Abstract
Intestinal cholesterol absorption is a key regulator of systemic cholesterol homeostasis. Excessive dietary cholesterol and its intestinal uptake lead to hypercholesterolemia, a major risk factor for cardiovascular disease. Intestinal cholesterol uptake is mediated by Niemann-Pick C1-like 1 (NPC1L1), a transmembrane protein localized in membrane microdomains (lipid rafts) enriched in gangliosides and cholesterol. The roles of gangliosides, such as monosialodihexosylganglioside (GM3) and its synthesizing enzyme GM3 synthase (GM3S), in NPC1L1-dependent cholesterol uptake have not been examined previously. Here, we examined NPC1L1-dependent cholesterol uptake in a cell model as well as in wild-type and apoE-deficient mice fed normal or high-cholesterol diets. We showed that NPC1L1-dependent cholesterol uptake was impaired in GM3S-deficient cells and that GM3S deficiency promoted resistance to hypercholesterolemia in both wild-type and apoE-deficient mice fed the high-cholesterol but not the normal diet. Our findings suggest that GM3 and related gangliosides are essential for NPC1L1-mediated intestinal cholesterol absorption and are potential targets for hypercholesterolemia therapy.
Keywords: gangliosides, monosialodihexosylganglioside, cholesterol absorption, hypercholesterolemia, lipid transport, Niemann-Pick C1-like 1
Cholesterol is an important component of cell membranes. It is a precursor for biosynthesis of steroid hormones and bile acids and is present in the circulatory system. Lowering cholesterol levels in plasma reduces the risk of coronary heart disease, a major cause of death in developed countries (1, 2). The transmembrane protein Niemann-Pick C1-like 1 (NPC1L1) plays an essential role in dietary cholesterol absorption and biliary cholesterol reabsorption (3–5). NPC1L1 mediates cellular cholesterol uptake through vesicular endocytosis and is a target of the cholesterol absorption inhibitor ezetimibe (6–10). NPC1L1 is localized in ganglioside-enriched membrane domains and requires lipid raft proteins flotillin-1 and -2 to create cholesterol-enriched membrane microdomains for efficient cholesterol uptake (11, 12).
Gangliosides (glycosphingolipids [GSLs] that contain at least one sialic acid) are enriched in the outer leaflet of plasma membranes and concentrated in specialized membrane microdomains, termed lipid rafts, that function as platforms for cell-cell interaction and cell signaling (13, 14). The ganglioside monosialodihexosylganglioside (GM3), synthesized by GM3 synthase (GM3S), is a precursor of a-, b-, and c-series gangliosides, interacts with transmembrane receptors such as the epidermal growth factor and insulin receptors, and regulates receptor functions by creating a specialized lipid environment (15, 16). There is often a close functional relationship between gangliosides and flotillins in membrane microdomain organization (17, 18); however, no study to date has addressed the role of gangliosides in NPC1L1-mediated cholesterol absorption. We demonstrate in the present study that i) NPC1L1-dependent cellular cholesterol uptake is inhibited in GM3S-deficient cells and ii) genetic hypercholesterolemia, diet-induced hypercholesterolemia, and the intestinal cholesterol absorption rate are reduced in GM3S-deficient mice. Our findings suggest that gangliosides, particularly GM3, are potential targets for hypercholesterolemia therapy.
MATERIALS AND METHODS
Animals
C57BL/6 mice and apoE-deficient mice (B6.KOR/stm Slc-Apoeshl) were from Japan SLC, Inc. (Hamamatsu, Japan). GM3S (St3gal5)-deficient mice were generated in our lab as described previously (19). To generate ApoEshl/GM3S+/− mice, ApoEshl mice were crossed with GM3S−/− mice.
ApoEshl/GM3S−/− mice and littermate controls were generated by heterozygous mating. Mice were analyzed for the GM3S genotype by PCR and for apoE protein expression by immunoblotting as described previously (20, 21). Mice were fed a regular chow diet (CE-2; CLEA Japan, Tokyo, Japan) or high-cholesterol diet (Research Diets; New Brunswick, NJ) ad libitum. All animal experiments were approved by appropriate institutional review board committees at Tohoku Medical and Pharmaceutical University.
Materials, antibodies, and plasmid
Cholesterol and lipoprotein-deficient serum were from Sigma-Aldrich (St. Louis, MO), methyl-β-cyclodextrin (MβCD) and compactin were from Tokyo Chemical Industry (Tokyo, Japan), and ezetimibe was from AdooQ Bioscience (Irvine, CA). Rabbit anti-NPC1L1 antibody was from Novus Biologicals (Littleton, CO). Alexa 594-conjugated goat anti-rabbit IgG was from Thermo Fisher Scientific (Waltham, MA). pCMV6-hNPC1L1-turboGFP plasmid vector was from OriGene (Rockville, MD).
Cell culture
HEK293T cells were cultured in DMEM (Nacalai Tesque, Kyoto, Japan) supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a 5% CO2 atmosphere. Cholesterol-depleting medium was DMEM containing 5% lipoprotein-deficient serum, 2 µM compactin, and 25 mM HEPES. Cholesterol-MβCD complex was prepared as described previously (22).
Generation of GM3S-deficient HEK293T cells
A single exon of the human ST3GAL5 (GM3S) gene containing the coding sequence for sialyl motif L was selected for the design of targeting guide RNAs using an online CRISPR design tool (23). Guide oligos (5′-CACCGCAAGACCTGTCGGCGCTGTG-3′ and 5′-AAACCACAGCGCCGACAGGTCTTGC-3′) were annealed and inserted into plasmid pSpCas9(BB) (Addgene, Cambridge, MA). The plasmid was introduced into HEK293T cells using Lipofectamine 2000 (Thermo Fisher) per the manufacturer’s instructions, clonal cell lines were obtained by single-cell cloning, and expression levels of gangliosides were evaluated by TLC.
Measurement of cellular cholesterol
Cells were seeded in DMEM in 60-mm dishes at a density of 8 × 105 cells/dish and transfected at 24 h with the indicated plasmid using Lipofectamine LTX (Thermo Fisher) per the manufacturer’s instructions. The medium was replaced by cholesterol-depleting medium at 48 h, cells were cultured overnight, and cholesterol-MβCD complex was added to the medium the next day. For ezetimibe treatment, cells were preincubated with 30 µM ezetimibe for 30 min, incubated with cholesterol-MβCD complex for 60 min, and washed with PBS, and total cellular lipids were extracted as described by Bligh and Dyer (24). Lipid extracts were dried by an N2 stream and resuspended in 1 ml 1% Triton X-100 in chloroform, chloroform was evaporated by an N2 stream, and detergent-solubilized lipids were resuspended in 1 ml distilled water. Total cholesterol and phospholipid concentrations were determined using LabAssayTM cholesterol and phospholipid test kits (Wako Pure Chemical Industries, Osaka, Japan).
Visualization of living cells and fluorescence quantification
For time-lapse fluorescence imaging, cells were seeded onto 35-mm glass-bottom dishes (Greiner Bio-One, Frickenhausen, Germany) coated with poly-l-lysine (Sigma-Aldrich) and transfected at 24 h. The medium was replaced by cholesterol-depleting medium at 48 h, and cells were cultured overnight. Cholesterol-MβCD complex was added to the medium the next day with or without 30 µM ezetimibe. Living cells were visualized by confocal laser scanning microscopy (FluoView FV1000; Olympus, Tokyo, Japan) for 60 min. The relative intensity of plasma membrane-localized NPC1L1-GFPturbo was quantified as described previously (6). The intensity at various time points was normalized relative to the intensity at time zero (defined as 100%). Fluorescence intensity was calculated using the FluoView software program (Olympus).
Blood cholesterol and lipoprotein analyses
Plasma total cholesterol was analyzed using the Cholesterol E-Test kit (Wako Pure Chemical Industries) per the manufacturer’s instructions. Serum lipoproteins were analyzed by an HPLC system at Skylight Biotech (Akita, Japan) according to the procedure described by Usui et al. (25).
Lipid and LC/MS/MS analyses
Lipids were analyzed as described previously (26). Intestinal samples were obtained by perfusing mice with saline and scraping off small-intestinal mucosa with a plastic spatula. LC/MS/MS analysis was performed as described previously (27).
Intestinal cholesterol absorption rate
The fecal dual-isotope ratio method (28) was used to determine the intestinal cholesterol absorption rate in 6- to 8-week-old ApoEshl mice and ApoEshl/GM3S−/− mice. Animals were orally gavaged with 100 µL corn oil containing [14C]cholesterol (1 µCi) and [3H]sitostanol (2 µCi), feces were collected once per day for 3 days, and [14C]cholesterol and [3H]sitostanol levels were determined.
Cholesterol feeding and immunohistochemical analysis of NPC1L1
The mice were fasted for 16 h and then gavaged with 200 µL corn oil containing 40 mg/ml cholesterol, anesthetized after 30 min, and perfused with saline. Intestinal tissues were removed, fixed in 4% paraformaldehyde in PBS, dehydrated in 30% sucrose overnight at 4°C, embedded in OCT compound (Sakura Finetek, Sendai, Japan), and frozen at −80°C. Sections (thickness: 8 µm) were prepared by cryostat, blocked with 3% BSA for 1 h, incubated with anti-NPC1L1 primary antibody (1:200) for 24 h at 4°C, washed twice with PBS, incubated with Alexa 594-conjugated secondary antibody (1:500) for 30 min at room temperature, and viewed by fluorescence microscopy (Axioskop2; Carl Zeiss, Göttingen, Germany).
Statistical analysis
Data were expressed as mean ± SD, and means were compared by Student’s t-test or ANOVA followed by Tukey’s post hoc test.
RESULTS
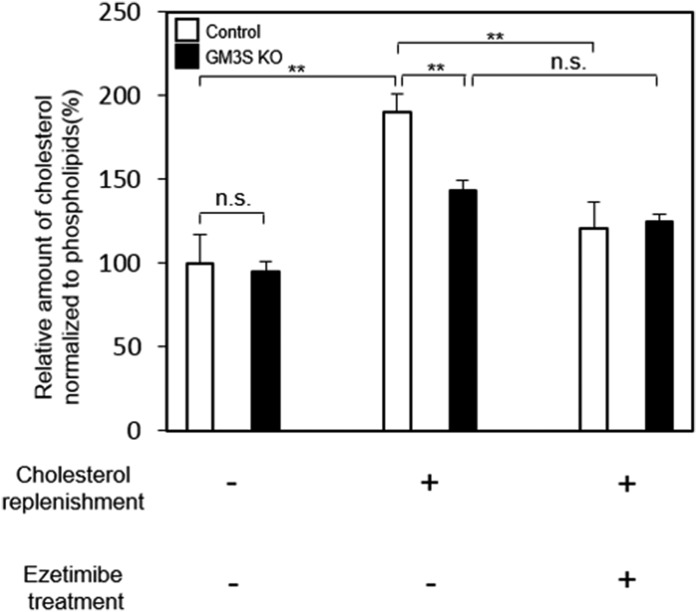
To evaluate the possible involvement of gangliosides (GM3) in NPC1L1-mediated cholesterol absorption, we first examined cholesterol content in control HEK293T and GM3S-deficient (GM3S KO) cells. The two cell lines were transfected with NPC1L1-GFPturbo, and cellular cholesterol content was measured. GM3S deficiency had no effect on endogenous cholesterol levels (Fig. 1). In NPC1L1-expressing control cells, the cholesterol level was increased by cholesterol supplementation, and the increase was blocked by pretreatment with ezetimibe. In NPC1L1-expressing GM3S KO cells, cholesterol uptake was significantly lower than in control cells, and ezetimibe pretreatment had no notable effect (Fig. 1). Previous studies indicate that the dynamic translocation of NPC1L1 between the cell surface and intracellular region is essential for NPC1L1-mediated cholesterol uptake and that cholesterol is required for the active endocytosis of NPC1L1 (6, 10).
Fig. 1.
GM3S deficiency inhibits cholesterol uptake via an NPC1L1-dependent pathway. NPC1L1-expressing control HEK293T cells and GM3S KO cells were incubated in cholesterol-depleting medium to reduce cellular cholesterol. For cholesterol replenishment, cholesterol-MβCD complex (30 µg/ml) was added directly to the medium, and cells were cultured for 60 min with or without ezetimibe pretreatment. Total lipid extraction was performed, and cholesterol and phospholipid contents were measured. Cholesterol content was normalized relative to phospholipid content. **P < 0.01.
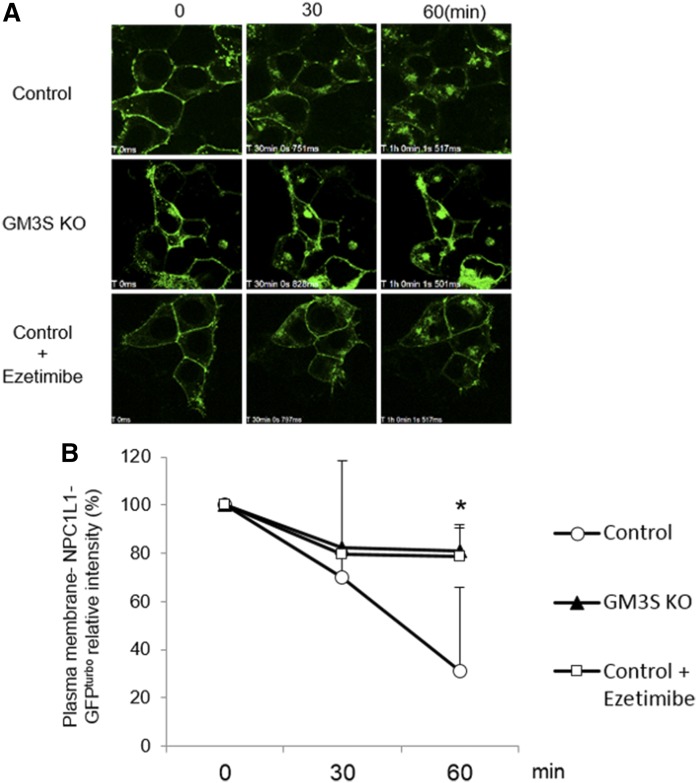
Next, experiments were performed to evaluate the effect of GM3S deficiency on NPC1L1 translocation. In control cells, cholesterol supplementation following cholesterol depletion resulted in the translocation of NPC1L1 from the plasma membrane to the intracellular region. In contrast, NPC1L1 translocation was much lower in GM3S KO cells and at a level similar to that of ezetimibe-treated cells (Fig. 2). These findings indicate the involvement of GM3 in NPC1L1-dependent cholesterol absorption.
Fig. 2.
Cholesterol-dependent internalization of NPC1L1-GFPturbo is ameliorated by GM3S depletion. A: Control HEK293T and GM3S KO cells were seeded in 0.001% poly-l-lysine-coated 35-mm glass-bottom dishes on day 0 and transfected with NPC1L1-GFPturbo on day 1. The medium was replaced with medium containing 5% lipoprotein-deficient serum and 2 µM compactin on day 2 to deplete cellular cholesterol, and cells were supplemented with 60 µg/ml cholesterol with or without ezetimibe treatment on day 3. Time-lapse images were taken by confocal microscopy. Representative images are shown. B: Quantification of plasma membrane-localized NPC1L1-GFPturbo in the cells shown in panel A. Intensity at time zero was defined as 100%. *P < 0.05 for comparison of control versus GM3S KO.
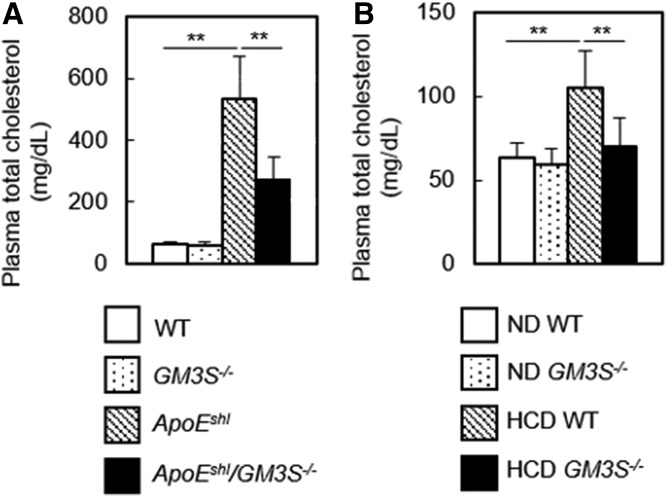
We accordingly hypothesized that experimentally induced hypercholesterolemia in GM3S KO (GM3S−/−) mice can be ameliorated by inhibiting NPC1L1-mediated intestinal cholesterol uptake. To test this hypothesis, we crossed apoE-deficient, spontaneously hyperlipidemic mice (ApoEshl) with GM3S−/− mice and examined plasma cholesterol levels. Plasma cholesterol was not significantly reduced in GM3S−/− mice, whereas levels in ApoEshl/GM3S−/− mice were strikingly lower than the high levels in ApoEshl mice (Fig. 3A). Next, we examined the possible resistance of GM3S−/− mice to diet- induced hypercholesterolemia. Plasma cholesterol levels were increased by a high-cholesterol diet in WT mice but not in GM3S−/− mice (Fig. 3B). These findings indicate that GM3S−/− mice were resistant to hypercholesterolemia induced by either apoE deficiency or a high-cholesterol diet.
Fig. 3.
Hypercholesterolemia is ameliorated in GM3S KO mice. A: Plasma total cholesterol was measured in four groups as indicated (n = 18–35 per group). B: Diet-induced hypercholesterolemia was normalized in GM3S KO mice. Plasma cholesterol levels were determined after 10 weeks of a normal diet (cholesterol-free; ND) or high-cholesterol diet (1.25% cholesterol; HCD) (n = 10–30 per group). **P < 0.01.
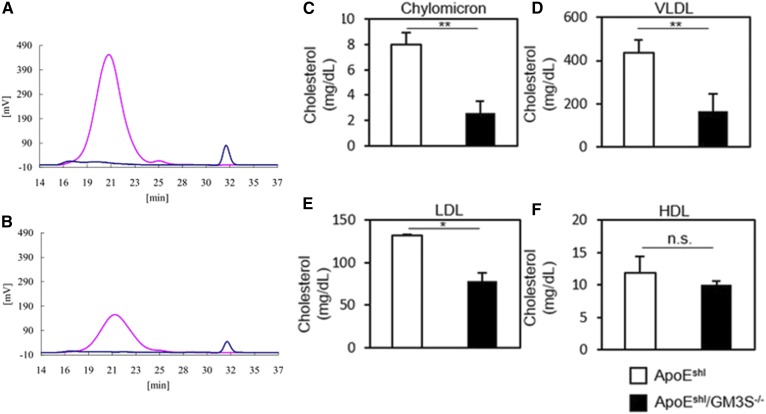
Plasma lipoprotein profiles were obtained for ApoEshl and ApoEshl/GM3S−/− mice. In ApoEshl/GM3S−/− mice, cholesterol content was significantly reduced in chylomicron, VLDL, and LDL fractions but not in the HDL fraction (Fig. 4). The reduction of cholesterol content was most striking for the chylomicron fraction (Fig. 4C), indicating defective intestinal cholesterol absorption in these mice.
Fig. 4.
Lipoprotein profiles of ApoEshl and ApoEshl/GM3S−/− mice. Lipoprotein-associated cholesterol levels in 16- to 18-week-old male mice were determined by HPLC. A, B: Representative HPLC patterns of (A) ApoEshl and (B) ApoEshl/GM3S−/− serum. A 5 μl serum sample was injected onto two tandem gel permeation columns and eluted with TSK eluent LP-1 at a flow rate of 0.7 ml/min. Pink lines represent cholesterol, and blue lines represent triglyceride. Serum total cholesterol and total triglyceride levels are 587 ± 65 and 59 ± 33 mg/dl (A) and 259 ± 75 and 35 ± 11 mg/dl (B), respectively. Lipoprotein subclasses determined from observed elution times are presented. C–F: Chylomicron, VLDL, LDL, and HDL, respectively (n = 3 per group). *P < 0.05 and **P < 0.01.
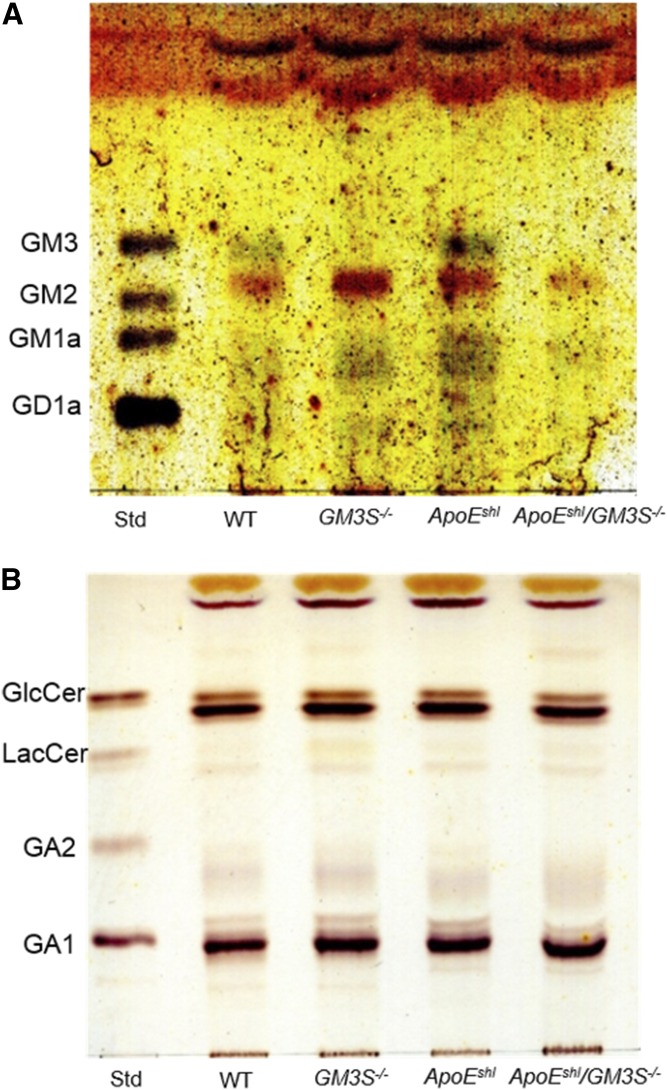
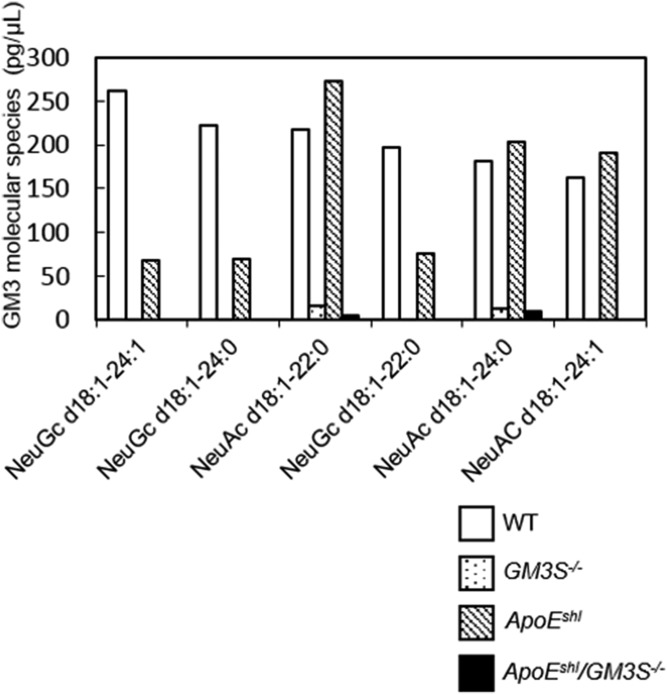
We next examined the GSL composition of intestinal mucosa, where NPC1L1-mediated cholesterol absorption occurs. It has been reported that intestinal GM3S expression level is high in neonatal mice and declines during the course of development (29). We detected GM3 expression in WT and ApoEshl mice by TLC and LC/MS/MS analyses. Trace amounts of GM3 molecular species were also detected in GM3S KO mice (Figs. 5A, 6), likely as the result of a newly identified transcriptional variant in GM3S−/− mice generated by targeting exon 3 of the GM3S gene (30). Levels of neutral GSLs in intestinal mucosa were not notably altered in GM3S−/− mice (Fig. 5B).
Fig. 5.
TLC analysis of ganglioside composition in mouse intestinal mucosa. Acidic (A) and neutral (B) GSLs equivalent to 2 or 1 mg intestinal mucosa wet weight, respectively, were applied to plates.
Fig. 6.
LC/MS/MS analysis of GM3 molecular species in mouse intestinal mucosa. Six species were detected (arranged according to abundance in WT mice).
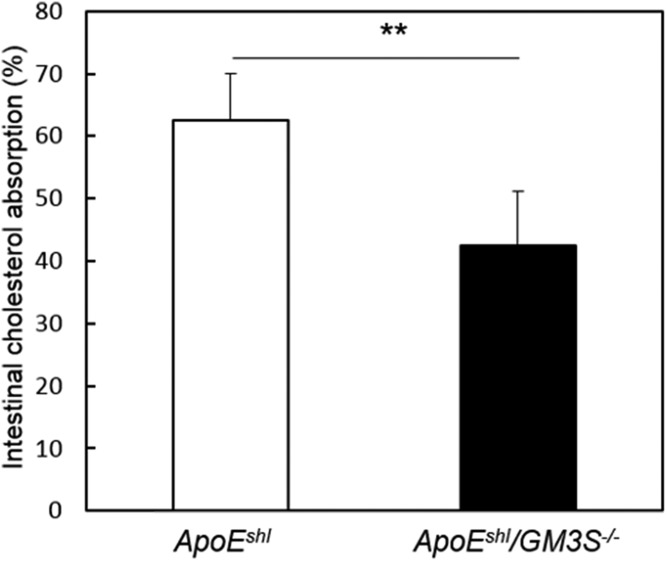
To test the hypothesis that resistance to hypercholesterolemia in GM3S−/− mice is due to impaired NPC1L1 function, we compared the intestinal cholesterol absorption rates of ApoEshl versus ApoEshl/GM3S−/− mice based on the oral administration of radiolabeled cholesterol. Uptake of cholesterol from the intestine was significantly lower in ApoEshl/GM3S−/− than in ApoEshl mice (Fig. 7).
Fig. 7.
GM3 plays an important role in intestinal cholesterol uptake. Mice (n = 5–6 per group) were orally gavaged with [14C]cholesterol and [3H]sitostanol, and the cholesterol absorption rate was determined by the fecal dual-isotope ratio method as described in Materials and Methods. **P < 0.01.
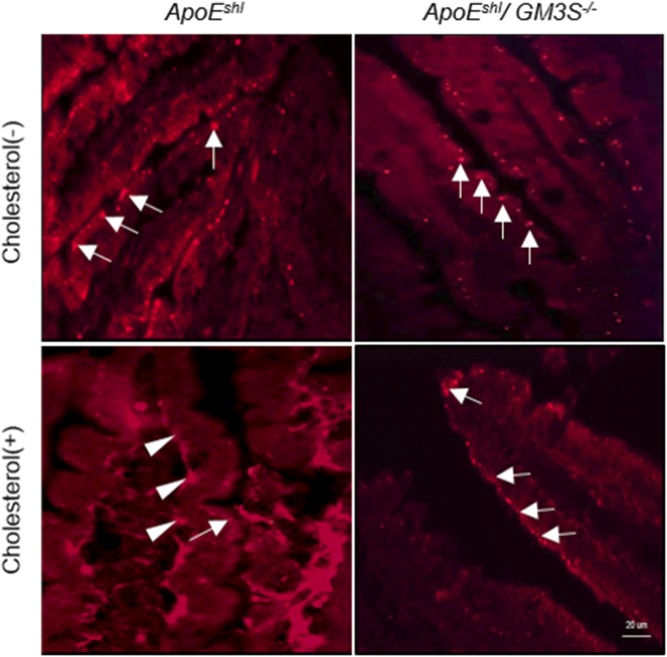
It has been demonstrated that the oral administration of cholesterol in mice induces the translocation of NPC1L1 from the intestinal epithelial surface to the intracellular region (31, 32). We examined the possibility that GM3S deficiency impairs cholesterol-dependent NPC1L1 translocation in vivo by immunostaining of intestinal NPC1L1. In both ApoEshl and ApoEshl/GM3S−/− mice, in the absence of cholesterol feeding, NPC1L1 localized mainly at the apical side of enterocytes (Fig. 8). Cholesterol feeding induced NPC1L1 internalization in ApoEshl but not in ApoEshl/GM3S−/− mice. Taken together, these findings clearly indicate that GM3 and/or related gangliosides are essential for NPC1L1-dependent intestinal cholesterol absorption.
Fig. 8.
Immunostaining of NPC1L1 in the small intestine of ApoEshl and ApoEshl/GM3S−/− mice with or without oral cholesterol administration. Frozen sections were stained with anti-NPC1L1 antibodies. Arrows: plasma membrane-localized NPC1L1. Arrowheads: internalized NPC1L1.
DISCUSSION
The protein NPC1L1 is known to be localized in detergent-resistant, ganglioside-enriched microdomains (11, 12, 32). The role of gangliosides in NPC1L1-dependent cholesterol absorption is unknown. Our previous studies have shown that GM3 plays key roles in certain metabolic disorders and that the inhibition of GM3 biosynthesis may help ameliorate metabolic imbalance (27, 33, 34). Results from the present study indicate that GM3S deficiency promotes resistance to hypercholesterolemia by inhibiting NPC1L1-mediated cholesterol uptake. NPC1L1-expressing GM3S KO cells displayed cholesterol uptake significantly lower than that of control cells (Fig. 1) and impairment of the cholesterol-dependent translocation of NPC1L1 from the plasma membrane to the intracellular region (Fig. 2). Consistent with these findings, GM3S−/− mice showed reductions of intestinal cholesterol uptake and cholesterol-dependent translocation of NPC1L1 (Figs. 7, 8). Plasma cholesterol levels in WT, GM3S−/−, ApoEshl, and ApoEshl/GM3−/− mice are summarized in Fig. 3A. GM3S-deficient mice were resistant to hypercholesterolemia induced by the high-cholesterol diet, and the hypercholesterolemia characteristic of ApoEshl mice was significantly ameliorated in ApoEshl/GM3S−/− mice. On the other hand, plasma cholesterol levels were similar for WT and GM3S−/− mice fed a normal diet. Taken together, these observations suggest functional involvement of GM3S in the exogenous pathways of cholesterol metabolism, including intestinal NPC1L1 activity.
Developmental changes in intestinal GSL composition have been found to be synchronized with expression levels of intestinal nutrient transporters (29). The knockdown of intestinal glucosylceramide synthase resulted in retarded growth and early death in mice because of defects in intestinal intracellular vesicular transport (35). These studies suggest that GSLs are physiologically important for intestinal nutrient absorption, but they did not address the role of GSLs in the NPC1L1 pathway. It has been shown that NPC1L1 requires a cholesterol-enriched membrane microdomain to function as a cholesterol transporter (11, 12, 32).
In the present study, cholesterol uptake by NPC1L1 was reduced in GM3S-deficient cells and mice. We therefore conclude that NPC1L1 requires not only cholesterol but also GM3 (or related gangliosides) to form functional membrane microdomains for cholesterol transport. Two possibilities can be considered: i) gangliosides interact directly with NPC1L1 via electrostatic interactions with multiple oligosaccharide chains to facilitate conformational change leading to translocation from lipid rafts to clathrin-coated pits, and ii) gangliosides are required for the association of NPC1L1 with proteins such as flotillins. The present findings provide novel insights into the mechanism of NPC1L1-mediated cholesterol absorption, which can be regulated by membrane lipid composition as well as by protein- protein interactions. The detailed mechanisms whereby GM3 and related gangliosides function in NPC1L1-mediated cholesterol absorption remain to be elucidated.
Gangliosides, particularly GM3, and its synthesizing enzyme GM3S appear to be potential targets for hypercholesterolemia therapy. Genetic variation in NPC1L1 is closely associated with interindividual variation in response to ezetimibe (36). Moreover, the loss of ezetimibe-binding mutations in the extracellular loop of NPC1L1 has been reported (9). Taken together, one can speculate that mutations in the region lead to unresponsiveness to the ezetimibe treatment. Membrane lipid modification such as GM3S inhibition can be used regardless of the binding affinity of compounds to the NPC1L1 and provide an alternative therapy for nonresponsive individuals.
Acknowledgments
The authors are grateful to all members of the Inokuchi and Fukase laboratories for valuable scientific discussions and technical support, to the Tohoku Medical and Pharmaceutical University Center for Laboratory Animal Science for their services, and to S. Anderson for English editing of the manuscript.
Footnotes
Abbreviations:
- GM3
- monosialodihexosylganglioside
- GM3S
- GM3 synthase
- GSL
- glycosphingolipid
- NPC1L1
- Niemann-Pick C1-like 1
- MβCD
- methyl-β-cyclodextrin
This study was supported by Ministry of Education, Culture, Sports, Science and Technology Research Grant-in-Aid 16H04767 (J.I.); grants-in-aid from the Mizutani Foundation for Glycoscience (J.I.), Takeda Science Foundation (J.I.), Fugaku Trust for Medicinal Research (J.I.), Naito Foundation (J.I.), Ono Medical Research Foundation (J.I.), and Uehara Memorial Foundation (J.I.); and the Ministry of Education, Culture, Sports, Science and Technology-supported Program for the Strategic Research Foundation at Private Universities.
REFERENCES
- 1.Shepherd J., Cobbe S. M., Ford I., Isles C. G., Lorimer A. R., Macfarlane P. W., McKillop J. H., and Packard C. J.. 1995. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N. Engl. J. Med. 333: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 2.Kreisberg R. A., and Oberman A.. 2002. Clinical review 141: lipids and atherosclerosis: lessons learned from randomized controlled trials of lipid lowering and other relevant studies. J. Clin. Endocrinol. Metab. 87: 423–437. [DOI] [PubMed] [Google Scholar]
- 3.Altmann S. W., Davis H. R., Zhu L-J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P. N., Maguire M., Golovko A., Zeng M., et al. 2004. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 303: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 4.Davis H. R., Zhu L-J., Hoos L. M., Tetzloff G., Maguire M., Liu J., Yao X., Iyer S. P. N., Lam M-H., Lund E. G., et al. 2004. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279: 33586–33592. [DOI] [PubMed] [Google Scholar]
- 5.Temel R. E., Tang W., Ma Y., Rudel L. L., Willingham M. C., Ioannou Y. A., Davies J. P., Nilsson L-M., and Yu L.. 2007. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Invest. 117: 1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge L., Wang J., Qi W., Miao H-H., Cao J., Qu Y-X., Li B-L., and Song B-L.. 2008. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 7: 508–519. [DOI] [PubMed] [Google Scholar]
- 7.Chang T-Y., and Chang C.. 2008. Ezetimibe blocks internalization of the NPC1L1/cholesterol complex. Cell Metab. 7: 469–471. [DOI] [PubMed] [Google Scholar]
- 8.Wang L-J., Song B-L.. 2012. Niemann-Pick C1-like 1 and cholesterol uptake. Biochim. Biophys. Acta. 1821: 964–972. [DOI] [PubMed] [Google Scholar]
- 9.Weinglass A. B., Kohler M., Schulte U., Liu J., Nketiah E. O., Thomas A., Schmalhofer W., Williams B., Bildl W., McMasters D. R., et al. 2008. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc. Natl. Acad. Sci. USA. 105: 11140–11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L., Bharadwaj S., Brown J. M., Ma Y., Du W., Davis M. A., Michaely P., Liu P., Willingham M. C., and Rudel L. L.. 2006. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J. Biol. Chem. 281: 6616–6624. [DOI] [PubMed] [Google Scholar]
- 11.Ge L., Qi W., Wang L-J., Miao H-H., Qu Y-X., Li B-L., and Song B-L.. 2011. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc. Natl. Acad. Sci. USA. 108: 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J-H., Ge L., Qi W., Zhang L., Miao H-H., Li B-L., Yang M., and Song B-L.. 2011. The N-terminal domain of NPC1L1 protein binds cholesterol and plays essential roles in cholesterol uptake. J. Biol. Chem. 286: 25088–25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakomori S. I. 2002. The glycosynapse. Proc. Natl. Acad. Sci. USA. 99: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons K., and Gerl M. J.. 2010. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 11: 688–699. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., and Yu R. K.. 2007. Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc. Natl. Acad. Sci. USA. 110: 19137–19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabayama K., Sato T., Saito K., Loberto N., Prinetti A., Sonnino S., Kinjo M., Igarashi Y., and Inokuchi J.. 2007. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl. Acad. Sci. USA. 104: 13678–13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saslowsky D. E., Cho J. A., Chinnapen H., Massol R. H., Chinnapen D. J. F., Wagner J. S., De Luca H. E., Kam W., Paw B. H., and Lencer W. I.. 2010. Intoxication of zebrafish and mammalian cells by cholera toxin depends on the flotillin/reggie proteins but not Derlin-1 or -2. J. Clin. Invest. 120: 4399–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohmi Y., Tajima O., Ohkawa Y., Yamauchi Y., Sugiura Y., Furukawa K., and Furukawa K.. 2011. Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: Elucidation by a series of ganglioside-deficient mutant mice. J. Neurochem. 116: 926–935. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa M., Go S., Takasaki K., Kakazu Y., Ohashi M., Nagafuku M., Kabayama K., Sekimoto J., Suzuki S., Takaiwa K., et al. 2009. Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc. Natl. Acad. Sci. USA. 106: 9483–9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagafuku M., Okuyama K., Onimaru Y., Suzuki A., Odagiri Y., Yamashita T., Iwasaki K., Fujiwara M., Takayanagi M., Ohno I., et al. 2012. CD4 and CD8 T cells require different membrane gangliosides for activation. Proc. Natl. Acad. Sci. USA. 109: E336–E342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushima Y., Hayashi S. I., and Tachibana M.. 1999. Spontaneously hyperlipidemic (SHL) mice: Japanese wild mice with apolipoprotein E deficiency. Mamm. Genome. 10: 352–357. [DOI] [PubMed] [Google Scholar]
- 22.Brown A. J., Sun L., Feramisco J. D., Brown M. S., and Goldstein J. L.. 2002. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol. Cell. 10: 237–245. [DOI] [PubMed] [Google Scholar]
- 23.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., and Zhang F.. 2013. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8: 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bligh E. G., and Dyer W. G.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 25.Usui S., Hara Y., Hosaki S., and Okazaki M.. 2002. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 43: 805–814. [PubMed] [Google Scholar]
- 26.Inokuchi J., Momosaki K., Shimeno H., Nagamatsu A., and Radin N. S.. 1989. Effects of D-threo- PDMP, an inhibitor of glucosylceramide synthetase, on expression of cell surface glycolipid antigen and binding to adhesive proteins by B16 melanoma cells. J. Cell. Physiol. 141: 573–583. [DOI] [PubMed] [Google Scholar]
- 27.Veillon L., Go S., Matsuyama W., Suzuki A., Nagasaki M., Yatomi Y., and Inokuchi J.. 2015. Identification of ganglioside GM3 molecular species in human serum associated with risk factors of metabolic syndrome. PLoS One. 10: e0129645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bietrix F., Lombardo E., Van Roomen C. P. A. A., Ottenhoff R., Vos M., Rensen P. C. N., Verhoeven A. J., Aerts J. M., and Groen A. K.. 2010. Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 leiden and low-density lipoprotein receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 30: 931–937. [DOI] [PubMed] [Google Scholar]
- 29.Yoneshige A., Sasaki A., Miyazaki M., Kojima N., Suzuki A., and Matsuda J.. 2010. Developmental changes in glycolipids and synchronized expression of nutrient transporters in the mouse small intestine. J. Nutr. Biochem. 21: 214–226. [DOI] [PubMed] [Google Scholar]
- 30.Shishido F., Uemura S., Nitta T., and Inokuchi J.. 2017. Identification of a new liver-specific c-type mRNA transcriptional variant for mouse ST3GAL5 (GM3/GM4 synthase). Glycoconj. J. 34: 651–659. [DOI] [PubMed] [Google Scholar]
- 31.Xie C., Zhou Z., Li N., Li B., and Song B-L.. 2012. Ezetimibe blocks the internalization of NPC1L1 and cholesterol in mouse small intestine. J. Lipid Res. 53: 2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P-S., Fu Z-Y., Zhang Y-Y., Zhang J-H., Xu C-Q., Ma Y-T., Li B-L., and Song B-L.. 2014. The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nat. Med. 20: 80–86. [DOI] [PubMed] [Google Scholar]
- 33.Nagafuku M., Sato T., Sato S., Shimizu K., Taira T., and Inokuchi J.. 2015. Control of homeostatic and pathogenic balance in adipose tissue by ganglioside GM3. Glycobiology. 25: 303–318. [DOI] [PubMed] [Google Scholar]
- 34.Inamori K. I., Ito H., Tamura Y., Nitta T., Yang X., Nihei W., Shishido F., Imazu S., Tsukita S., Yamada T., et al. 2018. Deficient ganglioside synthesis restores responsiveness to leptin and melanocortin signaling in obese KKAy mice. J. Lipid Res. 59: 1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jennemann R., Kaden S., Sandhoff R., Nordström V., Wang S., Volz M., Robine S., Amen N., Rothermel U., Wiegandt H., et al. 2012. Glycosphingolipids are essential for intestinal endocytic function. J. Biol. Chem. 287: 32598–32616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegele R.A., Guy J., Ban M. R., Wang J.. 2005. NPC1L1 haplotype is associated with inter-individual variation in plasma low-density lipoprotein response to ezetimibe. Lipids Health Dis.:4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]