Abstract
Purpose:
To compare the involvement of the retina with that of the choroid in ocular sarcoidosis (OS) using dual fluorescein angiography (FA)/indocyanine green angiography (ICGA).
Methods:
A retrospective study of 23 patients with the diagnosis of OS was performed. Angiographic signs were quantified following the established FA/ICGA scoring system for uveitis.
Results:
The choroid was predominantly involved in 19 (82.6%) patients or 87% (40/46) of the eyes, and the retina in 2 (8.7%) patients or 13% (6/46) of the eyes. The mean angiographic score was 7.15 ± 4.5 for the retina (FA) compared to 14.02 ± 4.86 for the choroid (ICGA) (P < 0.0001). In 13% (3/23) of patients, FA did not show retinal inflammation, whereas ICGA was strongly positive, revealing occult choroidal lesions.
Conclusion:
The choroid is preferentially involved in OS, for which ICGA is the examination of choice. There is a risk of underestimating the global ocular involvement and of missing choroidal involvement if only FA is used. FA/ICGA scoring system allows for quantitative assessment of inflammation in the posterior uvea that occurs in OS; therefore, the system can be useful to quantitatively monitor outcomes in clinical trials.
Keywords: Angiographic Score, Chorioretinitis, Fluorescein Angiography, Indocyanine Green Angiography, Ocular Sarcoidosis
INTRODUCTION
Sarcoidosis is a chronic multisystem granulomatous disease characterized by the presence of non-caseating granulomas in involved organs. The commonly affected organs are the lungs, skin, and eyes. The frequency of ocular involvement ranges from 25% to 80%. Most patients present between the ages of 20 and 40 years; however, children and the elderly can also be affected. Generally, ocular involvement appears early in the course of the disease and may co-exist with asymptomatic systemic disease or precede systemic involvement by several years. The criteria for the diagnosis of ocular sarcoidosis (OS) were described by the first International Workshop on Ocular Sarcoidosis (IWOS).[1] Such criteria may have a higher sensitivity in the diagnosis of OS. Retinal involvement in OS has been well described based on studies using clinical fundus examination and fluorescein angiography (FA).[2] However, choroidal involvement and the difference between the rate of retinal and choroidal involvement could not be determined until a reliable method – such as indocyanine green angiography (ICGA) – was available to assess choroiditis and a scoring system was established to precisely measure choroidal and retinal angiographic signs.[3] The aim of this work was to assess the involvement of retina and that of the choroid in OS using dual fluorescein angiography (FA) and indocyanine green angiography (ICGA).[3,4]
METHODS
A retrospective study of patients with the diagnosis of OS that were seen between 2000 and 2016 at the Centre for Ophthalmic Specialized Care in Lausanne, Switzerland was performed. The diagnosis of OS was based on a compatible history of uveitis and a positive biopsy; a positive broncho-alveolar lavage; bilateral hilar lymphadenopathy; or 2 of the following 3 laboratory abnormalities: elevated level of lysozyme, elevated level of angiotensin-converting enzyme, or increased polyclonal activation.[5] In addition, patients had to have negative QuantiFERON-TB Gold test. Patients presenting with sarcoidosis-related chorioretinitis, as defined above, who had dual FA/ICGA data available were included in the study. All patients underwent the complete work-up routinely applied to patients with uveitis. All patients in our center undergo a routine complete ocular assessment that, in addition to routine examinations such as Snellen visual acuity assessment, slit-lamp examination, applanation tonometry, and dilated fundoscopy, includes the following investigations: laser flare photometry (LFP), computerized visual field (VF) testing, optical coherence tomography (OCT), and dual FA and ICGA since it became available. Laser flare photometric assessment was performed using a Kowa FM-500 or Kowa FM-700 device (Kowa Company, Ltd., Electronics and Optics Division, Tokyo, Japan), and OCT was performed using OTI-Spectral OCT/SLO machine (OTI Inc., Toronto, Canada) or Heidelberg Spectralis OCT machine (Heidelberg Engineering Inc., Heidelberg, Germany). G1 program of the OCTOPUS 900 perimeter (Octopus 900, G Standard; Haag Streit, Bern, Switzerland) was used for VF assessment.
Angiography
FA and ICGA were performed using a standard protocol described previously.[6] Briefly, FA and ICGA were usually performed simultaneously. A Topcon 50 IA camera (Tokyo, Japan) coupled to an image digitalizing system (ImageNet, Topcon)) or a Heidelberg Retina Angiograph HRA 2 system (Heidelberg Engineering Inc., Heidelberg, Germany) were used to acquire the images. To exclude autofluorescence, pre-injection fluorescence was sought using the highest flash intensity used for ICGA. At the same time, red-free posterior pole frames were acquired. After a bolus injection of 4 mg of indocyanine green (ICG) (Cardiogreen; Peaselt, Lorei, Germany) diluted in 5 ml of an aqua solution, ICGA frames of the posterior pole were acquired for approximately 2 to 3 minutes (early phase angiogram). The ICG fluorescence pattern was analyzed in detail 12 ± 3 minutes after ICG injection (intermediate phase ICGA angiogram) by acquiring frames of the posterior pole and a minimum of eight frames of the whole periphery over 360°. At the end of the intermediate phase of ICGA, fluorescein angiography was performed. Early fluorescein angiographic frames of the posterior pole were acquired for 2 minutes. Between 4 and 7 minutes, 360° panoramic imaging of the periphery was performed (minimum eight frames), followed by acquisition of late fluorescein posterior pole frames at 10 minutes. The late ICG fluorescence pattern was analyzed 24 ± 4 minutes after ICG injection (late phase ICGA angiogram) in the same fashion as for the intermediate phase.
Angiographic Score
Fluorescein angiography and indocyanine green angiography were evaluated using a well-established dual fluorescein angiography/indocyanine green angiography scoring system.[3,4] Briefly, a maximum total score of 40 was assigned to the FA signs, which included optic disc hyperfluorescence (maximum score [MS] = 3), macular edema (MS = 4), retinal vascular staining and/or leakage (MS = 7), capillary leakage (MS = 10), retinal capillary nonperfusion, neovascularization of the optic disc and neovascularization elsewhere, pinpoint leaks, and retinal staining and/or subretinal pooling (MS = 16). A maximum total score of 40 was also assigned to the ICGA signs, which included early stromal vessel hyperfluorescence (MS = 3), choroidal vasculitis (fuzzy vessels) (MS = 6), dark dots or areas (excluding atrophy) (MS = 8), and optic disc hyperfluorescence (MS = 3). Each ICGA scoring item was counting twice because the number of items in ICGA was half the number of items in FA; this was to ensure the proportionality of the scores in both methods.
The patients were classified into 3 categories, namely (1) choroidal involvement > retinal involvement (FA score < ICGA score), (2) retina involvement > choroidal involvement (FA score > ICGA score), and (3) choroidal involvement = retinal involvement (FA score = ICGA score). A score <3 points for FA or ICGA was interpreted as background noise and considered negative for involvement of either of the two structures. Analysis of angiographic data was performed by two experienced angiography specialists (CPH and AE) in a masked fashion.
Standardization of Uveitis Nomenclature Vitreous Haze Scoring
Fundus images of all eyes were evaluated by two masked clinicians using the International SUN Vitreous Haze Score where the patient's fundus picture was compared to a standard set of pictures with progressive importance of vitreous haze scored from 0 to 4.[7]
Statistical Analysis
We calculated the proportion of patients with ocular sarcoidosis in relation to the total number of patients with uveitis and the mean age of patients. In addition, we calculated the average angiographic score at the time of diagnosis as well as best-corrected visual acuity (BCVA), intraocular pressure (IOP), LFP values, and FA and ICGA scores for all eyes. We also calculated the number of patients with a predominance of retinal involvement, those with predominance of choroidal involvement, as well as those with equal choroidal and retinal involvement. Furthermore, we calculated the number of cases with occult chorioretinal lesions only visible on ICGA or FA (scores <3). Finally, we calculated the vitreous haze score using the SUN scale[7] for each eye to evaluate its importance compared to that of angiographic scoring.
To compare these data, we used the paired t test. The inter-observer variation was assessed using the intraclass correlation coefficient. Statistical significance was set at P < 0.05. Analysis was performed using MedCalc (version 17.7.2 for Windows; MedCalc Software; Ostend, Belgium). The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and was approved by the institutional review board.
RESULTS
Demographic Characteristics
Among the 1325 patients with uveitis that were seen from 2000 to 2016 at the Centre for Ophthalmic Specialized Care, 55 (4.15%) had or were presumed to have ocular sarcoidosis. Of the 55 patients, 23 (19 women and 4 men; mean age, 50.34 ± 20.15 years [range, 26-80 years]) fulfilled the diagnostic criteria and were included in the study.
Visual Acuity, Intraocular Pressure, Intraocular Inflammation, and Type of Uveitis
At presentation, the mean BCVA was 0.93 ± 0.30 (0.067 ± 0.19 LogMAR) [Figure 1].
Figure 1.
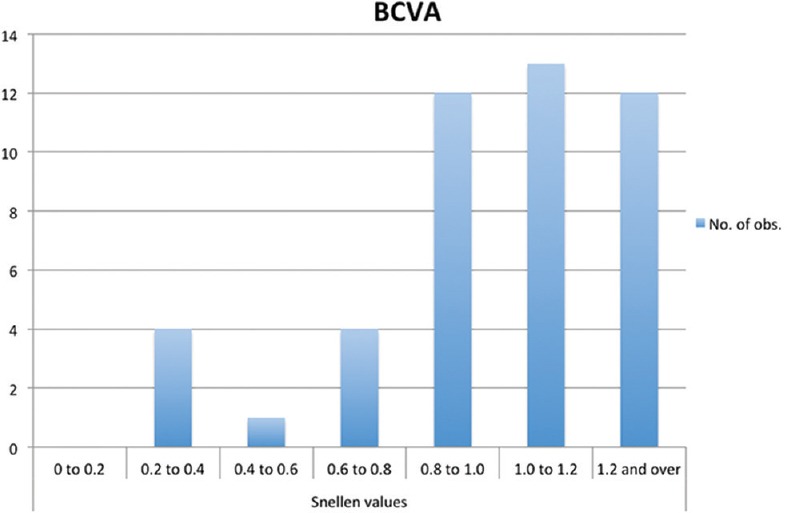
Bar graph showing best-corrected visual acuity at the time of diagnosis of ocular sarcoidosis. The majority of patients included had a visual acuity greater than 0.8.
The mean IOP at presentation was 14.59 ± 3.63 mm Hg.
Intraocular inflammation was measured using laser flare photometry, and the mean flare was 30 ± 38 ph/ms at presentation [Figure 2]. Ten patients presented with panuveitis with significant anterior uveitis. In the 13 remaining patients, uveitis was limited to the posterior segment (posterior uveitis). Patients with pure anterior uveitis without posterior involvement were not included.
Figure 2.
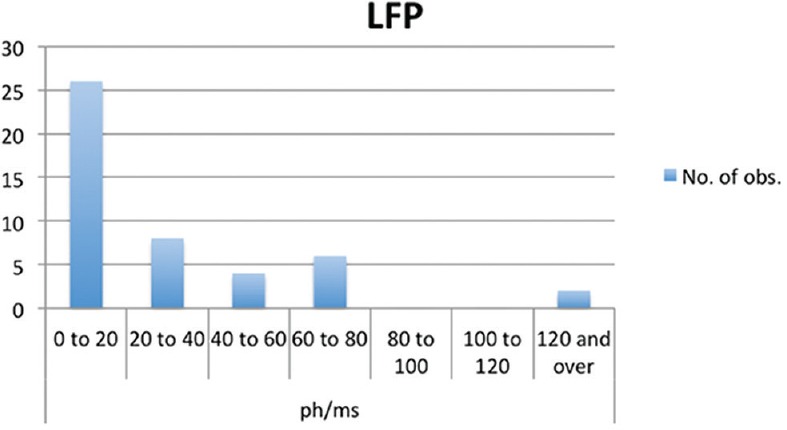
Bar graph showing results of laser flare photometry at the time of diagnosis of ocular sarcoidosis.
Fundus Findings
Fundus examination showed choroidal foci in only 14 of the 23 patients. Vasculitis was seen in 4 patients; one of whom had a typical string-of-pearls appearance of vasculitis. Disc swelling was seen in 4 of the 23 patients, and macroaneurysms were present in 3 of the 23 patients. Fundus examination showed posterior inflammation in no more than half of the patients, whereas FA/ICGA was positive in all patients and appeared to be essential in establishing the presence and determining the extent of the posterior inflammation.
Angiographic Findings
The intraclass correlation coefficient was 0.9848 for the angiographic score and 0.9595 for the vitreous haze classification; these values, showed there was a high reliability in inter-observers’ manual measurements. Dual FA/ICGA angiographic scoring was useful in establishing the degree of choroidal and retinal involvement in each patient and determining the percentage of patients (eyes) with more choroidal involvement, more retinal involvement, or equal involvement of the choroid and the retina. There was no significant difference in the mean angiographic scores between the two examiners (7 vs. 7.3 [P = 0.749] for FA score and 13.89 vs. 14.15 [P = 0.80] for ICG score). The choroid was predominantly involved in 19 patients (82.6%) [Figure 3] and the retina in 2 patients (8.7%) [Figure 4]. In two patients, one eye had predominant retinal involvement and the other eye had predominant choroidal involvement. Forty (87%) of the 46 eyes had more choroidal than retinal lesions, and 6 (13%) of the 46 eyes had more retinal than choroidal lesions [Figure 5].
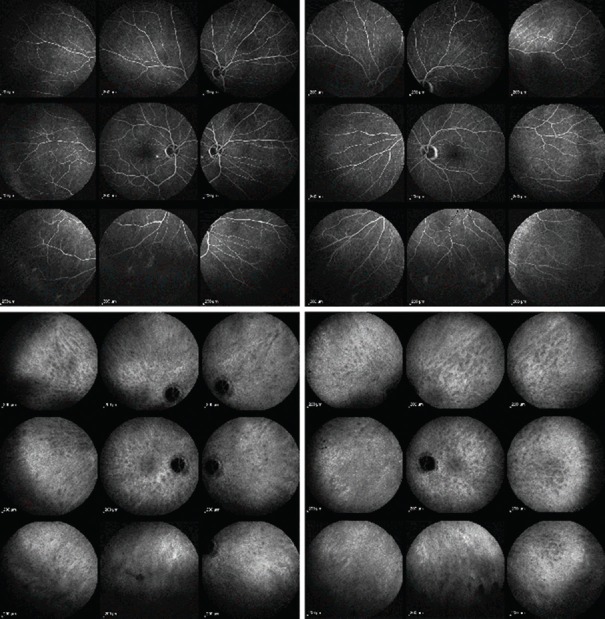
Figure 3.
Fluorescein and indocyanine green angiographic images of a 66-year-old man with ocular sarcoidosis showing predominant choroidal involvement. Fluorescein angiography (intermediate angiographic phase) showing macroaneurysms in the right eye (top left) with ocular sarcoidosis and appearing normal in the left eye (top right). The fluorescein angiographic score was 1 in the right eye and 0 in the left eye. Indocyanine green angiography (late phase) showing both eyes (bottom right and left) had numerous hypofluorescent dark dots, indicating presence of occult granulomas that cannot be seen on fundus imaging and fluorescein angiography. The indocyanine green angiographic score was 20 in both eyes.
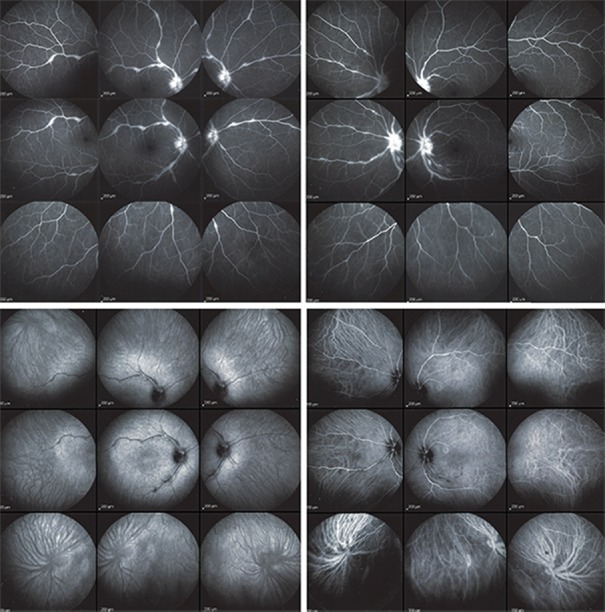
Figure 4.
Fluorescein and indocyanine green angiographic images of a 43-year-old woman with ocular sarcoidosis showing predominant retinal involvement. Fluorescein angiography (intermediate angiographic phase) showing optic disc hyperfluorescence and retinal vascular staining and leakage in both eyes (top right and left). The fluorescein angiographic score was 7 in the right eye and 6.5 in the left eye. Indocyanine green angiography (late phase) showing both eyes (bottom right and left) had small hypofluorescent dark areas along the retinal vessel producing a mask effect. The indocyanine green angiographic score was 2 in both eyes.
Figure 5.
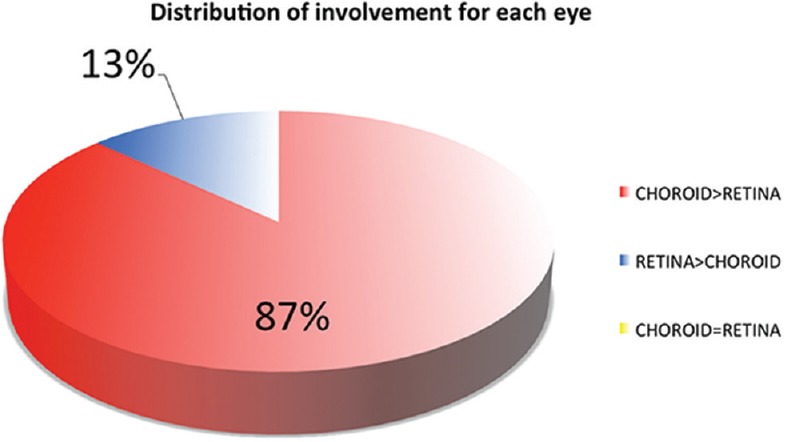
Pattern of involvement of each eye in sarcoidosis-related chorioretinitis. Forty (87%) of the 46 eyes had more choroidal than retinal lesions, and 6 (13%) of the 46 eyes had more retinal than choroidal lesions.
Eight eyes had negative FA scores (<3 points) but positive ICGA scores. In 2 patients, the score was negative in one eye. In 3 patients, the FA score was negative in both eyes. On the other hand, both eyes of one patient had negative ICGA scores and positive FA scores. Consequently, the number of patients with chorioretinitis (and a diagnosis) that would have been missed if only FA had been performed was 3 (11.5%) among the 26. If only ICGA had been performed, the number of undiagnosed cases would have been 1 (3.8%). When the mean angiographic scores were considered, it was also noted that choroidal involvement was significantly more prevalent than retinal involvement (14.02 ± 4.86 versus 7.15 ± 4.52 [P < 0.0001]) [Figure 6]. As described previously, we verified that lesions in both compartments developed independently and at random.[8]
Figure 6.
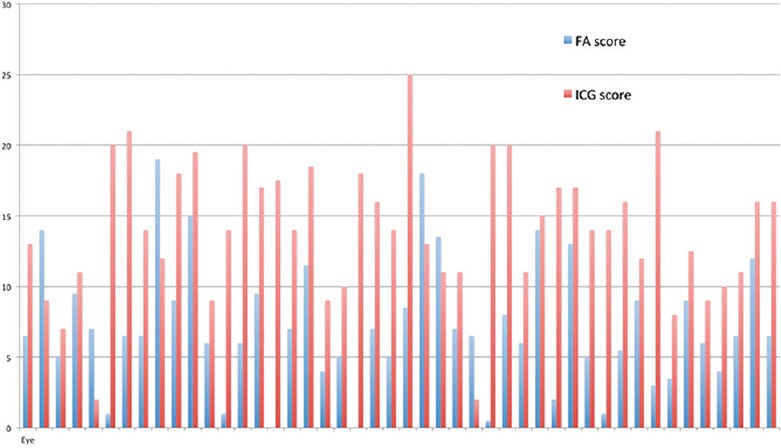
Bar graph showing pattern of eye involvement for each patient with sarcoidosis-related chorioretinitis at the time of diagnosis. Choroidal involvement (ICGA score) was significantly more prevalent than retinal involvement (FA score).
The most frequent angiographic signs were retinal vessel staining/leakage, capillary leakage, cystoid macular edema, and disc hyperfluorescence for FA. For ICGA, the two most frequent signs were fuzzy choroidal vessels and hypofluorescent dark dots (HDD).
Using the SUN criteria for vitritis, 80.4% (37/46) of the eyes had a vitreous haze score less than 1 [Figure 7]. Of the remaining 9 eyes, the vitreous score was 1 in 4 eyes, 2 in 4 eyes, and 3 in 1 eye.
Figure 7.
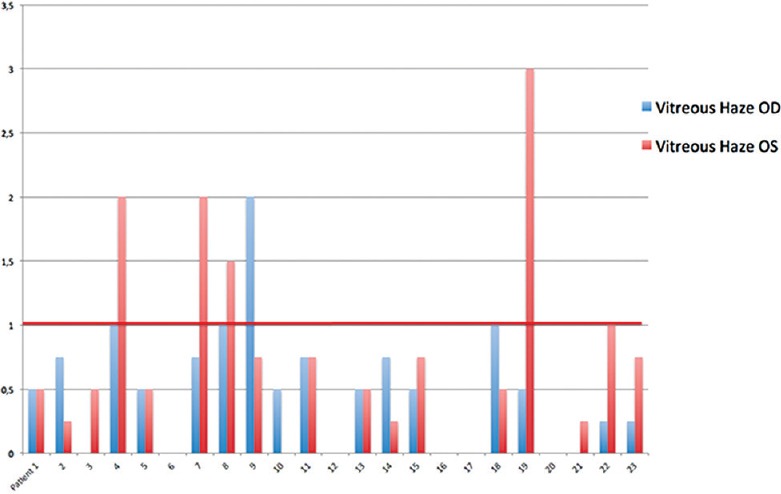
Bar graph showing vitreous haze at the time of diagnosis for each patient using Standardization of Uveitis Nomenclature scale. 80.4% (37/46) of the eyes had a vitreous haze score less than 1.
DISCUSSION
In this study, we performed an assessment of sarcoidosis-related chorioretinitis using dual fluorescein angiography/indocyanine green angiography (FA/ICGA). The main purpose was to compare between the involvement of the retina and that of the choroid. For the first time, we have determined specifically and objectively the amount of inflammatory angiographic lesions in both the retina and the choroid using the well-established dual FA/ICGA angiographic protocol and scoring system.[3,4] Furthermore, we determined the proportion of cases that were diagnosed with sarcoidosis-related chorioretinitis based on the criteria described by the first IWOS.[1] We showed that sarcoidosis-related chorioretinitis involves both the retinal and the choroidal structures independently, and lesions in one compartment are not a consequence of the lesions in the other compartment. This indicates that, unlike in Vogt-Koyanagi-Harada disease – where the choroidal stroma is selectively and exclusively generating inflammation with lesions in the other structures being the consequence of a spill-over of the choroidal inflammation[9,10]– any eye structure can be involved at random in ocular sarcoidosis; the ocular structures are innocent bystanders in which the systemic disease can produce lesions. The dual FA/ICGA angiographic scoring system also showed that choroiditis was predominant over retinitis, both in terms of intensity (14.00 versus 7.15) and proportion of cases (87%) affected. This indicates that ocular involvement can be underestimated and a diagnosis can be missed if only FA is performed without ICGA. Indeed, in three cases, both eyes had an FA score <3 (background signs), whereas the ICGA scores were 20, 20, 14, 14, 15, and 18; this meant that these patients would have been missed if only FA had been performed. If one type of angiography had been chosen the false-negative rate would have been twice less for ICGA than for FA.
In 2005, the SUN Working Group[7] proposed an adoption of universal criteria in reporting results to the Food and Drug Administration (FDA) and for use by pharmaceutical companies in designing study protocols. However, the Working Group did not integrate the new quantitative measurement methods. For posterior segment uveitis, the Working Group “rejuvenated” the 30-year-old clinical evaluation of vitreous haze (VH) (a subjective method comparing VH to a series of graded photographs [0-4]) as the main outcome measure for posterior uveitis trials.[11] Only 19.6% (9/46) of the eyes (haze ≥1) in the current study would have been included in a posterior uveitis trial based on the SUN/FDA criteria [Figure 7]. This outcome measure is very subjective, on one hand, and would exclude a substantial number of patients with sarcoidosis-related chorioretinitis, on the other hand. In contrast, dual FA/ICGA angiographic assessment provides the best global information, and it is probably the best modality to reach a diagnosis and to monitor disease progression or treatment. Indeed, using FA/ICGA allows one to obtain global information on chorioretinal inflammation at each time point. Moreover, it is much easier to study the evolution of the disease by using this combined score because the degree of inflammation is quantified, which, in addition to VA, IOP and flare, can be used to detect the improvement of the chorioretinal lesions. We could identify the details of each type of a lesion and determine its frequency in sarcoidosis-related chorioretinitis. In a recent study using dual FA/ICGA, similar findings were reported in patients with tuberculous chorioretinitis. The proportion of eyes with predominant choroidal involvement was 72% (39/54).[12] In contrast, a recent study in patients with Behçet's uveitis using the dual FA/ICGA scoring system showed that choroidal involvement (ICGA score) was insignificant and retinal involvement was highly preponderant over choroidal involvement in 100% (56/56) of the eyes.[13] This shows that FA/ICGA scoring is probably the best modality to date to evaluate and monitor posterior uveitis in practice and in clinical studies because it allows global and quantitative evaluation of inflammation in posterior uveitis.
In conclusion, this study assessed and showed for the first time the involvement of the retina and the choroid in sarcoidosis-related chorioretinitis. The choroid was preferentially involved, indicating that ICGA is the investigation of choice. There is a risk of underestimating the global ocular involvement and of missing the diagnosis if only FA is used. To evaluate correctly intraocular inflammation in sarcoidosis-related chorioretinitis and to conduct a better follow-up, the use of dual FA/ICGA is recommended.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Herbort CP, Rao NA, Mochizuki M. Members of Scientific Committee of First International Workshop on Ocular Sarcoidosis. International criteria for the diagnosis of ocular sarcoidosis: Results of the first international workshop on ocular sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17:160–169. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 2.Rothova A. Ocular involvement in sarcoidosis. Br J Ophthalmol. 2000;84:110–116. doi: 10.1136/bjo.84.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tugal-Tutkun I, Herbort CP, Khairallah M Angiography Scoring for Uveitis Working Group (ASUWOG) Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis) Int Ophthalmol. 2010;30:539–552. doi: 10.1007/s10792-008-9263-x. [DOI] [PubMed] [Google Scholar]
- 4.Tugal-Tutkun I, Herbort CP, Khairallah M, Mantovani A. Interobserver agreement in scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation. Ocul Immunol Inflamm. 2010;18:385–389. doi: 10.3109/09273948.2010.489730. [DOI] [PubMed] [Google Scholar]
- 5.Berthoud Kündig JF, Keller A, Herbort CP. Increase in polyclonal immunoglobulins: A possible useful aid in diagnosis of uveitis caused by sarcoidosis. Klin Monbl Augenheilkd. 1994;204:323–329. [PubMed] [Google Scholar]
- 6.Herbort CP, LeHoang P, Guex-Crosier Y. Schematic interpretation of indocyanine green angiography in posterior uveitis using a standard angiographic protocol. Ophthalmology. 1998;105:432–440. doi: 10.1016/S0161-6420(98)93024-X. [DOI] [PubMed] [Google Scholar]
- 7.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbort CP. Precise monitoring and differentiation of inflammatory events by indocyanine green (ICG) angiography in a case of recurrent posterior sarcoid uveitis. Ocul Immunol Inflamm. 2000;8:303–306. doi: 10.1076/ocii.8.4.303.6458. [DOI] [PubMed] [Google Scholar]
- 9.Bouchenaki N, Herbort CP. The contribution of indocyanine green angiography to the appraisal and management of Vogt-Koyanagi-Harada disease. Ophthalmology. 2001;108:54–64. doi: 10.1016/s0161-6420(00)00428-0. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi T, Horie S, Bouchenaki N, Ohno-Matsui K, Mochizuki M, Herbort CP, et al. Suboptimal therapy controls clinically apparent disease but not subclinical progression of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2010;30:41–50. doi: 10.1007/s10792-008-9288-1. [DOI] [PubMed] [Google Scholar]
- 11.Herbort CP, Jr, Tugal-Tutkun I, Neri P, Pavésio C, Onal S, LeHoang P. Failure to integrate quantitative measurement methods of ocular inflammation hampers clinical practice and trials on new therapies for posterior uveitis. J Ocul Pharmacol Ther. 2017;33(4):263–277. doi: 10.1089/jop.2016.0089. [DOI] [PubMed] [Google Scholar]
- 12.Massy R, Herbort CP. Contribution of dual fluorescein and indocyanine green angiography to the appraisal of presumed tuberculous chorioretinitis in a non-endemic area. J Ophthalmic Vis Res. 2017;12:30–38. doi: 10.4103/2008-322X.200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onal S, Uludag G, Oray M, Mengi E, Herbort CP, Akman M, et al. Quantitative analysis of structural alterations in the choroid of patients with active Behçet uveitis. Retina. 2018;38:828–840. doi: 10.1097/IAE.0000000000001587. [DOI] [PubMed] [Google Scholar]