Abstract
Optical coherence tomography angiography (OCTA) is a novel non-invasive imaging modality for 3-dimensional visualization of retinal and optic nerve capillary networks. In this article, a comprehensive review of relevant original articles in the PubMed database was performed using the search terms “diabetic retinopathy,” “diabetic macular edema,” “diabetes mellitus,” and “optical coherence tomography angiography.” OCTA was found to detect microvascular changes early in diabetes mellitus, even before they become clinically evident. Morphological and qualitative assessment of vascular changes can help to determine the pathophysiological processes, activity, treatment, and follow-up of diabetic retinopathy (DR). Vessel density and foveal avascular zone are the most investigated quantified indices shown to be early predictors of DR, correlated to DR severity and visual function, and useful in predicting response to treatment. OCTA has shown to be a promising alternative to fluorescein angiography in the management of DR. Further studies are warranted to determine the role of OCTA in the routine clinical management of DR.
Keywords: Deep Capillary Plexus, Diabetic Retinopathy, Foveal Avascular Zone, Ischemia, Optical Coherence Tomography Angiography, Superficial Capillary Plexus, Vessel Density
INTRODUCTION
The introduction of optical coherence tomography (OCT) in 1991[1] and its evolution has revolutionized retinal imaging. OCT is unique as it is comparable to histological microscopy in imaging the retina. Currently, OCT is an essential imaging modality which has no practical alternative in the objective and quantitative management of vitreoretinal diseases including diabetic maculopathy.[1,2] Unfortunately, OCT does not provide direct information regarding functional and dynamic changes in the retinal and choroidal structures and vasculature including velocity of blood flow, distinction between afferent arteries and efferent veins, or identification of vascular permeability changes. Therefore, fluorescein angiography (FA) and indocyanine green angiography (ICGA) still remain the standard imaging modalities to visualize blood vessels and the dynamic changes within the retinal vasculature.
FA and ICGA have limitations; they need intravenous dye administration, are time-consuming (up to 20 minutes), and are unable to provide topographic 3-dimensional (3-D) images. Moreover, the images are of low resolution and quantification of findings is difficult. The introduction of OCT angiography (OCTA) has resolved these issues and provides rapid, non-invasive, high-resolution 3-D images from the retinal and choroidal vasculature and structures. Furthermore, it provides reliable quantitative data.[3,4,5,6,7,8,9]
The OCTA technology compares consecutive, repeated scans and assumes that the sole moving objects in the retina are the blood cells inside the vessels. These changing contrasts are translated to blood vessels in the final images. The technology is oversensitive to minor eye movements and requires patient cooperation to maintain fixation during imaging, making image acquisition time unpredictable.[10] The introduction of higher speed scanners, eye trackers, and improved software protocols has significantly improved earlier problems.[10,11]
The OCTA maps provide angiograms from different segmentation slabs. The most commonly used slabs, typically provided automatically by the OCTA software, are the superficial capillary plexus (SCP) slab which is the capillary network embedded in the ganglion cell layer and/or the nerve fiber layer; the deep capillary plexus (DCP) slab that consists of the capillary network in the inner nuclear layer (INL), and the choriocapillaris (CC) slab. The outer retinal slab (photoreceptors) has no vasculature. In healthy eyes, a higher density of vasculature is observed in the deeper compared to the superficial layer. In addition, different customized slabs and manual or automatic settings can be utilized to generate user preferences. Manual segmented and projection resolved (PR) OCTA can distinguish a distinct vascular network from the SCP and the DCP which is called the middle capillary plexus (MCP).[12,13]
The hallmark of diabetic retinopathy (DR) is vascular changes involving different retinal layers. This may lead to visually devastating complications including macular edema, macular ischemia, and neovascularization. Until recently, FA was the only clinically available imaging modality to study different stages of DR. Using OCTA, it is now possible to visualize vascular, morphological, and distributional characteristics in different retinal layers. Although morphological and qualitative assessment of vascular changes can help us better understand the pathophysiological processes, determine the activity, enable appropriate treatment and follow-up of DR, many of the described features such as quantification of vascular dropout, vascular branching, vessel number, vessel tortuosity, and flow speed are still investigational. The vascular changes may be used in the physio-pathological assessment, prediction, diagnosis, grading, assessment of response to therapies, and follow up of DR.[3,4,5,6,7,8,9,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] Several studies have reported OCTA imaging in various retinal, choroidal, and optic nerve diseases.[45,46] We aimed to review the main findings, and discuss the applications and limitations of OCTA in DR.
METHODS
A comprehensive literature search was performed using the PubMed database for English-language publications using the keywords “Optical Coherence Tomography Angiography”, “diabetic retinopathy”, “diabetic macular edema”, and “diabetes mellitus” for original articles from January 2014 to December 2017. The relevant articles were studied, and key findings extracted.
RESULTS
Optical Coherence Tomography Angiography may Reveal Diabetic Retinopathy before it is Clinically Detectable
DR is prevalent among diabetics and is among the leading causes of blindness in developed countries.[2] Blindness is preventable in diabetics if diagnosed early in the course of the disease. Changes in the retinal microcirculation appear in diabetic patients before clinically visible retinopathy develops.[23] OCTA enables detection of retinal vascular abnormalities including areas of capillary nonperfusion, changes in foveal avascular zone (FAZ), and impairment of the choriocapillaris (CC) flow in diabetics with no apparent DR (NDR).[23,35,39] Interestingly, microvascular changes such as microaneurysms and tortuous beaded veins cannot distinguish eyes with diabetic retinopathy from healthy eyes. These changes are observed with similar frequency in both groups.[23]
FAZ metrics could be more easily measured by OCTA than FA. They are specially meant to provide cut-off points to differentiate healthy from diabetic eyes. Takase et al[35] demonstrated that FAZ area is enlarged in the SCP and DCP in diabetic eyes compared to healthy individuals before retinopathy develops [Table 1]. They suggested that OCTA may be able not only to detect diabetic eyes at a higher risk of retinopathy but also to screen for diabetes mellitus (DM) even before systemic diagnosis is made.[35]
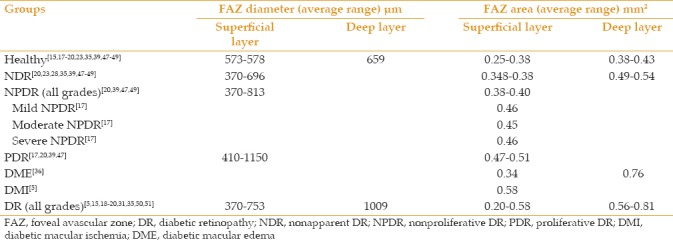
Table 1.
Summary of foveal avascular zone measurements by optical coherence tomography angiography in current literature
Optical Coherence Tomography Angiography Visualizes Morphological Changes in Diabetic Retinopathy
OCTA has the unique ability to visualize, quantify, and distinguish vascular and structural changes in all retinal and choroidal layers. Until now, OCTA has been used to image and describe many common and relatively rare types of vasculopathies in DR.[12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55,56,57] OCTA was comparable to clinical examination and FA to demonstrate vascular changes including microaneurysms (MAs), impaired perfusion, retinal edema, vascular loops, intraretinal microvascular abnormalities (IRMAs), and neovascularization (NVs).[40]
OCTA demonstrated that retinal vascular pathology including clustered capillaries, dilated capillary segments, tortuous capillaries, regions of capillary dropout, reduced capillary density, abnormal capillary loops, and FAZ enlargement are evident in both non-proliferative and proliferative diabetic retinopathy [Figures 1 and 2a–c].[39]
Figure 1.
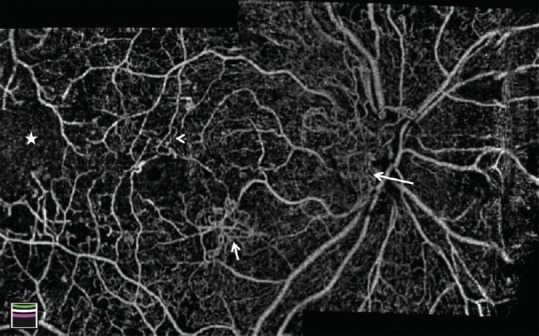
Montage optical coherence tomography angiography image of the optic disc and macula in a patient with diabetic retinopathy. Vascular changes are shown, including enlarged foveal avascular zone, optic disc neovascularization (long arrow), macular neovascularization (short arrow), microvascular tortuosity (arrowhead), and extensive capillary nonperfusion (star).
Figure 2.
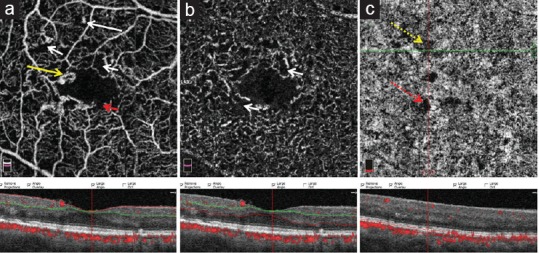
Optical coherence tomography images from the superficial capillary plexus (a), deep capillary plexus (b), and choriocapillaris (c) slabs with corresponding structural optical coherence tomography images in a patient with diabetic retinopathy. The figure shows microaneurysm (white long arrow), capillary non-perfusion (white short arrow), enlarged foveal avascular zone with a notch (red short arrow), focal microvascular tortuosity and dilation (yellow arrow), focal areas of true signal impairment (yellow dotted arrow) and shadow artifact (red dotted arrow).
MAs are focally dilated saccular or fusiform capillaries. On OCTA, these are reported as hyper-reflective spots which sometimes encircle the FAZ.[16,25,31] In OCTA, MAs reveal various morphologic patterns including fusiform, saccular, curved, and rarely, a coiled shape while in FA they appear as homogeneous hyperfluorescent dots [Figure 2a].[31] Although OCTA offers better visualization of MAs compared to FA,[16,31] the detection rate may be lower compared to FA.[31,33] Miwa et al[31] reported that the number of MAs is comparable in FA, OCTA, and SD-OCT images. Peres et al[27] demonstrated that the number of MAs in OCTA (both in SCP and DCP) and FA are statistically different and that MAs detected by OCTA are higher in number in DCP than SCP (P ≤ 0.001). Parravano et al[52] found that hyporeflective MAs on SD-OCT are more likely to be missed on OCTA. The blood flow dynamics in different types of MAs may explain these findings.[52] It remains unclear whether the OCTA has good sensitivity for the detection of MAs, in comparison to FA. Notably, the presence of MAs could not differentiate eyes with NDR from healthy eyes.[23] Fewer MAs in both SCP and DCP is associated with a better response of diabetic macular edema (DME) to anti-vascular endothelial growth factor (anti-VEGF) therapy.[36]
As OCTA can distinguish between the structural level of vascular lesions, it can uniquely differentiate retinal NVs from IRMAs or shunting vessels. It is not always possible to distinguish IRMA from RNV through clinical examination or FA.[16,19,26,39] The fact that RNV arises adjacent to the IRMA in 50% of cases adds to the value of OCTA in differentiating between IRMA and RNV.[22] Retinal and disc NVs are seen as flower-like interwoven vessels above the surface of the retina and the optic nerve.[16] In a series, 92% (11 out of 13) of RNVs were found to be adjacent to retinal capillary nonperfusion areas.[22] Hence, OCTA may help distinguish between severe non-proliferative (NPDR) from proliferative DR (PDR), and may help in close follow up of severe NPDR cases. OCTA is also able to distinguish optic disc neovascularization from optic nerve head vascular collaterals; the former enters the vitreous and forms a network of faint vessels while the latter are loops of small vessels that are distinct from radial peripapillary capillaries.[53,54,55,56]
Although OCTA does not provide functional information like leakage from new vessels, there are morphological clues that suggest active retinal neovascularization (RNVs). Ishibazawa et al[57] found that exuberant vascular proliferation (irregular proliferation of fine new vessels) in OCTA should be considered as a sign of active neovascularization.
Investigation of morphological clues in OCTA could reflect the functional and activity status of neovascular lesions which could be used to grade activity of vascular pathologies, to spot treatment targets, and to follow them up.
Foveal Avascular Zone in Diabetic Retinopathy
FAZ, a capillary-free area enclosed by foveal capillary circles, is located at the center of macula. Abnormalities in the structure or perfusion of this area profoundly affect vision. FAZ was first described in vivo by FA.[58] OCTA is considered superior to FA to define the central and parafoveal macular microvasculature, and to delineate FAZ, because it is not covered by fluorescein from dye leakage.[4] However, shadows from hemorrhage and macular edema may affect FAZ measurements by OCTA.[12,15,16,17,18,19,20,23,31,35,39,58] The shape and size of FAZ are found to be comparable using OCTA and FA.[16] FA cannot resolve the level of FAZ measurement; however, FAZ is found to have a larger area in both SCP and DCP using OCTA.[27] FAZ measurements cannot be compared using different OCTA devices; however, the same device could perform reliable, repeated measurements of FAZ.[59]
The normal FAZ is described as a well-defined round or oval area of absent vessel signals using OCTA. The border of the normal FAZ has no gaps, holes or interruption of the vascular network in both the superficial and deep plexuses. The longest FAZ diameter is in either the vertical or the horizontal axis.[19]
FAZ is enlarged in diabetic eyes as a result of loss of integrity of blood vessels.[19] In diabetics, the shape of FAZ is non-symmetrical due to gaps, holes, or notches of the capillary plexuses. The grading of FAZ disruption is correlated with DR severity[60,61] and visual function.[62] In contrast to normal eyes, the maximum diameter of FAZ in diabetic eyes is neither horizontal nor vertical.[19]
Vascular Changes in the Choroicapillaris in Diabetic Retinopathy
OCTA can visualize blood vessels as deep as the choriocapillaris (CC) and beyond. CC changes are not evident on clinical examination or FA. A better understanding of choroidal changes may help to predict disease progression and response to therapies.[63] Choi et al[39] utilized ultra-high speed swept source OCTA and documented CC flow impairment in NPDR and PDR. Figure 2c shows an OCTA image of diabetic retinopathy with both true and artifactual signal impairment in the choriocapillaris. Further studies using OCTA are warranted to elucidate changes in the choroidal vasculature in diabetic patients and their association with the grade and progression of DR, visual acuity, and response to therapies.
Quantitative Measurements in Diabetic Retinopathy
Quantitative assessment of vascular changes in DR is crucial to predict the grade, select preferred treatment, and follow-up of the efficacy of therapy. It helps to longitudinally assess the vascular remodeling processes which could enhance our knowledge about the pathophysiology of DR and can help in monitoring the efficacy of treatment.
Various vascular quantifications have been described including the area filled by binarized vessels (vessel area density = VD) or skeletonized vessels (vessel perfusion density = PD), vessel spacing or intercapillary area, length of the blood vessel based on the skeletonized OCTA (vascular length density = VLD or skeleton density = SD), vessel diameter index (VDI, calculated as VD divided by VLD), total length of vessels (vessel length fraction), vascular architecture, and branching [tortuosity and fractal dimension (FD)], and nonperfusion indexes (NPI).[12,17,19,34,44,64,65,66] To our knowledge, only VD indices and PD maps are available in some commercially available OCTA devices. SD, VD, FD, and VDI showed high reproducibility among graders. Repeatability was found satisfactory for SD, VD, FD, and VDI.[3,17,44] Therefore, vascular changes in DR may be characterized by SD, VD, FD, and VDI. A lower the SD, VD, and FD; and increased vascular spacing, VD, and FAZ size; were associated with more sever DR clinical scale.[17,44,62]
VD is mainly measured by manual (binarization, with or without skeletonization), or automated methods. There are significant differences between these methods. Although the automated method may distinguish diabetic changes as early as severe NPDR, other methods can detect significant VD changes only in the more advanced stages of retinopathy (PDR).[65] Repeated VD measurements using the same device is reliable; however, significant variability exists in measurements using different devices and methods.[59] Hence, comparisons should be made using the same type of device. Unlike previous belief, that DR involves the temporal area more than the nasal area; there is no preference for DR to involve the nasal or temporal area on VD assessment.[47]
VD is correlated with age and sex, which should be considered while interpreting results of studies.[66,67] VD as a vascular index is also correlated with retinal structural characteristics including retinal thickness and volume.[48] Reduced VD correlated with thinner macular ganglion cell/inner plexiform layer.[62] VD is both repeatable and reproducible in patients without DME.[68,69] However, there was a difference in VD measured with different patterns (3 × 3 mm vs. 6 × 6 mm) and locations (inner vs. outer ring) of scans.[69] VD decreases in both the deep and superficial layers in DR.[18,70] Some studies found that VD had a negative correlation with systemic indices like fasting blood sugar (FBS), postprandial blood sugar (PPBS), and glycosylated hemoglobin (HbA1c);[17,44,71] however, no correlation was found between VD and HbA1c or the duration of DM in other studies.[66,48] Hyperlipidemia, smoking and renal impairment also have been found to be negatively correlated with VD.[72]
Alterations in the microcirculation may precede clinically distinguishable neuroretinopathy in diabetics patients.[73] Some studies found that VD in the DCP (but not SCP or choriocapillaris) on OCTA was lower in diabetic patients without DR compared to non-diabetic individuals. They suggested that parafoveal capillary nonperfusion in DCP is an early sign of DR.[28,61,73,74] Other studies have shown that VD in the both DCP and SCP is different between diabetic patients and healthy controls.[44,64,70,72] Some studies found that vascular spacing and alterations in VD in SCP are more correlated with DR severity than VD in DCP, PD in SCP or FAZ area.[17,18,66]
VD may predict DR severity with a relatively high sensitivity and specificity, especially when the DCP is considered as a differentiator. VD was found to be negatively correlated with DR severity.[17,44,47,60,73,75] Adding a quantified vascular index to the current grading of severity of diabetic retinopathy makes the scaling measurable. This helps to identify patients at risk, and predict therapeutic efficacy, and helps in follow-up of patients.
It is not clear if VA and VD are correlated. In a small series, no correlation was found between VA and parafoveal or perifoveal VD.[3] However, in a larger study, there was a statistically significant negative correlation between the logMAR VA and the VD in the both SCP and DCP[50] whereas another study found a weak negative correlation between VD in SCP and VA.[66] Patients with DME and higher VD (particularly in DCP) have shown a better response to anti-VEGF therapy;[36] however, VD was found to be unchanged after therapy in a small series during a 1-month follow up.[29] Moreover, there was no change in the foveal and parafoveal VD after treatment with intravitreal dexamethasone implant (IDI) in a series of patients even after 120 days.[64]
Intercapillary spacing can distinguish non-perfusion areas earlier than VD.[75] In a study, the vessels were divided into small and large spacing based on normalized ratio of pixels. Large vessel spacing (in both DCP and SCP) has been found to be more sensitive than VD and FAZ area in the diagnosis of DR.[17] Large vessel spacing, particularly in SCP, has also been found to be associated with severity of DR.[17,18,75]
The extrafoveal avascular area may discriminate between early NPDR and healthy eyes.[76] An algorithm based on intercapillary space was more sensitive than vascular density-based methods to measure early capillary dropout or non-perfusion areas.[75] There was a significant association between progression of NPDR stages and superficial capillary plexus NPI. A significant correlation was found between NPI and BCVA. Besides, NPI was correlated with HbA1c in patients with NPDR.[61]
In one study, vessel tortuosity but not VD or FAZ area was shown to be an early differentiator of early NPDR from NDR, particularly in SCP. Vessel tortuosity increased with the increase in the stage of NPDR but decreased in PDR. FAZ area and acircularity in SCP was correlated with vessel tortuosity in 3 mm2 and 1.5 mm2 areas of SCP.[60] FD has been shown to be an early indicator of DR.[77] FD is reduced in both SCP and DCP in diabetic patients compared to healthy controls,[70,77] and the reduction may be more pronounced in DCP.[78] In contrast, some studies found that FD is increased in PDR cases in both SCP and DCP.[72] FD was found to be correlated with HbA1c and renal impairment.[72]
A novel algorithm was introduced to automatically detect dilated capillaries in DCP in DR with an accuracy comparable to clinical grading.[79] In a qualitative assessment of OCTA, the diameter of blood vessels decreased in DCP after treatment of DME.[64] Increased VDI was correlated with higher FBS.[62] It is noteworthy that the blood vessel caliber measurements may not be accurate with OCTA imaging.[80]
Perifoveal PD was significantly lower in all layers in patients with DR compared with healthy subjects.[81] Quantitative retinal vascular perfusion density values have been found to be comparable with the clinical grading of DR.[34,66,81] With progression of DR, capillary PD significantly decreases in most layers.[34,66,81,82] Besides, PD is reduced in the presence of DME[44,82] and does not change following anti-VEGF treatment.[82] PD is correlated with age,[66] hence cases and controls should be age-matched in clinical studies.
CC alterations are believed to contribute to the pathogenesis of DR. Non-perfusion areas in CC have been detected in patients with DR.[83] Although one study found that VD in CC is not different in diabetic patients compared with healthy individuals,[73] the flow void areas in CC may increase in size in the more severe stages of DR.[83] In patients without DME, CC non-perfusion area correlated with central retinal thickness as well as logMAR VA, but this was not the case in patients with DME. The extent of non-perfusion area corresponded to ellipsoid zone (EZ) distortion.[83] Choriocapillaris CD (CCCD) has been found to increase in response to therapy; however, changes could be due to the limitations of OCTA in the assessment of deeper layers.[64] Utilizing projection resolved (PR)-algorithm in the assessment of CCCD, which has been shown to be comparable to scanning electron microscopy, and provide higher quality images from deeper layers, may be helpful.[84]
FAZ size is correlated with age,[51,67,85] history of preterm birth,[86,87] vascular indices including VD and perfusion indices.[47,49,60] structure of the eye and retina like axial length, and retinal and macular ganglion cell/inner plexiform layer thickness.[49,62] It is important to point out that presence of CME may not influence the reproducibility and effectiveness of OCTA in the measurement of FAZ in SCP.[8,88] FAZ has been found to have no or the least correlation among other vascular indices with systemic indicators like HbA1c and duration of DM;[17,66] however, in one study, FAZ size was found to be correlated with HbA1c.[61] These should be confirmed in larger studies and considered in the design of studies and interpretation of results.
The FAZ diameter is not identical in SCP, MCP, and DCP.[12] There is no consensus on whether the FAZ changes are more profound in SCP or DCP.[12,17,20] While FAZ enlargement was more pronounced in the deep layer in DR in some studies.,[12,20] other studies found no difference in FAZ enlargement between the SCP and DCP.[17]
The Early Treatment of Diabetic Retinopathy Study (ETDRS) qualitatively evaluated macular ischemia using FA and found it to have predictive value for progression of disease.[89,90] Macular ischemia is associated with FAZ changes. FAZ metrics have been found to increase with progression of DR in most reports,[15,17,18,19,20,23,31,35,39,47,49,60,61] but one study reported no change in FAZ size as DR progresses.[73] Table 1 summarizes the mean area and diameter of FAZ in SCP and DCP in different grades of DR in different studies. Mean longest FAZ diameter in SCP increases from 573 microns in healthy individuals to up to 1150 microns in PDR and the same index in DCP increases from 659 microns in healthy individuals to up to 1009 microns in DR. Mean FAZ area in SCP increases from 0.25 mm2 in healthy individuals to up to 0.51 mm2 in PDR and 0.58 mm2 in cases with DMI; Mean FAZ area in DCP increases from 0.38 mm2 in healthy individuals to up to 0.81 mm2 in DR.[5,15,17,18,19,20,23,27,28,31,35,36,39,47,48,49,50,51] Other than the size of FAZ, alteration of the circular border and axis of FAZ can be quantitatively assessed.[49] Acircularity index and axis ratio of FAZ have also been shown to be correlated with severity of DR.[37,49,60] Therefore, FAZ assessment could be used for the grading of DR.
FAZ size (in both SCP and DCP) was found to be associated with visual function and VA in some studies.[19,50,51] however, this correlation was not found in another series.[3] Further large-scale investigations are required to elucidate this correlation.
Eyes with larger FAZ may be non-responders to laser therapy; they may benefit more from Anti-VEGF treatment.[36] Thus, FAZ assessment may help in selecting the appropriate therapeutic modality. No changes in FAZ size have been found after anti-VEGF or (IDI) treatment.[29,64,82]
Optical Coherence Tomography Angiography in Assessment of Diabetic Maculopathy
Diabetic maculopathy includes DME and diabetic ischemic maculopathy. Structural OCT may change the diagnosis and management of DME, as it provides quantifiable metrics;[1,2] however, it cannot directly distinguish patients with ischemic maculopathy who are usually refractory to either anti-VEGF or macular photocoagulation treatment. Therefore, evaluating the maculopathy is still dependent on FA as a gold standard imaging modality in many cases. Introducing OCTA as an alternative imaging modality may alleviate the need for FA in many cases.[5,91,92]
Diabetic macular ischemia (DMI) is defined as enlarged FAZ and capillary nonperfusion in the macula by FA in cases with or without diabetic macular edema.[15,91,92] Comparison of adaptive optics scanning laser ophthalmoscopy, OCT, FA, and OCTA revealed that non-perfusion area in DCP and reduced VD correspond to macular photoreceptor disruption in DMI cases.[5,14,15,21,91,92,93] In the area of the disrupted ellipsoid zone of the photoreceptor (EZ), CC layer had greater areas of flow void. Thus, alteration of choroidal circulation has a role in the pathogenesis of DR and DMI.[83]
OCTA is comparable to FA in assessing and detecting DMI.[5,21] The Early Treatment of Diabetic Retinopathy Study qualitatively evaluated macular ischemia using FA and found a predictive value for progression of the disease.[89,90] Quantitative assessments of macular ischemia utilized by OCTA could be used in the grading of DR.
DME is defined as thickening of macula corresponding to the areas of leakage in FA images. SD-OCT, and currently, OCTA can clearly show structural changes in DME in 3D images. Cystic spaces are sometimes hard to distinguish by FA as they may be covered by shadows; but in OCTA, cystoid spaces are oblong-shaped total flow void areas with smooth margins which do not follow the border of the neighboring capillaries; whereas areas of capillary non-perfusion have a lighter shade with irregular margins.[41] OCTA also reveals alteration in density and morphology of the microvasculature in SCP and DCP which improves understanding of the pathophysiology behind the edema.[44] A correlation has been shown between the MA number specifically in DCP and the presence of DME.[30] VD (in SCP and DCP), CCCD, and PD are reduced and FAZ is enlarged in the presence of DME.[18,20,36,44,64] While interpreting the results, one should consider that the presence of CME may influence reproducibility and effectiveness of OCTA in the measurement of FAZ and vascular indices, particularly in DCP.[8,88] Eyes with DME that showed outer plexiform layer (OPL) disruption, loss of integrity of the DCP, larger FAZ area, and more MAs in DCP had poor response to anti-VEGF treatment.[36] Size of FAZ and VD did not change following IDI and anti-VEGF therapy but a reduction in the caliber of DCP vessels and an increase in CCCD was observed.[29,64,82] OCTA distinguishes non-perfusion areas, FAZ alterations, cystic changes, and MAs; therefore, it may help clinicians better understand the pathophysiology and grading of DME, choose therapy, and follow up treatment efficacy.[41]
Optical Coherence Tomography Angiography Artifacts and Limitations
The clinical application of OCTA is limited by many factors. These include segmentation errors and lack of ideal automatic algorithms to resolve these issues;[42,94,95] the need for good patient fixation which can be challenging in the presence of DME or DMI;[96] sensitivity to minor eye movements and lack of commercial higher speed OCTA devices, optimal algorithms to correct motion artifacts;[6,97,98,99] projection artifacts that reduce repeatability and reliability of the assessment of deep layers;[13,76,84] small field of commercial OCTA devices;[100,101,102] and discrepancy between measurements using various commercial devices.[59]
Retinal and choroidal diseases disorganize the boundaries of structures and make automatic segmentation of the retina difficult. Therefore, images and calculated vascular indices are less reliable in the presence of pathology.[95] Artifacts including banding, motion, and segmentation have been found to be higher in eyes with ocular pathology. Also, choroidal diseases cause more segmentation errors in choriocapillaris slab than retinal diseases.[42] Manual segmentation is time-consuming and automatic segmentation typically fails to segment irregular layers appropriately. Advanced image processing helps to segment the retinal layers and to split up 3D flow data into different layers in the presence of pathology.[94,95]
Poor image quality may not influence the occurrence of segmentation error or motion artifacts; however, it increases projection and banding artifacts, which could decrease repeatability of measurements, particularly in DCP. Image quality should be noted while assessing measurements.[103]
One of the strengths of OCTA is its ability to assess vasculatures and structures of DCP separately from SCP; however, this is limited by projection artifacts from the superficial structures onto deeper layers. Projection-Resolved Optical Coherence Tomography Angiography (PR-OCTA) uses a novel reflectance-based projection-resolved (rbPR) algorithm that augments the flow signal and overcomes projection artifacts. This module is found to improve image resolution for the assessment of MCP, DCP, and CC.[13,76,84] Adaptive optics (AO) OCTA also improves axial and transverse resolution, reduces projection artifacts, and assists with proper segmentation. Thus, AO-OCTA improves tracking of smaller vessels and reliability of assessment of vascular indices.[104]
Motion artifacts due to eye movement result in poor quality images, stretch artifacts, and vessel doubling. Increasing scan speed reduces motion artifacts but should be balanced by blood flow speed; slow flow vessels may be ignored by faster scans.[99] An automated registration and selective merging (RSM) algorithm could improve image quality and resolve the few residual artifacts that remain after orthogonal registration.[98] Eye tracking systems significantly decrease motion artifacts; however, their use may lead to extended acquisition time which is challenging in a busy clinic practice.[6,97]
Utilization of OCTA is largely limited by the small field of images. Wavefront sensorless adaptive optics improves peripheral retinal imaging and generates a sharper wide field mosaic image.[105] Montage OCTA was found to be comparable or superior in some instances to FA and 8 × 8-mm OCTA to visualize vascular details.[106] Wide-field OCTA technology will be an important advancement which will enhance the utilization of OCTA by clinicians.[100,101,102]
Currently, swept source OCTA is available, with faster scan speed, wider scan field, denser scan pattern, safer higher energy, longer wavelength laser, and improved detection power in deeper layers compared to SD-OCTA.[39,107]
Another limitation of the currently available OCTA devices is the inability to measure the speed of blood flow. This has been measured using a novel variable interscan time analysis (VISTA) algorithm utilized in a non-commercial swept source OCTA.[78]
Although measurements by the same devices using the same methodology may be reliable and reproducible, significant variability exists between different devices and methods.[59] Therefore, measurements should not be compared using different devices. Moreover, a large normative data of vascular indices from normal and diseased eyes measured by each device should be collected to be used as reference values in the future.
SUMMARY
OCTA is a promising alternative or supplement to OCT and FA in the management of DR. There have been several advances using OCTA imaging in diabetic eyes, with an earlier detection of diabetic changes, better grading of DR, and more reliable quantitative measurements. Morphological and qualitative assessment of vascular changes adds to our knowledge about the pathophysiology of DR.
The most common scaling system for DR is the International Clinical Disease Severity Scale,[108] a descriptive scale based on clinical examination and grades of DR as non-proliferative (mild, moderate, severe) and proliferative. This scaling system is limited as it is prone to examiner errors including missing subtle details which may change the presumed severity and the inability to identify those who are at higher risk of progression to a more severe retinopathy. Furthermore, it may not correlat with visual function and may not help with selection of appropriate therapy or provide trackable measures in the treatment and follow-up of patients. OCTA can help to quantitatively grade changes and is presumed to be superior or complementary to current qualitative grading systems. Adding quantitative values including FAZ size to the current grading system may improve DR scaling system to a more trackable clinically applicable scaling.[109,110,111]
Investigations are currently on to analyze retinal images to improve the screening, grading, and follow up of DR. A major progress is deep learning system (DLS) technology, which has shown promise as a highly sensitive, specific, and accurate method to detect referable and vision-threatening DR. It may enable grading of DR, suggest appropriate therapy, and predict response to therapy based on retinal images.[112,113,114,115,116] Incorporating the OCTA imaging data into the DLS processing algorithms may improve sensitivity and specificity.[117]
Like any other imaging system, various artifacts are described in OCTA images which are caused by OCT image acquisition, intrinsic eye characteristics, motion, processing, and display strategies.[42,43] Therefore, special attention is required for interpretation of images, as artifacts may interfere with the diagnosis, classification, or measurement of lesions in OCTA images.[42]
Current clinical use of OCTA imaging in DR may be limited to the detection of the etiology of unexplained visual loss, and differentiation of disc and NV from IRMA and shunt vessels. Moreover, follow-up of rare cases with persistent NV despite full panretinal photocoagulation for possible neovascular growth, is easy to perform with OCTA. OCTA may also be helpful in the differentiation of choroidal neovascularization from diabetic macular edema associated with macular degeneration. Future studies are needed to further elucidate the role of OCTA in clinical practice.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–1781. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virgili G, Menchini F, Casazza G, Hogg R, Das RR, Wang X, et al. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD008081. doi: 10.1002/14651858.CD008081.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134:367–373. doi: 10.1001/jamaophthalmol.2015.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soares M, Neves C, Marques IP, Pires I, Schwartz C, Costa MÂ, et al. Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br J Ophthalmol. 2017;101:62–68. doi: 10.1136/bjophthalmol-2016-309424. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JM, Lima TT, Louzada RN, Rassi AT, Isaac DL, Avila M, et al. Diabetic macular ischemia diagnosis: Comparison between optical coherence tomography angiography and fluorescein angiography. J Ophthalmol 2016. 2016:3989310. doi: 10.1155/2016/3989310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khadamy J. Optical Coherence Tomography Angiography (OCTA) in Ophthalmology; Technology, Pros, Cons and Commercial Prototypes. JOJ Ophthal. 2017;2(5):555598. [Google Scholar]
- 7.de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA) Int J Retina Vitreous. 2015;1:5. doi: 10.1186/s40942-015-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill A, Cole ED, Novais EA, Louzada RN, de Carlo T, Duker JS, et al. Visualization of changes in the foveal avascular zone in both observed and treated diabetic macular edema using optical coherence tomography angiography. Int J Retina Vitreous. 2017;3:19. doi: 10.1186/s40942-017-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo S, Krawitz B, Efstathiadis E, Geyman L, Weitz R, Chui TY, et al. Imaging foveal microvasculature: Optical coherence tomography angiography versus adaptive optics scanning light ophthalmoscope fluorescein angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT130–OCT140. doi: 10.1167/iovs.15-18932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camino A, Zhang M, Gao SS, Hwang TS, Sharma U, Wilson DJ, et al. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed Opt Express. 2016;7:3905–3915. doi: 10.1364/BOE.7.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan HA, Mehmood A, Khan QA, Iqbal F, Rasheed F, Khan N, et al. A major review of optical coherence tomography angiography. Expert Rev Ophthalmol. 2017;12:373–385. [Google Scholar]
- 12.Park JJ, Soetikno BT, Fawzi AA. Characterization of the middle capillary plexus using optical coherence tomography angiography in healthy and diabetic eyes. Retina. 2016;36:2039–2050. doi: 10.1097/IAE.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang TS, Zhang M, Bhavsar K, Zhang X, Campbell JP, Lin P, et al. Visualization of 3 distinct retinal plexuses by projection-resolved optical coherence tomography angiography] in diabetic retinopathy. JAMA Ophthalmol. 2016;134:1411–1419. doi: 10.1001/jamaophthalmol.2016.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarinci F, Nesper PL, Fawzi AA. Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol. 2016;168:129–138. doi: 10.1016/j.ajo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cennamo G, Romano MR, Nicoletti G, Velotti N, de Crecchio G. Optical coherence tomography angiography versus fluorescein angiography in the diagnosis of ischaemic diabetic maculopathy. Acta Ophthalmol. 2017;95:e36–e42. doi: 10.1111/aos.13159. [DOI] [PubMed] [Google Scholar]
- 16.Yu S, Lu J, Cao D, Liu R, Liu B, Li T, et al. The role of optical coherence tomography angiography in fundus vascular abnormalities. BMC Ophthalmol. 2016;16:107. doi: 10.1186/s12886-016-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhanushali D, Anegondi N, Gadde SG, Srinivasan P, Chidambara L, Yadav NK, et al. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT519–OCT525. doi: 10.1167/iovs.15-18901. [DOI] [PubMed] [Google Scholar]
- 18.Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:3907–3913. doi: 10.1167/iovs.16-19570. [DOI] [PubMed] [Google Scholar]
- 19.Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S, et al. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1051–1058. doi: 10.1007/s00417-015-3148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di G, Weihong Y, Xiao Z, Zhikun Y, Xuan Z, Yi Q, et al. Amorphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2016;254:873–879. doi: 10.1007/s00417-015-3143-7. [DOI] [PubMed] [Google Scholar]
- 21.Bradley PD, Sim DA, Keane PA, Cardoso J, Agrawal R, Tufail A, et al. The evaluation of diabetic macular ischemia using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:626–631. doi: 10.1167/iovs.15-18034. [DOI] [PubMed] [Google Scholar]
- 22.de Carlo TE, Bonini Filho MA, Baumal CR, Reichel E, Rogers A, Witkin AJ, et al. Evaluation of preretinal neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2016;47:115–119. doi: 10.3928/23258160-20160126-03. [DOI] [PubMed] [Google Scholar]
- 23.de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–2370. doi: 10.1097/IAE.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 24.Hwang TS, Jia Y, Gao SS, Bailey ST, Lauer AK, Flaxel CJ, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina. 2015;35:2371–2376. doi: 10.1097/IAE.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, et al. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. Am J Ophthalmol. 2015;160:35–44. doi: 10.1016/j.ajo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Jia Y, Bailey ST, Hwang TS, McClintic SM, Gao SS, Pennesi ME, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015;112:E2395–E2402. doi: 10.1073/pnas.1500185112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peres MB, Kato RT, Kniggendorf VF, Cole ED, Onal S, Torres E, et al. Comparison of optical coherence tomography angiography and fluorescein angiography for the identification of retinal vascular changes in eyes with diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2016;47:1013–1019. doi: 10.3928/23258160-20161031-05. [DOI] [PubMed] [Google Scholar]
- 28.Simonett JM, Scarinci F, Picconi F, Giorno P, De Geronimo D, Di Renzo A, et al. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017;95:e751–e755. doi: 10.1111/aos.13404. [DOI] [PubMed] [Google Scholar]
- 29.Ghasemi Falavarjani K, Iafe NA, Hubschman JP, Tsui I, Sadda SR, Sarraf D, et al. Optical coherence tomography angiography analysis of the foveal avascular zone and macular vessel density after anti-VEGF therapy in eyes with diabetic macular edema and retinal vein occlusion. Invest Ophthalmol Vis Sci. 2017;58:30–34. doi: 10.1167/iovs.16-20579. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa N, Nozaki M, Takase N, Yoshida M, Ogura Y. New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Invest Ophthalmol Vis Sci. 2016;57:OCT348–OCT355. doi: 10.1167/iovs.15-18782. [DOI] [PubMed] [Google Scholar]
- 31.Miwa Y, Murakami T, Suzuma K, Uji A, Yoshitake S, Fujimoto M, et al. Relationship between functional and structural changes in diabetic vessels in optical coherence tomography angiography. Sci Rep. 2016;6:29064. doi: 10.1038/srep29064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanga PE, Papayannis A, Tsamis E, Stringa F, Cole T, D’Souza Y, et al. New findings in diabetic maculopathy and proliferative disease by swept-source optical coherence tomography angiography. Dev Ophthalmol. 2016;56:113–121. doi: 10.1159/000442802. [DOI] [PubMed] [Google Scholar]
- 33.Couturier A, Mané V, Bonnin S, Erginay A, Massin P, Gaudric A, et al. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina. 2015;35:2384–2391. doi: 10.1097/IAE.0000000000000859. [DOI] [PubMed] [Google Scholar]
- 34.Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35:2353–2363. doi: 10.1097/IAE.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 35.Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y, et al. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35:2377–2383. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Moon BG, Cho AR, Yoon YH. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology. 2016;123:2368–2375. doi: 10.1016/j.ophtha.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Krawitz BD, Mo S, Geyman LS, Agemy SA, Scripsema NK, Garcia PM, et al. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vision Res. 2017;139:177–186. doi: 10.1016/j.visres.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Bandello F, Corbelli E, Carnevali A, Pierro L, Querques G. Optical coherence tomography angiography of diabetic retinopathy. Dev Ophthalmol. 2016;56:107–112. doi: 10.1159/000442801. [DOI] [PubMed] [Google Scholar]
- 39.Choi W, Waheed NK, Moult EM, Adhi M, Lee B, De Carlo T, et al. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina. 2017;37:11–21. doi: 10.1097/IAE.0000000000001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsunaga DR, Yi JJ, De Koo LO, Ameri H, Puliafito CA, Kashani AH, et al. Optical coherence tomography angiography of diabetic retinopathy in human subjects. Ophthalmic Surg Lasers Imaging Retina. 2015;46:796–805. doi: 10.3928/23258160-20150909-03. [DOI] [PubMed] [Google Scholar]
- 41.de Carlo TE, Chin AT, Joseph T, Baumal CR, Witkin AJ, Duker JS, et al. Distinguishing diabetic macular edema from capillary nonperfusion using optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2016;47:108–114. doi: 10.3928/23258160-20160126-02. [DOI] [PubMed] [Google Scholar]
- 42.Ghasemi Falavarjani K, Al-Sheikh M, Akil H, Sadda SR. Image artefacts in swept-source optical coherence tomography angiography. Br J Ophthalmol. 2017;101:564–568. doi: 10.1136/bjophthalmol-2016-309104. [DOI] [PubMed] [Google Scholar]
- 43.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35:2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH, et al. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT362–OCT370. doi: 10.1167/iovs.15-18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falavarjani KG, Sarraf D. Optical coherence tomography angiography of the retina and choroid; current applications and future directions. J Curr Ophthalmol. 2017;29:1–14. doi: 10.1016/j.joco.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akil H, Falavarjani KG, Sadda SR, Sadun AA. Optical coherence tomography angiography of the optic disc; an overview. J Ophthalmic Vis Res. 2017;12:98–105. doi: 10.4103/2008-322X.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaizu Y, Nakao S, Yoshida S, Hayami T, Arima M, Yamaguchi M, et al. Optical coherence tomography angiography reveals spatial bias of macular capillary dropout in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:4889–4897. doi: 10.1167/iovs.17-22306. [DOI] [PubMed] [Google Scholar]
- 48.Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:190–196. doi: 10.1167/iovs.16-20531. [DOI] [PubMed] [Google Scholar]
- 49.Kim K, Kim ES, Yu SY. Optical coherence tomography angiography analysis of foveal microvascular changes and inner retinal layer thinning in patients with diabetes. Br J Ophthalmol. 2018;102:1226–1231. doi: 10.1136/bjophthalmol-2017-311149. [DOI] [PubMed] [Google Scholar]
- 50.Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124:235–244. doi: 10.1016/j.ophtha.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Balaratnasingam C, Inoue M, Ahn S, McCann J, Dhrami-Gavazi E, Yannuzzi LA, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123:2352–2367. doi: 10.1016/j.ophtha.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Parravano M, De Geronimo D, Scarinci F, Querques L, Virgili G, Simonett JM, et al. Diabetic microaneurysms internal reflectivity on spectral-domain optical coherence tomography and optical coherence tomography angiography detection. Am J Ophthalmol. 2017;179:90–96. doi: 10.1016/j.ajo.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 53.Singh A, Agarwal A, Mahajan S, Karkhur S, Singh R, Bansal R, et al. Morphological differences between optic disc collaterals and neovascularization on optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2017;255:753–259. doi: 10.1007/s00417-016-3565-x. [DOI] [PubMed] [Google Scholar]
- 54.Savastano MC, Federici M, Falsini B, Caporossi A, Minnella AM. Detecting papillary neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Acta Ophthalmol. 2018;96:321–323. doi: 10.1111/aos.13166. [DOI] [PubMed] [Google Scholar]
- 55.Jiang Y, Lim J, Degillio A. Profound macular ischemia on optical coherence tomography angiography in severe diabetic retinopathy. Ophthalmology. 2017;124:785. doi: 10.1016/j.ophtha.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 56.Falavarjani KG, Habibi A, Khorasani MA, Anvari P, Sadda SR. Time course of changes in optic disk neovascularization after a single intravitreal bevacizumab injection. Retina. 2018 Feb 19; doi: 10.1097/IAE.0000000000002107. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Ishibazawa A, Nagaoka T, Yokota H, Takahashi A, Omae T, Song YS, et al. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:6247–6255. doi: 10.1167/iovs.16-20210. [DOI] [PubMed] [Google Scholar]
- 58.Laatikainen L, Larinkari J. Capillary-free area of the fovea with advancing age. Invest Ophthalmol Vis Sci. 1977;16:1154–1157. [PubMed] [Google Scholar]
- 59.Magrath GN, Say EAT, Sioufi K, Ferenczy S, Samara WA, Shields CL, et al. Variability in foveal avascular zone and capillary density using optical coherence tomography angiography machines in healthy eyes. Retina. 2017;37:2102–2111. doi: 10.1097/IAE.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 60.Lee H, Lee M, Chung H, Kim HC. Quantification of retinal vessel tortuosity in diabetic retinopathy using optical coherence tomography angiography. Retina. 2018;38:976–985. doi: 10.1097/IAE.0000000000001618. [DOI] [PubMed] [Google Scholar]
- 61.Gozlan J, Ingrand P, Lichtwitz O, Cazet-Supervielle A, Benoudis L, Boissonnot M, et al. Retinal microvascular alterations related to diabetes assessed by optical coherence tomography angiography: A cross-sectional analysis. Medicine (Baltimore) 2017;96:e6427. doi: 10.1097/MD.0000000000006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang FY, Ng DS, Lam A, Luk F, Wong R, Chan C, et al. Determinants of quantitative optical coherence tomography angiography metrics in patients with diabetes. Sci Rep. 2017;7:2575. doi: 10.1038/s41598-017-02767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melancia D, Vicente A, Cunha JP, Abegão Pinto L, Ferreira J. Diabetic choroidopathy: A review of the current literature. Graefes Arch Clin Exp Ophthalmol. 2016;254:1453–1461. doi: 10.1007/s00417-016-3360-8. [DOI] [PubMed] [Google Scholar]
- 64.Toto L, D’Aloisio R, Di Nicola M, Di Martino G, Di Staso S, Ciancaglini M, et al. Qualitative and quantitative assessment of vascular changes in diabetic macular edema after dexamethasone implant using optical coherence tomography angiography. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061181. pii: E1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pedinielli A, Bonnin S, Sanharawi ME, Mané V, Erginay A, Couturier A, et al. Three different optical coherence tomography angiography measurement methods for assessing capillary density changes in diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2017;48:378–384. doi: 10.3928/23258160-20170428-03. [DOI] [PubMed] [Google Scholar]
- 66.Durbin MK, An L, Shemonski ND, Soares M, Santos T, Lopes M, et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135:370–376. doi: 10.1001/jamaophthalmol.2017.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coscas F, Sellam A, Glacet-Bernard A, Jung C, Goudot M, Miere A, et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT211–OCT223. doi: 10.1167/iovs.15-18793. [DOI] [PubMed] [Google Scholar]
- 68.You Q, Freeman WR, Weinreb RN, Zangwill L, Manalastas PIC, Saunders LJ, et al. Reproducibility of vessel density measurement with optical coherence tomography angiography in eyes with and without retinopathy. Retina. 2017;37:1475–1482. doi: 10.1097/IAE.0000000000001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lei J, Durbin MK, Shi Y, Uji A, Balasubramanian S, Baghdasaryan E, et al. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. 2017;135:1092–1098. doi: 10.1001/jamaophthalmol.2017.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zahid S, Dolz-Marco R, Freund KB, Balaratnasingam C, Dansingani K, Gilani F, et al. Fractal dimensional analysis of optical coherence tomography angiography in eyes with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:4940–4947. doi: 10.1167/iovs.16-19656. [DOI] [PubMed] [Google Scholar]
- 71.Falavarjani KG, Khadamy J, Amirkourjani F, Safi H, Modarres M. Macular thickness measurement in clinically significant macular edema before and after meal. J Curr Ophthalmol. 2015;27:125–128. doi: 10.1016/j.joco.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ting DS, Tan GS, Agrawal R, Yanagi Y, Sie NM, Wong CW, et al. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol. 2017;135:306–312. doi: 10.1001/jamaophthalmol.2016.5877. [DOI] [PubMed] [Google Scholar]
- 73.Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017;54:695–702. doi: 10.1007/s00592-017-0996-8. [DOI] [PubMed] [Google Scholar]
- 74.Cicinelli MV, Carnevali A, Rabiolo A, Querques L, Zucchiatti I, Scorcia V, et al. Clinical spectrum of macular-foveal capillaries evaluated with optical coherence tomography angiography. Retina. 2017;37:436–443. doi: 10.1097/IAE.0000000000001199. [DOI] [PubMed] [Google Scholar]
- 75.Schottenhamml J, Moult EM, Ploner S, Lee B, Novais EA, Cole E, et al. An automatic, intercapillary area-based algorithm for quantifying diabetes-related capillary dropout using optical coherence tomography angiography. Retina. 2016;36(Suppl 1):S93–S101. doi: 10.1097/IAE.0000000000001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang M, Hwang TS, Dongye C, Wilson DJ, Huang D, Jia Y, et al. Automated quantification of nonperfusion in three retinal plexuses using projection-resolved optical coherence tomography angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:5101–5106. doi: 10.1167/iovs.16-19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Q, Ma Q, Wu C, Tan F, Chen F, Wu Q, et al. Macular vascular fractal dimension in the deep capillary layer as an early indicator of microvascular loss for retinopathy in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2017;58:3785–3794. doi: 10.1167/iovs.17-21461. [DOI] [PubMed] [Google Scholar]
- 78.Bhardwaj S, Tsui E, Zahid S, Young E, Mehta N, Agemy S, et al. Value of fractal analysis of optical coherence tomography angiography in various stages of diabetic retinopathy. Retina. 2018;38:1816–1823. doi: 10.1097/IAE.0000000000001774. [DOI] [PubMed] [Google Scholar]
- 79.Dongye C, Zhang M, Hwang TS, Wang J, Gao SS, Liu L, et al. Automated detection of dilated capillaries on optical coherence tomography angiography. Biomed Opt Express. 2017;8:1101–1109. doi: 10.1364/BOE.8.001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghasemi Falavarjani K, Al-Sheikh M, Darvizeh F, Sadun AA, Sadda SR. Retinal vessel calibre measurements by optical coherence tomography angiography. Br J Ophthalmol. 2017;101:989–992. doi: 10.1136/bjophthalmol-2016-309678. [DOI] [PubMed] [Google Scholar]
- 81.Mastropasqua R, Toto L, Mastropasqua A, Aloia R, De Nicola C, Mattei PA, et al. Foveal avascular zone area and parafoveal vessel density measurements in different stages of diabetic retinopathy by optical coherence tomography angiography. Int J Ophthalmol. 2017;10:1545–1551. doi: 10.18240/ijo.2017.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michalska-Małecka K, Heinke Knudsen A. Optical coherence tomography angiography in patients with diabetic retinopathy treated with anti-VEGF intravitreal injections: Case report. Medicine (Baltimore) 2017;96:e8379. doi: 10.1097/MD.0000000000008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dodo Y, Suzuma K, Ishihara K, Yoshitake S, Fujimoto M, Yoshitake T, et al. Clinical relevance of reduced decorrelation signals in the diabetic inner choroid on optical coherence tomography angiography. Sci Rep. 2017;7:5227. doi: 10.1038/s41598-017-05663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Zhang M, Hwang TS, Bailey ST, Huang D, Wilson DJ, et al. Reflectance-based projection-resolved optical coherence tomography angiography [Invited] Biomed Opt Express. 2017;8:1536–1548. doi: 10.1364/BOE.8.001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: Quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:5780–5787. doi: 10.1167/iovs.16-20045. [DOI] [PubMed] [Google Scholar]
- 86.Falavarjani KG, Sarraf D, Tsui I. Optical coherence tomography angiography of the macula in adults with a history of preterm birth. Ophthalmic Surg Lasers Imaging Retina. 2018;49:122–125. doi: 10.3928/23258160-20180129-06. [DOI] [PubMed] [Google Scholar]
- 87.Falavarjani KG, Iafe NA, Velez FG, Schwartz SD, Sadda SR, Sarraf D, et al. Optical coherence tomography angiography of the fovea in children born preterm. Retina. 2017;37:2289–2294. doi: 10.1097/IAE.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 88.Tarassoly K, Miraftabi A, Soltan Sanjari M, Parvaresh MM. The relationship between foveal avascular zone area, vessel density, and cystoid changes in diabetic retinopathy: An optical coherence tomography angiography study. Retina. 2018;38:1613–1619. doi: 10.1097/IAE.0000000000001755. [DOI] [PubMed] [Google Scholar]
- 89.Fluorescein angiographic risk factors for progression of diabetic retinopathy. ETDRS report number 13.Early treatment diabetic retinopathy study research group. Ophthalmology. 1991;98:834–840. [PubMed] [Google Scholar]
- 90.Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11.Early treatment diabetic retinopathy study research group. Ophthalmology. 1991;98:807–822. [PubMed] [Google Scholar]
- 91.Coscas G, Lupidi M, Coscas F. Optical coherence tomography angiography in diabetic maculopathy. Dev Ophthalmol. 2017;60:38–49. doi: 10.1159/000459688. [DOI] [PubMed] [Google Scholar]
- 92.de Barros Garcia JM, Isaac DL, Avila M. Diabetic retinopathy and OCT angiography: Clinical findings and future perspectives. Int J Retina Vitreous. 2017;3:14. doi: 10.1186/s40942-017-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nesper PL, Scarinci F, Fawzi AA. Adaptive optics reveals photoreceptor abnormalities in diabetic macular ischemia. PLoS One. 2017;12:e0169926. doi: 10.1371/journal.pone.0169926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin X, Chao JR, Wang RK. User-guided segmentation for volumetric retinal optical coherence tomography images. J Biomed Opt. 2014;19:086020. doi: 10.1117/1.JBO.19.8.086020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang M, Wang J, Pechauer AD, Hwang TS, Gao SS, Liu L, et al. Advanced image processing for optical coherence tomographic angiography of macular diseases. Biomed Opt Express. 2015;6:4661–4675. doi: 10.1364/BOE.6.004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J, Rosen R. Optical coherence tomography angiography in diabetes. Curr Diab Rep. 2016;16:123. doi: 10.1007/s11892-016-0811-x. [DOI] [PubMed] [Google Scholar]
- 97.Lauermann JL, Treder M, Heiduschka P, Clemens CR, Eter N, Alten F, et al. Impact of eye-tracking technology on OCT-angiography imaging quality in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255:1535–1542. doi: 10.1007/s00417-017-3684-z. [DOI] [PubMed] [Google Scholar]
- 98.Camino A, Zhang M, Dongye C, Pechauer AD, Hwang TS, Bailey ST, et al. Automated registration and enhanced processing of clinical optical coherence tomography angiography. Quant Imaging Med Surg. 2016;6:391–401. doi: 10.21037/qims.2016.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmoll T, Singh AS, Blatter C, Schriefl S, Ahlers C, Schmidt-Erfurth U, et al. Imaging of the parafoveal capillary network and its integrity analysis using fractal dimension. Biomed Opt Express. 2011;2:1159–1168. doi: 10.1364/BOE2.001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Q, Lee CS, Chao J, Chen CL, Zhang T, Sharma U, et al. Wide-field optical coherence tomography based microangiography for retinal imaging. Sci Rep. 2016;6:22017. doi: 10.1038/srep22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghasemi Falavarjani K, Wang K, Khadamy J, Sadda SR. Ultra-wide-field imaging in diabetic retinopathy; an overview. J Curr Ophthalmol. 2016;28:57–60. doi: 10.1016/j.joco.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Poddar R, Migacz JV, Schwartz DM, Werner JS, Gorczynska I. Challenges and advantages in wide-field optical coherence tomography angiography imaging of the human retinal and choroidal vasculature at 1.7-MHz A-scan rate. J Biomed Opt. 2017;22:1–14. doi: 10.1117/1.JBO.22.10.106018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al-Sheikh M, Ghasemi Falavarjani K, Akil H, Sadda SR. Impact of image quality on OCT angiography based quantitative measurements. Int J Retina Vitreous. 2017;3:13. doi: 10.1186/s40942-017-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salas M, Augustin M, Ginner L, Kumar A, Baumann B, Leitgeb R, et al. Visualization of micro-capillaries using optical coherence tomography angiography with and without adaptive optics. Biomed Opt Express. 2017;8:207–222. doi: 10.1364/BOE.8.000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Polans J, Cunefare D, Cole E, Keller B, Mettu PS, Cousins SW, et al. Enhanced visualization of peripheral retinal vasculature with wavefront sensorless adaptive optics optical coherence tomography angiography in diabetic patients. Opt Lett. 2017;42:17–20. doi: 10.1364/OL.42.000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Carlo TE, Salz DA, Waheed NK, Baumal CR, Duker JS, Witkin AJ, et al. Visualization of the retinal vasculature using wide-field montage optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2015;46:611–616. doi: 10.3928/23258160-20150610-03. [DOI] [PubMed] [Google Scholar]
- 107.Miller AR, Roisman L, Zhang Q, Zheng F, Rafael de Oliveira Dias J, Yehoshua Z, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2017;58:1499–1505. doi: 10.1167/iovs.16-20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 109.Khadamy J. Can Foveal Avascular Zone (FAZ) Assessment By OCT Angiography, Used For Grading Of Diabetic Retinopathy? Advances in Ophthalmology & Visual System. 2016;5(3):00160. [Google Scholar]
- 110.Alipour SH, Rabbani H, Akhlaghi M, Dehnavi AM, Javanmard SH. Analysis of foveal avascular zone for grading of diabetic retinopathy severity based on curvelet transform. Graefes Arch Clin Exp Ophthalmol. 2012;250:1607–1614. doi: 10.1007/s00417-012-2093-6. [DOI] [PubMed] [Google Scholar]
- 111.Ahmad Fadzil M, Ngah NF, George TM, Izhar LI, Nugroho H, Adi Nugroho H, et al. Analysis of foveal avascular zone in colour fundus images for grading of diabetic retinopathy severity. Conf Proc IEEE Eng Med Biol Soc 2010. 2010:5632–5635. doi: 10.1109/IEMBS.2010.5628041. [DOI] [PubMed] [Google Scholar]
- 112.Ting DS, Cheung CY, Lim G, Tan GW, Quang ND, Gan A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211–2223. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prahs P, Radeck V, Mayer C, Cvetkov Y, Cvetkova N, Helbig H, et al. OCT-based deep learning algorithm for the evaluation of treatment indication with anti-vascular endothelial growth factor medications. Graefes Arch Clin Exp Ophthalmol. 2018;256:91–98. doi: 10.1007/s00417-017-3839-y. [DOI] [PubMed] [Google Scholar]
- 114.Ardiyanto I, Nugroho HA, Buana RL. Deep learning-based diabetic retinopathy assessment on embedded system. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:1760–1763. doi: 10.1109/EMBC.2017.8037184. [DOI] [PubMed] [Google Scholar]
- 115.Gargeya R, Leng T. Automated identification of diabetic retinopathy using deep learning. Ophthalmology. 2017;124:962–969. doi: 10.1016/j.ophtha.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 116.Wong TY, Bressler NM. Artificial intelligence with deep learning technology looks into diabetic retinopathy screening. JAMA. 2016;316:2366–2367. doi: 10.1001/jama.2016.17563. [DOI] [PubMed] [Google Scholar]
- 117.Sandhu HS, Eladawi N, Elmogy M, Keynton R, Helmy O, Schaal S, et al. Automated diabetic retinopathy detection using optical coherence tomography angiography: A pilot study. Br J Ophthalmol. 2018 doi: 10.1136/bjophthalmol-2017-311489. pii: bjophthalmol-2017-311489. [DOI] [PubMed] [Google Scholar]


