Abstract
Extracellular vesicles (EVs), such as exosomes and microvesicles, are small membrane-bound vesicles released by cells under various conditions. In a multitude of physiological and pathological conditions, EVs contribute to intercellular communication by facilitating exchange of material between cells. Rapidly growing interest is aimed at better understanding EV function and their use as biomarkers. The vast EV cargo includes cytokines, growth factors, organelles, nucleic acids (messenger and micro RNA), and transcription factors. A large proportion of research dedicated to EVs is focused on their microRNA cargo; however, much less is known about other EV constituents, in particular, eicosanoids. These potent bioactive lipid mediators, derived from arachidonic acid, are shuttled in EVs along with the enzymes in charge of their synthesis. In the extracellular milieu, EVs also interact with secreted phospholipases to generate eicosanoids, which then regulate the transfer of cargo into a cellular recipient. Eicosanoids are useful as biomarkers and contribute to a variety of biological functions, including modulation of distal immune responses. Here, we review the reported roles of eicosanoids conveyed by EVs and describe their potential as biomarkers.
Keywords: exosomes, microvesicles, eicosanoids, platelets, neutrophils, cancer
Extracellular vesicles (EVs) are membrane-bound vesicles that can be released from any cellular lineage, including eukaryotic, prokaryotic, and plant cells. They encompass microvesicles (also well known as microparticles or ectosomes), produced by plasma membrane outward budding and shedding; exosomes, stored in multivesicular bodies and secreted through the endosomal network; and larger vesicles known as apoptotic bodies generated during the vesiculation of apoptotic cells (Fig. 1) (1–4).
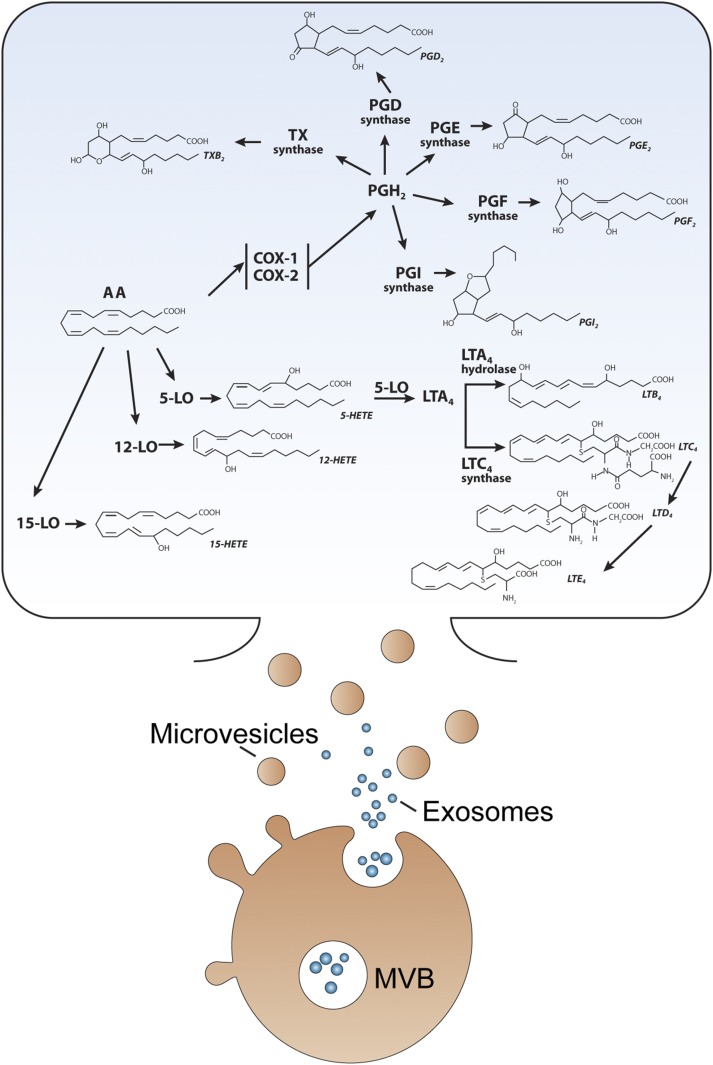
Fig. 1.
Eicosanoids and the associated enzymes are comprised in EVs. Cells produce different types of EVs, such as exosomes and microvesicles. Exosomes are stored in multivesicular bodies (MVBs) and released on cell activation, whereas microvesicles are generated by plasma membrane budding and shedding. Graphic representation of the metabolism of AA into eicosanoids. AA, arachidonic acid; COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; TX, thromboxane; 12-LO, 12-lipoxygenase; 5-LO, 5-lipoxygenase; LT, leukotriene; PG, prostaglandin; HETE ; hydroxyeicosatetraenoic acid.
The liberation of EVs implicates membrane trafficking pathways, such as the endosomal sorting complex required for transport (ESCRT) system, in the budding of vesicles in the lumen of endosomes, and fusion with the plasma membrane in the case of exosome release (3, 5). The ESCRT system can also participate in the release of microvesicles (6, 7), together with scramblase and flippase activities (8, 9). The EV content may vary according to the corresponding cellular source and may differ depending on the cell activation trigger involved. Moreover, the means of release also impacts the EV cargo, and observations from numerous studies confirm that certain proteins are enriched in exosomes while others appear enriched in microvesicles. For instance, transmembrane proteins such as tetraspanins (CD9, CD63, CD81), and tumor susceptibility gene 101 and Alix, accessory molecules from the endosomal sorting complex, are mostly associated with exosomes (10). Conversely, proteins of organelle origin, such as those from the endoplasmic reticulum, Golgi, mitochondria, or nucleus, are preferentially found in microvesicles and are rarely found in exosomes (10, 11).
Extracellular vesicles imperatively comprise a lipid moiety, and their cholesterol, sphingomyelin, phosphatidylserine, and glycosphingolipid content is richer than their cellular sources (11–16). The studies that originally reported their presence in blood determined that the EV membrane could support the coagulation cascade (2, 17). Hence, a proportion of microvesicles expose phosphatidylserine at the surface, which may facilitate the deposition of coagulation factors (14). Similarly to EVs circulating in blood, procoagulant activity was determined for EVs from various sources, including tumors, semen, cerebrospinal fluid, synovial fluid, and those present in saliva (18–24). However, the discovery of small noncoding RNA, the microRNA (miRNA), and the subsequent demonstration of actual transfer of miRNA from donor cell to recipient have greatly stimulated research on EVs as mediators of intercellular communication under physiological and pathological conditions (25, 26). As current research on EV-mediated intercellular communication is skewed toward understanding the miRNA contribution, the role of other members of the EV cargo is frequently overlooked.
Notwithstanding the potential of EV-associated miRNAs as biomarkers and their importance in the regulation of mRNA stability, the EV cargo is vast and includes other components (i.e., in addition to miRNA) that can be utilized as biomarkers and can explain the roles of EVs (27, 28). Hence, studies have identified cytokines, enzymes, growth factors, functional organelles (e.g., proteasome, mitochondria) and transcription factors in EVs, which are likely to play their part in EV-mediated functions (27–31).
With their phospholipid content, EVs represent a source of esterified fatty acids that can be released by phospholipases (32–34). The signaling molecules derived from arachidonic acid (AA) and other polyunsaturated fatty acids are called eicosanoids (32, 33). It is well established that eicosanoids are implicated in multiple biological functions, such as asthma, cancer, hemostasis, immunity, inflammation and reproduction (32, 33). Their biosynthesis implicates enzymatic and nonenzymatic processes (key processes illustrated in Fig. 1), which are conserved within EVs and can be induced by enzymes present in the EV bathing milieu (35). Hence, EVs are a highly potent source of eicosanoids such as prostaglandins (PGs) and leukotrienes (LTs) (35) with demonstrated activity in vitro and in vivo. The goal of this review is to highlight the importance of the eicosanoids pathway as shuttled by EVs derived from viable cells (exosomes and microvesicles).
PLATELET-DERIVED EVs
Platelet-derived EVs, originally called platelet dust and better known as platelet microparticles, account for the majority of circulating EVs in blood (17, 27, 36). Platelets release both exosomes and microvesicles, and can also shed EVs from the tip of long membrane protrusions when they are adhered on the endothelium and in the presence of blood flow (37–39). Given that megakaryocytes, the bone marrow cells that produce platelets, also release EVs, it has been suggested that the majority of the EVs in blood under healthy conditions actually originate from megakaryocytes (39–42). Platelet-derived EVs were the first to be reported. Thus, it is not surprising that the first demonstration of EV-mediated intercellular communication also involved EVs from platelets.
One key limiting factor in eicosanoid biosynthesis is the AA availability. Phospholipase A2s (PLA2s) are enzymes that hydrolyze phospholipids in the sn-2 position, thus liberating lysophospholipid and fatty acid (34, 43). If the released fatty acid is AA, it can be metabolized into eicosanoids. While some PLA2s are intracellular, others are secreted by cells and as such are ideally positioned to interact with EVs in the extracellular milieu. The family of secreted PLA2s (sPLA2s) includes 10 small (~ 14 kDa) soluble proteins (44, 45) classified into different groups according to their sequence homology, structure, and number and position of disulphide bonds (45). Secreted PLA2s from groups IIA, V, and X are among the most abundant and active enzymes (46). They present distinct substrate specificities, supporting the notion that they might not be isozymes (46–49).
Most, if not all, biological fluids, including blood/plasma, bronchoalveolar lavage fluid, cerebrospinal fluid, saliva, semen, synovial fluid, tears, and urine, contain both sPLA2s and EVs, suggesting a potential interaction between EVs and sPLA2s in vivo (23, 50–60). Hence, studies were undertaken to determine whether EVs could be used as substrates by sPLA2. Pioneer work by Barry and colleagues (61, 62) revealed that sPLA2 could release AA from platelet-derived EVs. AA transported by platelet-derived EVs was also capable of inducing mitogen-activating kinase cascade in a monocyte cell line EVs, which led to PG production (61). EVs might also contain intracellular PLA2s, such as cytosolic PLA2 (35), but these enzymes might not be as efficient at releasing AA from EVs than sPLA2; active caspases are present in EVs (63, 64), and given that caspases can cleave intracellular PLA2 (65), it might mitigate their activity in EVs.
Interestingly, it was found that cyclooxygenase and thromboxane synthase, contained in EVs, could metabolize AA into thromboxane, thereby promoting platelet activation and aggregation (62) (Fig. 2). Treatment of platelets with sPLA2 did not induce thromboxane production or aggregation, demonstrating a unique function for platelet-derived EVs that is absent in platelets (62). Thus, this seminal study demonstrates that enzymes are present in EVs and are capable of metabolizing lipid substrates into potent eicosanoids. An additional mechanism leading to platelet aggregation was also identified when platelet (and erythrocyte)-derived EVs were treated with a combination of sPLA2 and sphingomyelinase (66). In this case, EVs released lysophosphatidic acid, a novel lipid mediator at that time that has recognized high potency on platelets and endothelial cell functions (66).
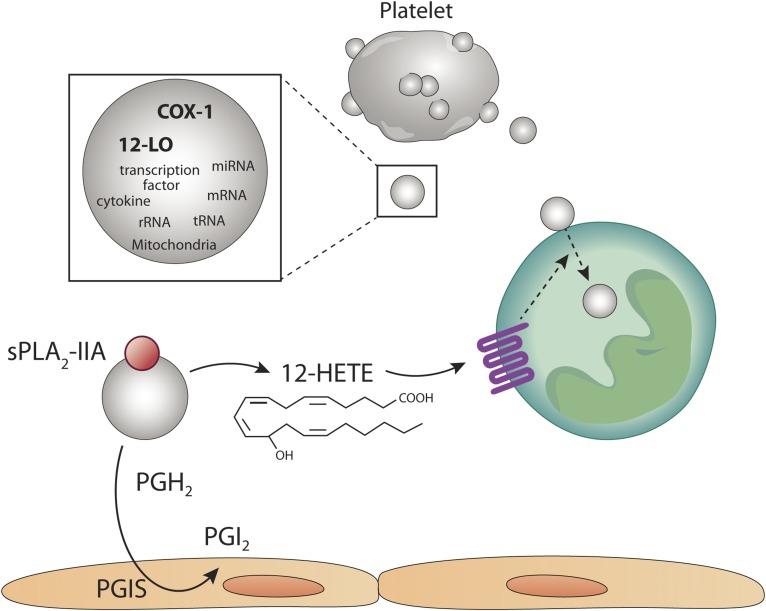
Fig. 2.
Platelet-derived EVs participate in intercellular communication through their eicosanoid content. Platelets are highly proficient at releasing EVs, such as microvesicles (microparticles). EVs from activated platelets contain a broad cargo, which includes cytokines, transcription factors, cytokines, mitochondria, and nucleic acid. Of importance is the content in active cyclooxygenase-1 (COX-1) and 12-lipoxygenase (12-LO). In the presence of secreted phospholipase A2 (sPLA2) in an inflammatory milieu, platelet-derived EVs generate arachidonic acid, which can be metabolized into prostaglandin H2 (PGH2) and subsequently into prostacyclin (PGI2) by the PGI synthase (PGIS) in endothelial cells. AA liberated by platelet-derived EVs can also undero lipoxygenation by the 12-LO itself present in platelet-derived EVs. Moreover, exogenous AA can be metabolized by platelet-derived EVs by 12-LO. 12-hydroxyeicosatetranoic acid (12-HETE) is generated and promotes the internalization of platelet EVs by neutrophils, thereby permitting efficient transfer of cargo. The internalization of platelet-EVs is therefore tightly regulated by the eicosanoid 12-HETE.
The AA liberated from sPLA2-treated EVs is also utilized by endothelial cells in a paracrine manner (62). In this case, prostacyclin is promptly produced by endothelial cells (62), suggesting that this pathway may counterbalance the activation of the vasculature triggered by thromboxane (Fig. 2). Note that although platelets can release AA in addition to unstable PGH2, which can also be metabolized in a paracrine manner by nearby cells independently of EVs (67), these in vitro experiments were the first to identify the role of potent lipid mediators shuttled by EVs in intercellular communication.
Evidence of concerted activities of sPLA2 and platelet EVs in intercellular communication was verified in vivo in the context of autoimmune arthritis (31). Platelet EVs were first identified in blood, but their small dimensions likely explain their presence in the extravascular milieu, such as in the synovial fluid of rheumatoid arthritis patients (30, 51, 52, 68–70) and in lymph (71). In rheumatoid arthritis, neutrophils predominate among other leukocytes in the inflamed joint fluid and display a prolonged lifespan and reduced migratory activities (72). These observations point to the accumulation of factor(s) in rheumatoid arthritis that promote neutrophil plasticity (72). Thus, it was hypothesized that factors conveyed by platelet EVs could reprogram neutrophils in rheumatoid arthritis.
In rheumatoid arthritis, sPLA2-IIA is overexpressed in joint lubricating synovial fluid and amplifies the disease (51, 73). Given the fact that sPLA2-IIA uses EVs as a substrate (44, 62, 66), it was verified whether platelet EVs were internalized in neutrophils and whether sPLA2-IIA could impact the internalization process. In this study (31), the authors utilized platelet-derived EVs, which, according to the centrifugation used and EV characterization, corresponded to microvesicles. Hence, in addition to EV markers such as those from the ESCRT system, the EVs contained miRNA, transcription factors, and sometimes even mitochondria (31). Interestingly, platelet EVs promptly associated with neutrophils, but remained on the neutrophil surface unless sPLA2-IIA was present. With the involvement of sPLA2-IIA, approximately 20 and 40 EVs localized in the neutrophil cytoplasm near the endoplasmic reticulum, Golgi apparatus, and lysosome within 30 and 60 min, respectively (31), pointing to the high efficiency of the process (Fig. 2).
In this study, Duchez et al. (31) conducted a complete lipidomic analysis of platelet EVs and examined the lipid mediators produced when in the presence of sPLA2-IIA. It was found that the product of 12-lipoxygenase (12-LO), 12(S)-HETE, is the dominant eicosanoid produced. Hence, platelet EVs shuttle 12-LO, and the incubation of EVs with exogenous AA preferentially leads to 12-HETE production, not thromboxane (31). Therefore, these observations illustrate that while platelets can predominantly produce the eicosanoids thromboxane and 12(S)HETE, in contrast, platelet EVs mainly generate 12(S)HETE. This unique feature of platelet EVs may be explained by the suicide inactivation of thromboxane synthase in EVs (74). Using 12-LO-deficient platelets and a pharmacological approach, the study then confirmed that 12(S)-HETE is the mediator generated by platelet EVs that dictates internalization in neutrophils and permits cargo transfer (31). While the intravenous injection of fluorescently labeled 12-LO+/+ platelet EVs in arthritic mice localized inside neutrophils in the disease joints, 12-LO−/− platelet EVs failed to accumulate inside neutrophils (31). Thus, intercellular communication from platelets to neutrophils is under the control of eicosanoids produced by EVs and implicates 12(S)-HETE. As the process requires sPLA2-IIA, this suggests that this pathway is highly regulated and occurs when neutrophils reach an inflammatory fluid, rich in sPLA2-IIA (Fig. 2). Whether this pathway is conserved among other cells is unknown, but given that 12-LO expression is mainly restricted to platelets (and platelet EVs), and given the involvement of the 12(S)-HETE receptor (BLT2) in the internalization process (31), it suggests that this mechanism is meant to promote internalization of platelet EVs, specifically in BLT2-expressing cells, such as neutrophils (75). As BLT2 is reported to contribute to arthritis (76), it is also tempting to speculate that its role is mediated by EV-derived 12(S)-HETE. Moreover, it is reported that 5(S)-12(S)-HETE is the most abundant eicosanoid in the synovial fluid of rheumatoid arthritis patients (77). However, no cells are known to be capable of producing this molecule because none express both 5-lipoxygenase (5-LO) and 12-LO. Given that platelets are devoid of 5-LO, but package 12-LO in their EVs (31), the transfer of 12-LO-containing EVs into 5-LO-expressing neutrophils may take place in synovial fluid and account for the presence of this unique eicosanoid.
The activity of sPLA2 toward the EV membrane is not unique to EVs derived from platelets. The sPLA2-IIA, V, and X enzymes were shown to be capable of hydrolyzing EVs, with different potency, from erythrocytes, thymocytes, endothelial cells, and those present in semen (prostasomes) (78). The hydrolysis by sPLA2 can generate fatty acids, but does not lead to clearance of EVs, as they remain detectable even in fluids rich (> 10 µg/ml) in sPLA2, such as in the synovial fluid of rheumatoid arthritis patients (51, 78). When mitochondria, comprised in certain EVs and released concomitantly with EVs, are hydrolyzed by sPLA2-IIA, there is generation of lyso-cardiolipin (30, 79). While cardiolipin is a phospholipid observed in bacterial membrane and mitochondria and is a recognized damage molecular pattern (80), the role of lyso-cardiolipin is unknown, but the products of the hydrolysis of mitochondrial membrane by sPLA2-IIA induces the production of neutrophil extracellular traps by neutrophils (30).
MYELOID CELL-DERIVED EVs AND LUNG INFLAMMATION
Proteomic and lipidomic approaches were undertaken to determine the machinery involved in eicosanoid synthesis in EVs from the RBL-2H3 cell line, which shares characteristics with both mast cells and basophils (13). Subra et al. (13) examined well-characterized exosomes isolated by differential centrifugation, and identified phospholipase C, phospholipase D, and three classes of PLA2, namely, the cytosolic calcium dependent cPLA2, calcium independent iPLA2, and sPLA2. Moreover, addition of GTP to exosomes induced the activation of PLA2 activity, elegantly demonstrating that enzymes encapsulated in exosomes can undergo further activation. Consistent with the presence of cyclooxygenase-1 and -2, prostaglandins were also detected in exosomes (13). Given that exosomes efficiently accumulated in endosomal compartments in an autocrine manner in RBL-2H3 (13), these observations suggest that the high (micromolar) concentrations of eicosanoids shuttled by exosomes may indeed mediate biological responses.
Leukotrienes are extremely potent lipid mediators involved in the pathogenesis of asthma and lung inflammation (81, 82). In a study of EVs produced by macrophages and dendritic cells (DCs), it was found that exosomes bear the necessary pathways for LT synthesis, including the 5-lipoxygnease activating protein, 5-LO, LTA4 hydrolase, and the LTC4 synthase (83) Similar observations were made in the study of circulating exosomes present in plasma (83). DCs and DC-derived exosomes both generate LTC4, a potent cysteinyl leukotriene involved in lung inflammation, when incubated in the presence of exogenous LTA4 (83). Unexpectedly, while macrophages preferentially produced LTB4, the exosomes from these macrophages promptly metabolized exogenous LTA4 into LTC4 (83). As lung epithelial cells can metabolize exogenous LTC4 into LTD4, the most potent mediator of bronchoconstriction, it can be suggested that exosomes shuttling LTC4 participate in this process (84). In contrast to platelet-EVs, which efficiently generate lipoxygenase products (31), exosomes from macrophages and DCs were poor producers of lipoxygenease products from AA (83), suggesting that the presence of LTA4-producing cells (or EVs) is limiting in this process. Together, these data reinforce the notion that the major eicosanoid production pathways found in exosomes can differ from those present in the producing cell.
Neutrophils are also an important source of heterogeneous EVs with reported bactericidal and inflammatory roles (85, 86). Moreover, they secrete LTB4, a highly potent neutrophil chemoattractant (81, 87, 88). In order to mediate its chemoattractant function, LTB4 must form a stable gradient. However, it has been established that LTB4 only forms transient gradients as a result of rapid diffusion due to its small size (89, 90). Studies on gradient formation of lipid modified Drosophilia morphogens, or the formation of palmityolated-Wnt gradients in Drosophilia embryogenesis and cAMP gradients in Dictyostelium, suggest a role for vesicles in the formation of gradients (91–93). Thus, it was hypothesized that neutrophils could also form an LTB4 gradient through EVs (94).
Majumdar et al. (94) determined that the majority of 5-LO is localized in multivesicular bodies in neutrophils chemotaxing toward the chemotactic peptide N-formylmethionyl-leucyl-phenylalanine, which mimics the formylated peptides released by bacteria. Multivesicular bodies associated with 5-LO are located near the nucleus (94), which is consistent with observations made in RBL-2H3 cells (13). The authors performed an extensive characterization of EVs using centrifugations on gradients, electron microscopy and biochemical approaches, and confirmed that exosomes were the EVs transporting 5-LO, in addition to 5-lipoxygnease activating protein, LTA4 hydrolase, and LTB4 (94). EVs, rich in LTB4, efficiently formed a gradient chemotactic response, and knocking down Rab27a or a neutral sphingomyelinase 2 using shRNA in a neutrophil-like cell line reduced exosome secretion, the release of LTB4, and directional motility (94). This breakthrough finding demonstrates that in order to mediate its chemoattractant activity, LTB4 must be released within EVs (Fig. 3). Furthermore, as neutral sphingomyelinase 2 depletion led to a near complete inhibition of exosome release, these intriguing observations suggest that exosome biogenesis in neutrophils mainly involves a ceramide-dependent mechanism and takes place independently of the ESCRT system.
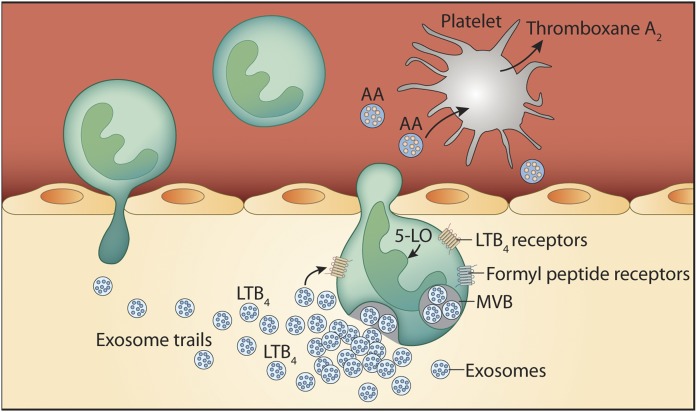
Fig. 3.
Neutrophils produce extracellular vesicles rich in eicosanoid components. When neutrophils are activated by the bacterial peptide N-formylmethionyl-leucyl-phenylalanine, they release exosomes containing leukotriene B4 (LTB4), which form a gradient. The gradient of LTB4 forms an “exosome trail” that extends from the EV-producing cell and is necessary in neutrophil recruitment to the site of inflammation. EVs from neutrophils also contain arachidonic acid (AA), which upon capture by platelets, is metabolized into thromboxane A2 (TxA2). Thromboxane A2 generated through the intercellular communication between neutrophils and platelets promotes inflammation and pathogen clearance in lungs in a bacterial infection model. 5-LO, 5-lipoxygenase; MVB, multivesicular body.
Although LTB4-containing EVs may act directly on neutrophils in both an autocrine and paracrine manner, neutrophil-derived EVs were also demonstrated to impact other neighboring lineages (95). The interaction of platelets and neutrophils is critical to the efficient innate immune response to bacterial infections (96, 97). The interactions of platelets and neutrophils implicate P-selectin and glycoprotein Ib (GPIb) on the platelet side, and P-selectin glycoprotein ligand-1 and Mac-1 on the neutrophil side (98–100). Fibrinogen can also bridge platelets and neutrophils together through glycoprotein IIbIIIa and Mac-1 (101, 102). Rossaint et al. (95) observed that platelet GPIb stimulates the release of EVs from neutrophils through Mac-1. Neutrophil-derived EVs were shown to be rich in AA content, and were internalized in platelets in a process involving GPIb and clathrin into compartments where cox-1 is localized (95). This process results in a very potent generation of thromboxane A2 by platelet cox-1, employing the AA from neutrophils shuttled by EVs (95) (Fig. 3). Of importance is the necessity for thromboxane A2 in neutrophil intravascular crawling and extravasation, required to combat lung infection. Hence, the depletion of platelets or the blockade of their interaction with neutrophils using a GPIb-blocking antibody reduced survival of mice infected with Escherichia coli that could be rescued by intravenous injection of neutrophil-derived EVs (95). Neutrophils release EVs containing LTB4 and its enzymatic machinery, but the blockade of GPIb did not impact the production of LTB4, suggesting that platelets are not involved in LTB4 synthesis in lung inflammation (95). Although these studies confirm that neutrophil-derived EVs are potent shuttles of eicosanoids that enable efficient innate immune response, it is not established whether platelet-derived EVs also shuttle eicosanoids to neutrophils in the context of lung inflammation.
TUMOR-DERIVED EVs
Tumor cells modify their environment in order to escape immune system surveillance and to promote growth (103). During tumor growth, there is expansion of myeloid-derived suppressor cells (MDSCs), thereby promoting tumor progression (104, 105). MDSCs accumulate in secondary lymphoid organs, blood, and the tumor itself and provide stroma and immune evasion (104, 105). How the tumor stimulates the generation of MDSC in the marrow is unclear, and studies were undertaken to determine whether intercellular communication through EVs was implicated (106). Exosomes, enriched by differential centrifugation from a tumor removed 21 days postinjection of a murine mammary adenocarcinoma cell line into mice, were injected intravenously into recipient mice (106). While they stimulated the generation of MDSC, assessed using Gr-1+ and CD11b+ markers, exosomes did not impact T-cells, natural killer cells, or B-cells (106). Exosomes even increased the size of tumors, pointing to their role in tumor growth through stimulation of MDSC. Of importance is that the authors identified PGE2 as a key molecule shuttled by exosomes involved in MDSC accumulation, and demonstrated that a PGE2-neutralizing antibody could impair expansion of MDSC (106).
Tumor-derived EVs can also directly impact cells from the immune system (103). EVs liberated by the intestinal epithelium in the mucus can migrate to the liver where they induce natural killer T-cell anergy (107). Anergy was induced by PGE2 transported by EVs and efficiently protected against liver inflammation in a hepatitis model when administered by gavage with EVs from the intestinal epithelium (107). Moreover, the microbiome in the intestine, more particularly the enterobacterium Bacterioides fragilis, mediates release of EVs from the epithelium, which convey sphingosine-1-phosphate and PGE2 (108). Sphingosine-1-phosphate and PGE2-containing EVs then recruit Th17 cells in the intestine (108). Of relevance to cancer is that Th17 recruitment, induced by EVs, favored the establishment of tumors in spontaneous and transplanted colon cancer mouse models (108). While these observations demonstrate a role for eicosanoids transported by EVs in the downregulation of the immune system in cancer, it is unknown whether similar mechanisms occur in the resolution phase of inflammation.
CONCLUSIONS AND PERSPECTIVES
Whereas lipids form the basis of the EV structure and affect EV stability or clearance in the whole organism (1, 27, 109, 110), EVs also shuttle bioactive lipid mediators and enzymes involved in their synthesis (27, 35). As the eicosanoid pathway differs from one cellular lineage to another, the fact that cells can exchange eicosanoid machinery can lead to generation of completely novel eicosanoid molecules. Furthermore, the examination of anti-inflammatory eicosanoids, such as the resolvins and lipoxins, in EVs may permit the identification of subtypes of EVs implicated in the resolution of inflammation (111). The appreciation of eicosanoid-containing EVs will lead to the discovery of novel molecules and functions for these lipid mediators and may shed light on eicosanoid pathways present in the extracellular milieu unique to certain pathological conditions.
Colossal efforts have been undertaken in recent years in order to better characterize EV subtypes (i.e., microvesicles vs. exosomes) and to ensure their authenticity (1, 10, 112). Certain markers have been proposed, but no perfect methodologies exist to easily discriminate EV subtypes and identify their compartment of origin. Determining how eicosanoids and enzymes are secreted may reveal new secretion pathways, independent of the canonical ESCRT system described thus far. How eicosanoids and associated enzymes are packaged in EVs is not well understood, but an understanding of the process may shed light on lipidomic approaches to further characterize EVs. For instance, mass spectrometry or infrared spectrometry (113) could be applied to differentiate subclasses of EVs. These approaches can also be utilized to determine eicosanoid-based biomarkers associated with EVs in certain pathologies.
The substrate for secreted phospholipases in the extracellular milieu was unclear until the discovery of eicosanoid machinery in EVs. EVs transport lipid mediators and related enzymes capable of producing large quantities (micromolar range) of eicosanoids that are not always redundant with those produced by the cell of origin, thus pointing to the relevance of these pathways. The fact that EVs contain a broad pool of components, such as cytokines, enzymes, miRNA, organelles, and transcription factors, in addition to eicosanoids, underlies their remarkable potential as key players in diverse biological process. Taking the EV cargo as a whole, continued research will serve to better define their actual significance.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- DC
- dendritic cell
- ESCRT
- endosomal sorting complex required for transport
- EV
- extracellular vesicle
- GPIb
- glycoprotein Ib
- 12-LO
- 12-lipoxygenase
- LT
- leukotriene
- MDSC
- myeloid-derived suppressor cell
- miRNA
- microRNA
- PG
- prostaglandin
- PLA2
- phospholipase A2
- sPLA2
- secreted PLA2
E.B. is the recipient of a New Investigator Award and a Foundation grant from the Canadian Institutes of Health Research (CIHR). There is no conflict of interest to disclose.
REFERENCES
- 1.Yáñez-Mó M., Siljander P. R., Andreu Z., Zavec A. B., Borras F. E., Buzas E. I., Buzas K., Casal E., Cappello F., Carvalho J., et al. 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 4: 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridger V. C., Boulanger C. M., Angelillo-Scherrer A., Badimon L., Blanc-Brude O., Bochaton-Piallat M. L., Boilard E., Buzas E. I., Caporali A., Dignat-George F., et al. 2017. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb. Haemost. 117: 1296–1316. [DOI] [PubMed] [Google Scholar]
- 3.Colombo M., Raposo G., and Thery C.. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30: 255–289. [DOI] [PubMed] [Google Scholar]
- 4.Record M., Subra C., Silvente-Poirot S., and Poirot M.. 2011. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 81: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez A. J., Maiuri P., Lafaurie-Janvore J., Divoux S., Piel M., and Perez F.. 2014. ESCRT machinery is required for plasma membrane repair. Science. 343: 1247136. [DOI] [PubMed] [Google Scholar]
- 6.Nabhan J. F., Hu R., Oh R. S., Cohen S. N., and Lu Q.. 2012. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA. 109: 4146–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurley J. H. 2015. ESCRTs are everywhere. EMBO J. 34: 2398–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beer K. B., Rivas-Castillo J., Kuhn K., Fazeli G., Karmann B., Nance J. F., Stigloher C., and Wehman A. M.. 2018. Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry. Proc. Natl. Acad. Sci. USA. 115: E1127–E1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii T., Sakata A., Nishimura S., Eto K., and Nagata S.. 2015. TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc. Natl. Acad. Sci. USA. 112: 12800–12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lötvall J., Hill A. F., Hochberg F., Buzas E. I., Di Vizio D., Gardiner C., Gho Y. S., Kurochkin I. V., Mathivanan S., Quesenberry P., et al. 2014. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 3: 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haraszti R. A., Didiot M. C., Sapp E., Leszyk J., Shaffer S. A., Rockwell H. E., Gao F., Narain N. R., DiFiglia M., Kiebish M. A., et al. 2016. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles. 5: 32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J. F., Kobayashi T., Salles J. P., Perret B., Bonnerot C., et al. 2004. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subra C., Grand D., Laulagnier K., Stella A., Lambeau G., Paillasse M., De Medina P., Monsarrat B., Perret B., Silvente-Poirot S., et al. 2010. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 51: 2105–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llorente A., Skotland T., Sylvanne T., Kauhanen D., Rog T., Orlowski A., Vattulainen I., Ekroos K., and Sandvig K.. 2013. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta. 1831: 1302–1309. [DOI] [PubMed] [Google Scholar]
- 15.Phuyal S., Skotland T., Hessvik N. P., Simolin H., Overbye A., Brech A., Parton R. G., Ekroos K., Sandvig K., and Llorente A.. 2015. The ether lipid precursor hexadecylglycerol stimulates the release and changes the composition of exosomes derived from PC-3 cells. J. Biol. Chem. 290: 4225–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skotland T., Ekroos K., Kauhanen D., Simolin H., Seierstad T., Berge V., Sandvig K., and Llorente A.. 2017. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Cancer. 70: 122–132. [DOI] [PubMed] [Google Scholar]
- 17.Wolf P. 1967. The nature and significance of platelet products in human plasma. Br. J. Haematol. 13: 269–288. [DOI] [PubMed] [Google Scholar]
- 18.Amin C., Mackman N., and Key N. S.. 2008. Microparticles and cancer. Pathophysiol. Haemost. Thromb. 36: 177–183. [DOI] [PubMed] [Google Scholar]
- 19.Wang J. G., Geddings J. E., Aleman M. M., Cardenas J. C., Chantrathammachart P., Williams J. C., Kirchhofer D., Bogdanov V. Y., Bach R. R., Rak J., et al. 2012. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 119: 5543–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas G. M., Brill A., Mezouar S., Crescence L., Gallant M., Dubois C., and Wagner D. D.. 2015. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J. Thromb. Haemost. 13: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández J. A., Heeb M. J., Radtke K. P., and Griffin J. H.. 1997. Potent blood coagulant activity of human semen due to prostasome-bound tissue factor. Biol. Reprod. 56: 757–763. [DOI] [PubMed] [Google Scholar]
- 22.Berckmans R. J., Nieuwland R., Tak P. P., Boing A. N., Romijn F. P., Kraan M. C., Breedveld F. C., Hack C. E., and Sturk A.. 2002. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 46: 2857–2866. [DOI] [PubMed] [Google Scholar]
- 23.Morel N., Morel O., Petit L., Hugel B., Cochard J. F., Freyssinet J. M., Sztark F., and Dabadie P.. 2008. Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J. Trauma. 64: 698–704. [DOI] [PubMed] [Google Scholar]
- 24.Berckmans R. J., Sturk A., van Tienen L. M., Schaap M. C., and Nieuwland R.. 2011. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 117: 3172–3180. [DOI] [PubMed] [Google Scholar]
- 25.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J. J., and Lotvall J. O.. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 26.Maroney P. A., Yu Y., Fisher J., and Nilsen T. W.. 2006. Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat. Struct. Mol. Biol. 13: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 27.Melki I., Tessandier N., Zufferey A., and Boilard E.. 2017. Platelet microvesicles in health and disease. Platelets. 28: 214–221. [DOI] [PubMed] [Google Scholar]
- 28.Kowal J., Arras G., Colombo M., Jouve M., Morath J. P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., and Thery C.. 2016. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 113: E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieudé M., Bell C., Turgeon J., Beillevaire D., Pomerleau L., Yang B., Hamelin K., Qi S., Pallet N., Beland C., et al. 2015. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci. Transl. Med. 7: 318ra200. [DOI] [PubMed] [Google Scholar]
- 30.Boudreau L. H., Duchez A. C., Cloutier N., Soulet D., Martin N., Bollinger J., Pare A., Rousseau M., Naika G. S., Levesque T., et al. 2014. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 124: 2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duchez A. C., Boudreau L. H., Bollinger J., Belleannee C., Cloutier N., Laffont B., Mendoza-Villarroel R. E., Levesque T., Rollet-Labelle E., Rousseau M., et al. 2015. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl. Acad. Sci. USA. 112: E3564–E3573. [Erratum. 2015. Proc. Natl. Acad. Sci. USA. 112: E6825.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harizi H., Corcuff J. B., and Gualde N.. 2008. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol. Med. 14: 461–469. [DOI] [PubMed] [Google Scholar]
- 33.Dennis E. A., and Norris P. C.. 2015. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 15: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami M., Taketomi Y., Miki Y., Sato H., Yamamoto K., and Lambeau G.. 2014. Emerging roles of secreted phospholipase A2 enzymes: the 3rd edition. Biochimie 107 Pt A: 105–113. [DOI] [PubMed] [Google Scholar]
- 35.Record M., Carayon K., Poirot M., and Silvente-Poirot S.. 2014. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 1841: 108–120. [DOI] [PubMed] [Google Scholar]
- 36.Arraud N., Linares R., Tan S., Gounou C., Pasquet J. M., Mornet S., and Brisson A. R.. 2014. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 12: 614–627. [DOI] [PubMed] [Google Scholar]
- 37.Heijnen H. F., Schiel A. E., Fijnheer R., Geuze H. J., and Sixma J. J.. 1999. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 94: 3791–3799. [PubMed] [Google Scholar]
- 38.Tersteeg C., Heijnen H. F., Eckly A., Pasterkamp G., Urbanus R. T., Maas C., Hoefer I. E., Nieuwland R., Farndale R. W., Gachet C., et al. 2014. FLow-induced PRotrusions (FLIPRs): a platelet-derived platform for the retrieval of microparticles by monocytes and neutrophils. Circ. Res. 114: 780–791. [DOI] [PubMed] [Google Scholar]
- 39.Boilard E., Duchez A. C., and Brisson A.. 2015. The diversity of platelet microparticles. Curr. Opin. Hematol. 22: 437–444. [DOI] [PubMed] [Google Scholar]
- 40.Flaumenhaft R., Mairuhu A. T., and Italiano J. E.. 2010. Platelet- and megakaryocyte-derived microparticles. Semin. Thromb. Hemost. 36: 881–887. [DOI] [PubMed] [Google Scholar]
- 41.Gitz E., Pollitt A. Y., Gitz-Francois J. J., Alshehri O., Mori J., Montague S., Nash G. B., Douglas M. R., Gardiner E. E., Andrews R. K., et al. 2014. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood. 124: 2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunin P., Penke L. R., Thon J. N., Monach P. A., Jones T., Chang M. H., Chen M. M., Melki I., Lacroix S., Iwakura Y., et al. 2017. Megakaryocytes compensate for Kit insufficiency in murine arthritis. J. Clin. Invest. 127: 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudo I., and Murakami M.. 2002. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 68–69: 3–58. [DOI] [PubMed] [Google Scholar]
- 44.Lambeau G., and Gelb M. H.. 2008. Biochemistry and physiology of mammalian secreted phospholipases A(2). Annu. Rev. Biochem. 77: 495–520. [DOI] [PubMed] [Google Scholar]
- 45.Murakami M., Taketomi Y., Sato H., and Yamamoto K.. 2011. Secreted phospholipase A2 revisited. J. Biochem. 150: 233–255. [DOI] [PubMed] [Google Scholar]
- 46.Singer A. G., Ghomashchi F., Le Calvez C., Bollinger J., Bezzine S., Rouault M., Sadilek M., Nguyen E., Lazdunski M., Lambeau G., et al. 2002. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 277: 48535–48549. [DOI] [PubMed] [Google Scholar]
- 47.Bezzine S., Koduri R. S., Valentin E., Murakami M., Kudo I., Ghomashchi F., Sadilek M., Lambeau G., and Gelb M. H.. 2000. Exogenously added human group X secreted phospholipase A(2) but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. J. Biol. Chem. 275: 3179–3191. [DOI] [PubMed] [Google Scholar]
- 48.Olson E. D., Nelson J., Griffith K., Nguyen T., Streeter M., Wilson-Ashworth H. A., Gelb M. H., Judd A. M., and Bell J. D.. 2010. Kinetic evaluation of cell membrane hydrolysis during apoptosis by human isoforms of secretory phospholipase A2. J. Biol. Chem. 285: 10993–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bezzine S., Bollinger J. G., Singer A. G., Veatch S. L., Keller S. L., and Gelb M. H.. 2002. On the binding preference of human groups IIA and X phospholipases A2 for membranes with anionic phospholipids. J. Biol. Chem. 277: 48523–48534. [DOI] [PubMed] [Google Scholar]
- 50.Aho V. V., Nevalainen T. J., and Saari K. M.. 2002. Group IIA phospholipase A2 content of basal, nonstimulated and reflex tears. Curr. Eye Res. 24: 224–227. [DOI] [PubMed] [Google Scholar]
- 51.Boilard E., Lai Y., Larabee K., Balestrieri B., Ghomashchi F., Fujioka D., Gobezie R., Coblyn J. S., Weinblatt M. E., Massarotti E. M., et al. 2010. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol. Med. 2: 172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boilard E., Nigrovic P. A., Larabee K., Watts G. F., Coblyn J. S., Weinblatt M. E., Massarotti E. M., Remold-O’Donnell E., Farndale R. W., Ware J., et al. 2010. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 327: 580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowton D. L., Seeds M. C., Fasano M. B., Goldsmith B., and Bass D. A.. 1997. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am. J. Respir. Crit. Care Med. 155: 421–425. [DOI] [PubMed] [Google Scholar]
- 54.Chalbot S., Zetterberg H., Blennow K., Fladby T., Grundke-Iqbal I., and Iqbal K.. 2009. Cerebrospinal fluid secretory Ca2+-dependent phospholipase A2 activity is increased in Alzheimer disease. Clin. Chem. 55: 2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cunningham T. J., Yao L., Oetinger M., Cort L., Blankenhorn E. P., and Greenstein J. I.. 2006. Secreted phospholipase A2 activity in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Neuroinflammation. 3: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escoffier J., Jemel I., Tanemoto A., Taketomi Y., Payre C., Coatrieux C., Sato H., Yamamoto K., Masuda S., Pernet-Gallay K., et al. 2010. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Invest. 120: 1415–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guervilly C., Lacroix R., Forel J. M., Roch A., Camoin-Jau L., Papazian L., and Dignat-George F.. 2011. High levels of circulating leukocyte microparticles are associated with better outcome in acute respiratory distress syndrome. Crit. Care. 15: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemoinne S., Thabut D., Housset C., Moreau R., Valla D., Boulanger C. M., and Rautou P. E.. 2014. The emerging roles of microvesicles in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 11: 350–361. [DOI] [PubMed] [Google Scholar]
- 59.Sellam J., Proulle V., Jungel A., Ittah M., Miceli Richard C., Gottenberg J. E., Toti F., Benessiano J., Gay S., Freyssinet J. M., et al. 2009. Increased levels of circulating microparticles in primary Sjogren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 11: R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sullivan R., and Saez F.. 2013. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 146: R21–R35. [DOI] [PubMed] [Google Scholar]
- 61.Barry O. P., Kazanietz M. G., Pratico D., and FitzGerald G. A.. 1999. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J. Biol. Chem. 274: 7545–7556. [DOI] [PubMed] [Google Scholar]
- 62.Barry O. P., Pratico D., Lawson J. A., and FitzGerald G. A.. 1997. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Invest. 99: 2118–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Böing A. N., Hau C. M., Sturk A., and Nieuwland R.. 2008. Platelet microparticles contain active caspase 3. Platelets. 19: 96–103. [DOI] [PubMed] [Google Scholar]
- 64.Abid Hussein M. N., Nieuwland R., Hau C. M., Evers L. M., Meesters E. W., and Sturk A.. 2005. Cell-derived microparticles contain caspase 3 in vitro and in vivo. J. Thromb. Haemost. 3: 888–896. [DOI] [PubMed] [Google Scholar]
- 65.Adam-Klages S., Schwandner R., Luschen S., Ussat S., Kreder D., and Kronke M.. 1998. Caspase-mediated inhibition of human cytosolic phospholipase A2 during apoptosis. J. Immunol. 161: 5687–5694. [PubMed] [Google Scholar]
- 66.Fourcade O., Simon M. F., Viode C., Rugani N., Leballe F., Ragab A., Fournie B., Sarda L., and Chap H.. 1995. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 80: 919–927. [DOI] [PubMed] [Google Scholar]
- 67.Boilard E., Larabee K., Shnayder R., Jacobs K., Farndale R. W., Ware J., and Lee D. M.. 2011. Platelets participate in synovitis via Cox-1-dependent synthesis of prostacyclin independently of microparticle generation. J. Immunol. 186: 4361–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cloutier N., Tan S., Boudreau L. H., Cramb C., Subbaiah R., Lahey L., Albert A., Shnayder R., Gobezie R., Nigrovic P. A., et al. 2013. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol. Med. 5: 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.György B., Szabo T. G., Turiak L., Wright M., Herczeg P., Ledeczi Z., Kittel A., Polgar A., Toth K., Derfalvi B., et al. 2012. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One. 7: e49726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marcoux G., Duchez A. C., Cloutier N., Provost P., Nigrovic P. A., and Boilard E.. 2016. Revealing the diversity of extracellular vesicles using high-dimensional flow cytometry analyses. Sci. Rep. 6: 35928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milasan A., Tessandier N., Tan S., Brisson A., Boilard E., and Martel C.. 2016. Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J. Extracell. Vesicles. 5: 31427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright H. L., Moots R. J., and Edwards S. W.. 2014. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 10: 593–601. [DOI] [PubMed] [Google Scholar]
- 73.Seilhamer J. J., Pruzanski W., Vadas P., Plant S., Miller J. A., Kloss J., and Johnson L. K.. 1989. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J. Biol. Chem. 264: 5335–5338. [PubMed] [Google Scholar]
- 74.Jones D. A., and Fitzpatrick F. A.. 1991. Thromboxane A2 synthase. Modification during “suicide” inactivation. J. Biol. Chem. 266: 23510–23514. [PubMed] [Google Scholar]
- 75.Yokomizo T., Kato K., Terawaki K., Izumi T., and Shimizu T.. 2000. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 192: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mathis S. P., Jala V. R., Lee D. M., and Haribabu B.. 2010. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J. Immunol. 185: 3049–3056. [DOI] [PubMed] [Google Scholar]
- 77.Giera M., Ioan-Facsinay A., Toes R., Gao F., Dalli J., Deelder A. M., Serhan C. N., and Mayboroda O. A.. 2012. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim. Biophys. Acta. 1821: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rousseau M., Belleannee C., Duchez A. C., Cloutier N., Levesque T., Jacques F., Perron J., Nigrovic P. A., Dieude M., Hebert M. J., et al. 2015. Detection and quantification of microparticles from different cellular lineages using flow cytometry. Evaluation of the impact of secreted phospholipase A2 on microparticle assessment. PLoS One. 10: e0116812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu Y. H., Dumlao D. S., Cao J., and Dennis E. A.. 2013. Assessing phospholipase A2 activity toward cardiolipin by mass spectrometry. PLoS One. 8: e59267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chakraborty K., Raundhal M., Chen B. B., Morse C., Tyurina Y. Y., Khare A., Oriss T. B., Huff R., Lee J. S., St Croix C. M., et al. 2017. The mito-DAMP cardiolipin blocks IL-10 production causing persistent inflammation during bacterial pneumonia. Nat. Commun. 8: 13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flamand N., Mancuso P., Serezani C. H., and Brock T. G.. 2007. Leukotrienes: mediators that have been typecast as villains. Cell. Mol. Life Sci. 64: 2657–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samuelsson B. 1983. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 220: 568–575. [DOI] [PubMed] [Google Scholar]
- 83.Esser J., Gehrmann U., D’Alexandri F. L., Hidalgo-Estevez A. M., Wheelock C. E., Scheynius A., Gabrielsson S., and Radmark O.. 2010. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 126: 1032–1040, 1040 e1031–1034. [DOI] [PubMed] [Google Scholar]
- 84.Lukic A., Ji J., Idborg H., Samuelsson B., Palmberg L., Gabrielsson S., and Radmark O.. 2016. Pulmonary epithelial cancer cells and their exosomes metabolize myeloid cell-derived leukotriene C4 to leukotriene D4. J. Lipid Res. 57: 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Timár C. I., Lorincz A. M., Csepanyi-Komi R., Valyi-Nagy A., Nagy G., Buzas E. I., Ivanyi Z., Kittel A., Powell D. W., McLeish K. R., et al. 2013. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 121: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dalli J., Montero-Melendez T., Norling L. V., Yin X., Hinds C., Haskard D., Mayr M., and Perretti M.. 2013. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol. Cell. Proteomics. 12: 2205–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Afonso P. V., Janka-Junttila M., Lee Y. J., McCann C. P., Oliver C. M., Aamer K. A., Losert W., Cicerone M. T., and Parent C. A.. 2012. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell. 22: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lämmermann T., Afonso P. V., Angermann B. R., Wang J. M., Kastenmuller W., Parent C. A., and Germain R. N.. 2013. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 498: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Udén A. M., Hafstrom I., and Palmblad J.. 1986. Relation to chemotactic factor gradients to neutrophil migration and orientation under agarose. J. Leukoc. Biol. 39: 27–35. [DOI] [PubMed] [Google Scholar]
- 90.Zsila F., Bikadi Z., and Lockwood S. F.. 2005. In vitro binding of leukotriene B4 (LTB4) to human serum albumin: evidence from spectroscopic, molecular modeling, and competitive displacement studies. Bioorg. Med. Chem. Lett. 15: 3725–3731. [DOI] [PubMed] [Google Scholar]
- 91.Greco V., Hannus M., and Eaton S.. 2001. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 106: 633–645. [DOI] [PubMed] [Google Scholar]
- 92.Entchev E. V., and Gonzalez-Gaitan M. A.. 2002. Morphogen gradient formation and vesicular trafficking. Traffic. 3: 98–109. [DOI] [PubMed] [Google Scholar]
- 93.Kriebel P. W., Barr V. A., Rericha E. C., Zhang G., and Parent C. A.. 2008. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J. Cell Biol. 183: 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Majumdar R., Tavakoli Tameh A., and Parent C. A.. 2016. Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biol. 14: e1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Rossaint J., Kuhne K., Skupski J., Van Aken H., Looney M. R., Hidalgo A., and Zarbock A.. 2016. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat. Commun. 7: 13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sreeramkumar V., Adrover J. M., Ballesteros I., Cuartero M. I., Rossaint J., Bilbao I., Nacher M., Pitaval C., Radovanovic I., Fukui Y., et al. 2014. Neutrophils scan for activated platelets to initiate inflammation. Science. 346: 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kolaczkowska E., and Kubes P.. 2013. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 98.Simon D. I., Chen Z., Xu H., Li C. Q., Dong J., McIntire L. V., Ballantyne C. M., Zhang L., Furman M. I., Berndt M. C., et al. 2000. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J. Exp. Med. 192: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y., Gao H., Shi C., Erhardt P. W., Pavlovsky A., Soloviev A. D., Bledzka K., Ustinov V., Zhu L., Qin J., et al. 2017. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbalpha. Nat. Commun. 8: 15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ehlers R., Ustinov V., Chen Z., Zhang X., Rao R., Luscinskas F. W., Lopez J., Plow E., and Simon D. I.. 2003. Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha. J. Exp. Med. 198: 1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flick M. J., LaJeunesse C. M., Talmage K. E., Witte D. P., Palumbo J. S., Pinkerton M. D., Thornton S., and Degen J. L.. 2007. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J. Clin. Invest. 117: 3224–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weber C., and Springer T. A.. 1997. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J. Clin. Invest. 100: 2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dunn G. P., Bruce A. T., Ikeda H., Old L. J., and Schreiber R. D.. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3: 991–998. [DOI] [PubMed] [Google Scholar]
- 104.Yang L., DeBusk L. M., Fukuda K., Fingleton B., Green-Jarvis B., Shyr Y., Matrisian L. M., Carbone D. P., and Lin P. C.. 2004. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 6: 409–421. [DOI] [PubMed] [Google Scholar]
- 105.Yan H. H., Pickup M., Pang Y., Gorska A. E., Li Z., Chytil A., Geng Y., Gray J. W., Moses H. L., and Yang L.. 2010. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70: 6139–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiang X., Poliakov A., Liu C., Liu Y., Deng Z. B., Wang J., Cheng Z., Shah S. V., Wang G. J., Zhang L., et al. 2009. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer. 124: 2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deng Z. B., Zhuang X., Ju S., Xiang X., Mu J., Liu Y., Jiang H., Zhang L., Mobley J., McClain C., et al. 2013. Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. J. Immunol. 190: 3579–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Deng Z., Mu J., Tseng M., Wattenberg B., Zhuang X., Egilmez N. K., Wang Q., Zhang L., Norris J., Guo H., et al. 2015. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nat. Commun. 6: 6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Happonen K. E., Tran S., Morgelin M., Prince R., Calzavarini S., Angelillo-Scherrer A., and Dahlback B.. 2016. The Gas6-Axl interaction mediates endothelial uptake of platelet microparticles. J. Biol. Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dasgupta S. K., Le A., Chavakis T., Rumbaut R. E., and Thiagarajan P.. 2012. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation. 125: 1664–1672. [DOI] [PubMed] [Google Scholar]
- 111.Serhan C. N., Chiang N., and Dalli J.. 2015. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin. Immunol. 27: 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coumans F. A. W., Brisson A. R., Buzas E. I., Dignat-George F., Drees E. E. E., El-Andaloussi S., Emanueli C., Gasecka A., Hendrix A., Hill A. F., et al. 2017. Methodological guidelines to study extracellular vesicles. Circ. Res. 120: 1632–1648. [DOI] [PubMed] [Google Scholar]
- 113.Lee J., Wen B., Carter E. A., Combes V., Grau G. E. R., and Lay P. A.. 2017. Infrared spectroscopic characterization of monocytic microvesicles (microparticles) released upon lipopolysaccharide stimulation. FASEB J. 31: 2817–2827. [DOI] [PubMed] [Google Scholar]