Abstract
Purpose:
To develop an infectious keratitis model using caprine (goat) corneas and to investigate the expression of virulence factors during infection.
Methods:
Goat eyes were surface-sterilized and dissected, and the corneas were placed on an agarose-gelatin solid support (0.5% in phosphate-buffered saline) in a 12-well culture plate containing 10% fetal bovine serum-supplemented culture medium for 3 weeks. Cell viability tests (trypan blue and MTT) were performed on the cultured corneas. Corneas were infected with Pseudomonas aeruginosa and Fusarium solani separately. Infection progression was observed via histological analysis and hematoxylin and eosin (H-E) staining. For Pseudomonas-infected corneas, expression of eight virulence genes (exoS, exoT, exoY, alpR, prpL, lasA, lasB, and algD) was determined via quantitative real-time PCR (qRT-PCR) at 48-h and 72-h time-points. For Fusarium-infected corneas, expression of five proteases (C7Z0E6, C7ZFW9, C7Z7U2, C7ZNV5, and C7YY94) was quantified via qRT-PCR at 2, 4, and 8 days after infection. Protease from infected corneas was detected via gelatin zymography.
Results:
Goat corneas with a viable epithelium could be maintained for 15 days. Pseudomonas infection progressed rapidly, and complete corneal degradation was observed on day 4 after infection. Fusarium infection progressed more slowly. Histological analysis and H-E staining of Fusarium-infected cornea revealed mycelia penetrating all layers of the cornea. qRT-PCR revealed expression of all eight virulence factors, and statistically significant difference in expression of prpL and alpR in Pseudomonas-infected corneas. Expression of C7ZNV5 was highest in Fusarium-infected corneas.
Conclusion:
Goat corneas can be used to evaluate the expression of virulence factors involved in Pseudomonas and Fusarium infection.
Keywords: Fusarium, Goat Cornea, Keratitis, Pseudomonas
INTRODUCTION
Keratitis is caused by a wide range of microorganisms, including fungi, bacteria, protozoa, and viruses. Bacterial keratitis can be caused by numerous bacterial species, of which the most common are Pseudomonas aeruginosa and Staphylococcus aureus.[1] Fungal keratitis is an important cause of blindness and visual impairment in tropical countries, and the most common causative agents are Fusarium spp. and Aspergillus spp.[2] Studies investigating pathogenesis, virulence, and host responses to bacterial and mycotic keratitis generally rely on in vivo murine models.[3,4] The murine model is one of the most commonly used models for studying bacterial and fungal infection due to the similarity of murine and human immune systems. However, ethical and logistical constraints associated with the use of animals in such experiments slow the progress of understanding in the field. Recently, there has been considerable interest in the use of ex vivo corneal models to study keratitis.[5,6,7] Such models are convenient in that they mimic the physiological conditions of corneas infected with pathogens. The use of canine, human, rabbit, and mouse corneas has been reported for the development of ex vivo model.[5,7] In the present study, an ex vivo corneal infection model was developed for Pseudomonas keratitis and Fusarium keratitis using goat corneas, and the presence of virulence factors (proteases) during infection was investigated using quantitative real-time PCR (qRT-PCR) and zymography at two stages of infection.
METHODS
Materials
Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), and antibiotics for corneal culture were obtained from Himedia Laboratories (Mumbai, India). Luria broth (LB) and potato dextrose agar (PDA) for the routine cultivation of P. aeruginosa and F. solani, respectively, were also purchased from Himedia Laboratories (Mumbai, India). Clinical isolates of P. aeruginosa strain PAO1 and F. solani strain CC61 were available in the laboratory. Other routine reagents were procured from Sigma–Aldrich (India) unless otherwise stated.
Goat Cornea Culture
Goat eyeballs were collected from an abattoir in a sterile beaker, and all subsequent procedures were performed under aseptic conditions. The eyeballs were washed five times with phosphate-buffered saline (PBS), and all extra tissue was removed. The eyeball was then incubated in 2.5% povidone iodine solution for 5 minutes, washed three times with PBS, and then incubated in 0.1% gentamicin for 15 minutes. After three more PBS washes, a scalpel was used to make an incision in the limbal region of the eyeball. Scissors were then used to further expand the incision and cut around the middle of the eye, bisecting the eye. The front half (the cornea) was incubated in culture media and maintained for the required time as detailed below. The dissected cornea was placed on an agarose-gelatin solid support in a culture plate containing 1 ml DMEM with 10% FBS and antibiotics (penicillin, 100 I. U./ml and streptomycin, 75 μg/ml) and gentamycin (35 μg/ml) and placed in a CO2 incubator at 37°C. The solid support was prepared using a sterilized solution of agarose (0.5%) and gelatin (0.5%) in PBS. Agarose-gelatin beads were made using surface-sterilized and stretched parafilm in a tube. The dimensions of the parafilm were 4 × 4 in.2, and surface sterilization was performed using 70% alcohol for 2 hours. The warm agarose-gelatin solution was poured into the excavated parafilm and left to cool. Upon cooling, the agarose-gelatin formed a concave bead with a diameter of approximately 3–4 cm. The cornea was placed on this bead, and the medium was added to the well so that the endothelial layer remained in direct contact with the bead, with the epithelial layer facing the air. The medium was changed after every 24 hours. The corneas were then either observed for the required period of time, used for various viability tests, or used for infection studies, as described below. Two pairs of corneas (n = 4) were used for viability tests, and five corneas (n = 5) were used for infection studies (two controls and three infected corneas). Each set of experiments was repeated three times. However, for standardization of infection load and viability tests, 20–25 pairs (n = 40 to 50) were used in the initial phase of the study (data not shown).
Cornea Viability
The viability of corneal epithelial cells was assessed using the trypan blue assay, and the viability of the entire cornea was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The trypan blue assay was performed after the separation of epithelial cells, and the MTT assay was performed using the whole cornea in the culture plate. Therefore, separate corneas were used for both the assays.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide Assay
Whole corneas were used in the MTT assays. Assays were performed at the required time-points using the EZcount TM MTT Cell Assay Kit (Himedia, Mumbai, India) in accordance with the manufacturer's protocol.
Trypan Blue Assay
The separation of epithelial cells was performed via a trypsin treatment. The cornea was treated with 0.25% trypsin for 12 hours at 37°C. After washing, the cornea was placed in PBS, and with the aid of a scalpel blade, the upper half of the cornea was gently scratched. The scratching was performed to separate some epithelial cells into the PBS. The PBS containing the cells was then moved to a micro-centrifuge tube and centrifuged at 6000 rpm (2857 × g) at 4°C for 20 minutes. The supernatant was discarded and the cells were resuspended in 100 μl of PBS; then, 100 μl of 0.4% trypan blue prepared in PBS was added to the cell suspension and it was incubated for 3 minutes. The cell suspension was loaded into a hemocytometer and examined immediately under a microscope. The number of stained cells (dead cells) and the total number of cells were counted manually and viability was calculated using the following formula:
% of viable cells = (number of viable cells × 100)/total number of cells.
Infection of corneas with Pseudomonas aeruginosa and Fusarium solani.
Corneas maintained as described above were infected with various numbers of colony forming units (CFUs) of either P. aeruginosa or spores of F. solani, in triplicate. P. aeruginosa was grown in LB, and bacteria were harvested by centrifugation and suspended in a known volume of saline. For P. aeruginosa, initially 106 CFU were used, and subsequently, the number was reduced to 100 ± 15 CFU, 242 ± 50 CFU, or 742 ± 50 CFU. A viable count for estimating the number of bacteria in the suspension was performed in triplicate at the time of each inoculation. A viable count was also performed from the infected cornea at each time-point, at the time of harvesting. Regular checks for the presence of pathogens in the medium in which each cornea was placed were performed to ensure that the pathogens were only growing on the cornea and not in the culture media. F. solani was grown on PDA for 10 days, and spores were harvested by flooding the plate with saline, followed by centrifugation. For F. solani, 106 spores were used initially; however, subsequently, all experiments were performed using 932 ± 46 spores. The presence of F. solani on the infected cornea and in the culture medium was assessed at regular time-points by streaking onto PDA plates.
A single gentle scratch was applied to the cornea with the aid of sterile forceps prior to infection with pathogens. Infection was performed by applying bacteria/spores with a sterile nichrome loop. Uninfected corneas (n = 2) were maintained along with the infected corneas in the same 12-well culture plate for each experiment. The progression of infection was monitored visually every day, as well as by keratome sectioning followed by H-E staining. Specimens for histological analysis (infected corneas) were fixed in 2% buffered paraformaldehyde for 15 minutes. Paraffin-embedded samples were sectioned at a thickness of 5 μm. Sections were harvested on silane-coated slides, and deparaffinized slices were processed for H-E staining. Stained sections were mounted in DPX (distyrene, a polystyrene), a plasticizer (tricresyl phosphate), and xylene and imaged using a Leica DM 750 light microscope and LasEZ software (Leica Microsystems, India).
Detection of Virulence Factors Using Quantitative Real-Time-Polymerase Chain Reaction
qRT-PCR was performed to quantify the expression of eight P. aeruginosa virulence genes and five F. solani protease genes. Total RNA was isolated from infected and uninfected corneas using the TRI Reagent® (Sigma, India) in accordance with the manufacturer's protocol. cDNA synthesis from 5 μg RNA was performed using the PrimeScript™ cDNA Synthesis Kit (TaKaRa, Japan) in accordance with the manufacturer's protocol. For qRT-PCR, amplifications were performed using the CFX 96 Real Time PCR product (BioRad Laboratories Inc., USA) in a 20-μl reaction using 2 μl cDNA, 10 μl SYBR Green I Master Mix (TaKaRa, Japan), and gene-specific primers at a concentration of 2.5 μM. The amplification was conducted at 95°C for 10 minutes before the first cycle, then 95°C for 15 seconds and 60°C for 60 seconds, which was repeated 35 times. Table 1 lists the genes investigated in the study, brief descriptions of their functions, and the primer sequences used. The 16S ribosomal RNA gene was used as the housekeeping control gene for Pseudomonas, and the glyceraldehyde 3-phosphate dehydrogenase gene was used as the housekeeping control gene for Fusarium. qRT-PCR calculations were performed using the △ CT method (Cycle Threshold) and data were reported as normalized expression compared to the housekeeping gene. delta CT shows the difference of expression between 2 genes; here difference between housekeeping gene and gene of interest.
Table 1.
The genes investigated in the current study, their known functions, and the primers used for quantitative real-time polymerase chain reaction
Detection of Protease from Infected Corneas
Infected corneas were crushed in PBS, total protein was quantified, and 50 μg was loaded on a gelatin zymography gel. Zymography was performed as previously described,[8] using gelatin as a substrate (0.1%) and 12% polyacrylamide (Sigma chemicals, USA).
Statistical Analysis
Data are expressed as mean ± SD and were compared using ANOVA and Fisher's least significant difference (LSD) to evaluate the significance of differences among various groups. Differences at P ≤ 0.05 were considered significant.
RESULTS
Caprine Cornea Culture
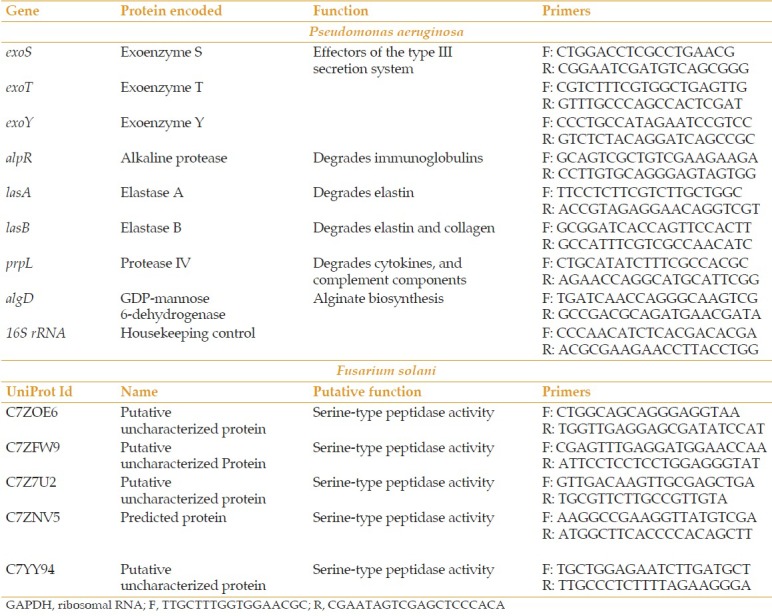
Dissected corneas were placed in 12-well culture plates for 15 days [Figure 1a], and the viability of intact corneas and epithelial cells was measured at various time-points. The viability of the cornea was maintained for up to 15 days [Figure 1b]. The trypan blue assay indicated that the maximum viability of epithelial cells on day 0 was 96%. By day 10 viability had reduced to 94%, and by day 15, it had reduced to 88% [Figure 1c]. Up to day 10 the corneas remained completely intact, but by day 15 they had lost their structural integrity. Therefore, corneas were not cultured beyond day 15.
Figure 1.
Ex vivo caprine cornea model. (a) Ex vivo caprine cornea maintained in dulbecco's modified eagle's medium at days 0, 5, 10, and 15. (b) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay to assess the viability of the entire cornea at days 0, 5, 10, and 15. (c) Epithelial cell viability assessment via the trypan blue assay.
Infection of Corneas with Pseudomonas aeruginosa
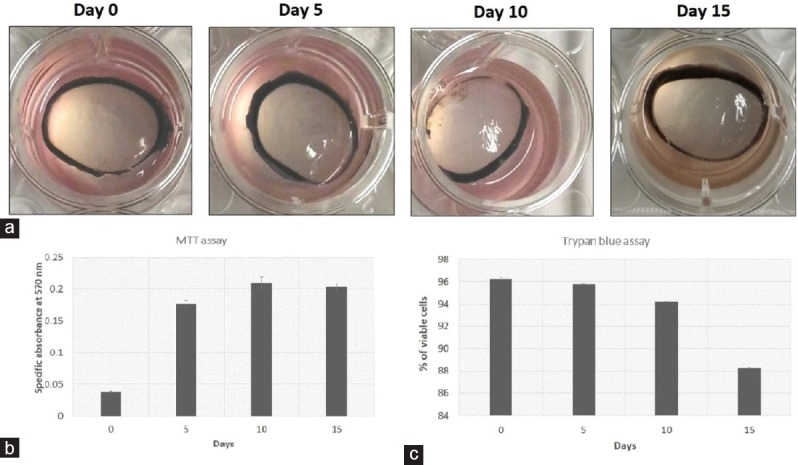
On the basis of the literature, infections were initially performed via the application of 106 CFU. However, under this bacterial load the entire cornea degraded within 48 hours (data not shown). Thus, administration of 100 ± 15 CFU, 242 ± 50 CFU, and 742 ± 50 CFU was investigated. Of these three, infection was only detectable after the administration of 742 CFU. Figure 2a shows the progression of infection at 24, 48, and 72 hr. Histological analysis of the infected corneas revealed reduced epithelium as infection progressed [Figure 2b]. By day 4, histological processing of corneas was no longer possible because of complete degradation.
Figure 2.
Cornea infection with Pseudomonas aeruginosa. (a) Infected caprine cornea showing haze compared to an uninfected cornea. (b) Histology of an infected cornea showing the presence of bacteria in the stroma. (c) Normalized expression of the virulence genes exoS, exoT, exoY, alpR, prpL, lasA, lasB, and algD quantified using quantitative real-time-polymerase chain reaction. (d) 10% gelatin zymography showing the presence of proteases at 48 and 72 h postinfection.
Figure 2 shows the expression of the virulence genes (exoS, exoT, exoY, alpR, prpL, lasA, lasB, algD) at 48 and 72 hr post-infection. At the 5% significance level (P ≤ 0.05, ANOVA, Fisher's LSD) the differences between the expression levels of all genes at 48 and 72 hr were statistically significant (PExoS = 0.34, PExoT < 0.001, PExoY = 0.001, PAlpR = 0.0003, PLasA = 0.04, PLasB = 0.0002, PprpL < 0.001, PAlgD = 0.0002.) The prpL gene exhibited the greatest change. Higher amounts of protease in conjunction with the progression of infection was confirmed via gelatin zymography gel analysis [Figure 2d]. Zymography also indicated the presence of more than one protease.
Infection of Corneas with Fusarium solani
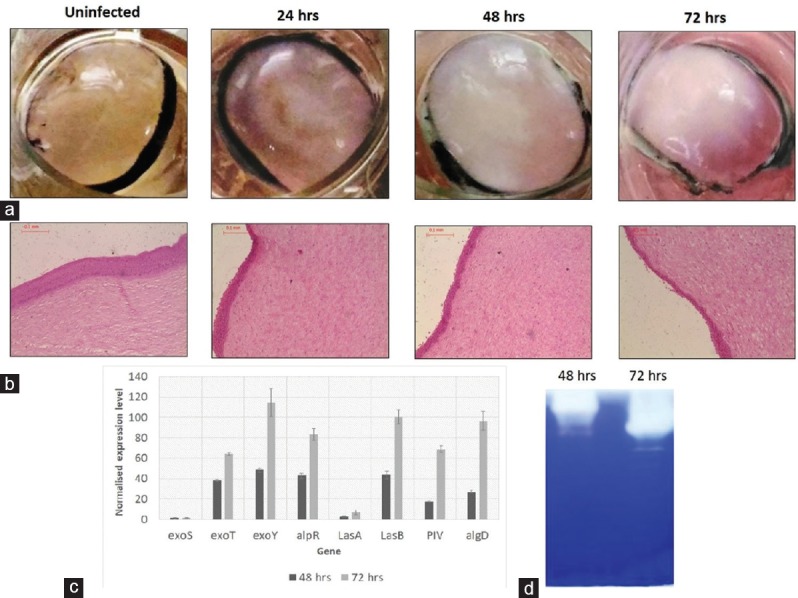
Corneas were infected with 932 ± 46 F. solani spores and observed every day up to day 10 after infection. Changes in morphology and transparency of the cornea with respect to the control are shown in Figure 3a. On every day up to day 10, the opacity of the cornea increased. Histological analysis of the infected corneas on days 2, 4, and 8 [Figure 3b] depicted progression of infection. On day 2, the epithelium was completely disrupted and the fungi could be seen penetrating the upper stroma. On day 4, the fungi were more concentrated in the upper half of the cornea and there was a greater presence of long hyphae compared to day 2. By day 8, half of the stroma was completely disrupted and the lower half also contained fungal hyphae. By day 10, histological processing of the cornea was no longer possible due to complete degradation. Because no well-established virulence factors have been reported in Fusarium keratitis, qRT-PCR was restricted to certain proteases. Preliminary gelatin zymography indicated the presence of high molecular weight protease that was inhibited by phenylmethylsulfonyl fluoride (PMSF; data not shown). Hence, five serine proteases were selected on the basis of their molecular weights and extracellular properties (signal peptide analysis). Figure 3c shows the relative expression of the proteases C7Z0E6, C7ZFW9, C7Z7U2, C7ZNV5, and C7YY94, of which C7Z0E6, C7Z7U2, and C7ZNV5 exhibited the highest levels of expression. At the 0.5% significance level (PC7Z0E6 < 0.001, PC7ZFW9 = 0.418, PC7Z7U2 = 0.0017, PC7ZNV5 = 0.0016, PC7YY94 = 0.48; ANOVA, Fisher's LSD) the difference between the expression of C7Z0E6 at days 4 and 8 was statistically significant, but the difference between days 2 and 4 was not. Expression of C7Z7U2 was upregulated at days 2, 4, and 8, and attained statistical significance. For C7ZNV5, the up regulation was statistically significant between day 2 and day 4 (P = 0.004) and between day 2 and day 8 (P = 0.0018), but not between day 4 and day 8 (P = 0.55). Gelatin zymography of infected corneas indicated the presence of more than one protease, and higher activity was seen on days 4 and 8 [Figure 3d].
Figure 3.
Cornea infection with Fusarium solani. (a) Infected caprine cornea showing haze compared to an uninfected cornea. (b) Histology of an infected cornea showing fungal hyphae in the stroma. (c) Normalized expression of the proteases C7Z0E6, C7ZFW9, C7Z7U2, C7ZNV5, and C7YY94 quantified using qRT-PCR. (d) 10% gelatin zymography showing increased proteases at days 2, 4, and 8 postinfection.
DISCUSSION
In the present study, an ex vivo corneal model of Pseudomonas and Fusarium keratitis using caprine corneas was developed, and the expression of specific virulence genes was evaluated. The first corneal cultures derived from human corneas were reported in 1977, in a study designed to investigate epithelial-endothelial interactions.[9] Subsequently, Richard et al (1991)[10] developed the “air/liquid” corneal organ culture model to study wound healing. They used the term air/liquid because the model mimics the in vivo situation wherein the epithelium is exposed to air whereas the endothelium is in contact with liquid (aqueous humor). In the aforementioned studies, corneas were maintained in vitro for 3 weeks without edema or other structural changes. In the present study, goat corneas remained viable for 2 weeks. The air/liquid human corneal organ culture is a valuable in vitro system for long-term maintenance of epithelial and endothelial integrity. A similar model using canine eyes was developed to investigate keratitis caused by herpes simplex virus.[5] In that model, corneas are placed epithelial-side-up in a fixed position, and culture medium is added to a level that alternately exposes the epithelium to air and liquid environments while rocking on a tilting platform. The model described in the present report is similar to the air/liquid model, with some changes. Goat corneas were used, and they were placed on a solid agarose support that promoted the maintenance of corneal shape. The advantages of using goat corneas are that no ethical approval is required, the cornea is large and comparable to that of humans, and they can be procured easily from abattoirs. Ex vivo models have been used to study keratitis caused by Candida,[11] Fusarium,[12] herpes simplex virus,[5] S. aureus, and P. aeruginosa.[7] The mouse corneal organ culture developed for Candida infection was used to study fungal adhesion mechanisms and drug screening.[11] Fusarium infection was modeled using human corneal buttons stabilized in an artificial anterior chamber (Refractive Technologies, Cleveland, OH).[12] Recently, rabbit corneas were used in an ex vivo model for Pseudomonas and Staphylococcus keratitis.[7] In that study, human and rabbit cornea ex vivo keratitis models were compared. Major differences between the present study and the earlier studies include the number of CFUs used to infect the cultures, and the duration of infection. Most studies have reported instigating in vitro corneal infections with 108 bacteria or spores. The total duration of experimental infection and investigation was 48 hr in most previous reports, whereas the course of infection was monitored for 3 days for bacteria and 10 days for fungi in the present study.
We were able to maintain the infections for longer periods because the initial numbers of bacteria/fungal spores used to instigate the in vitro infections were lower than those used in all previous reports—less than 1000. Additionally, when a higher load was used in the present study the entire cornea degraded within 72 hours (data not shown). Thus, future studies could be aimed at comparing all of the aforementioned ex vivo models (mouse, rabbit, goat, and human).
The expression levels of eight virulence genes, including those coding for proteases (alpR and prpL), elastases (lasA and lasB), T3SS effector proteins (exoS, exoT, and exoY), and biofilm (algD), were quantified at two time-points in P. aeruginosa-infected corneas (48 and 72 hr). All genes were found to be expressed in infected goat corneas as infections progressed (i. e., from 48 to 72 hr), and upregulation of all genes was observed. A number of studies have investigated potential correlations between the clinical manifestations of an infection and virulence factors encoded by pathogenic P. aeruginosa isolates causing keratitis.[13,14,15] However, information on collective expression of these genes during infection is lacking. Significant roles of proteases (alpR and prpL) during infection have been well documented using isogenic mutants in rabbit and mouse infection models.[16,17,18,19] Of these two proteases, protease IV appears to be more important for corneal degradation because most P. aeruginosa strains express protease IV.[20] Additionally, protease IV activity varies among P. aeruginosa strains, and a mutant specifically deficient in this activity produced alkaline protease and exhibited reduced corneal virulence.[20] It is also notable that alkaline protease alone was not capable of inducing corneal erosion when tested in a rabbit keratitis model.[13,21] P. aeruginosa devoid of alkaline protease produced varying disease scores in rabbit and mouse models, suggesting that the effects of alkaline protease are secondary to those of protease IV.[17] Comparisons of both proteases together are lacking, but it is evident that protease IV contributes to pathogenesis more. The data generated in the current study is concordant with this, in that there was a greater increase in protease IV compared to alkaline protease as infection progressed. Importantly, however, the fact that the initial expression of alkaline protease was higher suggests that its role may also be vital. While protease IV and alkaline protease mediate their effects directly, elastases (LasA and LasB) contribute to tissue damage by activating corneal matrix metalloproteinase-2.[22] LasB contributes directly to clinical score, but LasA is not a major corneal virulence factor.[13,23] In the current study, the expression of Las B was higher than that of LasA. With regard to the expression levels of the three effector proteins of the type III secretion system, ExoS, ExoT, and ExoY, that of ExoY was the highest. Their essential role in murine models of corneal infection is reportedly exerted by inducing neutrophil death and thereby inhibiting bacterial killing.[24] Clinical isolates of P. aeruginosa that secrete type III toxins (ExoS, ExoT, ExoU, and ExoY) have been associated with greater morbidity or mortality.[25] Very few studies have documented the co-expression of four effector proteins.[15,26] With regard to the expression of the three main effector molecules of P. aeruginosa, ExoS, ExoT, and ExoU, in clinical isolates causing keratitis, there was a high prevalence of isolates expressing ExoS and ExoT (84%), and less isolates expressed ExoU.[27] There are no reports describing the expression of ExoY in keratitis, and ExoY does not contribute significantly to disease in animal models of infection.[28] The pathogenesis of P. aeruginosa keratitis in ExoS-producing and ExoT-producing strains is almost entirely due to their ADP-ribosyltransferase activities, which weaken the host response by targeting the antibacterial activity of infiltrating neutrophils.[24] In the current study there was higher expression of exoY and exoT than exoS in infected corneas, and this observation warrants further detailed investigation in other clinical isolates. This discrepancy may be due to a lack of infiltrating neutrophils in explant corneas. Taken together, results suggest that proteases, elastases, and exotoxins all have a role in ocular pathology, but further studies are required in order to elucidate the collective effects of these elements, and the individual effects of each in the presence of the others. The current study emphasizes the importance of using convenient ex vivo model systems to study the expression of many virulence genes simultaneously.
In Fusarium-infected corneas, we studied the expression of five proteases. Secretion of hydrolytic enzymes such as proteases, lipases, and phospholipases are considered important virulence properties of pathogenic fungi.[29] Proteases from Fusarium spp. have been reported in species that are pathogenic to plants, but none from clinical isolates have been characterized.[30,31] Proteases from Fusarium causing keratitis have been reported occasionally, and most studies have quantified proteases from these isolates.[32] Studies focused on the characterization and identification of proteases produced by Fusarium spp. causing keratitis are lacking.
The whole genome sequence of F. solani is available. On the basis of molecular weight and inhibitor studies, five genes encoding extracellular serine protease were selected. qRT-PCR showed that one of these proteases (C7ZNV5) was expressed at high levels in infected corneas. Further studies to characterizing this protease are ongoing.
Fusarium keratitis studies using rat models have been conducted;[3,33] however, none have reported the expression of proteases. In a study using rabbits as the model organism, gelatin zymography reportedly identified the expression of protease, but thus far that expression has not been further characterized.[34] Moreover, sequence searches in NCBI and UniProtKB revealed the presence of many other extracellular proteases in the F. solani genome. These findings merit detailed studies focused on the characterization and role of proteases in keratitis. A major limitation of ex vivo models is that active immune responses are not involved and thus defense responses cannot be studied. It is also difficult to correlate ex vivo infection with actual clinical scores. Nevertheless, the expression of both bacterial and fungal virulence factors and antifungal agents can be studied using this model. Future studies are required in order to characterize the collective expression of virulence factors. Comparative genome analyses of five Fusarium spp. genomes have revealed that their genomes are compartmentalized into regions responsible for (1) essential functions, (2) host specificity, and (3) pathogen virulence.[35] The virulence genome is composed of a huge repertoire of genes for secondary metabolites, hydrolytic enzymes, and cell wall components, among other components. Because of their varying contributions to specific diseases and hosts, assigning definitive roles to virulence genes can be a challenging task using animal models. Given this, the utilization of ex vivo models to test large numbers of deletion strains to rule in/rule out certain factors may be highly informative.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15:321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Abou Shousha M, Santos AR, Oechsler RA, Iovieno A, Maestre-Mesa J, Ruggeri M, et al. Anovel rat contact lens model for Fusarium keratitis. Mol Vis. 2013;19:2596–2605. [PMC free article] [PubMed] [Google Scholar]
- 4.Marquart ME. Animal models of bacterial keratitis. J Biomed Biotechnol. 2011;2011:680642. doi: 10.1155/2011/680642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harman RM, Bussche L, Ledbetter EC, Van de Walle GR. Establishment and characterization of an air-liquid canine corneal organ culture model to study acute herpes keratitis. J Virol. 2014;88:13669–13677. doi: 10.1128/JVI.02135-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H, Kochevar IE, Behlau I, Zhao J, Wang F, Wang Y, et al. Antimicrobial blue light therapy for infectious keratitis: Ex vivo and in vivo studies. Invest Ophthalmol Vis Sci. 2017;58:586–593. doi: 10.1167/iovs.16-20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinnock A, Shivshetty N, Roy S, Rimmer S, Douglas I, MacNeil S, et al. Ex vivo rabbit and human corneas as models for bacterial and fungal keratitis. Graefes Arch Clin Exp Ophthalmol. 2017;255:333–342. doi: 10.1007/s00417-016-3546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lantz MS, Ciborowski P. Zymographic techniques for detection and characterization of microbial proteases. Methods Enzymol. 1994;235:563–594. doi: 10.1016/0076-6879(94)35171-6. [DOI] [PubMed] [Google Scholar]
- 9.Yanoff M, Cameron JD. Human cornea organ cultures: Epithelial-endothelial interactions. Invest Ophthalmol Vis Sci. 1977;16:269–273. [PubMed] [Google Scholar]
- 10.Richard NR, Anderson JA, Weiss JL, Binder PS. Air/liquid corneal organ culture: A light microscopic study. Curr Eye Res. 1991;10:739–749. doi: 10.3109/02713689109013868. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Chen H, Qu M, Wang Q, Yang L, Xie L, et al. Development of a novel ex vivo model of corneal fungal adherence. Graefes Arch Clin Exp Ophthalmol. 2011;249:693–700. doi: 10.1007/s00417-010-1601-9. [DOI] [PubMed] [Google Scholar]
- 12.Hua X, Yuan X, Di Pietro A, Wilhelmus KR. The molecular pathogenicity of Fusarium keratitis: A fungal transcriptional regulator promotes hyphal penetration of the cornea. Cornea. 2010;29:1440–1444. doi: 10.1097/ICO.0b013e3181d8383a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thibodeaux BA, Caballero AR, Marquart ME, Tommassen J, O’Callaghan RJ. Corneal virulence of Pseudomonas aeruginosa elastase B and alkaline protease produced by Pseudomonas putida. Curr Eye Res. 2007;32:373–386. doi: 10.1080/02713680701244181. [DOI] [PubMed] [Google Scholar]
- 14.Oka N, Suzuki T, Ishikawa E, Yamaguchi S, Hayashi N, Gotoh N, et al. Relationship of virulence factors and clinical features in keratitis caused by Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 2015;56:6892–6898. doi: 10.1167/iovs.15-17556. [DOI] [PubMed] [Google Scholar]
- 15.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR, et al. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 16.Engel LS, Hill JM, Caballero AR, Green LC, O’Callaghan RJ. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J Biol Chem. 1998;273:16792–16797. doi: 10.1074/jbc.273.27.16792. [DOI] [PubMed] [Google Scholar]
- 17.Hobden JA. Pseudomonas aeruginosa proteases and corneal virulence. DNA Cell Biol. 2002;21:391–396. doi: 10.1089/10445490260099674. [DOI] [PubMed] [Google Scholar]
- 18.Preston MJ, Seed PC, Toder DS, Iglewski BH, Ohman DE, Gustin JK, et al. Contribution of proteases and lasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect Immun. 1997;65:3086–3090. doi: 10.1128/iai.65.8.3086-3090.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel LS, Hobden JA, Moreau JM, Callegan MC, Hill JM, O’Callaghan RJ, et al. Pseudomonas deficient in protease IV has significantly reduced corneal virulence. Invest Ophthalmol Vis Sci. 1997;38:1535–1542. [PubMed] [Google Scholar]
- 20.Caballero A, Thibodeaux B, Marquart M, Traidej M, O’Callaghan R. Pseudomonas keratitis: Protease IV gene conservation, distribution, and production relative to virulence and other Pseudomonas proteases. Invest Ophthalmol Vis Sci. 2004;45:522–530. doi: 10.1167/iovs.03-1050. [DOI] [PubMed] [Google Scholar]
- 21.Pillar CM, Hazlett LD, Hobden JA. Alkaline protease-deficient mutants of Pseudomonas aeruginosa are virulent in the eye. Curr Eye Res. 2000;21:730–739. [PubMed] [Google Scholar]
- 22.Miyajima S, Akaike T, Matsumoto K, Okamoto T, Yoshitake J, Hayashida K, et al. Matrix metalloproteinases induction by pseudomonal virulence factors and inflammatory cytokines in vitro . Microb Pathog. 2001;31:271–281. doi: 10.1006/mpat.2001.0470. [DOI] [PubMed] [Google Scholar]
- 23.Alionte LG, Cannon BM, White CD, Caballero AR, O’Callaghan RJ, Hobden JA, et al. Pseudomonas aeruginosa LasA protease and corneal infections. Curr Eye Res. 2001;22:266–271. doi: 10.1076/ceyr.22.4.266.5509. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Karmakar M, Taylor PR, Rietsch A, Pearlman E. ExoS and ExoT ADP ribosyltransferase activities mediate Pseudomonas aeruginosa keratitis by promoting neutrophil apoptosis and bacterial survival. J Immunol. 2012;188:1884–1895. doi: 10.4049/jimmunol.1102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 26.Fleiszig SM, Zaidi TS, Preston MJ, Grout M, Evans DJ, Pier GB, et al. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karthikeyan RS, Priya JL, Leal SM, Jr, Toska J, Rietsch A, Prajna V. Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers. PLoS One. 2013;8:e64867. doi: 10.1371/journal.pone.0064867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat Rev Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurokawa CS, Sugizaki MF, Peraçoli MT. Virulence factors in fungi of systemic mycoses. Rev Inst Med Trop Sao Paulo. 1998;40:125–135. doi: 10.1590/s0036-46651998000300001. [DOI] [PubMed] [Google Scholar]
- 30.Olivieri F, Ma EZ. Characterization of an extracellular serine protease of Fusarium eumartii and its action on pathogenesis related proteins. Eur J Plant Pathol. 2002;108:63–72. [Google Scholar]
- 31.Barata RA, Andrade MH, Rodrigues RD, Castro IM. Purification and characterization of an extracellular trypsin-like protease of Fusarium oxysporum var.lini. J Biosci Bioeng. 2002;94:304–308. doi: 10.1263/jbb.94.304. [DOI] [PubMed] [Google Scholar]
- 32.Gharamah AA, Moharram AM, Ismail MA, Al-Hussaini AK. Bacterial and fungal keratitis in upper Egypt:In vitro screening of enzymes, toxins and antifungal activity. Indian J Ophthalmol. 2014;62:196–203. doi: 10.4103/0301-4738.116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu JL, Gao XR, Cui HP, Lang LL, Li Q, Liao X, et al. Experimental model of Fusarium solani keratitis in rats. Int J Ophthalmol. 2011;4:371–376. doi: 10.3980/j.issn.2222-3959.2011.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gopinathan U, Ramakrishna T, Willcox M, Rao CM, Balasubramanian D, Kulkarni A, et al. Enzymatic, clinical and histologic evaluation of corneal tissues in experimental fungal keratitis in rabbits. Exp Eye Res. 2001;72:433–442. doi: 10.1006/exer.2000.0971. [DOI] [PubMed] [Google Scholar]
- 35.Ma LJ, Geiser DM, Proctor RH, Rooney AP, O’Donnell K, Trail F, et al. Fusarium pathogenomics. Annu Rev Microbiol. 2013;67:399–416. doi: 10.1146/annurev-micro-092412-155650. [DOI] [PubMed] [Google Scholar]


