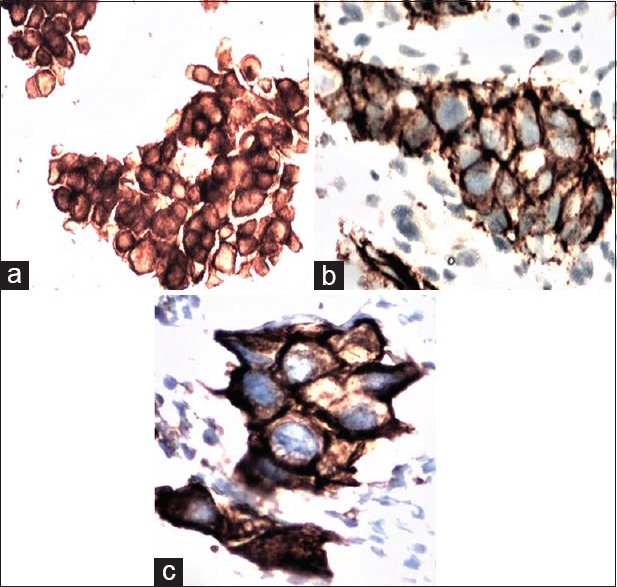
(a-c) (same case), IDC Grade-3 on FNAC, (a) Her-2neu in FNAC 3+, (ICC × 200); (b) Her-2neu 3+ in tissue section, (ICC × 400); and (c) Her-2neu 3+, (ICC × 400) cell block
Cytologic versus histologic grading
Cytologic (FNAC) grading was done in 250 ductal carcinomas of which 52 (20.8%), 149 (59.6%), and 49 (19.6%) were Grade-1, -2, and -3 tumours, respectively. Histologic grading was done in 177 cases of which 36 (20.3%), 115 (65.7%), 24 (13.5%) were Grade-1, -2, and -3 tumours, respectively. One hundred and seventy five cases were available for comparison of cytologic versus histologic gradings of which 98 (56%) cases showed cytohistologic concordance. Majority of the cases were interpreted as Grade-2 on both histopathology (65.7%) and FNAC (59.4%). Notably, 65.2% of Grade-2 ductal carcinomas on FNAC remained as “Grade-2 IDC” on histopathology as well.
Of the FNAC-interpreted Grade-2 tumours, 57.6% were ER positive and 53.7% PR positive. Only “3+ Her2” was taken as positive and 56% of such cases were cytologically Grade-2 IDCs and 18% were Grade-3 IDCs. Comparison of the histologic versus cytologic grading and IHC versus ICC for hormonal/Her-2 status was available in 153, 154, and 148 cases for ER, PR, and Her-2neu, respectively; of this, an absolute (100%) concordance was noted in 55 (35.94%), 54 (35.06%), and 35 (23.64%) cases, respectively. One hundred seventy tissue sections had both grading and Her-2 results, of which 63 (37.05%) had 3+ Her-2 expressions and 44/63 (69.84%) were Grade-2 IDCs.
DISCUSSION
Both FNAC and CNB are well-established procedures in the pre-operative diagnosis of breast cancer. ER positive tumours are known to show a better response to hormonal treatment.[3] Although ER status can readily be assessed on FNAC,[2] it has not been applied routinely for patient management.
ER status
Several authors[3,5,15] have compared the techniques of ICC and IHC in assessing the ER status. With regard to ER status, Zoppi et al.,[3] Jayaram and Elsayed,[5] and Nizzoli et al.[15] have reported a better correlation than in our study. However, Radhika and Prayaga[7] reported a much lower sensitivity (33%), specificity (75%), PPV (67%), NPV (43%), and concordance (50%) for ER than in our cases. While comparing ER expression status by ICC versus IHC, Stalhammar et al.[16] documented discordant results with a kappa value of 0.60. Our study had 36 false negatives (21.4%) which were probably due to low cellularity,[3] poor fixation, or in some cases sampling issues related to tumour heterogeneity.[3,5] Failure to detect a low positive ER expression and improper antigen retrieval are the other possible reasons.[17] False positivity in 15 (9%) of our cases is probably due to non-representative ER positive areas in the biopsy due to sampling error.[3,5]
PR is a surrogate marker for ER activity and is useful as an additional predictive factor for hormonal therapy in breast cancer.[18] Specificity of PR positivity between ICC and IHC in our cases are similar to that reported by Zoppi et al.[3] Although, some studies have reported a better correlation,[12,15] there also have been studies documenting a lower sensitivity (25%), specificity (33%), PPV (33%), NPV (25%), and concordance (29%) than ours.[7] When more than 10% tumour cells were taken as positive, Stalhammar et al.[16] noted a slightly better agreement than ours with a kappa value of 0.75. The factors similar to those noted for ER expression could be the reasons for our false negative (21.8%) and false positive (6.5%) cases as well.
Recent studies using ThinPrep and CBs have shown a high concordance between the two methods for both ER (98%) and PR (96%).[19] Monaco et al.[20] found similar results between ICC (liquid-based cytology) and IHC in their patients with primary and metastatic breast carcinomas. The concordance rate for ER, PR, and Her-2 localization was 81%, 65%, and 71%, respectively. They attributed the variability in staining pattern in their cases to tumour heterogeneity.
According to various cytopathologists, ER/PR status is better assessable on direct smears, while a CNB is preferable for Her-2 expression. However, most centres depend on fluorescent in-situ hybridization (FISH) for detecting Her-2 expression.[6] There has been only a limited literature with regard to ICC for Her-2neu assessment on direct smears. Our study compared Her-2 immunostaining on FNA smears (ICC) versus tissue sections (IHC); FNA smears versus CBs; and CBs versus tissue sections. In a study comparing the Her-2neu status by FISH and ICC/IHC techniques Troncone et al.[9] found FISH technique on FNAs to be superior (48% specific signals) to FISH on paraffin sections (43% specific signals). With regard to Her2-neu immunostaining (ICC vs. IHC), they found IHC (3+ staining in 41% cases) to be superior to ICC (3+ staining in 26.7% cases). As for their FISH results, the fresh cytologic material was found to be superior to paraffin tissue blocks. Pegolo et al.[21] used ThinPrep, with a good concordance for ER, PR, and Her-2neu. The kappa value for Her-2neu had a perfect agreement, which probably was due to the use of the cytopreservative solution for preservation of hormonal receptors.[21] Using ethanol as a fixative, taking utmost care in avoiding non-specific immunoreactive products and performing ICC on an autostainer, Moriki et al.[22] reported a good concordance between ICC and IHC. Corkill and Katz[23] found similar results with ICC on de-stained FNAC smears and IHC on tissue sections. Durgapal et al.[24] compared ICC and FISH on FNAC smears and IHC on histologic sections in 100 cases with a very good concordance. A relatively low concordance rate (70%) recorded in our study can be explained by the factors such as low cellularity, tumour heterogeneity, storage of air-dried FNAC smears, use of methanol as the fixative, manual staining procedure, and the use of different batches of antibodies. We had 10 (7.8%) false positive cases with ICC which may have occurred due to the reasons stated above and they may have been negative with IHC due to the small amount of representative tissue in core biopsies, exhausted block, and tumour heterogeneity. Beatty et al.[25] compared the Her-2 staining on FNAC smears fixed in ethanol, formalin, and ThinPrep preservatives with the formalin-fixed tissue sections. Discordant results in their study made them consider ICC to be unreliable for Her-2neu assessment. Bofin et al.[26] analysed Her-2neu status in 46 breast carcinomas by techniques such as Her-2 gene amplification and protein expression by FISH on FNA material, ICC on FNA smears, and IHC on paraffin-embedded tissue. Although, they noted that ICC was not more sensitive than IHC; the FISH results on smears were comparable to 3+ ICC. Various studies have stated that assessment of Her-2 receptor status is better in formalin-fixed tissue obtained by FNAC in the form of a CB than on direct FNAC smears. A study by Ferguson et al.[27] showed a sensitivity of 95%, 90%, and 88% for ER, PR, and Her-2, respectively, with a specificity of 100% for all three markers; they correlated ICC on alcohol-fixed smears and the corresponding CB or biopsy. Our study showed a moderate agreement between the two methods. Comparing IHC staining for hormone receptors and Her-2 in tissue sections with IHC on CB, Shabaik et al.[28] found CB to be reliable in predicting the markers with a 100% concordance for Her-2 with IHC on tissue sections. Though we did not record 100% concordance, in our study, the CBs were as good as tissue sections with 88.67% concordance. This may be particularly useful in metastatic lesions or inoperable cases where hormonal receptor and Her-2 analyses are required for planning therapy.
Cytologic grading of breast carcinoma has also taken a very important role, as quite often, the therapeutic decision is based on the pre-operative FNAC diagnosis. In our study, the highest degree of concordance (56%) was recorded with Grade-2 tumours. There was an overlap in grading between Grade-2 and Grade-3 tumours with 50% of the histologically Grade-3 tumours being interpreted as “Grade-2” on FNAC. The overall concordance between the cytologic and histologic grading systems in various studies ranged from 56.9% to 77.7%.[5,29,30,31] Frias et al.[4] found a statistically significant association between the two methods. The discrepancy between histologic and cytologic grading could possibly be due to the smaller number of tumour cells available on CNB as compared to FNAC; moreover, the same area may not have been represented in both FNAC and tissue sections. As the grading was done in a totally blinded manner, we believe that the chances of having a bias towards one particular grading system are unlikely.
FNAC of the breast is extensively used for pre-operative diagnosis of breast carcinoma, and in detecting inoperable, metastatic, or recurrent breast carcinomas. Tumour grading, morphologic types of tumour, and hormone receptor status are some of the prognostic indicators that are cytologically assessable. Only a limited number of studies have compared cytologic grade with Her-2 status. In our study, 56% of the positive cases were Grade-2 IDCs. Based on their experience, Bofin et al.[26] stated that Her-2 analysis by ICC was not easy to interpret in smears showing a predominant population of singly lying cells (like in most cases of Grade-3 IDCs) owing to the disrupted/damaged cell membrane, while it was easier to interpret smears from the Grade-1 and Grade-2 IDCs exhibiting an exclusive or a prominent population of cohesive clusters of neoplastic cells due to well-retained cell membrane. We agree with Garbar and Cure[32] who states in their review article that FNAC of palpable breast lesion still plays an important role in breast cancer evaluation if it is performed and evaluated by experienced cytopathologists.
CONCLUSION
Despite certain constraints, an assessment of predictive factors of breast carcinoma, like grading and evaluation of ER, PR, and Her-2neu status is fairly reliable on cytology, when performed on qualitatively superior FNA material from the primary breast lesions.
Financial support and sponsorship
Intramural funding for faculty project from Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kocjan G. Needle aspiration cytology of the breast: Current perspective on the role in diagnosis and management. Acta Med Croatica. 2008;62:391–400. [PubMed] [Google Scholar]
- 2.Denley H, Pinder SE, Elston CW, Lee AH, Ellis IO. Preoperative assessment of prognostic factor in breast cancer. J Clin Pathol. 2001;54:20–4. doi: 10.1136/jcp.54.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoppi JA, Rotunda AV, Sundlad AS. Correlation of immunocytochemical and immunohistochemical determination of estrogen and progesterone receptors in breast cancer. Acta Cytol. 2002;46:337–40. doi: 10.1159/000326731. [DOI] [PubMed] [Google Scholar]
- 4.Frias AR, Campora RG, Parra DM, Frias MJR, Cerezuela TR, Salaverri CO, et al. Robinson cytologic grading of invasive ductal breast carcinoma correlation with histologic grading and regional lymph node metastasis. Acta Cytol. 2005;49:149–53. doi: 10.1159/000326123. [DOI] [PubMed] [Google Scholar]
- 5.Jayaram G, Elsayed ME. Cytologic evaluation of prognostic markers in breast carcinoma. Acta Cytol. 2005;49:605–10. doi: 10.1159/000326247. [DOI] [PubMed] [Google Scholar]
- 6.Kocjan G, Bourgain C, Fassina A. The role of breast FNAC in diagnosis and clinical management: A survey of current practice. Cytopathology. 2008;19:271–8. doi: 10.1111/j.1365-2303.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 7.Radhika K, Prayaga AK. Estrogen and progesterone hormone receptor status in breast carcinoma: Comparison of immunocytochemistry and immunohistochemistry. Indian J Cancer. 2010;47:148–50. doi: 10.4103/0019-509X.63006. [DOI] [PubMed] [Google Scholar]
- 8.Sumiyoshi K, Shibayama Y, Akashi S, Nohara T, Iwamoto M, Kobayashi T, et al. Detection of human epidermal growth factor receptor2 protein and gene in fine needle aspiration cytology specimens and tissue sections from invasive breast cancer: Can cytology specimens take the place of tissue sections? Oncol Rep. 2006;15:803–8. [PubMed] [Google Scholar]
- 9.Troncone G, Panico L, Vetrani A, de Divitiis B, Zeppa P, Fulcinitti F. c-erbB-2 expression in FNAB smears and matched surgical specimens of breast cancer. Diagn Cytopathol. 1996;14:135–9. doi: 10.1002/(SICI)1097-0339(199603)14:2<135::AID-DC6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Shabaik A, Lin G, Peterson M, Hasteh F, Tipps A, Datnow B, et al. Reliability of Her2/neu, estrogen receptor and progesterone receptor testing by immunohistochemistry on cell block of FNA and serous effusions from patients with primary and metastatic breast carcinoma. Diagn Cytopathol. 2011;39:328–32. doi: 10.1002/dc.21389. [DOI] [PubMed] [Google Scholar]
- 11.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 12.Nizolli R, Bozetti C, Naldi N, Guazzi A, Gabrielli M, Michiara M. Comparison of the results of immunocytochemical assays for biologic variables on preoperative fine-needle aspirates and on surgical specimens of primary breast carcinomas. Cancer. 2000;90:61–6. [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond EH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Tazawa C, Miura H, Nakabayashi M, Moriya T, Sasano H. Evaluation of HER2 status in human breast carcinoma. Acta Histochem Cytochem. 2003;36:1–7. [Google Scholar]
- 15.Nizzoli R, Bozzetti C, Savoldi L, Manotti L, Naldi N, Camisa R. Immunocytochemical assay of estrogen and progesterone receptors in fine needle aspirates from breast cancer patients. Acta Cytol. 1994;38:933–8. [PubMed] [Google Scholar]
- 16.Stalhammar G, Rosin G, Fredriksson I, Bergh J, Hartman J. Low concordance of biomarkers in histopathological and cytological material from breast cancer. Histopathology. 2014;64:971–80. doi: 10.1111/his.12344. [DOI] [PubMed] [Google Scholar]
- 17.Kapila K, Anim JT, Francis IM, Al-Mulla F, George SS, Behbehani AI. Expression of estrogen receptor α and estrogen receptor β in fine needle aspirates of breast carcinoma. Acta Cytol. 2010;54:25–30. doi: 10.1159/000324962. [DOI] [PubMed] [Google Scholar]
- 18.Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, Roanh LD, et al. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: A validation study. Mod Pathol. 2004;17:1545–54. doi: 10.1038/modpathol.3800229. [DOI] [PubMed] [Google Scholar]
- 19.Domanski AM, Monsef N, Domanski HA, Grabau D, Ferno M. Comparison of the oestrogen and progesterone receptor status in primary breast carcinomas as evaluated by immunohistochemistry and immunocytochemistry: A consecutive series of 267 patients. Cytopathology. 2013;24:21–5. doi: 10.1111/j.1365-2303.2012.00997.x. [DOI] [PubMed] [Google Scholar]
- 20.Monaco SE, Wu Y, Teot LA, Cai G. Assessment of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status in the fine needle aspirates of metastatic breast carcinomas. Diagn Cytopathol. 2013;41:308–15. doi: 10.1002/dc.21841. [DOI] [PubMed] [Google Scholar]
- 21.Pegolo E, Machin P, Riosa F, Bassini A, Deroma L, Loreto CD. Hormone receptor and human epidermal growth factor receptor 2 status evaluation on Thinprep specimens from breast carcinoma: Correlation with histologic sections determination. Cancer Cytopathol. 2012;120:196–205. doi: 10.1002/cncy.20206. [DOI] [PubMed] [Google Scholar]
- 22.Moriki T, Takahashi T, Ueta S, Mitani M, Ichien M. Hormone receptor status and HER2/neu over expression determined by automated immunostainer on routinely fixed cytologic specimens from breast carcinoma: Correlation with histologic sections determinations and diagnostic pitfalls. Diagn Cytopathol. 2004;30:251–6. doi: 10.1002/dc.20007. [DOI] [PubMed] [Google Scholar]
- 23.Corkill ME, Katz R. Immunocytochemical staining of c-erbB-2 oncogene in fine-needle aspirates of breast carcinoma: A comparison with tissue sections and other breast cancer prognostic factors. Diagn Cytopathol. 1994;11:250–4. doi: 10.1002/dc.2840110311. [DOI] [PubMed] [Google Scholar]
- 24.Durgapal P, Mathur SR, Kalamuddin MD, Gupta SD, Parshad R, Julka PK. Assessment of Her-2/neu status using immunocytochemistry and fluorescence in situ hybridization on fine-needle aspiration cytology smears. Experience from a tertiary care centre in India. Diagn Cytopathol. 2014;42:726–31. doi: 10.1002/dc.23088. [DOI] [PubMed] [Google Scholar]
- 25.Beatty BG, Bryant R, Wang W, Ashikaga T, Gibson PC, Leiman G, et al. HER-2/neu detection in fine-needle aspirates of breast cancer fluorescence in situ hybridization and immunocytochemical analysis. Am J Clin Pathol. 2004;122:246–55. doi: 10.1309/N82C-TQ1J-0XEV-EFQB. [DOI] [PubMed] [Google Scholar]
- 26.Bofin AM, Ytterhus B, Martin C, O'Leary JJ, Hagmar BM. Detection and quantitation of Her-2 gene amplification and protein expression in breast carcinoma. Am J Clin Pathol. 2004;122:110–9. doi: 10.1309/8A2D-JFT0-7NE6-EWHE. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson J, Chamberlain P, Cramer HM, Wu HH. ER, PR, and Her2 immunocytochemistry on cell-transferred cytologic smears of primary and metastatic breast carcinomas: A comparison study with formalin-fixed cell blocks and surgical biopsies. Diagn Cytopathol. 2013;41:575–81. doi: 10.1002/dc.22897. [DOI] [PubMed] [Google Scholar]
- 28.Shabaik A, Lin G, Peterson M, Hasteh F, Tipps A, Datnow B, et al. Reliability of Her2/neu, estrogen receptor, and progesterone receptor testing by immunohistochemistry on cell block of FNA and serous effusions from patients with primary and metastatic breast carcinoma. Diagn Cytopathol. 2011;39:328–32. doi: 10.1002/dc.21389. [DOI] [PubMed] [Google Scholar]
- 29.Sinha SK, Sinha N, Bandyopadhyay R, Mondal SK. Robinson's cytological grading on aspirates of breast carcinoma: Correlation with Bloom Richardson's histological grading. J Cytol. 2009;26:140–3. doi: 10.4103/0970-9371.62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Einstien D, Omprakash P, Ganapathy H, Rahman S. Comparison of 3-tier cytological grading systems for breast carcinoma. ISRN Oncol 2014. 2014:252103. doi: 10.1155/2014/252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson IA, McKee G, Nicholson A, Jackson PA, Cook MG, D'Arcy J, et al. Prognostic value of cytological grading of fine-needle aspirates from breast carcinomas. Lancet. 1994;343:947–9. doi: 10.1016/s0140-6736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 32.Garbar C, Cure H. Fine-needle aspiration cytology can play a role in neoadjuvant chemotherapy in operable breast cancer. ISRN Oncol 2013. 2013:935796. doi: 10.1155/2013/935796. [DOI] [PMC free article] [PubMed] [Google Scholar]


