Abstract
Background
The purpose of this study was to analyze tear inflammatory cytokines of different subclasses of dry eye disease (DED) patients using Luminex technology.
Material/Methods
Forty-five DED patients including 20 Sjogren syndrome aqueous tear deficiency (SS-ATD) patients, 20 non-Sjogren syndrome aqueous tear deficiency (NSS-ATD) patients, 15 meibomian gland dysfunction (MGD) patients, and 15 normal participants were enrolled in this study. Concentrations of 11 inflammatory cytokines in tear samples of study participants were measured by Luminex assay; ELISA assay was further applied for validation.
Results
The levels of cytokines were mostly increased (TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-12P70, IL-13, IFN-γ, and MIP-1α) in DED patients compared with normal participants. And the levels of TNF-α, IL-6, IL-8, and IL-12P70 were significantly elevated in tears of the patient groups compared to tears of participants in the normal group (P<0.05). Statistical differences were also observed among the patient groups (SS-ATD, NSS-ATD, and MGD) for the level of IL-8 and TNF-α. The results of ELISA assay demonstrated the consistence with Luminex assay, confirming the practicality of Luminex technology for the analysis of multiple cytokines in DED patient tears.
Conclusions
The levels of inflammatory cytokines were mostly elevated in DED patients, and statistical differences of some cytokines were also found between SS-ATD, NSS-ATD, and MGD groups, suggesting that inflammatory cytokines could be potential supplements for the diagnosis of DED subclasses and therapeutic targets for DED patients.
MeSH Keywords: Cytokines, Dry Eye Syndromes, Inflammation, Meibomian Glands, Sjogren’s Syndrome
Background
Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface, this definition was produced by International Dry Eye Work Shop in 2007 [1]. The prevalence of dry eye disease (DED) in larger epidemiological studies was reported to be over 25%; Asian dry eye morbidity rates include China’s incidence of about 20–36% [2]. Due to the differences in clinical symptoms, DED can be classified into aqueous tear deficiency (ATD) and evaporative dry eye, which also called meibomian gland dysfunction (MGD). ATD has 2 major subclasses, Sjogren syndrome dry eye (SS-ATD) and non-Sjogren syndrome dry eye (NSS-ATD) [1].
Although the clinical manifestations of DED are varied, the pathophysiological changes are similar. Inflammation was been acknowledged as the most important factor in the pathogenesis of DED [3]. The infiltration of a large number of lymphocytes in the lacrimal gland and ocular surface as well as the release of inflammatory cytokines can lead to immune-related inflammation, moreover, the production of inflammatory cytokines can damage the normal nerve conduction of tear secretion and affect the quality and quantity of tear secretion, forming a vicious circle [4,5]. A series of studies demonstrated that the levels of various cytokines including TNF-α, IL-1α, IL-1β, IL-6, IL-10, IL-8, and MIP-1α in tears of DED patients were significantly higher than those in normal people [6–8]. Studies also have suggested that the overexpression of these cytokines inhibit the release of neurotransmitters, and the activity of sensory nerves on the ocular surface thus weaken the secretion of lacrimal glands. Furthermore, the cytokines levels were proven to be positively related to the severity of DED [9].
Luminex is a new type of biomolecular detection technology that can simultaneous detect multi-biomolecules such as antigen, antibody, protein, and nucleic acid) by flow cytometry [10]. It has emerged that it not only offers the benefits of enzyme-linked immunosorbent assay (ELISA), but also enables the advantages of high throughput, high sensitivity, smaller sample volumes, and lower cost, which are suitable for multiple samples analysis [11]. In addition, comprehensive monitoring and evaluation of multiple cytokines with Luminex can better reflect the overall changes in the occurrence and development of the disease compared to just monitoring a single cytokine or several cytokines. For DED, the cytokine network formed by the interaction of multiple cytokines has a crucial effect on the process of inflammation. However, studies that focused on the correlation of different kinds of DED and multiple cytokine with Luminex assay have been limited [12–14]. Moreover, these studies rarely focused on the analysis of inflammatory cytokines in different subpopulations of DED.
In our current study, the Luminex technique was applied for the investigation of inflammatory cytokines in DED patient tears including TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-12P70, IL-13, IFN-γ, EGF, and MIP-1α. Besides, we systematically classified DED patients into 3 subclasses: SS-ATD, NSS-ATD, and MGD, in order to evaluate the differential expression in different subgroups of DED.
Material and Methods
Patients
The study comprised 70 volunteers: 20 SS-ATD patients, 20 NSS-ATD patients, 15 MGD patients, and 15 normal participants. Our study was approved by the medical ethics committee of Zhejiang Provincial People’s Hospital (2017KY002), and informed consent was obtained from all participants. All participants completed a symptom questionnaire which was modified based on the McMonnies CW’s questionnaire which has been clinically tested and proven to have 92% sensitivity and 95% specificity for dry eye diagnosis; symptom score >30 and <30 corresponded to the experimental group and the normal group, respectively. Groups were divided according to the criteria of the American College of Rheumatologist for Sjogren patients. All enrolled volunteers were further measured for conjunctival staining with tear breakup time (TBUT), corneal staining with fluorescein, and Schirmer I test [1,7]. The control group was included in the standard: without dry eye history and eye disease, no history of ocular surgery, corneal contact lens, ocular allergies. The Schirmer I test > 10 mm/5 min and TBUT >10 sec [1]. DED was diagnosed and included in the standard: the stability of lacrimal film was measured, and TBUT <10 sec regarded as unstable. The second step in the diagnosis of patients with unstable lacrimal film and included: lacrimal line height (not less than 0.3 mm), Schirmer I test (normal >10 mm/5 min), phenolic cotton filament test (normal reddish part >9 mm/15 sec), fluorescein clearance test (normally disappeared after 20 min). If the results of these tests were reduced, then further testing was done. The patients were further examined with serum antibodies (anti-DNA antibodies, anti-ENA antibodies, rheumatoid factors), Schirmer II test (normal >10 mm/5 min), rose bengal staining (<3, positive), and fluorescein staining in the exposed area of eyelid fissure (moderate to severe staining were considered positive). If these tests were positive, patients were classified into the SS-ATD group (20 patients). If negative, patients belonged to NSS-ATD group (20 patients). If the results of tear formation and tear distribution were normal, but there was no lipid secretion or more abnormal secretion after compression of tarsal gland, which means meibomian gland dysfunction and could be preliminarily diagnosed as evaporative dry eye and included in the MGD group (15 patients) [15].
Tears collection
All study participants (patient group and control group) had tear secretion collected at 17: 00 pm in a quiet room with weak light without surface anesthetic, throughout the study course. The tears samples were collected from marginal tear strip of the lateral lower lid with capillary tube disinfected with 75% alcohol under the slit lamp. Tear samples were collected for each eye with 100 μL and immediately placed in a tube containing 300 μL 1% BSA solution and stored in a deep cryogenic refrigerator at −86°C to avoid repeated freezing and thawing.
Luminex assay
A Luminex 200™ System (Luminex, Austin, TX, USA) was used to measure the cytokine levels of tear samples with analyses performed in triplicate. The cytokines included TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-12P70, IL-13, IFN-γ, EGF, and MIP-1α. The concentration values were obtained from the mean fluorescent intensity (MFI) by using Luminex200 IS V2.1 Software. Standard curves were generated from the reference cytokine gradient concentrations; the concentrations of these cytokines in tear samples were calculated from the standard curves.
ELISA assay
Four cytokines (TNF-α, IL-6, IL-8, and IL-12P70) in tear samples of 70 study participants were further detected using the ELISA assay (R&D Systems, Minneapolis, MN, USA). Analyses were performed according to the manufacturer instructions for each ELISA kit, in triplicate. The ELISA was performed as previously described [16].
Statistical analysis
SPSS 19.0 software was used for statistical analysis. Concentration of the cytokines was expressed as mean ± standard deviation (SD), and statistical significance among the groups was determined using the one-way ANOVA with Bonferroni post hoc test, P<0.05 was considered statistically significant.
Results
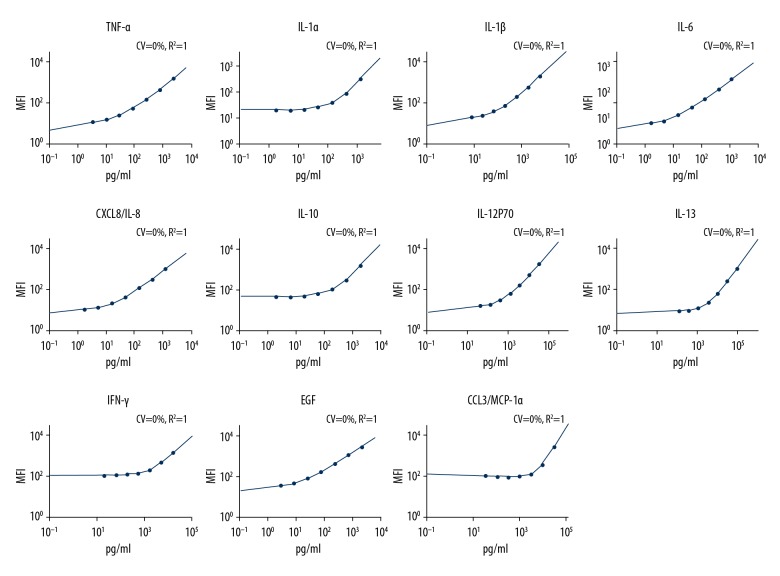
In this study, tear samples were collected from 70 participants including DED patients (SS-ATD, NSS-ATD, and MGD), and normal participants. Eleven cytokines (TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-12P70, IL-13, IFN-γ, EGF, and MIP-1α) were determined with the Luminex assay, standard curves of each cytokine are shown in Figure 1. Concentration values with Luminex assay of the 4 groups are shown in Table 1. Most of the cytokines had the highest expression in the SS-ATD group, followed by the NSS-ATD or MGD group, and finally the lowest levels were found in normal control participants.
Figure 1.
Standard curves of each cytokine in tears detected with Luminex assay. The corresponding standard curves of TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-12P70, IL-13, IFN-γ, EGF, and MIP-1α was given in turn.
Table 1.
Levels of tear inflammatory cytokines from dry eye and normal control patients with luminex assay (mean ±SD, pg/mL).
| Cytokine | Normal control | SS-ATD group | NSS-ATD group | MGD group |
|---|---|---|---|---|
| TNF-α | 9.08±6.85 | 25.80±11.81# | 16.45±9.04#* | 16.66±8.93#* |
| IL-1α | 27.19±25.77 | 49.98±58.23# | 43.24±40.71 | 40.57±42.62 |
| IL-1β | 7.83±6.83 | 11.6±8.12 | 14.85±14.96 | 14.01±10.24# |
| IL-6 | 3.79±4.31 | 9.46±9.16# | 6.78±7.63 | 9.15±9.98# |
| IL-8 | 74.39±30.36 | 412.13±107.59# | 253.16±94.29#* | 136.49±48.74#*$ |
| IL-10 | 4.46±3.49 | 6.63±3.43 | 6.38±4.32 | 4.13±3.25* |
| IL-12P70 | 134.04±77.03 | 211.12±70.38# | 162.11±71.35 | 196.81±56.12# |
| IL-13 | 1408.17±822.13 | 1684.66±2324.36 | 1592.31±1025.37 | 1398.19±952.7 |
| IFN-γ | 80.69±47.94 | 125.1±50.29 | 119.08±53.31 | 147.1±103.31# |
| EGF | 89.76±120.71 | 63.70±92.82 | 164.52±159.73 | 136.28±183.22 |
| MIP-1α | 1153.35±746.03 | 1743.86±832.74# | 1334.29±585.04 | 1209.26±851.27* |
Versus normal control,
P<0.05; versus SS-ATD group,
P<0.05; versus NSS-ATD group,
P<0.05.
The concentrations of TNF-α were significantly higher in the tear samples from the SS-ATD, NSS-ATD, and MGD groups compared with the normal group (P<0.05). In addition, significant differences were observed between the SS-ATD group and the NSS-ATD group (P=0.038), as well as the SS-ATD group and the MGD group (P=0.042). A tendency for the DED group levels to be higher than the levels of the normal control group was persists for IL-1 (IL-1α and IL-1β). There was a significant difference in IL-1α level between the SS-ATD group and the normal group (P<0.05), as well as a significant difference in IL-1β level between the MGD group and the normal group (P<0.05). For the concentrations of IL-6, the result showed that the SS-ATD, NSS-ATD, and MGD groups had higher levels than the normal group, with significant differences between the SS-ATD group and the normal group (P<0.05), as well as between the MGD group and the normal group (P<0.05). For the level of IL-8, Table 1 shows that the SS-ATD, NSS-ATD and MGD groups had significantly higher levels than the normal group (P<0.05). Furthermore, it is noteworthy that significant differences in IL-8 levels were also found between the SS-ATD group and the NSS-ATD group (P=0.000), the SS-ATD group and the MGD group (P = 0.000), as well as the NSS-ATD group and the MGD group (P=0.000). The tear concentrations of the IL-10 were higher in the SS-ATD group and the NSS-ATD group compared with the normal group, and the MGD group levels were significantly lower than found in the SS-ATD group (P<0.05).
The tear concentrations of the Th1 inducing cytokine IL-12P70, cytokines produced by Th1 (IFN-γ), and Th2 (IL-13) were also evaluated. The concentrations of IL-12P70 were significantly higher in the SS-ATD group and the MGD group compared with the control group (P<0.05). There was no significant difference among the groups for the concentrations of IL-13. The level of IFN-γ in the MGD group was statistically different compared with normal control group (P<0.05). For EGF, the concentration in the SS-ATD group was lower than the normal control group; the NSS-ATD group and the MGD group had higher levels than the normal group. The concentration of MIP-1α in the SS-ATD group was significantly higher than in the normal control group and the MGD group (P<0.05).
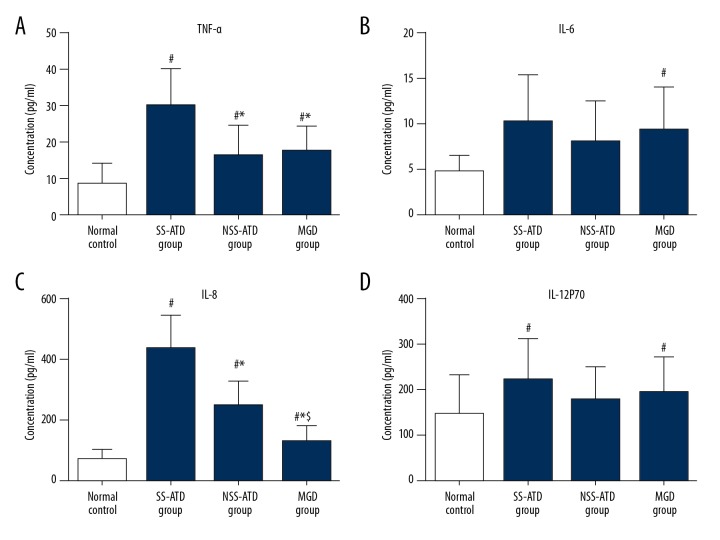
The concentrations of 4 cytokines (TNF-α, IL-6, IL-8, and IL-12P70) with ELISA assay are shown in Table 2 and Figure 2. The concentrations of these 4 cytokines determined by ELISA were consisted with the Luminex assay. The concentrations of TNF-α in the SS-ATD group, NSS-ATD group, and MGD group were all significantly higher than found in the normal control group (P<0.05, Figure 2A). The levels of TNF-α in the NSS-ATD group and the MGD group were significantly lower than found in the SS-ATD group (P=0.005, P=0.014, respectively). The concentration of IL-6 in the SS-ATD group and the MGD group was statistical different from the normal control group (P<0.05, Figure 2B). The concentrations of IL-8 in the 3 DED groups (SS-ATD, NSS-ATD, and MGD) were significantly higher than in the normal control group (P<0.05, Figure 2C). In addition, statistical differences could be reserved among these 3 subgroups of DED, with P value of 0.000 between the SS-ATD and the NSS-ATD groups (P=0.000), the SS-ATD group and MGD group (P=0.000), as well as the NSS-ATD group and the MGD group (P=0.000). For the IL-12P70 concentration, we detected a statistical difference between SS-ATD group and the MGD group compared with the normal group (Figure 2D).
Table 2.
Levels of tear inflammatory cytokines TNF-α, IL-6, IL-8, and IL-12P70 from dry eye and normal control patients with ELISA assay (mean ±SD, pg/mL).
| Cytokine | Normal Control | SS-ATD group | NSS-ATD group | MGD group |
|---|---|---|---|---|
| TNF-α | 8.29±5.79 | 29.63±10.42# | 16.11±8.51#* | 17.39±6.99#* |
| IL-6 | 4.83±1.69 | 10.19±5.14# | 8.04±4.36 | 9.39±4.57# |
| IL-8 | 67.21±32.43 | 433.51±111.40# | 245.72±78.75#* | 129.57±50.37#*$ |
| IL-12P70 | 147.17±86.18 | 225.12±88.46# | 179.44±69.99 | 195.86±75.04# |
Versus normal control,
P<0.05; versus SS-ATD group,
P<0.05; versus NSS-ATD group,
P<0.05.
Figure 2.
Concentrations of TNF-α (A), IL-6 (B), IL-8 (C), and IL-12P70 (D) from dry eye and normal control patients were determined by ELISA assay. Versus normal control, # P<0.05; versus SS-ATD group, * P<0.05; versus NSS-ATD group, $ P<0.05.
Discussion
DED is a complex and insidious pathology with a high level of prevalence among the human population [17]. Although the etiology of DED varies and the pathological mechanism is complicated, a common consensus is that the involvement of inflammation as an integral part of DED. Abundant evidences from animal and clinical studies has shown that upregulated of inflammatory cytokines in tears and conjunctival epithelium can result in more severe forms of DED [6,13,18,19]. In light of the important role in the process of inflammation, there is growing interests on the identification of cytokines that are specifically involved in the pathogenesis of DED. If particular, if cytokines are found to be associated with differences in the severity of the disease, then the expression of one or more of those cytokines could be used as a potential biomarker or therapeutic target of DED. Anti-inflammatory and lipid abnormalities therapies are currently the main treatment for DED, depending on the typical signs and symptoms [20]. There are an increasing number of clinical trials of anti-inflammatory drugs like ciclosporin A (Cs A), fluorometholone, and diclofenac for the clinical treatment of DED patients [21–23].
According to our study of 11 cytokines, the levels were mostly increased in DED patients (TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-12, IL-13, IFN-γ, and MIP-1α), and these findings were consisted with the previous published reports. In addition, ELISA assay further applied to measure the same cytokines found results consistent with Luminex assay results. TNF-α, IL-6, IL-8, and IL-12P70 were detected at higher concentrations in the SS-ATD group, NSS-ATD group, and MGD group. And, more importantly, statistical differences were also observed among patient groups, of the same cytokines, TNF-α and IL-8. For the concentration of TNF-α, the NSS-ATD group and MGD group all had statistical differences with the SS-ATD group. And for IL-8, there were statistical differences between each group.
TNF-α, mainly secreted by macrophages, plays a key role in many physiological and pathological processes such as inflammatory response, cellular immunity, and tumor immunity. The biological effects of TNF-α were induced by the interactions with either of 2 distinct receptors, TNF receptor1 (TNFR1, and TNF receptor2 (TNFR2) on the cell surface [24]. Both receptors trigger several signal transduction pathways, including apoptosis mediated by caspase family, the activation of NF-κB and JNK mediated by TRAF [25–27]. TNFR1 and TNFR2 perform their functions in a cooperative rather than independent manner. There are various regulation mechanisms existing in the 3 signal transduction pathways, which contribute to the coordinative effect of TNF-α. High expression of TNF-α can initiate a series of inflammatory reactions on the surface of the eye, inducing the occurrence and development of the DED. Series studies demonstrated that TNF-α was increased in tears and conjunctival epithelium of patients suffering from DED, and TNF-α levels were significantly correlated with ocular surface parameters and Schirmer I test [28]. Besides, some researchers suggest that TNF-α blockers can effectively suppressed lacrimal gland and corneal inflammation by suppressing other cytokines.
In addition, many members of the IL family are also involved in the inflammation of DED, including IL-1, IL-6, IL-8, IL-10, IL-12, and IL-13. The levels of these ILs in DED patient tears were correlated inversely with TBUT and Schirmer test scores. The injection of IL-1 could induce reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation on the mouse lacrimal gland [29]. Besides, in IL-1 receptor-1 (IL-1R1)-deficient (KO) mice with experimental dry eye disease, the expression of inflammatory cytokines TNF-α, IL-1α, IL-1β, and IL-6 in the corneal epithelium and conjunctiva were significantly decreased, indicated that IL-1 signaling is partly responsible for release of inflammatory cytokines [30]. The levels of IL-6 in DED patient tears were correlated inversely with ocular surface parameters like systemic symptom score, TBUT, and Schirmer I test score [31]. IL-6 was also regarded as a main characteristic for the immune response in human microbial keratitis and pediatric lacrimal duct obstruction. Amplification of IL 8 by resident tissues is an important mechanism for directing leukocytes migration, especially through avascular tissues like cornea. IL-8 also has strong attractive chemotactic effects to T cells and neutrophil cells, massive infiltration and activation of T lymphocytes leading to the damage of the lacrimal gland and ocular surface tissues through cytotoxicity and apoptosis [32]. High levels of IL-8 can be potent signal causing the typical signs of DED. And in our study, except for the elevated levels observed in DED patient groups, significant differences also can be found between subgroups of DED patients, indicated the involvement of different inflammatory processes as causes of different DED subgroups. IL-10, as a kind of anti-inflammation cytokine, is essential for promoting immune response from tissue epithelia to relieve the injury and facilitating the tissue-healing process in injuries caused by infection or inflammation. The study also found that IL-10 showed a stronger correlation with ocular surface parameters and several chronic ocular systemic chronic graft-versus-host disease (GVHD) severity scales [33].
Besides, in the present study, the levels of Th1 type cytokines IL-12, IFN-γ, and Th2 type cytokine IL-13 were also found to be increased in DED patient groups compared with the normal group. IL-12, an important immunoregulatory cytokine mainly produced by macrophages and dendritic cells, plays a vital role in initiation and progression of Th-1-type responses that produce IFN-γ [34]. The production of IL-12 mediated through toll-like receptors (TLR) can be augmented in the presence of pro-inflammatory signaling such as IFN-γ and IL-4. There are 2 forms of IL-12, IL-12p70 for active form and IL-12P40 for inactive form, and the elevated of IL-12p70 also can be observed in many inflammatory related diseases like DED. IFN-γ, as mentioned before, was secreted by Th-1, has specifically association with tear hyperosmolarity, severity of conjunctival pathology, and key clinical parameters of DED [35]. Moreover, elevated tear IFN-γ levels have been reported in heterogeneous populations of DED patients. The Th2 cytokine IL-13 is a predominant Th2 cytokines in ocular surface and has a homeostatic function in modulate goblet cell differentiation and density in DED. Th1cytokines IFN-γ has also been found to suppress IL-13 signaling and promotes apoptosis and squamous metaplasia of the ocular surface epithelia in mice.
Some previous studies have demonstrated the role of MIP-1α in dysfunctional tear syndrome, the increasing level of MIP-1α has correlation with parameters for meibomian gland dysfunction (MGD) in congenital aniridia, including increasing atrophy of meibomian glands, and shorter break-up time of the tear film [36]. EGF, a pleiotropic cytokine secreted by the lacrimal gland, is essential for maintaining homeostasis of the ocular surface epithelia and to stimulate proliferation, migration, adhesion of corneal epithelial cells during wound healing. However, when EGF concentration exceeds a certain high level, it is likely to promote corneal hypertrophy. In present study, tear EGF we detected was higher in MGD than in the control and was lower in SS than in the control. There is similar conclusion in the former study by Rao et al., the study found that tear EGF concentration was increased in eyes with corneal subepithelial fibrosis and MG orifice metaplasia, suggesting tear EGF may promote the development of corneal subepithelial fibrosis and lid margin changes [37].
Lots of studies were focused on cytokine profiles of DED patients, while the disease was rarely classified and limited reports have been published involving the classification of DED of those cytokines. Besides, the related reports of subclasses of DED mostly were meibomian gland dysfunction and Sjogren syndrome dry eye, but few data focused on the non-Sjogren’s DED of inflammatory cytokines. In this study, the SS-ATD, NSS-ATD, and MGD patients were comprehensive involved for the analysis of the inflammatory cytokines, statistical difference of certain cytokines observed among these patient groups indicated that the level of cytokines could be a potential supplement for the diagnosis of DED subpopulations as well as a biomarker to differentiate SS-ATD, NSS-ATD, and MGD.
Conclusions
The current study developed a Luminex technology to evaluate cytokine levels including TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-12P70, IL-13, IFN-γ, EGF, and MIP-1α in 3 subclasses of DED patient tear samples and that were further confirmed with ELISA assay. The multiplexed Luminex assay was proven to be an ideal method for detection of various cytokines in tear samples, with high-sensitivity and high-throughput characteristics, which is potentially suitable for use in medical research. The levels of these cytokines were mostly increased in DED patients (TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-12P70, IL-13, IFN-γ, and MIP-1α) compared with normal participants. And, more importantly, statistical differences were also observed among the subclasses of DED for the IL-8 and TNF-α, suggesting that cytokines could be a potential supplement for the diagnose of DED subpopulations and therapeutic targets for SS-ATD, NSS-ATD, and MGD patients. Our study has potential impact on the research of the dry eye; the systematic analysis of inflammatory cytokines might lead to greater control of inflammation reaction in dry eye treatment. Further studies are need to evaluate more patients and a large population in order to co-validate the data and eventually develop a panel to be used for patients with dry eye disease.
Footnotes
Conflict of interest
None.
Source of support: Funding for this work was supported by the Technology Department of Zhejiang Province of China (NO: 2017C37079)
References
- 1.The definition and classification of dry eye disease: report of the definition and classification Subcommittee of the International Dry Eye Work Shop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–82. doi: 10.3238/arztebl.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudouin C, Irkeç M, Messmer EM, et al. Clinical impact of inflammation in dry eye disease: Proceedings of the odissey group meeting. Acta Ophthalmologica. 2018;96(2):111–19. doi: 10.1111/aos.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okuma A, Hoshino K, Ohba T, et al. Enhanced apoptosis by disruption of the STAT3-IκB signaling pathway in epithelial cells induces Sjögren’s syndrome-like autoimmune disease. Immunity. 2013;38(3):450–60. doi: 10.1016/j.immuni.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Maliborski A, Różycki R. Diagnostic imaging of the nasolacrimal drainage system. Part I. Radiological anatomy of lacrimal pathways. Physiology of tear secretion and tear outflow. Med Sci Monit. 2014;20(20):628–38. doi: 10.12659/MSM.890098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal R, Balne PK, Veerappan A, et al. A distinct cytokines profile in tear film of dry eye disease (DED) patients with HIV infection. Cytokine. 2016;88:77–84. doi: 10.1016/j.cyto.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Carreño E, Portero A, Herreras JM, et al. Cytokine and chemokine tear levels in patients with uveitis. Acta Ophthalmologica. 2017;95(5):e405–14. doi: 10.1111/aos.13292. [DOI] [PubMed] [Google Scholar]
- 8.Tong L, Wong TY, Cheng Y. Level of tear cytokines in population-level participants and correlation with clinical features. Cytokine. 2018;110:452–58. doi: 10.1016/j.cyto.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan S, Miller WL, Mcdermott AM. Conjunctival cytokine expression in symptomatic moderate dry eye subjects. Invest Ophthalmol Vis Sci. 2006;47(6):2445–50. doi: 10.1167/iovs.05-1364. [DOI] [PubMed] [Google Scholar]
- 10.Khalifian S, Raimondi G, Brandacher G. The use of luminex assays to measure cytokines. J Invest Dermatol. 2015;135(4):e31. doi: 10.1038/jid.2015.36. [DOI] [PubMed] [Google Scholar]
- 11.Marleen JA, Simmelinka K, Vennegoor A, et al. The impact of pre-analytical variables on the stability of neurofilament proteins in CSF, determined by a novel validated Single Plex Luminex assay and ELISA. J Immunol Methods. 2014;402:4–9. doi: 10.1016/j.jim.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Le GX, Quah J, Tong L, et al. Human tear analysis with miniaturized multiplex cytokine assay on “wall-less” 96-well plate. Mol Vis. 2015;21:1151–61. [PMC free article] [PubMed] [Google Scholar]
- 13.Dionne K, Redfern RL, Nichols JJ, et al. Analysis of tear inflammatory mediators: A comparison between the microarray and Luminex methods. Mol Vis. 2016;22:177–88. [PMC free article] [PubMed] [Google Scholar]
- 14.Lafrance MW, Kehinde LE, Fullard RJ. Multiple cytokine analysis in human tears: An optimized procedure for cytometric bead-based assay. Curr Eye Res. 2008;33(7):525–44. doi: 10.1080/02713680802190085. [DOI] [PubMed] [Google Scholar]
- 15.Giannaccare G, Vigo L, Pellegrini M, et al. Ocular surface workup with automated noninvasive measurements for the diagnosis of meibomian gland dysfunction. Cornea. 2018;37(6):740–45. doi: 10.1097/ICO.0000000000001500. [DOI] [PubMed] [Google Scholar]
- 16.Baldofski S, Hoffmann H, Lehmann A, et al. Enzyme-linked immunosorbent assay (ELISA) for the anthropogenic marker isolithocholic acid in water. J Environ Manage. 2016;182:612–19. doi: 10.1016/j.jenvman.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Villatoro AJ, Fernã n V, Claros S, et al. Regenerative therapies in dry eye disease: from growth factors to cell therapy. Int J Mol Sci. 2017;18(11) doi: 10.3390/ijms18112264. pii: E2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mrugacz M, Ostrowska L, Bryl A, et al. Pro-inflammatory cytokines associated with clinical severity of dry eye disease of patients with depression. Adv Med Sci. 2017;62(2):338–44. doi: 10.1016/j.advms.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Tong L, Wong TY, Cheng Y. Level of tear cytokines in population-level participants and correlation with clinical features. Cytokine. 2018;110:452–58. doi: 10.1016/j.cyto.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguezpomar C, Pintor J, Colligris B, et al. Therapeutic inhibitors for the treatment of dry eye syndrome. Expert Opin Pharmacother. 2017;18(4):1855–65. doi: 10.1080/14656566.2017.1403584. [DOI] [PubMed] [Google Scholar]
- 21.Jung HH, Ji YS, Sung MS, et al. Long-term outcome of treatment with topical corticosteroids for severe dry eye associated with Sjogren’s syndrome. Chonnam Med J. 2015;51(1):26–32. doi: 10.4068/cmj.2015.51.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van WA, Pandhi S, Nixon RM, et al. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: A network meta-analysis. Arthritis Res Ther. 2015;17(1):66. doi: 10.1186/s13075-015-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal P, Rupenthal ID. Modern approaches to the ocular delivery of cyclosporine A. Drug Discov Today. 2016;21(6):977–88. doi: 10.1016/j.drudis.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Idriss HT, Naismith JH. TNFα and the TNF receptor superfamily: Structure function relationship(s) Microsc Res Tech. 2015;50(3):184–95. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Ni HM, Mcgill MR, Chao X, et al. Caspase inhibition prevents tumor necrosis factor-α-induced apoptosis and promotes necrotic cell death in mouse hepatocytes in vivo and in vitro. Am J Pathol. 2016;186(10):2623–36. doi: 10.1016/j.ajpath.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver MM, Fuchs D, Tagscherer KE, et al. Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-α-dependent necroptosis driven by RIP1 kinase and NF-κB. Oncogene. 2015;35(26):3399–409. doi: 10.1038/onc.2015.398. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Zheng FP, Zhan YS, et al. Tumor necrosis factor-α mediates JNK activation response to intestinal ischemia-reperfusion injury. World J Gastroenterol. 2013;19(30):4925–34. doi: 10.3748/wjg.v19.i30.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sang YL, Han SJ, Sang MN, et al. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients. Am J Ophthalmol. 2013;156(2):247–53. doi: 10.1016/j.ajo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007;84(5):894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayanan S, Corrales RM, Farley W, et al. Interleukin-1 receptor-1-deficient mice show attenuated production of ocular surface inflammatory cytokines in experimental dry eye. Cornea. 2008;27(7):811–17. doi: 10.1097/ICO.0b013e31816bf46c. [DOI] [PubMed] [Google Scholar]
- 31.Yoon KC, Jeong IY, Park YG, et al. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26(4):431–37. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]
- 32.Xia L, Zhang S, Zhou J, et al. A crucial role for B and T lymphocyte attenuator in preventing the development of CD4+ T cell-mediated herpetic stromal keratitis. Mol Vis. 2010;16:2071–83. [PMC free article] [PubMed] [Google Scholar]
- 33.Won JJ, Soo Jung H, Kyung SM, et al. Tear cytokines as biomarkers for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(12):2079–85. doi: 10.1016/j.bbmt.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Wang Q. Factors determining the formation and release of bioactive IL-12: regulatory mechanisms for IL-12p70 synthesis and inhibition. Biochem Biophys Res Commun. 2008;372(4):509–12. doi: 10.1016/j.bbrc.2008.05.081. [DOI] [PubMed] [Google Scholar]
- 35.Jackson DC, Zeng W, Wong CY, et al. Tear interferon-gamma as a biomarker for evaporative dry eye disease. Invest Ophthalmol Vis Sci. 2016;57(11):4824–30. doi: 10.1167/iovs.16-19757. [DOI] [PubMed] [Google Scholar]
- 36.Landsend ECS, Utheim OA, Pedersen HR, et al. The level of inflammatory tear cytokines is elevated in congenital aniridia and associated with meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2018;59(5):2197–204. doi: 10.1167/iovs.18-24027. [DOI] [PubMed] [Google Scholar]
- 37.Rao K, Farley WJ, Pflugfelder SC. Association between high tear epidermal growth factor levels and corneal subepithelial fibrosis in dry eye conditions. Invest Ophthalmol Vis Sci. 2010;51(2):844–49. doi: 10.1167/iovs.09-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]