Abstract
Platelet-activating factor (PAF) is a potent inflammatory mediator that exerts its actions via the single PAF receptor (PAF-R). Cells that biosynthesize alkyl-PAF also make abundant amounts of the less potent PAF analogue acyl-PAF, which competes for PAF-R. Both PAF species are degraded by the plasma form of PAF acetylhydrolase (PAF-AH). We examined whether cogenerated acyl-PAF protects alkyl-PAF from systemic degradation by acting as a sacrificial substrate to enhance inflammatory stimulation or as an inhibitor to dampen PAF-R signaling. In ex vivo experiments both PAF species are prothrombotic in isolation, but acyl-PAF reduced the alkyl-PAF-induced stimulation of human platelets that express canonical PAF-R. In Swiss albino mice, alkyl-PAF causes sudden death, but this effect can also be suppressed by simultaneously administering boluses of acyl-PAF. When PAF-AH levels were incrementally elevated, the protective effect of acyl-PAF on alkyl-PAF-induced death was serially decreased. We conclude that, although acyl-PAF in isolation is mildly proinflammatory, in a pathophysiological setting abundant acyl-PAF suppresses the action of alkyl-PAF. These studies provide evidence for a previously unrecognized role for acyl-PAF as an inflammatory set-point modulator that regulates both PAF-R signaling and hydrolysis.
Keywords: PAF analogue, PAF acetylhydrolase, platelet aggregation, PAF-like lipids
Platelet-activating factor (PAF), a potent ether phospholipid, possesses both pro- and anti-inflammatory properties revolving around inflammation (1–3). In the literature, the term PAF generally refers to 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (alkyl-PAF) (1, 3). This is the most active form of biosynthetic PAF among all homologous species of phospholipids studied to date (4). A pharmacologically less active PAF species that is made concomitantly with alkyl-PAF is the acyl analogue of PAF, chemically identified as 1-acyl-2-acetyl-sn-glycero-3-phosphocholine (acyl-PAF) (5–11). In many cell types acyl-PAF is made in far greater abundance than alkyl-PAF when PAF biosynthetic machinery is appropriately activated (6, 11–15). In addition, nonenzymatic free-radical attack on polyunsaturated fatty acids esterified in the sn-2 position of glycerophosphocholines (GPCs) makes several truncated analogues of PAF, termed PAF-like lipids or PAF mimetics (16, 17). These are formed both in alkyl and acyl versions, which have variable potencies in activating the single PAF receptor (PAF-R) (16–20). Thus, PAF-R ligands are structurally diverse. However, all diverse PAF species and PAF-like lipids serve as substrates for the plasma form of PAF acetylhydrolase (PAF-AH), the major and best characterized isoform of the PAF-AH esterase family (21–24). Because of its ability to hydrolyze proinflammatory PAF and PAF-like lipids, PAF-AH is commonly considered as an anti-inflammatory enzyme (25–28); however, this notion has been disputed (29, 30). Several attempts by investigators to curtail PAF signaling by using an anti-PAF strategy have not yielded promising results. Furthermore, dynamic variation in endogenous PAF-AH levels has been noted in healthy (31) and critically ill (32–34) individuals, as well as in animal models of inflammation (35). The interplay between alkyl-PAF, acyl-PAF, PAF-AH, and PAF-R in vivo is complex, and the outcomes of these interactions have not been well delineated.
In this study, we used Swiss albino mice and isolated human platelets to provide experimental evidence that acyl-PAF functions as an endogenous modulator of PAF-R signaling (3, 36). Given that PAF-R can recognize multiple ligands with variable potencies and that PAF-AH can recognize multiple endogenous phospholipid substrates, we examined whether increasing the level of acyl-PAF could change the degree of PAF-R activation and whether increasing the levels of PAF-AH just above the circulating levels could provide clues regarding the enigmatic role of these lipid mediators in inflammation.
MATERIALS AND METHODS
Synthesis of acyl-PAF from lysophosphatidylcholine
Acyl-PAF was synthesized using the method previously described by Androulakis et al. (37). Lysophosphatidylcholine (lyso-PC) (type 2, from egg yolk, containing approximately 66% palmitic acid and 33% stearic acid at the sn-1 position of GPC) was purchased from Sigma-Aldrich (St. Louis, MO). Briefly, lyso-PC was acylated by combining 1 mg lyso-PC with acetic anhydride (0.625 ml) in the presence of dry benzene (0.125 ml) and stirring overnight at 37°C. The synthesized acyl-PAF was then extracted with the use of the Bligh and Dyer method (38). Briefly, the lipids were extracted in chloroform and methanol (acidified with 0.3% acetic acid) at a ratio of 1:2. After being vigorously mixed, the monophasic solution was split by adding chloroform and 0.1 M sodium acetate (1:1), resulting in the formation of a lower hydrophobic phase and an upper aqueous phase. The extracted acyl-PAF in the lower organic phase was evaporated under a stream of nitrogen and stored in methanol at –20°C. Synthesis of acyl-PAF was confirmed by thin-layer chromatography (precoated silica gel 60 F254). Alkyl-PAF and lyso-PAF (C16: predominantly hexadecyl at the sn-1 position of GPC catalog nos. 840009P and 840008P respectively) were purchased from Avanti Polar Lipids (Alabaster, AL). Alkyl-PAF, lyso-PAF, lyso-PC (stock solution: 5 mg/ml in methanol), and acyl-PAF (stock solution: 26 mg/ml in methanol) were spotted onto a silica gel plate (40 µg each) and allowed to migrate in the solvent system (chloroform-methanol-water; 65:35:6; v/v/v) (39). After the plates were dried, they were developed with iodine vapors in a closed chamber. Acyl-PAF was also quantified by lipid phosphorous with the use of the Bartlett assay (40). All experiments were also repeated using commercial preparations of acyl-PAF (1-O-palmitoyl-2-O-acetyl-sn-glycero-3-phosphorylcholine) (Enzo Life Sciences-BML-L134-0005, Inc., Farmingdale, NY).
Mice
The male and female Swiss albino mice used for this study were 8–10 weeks old and weighed 20–25 g. They were housed with adequate ventilation, food, and water (available ad libitum) and monitored for the experimental period. All experiments were approved by the Institutional Animal Ethical Committee of the University of Mysore(approval no. UOM/IAEC/13/2016).
The C57BL/6 wild-type mice expressing PAF-R were obtained from Wright State University, and the PAF-R−/− mice with a C57BL/6 background were a generous gift from Professor Takao Shimizu (University of Tokyo). All experiments with backcrossed C57BL/6 mice were performed at J.B.T. and R.P.S.’s laboratory, and the procedures were approved by the Institutional Animal Care and Use Committee of Wright State University.
Alkyl-PAF-induced mortality in Swiss albino mice
The intraperitoneal injection of alkyl-PAF was performed as previously described (41). Briefly, Swiss albino mice were assigned into six groups comprising six mice each. A stock solution of alkyl-PAF (5 mg/ml) was made in absolute methanol and stored at –20°C. An aliquot from the stock was dried under nitrogen and reconstituted in sterile PBS containing 0.1% human serum albumin (HSA; Baxter Healthcare, Glendale, CA) before use in siliconized tubes. The mice were intraperitoneally injected with 100, 112.5, 125, 200, or 250 μg/kg alkyl-PAF in a total volume of 0.5 ml, and the control mice received the vehicle used in the alkyl-PAF preparation.
Intraperitoneal injection of alkyl-PAF or acyl-PAF in wild-type C57BL/6 mice and PAF-R−/− mice
Wild-type C57BL/6 mice and PAF-R−/− mice were assigned to groups of six. The required amounts of stock acyl-PAF or alkyl-PAF in methanol were aliquoted into separate siliconized glass tubes, evaporated under a stream of nitrogen, reconstituted in sterile PBS containing 0.1% HSA, and injected intraperitoneally in a total volume of 0.5 ml. Control mice received the vehicle used for the acyl-PAF or alkyl-PAF injections.
Effect of acyl-PAF pretreatment on alkyl-PAF-induced mortality in Swiss albino mice
Swiss albino mice were assigned into 13 groups comprising 6 mice per group. A stock solution of synthesized acyl-PAF (26 mg/ml) in methanol was aliquoted into separate siliconized glass tubes, evaporated under a stream of nitrogen, and reconstituted in sterile PBS containing 0.1% HSA. The mice were pretreated with 2.5, 5.0, 7.5, 8.75, 10, 12.5, 25, or 50 mg/kg acyl-PAF. After 3 h, the mice were then challenged with a lethal dose of alkyl-PAF (250 µg/kg). The control mice received the abovementioned doses of acyl-PAF or alkyl-PAF alone in PBS containing 0.1% HSA.
Effect of WEB-2086 on alkyl-PAF-induced death in Swiss albino mice
WEB-2086 was a generous gift from Boehringer Ingelheim Pharmaceuticals (Ridgefield, CT). A stock solution of WEB-2086 (30 mg/ml) was prepared in DMSO, and aliquots were diluted with sterile PBS to 0.5 ml. Five groups of Swiss albino mice were injected with WEB-2086 (1, 5, 10, 20, or 30 mg/kg) and then challenged 3 h later with a lethal dose of alkyl-PAF (250 μg/kg). The control mice received only the vehicle used in the preparation of WEB-2086. The concentration of DMSO never exceeded 0.2% in these experiments.
Reactivation of PAF-R for alkyl-PAF-induced lethality in Swiss albino mice pretreated with acyl-PAF or WEB-2086
Swiss albino mice weighing 20–25 g were assigned to 4 groups comprising 6 mice each. The mice were injected intraperitoneally with acyl-PAF (12.5 mg/kg) and then challenged with a lethal dose of alkyl-PAF (250 μg/kg) at 3, 5, 7.5, or 10 h after the initial acyl-PAF injection. In a parallel experiment, 7 groups of mice were injected with WEB-2086 (30 mg/kg) for various time points such as 3, 5, 10, 15, 30, and 45 h, followed by a lethal dose of alkyl-PAF. The mice were monitored for survival for up to 6 days.
Comparison of the inhibitory actions of acyl-PAF, lyso-PC, and lyso-PAF on alkyl-PAF-induced death in Swiss albino mice
Swiss albino mice were assigned to different groups comprising six mice each. The mice were injected intraperitoneally with acyl-PAF, lyso-PC, or lyso PAF (12.5 mg/kg) 3 h before being challenged with a lethal dose of alkyl-PAF (250 μg/kg). The mice were then monitored for survival for up to 6 days.
Effect of exogenous recombinant PAF-AH pretreatment on alkyl-PAF-induced lethality in the presence of acyl-PAF
Recombinant PAF-AH (rPAF-AH) was a generous gift from Icos Corporation (Bothell, WA). Swiss albino mice weighing 20–25 g were assigned to 8 groups comprising 6 mice each. The mice were injected with rPAF-AH (0.5, 1, 3, or 5 µg per mouse) 30 min before they received an intraperitoneal injection of both alkyl-PAF (250 µg/kg) and acyl-PAF (12.5 or 25 mg/kg). Control mice received only a coinjection of alkyl-PAF (250 µg/kg) and acyl-PAF (12.5 or 25 mg/kg). The mice were then monitored for survival for up to 6 days.
Human platelet aggregation
Blood was drawn from healthy volunteers with informed consent. All experiments involving human blood samples were approved by the University of Mysore Institutional Human Ethics Committee, University of Mysore (approval no. IHEC-UOM No: 117 Ph.D/2015-16). BN-52021 was obtained from Enzo Life Sciences, Inc. A stock solution (10 mM) of WEB-2086 and BN-52021 was prepared in DMSO, and a working solution (1 mM) was diluted with saline and used in aggregation studies. Platelet-rich plasma (PRP) was isolated from the blood of healthy volunteers using the method previously described by Zhou et al. (42). Briefly, blood was drawn into citrated tubes (1:9; citrate-blood), and the tubes were centrifuged for 15 min at 45 g at 28°C to obtain PRP. The remaining cells were settled by centrifuging the tubes at 2,200 g for 15 min, and the platelet-poor plasma obtained was used for setting the blank and diluting the PRP. Platelet aggregation was performed with the use of 4 × 108 platelets/ml in a final volume of 250 μl with stirring at 1,200 rpm at 37°C for up to 6 min. An aliquot of alkyl-PAF taken from a 10 mM stock (in methanol) was evaporated under a stream of nitrogen and reconstituted in PBS containing 0.1% HSA, yielding a 1 mM working solution. Using this solution, aggregation was initiated by adding alkyl-PAF (8 µM final concentration) to the PRP. The aggregation effects of acyl-PAF were determined by using the same protocol, but a concentration of 800 µM acyl-PAF was needed to induce effects similar to those of alkyl-PAF.
To determine the effect of PAF-R antagonists on platelet aggregation mediated by acyl-PAF or alkyl-PAF, PRP was pretreated with WEB-2086 (10 μM) or BN-52021 (10 μM) for 5 min and then exposed to acyl-PAF (800 µM) or alkyl-PAF (8 µM). In all aggregation studies, the final concentrations of DMSO never exceeded 0.1%. The dampening effect of acyl-PAF on PAF-R was studied by pretreating or simultaneously exposing platelets with the stated amounts of acyl-PAF (5 nM to 800 μM) and then stimulating platelets with alkyl-PAF (8 µM). In some of the aggregation experiments, alkyl-PAF or acyl-PAF was first predigested with various concentrations of rPAF-AH (stock solution: 4 µg/ µl) for 30 min at 37°C and then monitored for its ability to induce aggregation. In some experiments, the effect of rPAF-AH on aggregation induced by acyl-PAF or alkyl-PAF was determined by treating PRP simultaneously with either alkyl-PAF (8 µM) or acyl-PAF (800 µM) and rPAF-AH (50–200 ng). All assays were performed by using a Chrono-log platelet aggregometer (Chrono-Log Corp.., Havertown, PA), and the data were recorded by using AGROLINK software.
LC-MS analysis of phospholipids during platelet aggregation
PRP was stimulated with combinations of acyl-PAF (800 µM), alkyl-PAF (8 µM), or both in the absence or presence of rPAF-AH (100 ng per assay). Aggregation was stopped after 3 min by the addition of acidified methanol-chloroform (1:2; v/v). Total lipids were extracted by using the Bligh and Dyer method (38), dried under nitrogen, and then reconstituted in methanol. A methanolic aliquot was used to quantify acyl-PAF, alkyl-PAF (C16: predominantly hexadecyl at the sn-1 position of GPC), lyso-PC, and lyso-PAF as described previously (16, 43, 44). Products or substrates present at the end of the third minute during platelet aggregation were measured relative to C16:0-d4-PAF (Cayman Chemical, Ann Arbor, MI) and are expressed as the mean number of nanomoles per assay from two independent experiments.
Statistics
All in vivo experiments were performed with groups of six mice, and individual experiments were repeated at least three times. Representative results from the three individual experiments are presented. In the case of the platelet aggregation studies, one set of experiments was carried out using platelets from the same donor and then repeated with platelets from at least three different donors.
RESULTS
Acyl-PAF suppresses alkyl-PAF-induced mortality
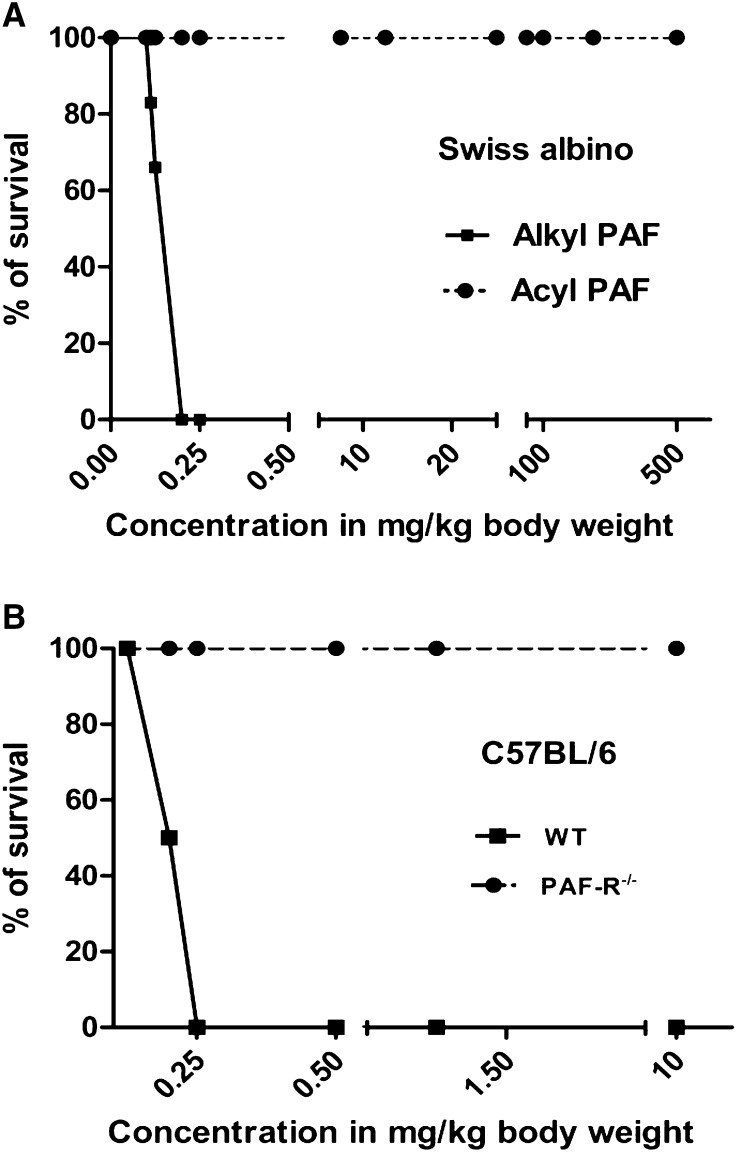
We have shown previously that the intraperitoneal injection of alkyl-PAF causes sudden death in Swiss albino mice and that this effect could be inhibited by pretreating the mice with a PAF-R antagonist or rPAF-AH (41). To reconfirm that these effects are due to direct PAF-R activation, we also used C57BL/6 wild-type and PAF-R−/− mice to determine whether the lethal effects of alkyl-PAF in fact result from the single PAF-R (Fig. 1A). The wild-type mice were completely susceptible to death induced by alkyl-PAF, whereas the PAF-R−/− mice were unaffected by alkyl-PAF, even at a dose 40 times higher than the lethal dose used for wild-type mice (Fig. 1B).
Fig. 1.
Effects of alkyl-PAF and acyl-PAF on mortality in mice. A: Dose-dependent effects of alkyl-PAF (solid line) and acyl-PAF (broken line) on the survival of Swiss albino mice. Alkyl-PAF caused sudden death in Swiss albino mice, whereas acyl-PAF did not induce death in these mice at the doses tested (although unphysiologic, acyl-PAF alone can also cause death at 1 g/kg body weight; data not shown). B: The deletion of PAF-R in C57BL/6 mice protected the mice from alkyl-PAF-induced death, whereas wild-type mice were sensitive to alkyl-PAF. The best representative results of three individual experiments are shown.
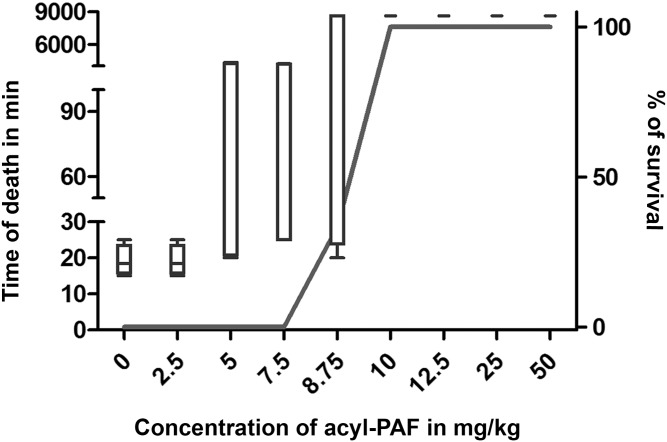
Acyl-PAF has historically been considered to be a pharmacologically less active analogue of PAF or even an inactive analogue of PAF (45). Therefore, using Swiss albino mice as a model (41), we also tested the hypothesis that acyl-PAF is simply a less functional analogue than alkyl-PAF. When we injected increasing amounts of acyl-PAF (2.5–500 mg/kg) alone, none of the mice died (Fig. 1A). Three hours later, when these mice received a lethal dose of alkyl-PAF (250 µg/kg), mice that received acyl-PAF at doses between 10 and 50 mg/kg were completely protected from alkyl-PAF-induced death (Fig. 2). In mice that received acyl-PAF at a dose above 100 mg/kg, the protection offered by acyl-PAF progressively declined (data not shown). In another experiment, we found that injecting 1 g/kg acyl-PAF alone caused death in all of the mice within 30 min, defining the upper limit of acyl-PAF-induced lethality (data not shown). We also found that a minimum dose of 30 mg/kg WEB-2086, the most potent commercially available PAF-R antagonist, was needed to provide the same level of protection provided by 12.5 mg/kg acyl-PAF (Table 1, Fig. 2). These experiments suggest that low concentrations of the pharmacologically less active acyl-PAF and possibly other acyl-PAF-like lipids described in the literature (1, 3, 16) may dampen PAF-R signaling.
Fig. 2.
Effect of acyl-PAF on PAF-R dampening in alkyl-PAF-mediated lethality in Swiss albino mice. Mice (20–25 g) were divided into 9 groups containing 6 mice each. In this experiment each group of mice was injected with the indicated amount of acyl-PAF (2.5–50 mg/kg) in a total volume of 0.5 ml PBS containing 0.1% HSA. After 3 h, the same mice were injected with a lethal dose of alkyl-PAF (250 µg/kg), and survival time was monitored. The control group received only alkyl-PAF at a dose of 250 µg/kg, and all of the mice died between 15 and 20 min. The survival of mice was monitored for up to 6 days. The open bar represents the time of death in minutes, and the solid line represents the percentage survival of individual groups of mice. The best representative results of three individual experiments are shown.
TABLE 1.
Protective effect of WEB-2086 on alkyl-PAF-mediated lethality in Swiss albino mice
| Group | First Injection | Second Injection | Deaths/Total | Survival (%) |
| 1 | Vehicle I (PBS containing 0.1% HSA) | — | 0/6 | 100 |
| 2 | Vehicle II (DMSO + PBS; for WEB-2086) | — | 0/6 | 100 |
| 3 | Alkyl-PAF (250 µg/kg) | — | 6/6 (all died between 15 and 20 min) | 0 |
| 4 | WEB-2086 (1 mg/kg) | Alkyl-PAF (250 μg/kg) | 6/6 (all died between 20-30 min) | 0 |
| 5 | WEB-2086 (5 mg/kg) | Alkyl-PAF (250 μg/kg) | 4/6 (4 died between 20 and 35 min) | 33.33 |
| 6 | WEB-2086 (10 mg/kg) | Alkyl-PAF (250 μg/kg) | 1/6 (1 died between 50 and 60 min) | 83.33 |
| 7 | WEB-2086 (20 mg/kg) | Alkyl-PAF(250 μg/kg) | 1/6 (1 died between 60 and 65 min) | 83.33 |
| 8 | WEB-2086 (30 mg/kg) | Alkyl-PAF(250 μg/kg) | 0/6 | 100 |
Mice weighing between 20 and 25 g were assigned into eight groups, each of which contained 6 mice. Mice were injected intraperitoneally with the indicated doses of the PAF-R antagonist WEB-2086. Three hours later, they were challenged with a lethal dose of alkyl-PAF (250 μg/kg) in a final volume of 0.5 ml PBS containing 0.1% HSA. Control mice received the vehicle used in the preparation of WEB-2086 (DMSO + PBS). The concentration of DMSO never exceeded 0.2% in any of the experiments. Survival was monitored for up to 6 days.
In a separate set of experiments, we also tested whether lyso-PC or lyso-PAF (products of PAF-AH action on acyl-PAF and alkyl-PAF, respectively) could substitute for acyl-PAF and mediate protection against alkyl-PAF-induced lethality at a dose equivalent to the effective dose of acyl-PAF (46). Lyso-PC did not protect mice from alkyl-PAF-induced death at the dose tested, although a delay in death was observed. In contrast, an equivalent dose of lyso-PAF prevented not only 50% of alkyl-PAF-induced death but also prolonged alkyl-PAF-mediated lethality even further (Table 2). These experiments showed that lyso-PC and lyso-PAF have differential effects on alkyl-PAF-induced lethality (Table 3).
TABLE 2.
Comparison of the relative inhibitory actions of acyl-PAF, lyso-PC, and lyso-PAF on alkyl-PAF-induced death in Swiss albino mice
| Group | First Injection | Second Injection | Deaths/Total | Survival (%) |
| 1 | Vehicle (PBS containing 0.1% HSA) | — | 0/6 | 100 |
| 2 | Alkyl-PAF (250 μg/kg) | — | 6/6 (all died between 15 and 20 min) | 0 |
| 3 | Acyl-PAF (12.5 mg/kg) | Alkyl-PAF (250 μg/kg) | 0/6 | 100 |
| 4 | Lyso-PC (12.5 mg/kg) | Alkyl-PAF (250 μg/kg) | 6/6 (1 died within 20 min; 2 died between 15 and 16 h; 1 died between 20 and 21 h; and 2 died between 40 and 43 h) | 0 |
| 5 | Lyso-PAF (12.5 mg/kg) | Alkyl-PAF (250 μg/kg) | 3/6 (1 died between 15 and 16 h; 1 died between 30 and 36 h; and 1 died between 40 and 41 h) | 50 |
Swiss albino mice weighing between 20 and 25 g were assigned into 5 groups, each of which contained 6 mice. Mice were injected with acyl-PAF or lyso-PC or lyso-PAF (12.5 mg/kg) in a final volume of 0.5 ml PBS containing 0.1% HSA 3 h prior to injecting a lethal dose of alkyl-PAF (250 μg/kg). Control mice received the vehicle used in the preparation of acyl-PAF or lyso-PAF or lyso-PC (0.5 ml PBS containing 0.1% albumin). Survival was monitored for up to 6 days.
TABLE 3.
Effect of exogenous rPAF-AH on acyl-PAF-mediated protection
| rPAF-AH (µg) | Coinjection of 12.5 mg/kg Acyl-PAF + 250 µg/kg Alkyl-PAF (Deaths/Total) | Coinjection of 25 mg/kg Acyl-PAF + 250 µg/kg Alkyl-PAF (Deaths/Total) |
| — | 0/6 | 0/6 |
| 0.5 | 1/6 (1 died between 20 and 30 min) | 0/6 |
| 1 | 6/6 (3 died between 20 and 30 min and 3 died between 13 and 16 h) | 6/6 (1 died between 50 and 65 min and 5 died between 15 and 18 h) |
| 3 | 2/6 (2 died between 40 and 45 min) | 0/6 |
| 5 | 0/6 | 0/6 |
Mice were divided into 10 groups, each of which contained 6 mice. Mice were injected with various doses of rPAF-AH (0.5, 1, 3, or 5 μg) 30 min before the coinjection of alkyl-PAF (250 μg/kg) and acyl-PAF (12.5 or 25 mg/kg) in a final volume of 0.5 ml PBS containing 0.1% HSA. Control mice received only the coinjection of alkyl-PAF (250 μg/kg) and acyl-PAF (12.5 or 25 mg/kg).
Reactivation of PAF-R for alkyl-PAF-induced lethality in Swiss albino mice pretreated with acyl-PAF or WEB-2086
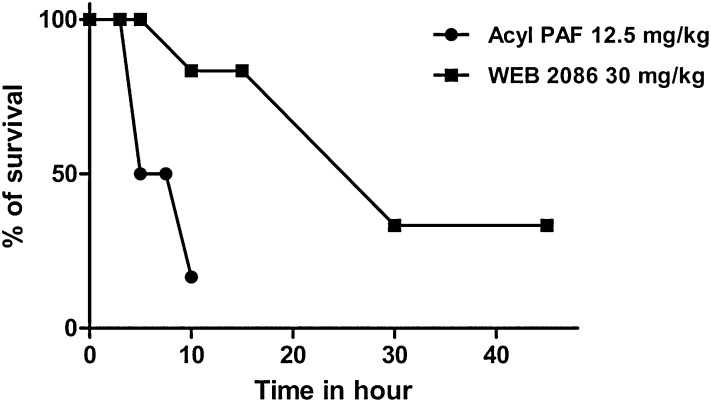
Given our observation that acyl-PAF has a dampening effect on PAF-R activation, we next determined the duration of acyl-PAF-induced dampening action by assessing the reactivation of PAF-R in Swiss albino mice at various time points. Briefly, we first injected mice with acyl-PAF at a fixed dose of 12.5 mg/kg, which was chosen based on our previous experiments (Fig. 2). We then challenged the mice with a lethal dose of alkyl-PAF (250 µg/kg) at 3, 5, 7.5, or 10 h after the initial PAF-R dampening. When pretreated with acyl-PAF, none of the mice died after being challenged 3 h later with a lethal dose of alkyl-PAF. However, 50% of the mice died when challenged 5 or 7.5 h later, and 83% died when challenged 10 h later (Fig. 3). Thus, the complete PAF-R dampening effects lasted for only 3 h when acyl-PAF was used as the dampening agent. A different scenario was observed when WEB-2086 (30 mg/kg) was used as the PAF-R dampening agent. None of the mice died when they were pretreated with WEB-2086 and then challenged with a lethal dose of alkyl-PAF 5 h later. Only 17% died when challenged 10 h after the WEB-2086 pretreatment. Even in the groups that received the alkyl-PAF injection 30 or 45 h after the WEB-2086 pretreatment, 33% of the mice still survived (Fig. 3).
Fig. 3.
Reactivation of PAF-R in Swiss albino mice pretreated with acyl-PAF or WEB-2086. Swiss albino mice (20–25 g) were assigned into 13 groups containing 6 mice each. Each group of mice was pretreated with acyl-PAF (12.5 mg/kg) or WEB-2086 (30 mg/kg) for various time intervals (0–45 h), followed by a lethal dose of alkyl-PAF (250 μg/kg) in a final volume of 0.5 ml PBS containing 0.1% HSA. Control mice received the vehicle used in the preparation of acyl-PAF or alkyl-PAF (PBS containing 0.1% albumin) or WEB-2086 (DMSO + PBS). The survival of mice was monitored for up to 6 days. The best representative results of three individual experiments are shown.
Effect of elevating the circulating levels of PAF-AH on acyl-PAF-mediated PAF-R dampening in Swiss albino mice
In the next series of experiments, we determined the effect of exogenous rPAF-AH on the acyl-PAF-induced dampening of PAF-R. Control mice were coinjected with both acyl-PAF (12.5 mg/kg) and alkyl-PAF (250 µg/kg). As expected, none of the mice died. We then injected 4 groups of mice with increasing doses of rPAF-AH (0.5, 1, 3, or 5 μg per mouse) 30 min before challenging them with the same coinjection of PAF analogues (Table 3). In the group of mice that received rPAF-AH at a dose of 0.5 µg, only 17% of the mice died, with the single death occurring within 30 min of the coinjection. When the rPAF-AH dose was increased to 1 µg, 50% of the mice died between 20 and 30 min after the coinjection, whereas the rest died between 13 and 16 h. When the rPAF-AH dose was increased to 3 µg, only 33.3% of the mice died, with the deaths occurring between 40 and 45 min after the coinjection. Finally, when the rPAF-AH dose was increased to 5 µg, none of the mice died. We repeated the same set of experiments but used double the dose of acyl-PAF (25 mg/kg) and kept the alkyl-PAF level the same for the coinjection (Table 3). Only the mice that received rPAF-AH at a dose of 1 µg died after the coinjection; however, the deaths of all of these mice were delayed. These experiments clearly indicate that PAF-AH preferentially hydrolyzes the abundant acyl-PAF over alkyl-PAF; thus, the sheer abundance of acyl-PAF makes it a readily available target for PAF-AH hydrolysis that competes with alkyl-PAF.
Human platelet aggregation with acyl-PAF, alkyl-PAF, and rPAF-AH
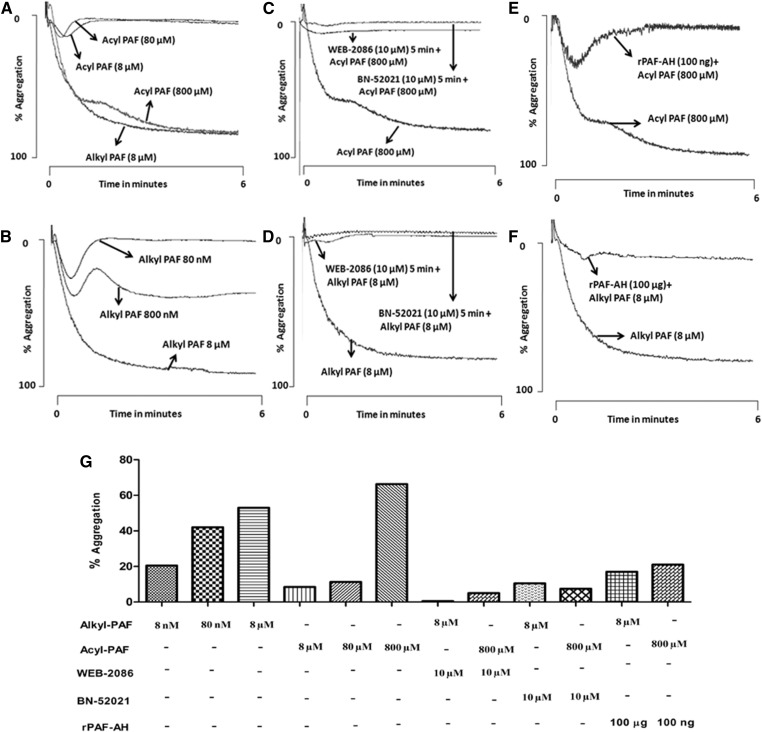
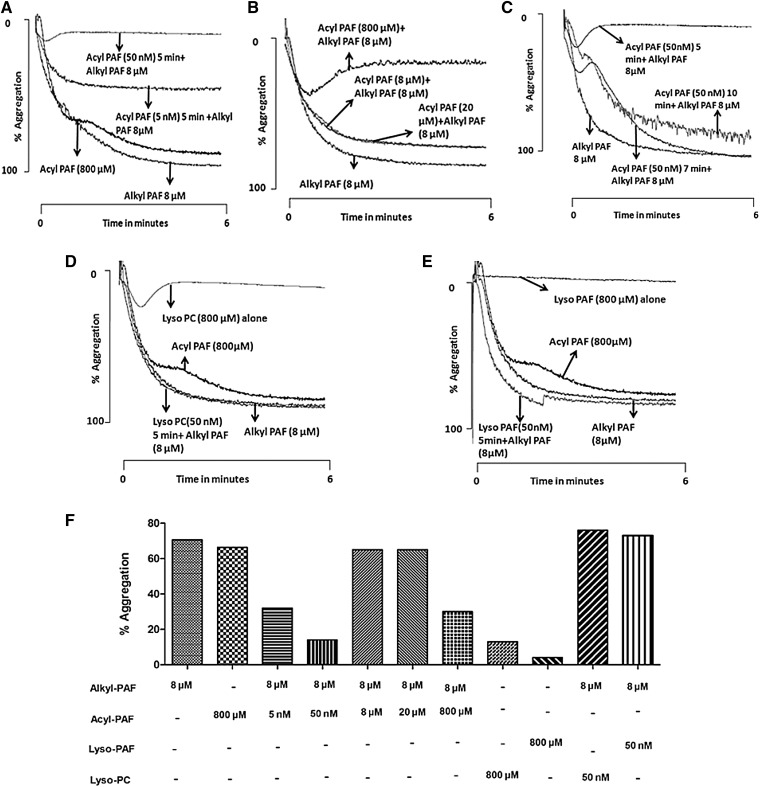
Our foregoing animal studies suggested that acyl-PAF acts as an antagonist of alkyl-PAF-mediated death via PAF-R stimulation. We confirmed this finding by using isolated human cells. For these experiments, we chose to use human platelets, which express functional PAF-R, the activation of which can easily be monitored with platelet aggregometry. Human platelets aggregated in response to both acyl-PAF and alkyl-PAF (Fig. 4A, B). Alkyl-PAF induced concentration-dependent platelet aggregation, with complete aggregation occurring at the 8 µM concentration (although this concentration varied among platelet donors, as with other stimuli) (Fig. 4A). To attain the same level of aggregation, a concentration of 800 µM acyl-PAF was required (Fig. 4B). Therefore, in isolated cells, acyl-PAF was 100 times less potent than its alkyl-PAF counterpart, a difference in potency that varied from that observed in vivo (Fig. 2). Platelet aggregation induced by either acyl-PAF or alkyl-PAF was inhibited when the platelets were preincubated with WEB-2086 or BN-52021, two structurally different PAF-R antagonists (Fig. 4C, D). Predigestion with rPAF-AH also abolished the ability of both PAF homologues to induce platelet aggregation, although the amount of rPAF-AH required to abolish the observed platelet aggregation with acyl-PAF was less than that required for alkyl-PAF (Fig. 4E, F).
Fig. 4.
Acyl-PAF is less potent than alkyl-PAF in inducing human platelet aggregation via PAF-R. The defined amounts of acyl-PAF (A) or alkyl-PAF (B) were taken from their respective methanolic stocks in separate siliconized glass tubes, evaporated under a stream of nitrogen, and reconstituted in PBS containing 0.1% HSA. Platelet aggregation was initiated with the use of human PRP (4 × 108 platelets/ml) in a fixed volume of 250 μl with various concentrations of alkyl-PAF (A) (80 or 800 nM or 8 μM) and acyl-PAF (B) (8, 80, or 800 μM). In some experiments (C, D), PRP was preincubated with WEB-2086 (10 μM) or BN-52021 (10 μM) for 5 min before stimulating with alkyl-PAF (C) or acyl PAF (D) at a final concentration of 8 μM and 800 μM, respectively. In parallel experiments (E, F), alkyl-PAF (E) or acyl-PAF (F) predigested with rPAF-AH for 30 min at 37°C was used as a platelet agonist under similar conditions. A bar graph shows the percentage platelet aggregation (G). In all aggregation experiments, either alkyl-PAF (8 µM) or acyl-PAF (800 µM) was used to induce the response of platelets. Each set of experiments was carried out with the use of platelets from the same donor and then repeated with platelets from at least three different donors. All assays were performed with the use of a Chrono-log platelet aggregometer, and traces were recorded with the use of AGROLINK software.
Exposing human platelets to a concentration as low as 50 nM acyl-PAF made them unable to respond to a subsequent challenge with alkyl-PAF (Fig. 5A), a result comparable to that observed for the in vivo experiment reported in Fig. 2. Simultaneous stimulation with both acyl-PAF and alkyl-PAF did not superactivate the platelets but rather dampened the aggregation (Fig. 5B). We then measured the time required by the acyl-PAF-pretreated platelets to regain sensitivity to alkyl-PAF, as we did in our animal studies shown in Fig. 3. We found that the acyl-PAF-pretreated platelets regained sensitivity to alkyl-PAF in 7–10 min (Fig. 5C) (although this result also varied from donor to donor), indicating that the acyl-PAF-mediated dampening actions of PAF-R are temporary. As shown in Fig. 5D and E, lyso-PAF or lyso-PC alone failed to aggregate platelets at a dose equivalent to the effective dose of acyl-PAF and were ineffective in dampening PAF-R for further stimulation with alkyl-PAF.
Fig. 5.
Dampening of PAF-R activation by acyl-PAF, lyso-PC, and lyso-PAF and the subsequent reactivation of PAF-R on human platelets. A: The stated amounts of acyl-PAF (5 or 50 nM) were prepared as described previously, incubated for 5 min with PRP (4 × 108platelets/ml), and then stimulated with alkyl-PAF (8 μM) to induce aggregation. In some experiments (B), platelets were costimulated with alkyl-PAF (8 μM) and acyl-PAF (8, 20, or 800 μM). C: PRP was preincubated for various time periods such as 5, 7, or 10 min with a fixed amount of acyl-PAF (50 nM) before the addition of alkyl-PAF (8 μM). In some experiments (D, E), PRP was pretreated with lyso-PAF (50 nM) (D) or lyso-PC (50 nM) (E) for 5 min and then monitored for aggregation after the addition of alkyl-PAF (8 μM). The effect of lyso-PAF alone (D) or lyso-PC alone (E) on PRP aggregation was also measured. Each set of experiments used platelets from the same donor and then was repeated with platelets from at least three different donors. In each aggregation panel, alkyl-PAF (8 µM) and acyl-PAF (800 µM) were used to monitor the response of platelets. The percentage platelet aggregation is shown in the bar graph (F). All assays were performed with the use of a Chrono-log platelet aggregometer, and traces were recorded with the use of AGROLINK software.
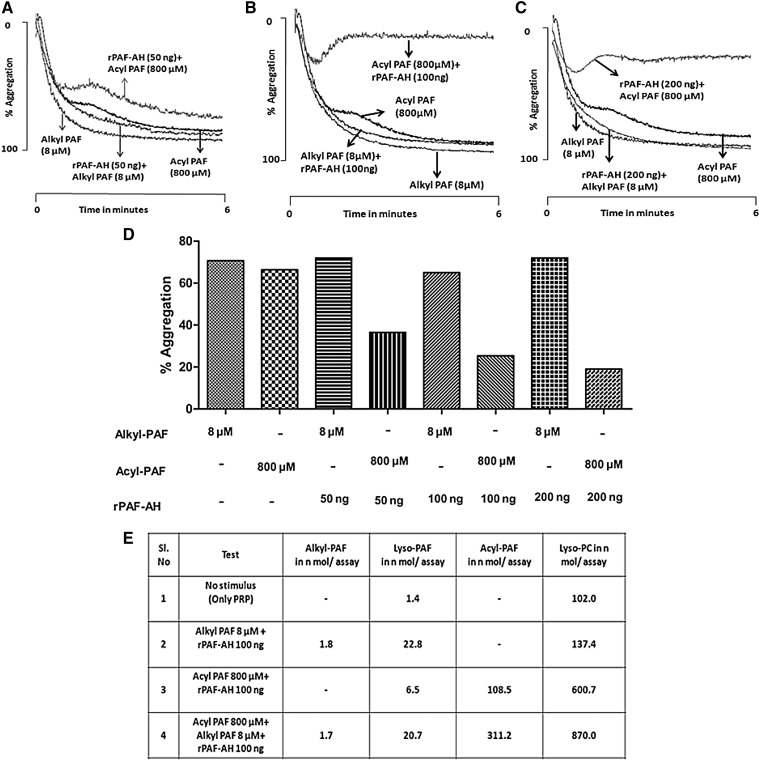
Furthermore, to examine the ability of rPAF-AH to hydrolyze acyl-PAF versus alkyl-PAF, we simultaneously exposed platelets to acyl-PAF (800 µM) or alkyl-PAF (8 µM) with varying amounts (50–200 ng per assay) of rPAF-AH (Fig. 6A–C). We found that the effect of rPAF-AH on hydrolysis was more pronounced for acyl-PAF than for alkyl-PAF. LC-MS analysis of these phospholipids during platelet aggregation stimulated with a combination of acyl-PAF (800 µM), alkyl-PAF (8 µM), or both, in the presence or absence of rPAF-AH (100 ng/ assay), showed that the lyso-PC released from acyl-PAF (800 µM) hydrolysis was greater than the amount of lyso-PAF released from alkyl-PAF (8 µM) hydrolysis during the hydrolytic reaction by rPAF-AH (Fig. 6E). In all aggregation studies, for each set of experiments, either alkyl-PAF (8 µM) or acyl-PAF (800 µM) was used to confirm the responsiveness of platelets.
Fig. 6.
Preferential hydrolysis of acyl-PAF over alkyl-PAF by rPAF-AH and the quantification of phospholipid substrates and products of rPAF-AH actions with the use of LC-MS. Human PRP (4 × 108 platelets/ml) in a fixed volume of 250 μl was simultaneously stimulated with alkyl-PAF or acyl-PAF at a final concentration of 8 μM and 800 μM, respectively, in the presence of rPAF-AH at three different concentrations, 50 ng (A), 100 ng (B), and 200 ng (C), and monitored for aggregation. Each set of experiments was carried out with the use of platelets from the same donor and then repeated with platelets from at least three different donors, showing similar aggregation traces. In each aggregation panel, alkyl-PAF (8 µM) or acyl-PAF (800 µM) was used to test the sensitivity of platelets. The percentage platelet aggregation is represented in the bar graph from each experiment (D). PRP was stimulated with respective combinations of acyl-PAF (800 µM), alkyl-PAF (8 µM), or both in the presence and absence of rPAF-AH (100 ng per assay). Aggregation was stopped after 3 min, and lipids were extracted and subjected to LC-MS analysis for the presence of acyl-PAF, lyso-PC, lyso-PAF, and alkyl-PAF (E) relative to a d4 PAF standard. All assays were performed with the use of a Chrono-log platelet aggregometer, and traces were recorded with the use of AGROLINK software.
DISCUSSION
Activation of PAF-R by PAF and structurally related PAF analogues plays a critical role in many inflammatory disorders (1, 3, 47). Almost all cell types studied to date synthesize not only alkyl-PAF but also the structurally related acyl-PAF, which is produced in greater abundance (6, 11–15). For example, endothelial cells that use cell-associated PAF in the initial steps of inflammation make more acyl-PAF than alkyl-PAF (6). Although acyl-PAF can have proinflammatory effects, it was a less potent activator of PAF-R than alkyl-PAF was in both our in vivo (Fig. 1A) and in vitro (Fig. 4B) experiments.
We have previously shown that the intraperitoneal injection of alkyl-PAF causes sudden death in Swiss albino mice by activating PAF-R (41). In this study, we confirmed this phenomenon with PAF-R-null mice (Fig. 1B). We repeated the previous experiments with C57BL/6 mice because PAF-R−/− mice are not available in a Swiss albino background. We found that alkyl-PAF caused PAF-R-mediated death in these mice in a similar dose and time frame to that observed in the Swiss albino mice (41). As expected, PAF-R−/− mice showed resistance for alkyl-PAF even when a dose 40-fold higher than the lethal dose was given.
With this background in mind, we designed our study to examine the effect of the abundant PAF structural analogue acyl-PAF on PAF-R activation. We found that acyl-PAF by itself was not toxic to the mice under physiologic conditions unless it was given at a supraphysiologic dose (data not shown). Intriguingly, we found that acyl-PAF at a dose of 10–50 mg/kg protected mice from alkyl-PAF-induced mortality (Fig. 2). The inhibitory effects of acyl-PAF were then compared with those of the potent, synthetic PAF-R antagonist WEB-2086 (Fig. 3). We found that acyl-PAF displayed inhibitory effects when used at a dose of 12.5 mg/kg, whereas for WEB-2086, a minimum dose of 30 mg/kg was required to achieve the same effect. This suggests that acyl-PAF is a better antagonist of PAF-R-induced lethality than the well-studied antagonist WEB-2086 (Table 1, Fig. 2).
We next sought to define the duration of protection exerted by acyl-PAF and found that, 5 h after being pretreated with acyl-PAF, mice exhibited a loss of protection, indicating that the reactivation of PAF-R in response to alkyl-PAF had occurred (Fig. 3). Thus, the protection provided by acyl-PAF from alkyl-PAF-induced lethality was temporary and quite different from the protection provided by WEB-2086 (Fig. 3). One possible explanation for the short duration of protection provided by acyl-PAF is its susceptibility to degradation by endogenous PAF-AH, which results in the reactivation of PAF-R for subsequent injections of alkyl-PAF. On the other hand, WEB-2086 contains no ester bonds, is not a substrate for PAF-AH, and may follow a different P-glycoprotein-dependent slower catabolic route (48). We observed that repeated injection of acyl-PAF did not protect against alkyl-PAF-induced animal death under our experimental conditions, suggesting that the continuous presence of acyl-PAF may not serve as a natural antagonist for PAF-R (data not shown).
Our in vivo findings were recapitulated in our in vitro experiments by using platelet aggregometry (Fig. 5A, B). In these experiments, exposing platelets to 50 nM acyl-PAF was sufficient to block the aggregation normally induced by alkyl-PAF (8 μM). This suggested that acyl-PAF dampens PAF-R activation induced by subsequent exposure to alkyl-PAF. Simultaneously exposing platelets to both acyl-PAF and alkyl-PAF did not superactivate the platelets; rather, it diminished the ability of the platelets to aggregate (Fig. 5B). Consistent with the results reported in the literature (6, 11, 49), we have found acyl-PAF to be 100-fold less potent than alkyl-PAF in in vitro studies (16, 46), including the current platelet aggregation studies (Fig. 4B). However, when we used Swiss albino mice as a model, we found that acyl-PAF was 2,000-fold less potent than alkyl-PAF (Fig. 2). This is not surprising because these two experimental readouts are different. Moreover, murine platelets are less sensitive to PAF than human platelets (50). The possibility remains that the other cell types expressing PAF-R may mediate these effects to PAF analogues in murine models.
When we tested for the activation of PAF-R by pretreating mice or human platelets with phospholipids such as lyso-PC or lyso-PAF at the same dose used for acyl-PAF (Table 2, Fig. 5D, E), the results showed that acyl-PAF preferentially dampened PAF-R more than lyso-PC did. Although not experimentally tested yet, we believe that the better dampening effect exhibited by lyso-PAF over lyso-PC observed in the mice may have been due to partial reacetylation of lyso-PAF to alkyl-PAF or possibly to rapid interactions between lyso-PAF and PAF-R than to lyso-PC, given that lyso-PAF contains an ether linkage, an essential feature for PAF-R in recognizing its ligands.
The major PAF-catabolizing enzyme in plasma is PAF-AH, which does not discriminate between sn-2 residues of acyl-PAF and alkyl-PAF. This enzyme has been described as both a pro-and anti-inflammatory enzyme (29, 30), although its potential anti-inflammatory role has been questioned (3, 36). Targeting PAF-AH by using its selective inhibitor darapladib was promising in specific animal models of inflammation, but this approach failed to reduce the risk of atherosclerotic lesion formation in clinical trials (51, 52). Dynamic variation in this enzyme has been seen in both humans and experimental animal models (31). Moreover, the plasma form of PAF-AH is susceptible to oxidative modification (53), and it is regulated at the transcription level by multiple mediators (54), suggesting a dynamic role for this enzyme in maintaining levels of acyl-PAF, alkyl-PAF, and PAF mimetics. For this reason, we studied the effect of PAF-AH levels on dampening the activation of PAF-R in the presence of acyl-PAF by progressively increasing the circulating levels of PAF-AH to mimic the dynamic variation observed in both physiologic and pathologic settings (31). Prior work (55) has not titrated PAF-AH levels but instead used overwhelming doses of rPAF-AH, which hydrolyzes both acyl-PAF and alkyl-PAF. Progressively increasing the level of circulating PAF-AH by administering exogenous rPAF-AH caused the mice to die sooner than the control mice that received only a coinjection of acyl-PAF and alkyl-PAF (Table 3). The results of our LC-MS analysis also suggested the preferential hydrolysis of the abundant substrate acyl-PAF rather than alkyl-PAF. Such a scenario may allow alkyl-PAF to accumulate and manifest its effects on PAF-R during disease progression.
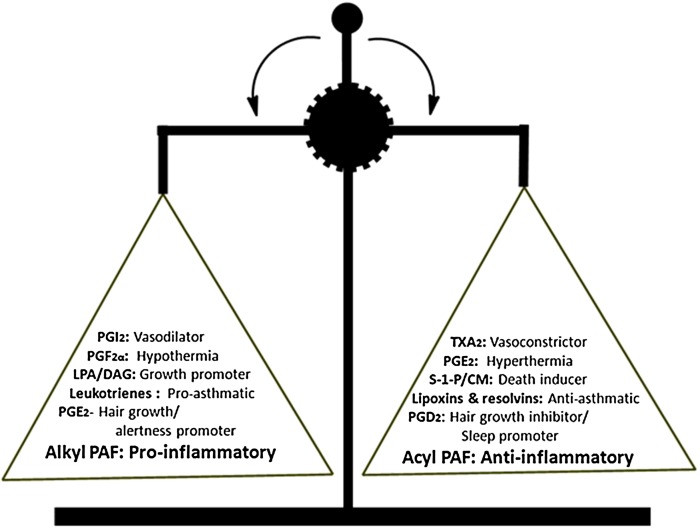
Finally, our results suggest that alkyl-PAF promotes inflammation, whereas acyl-PAF dampens it. Therefore, this lipid mediator pair can be added to those that maintain homeostatic balance (Fig. 7). Moreover, acyl-PAF acts as an inflammatory set-point modulator that regulates both PAF-R signaling and hydrolysis. In contrast to the other lipid pairs listed in Fig. 7 that exert their opposing actions through different receptors, alkyl-PAF and acyl-PAF exert their actions through a single canonical PAF-R, making them a distinct class of lipid mediators of homeostasis. In view of our current findings, PAF biology continues to be a challenge for both academicians and clinicians alike in unraveling the enigmatic role of this lipid mediator in health and disease.
Fig. 7.
Lipid mediator balance. Pairs of lipid mediators/eicosanoids with opposing biological actions are enlisted. According to our findings, the lipid mediators alkyl-PAF and acyl-PAF may be added to this growing list. Alkyl-PAF functions as a promoter of inflammation, whereas acyl-PAF dampens inflammation in isolation. However, unlike the other lipid mediator pairs shown, alkyl-PAF and acyl-PAF exert their actions through a single PAF receptor. CM, ceramide; DAG, diacylglycerol; LPA, lysophosphatidic acid; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; PGI2, prostaglandin I2; S-1-P, sphingosine 1-phosphate; TXA2, thromboxane A2.
Acknowledgments
The authors thank the Cleveland Clinic Lerner Research Institute for the LC-MS analysis and the Texas Heart Institute for editorial assistance. The Department of Studies in Biochemistry, University of Mysore, received financial assistance from the Special Assistance Programme (SAP), and the Vision Group of Science & Technology (VGST), Government of Karnataka, India.
Footnotes
Abbreviations:
- GPC
- glycerophosphocholine
- HSA
- human serum albumin
- lyso-PC
- lysophosphatidylcholine
- PAF
- platelet-activating factor
- PAF-AH
- platelet-activating factor acetylhydrolase
- PAF-R
- platelet-activating factor receptor
- PRP
- platelet-rich plasma
- rPAF-AH
- recombinant platelet-activating factor acetylhydrolase
This work was supported by University Grants Commission India Grant 41-1284/2012(SR), the Vision Group on Science and Technology, Government of Karnataka, India (G.K.M), National Institutes of Health Grants HL062996 (J.B.T.) and KK22ES023850 (R.P.S.), and US Department of Veterans Affairs Grant 510BX000853 (J.B.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government. V.H.C., C.C.L., and M.S.S. were supported by the University Grants Commission (UGC), India, for Basic Science Research Fellowship (BSR), K.V.A. by a National Fellowship for Higher Education (NFHE), S.P.J. by a Non-NET fellowship, and G.K.M. by Major Research Project [MRP: 41-1284/2012(SR)]. The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Prescott S. M., Zimmerman G. A., Stafforini D. M., and McIntyre T. M.. 2000. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69: 419–445. [DOI] [PubMed] [Google Scholar]
- 2.Ishii S., and Shimizu T.. 2000. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 39: 41–82. [DOI] [PubMed] [Google Scholar]
- 3.Marathe G. K., Pandit C., Lakshmikanth C. L., Chaithra V. H., Jacob S. P., and D’Souza C. J. M.. 2014. To hydrolyze or not to hydrolyze: the dilemma of platelet-activating factor acetylhydrolase. J. Lipid Res. 55: 1847–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Flaherty J. T., Salzer W., Cousart S., McCall C., Piantadosi C., Surles J., Hammett M. J., and Wykle R. L.. 1983. Platelet-activating factor and analogues: comparative studies with human neutrophils and rabbit platelets. Res. Commun. Chem. Pathol. Pharmacol. 39: 291–309. [PubMed] [Google Scholar]
- 5.Sturk A., Schaap M. C., Prins A., ten Cate J. W., and van den Bosch H.. 1989. Synthesis of platelet-activating factor by human blood platelets and leucocytes. Evidence against selective utilization of cellular ether-linked phospholipids. Biochim. Biophys. Acta. 993: 148–156. [DOI] [PubMed] [Google Scholar]
- 6.Whatley R. E., Clay K., Chilton F., Triggiani M., Zimmerman G. A., McIntyre T. M., and Prescott S. M.. 1992. Relative amounts of 1-O-alkyl-and 1-acyl-2-acetyl-sn-glycero-3-phosphocholine in stimulated endothelial cells. Prostaglandins. 43: 21–29. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa Y., Sugai M., Karasawa K., Tokumura A., Tsukatani H., Setaka M., and Shoshichi N.. 1992. Possible influence of lysophospholipase on the production of 1-acyl-2- cetylglycerophosphocholine in macrophages. Biochim. Biophys. Acta. 1126: 277–285. [DOI] [PubMed] [Google Scholar]
- 8.Mueller H. W., Nollert M. U., and Eskin S. G.. 1991. Synthesis of 1-acyl-2-[3H] acetyl-sn-glycero-3-phosphocholine, a structural analog of platelet activating factor, by vascular endothelial cells. Biochem. Biophys. Res. Commun. 176: 1557–1564. [DOI] [PubMed] [Google Scholar]
- 9.Pinckard R. N., Woodard D. S., Showell H. J., Conklyn M. J., Novak M. J., and McManus L. M.. 1994. Structural and (patho) physiological diversity of PAF. Clin. Rev. Allergy. 12: 329–359. [DOI] [PubMed] [Google Scholar]
- 10.Travers J. B., Harrison K. A., Johnson C. A., Clay K. L., Morelli J. G., and Murphy R. C.. 1996. Platelet-activating factor biosynthesis induced by various stimuli in human HaCaT keratinocytes. J. Invest. Dermatol. 107: 88–94. [DOI] [PubMed] [Google Scholar]
- 11.Tokumura A., Kamiyasu K., Takauchi K., and Tsukatani H.. 1987. Evidence for existence of various homologues and analogues of platelet activating factor in a lipid extract of bovine brain. Biochem. Biophys. Res. Commun. 145: 415–425. [DOI] [PubMed] [Google Scholar]
- 12.Triggiani M., Fonteh A. N., and Chilton F.. 1992. Factors that influence the proportions of platelet-activating factor and 1-acyl-2-acetyl-sn-glycero-3-phosphocholine synthesized by the mast cell. Biochem. J. 286: 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chap H., Mauco G., Simon M., Benveniste J., and Douste-Blazy L.. 1981. Biosynthetic labelling of platelet activating factor from radioactive acetate by stimulated platelets. Nature. 289: 312–314. [DOI] [PubMed] [Google Scholar]
- 14.Tokumura A., Takauchi K., Asai T., Kamiyasu K., Ogawa T., and Tsukatani H.. 1989. Novel molecular analogues of phosphatidylcholines in a lipid extract from bovine brain: 1-long-chain acyl-2-short-chain acyl-sn-glycero-3-phosphocholines. J. Lipid Res. 30: 219–224. [PubMed] [Google Scholar]
- 15.Weintraub S. T., Satsangi R. K., Sprague E. A., Prihoda T. J., and Pinckard R. N.. 2000. Mass spectrometric analysis of platelet-activating factor after isolation by solid-phase extraction and direct derivatization with pentafluorobenzoic anhydride. J. Am. Soc. Mass Spectrom. 11: 176–181. [DOI] [PubMed] [Google Scholar]
- 16.Marathe G. K., Davies S. S., Harrison K. A., Silva A. R., Murphy R. C., Neto H. C. C. F., Prescott S. M., Zimmerman G. A., and McIntyre T. M.. 1999. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J. Biol. Chem. 274: 28395–28404. [DOI] [PubMed] [Google Scholar]
- 17.Marathe G. K., Harrison K. A., Murphy R. C., Prescott S. M., Zimmerman G. A., and McIntyre T. M.. 2000. Bioactive phospholipid oxidation products. Free Radic. Biol. Med. 28: 1762–1770. [DOI] [PubMed] [Google Scholar]
- 18.Pontsler A. V., Hilaire A. S., Marathe G. K., Zimmerman G. A., and McIntyre T. M.. 2002. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor γ and oxidized alkyl phospholipids from oxidized low density lipoprotein. J. Biol. Chem. 277: 13029–13036. [DOI] [PubMed] [Google Scholar]
- 19.Subbanagounder G., Leitinger N., Schwenke D. C., Wong J. W., Lee H., Rizza C., Watson A. D., Faull K. F., Fogelman A. M., and Berliner J. A.. 2000. Determinants of bioactivity of oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 20: 2248–2254. [DOI] [PubMed] [Google Scholar]
- 20.Chen R., Chen X., Salomon R. G., and McIntyre T. M.. 2009. Platelet activation by low concentrations of intact oxidized LDL particles involves the PAF receptor. Arterioscler. Thromb. Vasc. Biol. 29: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stafforini D. M., McIntyre T. M., Carter M., and Prescott S. M.. 1987. Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J. Biol. Chem. 262: 4215–4222. [PubMed] [Google Scholar]
- 22.Tjoelker L. W., Eberhardt C., Unger J., Le Trong H., Zimmerman G. A., McIntyre T. M., Stafforini D. M., Prescott S. M., and Gray P. W.. 1995. Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad. J. Biol. Chem. 270: 25481–25487. [DOI] [PubMed] [Google Scholar]
- 23.Stafforini D. M. 2009. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2). Cardiovasc. Drugs Ther. 23: 73–83. [DOI] [PubMed] [Google Scholar]
- 24.McIntyre T. M., Prescott S. M., and Stafforini D. M.. 2009. The emerging roles of PAF acetylhydrolase. J. Lipid Res. 50: S255–S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tjoelker L. W., and Stafforini D. M.. 2000. Platelet-activating factor acetylhydrolases in health and disease. Biochim. Biophys. Acta. 1488: 102–123. [DOI] [PubMed] [Google Scholar]
- 26.Castro Faria Neto H. C., Stafforini D. M., Prescott S. M., and Zimmerman G. A.. 2005. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Mem. Inst. Oswaldo Cruz. 100 (Suppl. 1): 83–91. [DOI] [PubMed] [Google Scholar]
- 27.Bazan N. G. Inflammation. A signal terminator. 1995. Nature. 374:501–502. [DOI] [PubMed] [Google Scholar]
- 28.Jeong Y-I., Jung I. D., Lee C-M., Chang J. H., Chun S. H., Noh K. T., Jeong S. K., Shin Y. K., Lee W. S., Kang M. S., et al. 2009. The novel role of platelet-activating factor in protecting mice against lipopolysaccharide-induced endotoxic shock. PLoS One. 4: e6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karabina S-A., and Ninio E.. 2006. Plasma PAF-acetylhydrolase: an unfulfilled promise? Biochim. Biophys. Acta. 1761: 1351–1358. [DOI] [PubMed] [Google Scholar]
- 30.Chen C-H. 2004. Platelet-activating factor acetylhydrolase: is it good or bad for you? Curr. Opin. Lipidol. 15: 337–341. [DOI] [PubMed] [Google Scholar]
- 31.Stafforini D. M., Numao T., Tsodikov A., Vaitkus D., Fukuda T., Watanabe N., Fueki N., McIntyre T. M., Zimmerman G. A., Makino S., et al. 1999. Deficiency of platelet-activating factor acetylhydrolase is a severity factor for asthma. J. Clin. Invest. 103: 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vadas P., Gold M., Perelman B., Liss G. M., Lack G., Blyth T., Simons F. E., Simons K. J., Cass D., and Yeung J.. 2008. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N. Engl. J. Med. 358: 28–35. [DOI] [PubMed] [Google Scholar]
- 33.Claus R. A., Russwurm S., Dohrn B., Bauer M., and Lösche W.. 2005. Plasma platelet-activating factor acetylhydrolase activity in critically ill patients. Crit. Care Med. 33: 1416–1419. [DOI] [PubMed] [Google Scholar]
- 34.Imaizumi T. A., Stafforini D. M., Yamada Y., McIntyre T. M., Prescott S. M., and Zimmerman G. A.. 1995. Platelet-activating factor: a mediator for clinicians. J. Intern. Med. 238: 5–20. [DOI] [PubMed] [Google Scholar]
- 35.Gomes R. N., Bozza F. A., Amâncio R. T., Japiassú A. M., Vianna R. C., Larangeira A. P., Gouvêa J. M., Bastos M. S., Zimmerman G. A., Stafforini D. M., et al. 2006. Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock. 26: 41–49. [DOI] [PubMed] [Google Scholar]
- 36.Stafforini D. M., and Zimmerman G. A.. 2014. Unraveling the PAF-AH/lp-PLA2 controversy. J. Lipid Res. 55: 1811–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Androulakis N., Durand H., Ninio E., and Tsoukatos D. C.. 2005. Molecular and mechanistic characterization of platelet-activating factor-like bioactivity produced upon LDL oxidation. J. Lipid Res. 46: 1923–1932. [DOI] [PubMed] [Google Scholar]
- 38.Bligh E. G., and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 39.Marathe G. K., Krishnakanta T., and D’Souza C. J. M.. 1990. PAF-acether (PAF) in Ehrlich ascites tumour cells. J. Lipid Mediat. 2: 257–262. [PubMed] [Google Scholar]
- 40.Bartlett G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234: 466–468. [PubMed] [Google Scholar]
- 41.Jacob S. P., Lakshmikanth C. L., Chaithra V. H., Kumari T. R. S., Chen C-H., McIntyre T. M., and Marathe G. K.. 2016. Lipopolysaccharide cross-tolerance delays platelet-activating factor-induced sudden death in Swiss albino mice: involvement of cyclooxygenase in cross-tolerance. PLoS One. 11: e0153282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou L., and Schmaier A. H.. 2005. Platelet aggregation testing in platelet-rich plasma: description of procedures with the aim to develop standards in the field. Am. J. Clin. Pathol. 123: 172–183. [DOI] [PubMed] [Google Scholar]
- 43.Latchoumycandane C., Marathe G. K., Zhang R., and McIntyre T. M.. 2012. Oxidatively truncated phospholipids are required agents of tumor necrosis factor α (TNFα)-induced apoptosis. J. Biol. Chem. 287: 17693–17705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lakshmikanth C. L., Jacob S. P., Kudva A. K., Latchoumycandane C., Yashaswini P. S., Sumanth M. S., Goncalves-de-Albuquerque C. F., Silva A. R., Singh S. A., Castro-Faria-Neto H. C., et al. 2016. Escherichia coli Braun Lipoprotein (BLP) exhibits endotoxemia-like pathology in Swiss albino mice. Sci. Rep. 6: 34666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demopoulos C. A., Pinckard R., and Hanahan D. J.. 1979. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 254: 9355–9358. [PubMed] [Google Scholar]
- 46.Marathe G. K., Silva A. R., de Castro Faria Neto H. C., Tjoelker L. W., Prescott S. M., Zimmerman G. A., and McIntyre T. M.. 2001. Lysophosphatidylcholine and lyso-PAF display PAF-like activity derived from contaminating phospholipids. J. Lipid Res. 42: 1430–1437. [PubMed] [Google Scholar]
- 47.Shimizu T. 2009. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 49: 123–150. [DOI] [PubMed] [Google Scholar]
- 48.Leusch A., Volz A., Müller G., Wagner A., Sauer A., Greischel A., and Roth W.. 2002. Altered drug disposition of the platelet activating factor antagonist apafant in mdr1a knockout mice. Eur. J. Pharm. Sci. 16: 119–128. [DOI] [PubMed] [Google Scholar]
- 49.Pinckard R. N., Showell H., Castillo R., Lear C., Breslow R., McManus L., Woodard D. S., and Ludwig J. C.. 1992. Differential responsiveness of human neutrophils to the autocrine actions of 1-O-alkyl-homologs and 1-acyl analogs of platelet-activating factor. J. Immunol. 148: 3528–3535. [PubMed] [Google Scholar]
- 50.Hanahan D. J. 1986. Platelet activating factor: a biologically active phosphoglyceride. Annu. Rev. Biochem. 55: 483–509. [DOI] [PubMed] [Google Scholar]
- 51.White H. D., Held C., Stewart R., Tarka E., Brown R., Davies R. Y., Budaj A., Harrington R. A., Steg P. G., Ardissino D., et al. 2014. Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med. 370: 1702–1711. [DOI] [PubMed] [Google Scholar]
- 52.Hassan M. 2015. STABILITY and SOLID-TIMI 52: lipoprotein associated phospholipase A2 (Lp-PLA2) as a biomarker or risk factor for cardiovascular diseases. Glob. Cardiol. Sci. Pract. 2015: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacRitchie A. N., Gardner A. A., Prescott S. M., and Stafforini D. M.. 2007. Molecular basis for susceptibility of plasma platelet-activating factor acetylhydrolase to oxidative inactivation. FASEB J. 21: 1164–1176. [DOI] [PubMed] [Google Scholar]
- 54.Cao Y., Stafforini D. M., Zimmerman G. A., McIntyre T. M., and Prescott S. M.. 1998. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. J. Biol. Chem. 273: 4012–4020. [DOI] [PubMed] [Google Scholar]
- 55.Henderson W. R., Lu J., Poole K. M., Dietsch G. N., and Chi E. Y.. 2000. Recombinant human platelet-activating factor-acetylhydrolase inhibits airway inflammation and hyperreactivity in mouse asthma model. J. Immunol. 164: 3360–3367. [DOI] [PubMed] [Google Scholar]