Abstract
The initial studies of the metabolism of arachidonic acid (AA) by the cytochrome P450 (P450) hemeproteins sought to: a) elucidate the roles for these enzymes in the metabolism of endogenous pools of the FA, b) identify the P450 isoforms involved in AA epoxidation and ω/ω-1 hydroxylation, and c) explore the biological activities of their metabolites. These early investigations provided a foundation for subsequent efforts to establish the physiological relevance of the AA monooxygenase and its contributions to the pathophysiology of, for example, cancer, diabetes, hypertension, inflammation, nociception, and vascular disease. This retrospective analyzes the history of some of these efforts, with emphasis on genetic studies that identified roles for the murine Cyp4a and Cyp2c genes in renal and vascular physiology and the pathophysiology of hypertension and cancer. Wide-ranging investigations by laboratories worldwide, including the authors, have established a better appreciation of the enzymology, genetics, and physiologic roles for what is now known as the third branch of the AA cascade. Combined with the development of analytical and pharmacological tools, including robust synthetic agonists and antagonists of the major metabolites, we stand at the threshold of novel therapeutic approaches for the treatment of renal injury, pain, hypertension, and heart disease.
Keywords: eicosanoids, diseases, genetics, kidney, endothelial cells, PPAR
A BRIEF HISTORIC PERSPECTIVE
The pursuit of science is a journey with many destinations
Our exploration of roles for the cytochrome P450 (P450) enzyme system in arachidonic acid (AA) metabolism and bioactivation became part of an exciting, challenging, and rewarding journey that allowed us to pursue goals that at times seemed unrealistic and/or nearly impossible, but with the help and example of many collaborators contributed to our growth as scientists and individuals. Our journey started in 1981 with reports by others and us that microsomal and purified forms of P450 catalyzed the NADPH-dependent oxidation of AA to hydroxy-AA, dihydroxy-AA (1–3), and epoxy-AA products (4). These initial observations were judged by some in the P450 and AA fields to be another example of the catalytic versatility of the P450 enzyme system and, consequently, of limited and questionable physiological relevance and not worth pursuing. For others, the structural simplicity of the metabolites generated by the P450 enzymes was yet more reason to dismiss them as inconsequential. However, the established importance of AA as biosynthetic precursor for several mediators of cell, organ, and body physiology enticed us to explore the functional significance of these novel P450 catalyzed reactions. With the help and know-how of colleagues in the Departments of Physiology and Medicine at University of Texas Health Science Center at Dallas (UTHSCD) (now UT Southwestern), we initiated studies of the potential biological significance of the P450-derived eicosanoids soon after their structural characterization and syntheses. These initial efforts identified the epoxyeicosatrienoic acids (EETs) as potential participants in Na+ and K+ transport by the distal nephron (5), the release of Ca2+ from intracellular stores (5), and the secretion of peptide hormones from cell and tissue preparations (5). These data provided a foundation to subsequent investigations of the involvement of the P450 AA monooxygenase (AA monooxygenase) metabolites in vascular and renal physiology. Thanks to the recognition of the novelty and potential importance of these studies by the staff and reviewers at the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of General Medical Sciences, critical financial support was provided to our laboratories to initiate the chemical, biochemical, and functional characterizations of the AA monooxygenase pathway. It is worth mentioning that the NIH funded the pursuit of a novel hypothesis that, at the time, had limited supporting preliminary data and was not widely accepted. One can only speculate if that would be the case today.
As we initiated these studies, priority was placed upon substantiating whether: a) the AA monooxygenase participated in the metabolism of endogenous pools of AA, i.e., it is a formal metabolic pathway; b) its metabolites were biologically active; and c) the enzymes and products of the AA monooxygenase were physiologically and/or pathophysiologically relevant. To achieve these goals, we focused first on completing the structural and stereochemical elucidation of all P450-derived primary AA metabolites; second, developing methods for their total synthesis and their identification and quantification in biological samples; and third, initiating wide-ranging analyses of their potential biological activities. With evidence of biological significance, we would then proceed to the identification of functionally relevant P450 isoforms, the characterization of their enzymatic properties, organ/tissue expression and regulation, roles in animal models of genetically determined organ dysfunction, and, ultimately, their physiological/pathophysiological relevance and potential biomedical significance. Overall progress in this field of studies has followed more or less the above sequence, with work from now many different laboratories worldwide contributing to the identification of new functionalities for AA monooxygenase ranging from, for example, hypertension, vascular diseases, cancer, diabetes, inflammation, to nociception.
It is not our intent in this retrospective to be comprehensive; therefore, the reader is referred to several excellent in-depth reviews of the pertinent literature (5–16). Instead, we provide a more or less temporal sequence of events and a personal view of the crucial observations and concepts, especially in renal and vascular physiology/pathophysiology, that helped bring this area of study to its present status as the third branch of the AA cascade. Along the way, we reflect upon almost four decades of our own studies while recognizing the many pioneering and creative contributions from generous and supportive scientists, including Drs. John C. McGiff, Michal L. Schwartzman, Richard Roman, David Harder, John Imig, William Campbell, and Wen-Hui Wang and associates. Their efforts were instrumental in the identification of functional roles for the P450 AA metabolites and in the establishment of the AA monooxygenase as a physiologically and pathophysiologically relevant metabolic pathway.
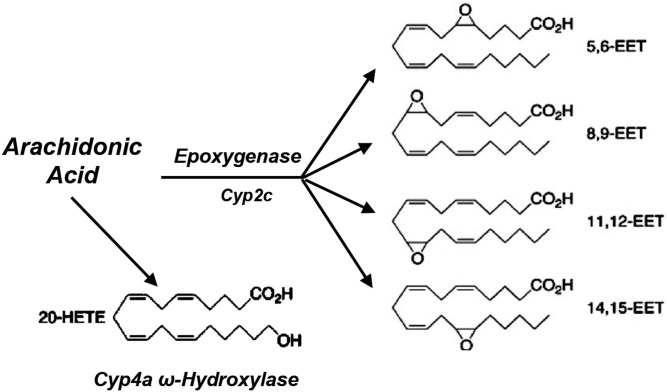
The P450 gene superfamily codes for an extensive group of catalytic heterogeneous hemeproteins that, based on amino acid sequence identities, are organized into families and subfamilies (≥40% and ≥60% identity, respectively), with approximately 57 genes identified in humans (17). As typical monooxygenases, P450s catalyze the insertion of an oxygen atom from molecular oxygen into their substrates and the release of the other oxygen atom as water. Over the last 60 years, these enzymes have been the subjects of intensive studies that led to the establishment of their toxicological and pharmacological importance (17). However, the recognition that P450 was competent to metabolize AA stimulated studies of its functional roles and relevance to human physiology and pathophysiology. Our interest in the participation of P450 in the oxidation of AA was an unanticipated outcome of experiments investigating the involvement of endogenous FA hydroperoxides as oxygen donors during the oxidation of polyaromatic hydrocarbons by microsomal fractions. While searching for precursors for these putative lipid hydroperoxides, with Nicholas Chacos, a UTHSCD graduate student, we incubated rat liver microsomes with radiolabeled AA in the presence of NADPH. Chromatographic analyses of the resulting organic extracts revealed that liver P450s metabolized AA, in an NADPH-dependent fashion, to oxygenated products that included hydroxy- and epoxy-AA metabolites (1, 4). While debating whether to continue with these studies, we started a productive and long-standing collaboration with Dr. John R. Falck, a member of the UTHSC faculty. John shared with us his knowledge of the chemistry and biological significance of the many products of AA cascade and was instrumental in convincing us to pursue studies of roles for the P450 enzymes in the metabolism and bioactivation of AA. The ensuing structural characterization of metabolites generated by microsomal and purified P450 isoforms showed that the AA monooxygenase (Fig. 1) consisted of two major branches: a) the AA ω/ω-1-hydroxylase that catalyzes mostly hydroxylations at or near the AA terminal ω and ω-1 carbons to yield 20-HETE and 19-HETE (5), and b) the AA epoxygenase that epoxidizes AA to generate the 5,6-; 8,9-; 11,12-; and 14,15-EETs (5). Subsequently, we demonstrated that EETs could be enzymatically hydrated by the cytosolic form of soluble epoxide hydrolase (sEH) to furnish the corresponding vic-dihydroxyeicosatrienoic acids or dihydroxyeicosatrienoic acids (DHETs) (18), initially identified by E. H. Oliw and J. A. Oates in their 1981 report (2).
Fig. 1.
The AA monooxygenase.
METABOLITE CHARACTERIZATION, CHEMICAL SYNTHESIS, AND ENZYMOLOGY
The synthesis of homochiral and racemic standards of 5,6-; 8,9-; 11,12-; and 14,15-EET (19–21), 19- and 20-HETE (22), and 5,6-; 8,9-; 11,12-; and 14,15-DHET (23) was followed by the rapid development of methods for their chromatographic resolution (5), stereochemical analysis (24), ELISA (25), and mass spectral identification and quantification (5). The availability of synthetic metabolites opened the door to wide-ranging interrogations of their biological activities and the characterization of P450 isoform-dependent regio and/or stereo-selective AA metabolism (5). Abundant enzymatic, biochemical, immunological, genetic, and functional data identified P450s of the CYP2 and CYP4 families as the predominant and functionally relevant AA epoxygenases and ω/ω-1 hydroxylases, respectively, in most organ tissues (5–7, 10, 11).
The CYP4A enzymes show extensive functional and sequence homology across species and are high selectivity toward the ω/ω-1 hydroxylation of FAs (26). The ω-hydroxylation of AA to 20-HETE by CYP4A isoforms is common to several organs (5, 6, 10, 14, 27–32); however, it is in kidney where it is prevalent and has been assigned important functions (6, 7, 10, 14). Several members of the CYP4F subfamily are known to catalyze ω/ω-1 hydroxylation of AA as well as the metabolism of several eicosanoids and drugs (6, 10, 14, 33). A predominant role for human CYP4F2 in renal 20-HETE biosynthesis has been proposed (29). Table 1 shows a summary of CYP4 proteins so far characterized as 20-HETE synthases.
TABLE 1.
Members of the CYP4 and CYP2 subfamilies characterized as 20-HETE and EET synthases, respectively, in human and rodent tissues
| 20-HETE Synthases | EET Synthases | |||
| CYP4A | CYP4F | CYP2C | CYP2J | |
| Human | 4A11 | 4F2 | 2C8,2C9 | 2J2 |
| Rat | 4A1,4A2,4A8 | 4F1,4F4 | 2C11,2C23 | 2J4 |
| Mouse | 4a12s,4a12b | 4f14,4f15 | 2c29,2c44 | 2j3 |
Members of the CYP2C and CYP2J subfamilies are recognized as the major AA epoxygenases in organs such as liver, kidney, lung, brain, and vascular endothelium (5–9, 11–15) (Table 1). Rat CYP2C23 and mouse Cyp2c44, the predominant AA epoxygenases in the rat and mouse kidney and endothelium (11, 35–37), are unique in that: a) they display high interspecies amino acid sequence identity, but limited intraspecies homology toward other CYP2C subfamily members; b) they epoxidize AA with high regio and stereoselectivity (5, 35); and c) their kidney expression is regulated by dietary salt (5, 7, 11). Human CYP2C8 and CYP2C9 were recognized as renal and endothelial epoxygenases, respectively (36, 37), and shown to participate in vascular relaxation (5–9, 12). Finally, there is genetic evidence for the participation of Cyp2j5 in estrogen metabolism, but not AA epoxidation (38).
BIOLOGICAL SIGNIFICANCE
The AA monooxygenase: a member of the endogenous AA metabolic cascade
Because asymmetric synthesis is a hallmark of biosynthetic origin, the identification of endogenous enantiomerically enriched (scalemic) EETs in rodent and human organs, plasma, and urine (5) established the AA epoxygenase as a formal metabolic pathway. Studies of the effects of P450 inducers on the levels and properties of the endogenous EETs confirmed a role for P450 in their biosynthesis (5). During these early studies, we learned that better than 90% of the EETs in most plasma and organ tissues were present esterified to the sn-2 position of glycerophospholipids (5, 39). The fact that these EET-phospholipids are biosynthesized from endogenous precursors and under physiological conditions (39) suggested that they could serve as an intracellular reservoir of EETs that could be released upon hydrolysis of existing EET-phospholipids (5, 11). Based on sEH bias toward the hydration of the EET enantiomers present in liver and kidney, we proposed a role for the enzyme in the in vivo hydration of endogenous EETs (40). Since these early studies, Dr. Bruce Hammock and collaborators recognized the potential that sEH inhibition had as a maneuver to control cellular EET levels and function and identified the enzyme as a promising target for drug development (16).
The detection and quantification of endogenously generated 20-HETE was facilitated by the properties of the AA molecular template, in which oxidations at its less thermodynamically reactive C17 through C20 carbons, with segregation of its more reactive olefins and bis-allylic methylene carbons, are necessarily enzymatic and not artifactual. The urine excretion of 20-HETE as a glucuronic acid conjugate was demonstrated (41), a fact shown to be the case in nearly all urine samples so far analyzed (5, 6, 10).
The metabolites of the AA monooxygenase are bioactive
Because of the limited availability of the AA monooxygenase metabolites from natural sources, most of the studies of their biological activities utilized synthetic metabolites, enzyme inhibitors, and EET or 20-HETE functional analogs or antagonists. Later, advances in the characterization of specific EET and 20-HETE synthases and of cDNA transfection techniques allowed for studies of transgene/promoter-mediated changes in enzyme expression, product biosynthesis, and functional responses. From these efforts, the number of biological activities ascribed to the AA monooxygenase has steadily grown to include, among other things, vascular, renal, cerebral, and pulmonary function (5–15). As mentioned, we highlight here a few studies that, in our view, contributed to the recognition of the AA monooxygenase as an important source of lipidic autacoids.
In our early search for ways to determine whether the AA monooxygenase pathway had biological significance, we thought that because metabolites of AA such as prostanoids were known to play functional roles in kidney physiology, the organ appeared as a reasonable choice to initiate these studies. Soon after synthetic EETs became available, Dr. Harry Jacobson, a member of the UTHSCD Nephrology Division, showed that 5,6- and 14,15-EET inhibit Na+ and K+ transport when added to the lumen of isolated perfused rabbit cortical collecting tubules (5). Although at that moment, the functional significance of these results was not obvious to us, it did provide important experimental support that: a) helped us to secure funding from the NIDDK Institute for a program project grant that supported our studies uninterruptedly from 1985 through 2014, b) was instrumental in moving the AA monooxygenase from a biochemical curiosity to a functionally significant metabolic pathway, and c) identified renal physiology as a subject of efforts to characterize the physiological/pathophysiological significance of the P450-mediated pathway of AA bioactivation.
Subsequent studies by different investigators have identified functional roles for the EETs in cell Ca2+ mobilization, renal Na+ excretion, nociception, epidermal growth factor (EGF) signaling, mitogenic kinase activation, cell replication, vascular endothelial growth factor (VEGF)-stimulated angiogenesis, NFkB subunit signaling, tumorigenesis ,and metastatic growth (5–15, 36, 42–44). The EETs were first identified as vasoactive lipids in 1987 by groups associated with Drs. Kenneth G. Proctor (University of Tennessee) (45) and John McGiff (New York Medical College) (46). Soon after these seminal reports, the vasoactive properties of the EETs were linked to the activation of vascular smooth muscle calcium-sensitive K+ channels (6, 8, 47, 48). Subsequently, in 1996, William B. Campbell, PhD, and collaborators identified the 11,12- and 14,15-EET generated by the vascular endothelium, as endothelium-derived hyperpolarizing factors (EDHFs) by showing that they activated vascular smooth muscle cell calcium-sensitive 256 pS maxi-K channels (BKCa), resulting in cell hyperpolarization and vasodilation (49). Collectively, these studies provided a mechanistic explanation for the vasoactive properties of the epoxygenase metabolites. Since then, the EETs have been shown to mediate vasodilation by mediators such as bradykinin, acetylcholine, and adenosine (6–8, 12, 14).
Our initial, and perhaps somewhat naïve, forays into pharmacology/physiology had a steep learning curve. William (Bill) Campbell, who at the time was on the faculty at UTHSCD and already a prominent authority on eicosanoids and vasoactive compounds, offered to evaluate the EETs in his bovine aortic ring assay. We gathered in Bill’s lab one evening and gave him a 10 μM solution of synthetic 14,15-EET to test its vasoactive properties. To everyone’s delight, the pen on the chart recorder registered maximum relaxation almost immediately. The sample was returned to the chemistry student who had prepared it with instructions to dilute the sample 10-fold. Again, a maximum response, and again the sample was diluted another 10-fold. Following many more rounds with the same result, it was now late evening, and Bill finally asked the chemist, “What are you using as the solvent?” Benzene came as the answer. By then it was nearly midnight, so all we could do was laugh and go home. Although this experiment was a failure, it was repeated properly and successfully a few days later. Together with additional studies, a few years later, Bill formulated his now widely accepted hypothesis that EETs are EDHFs in some mammalian species, including man.
Multiple functional roles are reported for 20-HETE (6, 10, 14), including regulation of renal Na+/K+ ATPase activity (10), Ca2+ and Cl- fluxes (6, 10, 14), vascular remodeling (10), and tumor metastasis (50), as well as hormonal signaling by vasopressin, angiotensin, norepinephrine, EGF, and VEGF (6, 10, 12, 14). Recent studies identified: a) GPR75, a G-protein coupled receptor (Gq), as the 20-HETE receptor that mediates its effects on vascular function and blood pressure (BP) control (51); and b) the long-chain FA receptor one (FFAR1) that, upon 20-HETE binding, mediates autocrine glucose-stimulated insulin secretion by pancreatic β-cells (52). Consistent with the often-opposing biological properties of 20-HETE and EETs, 20-HETE: i) is a vasoconstrictor of small arterioles, including the cerebral and renal microcirculation, and mediates the activity of prohypertensive hormones (6, 10, 14); and ii) modulates renal and cerebral microcirculation responses to perfusion pressure in vitro and blood flow in vivo (9, 10, 14, 15). Furthermore, at difference with the EETs, 20-HETE is formed by vascular smooth muscle cells and functions as anti-EDHF by inhibiting BKCa and triggering cell depolarization and vasoconstriction (10, 14, 53); effects have been attributed to binding to its GPR75 receptor (51). Summarizing, EETs and 20-HETE can dilate or constrict the brain and kidney microcirculation, respectively, alter vascular resistance and blood flow and, in the kidney glomerular filtration rates, and Na+ excretion (10, 14).
PHYSIOLOGICAL/PATHOPHYSIOLOGICAL SIGNIFICANCE
The initial biological activities ascribed to the enzymes and products of the AA monooxygenase provided important information regarding their potential functional roles; however, due to their mostly in vitro and descriptive nature, their physiological and/or pathophysiological relevance remained uncertain. Many of the early functional studies targeted the kidney because it shows significant CYP4A and CYP2C expression (5–7, 10, 14) and synthesizes EETs and 20-HETE in vivo (5–7, 10, 14), and the transport and vascular effects of these eicosanoids suggested roles for them in BP regulation (6, 7, 10, 14). The proposal of a role for AA monooxygenase in the pathophysiology of experimental hypertension (54) was a decisive milestone that made possible studies of associations between genetically determined functional phenotypes and the expression and/or activity of individual P450s and inspired some of the most important advances in this area of research (6–15). From integrations of functional responses to P450 metabolites and correlations between their biosynthesis and the development of hypertension in rat models of genetically determined hypertension, pro hypertensive and antihypertensive roles were proposed for the CYP4A ω-hydroxylases and the CYP2C epoxygenases (7, 10, 11, 14). Since those early pioneering studies, the enzymes and products of AA monooxygenase have been implicated in the pathophysiology of diseases such as hypertension, cancer, and diabetes, as well as inflammation, ischemic reperfusion injury, and vascular dysfunction (6–15). We will focus primarily on studies of experimental hypertension and cancer, two diseases for which there is unequivocal genetic data supporting the involvement of specific CYP4A and CYP2C genes and their products.
The kidney AA monooxygenases and experimental hypertension
The kidneys play crucial roles in the control of plasma volume, and sodium concentrations and alterations in renal hemodynamics and/or Na+ transport are common features of prevalent forms of human hypertension (6, 10, 14). The renin-angiotensin-aldosterone and Kallikrein-Bradykinin hormonal systems are established regulators of the renal vasculature, pressure natriuresis, and systemic BP with tubular Na+ transport serving as the final effector of their roles in plasma Na+ and volume homeostasis (6, 7, 10, 14). Studies with the SHR/WKY rat model of genetically determined spontaneous hypertension led investigators at the New York Medical College (Valhalla, NY) to propose a role for kidney P450s in the pathophysiology of hypertension based on: a) temporal correlations between the onset of SHR hypertension and increases in renal CYP4A expression and 20-HETE biosynthesis (10, 54), b) the reno-vascular responses to 20-HETE administration (10, 14), c) the normotensive effects of SnCl2-mediated P450 depletion (54), and d) the pressure-lowering effects of CYP4A antisense nucleotide administration (10). This pioneering proposition stimulated broad investigations into the associations between BP phenotypes and structural and/or regulatory changes in P450 gene(s) function and/or expression. For instance, in the Dahl rat model of genetically determined salt sensitive hypertension, the CYP4A2 20-HETE synthase was assigned antihypertensive roles based upon: a) the in vitro natriuretic properties of 20-HETE (10, 14), b) inhibitor studies (6, 14), and c) differences between Dahl salt-sensitive (SS) and salt-resistant (SR) rats in CYP4A2 expression and 20-HETE biosynthesis (6, 14). Alternatively, antihypertensive roles for the kidney CYP2C23 epoxygenase and the EETs were proposed based on the facts that: a) CYP2C23 and urinary EET excretion are upregulated by excess dietary salt intake (7); b) inhibition of the CYP2C23 epoxygenase reduces urinary EET excretion and causes SS hypertension (7); c) Dahl SR rats on a high-salt (HS) diet increase renal EET biosynthesis and remain normotensive, whereas SS animals fail to do so and become hypertensive (7); and d) the identification of 11,12-EET as a selective inhibitor of the kidney epithelial sodium channel (ENaC) gating and sodium reabsorption in rat collecting ducts (CDs) (55). However, the multigenic and complex nature of the SHR and Dahl SS pressure phenotypes precluded unequivocal identifications of the role(s) played by distinct P450 genes and/or proteins in BP control. Advancements in gene-targeting techniques led us to develop mouse lines carrying globally disrupted copies of the Cyp4a14 (56), Cyp4a10 (57), and Cyp2c44 (58) genes, a line of Cyp2c44 CD conditional KOs as models of P450 dysfunction (59), as well as transgenic mice overexpressing the Cyp4a12a 20-HETE synthase (60). Given the complexity of the P450 gene superfamily, selecting a functionally meaningful gene for disruption carried a degree of uncertainty that we narrowed by initiating these efforts with the Cyp4a mouse subfamily based on: 1) the identification of rat CYP4A2 as prohypertensive (10, 14), 2) the subfamily evolutionary conservation and selectivity toward FA hydroxylation (26), and 3) its functional specialization as illustrated by the fact that the human genome contains two CYP4A genes, but only one is catalytically active (11, 28). During the characterization of the Cyp4a gene cluster, we identified two Cyp4a12 genes with high genomic sequence identity and, after several attempts, failed to generate a line of Cyp4a12(−/−) mice. Subsequently, the proteins encoded by these genes were identified as Cyp4a12a and Cyp4a12b (31). The hypertensive phenotype of Cyp4a10(−/−) (57) mice simplified the choice of the Cyp2c44 gene for disruption (58).
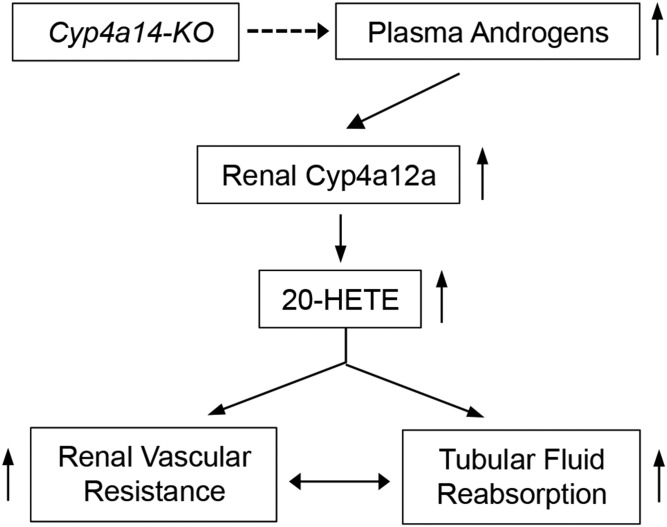
The Cyp4a14 gene and androgen-sensitive hypertension.
Disruption of the Cyp4a14 gene (4a14-KO) causes a type of hypertension that is male-specific and associated with increases in plasma androgens, the kidney expression of Cyp4a12a, and urinary 20-HETE (Fig. 2) (11, 56). Castration lowers renal Cyp4a12a expression and normalizes the pressures of hypertensive 4a14-KO mice, whereas the administration of androgens to castrated 4a14-KO mice restores Cyp4a12a expression and the hypertensive phenotype (11, 56). Furthermore, androgen administration raises the BPs of female mice, regardless of their Cyp4a14 genotype (11, 56). Male 4a14-KO mice showed increased renal afferent arteriole resistance and a compromised microvascular autoregulatory capacity, implicating alterations in renal hemodynamics (11, 56) and proximal tubule fluid reabsorption (Fig. 2) (61) as determining factors of the animal’s hypertensive phenotype. The demonstration that Cyp4a12a transgenic mice with increased kidney Cyp4a12a expression and 20-HETE biosynthesis show androgen-independent hypertension (10, 60) confirmed that the pressure phenotype of 4a14-KO mice was 20-HETE-dependent and not a direct result of changes in plasma androgen levels. Furthermore, 20-HETE receptor antagonists (51) reversed the hypertensive phenotypes of 4a14-KO and Cyp4a12a transgenic mice (60). Of special interest is the fact that Cyp4a14 does not metabolize AA or androgens to measurable extents (11, 31, 56); hence, the pressure effects of the Cyp4a14 gene are mediated, indirectly, by the upregulated expression of the renal Cyp4a12a 20-HETE synthase (Fig. 2) (10, 11, 56). The hypertensive phenotype of male 4a14-KO provided the first unequivocal evidence of a role for a P450 gene in the regulation of systemic BPs, as well as a yet-to-be-characterized role for Cyp4a14 gene product(s) in the control of plasma androgens. It is of interest that our initial identification of the sexually dimorphic hypertensive phenotype of 4a14-KO mice was more or less fortuitous and resulted from a colleague’s request to use our 4a14-KOs to check his BP measurement instrument. After several attempts, he reported back that the animals were not as hypertensive as we thought. Because at that moment we didn’t have enough males to spare, we had instead given him a few females, which, as we latter corroborated, were not hypertensive.
Fig. 2.
The Cyp4a14 gene and the pathophysiology of sexually dimorphic hypertension (56). Arrows pointing up denote increases in expression or functional responses. The dashed arrow indicates that the Cyp4a14 gene regulates plasma androgens by unknown mechanism(s).
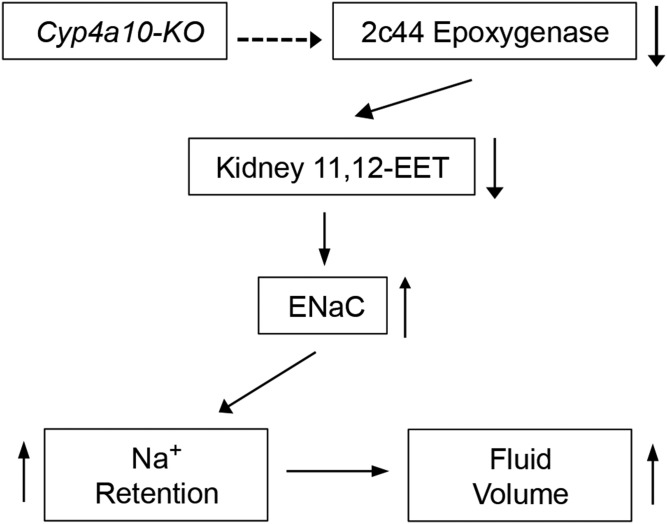
The Cyp4a10 gene and dietary SS hypertension.
Male and female Cyp4a10(−/−) (4a10-KO) mice on low-salt (LS) diets (0.05% NaCl) are normotensive, but become hypertensive when fed diets containing normal-salt (NS) (0.3% NaCl) or HS (8% NaCl) (57). At difference with 4a14-KO mice, disruption of the Cyp4a10 gene did not alter renal Cyp4a12a expression or 20-HETE biosynthesis, but, unexpectedly, led to reductions in renal Cyp2c44 expression and EET biosynthesis (Fig. 3) (57). In contrast with their negative effects on the liver and endothelial expression of CYP2C epoxygenases (36, 62), ligands to the PPARα increased kidney Cyp2c44 expression and EET biosynthesis and normalized the pressures of hypertensive 4a10-KO mice (57). Hypertensive 4a10-KO mice showed reductions in urine Na+ and K+ excretion and fluid retention (Fig. 3) (57), a phenotype reminiscent of early reports that EETs inhibited Na+ and K+ excretion by perfused rabbit CDs (5) and that 11,12-EET was a potent inhibitor of the renal ENaCs (55). Moreover, and as shown by Dr. WenHui Wang (Medical College of New York), patch clamp analyses confirmed the presence of a constitutively hyperactive ENaC in CDs dissected from 4a10-KO mice (57). The demonstration that amiloride inhibition of ENaC gating normalized the pressures of hypertensive 4a10-KO mice and that 11,12-EET, but not 20-HETE, inhibited ENaC gating showed that: a) a hyperactive ENaC was a cause of the animals hypertensive phenotype (11, 57), and b) deficiencies in EET-dependent ENaC inhibition and increased distal Na+ reabsorption mediate the SS hypertensive phenotype of 4a10-KO mice (Fig. 3).
Fig. 3.
The Cyp4a10 gene and the pathophysiology of SS hypertension (57). Arrows pointing up or down denote increases or reductions in expression or functional responses, respectively. The dashed arrow indicates that the Cyp4a10 gene regulates the SS expression of renal Cyp2c44 by unknown mechanisms.
The characterization of 4a14-KO and 4a10-KO mice corroborated the prohypertensive and antihypertensive responses attributed to members of the rat CYP4A subfamily (6, 14) and showed that the pressure effects of these genes were independent of the ability of their encoded proteins to metabolize AA, but, instead, rely on: a) upregulated kidney Cyp4a12a expression, increased 20-HETE synthesis, and renal vasoconstriction, or b) downregulated kidney Cyp2c44 epoxygenase expression, reduced EET synthesis, and increased distal Na+ reabsorption. The relevance of these CYP4A-mediated effects to human hypertension is evident in the observation that a T8590C variant of the CYP4A11 gene coding for a reduced activity 20-HETE synthase is associated with the prevalence of hypertension in humans (10, 28) and has been linked to SS hypertension (63).
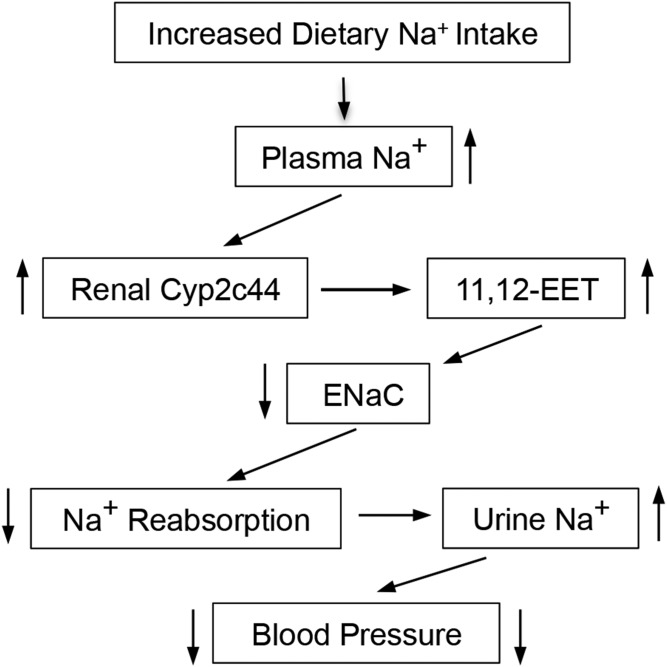
The Cyp2c44 gene and dietary SS hypertension.
The demonstration that mice carrying aglobally disrupted Cyp2c44 gene (2c44-KO) become hypertensive when fed HS diets provided unequivocal evidence of a role for the Cyp2c44 epoxygenase in BP control (58). Male and female WT and 2c44-KO mice on NS diets are normotensive. On the other hand, WT mice on HS diets remain essentially normotensive, but under similar conditions, 2c44-KO animals become severely hypertensive (58). Whereas WT animals on HS diets increase their urinary excretion of epoxygenase metabolites by nearly 3-fold, 2c44-KO mice, lacking a salt-inducible Cyp2c44 epoxygenase, are unable to do so, showing that their hypertensive phenotype is associated with reductions in EET biosynthesis (58). As with 4a10-KO mice, electrophysiology studies in dissected CDs demonstrated that 2c44-KO mice had a hyperactive ENaC and increased inward sodium currents (58). Normalization of the pressure of hypertensive 2c44-KO mice by the administration of amiloride or EET functional analogs showed that a lack of Cyp2c44 mediated EET biosynthesis, ENaC dysfunction, and increased distal Na+ reabsorption were a cause of the animals’ hypertensive phenotype (12, 58). Together, these studies identified a functional role for the Cyp2c44 epoxygenase in the control of plasma sodium concentrations and volume and for its 11,12-EET metabolite as an in vivo natriuretic lipid (Fig. 4) (58). Evidence that 11,12-EET inhibits ENaC gating regardless of mouse Cyp4a10 or Cyp2c44 genotype proved that disruption of these genes does not affect ENaC’s intrinsic properties, but rather its EET-mediated regulation (11). An examination of mechanism(s) by which EETs inhibited ENaC activity revealed that in WT animals, as is with EGF (64), the EETs stimulated the ERK1/2-catalyzed inhibitory threonine phosphorylation of the ENaC β and γ subunits (11, 58, 64). In contrast, EET-mediated ERK1/2 activation in 2c44-KO mice is impaired, leading to reductions in ENaCβ and ENaCγ phosphorylation and a hyperactive channel (11, 58). It has been reported that EETs mediate the activation of protein kinases such as phosphatidylinositol-3 kinase (PI-3K), ERK1/2, and the upstream protooncogene tyrosine kinase (c-Src), all known targets of EGF binding to its receptor (11, 65).
Fig. 4.
The renal Cyp2c44 is a dietary SS pronatriuretic and antihypertensive epoxygenase (58). Arrows pointing up or down denote increases or reductions in expression or functional responses, respectively.
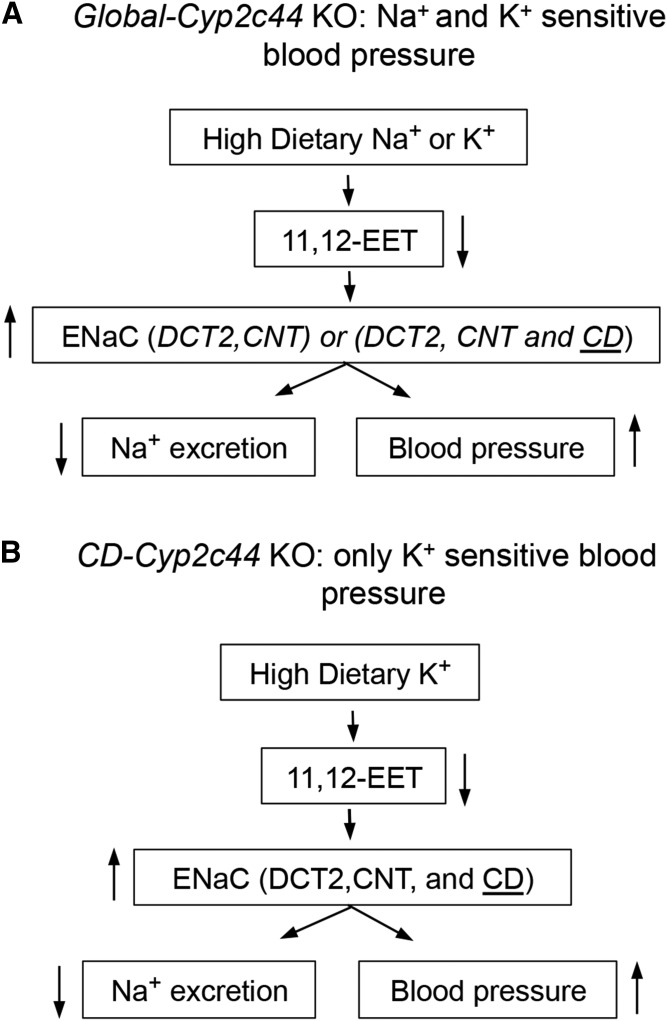
Apical Na+ and K+ transport by the principal cells of the aldosterone-sensitive distal nephron (ASDN) is compartmentalized along the distal convoluted (DCTs) (proximal DCT1 and distal DCT2) and connecting tubules (CNTs) and the CD, and mediated by ENaC, the outer medullary K+ (ROMK), and BKCa channels (7, 14, 59, 66–69). An interesting feature of the global 2c44-KO mice phenotype is that: a) as it is with HS diets, they become hypertensive when fed high-potassium (HK)-containing diets (2.5–4% KCl; 5–10 days) (Fig. 5) (70), b) show a hyperactive CD ENaC, and c) amiloride normalizes their BP when on a HK diet (70). All of the above points to ENaC dysfunction as a cause of the animals HK sensitive hypertensive phenotype (70).
Fig. 5.
The Cyp2c44 gene and dietary NaCI- and/or KCI-sensitive hypertension on mice carrying a Cyp2c44 gene disrupted globally (58) (A), or in the CD segment of the ASDN (59) (B). Arrows pointing up or down denote increases or reductions in expression or functional responses.
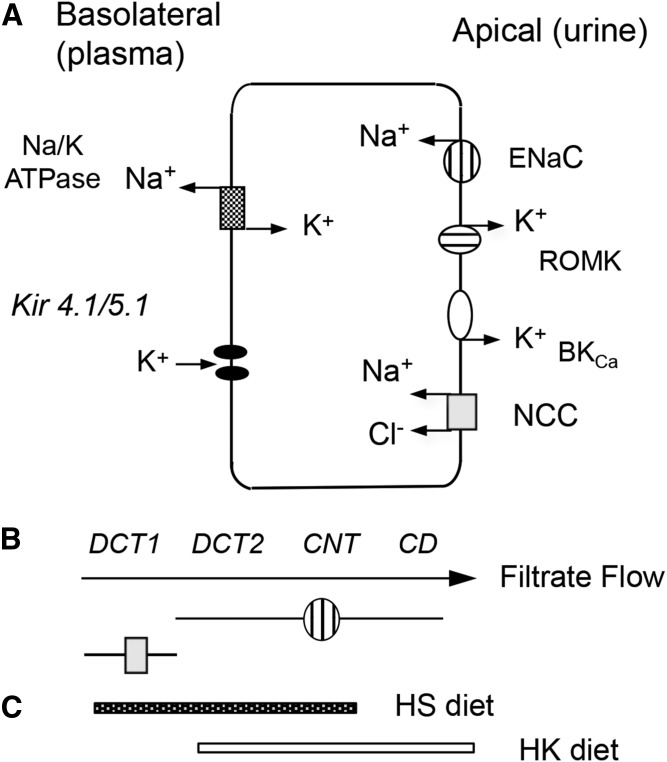
To explore the uniqueness of the CD in the Cyp2c44-KO hypertensive phenotypes, we utilized a CD selective HoxB7 promoter (71) to drive the expression of the Cre-recombinase, disrupt a Cyp2c44flx/flx gene, and generate mice lines carrying a disrupted Cyp2c44 gene only in their CDs (2c44-CD-KO) (11, 59). Importantly, at difference with global 2c44-KO mice (11, 58), the 2c44-CD-KO animals became hypertensive when fed HK, but not HS, diets (Fig. 5) (11, 59). Studies with Dr. WenHui Wang’s laboratory (11, 59) demonstrated that: a) as with 2c44-KO animals, amiloride normalized the pressures of hypertensive 2c44-CD-KO mice (59); b) quantitative PCR analyses of Cyp2c44 expression along the ASDN from WT mice showed that HS diets upregulated Cyp2c44 expression in the thick ascending limb, DCT2, and CNT, but not in the CD (Fig. 6) (59); and c) HK diets induced Cyp2c44 expression in the DCT, CNT, and, importantly, the CD (Fig. 6) (59). It was then concluded that lack of a HK-sensitive Cyp2c44 epoxygenase in the CD of the 2c44-CD-KO enhances ENaC activity, increases sodium inward currents, and raises BP (Fig. 5) (11, 59). Consequently, it appears that: a) upregulated Cyp2c44 expression in the DCT2 and CNT segments is sufficient to prevent excessive sodium absorption during a high sodium intake (Figs. 5, 6); b) conversely, mice on HK diets upregulate Cyp2c44 expression in their CDs, and ENaC in that region becomes a major player in maintaining sodium homeostasis (Figs. 5, 6); and c) the HS- and HK-sensitive phenotypes of mice carrying a globally disrupted Cyp2c44 gene are associated with lack of a HS- or HK-sensitive Cyp2c44 epoxygenase, reduced biosynthesis of ENaC inhibitory 11,12-EET, and a hyperactive ENaC in the DCT2, CNT, and CD segments of the ASDN (Figs. 5, 6) (11). This differential regulation of Cyp2c44 expression and EET biosynthesis by dietary Na+ or K+ along the ASDN indicates that the effects of these cations on ENaC-mediated Na+ transport are spatially and functionally segmented (Fig. 6).
Fig. 6.
Schematic, nonscale, representations of an ASDN principal cell showing in its apical membrane: ENaC (hatched circle), ROMK (hatched oval), BKca (empty oval), and NCC (gray square), and basolateral membrane: NafK ATPase and inward rectifying Kir 4.1/4.5 K+ channels (A); the zonal distribution of principal cells expressing ENaC and NCC along a segment of the ASDN (B); and the effects of HS- or HK-containing diets in the zonal upregulation of the Cyp2c44 epoxygenase and the biosynthesis of ENaC inhibitory EETs (C) (59).
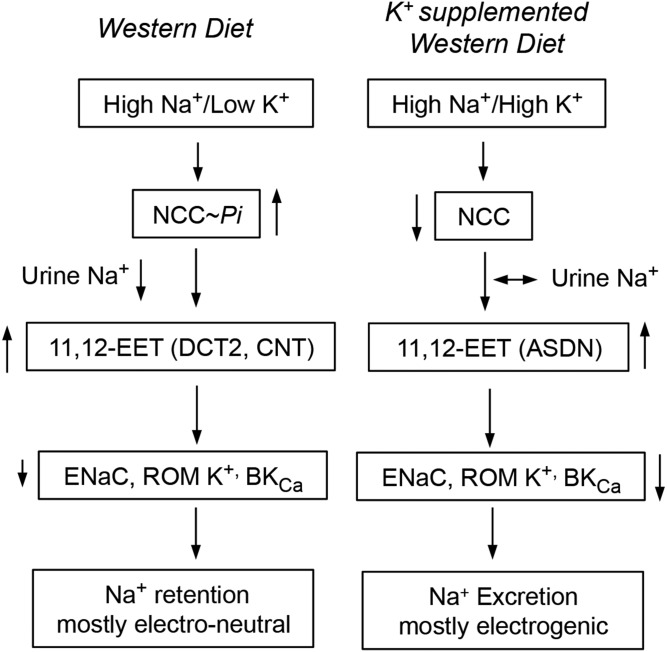
The ASDN accounts for between 5% and 10% of renal Na+ reabsorption, and, yet, it plays central roles in the fine-tuning and regulation of plasma Na+ concentrations and, as mentioned earlier, is a final target for hormones such as angiotensin and bradykinin, known to regulate glomerular hemodynamics and tubular Na+ transport (6, 10–12, 14). In the DCT1, the Na+/Cl- cotransporter (NCC) mediates electro-neutral Na+ reabsorption (Fig. 6) (72–74), whereas electrogenic Na+/K+ exchange by ENaC, the ROMK, and BKCa channels, occurs predominantly in the DCT2, CNT, and CD (Fig. 6) (59, 66–69). Dietary K+ loading inhibits NCC (74), reduces NCC-mediated delivery of Na+ to the DCT2, CNT, and CD segments, and facilitates electrogenic Na+/K+ exchange by ENaC and the ROMK and BKCa channels along those segments of the ASDN (Figs. 6, 7) (66–70). In contrast, dietary-induced reductions in plasma K+ concentration stimulates the WNK-SPAK/OSR1 kinase cascade-mediated phosphorylation and activation of NCC (75), raises electroneutral Na+ absorption and its delivery to the more distal DCT, CNT, and CD segments (Figs. 6, 7) (69–71, 73, 74). Thus, by controlling electroneutral Na+ transport and delivery to CNT and CD segments, NCC regulates electrogenic Na+/K+ fluxes in the ASDN. By its effects on: a) the partition between electroneutral (NCC-mediated) and electrogenic (ENaC-mediated) Na+ transport along the ASDN (Fig. 7), and b) the EET-dependent regulation of ENaC gating, dietary K+ contributes to plasma volume balance and the control of systemic BP (67–73).
Fig. 7.
By its effects on NCC activity, dietary K+ regulates the partition between electroneutral (NCC-driven) and electrogenic Na+ (ENaC-driven) Na+ excretion. Arrows pointing up or down denote increases or reductions in expression or function.
The studies discussed identified a physiological, antihypertensive function for the Cyp2c44 epoxygenase in Na+ transport along the ASDN and for the EETs as natriuretic lipids (Figs. 4, 5). Additionally, they provided a molecular mechanism to explain the known antihypertensive effects associated with dietary K+ supplementation (Fig. 7). Although effective treatments are available for the management of most common forms of human hypertension, the disease is usually diagnosed at its chronic stage with devastating renal, cardiovascular, and cerebral consequences, and, therefore, there are urgent needs for preventive maneuvers to ameliorate the negative consequences of chronic hypertension. Abundant epidemiological studies show that dietary K+ supplementation can be used as an efficient and inexpensive maneuver that could be implemented on a population basis in order to minimize the negative consequences of undiagnosed, untreated high BP (76–79).
Summarizing, the pressure phenotypes of 4a10-KO, 4a14-KO, and 2c44-KO mice can be described as arising from either indirect or direct gene-dependent mechanisms. Indirect_mechanisms are those mediated by effects of the targeted gene on the expression of alternate genes. Examples of this are the Cyp4a10(−/−) and Cyp4a14(−/−) mice. In the former, SS hypertension results from a Cyp4a10 gene product(s)-dependent downregulation of Cyp2c44 epoxygenase renal expression, reduced urinary EET levels, and a hyperactive ENaC. In the latter, sexually dimorphic hypertension results from a Cyp4a14 gene product(s)-dependent, androgen-mediated, upregulation of the kidney Cyp4a12a 20-HETE synthase, increases in 20-HETE biosynthesis, and changes in renal hemodynamics and tubular transport. Direct mechanisms result from a lack of the protein(s) encoded by the targeted gene. This is the case with 2c44-KO mice, in which absence of a functional Cyp2c44 epoxygenase reduces renal EET biosynthesis and increases distal sodium reabsorption. The mechanism(s) by which Cyp4a14 gene product(s) regulate plasma androgens and, thus, Cyp4a12a expression and 20-HETE syntheses, remains undetermined. Also, it remains to be established how the Cyp4a10 gene or HS and HK diets control the kidney expression of the Cyp2c44 epoxygenase and EET synthesis. These murine gene-dependent direct or indirect effects on functional phenotypes could be of potential importance in efforts to identify human genes involved in the prevalence or pathophysiology of a given disease and to determine their mechanism of action.
The AA monooxygenase and the pathophysiology of human hypertension.
The hypertensive phenotypes of Cyp4a KO mice identified CYP4A11 as a candidate gene for studies of its role in human hypertension and led to the establishment of associations between a functional variant of human CYP4A11 (T8590C) and essential hypertension in Caucasian American and German and African-American cohorts (10, 28, 80, 81). Furthermore, carriers of the 8590C allele show increased diastolic BP and reductions in 20-HETE urinary excretion (82), and their BPs are dietary salt sensitivity (63). In the African American Study of Kidney Disease cohort, homozygosis for the 8590C allele was associated with increased progression to end-stage renal disease among hypertensive males with nephrosclerosis and proteinuria (81). A guanine to adenine polymorphism in the human CYP4F2 gene coding for a protein with reduced 20-HETE synthase activity has been associated with increases in urinary 20-HETE levels and BPs (10, 83).
Although still in their early stages, the potential clinical significance of these findings opened the door to what we believe to be the next important chapter in the studies of the AA monooxygenase: the definition of its role in human kidney physiology and disease. This is of importance because, for example, the molecular bases of prevalent forms of hypertension remain uncertain, and, therefore, its early diagnosis and treatment remains largely symptomatic. The identification of new pathways/genes involved in BP variations should lead to novel diagnostic and therapeutic targets for hypertension. Early detection and treatment are needed to prevent the dangerous and profound consequences of chronic hypertension.
The Cyp2c44 epoxygenase and experimental carcinogenesis
The involvement of CYP2C epoxygenases in tumor angiogenesis and the pathophysiology of cancer were suggested by reports identifying: a) the EETs as proangiogenic lipids (84–88) and the human CYP2C8 and CYP2C9 epoxygenases as mediators of VEGF signaling (86, 87); and b) roles for the epoxygenases and the EETs in tumor growth and metastasis (36, 42–44). The participation of the Cyp2c44 epoxygenase in VEGF signaling was inferred from studies in cultured lung endothelial cells showing that cells from WT, but not 2c44-KO, mice responded to VEGF by increasing Cyp2c44 expression, 11,12- and 14,15-EET biosynthesis, ERK, and protein kinase B (Akt) kinase phosphorylation, and cell proliferation and tubulogenesis (36). It was the convergence of these and earlier studies by Raymond C. Harris, MD, and collaborators (Vanderbilt University) on the epoxygenases and EGF signaling (65) that prompted us and Ambra Pozzi, PhD, a Vanderbilt colleague and experienced tumor biologist, to explore the participation of the Cyp2c44 epoxygenase in the pathophysiology of tumor angiogenesis and the consequences that a dysfunctional proangiogenic Cyp2c44 epoxygenase (2c44-KO mice) had in tumor growth, progression, and metastasis (36, 42). These studies had as additional goals to determine whether the downregulation of endothelial Cyp2c44 expression and EET biosynthesis could mimic the phenotypes of 2c44-KO mice and whether this could serve as an effective therapeutic tool.
The Cyp2c44 epoxygenase and its EET metabolites were shown to be a requirement for primary tumor angiogenesis and growth in xenograft experiments in which WT and 2c44-KO mice were injected subcutaneously with large T antigen/Myc-transformed p60.5 fibroblasts, whereas lack of a host endothelial Cyp2c44 epoxygenase resulted in marked reductions in tumor number and weight, and impairments in tumor angiogenesis and vascularization (36). Moreover, administration of EET analogs to 2c44-KO mice, prior to p60.5 cell injections, reversed their tumorigenic phenotype, thus corroborating that it was EET-mediated (42). The fact that cultured p60.5 cells do not synthesize EETs nor do they proliferate in response to EETs confirmed the role of the host endothelial Cyp2c44 epoxygenase in tumorigenesis (36). Alternatively, the overexpression of a Cyp2J2 epoxygenase transgene in tumor cells, via adenovirus-mediated transfection, induced tumorigenesis and metastatic growth, suggesting that in these experiments, angiogenesis was secondary to the tumor growth-promoting effects of Cyp2J2 (43). Similarly, endothelial cell CYP2C8 epoxygenase transfection and sEH inhibition experiments were shown to promote primary tumor growth and metastasis (44).
The PPARα-ligand mediated downregulation of hepatic (62), but not renal, CYP2C epoxygenase expression (57), and the potential usefulness of this class of drugs as an antitumor therapy led us to investigate the effects of Wyeth14643 (Wy), a selective PPARα ligand, on endothelial Cyp2c44 expression and tumorigenic activity (36, 42, 86). This was a highly attractive concept because PPARα ligands have been extensively utilized in clinical medicine for the treatment of plasma hyperlipidemia and found to be safe and well tolerated. Initial analysis of the effects of Wy on cultured mouse lung endothelial cells showed that it downregulated Cyp2c44 epoxygenase expression and inhibited cell replication and tubulogenic activity; such responses were absent in cells isolated from PPARα(−/−) (PPAR-KO) (36, 86). Notably, treatment with Wy, prior to p60.5 cell injections, resulted in significant reductions in tumor vascularization, number, and size in WT mice, but not PPAR-KO animals (36, 86). These studies proved that the antitumor properties of Wy require a functional, host endothelial cell PPARα receptor. Soon after these studies, the antitumorigenic properties of PPARα ligands were confirmed, but attributed to changes in VEGF and thrombospondin regulation (89).
Xenograft models of tumorigenesis do not address events involved in tumor initiation and growth; limitations that are missing in the transgenic KRasLA2 mouse model of spontaneous primary non-small-cell lung cancer (NSCLC) (42). At about 1 month of age, transgenic KRAsLA2 mice develop a few small tumors in their lung surfaces, but within the next 3–5 months, their number and size increase substantially (42). In these animals, after 2 months of treatment, Wy significantly reduced the number and sizes of NSCLC tumors present in the lungs of 5-month-old mice, regardless of whether treatment was initiated 1 or 3 months after birth (42). These results indicated that, after tumor initiation, the beneficial effects of Wy were long lasting and independent of the length of treatment.
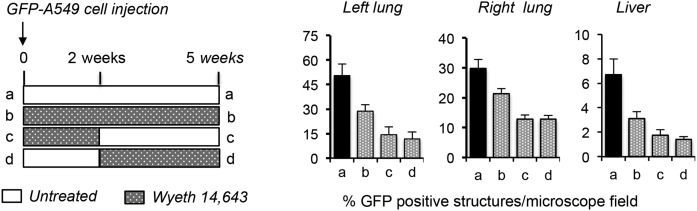
The orthotopic model of human lung cancer, where human NSCLC cells are injected into the lungs of immunocompromised mice, recapitulates the steps of tumor progression seen in most human cancers, including focal growth, vascular invasion, and metastasis. In this model, human A549 NSCLCs are injected in either the left or right lung, and, after the first 2 weeks, mice develop visible tumors that, within the next 3 weeks, metastasize to the other lung and the liver (42). To investigate whether the Wy could reduce and/or prevent NSCLC metastatic growth, our colleague, Dr. Ambra Pozzi, injected GFP-A549 cells expressing green fluorescent protein (GFP) into the left lung parenchyma of immunocompromised nude mice (42). As shown in Fig. 8, the animals were then divided into groups of untreated (a), treated immediately after cell injection with WY for 5 weeks (b) or 2 weeks (c), and mice in which the Wy treatment was initiated 2 weeks after cell injection (d). All animals were euthanized 5 weeks later, their lungs and livers were collected, and their tumor load was evaluated by fluorescence microscopy (42). Compared with the untreated animals in group a, Wy reduced significantly primary (left lung) and metastatic (right lung and liver) NSCLC growth, a response that was independent of the time (groups b and c vs. d) or the length of Wy administration (group b vs. c and d) (Fig. 8). Most importantly, its beneficial effects persisted also even after its withdrawal (group c) (Fig. 8).
Fig. 8.
Wy ameliorates human NSCLC primary and metastatic growth. GFP-A459 cells were injected into the left lungs of athymic mice, and animals were then separated into an untreated group (a) or groups treated with Wy immediately after injection (groups b and c) or 2 weeks after (group d). For group c, Wy treatment was stopped 2 weeks after initiation. All mice were euthanized 5 weeks after the cell injections, and the number of primary (left lung) and metastatic (right lung and liver) tumors were determined in the dissected organs by the number of surface GFP-positive structures/microscopic field. n = 6 mice/group (42).
Tumor metastasis, a devastating and common complication of human cancers, continues to be a serious clinical challenge with few positive outcomes. The lack of anticancer approaches that are effective, show limited negative side effects, and result in positive outcomes, underscores the urgent need for new therapeutic strategies. Tumor growth and invasiveness are dependent on the blood supply, and, therefore, the identification of effective antagonist and inhibitors of angiogenesis continues to be a promising target for current efforts to develop more effective anticancer therapies. It would appear that downregulation of human endothelial epoxygenase(s) expression protects against primary and metastatic tumor growth, whereas, in contrast, increasing endothelial EET levels by either tumor or endothelial cell CYP2 epoxygenase transfection or sEH inhibition promotes primary tumor growth and metastasis (43, 44). The identification by immunofluorescence and mass spectral imaging of CYP2C9 as the endothelial epoxygenase in several human tumors (36) should facilitate studies to validate its usefulness as a target for future antitumor, antimetastatic approaches for the treatment of human cancer. In this regard, bezafibrate, a PPARα ligand in clinical use known to be well tolerated does show antitumor and antimetastatic properties similar to those of Wy (42).
The documentation of the EETs as proangiogenic and pro-tumorigenic lipids raised pertinent questions regarding long-term risks that might be associated with efforts to use inhibitors of EET inactivation via enzymatic hydration or EET analogs as antihypertensive, antiinflammatory, and renoprotective drugs, because they could emulate or potentiate EET mitogenic and tumorigenic activities. Although this is a valid concern, it must be noted that there is as yet no evidence that the EETs play a part in the biology of tumor initiation. Most studies of the effects of EET on tumor growth have utilized cultured cancer cell lines (36, 42–44) or analyzed in vivo changes in tumor growth and metastasis resulting from tumor overexpression of CYP2 transgenes (43, 44) and, therefore, do not address whether EETs have an effect on tumor initiation, a required first step in carcinogenesis. Nevertheless, setting aside any possible preventive value, targeting the CYP2C epoxygenases and the EETs does appear to offer a potentially valuable therapeutic alternative for cancer treatment.
DRUG DEVELOPMENT AND THERAPEUTIC APPROACHES
The AA monooxygenase metabolites, in common with most eicosanoids, are metabolically and chemically labile. Autooxidation, epoxide hydration, chain elongation, β- and/or ω-oxidation, esterification, secondary metabolism by enzymes of the AA cascade, olefin reduction, glucuronidation, or some combination of these conspire to trammel both investigational studies of the natural metabolites and their potential clinical utility (82). Elucidation of the structure-activity relationships of the monooxygenase metabolites, mainly in collaboration with the laboratories of Roman, Campbell, Imig, and Schwartzman, also led to the discovery of agonist and antagonist structural variants that have been widely exploited in biochemical and physiological contexts. Generally, these early pharmacological tools retained a recognizable eicosanoid scaffold and were preferred in many cases to P450 biosynthesis inhibitors in the belief that they confer greater specificity (90). In the case of metabolite antagonists, they can more effectively compensate for release of P450 metabolites from cellular reservoirs, which might require up to 24 h to deplete using P450 inhibitors (91).
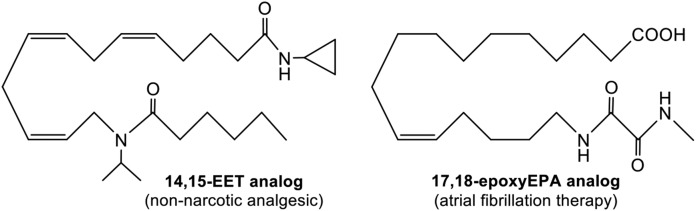
Second- and third-generation metabolite analogs systematically introduced bioisosteres to replace the epoxide, carboxylate, hydroxyl, and reactive carbons, resulting in long-lived and orally bioavailable analogs with promising drug-like properties (92, 93). Additionally, novel drug-delivery technology has been pioneered specifically for extended-life oral applications. Undeniably, there is great anticipation and sense of validation, as some of these analogs currently advance through human clinical trials, e.g., CMX020, a nonnarcotic therapy for mild to severe pain, from Cytometix (Milwaukee, WI); and OMEICOS Therapeutic’s (Berlin, Germany) 17,18-epoxyEPA analogs for management of atrial fibrillation (Fig. 9).
Fig. 9.
Monooxygenase-inspired drugs in clinical trials.
Recently, GPR75 was proposed as a 20-HETE receptor (51) and GPR40 as a low-affinity EET receptor in vascular cells (94). If confirmed, these reports hold far-reaching significance and might portend a new golden age for the P450 eicosanoid field. The search for other receptors, including those that recognize a specific regioisomer or PUFA class, would likely intensify, and future drug-development programs would be able to screen large compound libraries and apply high-throughput techniques to identify structurally unique agonist and antagonist scaffolds. Alternatively, a better understanding of P450 eicosanoid receptors could lead to new opportunities for diagnosis and pathway control.
PENDING ISSUES AND SPECULATIONS ABOUT THE FUTURE
Abundant evidence from laboratories in the United States and abroad validates the contention that the epoxygenase and ω-hydroxylase branches of the AA monooxygenase make important contributions to cell, organ, and body physiology and that alterations in P450 expression, enzymatic activity, metabolite signaling, or activation/inactivation are associated with the pathophysiology of renal, vascular, cerebral, and metabolic diseases. Given these advances, the next challenge, i.e., the definition of the significance of the AA monooxygenase pathway and its metabolites to human disease, will require unequivocal identifications of the physiologically relevant human CYP2 and CYP4 genes and enzymes, their tissue-specific expression, regulatory control, signaling mechanism, and pathophysiological roles. These efforts should serve as a prelude to drug-development efforts that target human epoxygenases and ω-hydroxylases as novel therapies for the treatment of cardiovascular, cerebral, and renal diseases, hypertension, diabetes, cancer, CNS disorders, and inflammation. Among issues to be address we draw attention to the following.
The characterization of mechanism(s) by which the AA monooxygenase metabolites elicit functional responses
As mentioned, the recognition that GPR75 functions as a 20-HETE receptor identified a new, 20-HETE-specific target for drug design (51). In contrast, after attempts by different groups, selective binding and trans-membrane signaling has been reported only for 14(R),15(S)-EET in guinea pig mononuclear and human U937 cells (95, 96). G proteins, mitogenic kinases/phosphatases, cAMP-kinase A, prostanoids, PPAR nuclear receptors, and direct effects on ion-channel activity have been proposed as mediators of EET signaling (6, 8, 10, 14, 94); however, a membrane receptor capable of selective high-affinity EET binding and trans-membrane signaling has yet to be unequivocally identified. Complicating these efforts is the ready migration or transport of exogenously added EETs across cell membranes, as shown by their rapid esterification into cellular glycerolipids (39), raising the possibility that extracellular and/or intracellular events could mediate EET functional effects. As initially proposed (11), extracellular signaling would involve EET interactions with cognate receptors capable of trans-membrane signaling and amplification or, alternatively, direct EET effects on the signaling properties of hormone receptors such as reported for the EGF receptor (5, 65). Intracellular signaling would involve EET crossing the cell membrane barrier by diffusion, carrier plasma lipoprotein uptake, or yet-to-be-determined mechanisms. Alternatively, and perhaps more likely, the EETs could originate from phospholipase A2 catalyzed release of AA followed by P450-mediated epoxidation (5, 11). The thus-formed intracellular EETs could signal by: a) facilitating physical interactions between intracellular protein kinases (MAPKs, Src, PI-3K, Akt, ERK, protein phosphatases, adenylyl- and/or guanylyl cyclase, G proteins, etc.) with their substrates; b) direct effects on the activities of enzymatic pathways such as those catalyzed by mitogenic kinases; or c) effects on ion-channel/transporter phosphorylation/dephosphorylation. There is already convincing evidence for the participation of endogenously generated 11,12- and/or 14,15-EET in EGF-stimulated epithelial cell proliferation and the activation of PI-3K, ERK, and c-Src kinases (5, 11, 65), as well as VEGF-mediated increases in endothelial cell proliferation, tubulogenic activity, and ERK and Akt activation; all in a Cyp2c44 epoxygenase-dependent fashion (11, 36, 88). As discussed, the EETs were shown to inhibit ENaC gating by activating the ERK-mediated inhibitory phosphorylation of the channel (11, 58). In summary, if these types of intracellular actions were unequivocally established, they would add the EETs to the list of known intracellular signal effector molecules such as, for example, cAMP and cGMP nucleotides, inositol-phosphates, and NO. This would represent a conceptual breakthrough as well as help rationalize many of the multiple and pleotropic functional effects of EETs.
The nature and source of the active EET-mediators
Although rarely recognized, more than 90% of the EETs present endogenously in rodent and human tissues and plasma are found as esters of phosphatidyl-choline, -ethanolamine, and -inositol (5). As proposed earlier (11), these epoxy-phospholipids, generated enzymatically by stereoselective acylation of EET-CoA derivatives into lysolipids (39), raise important issues, inter alia: a) their function as a source of bioactive EETs that can be made available independently of P450-catalyzed AA epoxidation; b) the existence of selective, hormonally sensitive, epoxy-phospholipid A2 lipases responsible for the release of functionally important EETs that could participate in intracellular signaling as proposed above; c) whether the EET moiety confers unique functional properties to these phospholipids and whether epoxidation serves as a marker for cells to distinguish functionally important phospholipids from merely bulk or structural phospholipid pools; and d) are the EET-containing phospholipids or their diglyceride or monoglyceride metabolic products signaling molecules or simple reservoirs of bioactive EETs. It will be recalled that the 2-(11,12- and/or 14,15-epoxyeicosatrienoyl)-glycerides present in rat brain, kidney, and spleen are known to bind and activate the CB1 and CB2 cannabinoid receptor subtypes (97).
The identification of P450 genes and proteins responsible for the endogenous synthesis of bioactive EETs and 20-HETE
Unlike the cyclooxygenase and lipoxygenase members of the AA cascade, P450s belong to an extended family of hemeproteins that display significant degrees of intrafamily sequence homology (17). This fact complicates the unequivocal identification of physiologically relevant P450 genes and proteins. Members of the CYP2C and CYP2J, and CYP4A and CYP4F gene subfamilies noted above, have been characterized as AA epoxygenase or ω-hydroxylases, respectively. However, most of these assignments are based on in vitro enzymatic activities, immunological and/or RNA based studies of expression levels, genetic associations, inhibitor studies, or functional responses to transgene-induced changes in expression (5–14). Current advances in gene-targeting methods should hasten future developments of models of monogenic P450 dysfunction and lead to further progress in this important area. The identification of the functionally relevant human EET/20-HETE synthases is needed for analyses of correlations between pathophysiological conditions and alterations in the genes coding for these enzymes and/or involved in their expression, as well as for the development of novel strategies for disease diagnosis and/or treatment.
The characterization of mechanisms controlling the expression of selected AA epoxygenases and ω-hydroxylases in an organ-/tissue-/cell-specific manner
The direct and indirect mechanisms by which P450 genes control functional phenotypes illustrates the importance of a gene-dependent regulation of different (and at times unrelated) genes in the development of functional phenotype(s). As discussed, the Cyp4a10 and Cyp4a14 genes regulate, by yet-unknown mechanism(s), the expression of antihypertensive or prohypertensive Cyp2c44 or Cyp4a12 proteins, respectively, and their pressure responses to changes in dietary salt or plasma androgen levels. This added level of complexity underscores the potential clinical relevance of gene-determined alterations in protein function and/or the regulatory control of alternate genes that could be relevant for studies of links between P450 gene variants and pathophysiological conditions. Furthermore, gene-mediated dysfunction can be masked or enhanced by coexistent morbidities or be detectable under stress or nonstress conditions. Under LS or NS intake conditions, 4a10-KO and 2c44-KO mice show no overt functional phenotypes; however, roles for these genes in BP control or tumor angiogenesis are reveled under the stress imposed by increases in dietary salt intake (11) or processes associated with tumorigenesis (36). At difference with 4a10-KO and 2c44-KO mice (57, 58), the hypertensive phenotype of the 4a14-KO animals is evident in the absence of experimental manipulation (56).
It is of interest that the BPs of carriers of the CYP4A11 8590C allele are dietary SS (63), as is with 4a10-KO mice (57); however, a clear relationship between this genotype, BP, and urinary 20-HETE excretion is yet to be established (82). It remains to be determined whether CYP2C8 and/or CYP2C9 derived EETs play a role in the SS hypertensive phenotype identified for the variant CYP4A11 genotype, as well as the prevalence and severity of human hypertension in double carriers of the CYP4A11 8590C allele and CYP2C8*3/*3 or CYP2C9*2/*2 reduced EET synthase activity variants (98). As mentioned, a polymorphism in the human CYP4F2 gene has been associated with increases in BP and the urinary excretion of 20-HETE (10, 83). However, the variant gene codes for a protein with reduced 20-HETE synthase activities due to a valine-to-methionine substitution at residue 433 (V433M) (10, 83). This discrepancy between a reduced 20-HETE synthase activity and increased 20-HETE urinary excretion in carriers of the CYP4F2 G/C variant may be similar to what has been reported for hypertensive 4a10-KO mice (11, 57) and suggests roles for the CYP4F2 gene in the regulation of alternate prohypertensive or antihypertensive gene(s).
Finally, the biochemical segmentation of the CYP2C and CYP4A AA epoxygenases and ω-hydroxylases expression along the nephron have been reported (6, 10, 14), as have their renovascular and nephron segment selective upregulation or downregulation by hormones, autacoids, dietary salt/potassium intake, and disease states, such as diabetes, hypertension, cancer, and inflammation (6–15). However, there is a paucity of information regarding regulatory mechanisms that determine organ, tissue, and cell-type selective expression of functionally significant P450 isoforms. This is especially relevant to roles in kidney physiology/pathophysiology, where the functional and biochemical segmentation along the length of the nephron is well known. Most of the data regarding zonal expression of CYP2C, CYP2J, CYP4A, and CYP4F isoforms in rodent kidneys are based on either immunological, mRNA in situ hybridization, or PCR amplification data (6, 10, 14, 59) Although useful in distinguishing P450s from different gene families, these methods are limited when applied to P450s of the same gene subfamily because, in most cases, they share extensive open reading frame nucleotide as well as amino acid sequence homology. Some of these limitations could be addressed by, for example, targeting mRNA untranslated sequences, developing peptide antibodies raised against unique protein segments, performing differential analyses before and after gene deletion or antisense nucleotide inhibition, or, when the methodology becomes more widely available, diagnostic peptide imaging of tissue sections by mass spectroscopy (36).
Acknowledgments
The authors thank Elaine and Antonieta for helping us to keep the hope and the dream alive. The authors also thank John C. McGiff, MD; because of his contributions, courage, and foresight, the arachidonate monooxygenase gained the recognition that it enjoys today as a physiologically important metabolic pathway. Finally, none of the work summarized would have been possible without the contributions of the many fellows, students, and technical assistants with whom the authors have interacted; they made the authors’ journey not only more productive, but also substantially more gratifying. The authors thank Drs. Frank Gonzalez (National Cancer Institute-The National Institutes of Health) and Darryl Zeldin (National Institute of Environmental Health Sciences) for sharing their PPARα (−/−) and Cyp2c44(flx/flx) mice.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- Akt
- protein kinase B
- ASDN
- aldosterone-sensitive distal nephron
- BP
- blood pressure
- CD
- collecting duct
- CNT
- connecting tubule
- DCT
- distal convoluted tubule
- EET
- epoxyeicosatrienoic acid
- EGF
- epidermal growth factor
- ENaC
- epithelial sodium channel
- HK
- high potassium
- HS
- high salt
- LS
- low salt
- NCC
- Na+/Cl- co-transporter
- NS
- normal salt
- NSCLC
- non-small-cell lung cancer
- P450
- cytochrome P450
- PI-3K
- phosphatidylinositol-3 kinase
- ROMK
- renal outer medullary potassium channel
- sEH
- soluble epoxide hydrolase
- Src
- protooncogene tyrosine kinase
- SS
- salt sensitive
- UTHSCD
- University of Texas Health Science Center at Dallas
- VEGF
- vascular endothelial growth factor
- Wy
- Wyeth14643
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P01-038226 (J.H.C. and J.R.F.); National Institute of General Medical Sciences Grants R01-037922 (J.H.C.) and R01-031278 (J.R.F.); National Institutes of Health Grant LR01-0139793 (J.R.F.); Welch Foundation Grant I-0011 (J.R.F.); and the Dr. Ralph and Marian Falk Medical Research Trust (J.R.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Capdevila J., Chacos N., Werringloer J., Prough R. A., and Estabrook R. W.. 1981. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc. Natl. Acad. Sci. USA. 78: 5362–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliw E. H., and Oates J. A.. 1981. Oxygenation of arachidonic acid by hepatic microsomes of the rabbit. Mechanism of biosynthesis of two vicinal dihydroxyeicosatrienoic acids. Biochim. Biophys. Acta. 666: 327–340. [DOI] [PubMed] [Google Scholar]
- 3.Morrison A. R., and Pascoe N.. 1981. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc. Natl. Acad. Sci. USA. 78: 7375–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chacos N., Falck J. R., Wixtrom C., and Capdevila J.. 1982. Novel epoxides formed during the liver cytochrome P-450 oxidation of arachidonic acid. Biochem. Biophys. Res. Commun. 104: 916–922. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila J. H., Falck J. R., and Harris R. C.. 2000. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of the arachidonate monooxygense. J. Lipid Res. 41: 163–181. [PubMed] [Google Scholar]
- 6.Roman R. J. 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 7.Capdevila J. H. 2007. Regulation of ion transport and blood pressure by cytochrome P450 moooxygenases. Curr. Opin. Nephrol. Hypertens. 16: 465–470. [DOI] [PubMed] [Google Scholar]
- 8.Pfister S. L., Gauthier K. M., and Campbell W. B.. 2010. Vascular pharmacology of epoxyeicosatrienoic acids. Adv. Pharmacol. 60: 27–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imig J. D., Simpkins A. N., Renic M., and Harder D. R.. 2011. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev. Mol. Med. 13: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C. C., Gupta T., Garcia V., Ding Y., and Schwartzman M. L.. 2014. 20-HETE and blood pressure regulation. Clinical implications. Cardiol. Rev. 22: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capdevila J. H., Wang W. H., and Falck J. R.. 2015. Arachidonic acid monooxygenase: genetic and biochemical approached to physiological/pathophysiological relevance. Prostaglandins Other Lipid Mediat. 120: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imig J. D. 2016. Epoxyeicosatrienoic acids and 20-hydroxyeicosatetraenoic acid on endothelial and vascular function. Adv. Pharmacol. 77: 105–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luther J. M., and Brown N. J.. 2016. Epoxyeicosatrienoic acids and glucose homeostasis in mice and men. Prostaglandins Other Lipid Mediat. 125: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan F., and Roman R. J.. 2017. Effect of cytochrome P450 metabolites of arachidonic acid in nephrology. J. Am. Soc. Nephrol. 28: 2845–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis C. M., Liu X., and Alkayed N. J.. 2017. Cytochrome P450 eicosanoids in cerebrovascular function and disease. Pharmacol. Ther. 179: 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner K. M., McReynolds C. B., Schmidt W. K., and Hammock B. D.. 2017. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol. Ther. 180: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guengerich F. P. 2006. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 8: E101–E111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chacos N., Capdevila J. H., Falck J. R., Manna S., Wixtrom C., Gill S. S., Hammock B. D., and Estabrook R. W.. 1983. The reaction of arachidonic acid epoxides with a cytosolic epoxide hydrolase. Arch. Biochem. Biophys. 223: 639. [DOI] [PubMed] [Google Scholar]
- 19.Moustakis C. A., Viala J., Capdevila J., and Falck J. R.. 1985. Total synthesis of the Cytochrome P-450 epoxygenase metabolites 5(R),6(S)-, 5(S),6(R)-, and 14(R),15(S)-epoxyeicosatrienoic acid (EET) and hydration products 5(R),6(R)- and 14(R),15(R)-dihydroxyeicosatrienoic acid (DHET). J. Am. Chem. Soc. 107: 5283–5285. [Google Scholar]
- 20.Corey E. J., Niwa H., and Falck J. R.. 1979. Selective epoxidation of eicosa-cis-5,8,11,14-tetraenoic (arachidonic) acid and eicosa-cis-8,11,14-trienoic acid. J. Am. Chem. Soc. 101: 1586–1587. [Google Scholar]
- 21.Mosset P., Yadagiri P., Lumin S., Capdevila J., and Falck J. R.. 1986. Arachidonate epoxygenase: total synthesis of both enantiomers of 8,9- and 11,12-epoxyeicosatrienoic acid. Tetrahedron Lett. 27: 6035–6038. [Google Scholar]
- 22.Manna S., Falck J. R., Chacos N., and Capdevila J.. 1983. Synthesis of arachidonic acid metabolites produced by purified kidney cortex microsomal cytochrome P-450. Tetrahedron Lett. 24: 33–36. [Google Scholar]
- 23.Chauhan K., Aravind S., Lee S-G., Falck J. R., and Capdevila J. H.. 1994. vic-Diol chirons: enantiospecific synthesis of 11,12- and 14,15-dihydroxyeicosatrienoic acids. Tetrahedron Lett. 35: 6791–6794. [Google Scholar]
- 24.Hammonds T. D., Blair I. A., Falck J. R., and Capdevila J. H.. 1989. Resolution of epoxyeicosatrienoate enantiomers by chiral phase chromatography. Anal. Biochem. 182: 300–303. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki D. M., Yuan Y., Gikas K., Reddy M., Falck J. R., Nithipatikom K., Campbell W. B., and Callewaert D. M.. 2002. Development of enzyme immunoassays for 5,6-, 8,9-, 11,12-, and 14,15-EETs and the corresponding DHETs. In Advances in Experimental Medicine and Biology. Vol. 507. Honn et al., editors. Kluwer Academic/Plenum Publishers, London. 531–536. [DOI] [PubMed] [Google Scholar]
- 26.Okita R. T., and Okita J. R.. 2001. Cytochrome P450 fatty acid omega hydroxylases. Curr. Drug Metab. 2: 265–281. [DOI] [PubMed] [Google Scholar]
- 27.Imaoka S., Ogawa H., Kimura S., and Gonzalez F. J.. 1993. Complete cDNA sequence and cDNA-directed expression of CYP4A11, a fatty acid omega-hydroxylase expressed in human kidney. DNA Cell Biol. 12: 893–899. [DOI] [PubMed] [Google Scholar]
- 28.Gainer J. V., Bellamine A., Dawson E. P., Womble K. E., Grant S. W., Wang Y., Cupples A., Guo C. Y., Demissie S., O’Donnell C. J., et al. 2005. A functional variant of CYP4A11 20-HETE synthase is associated with essential hypertension. Circulation. 111: 63–69. [DOI] [PubMed] [Google Scholar]
- 29.Lasker J. M., Chen W. B., Wolf I., Bloswick B. P., Wilson P. D., and Powell P. K.. 2000. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid in human kidney. J. Biol. Chem. 275: 4118–4126. [DOI] [PubMed] [Google Scholar]
- 30.Albertolle M. E., Kim D., Nagy L. D., Yun C-H., Pozzi A., Savas U., Johnson E. F., and Guengerich F. P.. 2017. Heme-thiolate sulfenylation of human cytochrome P450 4A11 functions as a redox switch for catalytic inhibition. J. Biol. Chem. 292: 11230–11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller D. N., Schmidt C., Barbosa-Sicard E., Wellner M., Gross V., Hercule H., Markovic M., Honeck H., Lutf F. C., and Schunck W. H.. 2007. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hdroxyeicosatetraenoic acid formation. Biochem. J. 403: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helvig C., Dishman E., and Capdevila J. H.. 1998. Molecular, enzymatic and regulatory characterization of rat kidney cytochromes P450 4A2 and 4A3. Biochemistry. 37: 12546–12558. [DOI] [PubMed] [Google Scholar]
- 33.Kalsotra A., and Strobel H. W.. 2006. Cytochrome P450 4F subfamily: at the crossroads of eicosanoid and drug metabolism. Pharmacol. Ther. 112: 589–611. [DOI] [PubMed] [Google Scholar]
- 34.Bardowell S. A., Duan F., Manor D., Swanson J. E., and Parker R. S.. 2012. Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism. J. Biol. Chem. 287: 26077–26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeLozier T. C., Tsao C. C., Coulter S. J., Foley J., Bradbury J. A., Zeldin D. C., and Goldstein J. A.. 2004. CYP2C44, a new murine CYP2C that metabolizes arachidonic acid to unique stereospecific products. J. Pharmacol. Exp. Ther. 310: 845–854. [DOI] [PubMed] [Google Scholar]
- 36.Pozzi A., Popescu V., Yang S., Mei S., Shi M., Puolitaival S. M., Capriolo R. M., and Capdevila J. H.. 2010. The anti-tumorigenic properties of peroxisomal proliferator-activated receptor α are arachidonic acid epoxygenase mediated. J. Biol. Chem. 285: 12840–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeldin D. C., DuBois R. N., Falck J. R., and Capdevila J. H.. 1995. Molecular cloning and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch. Biochem. Biophys. 322: 76–86. [DOI] [PubMed] [Google Scholar]
- 38.Athirakul K., Bradbury J. A., Graves J. P., DeGraff L. M., Ma J., Zhao Y., Couse J. F., Quigley R., Harder D. R., Zhao X., et al. 2008. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J. 22:4096–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karara A., Dishman E., Falck J. R., and Capdevila J. H.. 1991. Endogenous epoxyeicosatrienoyl- phospholipids. A novel class of cellular glycerolipids containing epoxidized arachidonate moieties. J. Biol. Chem. 266: 7561–7569. [PubMed] [Google Scholar]
- 40.Zeldin D. C., Kobayashi J., Falck J. R., Winder B. S., Hammock B. D., Snapper J. R., and Capdevila J. H.. 1993. Regio and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydratase. J. Biol. Chem. 268: 6402. [PubMed] [Google Scholar]
- 41.Prakash C., Zhang J. Y., Falck J. R., Chauhan K., and Blair I. A.. 1992. 20-hydroxyeicosatetraenoic acid is excreted as a glucuronide conjugate in humanurine. Biochem. Biophys. Res. Commun. 185: 728–733. [DOI] [PubMed] [Google Scholar]
- 42.Skrypnyk N., Chen X., Hu W., Su Y., Mont S., Yang S., Gangadhariah M., Wei S., Falck J. R., Jat J. L., et al. 2014. PPARα activation can help prevent and treat non-small cell lung cancer. Cancer Res. 74: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang J. G., Ning Y. G., Chen C., Ma D., Liu Z. J., Yang S., Zhiu J., Xiao X., Zhang X. A., Edin M. L., et al. 2007. Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 67: 6665–6674. [DOI] [PubMed] [Google Scholar]
- 44.Panigrahy D., Mathew E. L., Lee C., Sui H., Bielenberg D. R., Butterfield C., Barnés C., Mammoto A., Mammoto T., Ayala C., et al. 2012. Epoxyeicosatrienoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Invest. 122: 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Proctor K. G., Falck J. R., and Capdevila J.. 1987. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ. Res. 60: 50–59. [DOI] [PubMed] [Google Scholar]
- 46.Carroll M. A., Schwartzman M., Capdevila J., Falck J. R., and McGiff J. C.. 1987. Vasoactivity of arachidonic acid epoxides. Eur. J. Pharmacol. 138: 281–283. [DOI] [PubMed] [Google Scholar]
- 47.Gebremedhin D., Ma Y. H., Falck J. R., Roman R. J., VanRollins M., and Harder D. R.. 1992. Mechanism of action of cerebral epoxyeicosatrienic acids on cerebral arterial smooth muscle. Am. J. Physiol. 263: H519–H525. [DOI] [PubMed] [Google Scholar]
- 48.Hu S., and Kim H. S.. 1993. Activation of K+ channel in vascular smooth muscles by cytochrome P450 metabolites of arachidonic acid. Eur. J. Pharmacol. 230: 215–221. [DOI] [PubMed] [Google Scholar]
- 49.Campbell W. B., Gebremedhin D., Pratt P. F., and Harder D. R.. 1996. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 78: 415–423. [DOI] [PubMed] [Google Scholar]
- 50.Chen X. W., Yu T. J., Znahg J., Li Y., Chen H. L., Yang G. F., Yu W., Duan C. F., Tang H. L., Qiu M., et al. 2017. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene. 36: 5045–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia V., Gilani A., Shkolnik B., Pandey V., Zhang F. F., Dakarapu R., Gandham S. K., Reddy R., Graves J. P., Gruzdev A., et al. 2017. 20-HETE signals through G-protein-coupled receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Circ. Res. 120: 1776–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tunaru S., Bonnavion R., Brandenburger I., Preussner J., Thomas D., Scholich K., and Offermanns S.. 2018. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1 Nat Commun. 9: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou A. P., Fleming J. T., Falck J. R., Jacobs E. R., Gebremedhin D., Harder D. R., and Roman R. J.. 1996. 20-HETE is an endogenous inhibitor of the large conductance Ca2+-activated K+ channel in renal arterioles. Am J Physiol. 270:R228–R237. [DOI] [PubMed] [Google Scholar]
- 54.Sacerdoti D., Escalante B., Abraham N. G., McGiff J. C., Levere R. D., and Schwartzman M. L.. 1989. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 243: 388–390. [DOI] [PubMed] [Google Scholar]
- 55.Wei Y., Lin D. H., Kemp R., Yaddanapudi G. S., Nasjletti A., Falck J. R., and Wang W. H.. 2004. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent pathways. J. Gen. Physiol. 124: 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holla V. R., Adas F., Imig J. D., Zhao Z., Proce E., Olsen N., Kovacs W. T., Magnuson M. A., Keeney D. S., Bryer M. D., et al. 2001. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases causes hypertension. Proc. Natl. Acad. Sci. USA. 98: 5211–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakagawa K., Holla V. R., Wei Y., Wang W. H., Gatica A., Wei S., Mei S., Miller C. M., Cha D. R., Price E., et al. 2006. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J. Clin. Invest. 116: 1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capdevila J. H., Pidkovka N., Mei S., Gong Y., Falck J. R., Imig J. D., Harris R. C., and Wang W.. 2014. The Cyp2c44 epoxygenase regulates epithelial sodium channel activity and the blood pressure responses to increased dietary salt. J. Biol. Chem. 289: 4377–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W. H., Zhang C., Lin D. H., Wang L., Graves J. P., Zeldin D. C., and Capdevila J. H.. 2014. Cyp2c44 epoxygenase in the collecting duct is essential for the high K+ intake induced anti-hypertensive effect. Am. J. Physiol. Renal Physiol. 307: F453–F460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu C-C., Mei S., Cheng J., Ding Y., Weidenhammer A., Garcia V., Zhang F., Gotlinger K., Manthati V. L., Falck J. R., et al. 2013. Androgen sensitive hypertension associates with upregulated vascular CYP4A12–20-HETE synthase. J. Am. Soc. Nephrol. 24: 1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quigley R., Chakravarty S., Zhao X., Imig J. D., and Capdevila J. H.. 2009. Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron Physiol. 113: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corton J. C., Fan L. Q., Brown S., Anderson S. P., Bocos C., Cattley R. C., Mode A., and Gustafsson J. Å.. 1998. Down-regulation of cytochrome P450 2C family members and positive acute-phase response gene expression by peroxisomal proliferator chemicals. Mol. Pharmacol. 54: 463–473. [DOI] [PubMed] [Google Scholar]
- 63.Williams J. S., Hopkins P. N., Jeunemaitre X., and Brown N. J.. 2011. CYP4A11 T8590C polymorphism, salt-sensitive hypertension, and renal blood flow. J. Hypertens. 29: 1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen J. P., and Cotton C. U.. 2003. Epidermal growth factor inhibits amiloride-sensitive sodium absorption in renal collecting ducts. Am. J. Physiol. Renal Physiol. 284: F57–F64. [DOI] [PubMed] [Google Scholar]
- 65.Chen JK, Capdevila JH, Harris RC.. 2002. Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells. Proc Natl Acad Sci USA 99:6029–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rieg T., Vallon V., Sausbier M., Sausbier U., Kaissling B., Ruth P., and Osswald H.. 2007. The role of the BK channel in potassium homeostasis and flow-induce renal potassium excretion. Kidney Int. 72: 566–573. [DOI] [PubMed] [Google Scholar]
- 67.Penton D., Czogalla J., and Loffing J.. 2015. Dietary potassium and the renal control of salt balance and blood pressure. Pflugers Arch. 467: 513–530. [DOI] [PubMed] [Google Scholar]
- 68.Welling P. A. 2016. Roles and regulation of renal K channels. Annu. Rev. Physiol. 78: 415–435. [DOI] [PubMed] [Google Scholar]
- 69.McDonough A. A., and Youn J. H.. 2017. Potassium homeostasis: the knowns, the unknowns, and the health benefits. Physiology (Bethesda). 32: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun P., Antoun J., Lin D-H., Yue P., Gotlinger K. H., Capdevila J. H., and Wang W. H.. 2012. Cyp2c44-epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake. Hypertension. 59: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kobayashi A., Kwan K. M., Carroll T. J., McMahon A. P., Mendelsohn C. L., and Behringer R. R.. 2005. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 132: 2809–2823. [DOI] [PubMed] [Google Scholar]
- 72.Moes A. D., van der Lubbe N., Zietse R., Loffing J., and Hoorn E. J.. 2014. The sodium chloride cotransporter SLC12A3: new roles in sodium, potassium, and blood pressure regulation. Pflugers Arch. 466: 107–118. [DOI] [PubMed] [Google Scholar]
- 73.Verouti S. N., Boscardin E., Hummler E., and Frateschi S.. 2015. Regulation of blood pressure and renal function by NCC and ENaC: lessons from genetically engineered mice. Curr. Opin. Pharmacol. 21: 60–72. [DOI] [PubMed] [Google Scholar]
- 74.Vallon V., Schroth J., Lang F., Kuhl D., and Uchida S.. 2009. Expression and phosphorylation of the Na+-Cl-- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am. J. Physiol. Renal Physiol. 297: F704–F712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castañeda-Bueno M., Cervantes-Pérez L. G., Vásquez N., Uribe N., Kantesaria S., Morla L., Bobadilla N. A., Doucet A., Alesi D. R., and Gamba G.. 2012. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc. Natl. Acad. Sci. USA. 109: 7929–7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khaw K. T., and Barret-Connor E.. 1987. Dietary potassium and stroke-associated mortality: a 12-year prospective population study. N. Engl. J. Med. 316: 235–240. [DOI] [PubMed] [Google Scholar]
- 77.Chen Q., Turban S., Miller E. R., and Appel L. J.. 2012. The effects of dietary patterns on plasma renin activity: results from the Dietary Approaches to Stop Hypertension. J. Hum. Hypertens. 26: 664–669. [DOI] [PubMed] [Google Scholar]
- 78.Mente A., O’Donnell M. J., Rangarajan S., McQueen M. J., Poirier P., Wielgosz A., Wielgosz A., Morrison H., Li W., Wang X., et al. 2014. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 371: 601–611. [DOI] [PubMed] [Google Scholar]
- 79.Kieneker L. M., Bakker S. J. L., de Boer R. A., Navis G. J., Gansevoort R. T., and Joosten M. M.. 2016. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 90: 888–896. [DOI] [PubMed] [Google Scholar]
- 80.Mayer B., Lieb W., Gotz A., Konig I. R., Aherrahrou Z., Doering A., Loewel H., Hense H. W., Schunkert H., and Erdmann J.. 2005. Association of the T8590C polymorphism of CYP4A11 with hypertension in the MONICA Augsburg echocardiographic substudy. Hypertension. 46: 766–771. [DOI] [PubMed] [Google Scholar]
- 81.Gainer J. V., Lipkowitz M. S., Yu C., Waterman M. R., Dawson E. P., Capdevila J. H., and Brown N. J.. 2008. The AASK Study Group. Association of a CYP4A11 variant and blood pressure in black man. J. Am. Soc. Nephrol. J. Am. Soc. Nephrol. 19: 1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laffer C. L., Gainer J. V., Waterman M. R., Capdevila J. H., Laniado-Schwartzman M., Nasjletti A., Brown N. J., and Elijovich F.. 2008. The T8590C polymorphism of CYP4A11 and 20 hydroxyeicosatetraenoic acid in essential hypertension. Hypertension. 51: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ward N. C., Tsai I. J., Barden A., vanBockxmeer F. M., Puddey I. B., Hodgson J. M., and Croft K. D.. 2008. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HET excretion and blood pressure. Hypertension. 51: 1393–1398. [DOI] [PubMed] [Google Scholar]
- 84.Medhora M., Daniels J., Mundey K., Fisslthaler B., Busse R., Jacobs E. R., and Harder D. R.. 2003. Epoxygenase driven angiogenesis in human lung microvascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 284: H215–H224. [DOI] [PubMed] [Google Scholar]
- 85.Fleming I. 2007. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins other Lipid Mediat. 82:60–67. [DOI] [PubMed] [Google Scholar]
- 86.Pozzi A., Ibanez M. R., Gatica A. E., Yang S., Wei S., Mei S., Falck J. R., and Capdevila J. H.. 2007. Peroxisomal proliferator-activated receptor-α-dependent inhibition of endothelial cell proliferation and tumorigenesis. J. Biol. Chem. 282: 17685–17695. [DOI] [PubMed] [Google Scholar]
- 87.Webler A. C., Michaelis U. R., Popp R., Barbosa-Sicard E., Murugan A., Falck J. R., Fisslthaler B., and Fleming I.. 2008. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am. J. Physiol. Cell Physiol. 295: C1292–C1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang S., Wei S., Pozzi A., and Capdevila J. H.. 2009. The arachidonic acid epoxygenase is a component of the signaling mechanisms responsible for VEGF-stimulated angiogenesis. Arch. Biochem. Biophys. 489: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Panigrahy D., Kaipainen A., Huang S., Butterfield C. E., Barnés C. M., Fannon M., Laforme A. M., Chaponis D. M., Folkman J., and Kieran M. W.. 2008. PPARα agonist fenofibrate suppress tumor growth through direct and indirect angiogenesis inhibition. Proc. Natl. Acad. Sci. USA. 105: 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falck J. R., Kodela R., Manne R., Atcha K. R., Puli N., Dubasi N., Manthati V. L., Capdevila J. H., Yi X-Y., Goldman D. H., et al. 2009. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble hydrolase inhibition. J Med Chem. 52:5069–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alonso-Galicia M., Falck J. R., Reddy K. M., and Roman R. J.. 1999. 20-HETE agonists and antagonists in the renal circulation. Am. J. Physiol. 277: F790–F796. [DOI] [PubMed] [Google Scholar]
- 92.Carroll M. A., Balazy M., Huang D-D., Rybalova S., Falck J. R., and McGiff J. C.. 1997. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int. 51: 1696–1702. [DOI] [PubMed] [Google Scholar]
- 93.Falck J. R., Koduru S. R., Mohapatra S., Manne R., Atcha K. R., Manthati V. L., Capdevila J. H., Christian S., Imig J. D., and Campbell W. B.. 2014. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates: carboxylate modifications. J. Med. Chem. 57: 6965–6972. [Erratum. 2014. J. Med. Chem. 57: 9218.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park S. K., Herrnreiter A., Pfister S. L., Gauthier K. M., Falck B. A., Falck J. R., and Campbell W. B.. 2018. GPR40 is a low affinity epoxyeicosatrienoic acid receptor in vascular cells. J. Biol. Chem. 293: 10675–10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong P. Y-K., Lai P-S., Shen S-Y., Belosludtsev Y. Y., and Falck J. R.. 1997. Post-receptor signal transduction and regulation of 14(R), 15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J. Lipid Mediat. Cell Signal. 16: 155–169. [DOI] [PubMed] [Google Scholar]
- 96.Wong P. Y-K., Lai P-S., and Falck J. R.. 2000. Mechanism and signal transduction of 14(R), 15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in guinea pig monocytes. Prostaglandins Other Lipid Med. 62: 321–333. [DOI] [PubMed] [Google Scholar]
- 97.Chen J. K., Chen J., Imig J. D., Wei S., Hachey D. L., Guthi J. S., Falck J. R., Capdevila J. H., and Harris R. C.. 2008. Identification of novel endogenous cytochrome P450 arachidonate metabolites with high affinity for cannabinoid receptors. J. Biol. Chem. 283: 24514–24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lundblad M. S., Stark K., Eliasson E., Oliw E., and Rane A.. 2005. Biosynthesis of epoxyeicosatrienoic acids varies between polymorphic CYP2C enzymes. Biochem. Biophys. Res. Commun. 327: 1052–1057. [DOI] [PubMed] [Google Scholar]