Abstract
PARKIN (E3 ubiquitin ligase PARK2), PINK1 (PTEN induced kinase 1) and DJ-1 (PARK7) are proteins involved in autosomal recessive parkinsonism, and carcinogenic processes. In damaged mitochondria, PINK1’s importing into the inner mitochondrial membrane is prevented, PARKIN presents a partial mitochondrial localization at the outer mitochondrial membrane and DJ-1 relocates to mitochondria when oxidative stress increases. Depletion of these proteins result in abnormal mitochondrial morphology. PINK1, PARKIN, and DJ-1 participate in mitochondrial remodeling and actively regulate mitochondrial quality control. In this review, we highlight that PARKIN, PINK1, and DJ-1 should be regarded as having an important role in Cancer Biology. The STRING database and Gene Ontology (GO) enrichment analysis were performed to consolidate knowledge of well-known protein interactions for PINK1, PARKIN, and DJ-1 and envisage new ones. The enrichment analysis of KEGG pathways showed that the PINK1/PARKIN/DJ-1 network resulted in Parkinson disease as the main feature, while the protein DJ-1 showed enrichment in prostate cancer and p53 signaling pathway. Some predicted transcription factors regulating PINK1, PARK2 (PARKIN) and PARK7 (DJ-1) gene expression are related to cell cycle control. We can therefore suggest that the interplay among PINK1/PARKIN/DJ-1 network during mitochondrial quality control in cancer biology may occur at the transcriptional level. Further analysis, like a systems biology approach, will be helpful in the understanding of PINK1/PARKIN/DJ-1 network.
Keywords: mitochondrial quality control, oxidative stress, PARKIN, PINK1, DJ-1, cancer biology, protein-protein interactions
Mitochondria are fundamental in numerous cellular functions. The oxidation of carbon sources, the synthesis of adenosine triphosphate, ATP, the generation of reactive oxygen species (ROS), apoptosis, calcium homeostasis, and heme synthesis, among other primordial processes, depend on them. In this same way, mitochondrial dysfunctions are described as the etiological origin of numerous pathologies in mammals such as neurodegenerative diseases, cancer, diabetes, and anemia. One of the main reasons for mitochondrial dysfunction is oxidative stress. Among the damages suffered by the cells are the modification of lipids, alteration of nucleic acids, and damage to proteins. In this review, we will present the most relevant aspects of oxidative stress in mitochondrial biology and the mechanisms of quality control of mitochondria where PARKIN, PINK1, and DJ-1 proteins are involved. In doing so, we try to understand how is the interaction of the network PINK1/PARKIN/DJ-1 during the loss of homeostasis in quality control and how this is associated with carcinogenesis.
1. Biochemistry of Reactive Oxygen and Nitrogen Species (ROS/RNS)
Diatomic oxygen (O2) has a high redox potential making it an oxidizing agent capable of accepting electrons from reduced substrates [1]. The reactive oxygen species (ROS) generated in the mitochondrial network primarily originate from O2•−, and from its acidic conjugate hydroperoxyl radical HO2• which is soluble in membranes [2,3]. However, hydroxyl (OH•), carbonate (CO3•−), peroxyl (RO2•), and alkoxyl (RO•) radicals are also potential originators [4]. Mitochondrial O2•− production by reduction of O2 single electron is thermodynamically preferred, even by relatively oxidizing redox couples, due to being an event that occurs within the proteins’ redox-active prosthetic groups. However, mitochondrial O2•− production is also achieved when electron carriers such as CoQH2 (NADH, NADPH, Glutathione) are bound to proteins hence, driving the kinetic factors determine the O2•− production [5,6]. Additionally, transition metals like Copper (Cu), Iron (Fe) and Zinc (Zn) destabilize spin states at the outer orbital electrons in O2 and generate ROS such as O2•−, hydrogen peroxide (H2O2) and hydroxyl radical (OH•) [1]. By contrast, Fenton reactions are accountable for the most reactive hydroxyl radical OH• originating from H2O2 (Table 1) [4,7]. O2•− and H2O2 can be generated from major sites at high rates both in the mitochondrial matrix and in the cytosolic side of the mitochondrial inner membrane. However, mitochondrial and cytosolic scavenging of O2•− and H2O2 are also very powerful, for instance, four sets of dynamic balance between production and consumption rates regulate the levels of O2•− and H2O2 [6].
Table 1.
Main equations involved in free radical biochemistry.
| Metal-Catalyzed Haber-Weiss Reaction |
|---|
| (1) |
| (2) |
| (3) |
(1) Reduction of ferric ion to ferrous, (2) Fenton reaction, (3) The net reaction. O2, Diatomic oxygen; O2•−, Superoxide; H2O2, Hydrogen Peroxide, OH•, Hydroxyl radical.
Under hypoxic conditions, the mitochondrial respiratory chain also produces nitric oxide (NO•) that in turn can lead to the production of further reactive nitrogen species (RNS) [1]. Similarly, NO• can react with O2•− to form strong oxidant peroxynitrite (ONOO−) [1]. It is therefore reasonable to suggest that the best-understood function of mitochondrial ROS and NO• is their role in oxygen sensing [1], whereby high oxygen levels would favor O2•−, while low oxygen levels would favor NO•; however, intermediate oxygen levels would prefer ONOO− [1]. Nitrosative and oxidate stresses have been identified in aging and several diseases, including neurodegenerative diseases and cancer. Cancer cells are skilled at upregulating survival signaling pathways and must respond to the high levels of NO and ROS, which are byproducts of their growth.
2. How are Free Radicals Produced in the Mitochondria?
A mitochondrion is characterized by a complex double membrane structure housing cristae (formation of cavities and folds within the inner mitochondrial (IMM) membrane whose longitudinal axis is at right angles to the inner boundary layer) and by continuous tubules of the mitochondrial reticulum network [6,8,9]. The respiratory chain is usually considered one of the primary sources of ROS. The oxidative phosphorylation (OXPHOS) housed in the IMM, plays a crucial role in energy production and the generation of ROS [10]. The OXPHOS system comprises of the electron transport chain (ETC) formed by four respiratory complexes (Complex I, NADH dehydrogenase; Complex II, succinate dehydrogenase; Complex III, ubiquinol cytochrome c reductase; and Complex IV, cytochrome c oxidase) plus the mobile electron carriers (e.g., cytochrome c and coenzyme Q) [11]. The OXPHOS machinery is organized into higher-order complexes called respiratory supercomplexes or respirosomes, which are formed by the agglomeration of complexes I, III, and IV in the membrane of mitochondria in different configurations. These supercomplexes or respirosomes form functional units in the ETC through which electrons are channeled [12,13].
Within the Krebs cycle, ETC and β-oxidation in mammalian mitochondria a minimum of ten different sites for O2•−/H2O2 production have so far been identified. Complex I and complex III are the most critical mitochondrial O2•− producers but other sites have also been acknowledged, including pyruvate dehydrogenase, glycerol phosphate dehydrogenase, and α-ketoglutarate dehydrogenase and ETF-CoQ reductase [14]. The factors managing mitochondrial ROS levels are associated to their own generation and removal rate, while the regulation of the concentration of redox species responsible for electron leaking and ROS generation is mediated by the redox potential of the NAD+/NADH coupling degree and the proton-motive force (Δp) [7,15]. In turn, these events are regulated by the redox supply to the respiratory chain achieved via the number of couplings made and constraints encountered during the electron transfer [7,15]. Superoxide Dismutase (MnSOD) in the mitochondrial matrix catalyzes the dismutation of O2•− very rapidly into non-radical H2O2 which is regarded as a stable species as well as a primordial mediator in redox regulations by implementing relay or diffusion through the redox buffer system [16,17]. It is also worth mentioning that the Superoxide Dismutase (CuZnSOD) in the mitochondrial intermembrane space and cytosol dismutes O2•− to H2O2 [16,17].
In mammals, complex I is the entry point for electrons from NADH into the respiratory chain. It is also a ~1 MDa complex comprising 45 proteins, seven of which are mitochondrially encoded subunits [18,19]. Complex I produce large amounts of O2•− by two mechanisms: (1) high mitochondrial matrix NADH/NAD+ ratio, leading to a reduced flavin mononucleotide site on complex I, and (2) when electron donation to the coenzyme Q (CoQ) pool is accompanied by a high Δp and a lack of ATP synthesis leading to a reversal in the electron transfer (Re-t) [5]. So far, research findings seems to point out at Re-t mediated O2•− production rate as being the highest ever found in mitochondria [20]. Under mitochondrial physiological conditions, complex III is yet another originator of O2•− however, this source is minor compared that achieved by complex I [17,21]. Nevertheless, in conditions where complex I is either absent or low then O2•− production by complex III assumes a more significant role [5]. For mitochondria that are either actively synthesizing ATP or utilizing Δp for other functions (state 3), both a low Δp and an oxidized NADH pool prevent O2•− production by Re-t and significantly decrease it at the flavine mononucleotide of complex I. Although, O2•− production rate is almost minimal in state 3, this particular state might be the driver of the highest biological grade and be accountable for the cumulative mitochondrial oxidative damage and the enormous tissue damage as found in the absence of MnSOD. Additionally, at low oxygen concentration complex IV (CIV) produces NO• from nitrite (NO2−) [22]. Inhibition of complex IV may facilitate ROS production by complexes I or III [10].
3. Mitochondrial Quality Control Systems
Within the human mitochondrial matrix, 495 proteins have thus far been identified [23]. Many of the coordinated protein quality control systems are localized in the mitochondrial matrix. These control systems encompass the Lon, ClpXP, and m-AAA proteases, which are proteases of AAA+ conserved superfamily. The latter have been suggested to be highly involved in important events of the mitochondrial proteins, found in the matrix and the inner membrane, including degradation, processing, and supervision of the proteins assembly. The inner membrane is in turn involved in oxidative phosphorylation, mitochondrial protein synthesis, mitochondrial network dynamics, and nucleoid function. The quality control proteases are in turn upregulated by a variety of mitochondrial stressors, including oxidative stress, unfolded protein stress and imbalances in the assembly of respiratory complexes [24].
The mitochondria have highly developed networks that are kept in good working order by the contrasting events of fusion and fission [25,26]. Each fusion event requires two reactions: an initial one triggered at the outer membrane followed by reaction of the inner membrane [27,28]. In mammals, these reactions are catalyzed by dedicated GTP-ases (Mfn1, Mfn2, and OPA1) [29,30]. By contrast, mitochondrial fission is a separation event in which a mitochondrion is split into two organelles [31]. In mammals, this event is moderated by Drp1, a cytosolic dynamin-like protein that constricts the mitochondrial membrane and promotes mitochondrial fragmentation (fission) [32,33]. Drp1 binds to mitochondrial fission protein 1 (Fis1) at the mitochondrial outer membrane. Preservation of a fit and functional mitochondrial network is vital in the adequate response to physiological changes and stressful environments. Unfortunately, due to exposure to high levels of ROS in their role in energy production, mitochondria is a damage-prone structure [6]. Mitochondria have developed elaborate mechanisms of quality control regulated by two opposing events: the removal of damaged organelles or their components and mitochondrial biogenesis to ensure that the proper quantity of functional mitochondria is available to counter the cellular stresses. The elimination of whole mitochondria is accomplished by a selective form of autophagy called mitophagy [34,35]. Fusion, fission, and mitophagy are important moderators of mitochondrial turnover and homeostasis; the combination of these processes shape the cell’s survival decisions [36,37].
In this review, we focus on the regulatory processes of proteins PINK1, PARKIN, and DJ-1 specifically during mitochondrial quality control due to its importance in cancer biology. PARKIN (Parkin RBR E3 Ubiquitin Protein Ligase, PARK2 gene), PINK1 (PTEN Induced Putative Kinase 1, PINK1 gene), and DJ-1 (Parkinsonism Associated Deglycase, PARK7 gene) are proteins involved in autosomal recessive parkinsonism [38,39,40,41], and modulators of carcinogenic processes [42,43,44,45,46,47].
In damaged mitochondria, PINK1’s importing into the inner mitochondrial membrane is prevented, PARKIN presents a partial mitochondrial localization at the outer mitochondrial membrane and DJ-1 relocates to mitochondria when oxidative stress increases [48,49,50,51,52,53,54,55,56]. These proteins actively regulate mitochondrial quality control [48,51,52,56,57,58] and are involved in mitochondrial remodeling [53,59,60,61] hence, their depletion or even loss is a cause of changes in the mitochondria’s morphology [53,55]. The interaction of PINK1 and PARKIN regulates mitochondrial dynamics [62,63,64,65,66,67] and also mediates mitochondrial quality control [51,56,59,60,61,62]. They are proteins that mediate mitophagy of malfunctioning mitochondria when they cooperate with each other [68,69]. Interestingly, PINK1 and PARKIN’s reciprocity aids the regulation of mitochondrial morphology via mitochondrial fission and fusion, and this in turn has an impact on mitochondrial quality control [59,61,64,67]. Only recently, DJ-1 was found to have a role in mitochondrial quality control [57,58,70]; it brings a protective function by aiding mitochondrial homeostasis or by responding to oxidative stresses [71,72].
3.1. PINK1′s Role in Mitochondrial Quality Control
PINK1 gene encodes PTEN Induced Putative Kinase 1, a serine/threonine kinase homologous to kinases that are moderated by calcium and/or calmodium [40]. The exogenous expression of PTEN (Phosphatase and Tensin Homolog), a primary tumor suppressor, upregulates PINK1 [73]. PINK1 has been found in mitochondria in all cell types from rat and human brain tissue [74]. PINK1 protein spans the outer mitochondrial membrane (OMM). It has a topology in which the kinase domain in the C-terminal faces the cytoplasm while the N-terminal tail contained inside the mitochondria, near the canonical N-terminal mitochondrial targeting signal (MTS), is located in the transmembrane (TM) domain [55]. This domain is necessary for PINK to attach to the mitochondrial membrane with its kinase domain facing the cytoplasm [55]. PINK also displays neuroprotective roles including stabilization of the mitochondrial membrane potential; halting the release of apoptogenic factors such as cytochrome c; inhibiting O2•− production [59,64,75,76,77]. The role of PINK1 in mitochondrial dynamics was first identified in studies done in a Drosophila model in which lack of PINK1 resulted in adverse conditions for mitochondria’s post-mitotic tissues [59].
The study of silencing PINK1 gene expression in different models, including cultured mammalian cells, Drosophila, mouse and primary fibroblasts in a human subject with mutated PINK1 revealed a highly abnormal morphology presenting loss of membrane potential and increased oxidative stress; thus, associating PINK1 with mitochondrial homeostasis control [61,64,67,70,77,78,79,80,81]. Surprisingly, these alterations in mitochondrial physiology were found to be revoked by either a fluctuating or steady expression of PARKIN suggesting that PARKIN is a downstream effector of PINK1 in the regulation of mitochondrial homeostasis [64]. Not only was the induction of mitochondrial fragmentation and autophagy of PINK1 knockdown rescued with PARKIN, its overexpression caused an augmented autophagic and mitophagy response [62]. The latter was also reported recently in a study focusing on PARKIN’s role in autophagic cleansing of depolarized mitochondria [51,56].
PINK1 supports mitochondrial membrane potential; oxidative phosphorylation [82,83,84] complex I activity [84]; ROS and Ca2+ balance [85]; protects from mitochondrial stress [62,63,83,86], regulates mitochondrial dynamics [61]; key regulator of mitophagy [87,88]. It is also involved in the upkeep of healthy mitochondrial networks which in turn allows for the migration of mitochondria to synapses via Miro and Milton, kinesin adaptor-like proteins [89].
The appearance of unfolded proteins in the matrix acts as a sensor that promotes PINK1 buildup in mitochondria with healthy bioenergetics; thus, causing PARKIN migration and mitophagy; eventually, reducing the amount of unfolded protein. Likewise, silencing of the LONP1 protease enhances PINK1 accumulation [90]. While PINK build up is the precursor of mitophagy as a quality control process, PARKIN assumes a cleansing role by removing defective mitochondria via autophagy [51,52,56,91].
3.2. PARKIN’s Role in Mitochondrial Quality Control.
PARKIN is a protein, the after encoded by PARK2 gene, that acts as a E3 ubiquitin ligase, it is part of the ubiquitin-proteasome system [92,93]. It is typically found in the cytoplasma of most cells but would translocate to mitochondria when the organelle is damaged [94,95].
A variety of human cancers present alterations in chromosome 6q25–27 [92,96]; PARKIN is a potential tumor suppressor gene for that chromosome [97]. Thus, PARKIN mutations resulting in changes in this chromosome are potentially a risk factor for neurodegeneration and cancer.
The phosphorylation of serine 65 (S65) amino acid residue in the Ubl domain in PARKIN mediated by PINK1 is critical for its recruitment to the mitochondrial outer membrane [94,98]. The same phosphorylation also generates a type of phospo-ubiquitin (pUb) [94,99,100] that triggers morphologic changes leading to the release of the UbI domain. The latter allows for PARKIN’s center to be phosphorylated by PINK1 [101,102,103]. Mutations of the S65 domain in PARKIN produces physiologically inactive proteins that alter the mitochondria’s ability to recruit PARKIN; hence, producing a misbalance in cleansing activities such as mitophagy [104]. Thus, the protein PARKIN presents a basal auto-inhibited state, for its enzymatic activity in mitophagy requires the activation through the S65 phosphorylation, although it is not clear if the S65 phosphorylation step activation is necessary for PARKIN mediated ubiquitination in non-mitophagy events [105].
The S-nitrosylation of PARKIN inhibits its neuroprotective ability as found in the brains of patients with PD [106]. This modification arises, after its reaction with nitric oxide (NO) and as a consequence producing SNO-PARKIN [107,108]. E3 ubiquitin ligase is then briefly stimulated before it becomes wholly dysfunctional, initiating a atypical protein production [18,19].
PARKIN is involved in mitochondrial homeostasis however; it is not active in the cytosol. PARKIN’s translocation to mitochondria by a decrease in mitochondrial membrane potential depends on PINK1 expression [54]. In ROS-driven processes, PARKIN translocates to mitochondria [109] of primary neurons; by contrast, it translocates to the uncoupled mitochondria of tumor cell lines [110], and it participates in the clearance of damaged mitochondria under PINK1’s influence [51,54,56,91,111,112]. The importance of the PINK1/PARKIN interactions in mitochondrial quality control in neurodegeneration has been elegantly reviewed by Cummins and Götz (2018) [113]. The PINK1-PARKIN pathway mediates the mitophagy of dysfunctional mitochondria in distal neuronal axons [114]. The detection of phosphorylated ubiquitin in the brains of Mutator mice that accumulates dysfunctional mitochondria indicates the activation of PINK1-Parkin [115]. The loss of Parkin in the Mutator mice cause degeneration of dopaminergic neurons and motor deficit. This work demonstrates a role of endogenous Parkin in the preservation of dopaminergic neurons [115].
3.3. DJ-1 in Mitochondrial Quality Control
The locus of the PARK7 gene, found on the short arm of chromosome 1 (1p36.23); encodes the DJ-1 protein. The latter is a multifunctional protein, initially identified as an oncogene [116] due to its increased presence in carcinomas such as melanoma and breast cancer [117], well preserved in species yet highly standing in most cells and tissues in the body [118]. DJ-1 is participating in cell survival [119], apoptosis [120], transcriptional regulation [121,122], and oxidative stress mechanisms [70,121,123], among others. DJ-1 localizes mainly in the cytoplasm at the basal condition; but is also found in the nucleus and mitochondria [124]. During oxidative stress, DJ-1 is translocated to the mitochondria for its protective role [49,121]. During the S-phase of the cell cycle, with the aid of growth factors and oxidative stress, DJ-1 translocates to the nucleus [124,125].
Early-onset of PD results after homozygous loss-of-function mutations in the PARK7 gene [38]. Several studies have provided strong evidence that DJ-1 safeguards neurons against cell death due to oxidative stresses [72,126]. This protective function is either achieved by gathering antioxidants or acting as a redox sensor [71,72,127,128].
DJ-1 deficiency in DJ-1−/− mice results in elevated ROS levels and a fragmented mitochondrial phenotype in primary cortical neurons; mouse embryonic fibroblasts (MEFs) and brain; striatum [129]. DJ-1-dependent mitochondrial fragmentation was related to a decreased in MFN1 fusion protein levels, and mitochondrial fusion rates [129]. This phenotype can be rescued by PINK1 and PARKIN resulting in increased autophagic activity [66,129]. DJ-1 integrates into the classical PINK1/PARKIN pathway by modulating PARKIN translocation/mitophagy and responding to PINK1/PARKIN by increasing the level of mitochondria activity during oxidative stress [66,109].
4. Relation of PINK-1/PARKIN/DJ-1 Network in Cancer Biology
Cancer is characterized by extensive cellular proliferation, while premature cell death marks neurodegeneration. Both processes share biological pathways differentially regulated [130,131]. Remarkably, patients with PD have presented low cancer incidence [132,133,134,135,136,137,138,139]. The evidence exposes a different pattern of cancer in certain neurological conditions suggesting that neurological diseases may prevent cancer [132]. Unfortunately, comorbidity (presence of new diseases about an index disease) in medical health investigation and practice is comparatively low with that of individual diseases [140,141]. Many factors can explain this duality including biological ones, as opposed to genes and pathways, as well as inorganic ones, such as behaviors, characteristic patterns or effects of medication. Past observational studies have shown the “comorbidity of cancer and disorders of the central nervous system (CNS)” [142], while newer studies suggest a lower incidence of some types of cancer in certain pre-existing CNS disorders [133,143]. The latter has been termed “inverse cancer comorbidity” and has been reported in individuals with Schizophrenia and PD, mainly for colorectal and prostate cancers [144]. Contradictorily, melanoma and breast cancer display high incidence in patients with PD [145]; however, this could be linked to the presence of malfunctioning autophagy in the two conditions [116,146,147].
Defects in autophagy have been linked to neurodegenerative diseases [148,149,150,151]. Some studies have shown that it inhibits tumor initiation, but in the presence of hypoxia and/or metabolic stress this role is reversed and instead supports the survival of existing tumors [152]. Interestingly, in melanoma the autophagy genes Beclin1 and LC3 have been found to be decreased [153], yet melanoma cells show high levels of autophagy [154].
4.1. PARKIN Signaling in Cancer Biology
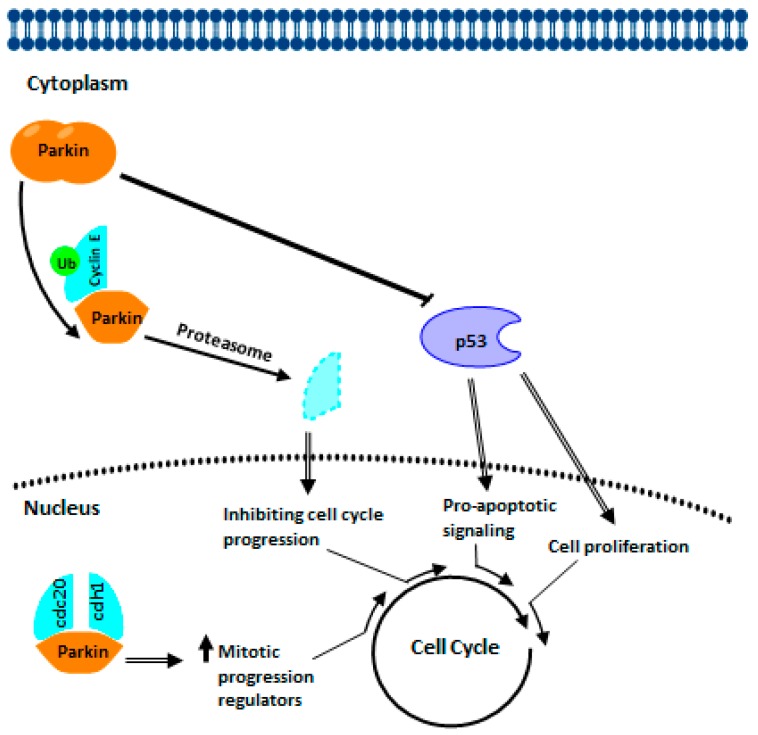
Many types of human cancers downregulate PARKIN expression [155,156]. The PARK2 gene is a tumor suppressor; hence, it is a target of research in a variety of cancers, while mutations in PARK2 in somatic cells contribute to oncogenesis [46,93,157]. Remarkably, PARKIN behaves as an “ubiquitin-protein ligase” having cyclin E (G1 cyclin) as one of their well-recognized substrates (Figure 1). The deficiency of PARKIN is associated with the accumulation of these types of cyclins in breast cancer development [156]. Overexpression of PARKIN represses cell growth by degradation of cyclin E mediated by the ubiquitin [157,158]. In colon and brain cancer cells with dysfunctional PARKIN show alterations in the proteolysis of cyclin E [92,159]. Besides, PARKIN regulates mitosis through the interaction with Cdc20 and Cdh1 (Figure 1); these are coactivators of the anaphase-promoting complex/cyclosome [160]. Studies performed by Tay et al. [156] found that restoring PARKIN’s expression in MCF7 breast cancer cells reduce their growth rate and motility. The former is likely due to the cells’ sudden cessation during the G1 phase of the cell cycle.
Figure 1.
Graphical representation of the interactions of the PARKIN proteins. The directionality and the nature of the interactions (activation or inhibition) are shown as they are believed to exist in a healthy state.
Interestingly, a microarray analysis of MCF7 cells expressing functional PARKIN exposed an important increment in the expression of cyclin-dependent kinase 6 (CDK6) [161]. PARKIN overexpression in cancer cells results in a significant decrement in their growth rate [92]. Clearly, CDK6 are now well known for downregulating the growth of breast cancer cells, suggesting a potential tumor-suppressing mechanism of PARKIN on breast cancer cells [156]. Additionally, in breast cancer, PARKIN stabilizes microtubules and rises cancer vulnerability to oncologic drugs [162]. In HeLa cells, a human cervical cancer line, the expression of PARKIN modulates caspase activity and survivin protein; these promote susceptibility to TNFα-induced cell death [163]. In the presence of functional PARKIN, via cyclin E downregulation and AKT signaling, glioma cells decrease their proliferation [92]. In other words, activation of the PARKIN pathway is an indicator of “survival prognosis” [92]. Notably, PARK2 gene expression is often decreased in human cancers, leading to a dysregulated PI3K (phosphatidylinositol 3-kinase) signaling. These outcomes were contingent on the appearance of PTEN, suggesting that loss of PARKIN, directly or indirectly, impaired the tumor suppressor activity of PTEN [164].
4.2. PINK1 Signaling in Cancer Biology
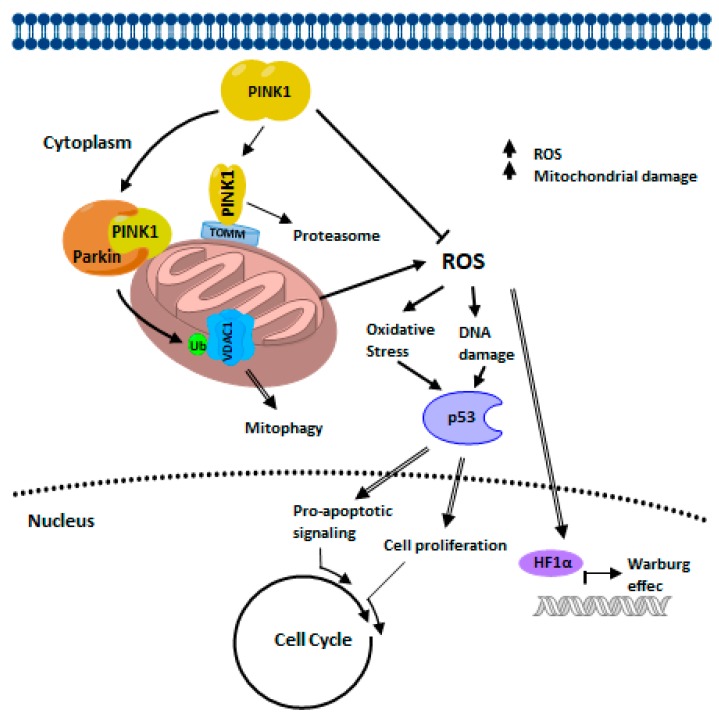
PINK’s role in cancer cell biology is not well understood. The Figure 2 show a graphical representation of the interactions of the PINK1 protein, as they are believed to exist in a healthy state. The majority of the work done so far has been related to PINK’s role in mitochondrial function in PD however, many of the experiments in those studies were carried out in cancer cell lines [54,56,67,91,110,165,166]. Significant findings in this field of research suggest that PINK’s absence in cancer cells promotes fission which in turn results in higher number of fragmented mitochondria [45,62,63]. It was also found that PINK’s expression was higher in mice cancer cell lines that have the higher metastatic potential [167]. Notably, PTEN family may participate in multiple distinct cellular functions, and the isoform PTENα is essential for mitochondrial energy metabolism. Cells lacking PTENα show a reduced level of PINK1 expression [168]. Interestingly, the deletion of PINK1 in immortalized mouse embryonic fibroblasts (MEFs) have been found to reduce cancer-associated phenotypes, and this was seen to be reversed by the overexpression of human PINK1. The knockout mice for PINK1 present significant defects in cell cycle progression, this indicates that PINK1 has tumor-promoting properties [169].
Figure 2.
Graphical representation of the interactions of the PINK proteins. The directionality and the nature of the interactions (activation or inhibition) are shown as they are believed to exist in a healthy state.
High glycolysis and impaired mitochondrial metabolism characterize the metabolic reprogramming of cancer cells. Loss of PINK1 in glioblastoma contributes to the Warburg effect through regulators of aerobic glycolysis [170]. Mainly, in Human brain tumors with poor patient survival deletion of PINK1 in association with regulators of aerobic glycolysis give rise to the Warburg effect [170].
4.3. DJ-1 Signaling in Cancer Biology
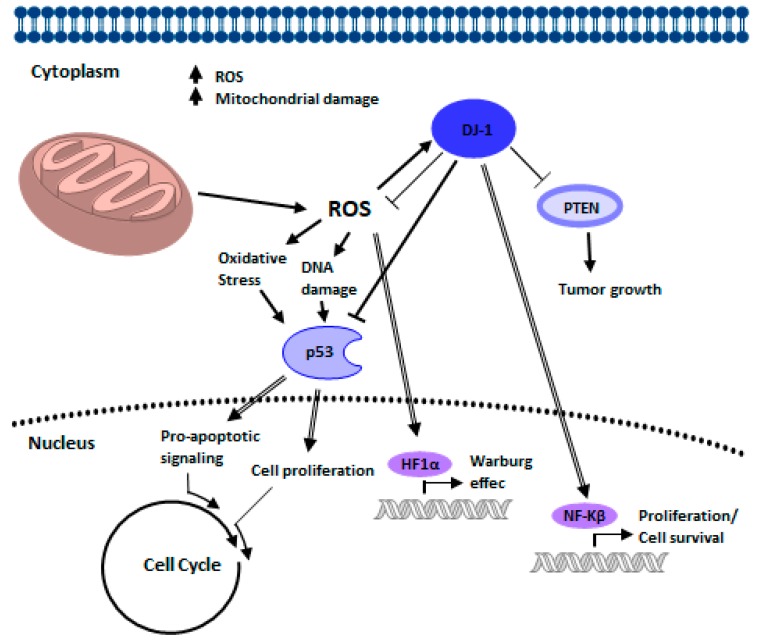
Curiously, DJ-1 was isolated for the first time in 1997 when studying c-Myc-binding proteins (proto-oncogene) [125], although DJ-1 does not interact directly with c-Myc, the PARK7 gene in combination with H-Ras transform mice NIH3T3 cells into cancerogenic ones [125]. Some understanding in the relationship between DJ-1 and cancer has been gathered [43,58], DJ-1 is now known to act as a mitogen-dependent oncogene [125,171,172] (Figure 3). Precise interactions between the biochemical function of DJ-1 and its subcellular localization are yet to be elucidated; however, the DJ-1 malfunction has been linked with cancer onset. Various malignant tumor cells, including prostate cancer, non-small cell lung cancer, primary lung cancer, laryngeal cancer, ovarian carcinoma, cervical cancer, and endometrial cancer, show a high DJ-1 expression [38,173,174,175]. DJ-1 plays a role in increasing cell proliferation and metastasis [176,177]. DJ-1 knockdown in cancer cells significantly reduces in vitro cell proliferation and migration, and in vivo tumor growth [47]. DJ-1 promotes oncogenesis mediating the cell survival supported by upregulation of protein kinase B (PKB)/Akt [125,178]. The studies conducted by Lin et al. [179], in surgical medulloblastoma tissue specimens and paired tumor-adjacent tissue specimens, it was observed that tumor cells had a high DJ-1 expression and this in turn was linked to high growth rate and undifferentiated tumors, while metastasis stage tumors and high-risk tumors showed a high p-Akt expression. Also, DJ-1 antagonize the tumor suppressor PTEN, inhibiting PTEN gene activity and promoting tumor cells proliferation [178]. The loss or decrease of PTEN seems to be a joint event in many types of tumors. Downregulation of PTEN and high expression of DJ-1 correlate with poor prognosis in gastric carcinoma [174]. Moreover, DJ-1 interacts with HER3, this protein plays a crucial role in cell proliferation and survival [180]. Neuregulin (NRG) binding to HER3 induces heterodimerization of HER3 with other EGFR family receptors, resulting in its phosphorylation. Phosphotyrosines of HER3 provide binding sites for PI3K and other HER3 interacting proteins [181,182], which mediate activation of the PI3K/AKT and Ras/Raf/MAPK pathways [183,184,185]. The overexpression of DJ-1 in cancer cells increased the level of HER3 and promoted proliferation and tumor growth [47]. Tumor tissues of breast cancer patients have shown co-expression of HER3 and DJ-1. Thus, high expression of DJ-1 in breast cancer cells predicts elevated HER3 signaling [47]. Furthermore, DJ-1 regulated the transcription factor nuclear factor-κβ (NF-κβ) enhancing its nuclear translocation and cell survival [186]. NF-κβ is critical in cancer development [187,188]. DJ-1 also directly interacts with Cezanne, which networks with NF-κβ reducing its nuclear translocation working as a physiological inhibitor of its transcriptional activity [186].
Figure 3.
Graphical representation of the interactions of the PINK proteins. The directionality and the nature of the interactions (activation or inhibition) are shown as they are believed to exist in a healthy state.
Remarkably, the striatin family SG2NA protein was first characterized as an autoantigen in a cancer patient and described as an oncogene [189,190]. Like DJ-1, SG2NA was also found to be an oncogene that in cooperation with ras transforms NIH3T3 cells [125]. DJ-1 and Akt are recruited to the plasma membrane and mitochondria by SG2NA, to protect cells from oxidative stress [191]. Interestingly, DJ-1 isoform in serum with isoelectric point (pI) of 6.3 is prevalent in breast cancer patients, suggesting that these proteins can act as potential markers for breast cancer [192]. Intriguingly, studies of multidrug resistance (MDR) in humans originating from chemotherapy in cancer treatment have identified that silencing DJ-1 successfully reverses MDR [193].
5. Protein-Protein Interactions Analysis of PINK1/PARKIN/DJ-1 Network
The STRING database, complemented with heuristic methods of association and analysis, was used to consolidate known and predicted protein-protein association for PINK1, PARKIN, and DJ-1. The STRING (search tool for the retrieval of interacting genes) database generates a network of protein interactions from high-throughput experimental data, literature, and predictions based on genomic context analysis [194,195]. Interactions in STRING are derived from five main sources: Genomic Context Predictions, High-throughput Lab Experiments, (Conserved) Co-Expression, Automated Textmining and Previous Knowledge in Databases.
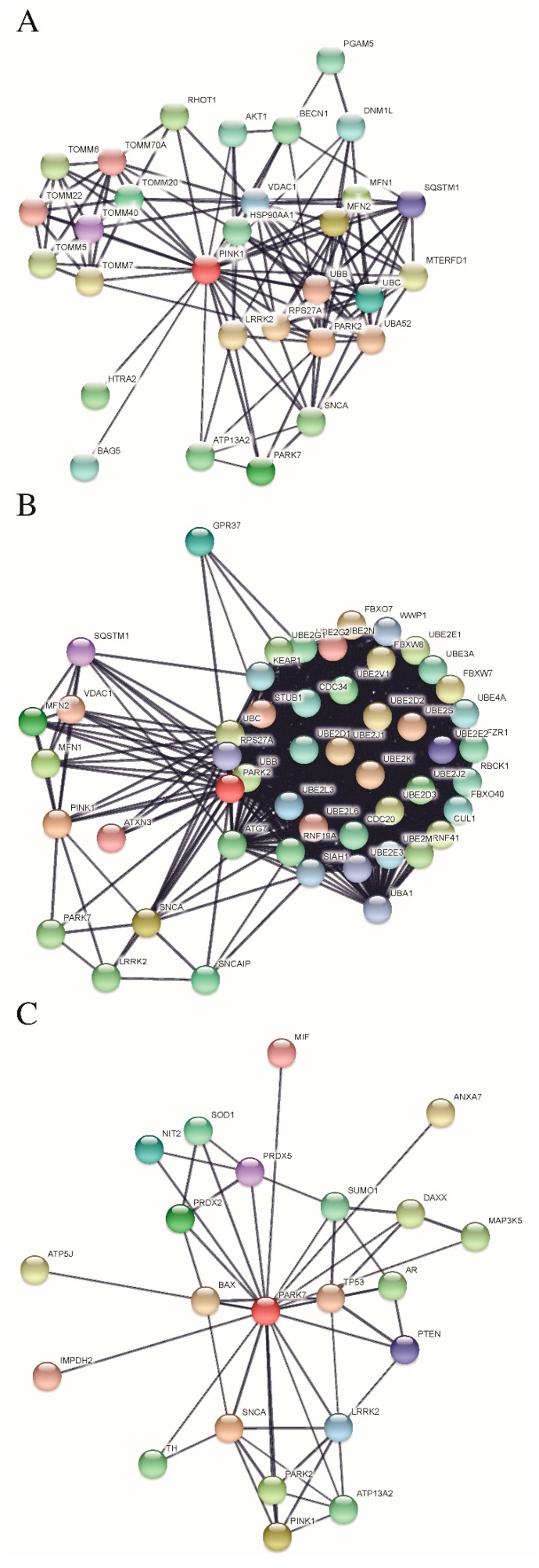
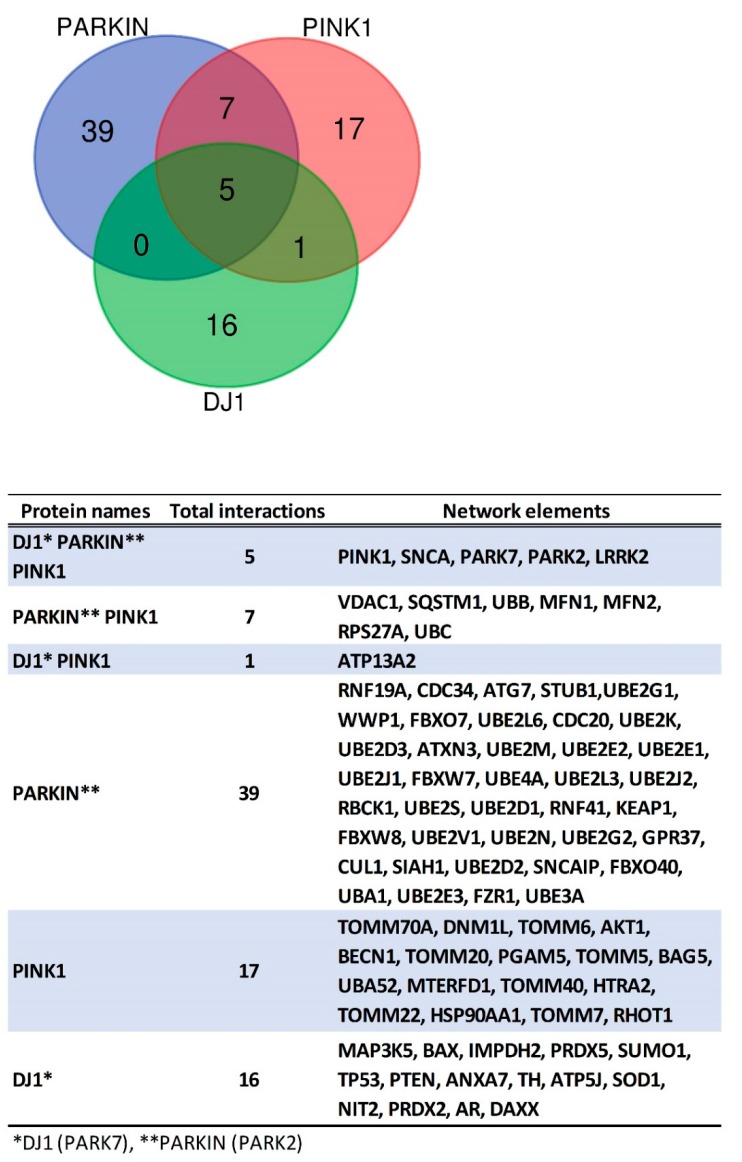
The top predicted functional partners of PINK1, PARKIN, and DJ-1 are shown in Figure 4. A Venn diagram, displaying a three-way interaction network, was assembled by implementing the Bioinformatics and Evolutionary Genomics Tool [196]. The three proteins interacted with each other and with SNCA (alpha Synuclein) and LRRK2 (Leucine-rich repeat kinase 2) (Figure 5). On the one hand, the protein SNCA induces fibrillization of microtubule-associated protein TAU while neuronal responsiveness to various apoptotic stimuli was reduced. On the other hand, the protein LRRK2 positively regulates autophagy through a calcium-dependent activation of CaMKK/AMPK signaling pathway. PARKIN and PINK1 are interacting in the network with VDAC1 (Voltage-dependent anion channel 1), SQSTM1 (Sequestosome 1), UBB (Ubiquitin B), MFN1 (Mitofusin 1), MFN2 (Mitofusin 2), RPS27A (Ribosomal protein S27a; Ubiquitin) and UBC (Ubiquitin C). In the case of DJ-1 and PINK1 interactions were established with ATP13A2 (ATPase type 13A2). Figure 5 shows the predicted functional partners of PINK1, PARKIN, and DJ-1. It is important to acknowledge that in the interaction analysis of protein PARKIN maybe included the interactions with the autoinhibited PARKIN and with the activated PARKIN. We trust that all the possible interactions with the autoinhibited or activated PARKIN might have a biological function.
Figure 4.
STRING analysis reveals protein interaction networks of the proteins DJ-1 (PARK7) (A), PINK1 (B) and PARKIN (PARK2) (C) with other proteins. The interaction network was created with STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) database version 10.5. A high confidence cutoff of 0.7 was implemented in this work. In the resulting protein association network, proteins are presented as nodes which are connected by lines whose thickness represents the confidence level (0.7–0.9).
Figure 5.
Venn diagram of PINK1/PARKIN/DJ-1 network. This was created with the Bioinformatics and Evolutionary Genomics tool.
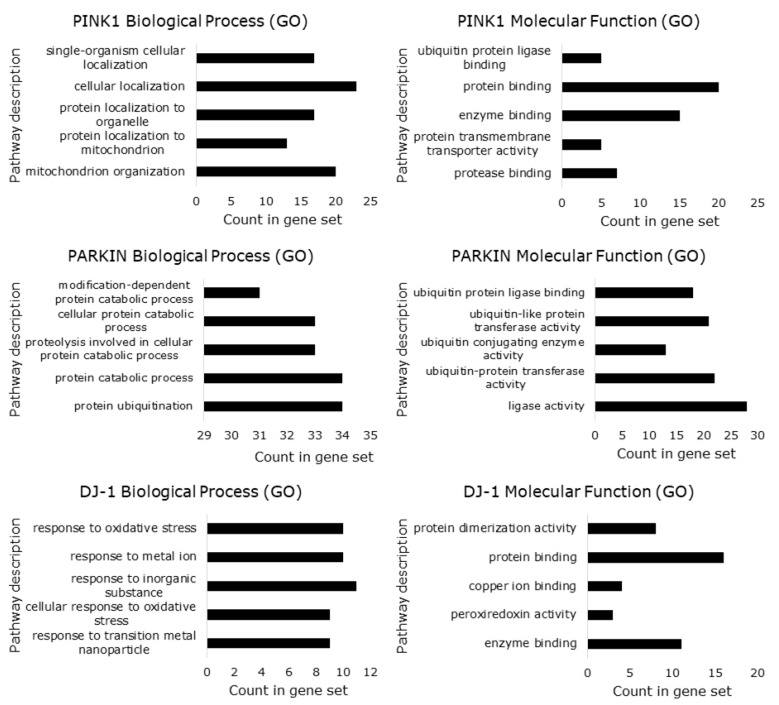
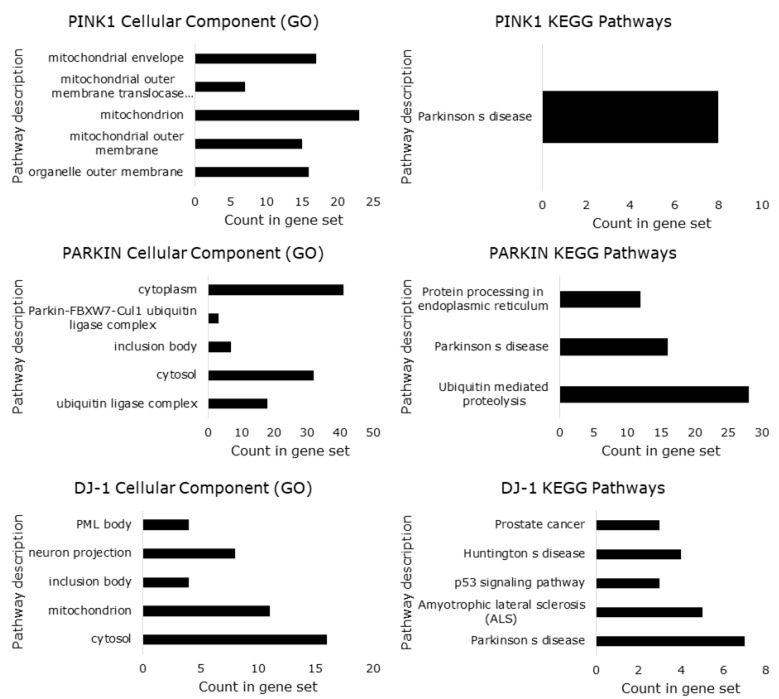
A gene ontology (GO) analysis [195] was performed to establish the role of enrichment in biological processes, molecular function, and cellular components in the interaction networks of PINK1, PARKIN, and DJ-1. The enrichment analysis showed that for biological processes in the PINK1 system most of the genes were enriched at a “cellular localization” and at a “mitochondrion organization” level (Figure 6). For the PARKIN network, most of the genes show enrichment in “protein ubiquitination” and “catabolic process” (Figure 6). “Response to an inorganic substance (metal ion)” and “response to oxidative stress” were higher for DJ-1 network (Figure 6). The typical molecular functions were protein binding for PINK1 and DJ-1 network, and ligase activity for PARKIN network (Figure 6). The cellular component analysis showed that most of the genes were higher in mitochondrion for the PINK1 system, cytoplasm for the PARKIN network, and cytosol and mitochondrion for the DJ-1 network (Figure 7). The enrichment analysis of the KEGG pathways showed that the PINK1/PARKIN/DJ-1 network was Parkinson disease (Figure 7). Only the protein DJ-1 showed enrichment in prostate cancer and p53 signaling pathway (Figure 7). This kind of analysis will be useful in the understanding of the interplay among PINK1, PARKIN, and DJ-1 in cancer biology.
Figure 6.
Gene ontology enrichment analysis for biological processes and molecular function in the interaction networks of PINK1, PARKIN, and DJ-1. The numbers on the horizontal axis represent the enrichment score. A high enrichment score related to the genes were found more frequently in the ontology.
Figure 7.
Gene ontology enrichment analysis for cellular component and KEGG pathways in the interaction networks of PINK1, PARKIN, and DJ-1. The numbers on the horizontal axis represent the enrichment score. A high enrichment score related to the genes were found more frequently in the ontology.
6. Predicted Transcription Factor Regulating PINK1, PARK2 and PARK7 Gene Expression
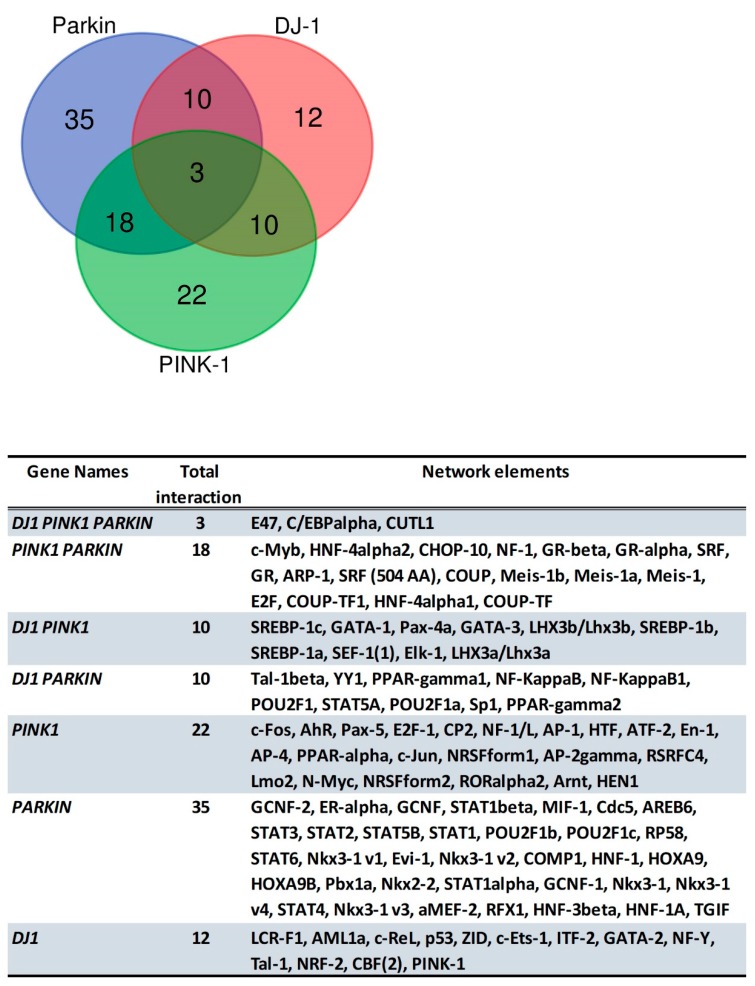
There are many predicted transcription factors regulating PINK1, PARK2 (PARKIN) and PARK7 (DJ-1) gene expression. However, some transcription factors related to cell cycle control are as follows: for PINK1, AP-1, and c-Jun; for PARK7, SP1, p53, NF-Kappaβ, and NF-Kappaβ1; for PARK2, NF-Kappaβ, NF-Kappaβ1, STAT1, STAT1α, STAT1β, STAT14, GR-β, GR-α, GR and E2F (Figure 8). The Venn diagram in Figure 5 presents all possible logical relations between the predicted transcription factors for PINK1, PARK2 (PARKIN) and PARK7 (DJ-1). The Venn diagram of predicted transcription factor allowed us to recognize three common transcription factors for PINK1, PARK2, and PARK7. These transcription factors are E47, C/EBPα and CUTL1, and may play a role in the interplay between PINK1, PARKIN, and DJ-1 (Figure 8). These transcription factors related to the cell cycle suggests that the interaction among PINK1/PARKIN/DJ-1 network during mitochondrial quality control in cancer biology may be regulated at the transcriptional level. Further analysis, like a systems biology approach, will be helpful in the understanding of the PINK1/PARKIN/DJ-1 network.
Figure 8.
Venn diagram that presents all possible logical relations between the predicted transcription factors for the PINK, PARKIN and PARK7. The diagram was created with the Bioinformatics and Evolutionary Genomics tool.
7. Conclusions
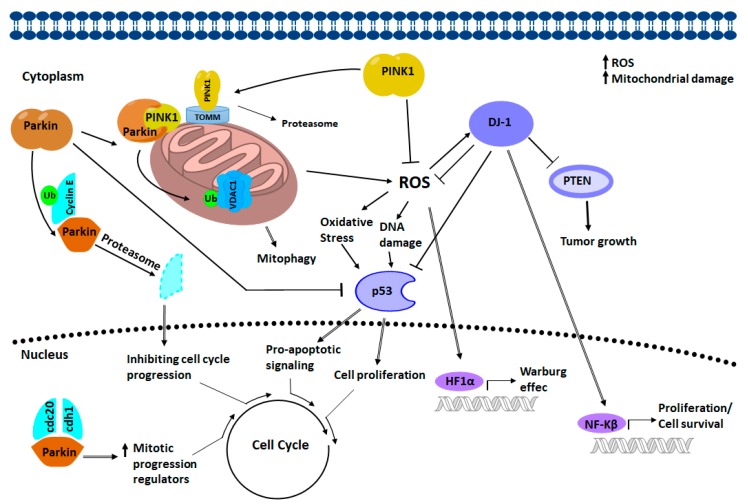
There is an interplay between DJ-1, PINK1, and PARKIN in cancer biology (Figure 9). During late anaphase to G1 phase, PARKIN interacts with the co-activators of APC/C, Cdc20 and Cdh1 thus, forming a complex that increases the expression of regulators of mitotic progression which in turn suppresses the progression of the cell cycle. Also, PARKIN represses cyclin E levels, also through ubiquitination (Ub), by inhibiting the progression of the cell cycle, stopping in G1 phase. PARKIN repressed p53 levels through its interaction with the TP53 promoter region, which could help maintain controlled pro-apoptotic signaling. DJ-1 also represses the expression of TP53, as well as p53 protein levels. The reduction in the expression of PINK1 causes the stabilization of HIF1A through the elevation of ROS. Cancer cells increase aerobic glycolysis through the Warburg effect and produce higher levels of oxidative stress through ROS. Through multiple mechanisms, DJ-1 helps minimize the damage caused by oxidative stress. During cancer development, DJ-1 delivers cytoprotection by independent mechanisms such as NF-κβ and PTEN. DJ-1 activates NF-κβ and inhibits tumor suppressor activity activated by PTEN, leading to cell survival and tumor growth. The mutation or altered expression of these associated genes in cancer interrupts the checkpoints and balances that maintain cellular integrity and that protect against inappropriate cell proliferation. Many of these interruptions converge and drive the cell cycle. The result is determined by whether the cell is capable of mitosis, in which case an over proliferation occurs, or whether the cell is postmitotic, in which case cell death occurs.
Figure 9.
Representation of PARKIN/PINK1/DJ-1 network. The directionality and the nature of the interactions (activation or inhibition) are shown as they are believed to exist in a healthy state. PARKIN interacts with PINK1 to promote the removal of defective mitochondria through the ubiquitination of the voltage-dependent anionic channel 1 (VDAC1), a component of the mitochondrial permeability transition pore that is involved in apoptosis, contributing to limit the damage produced by ROS.
The STRING database and Gene Ontology (GO) enrichment analysis were performed to consolidate known and predicted protein-protein association with PINK1, PARKIN, and DJ-1. The enrichment analysis of KEGG pathways showed that the PINK1/PARKIN/DJ-1 network show Parkinson disease as the main feature. Only the protein DJ-1 showed enrichment in prostate cancer and p53 signaling pathway. Some predicted transcription factors regulating PINK1, PARK2 (PARKIN) and PARK7 (DJ-1) gene expression are related to cell cycle control. We suggest that the interplay among PINK1/PARKIN/DJ-1 network during mitochondrial quality control in cancer biology may occur at the transcriptional level. Further analysis, like a systems biology approach, will be helpful in the understanding of PINK1/PARKIN/DJ-1 network.
Acknowledgments
We acknowledge the support given by “Vicerrectoría de Investigación y Postgrado” from “Universidad Autónoma de Chile”.
Author Contributions
The following statements are specifying the author contributions “Conceptualization, C.S. and L.M.R.; Methodology, C.S. and L.M.R.; Software, C.S. and L.M.R.; Formal Analysis, C.S., P.R.-H. and L.M.R.; Investigation, C.S., P.R.-H. and L.M.R.; Resources, C.S. and L.M.R.; Data Curation, C.S. and L.M.R.; Writing-Original Draft Preparation, C.S., P.R.-H. and L.M.R.; Writing-Review & Editing, C.S., P.R.-H. and L.M.R.; Visualization, L.M.R.; Supervision, L.M.R.; Project Administration, L.M.R.; Funding Acquisition, L.M.R.”.
Funding
This research was funded by FONDECYT grant number 11130192.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Poyton R.O., Ball K.A., Castello P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Fontmorin J.M., Burgos Castillo R.C., Tang W.Z., Sillanpää M. Stability of 5,5-dimethyl-1-pyrroline-n-oxide as a spin-trap for quantification of hydroxyl radicals in processes based on fenton reaction. Water Res. 2016;99:24–32. doi: 10.1016/j.watres.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 5.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plecitá-Hlavatá L., Ježek P. Integration of superoxide formation and cristae morphology for mitochondrial redox signaling. Int. J. Biochem. Cell Biol. 2016;80:31–50. doi: 10.1016/j.biocel.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Ježek P., Hlavatá L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Kühlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015;13:89. doi: 10.1186/s12915-015-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlattner U., Tokarska-Schlattner M., Rousseau D., Boissan M., Mannella C., Epand R., Lacombe M.-L. Mitochondrial cardiolipin/phospholipid trafficking: The role of membrane contact site complexes and lipid transfer proteins. Chem. Phys. Lipids. 2014;179:32–41. doi: 10.1016/j.chemphyslip.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Huttemann M., Lee I., Pecinova A., Pecina P., Przyklenk K., Doan J.W. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J. Bioenerg. Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- 11.Wittig I., Carrozzo R., Santorelli F.M., Schägger H. Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. BBA Bioenerg. 2006;1757:1066–1072. doi: 10.1016/j.bbabio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Schägger H., Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornhövd C., Vogel F., Neupert W., Reichert A.S. Mitochondrial membrane potential is dependent on the oligomeric state of f1f0-atp synthase supracomplexes. J. Biol. Chem. 2006;281:13990–13998. doi: 10.1074/jbc.M512334200. [DOI] [PubMed] [Google Scholar]
- 14.Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Orr A.L., Brand M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenaz G. The mitochondrial production of reactive oxygen species: Mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–164. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- 16.Andreyev A.I., Kushnareva Y.E., Starkov A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 17.Cadenas E., Davies K.J.A. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 18.Hirst J., Carroll J., Fearnley I.M., Shannon R.J., Walker J.E. The nuclear encoded subunits of complex I from bovine heart mitochondria. BBA Bioenerg. 2003;1604:135–150. doi: 10.1016/S0005-2728(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 19.Sazanov L.A. Respiratory complex I: Mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry. 2007;46:2275–2288. doi: 10.1021/bi602508x. [DOI] [PubMed] [Google Scholar]
- 20.Lambert A.J., Brand M.D. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH: Ubiquinone oxidoreductase (complex I) J. Biol. Chem. 2004;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- 21.Santiago A.P.S.A., Chaves E.A., Oliveira M.F., Galina A. Reactive oxygen species generation is modulated by mitochondrial kinases: Correlation with mitochondrial antioxidant peroxidases in rat tissues. Biochimie. 2008;90:1566–1577. doi: 10.1016/j.biochi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Castello P.R., David P.S., McClure T., Crook Z., Poyton R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Rhee H.-W., Zou P., Udeshi N.D., Martell J.D., Mootha V.K., Carr S.A., Ting A.Y. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science. 2013;339:1328–1331. doi: 10.1126/science.1230593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goard C.A., Schimmer A.D. Mitochondrial matrix proteases as novel therapeutic targets in malignancy. Oncogene. 2014;33:2690–2699. doi: 10.1038/onc.2013.228. [DOI] [PubMed] [Google Scholar]
- 25.Parra V., Verdejo H., del Campo A., Pennanen C., Kuzmicic J., Iglewski M., Hill J.A., Rothermel B.A., Lavandero S. The complex interplay between mitochondrial dynamics and cardiac metabolism. J. Bioenerg. Biomembr. 2011;43:47–51. doi: 10.1007/s10863-011-9332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westermann B. Merging mitochondria matters: Cellular role and molecular machinery of mitochondrial fusion. EMBO Rep. 2002;3:527–531. doi: 10.1093/embo-reports/kvf113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra P., Chan D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:634. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 29.Delettre C., Lenaers G., Griffoin J.-M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E., et al. Nuclear gene opa1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 2000;26:207. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 30.Santel A., Fuller M.T. Control of mitochondrial morphology by a human mitofusin. J. Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 31.Richter V., Singh A.P., Kvansakul M., Ryan M.T., Osellame L.D. Splitting up the powerhouse: Structural insights into the mechanism of mitochondrial fission. Cell. Mol. Life Sci. 2015;72:3695–3707. doi: 10.1007/s00018-015-1950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y., Jeong S.-Y., Karbowski M., Smith C.L., Youle R.J. Roles of the mammalian mitochondrial fission and fusion mediators fis1, drp1, and opa1 in apoptosis. Mol. Biol. Cell. 2004;15:5001–5011. doi: 10.1091/mbc.e04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labrousse A.M., Zappaterra M.D., Rube D.A., van der Bliek A.M. C. elegans dynamin-related protein drp-1 controls severing of the mitochondrial outer membrane. Mol. Cell. 1999;4:815–826. doi: 10.1016/S1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 34.Lemasters J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuv. Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 35.Pickles S., Vigié P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liesa M., Shirihai O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youle R.J., van der Bliek A.M. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonifati V., Rizzu P., Squitieri F., Krieger E., Vanacore N., van Swieten J.C., Brice A., van Duijn C.M., Oostra B., Meco G., et al. Dj-1 (park7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol. Sci. 2003;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- 39.Kitada T., Asakawa S., Matsumine H., Hattori N., Minoshima S., Shimizu N., Mizuno Y. Positional cloning of the autosomal recessive juvenile parkinsonism (ar-jp) gene and its diversity in deletion mutations. Parkinsonism Relat. Disord. 1999;5:163–168. doi: 10.1016/S1353-8020(99)00032-2. [DOI] [PubMed] [Google Scholar]
- 40.Valente E.M., Abou-Sleiman P.M., Caputo V., Muqit M.M.K., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A.R., Healy D.G., et al. Hereditary early-onset parkinson’s disease caused by mutations in pink1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 41.Valente E.M., Salvi S., Ialongo T., Marongiu R., Elia A.E., Caputo V., Romito L., Albanese A., Dallapiccola B., Bentivoglio A.R. Pink1 mutations are associated with sporadic early-onset parkinsonism. Ann. Neurol. 2004;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- 42.Bernardini J.P., Lazarou M., Dewson G. Parkin and mitophagy in cancer. Oncogene. 2016;36:1315. doi: 10.1038/onc.2016.302. [DOI] [PubMed] [Google Scholar]
- 43.Cao J., Lou S., Ying M., Yang B. Dj-1 as a human oncogene and potential therapeutic target. Biochem. Pharmacol. 2015;93:241–250. doi: 10.1016/j.bcp.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Gong Y., Schumacher S.E., Wu W.H., Tang F., Beroukhim R., Chan T.A. Pan-cancer analysis links park2 to bcl-xl-dependent control of apoptosis. Neoplasia. 2017;19:75–83. doi: 10.1016/j.neo.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Flanagan C.H., O’Neill C. Pink1 signalling in cancer biology. BBA Rev. Cancer. 2014;1846:590–598. doi: 10.1016/j.bbcan.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Xu L., Lin D., Yin D., Koeffler H.P. An emerging role of park2 in cancer. J. Mol. Med. 2014;92:31–42. doi: 10.1007/s00109-013-1107-0. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S., Mukherjee S., Fan X., Salameh A., Mujoo K., Huang Z., Li L., Salazar G.T., Zhang N., An Z. Novel association of dj-1 with her3 potentiates her3 activation and signaling in cancer. Oncotarget. 2016;7:65758–65769. doi: 10.18632/oncotarget.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherra S.J., III, Dagda R.K., Tandon A., Chu C.T. Mitochondrial autophagy as a compensatory response to pink1 deficiency. Autophagy. 2009;5:1213–1214. doi: 10.4161/auto.5.8.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Junn E., Jang W.H., Zhao X., Jeong B.S., Mouradian M.M. Mitochondrial localization of dj-1 leads to enhanced neuroprotection. J. Neurosci. Res. 2009;87:123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lev N., Ickowicz D., Melamed E., Offen D. Oxidative insults induce dj-1 upregulation and redistribution: Implications for neuroprotection. Neurotoxicology. 2008;29:397–405. doi: 10.1016/j.neuro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Narendra D., Tanaka A., Suen D.-F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narendra D., Tanaka A., Suen D.-F., Youle R.J. Parkin-induced mitophagy in the pathogenesis of parkinson disease. Autophagy. 2009;5:706–708. doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- 53.Silvestri L., Caputo V., Bellacchio E., Atorino L., Dallapiccola B., Valente E.M., Casari G. Mitochondrial import and enzymatic activity of pink1 mutants associated to recessive parkinsonism. Hum. Mol. Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- 54.Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R.L.A., Kim J., May J., Tocilescu M.A., Liu W., Ko H.S., et al. Pink1-dependent recruitment of parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou C., Huang Y., Shao Y., May J., Prou D., Perier C., Dauer W., Schon E.A., Przedborski S. The kinase domain of mitochondrial pink1 faces the cytoplasm. Proc. Natl. Acad. Sci. USA. 2008;105:12022–12027. doi: 10.1073/pnas.0802814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narendra D.P. Parkin/pink1 pathway for the selective isolation and degradation of impaired mitochondria. In: Buhlman L.M., editor. Mitochondrial Mechanisms of Degeneration and Repair in Parkinson’s Disease. Springer International Publishing; Cham, Switzerland: 2016. pp. 159–182. [Google Scholar]
- 57.Gonzalez-Polo R.A., Niso-Santano M., Moran J.M., Ortiz-Ortiz M.A., Bravo-San Pedro J.M., Soler G., Fuentes J.M. Silencing dj-1 reveals its contribution in paraquat-induced autophagy. J. Neurochem. 2009;109:889–898. doi: 10.1111/j.1471-4159.2009.06020.x. [DOI] [PubMed] [Google Scholar]
- 58.Vasseur S., Afzal S., Tardivel-Lacombe J., Park D.S., Iovanna J.L., Mak T.W. Dj-1/park7 is an important mediator of hypoxia-induced cellular responses. Proc. Natl. Acad. Sci. USA. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark I.E., Dodson M.W., Jiang C., Cao J.H., Huh J.R., Seol J.H., Yoo S.J., Hay B.A., Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 60.Deng H., Dodson M.W., Huang H., Guo M. The parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poole A.C., Thomas R.E., Andrews L.A., McBride H.M., Whitworth A.J., Pallanck L.J. The pink1/parkin pathway regulates mitochondrial morphology. Proc. Natl. Acad. Sci. USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dagda R.K., Cherra S.J., III, Kulich S.M., Tandon A., Park D., Chu C.T. Loss of pink1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dagda R.K., Zhu J., Chu C.T. Mitochondrial kinases in parkinson’s disease: Converging insights from neurotoxin and genetic models. Mitochondrion. 2009;9:289–298. doi: 10.1016/j.mito.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Exner N., Treske B., Paquet D., Holmstrom K., Schiesling C., Gispert S., Carballo-Carbajal I., Berg D., Hoepken H.-H., Gasser T., et al. Loss-of-function of human pink1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y., Ouyang Y., Yang L., Beal M.F., McQuibban A., Vogel H., Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc. Natl. Acad. Sci. USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamp F., Exner N., Lutz A.K., Wender N., Hegermann J., Brunner B., Nuscher B., Bartels T., Giese A., Beyer K., et al. Inhibition of mitochondrial fusion by α-synuclein is rescued by pink1, parkin and dj-1. EMBO J. 2010;29:3571–3589. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lutz A.K., Exner N., Fett M.E., Schlehe J.S., Kloos K., Lämmermann K., Brunner B., Kurz-Drexler A., Vogel F., Reichert A.S., et al. Loss of parkin or pink1 function increases drp1-dependent mitochondrial fragmentation. J. Biol. Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y., Dorn G.W. Pink1-phosphorylated mitofusin 2 is a parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong G., Song M., Csordas G., Kelly D.P., Matkovich S.J., Dorn G.W. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science. 2015;350 doi: 10.1126/science.aad2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCoy M.K., Cookson M.R. Dj-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy. 2011;7:531–532. doi: 10.4161/auto.7.5.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim R.H., Smith P.D., Aleyasin H., Hayley S., Mount M.P., Pownall S., Wakeham A., You-Ten A.J., Kalia S.K., Horne P., et al. Hypersensitivity of dj-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park J., Kim S.Y., Cha G.H., Lee S.B., Kim S., Chung J. Drosophila dj-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 73.Unoki M., Nakamura Y. Growth-suppressive effects of bpoz and egr2, two genes involved in the pten signaling pathway. Oncogene. 2001;20:4457. doi: 10.1038/sj.onc.1204608. [DOI] [PubMed] [Google Scholar]
- 74.Gandhi S., Muqit M.M.K., Stanyer L., Healy D.G., Abou-Sleiman P.M., Hargreaves I., Heales S., Ganguly M., Parsons L., Lees A.J., et al. Pink1 protein in normal human brain and parkinson’s disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- 75.Petit A., Kawarai T., Paitel E., Sanjo N., Maj M., Scheid M., Chen F., Gu Y., Hasegawa H., Salehi-Rad S., et al. Wild-type pink1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by parkinson disease-related mutations. J. Biol. Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- 76.Wang H.-L., Chou A.-H., Yeh T.-H., Li A.H., Chen Y.-L., Kuo Y.-L., Tsai S.-R., Yu S.-T. Pink1 mutants associated with recessive parkinson’s disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol. Dis. 2007;28:216–226. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Wood-Kaczmar A., Gandhi S., Yao Z., Abramov A.S.Y., Miljan E.A., Keen G., Stanyer L., Hargreaves I., Klupsch K., Deas E., et al. Pink1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/annotation/17d5aaa1-c6d8-4aad-a9a4-56b2c1220c83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gautier C.A., Kitada T., Shen J. Loss of pink1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc. Natl. Acad. Sci. USA. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park J., Lee S.B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M.H., Kim J.M., et al. Mitochondrial dysfunction in drosophila pink1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 80.Wang D., Qian L., Xiong H., Liu J., Neckameyer W.S., Oldham S., Xia K., Wang J., Bodmer R., Zhang Z. Antioxidants protect pink1-dependent dopaminergic neurons in drosophila. Proc. Natl. Acad. Sci. USA. 2006;103:13520–13525. doi: 10.1073/pnas.0604661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y., Gehrke S., Imai Y., Huang Z., Ouyang Y., Wang J.-W., Yang L., Beal M.F., Vogel H., Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused inactivation of drosophila pink1 is rescued by by parkin. Proc. Natl. Acad. Sci. USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amo T., Sato S., Saiki S., Wolf A.M., Toyomizu M., Gautier C.A., Shen J., Ohta S., Hattori N. Mitochondrial membrane potential decrease caused by loss of pink1 is not due to proton leak, but to respiratory chain defects. Neurobiol. Dis. 2011;41:111–118. doi: 10.1016/j.nbd.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 83.Gautier C.A., Giaime E., Caballero E., Núñez L., Song Z., Chan D., Villalobos C., Shen J. Regulation of mitochondrial permeability transition pore by pink1. Mol. Neurodegener. 2012;7:22. doi: 10.1186/1750-1326-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morais V.A., Verstreken P., Roethig A., Smet J., Snellinx A., Vanbrabant M., Haddad D., Frezza C., Mandemakers W., Vogt-Weisenhorn D., et al. Parkinson’s disease mutations in pink1 result in decreased complex i activity and deficient synaptic function. EMBO Mol. Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heeman B., Van den Haute C., Aelvoet S.-A., Valsecchi F., Rodenburg R.J., Reumers V., Debyser Z., Callewaert G., Koopman W.J.H., Willems P.H.G.M., et al. Depletion of pink1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J. Cell Sci. 2011;124:1115–1125. doi: 10.1242/jcs.078303. [DOI] [PubMed] [Google Scholar]
- 86.Pridgeon J.W., Olzmann J.A., Chin L.-S., Li L. Pink1 protects against oxidative stress by phosphorylating mitochondrial chaperone trap1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deas E., Wood N.W., Plun-Favreau H. Mitophagy and parkinson’s disease: The pink1–parkin link. BBA Mol. Cell Res. 2011;1813:623–633. doi: 10.1016/j.bbamcr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shiba-Fukushima K., Imai Y., Yoshida S., Ishihama Y., Kanao T., Sato S., Hattori N. Pink1-mediated phosphorylation of the parkin ubiquitin-like domain primes mitochondrial translocation of parkin and regulates mitophagy. Sci. Rep. 2012;2:1002. doi: 10.1038/srep01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nadtochiy S.M., Tompkins A.J., Brookes P.S. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: Implications for pathology and cardioprotection. Biochem. J. 2006;395:611–618. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jin S.M., Youle R.J. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by pink1 to induce park2/parkin-mediated mitophagy of polarized mitochondria. Autophagy. 2013;9:1750–1757. doi: 10.4161/auto.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narendra D.P., Jin S.M., Tanaka A., Suen D.-F., Gautier C.A., Shen J., Cookson M.R., Youle R.J. PINK1 is selectively stabilized on impaired mitochondria to activate parkin. PLoS Biol. 2010;8:e100298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Veeriah S., Taylor B.S., Meng S., Fang F., Yilmaz E., Vivanco I., Janakiraman M., Schultz N., Hanrahan A.J., Pao W., et al. Somatic mutations of the parkinson’s disease-associated gene park2 in glioblastoma and other human malignancies. Nat. Genet. 2010;42:77–82. doi: 10.1038/ng.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rankin C.A., Joazeiro C.A.P., Floor E., Hunter T. E3 ubiquitin-protein ligase activity of parkin is dependent on cooperative interaction of ring finger (triad) elements. J. Biomed. Sci. 2001;8:421–429. doi: 10.1007/BF02255952. [DOI] [PubMed] [Google Scholar]
- 94.Kazlauskaite A., Kondapalli C., Gourlay R., Campbell D.G., Ritorto M.S., Hofmann K., Alessi D.R., Knebel A., Trost M., Muqit M.M.K. Parkin is activated by pink1-dependent phosphorylation of ubiquitin at ser65. Biochem. J. 2014;460:127–141. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bouman L., Schlierf A., Lutz A.K., Shan J., Deinlein A., Kast J., Galehdar Z., Palmisano V., Patenge N., Berg D., et al. Parkin is transcriptionally regulated by ATF4: Evidence for an interconnection between mitochondrial stress and er stress. Cell Death Differ. 2011;18:769–782. doi: 10.1038/cdd.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Millikin D., Meese E., Vogelstein B., Witkowski C., Trent J. Loss of heterozygosity for loci on the long arm of chromosome-6 in human-malignant melanoma. Cancer Res. 1991;51:5449–5453. [PubMed] [Google Scholar]
- 97.Cesari R., Martin E.S., Calin G.A., Pentimalli F., Bichi R., McAdams H., Trapasso F., Drusco A., Shimizu M., Mascillo V., et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc. Natl. Acad. Sci. USA. 2003;100:5956–5961. doi: 10.1073/pnas.0931262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kondapalli C., Kazlauskaite A., Zhang N., Woodroof H.I., Campbell D.G., Gourlay R., Burchell L., Walden H., Macartney T.J., Deak M., et al. Pink1 is activated by mitochondrial membrane potential depolarization and stimulates parkin e3 ligase activity by phosphorylating serine 65. Open Biol. 2012;2:120080. doi: 10.1098/rsob.120080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koyano F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M., Kimura Y., Tsuchiya H., Yoshihara H., Hirokawa T., et al. Ubiquitin is phosphorylated by pink1 to activate parkin. Nature. 2014;510:162. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 100.Kane L.A., Lazarou M., Fogel A.I., Li Y., Yamano K., Sarraf S.A., Banerjee S., Youle R.J. Pink1 phosphorylates ubiquitin to activate parkin e3 ubiquitin ligase activity. J. Cell Biol. 2014;205:143. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wauer T., Komander D. Structure of the human parkin ligase domain in an autoinhibited state. EMBO J. 2013;32:2099–2112. doi: 10.1038/emboj.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trempe J.-F., Sauvé V., Grenier K., Seirafi M., Tang M.Y., Ménade M., Al-Abdul-Wahid S., Krett J., Wong K., Kozlov G., et al. Structure of parkin reveals mechanisms for ubiquitin ligase activation. Science. 2013;340:1451. doi: 10.1126/science.1237908. [DOI] [PubMed] [Google Scholar]
- 103.Gladkova C., Maslen S.L., Skehel J.M., Komander D. Mechanism of parkin activation by pink1. Nature. 2018;559:410–414. doi: 10.1038/s41586-018-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ordureau A., Sarraf S.A., Duda D.M., Heo J.-M., Jedrykowski M.P., Sviderskiy V., Olszewski J.L., Koerber J.T., Xie T., Beausoleil S.A., et al. Quantitative proteomics reveal a feed-forward model for mitochondrial parkin translocation and ub chain synthesis. Mol. Cell. 2014;56:360–375. doi: 10.1016/j.molcel.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang C.-W., Hang L., Yao T.-P., Lim K.-L. Parkin regulation and neurodegenerative disorders. Front. Aging Neurosci. 2015;7:248. doi: 10.3389/fnagi.2015.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vandiver M.S., Paul B.D., Xu R., Karuppagounder S., Rao F., Snowman A.M., Ko H.S., Lee Y.I., Dawson V.L., Dawson T.M., et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakamura T., Lipton S.A. Emerging roles of s-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid. Redox Signal. 2007;10:87–102. doi: 10.1089/ars.2007.1858. [DOI] [PubMed] [Google Scholar]
- 108.Sunico C., Nakamura T., Rockenstein E., Mante M., Adame A., Chan S., Newmeyer T., Masliah E., Nakanishi N., Lipton S. S-nitrosylation of parkin as a novel regulator of p53-mediated neuronal cell death in sporadic parkinson’s disease. Mol. Neurodegener. 2013;8:29. doi: 10.1186/1750-1326-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joselin A.P., Hewitt S.J., Callaghan S.M., Kim R.H., Chung Y.-H., Mak T.W., Shen J., Slack R.S., Park D.S. Ros-dependent regulation of parkin and dj-1 localization during oxidative stress in neurons. Hum. Mol. Genet. 2012;21:4888–4903. doi: 10.1093/hmg/dds325. [DOI] [PubMed] [Google Scholar]
- 110.Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C.A., Sou Y., Saiki S., Kawajiri S., Sato F., et al. Pink1 stabilized by mitochondrial depolarization recruits parkin to damaged mitochondria and activates latent parkin for mitophagy. J. Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim Y., Park J., Kim S., Song S., Won S.-K., Lee S.-H., Kitada T., Kim J.-M., Chung J. Pink1 controls mitochondrial localization of parkin through direct phosphorylation. Biochem. Bioph. Res. Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 112.Geisler S., Holmstroem K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. Pink1/parkin-mediated mitophagy is dependent on vdac1 and p62/sqstm1. Nat. Cell Biol. 2010;12:119–U170. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 113.Cummins N., Götz J. Shedding light on mitophagy in neurons: What is the evidence for pink1/parkin mitophagy in vivo? Cell. Mol. Life Sci. 2018;75:1151–1162. doi: 10.1007/s00018-017-2692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ashrafi G., Schlehe J.S., LaVoie M.J., Schwarz T.L. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires pink1 and parkin. J. Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pickrell A.M., Huang C.-H., Kennedy S.R., Ordureau A., Sideris D.P., Hoekstra J.G., Harper J.W., Youle R.J. Endogenous parkin preserves dopaminergic substantia nigral neurons following mitochondrial DNA mutagenic stress. Neuron. 2015;87:371–381. doi: 10.1016/j.neuron.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan T., Li X., Jankovic J. The association between parkinson’s disease and melanoma. Inter. J. Cancer. 2011;128:2251–2260. doi: 10.1002/ijc.25912. [DOI] [PubMed] [Google Scholar]
- 117.Maita C., Tsuji S., Yabe I., Hamada S., Ogata A., Maita H., Iguchi-Ariga S.M.M., Sasaki H., Ariga H. Secretion of dj-1 into the serum of patients with parkinson’s disease. Neurosci. Lett. 2008;431:86–89. doi: 10.1016/j.neulet.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 118.Pei X., Wu T., Li B., Tian X., Li Z., Yang Q. Increased expression of macrophage migration inhibitory factor and dj-1 contribute to cell invasion and metastasis of nasopharyngeal carcinoma. Int. J. Med. Sci. 2014;11:106–115. doi: 10.7150/ijms.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aron L., Klein P., Pham T.-T., Kramer E.R., Wurst W., Klein R. Pro-survival role for parkinson’s associated gene dj-1 revealed in trophically impaired dopaminergic neurons. PLoS Biol. 2010;8:e1000349. doi: 10.1371/journal.pbio.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cookson M.R. Pathways to parkinsonism. Neuron. 2003;37:7–10. doi: 10.1016/S0896-6273(02)01166-2. [DOI] [PubMed] [Google Scholar]
- 121.Usami Y., Hatano T., Imai S., Kubo S., Sato S., Saiki S., Fujioka Y., Ohba Y., Sato F., Funayama M., et al. Dj-1 associates with synaptic membranes. Neurobiol. Dis. 2011;43:651–662. doi: 10.1016/j.nbd.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 122.Fan J., Ren H., Jia N., Fei E., Zhou T., Jiang P., Wu M., Wang G. Dj-1 decreases bax expression through repressing p53 transcriptional activity. J. Biol. Chem. 2008;283:4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- 123.Clements C.M., McNally R.S., Conti B.J., Mak T.W., Ting J.P.-Y. Dj-1, a cancer- and parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator nrf2. Proc. Natl. Acad. Sci. USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim S.-J., Park Y.-J., Hwang I.-Y., Youdim M.B.H., Park K.-S., Oh Y.J. Nuclear translocation of dj-1 during oxidative stress-induced neuronal cell death. Free Radic. Biol. Med. 2012;53:936–950. doi: 10.1016/j.freeradbiomed.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 125.Nagakubo D., Taira T., Kitaura H., Ikeda M., Tamai K., Iguchi-Ariga S.M.M., Ariga H. Dj-1, a novel oncogene which transforms mouse nih3t3 cells in cooperation withras. Biochem. Biophys. Res. Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 126.Aleyasin H., Rousseaux M.W.C., Phillips M., Kim R.H., Bland R.J., Callaghan S., Slack R.S., During M.J., Mak T.W., Park D.S. The parkinson’s disease gene dj-1 is also a key regulator of stroke-induced damage. Proc. Natl. Acad. Sci. USA. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andres-Mateos E., Perier C., Zhang L., Blanchard-Fillion B., Greco T.M., Thomas B., Ko H.S., Sasaki M., Ischiropoulos H., Przedborski S., et al. Dj-1 gene deletion reveals that dj-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taira T., Saito Y., Niki T., Iguchi-Ariga S.M.M., Takahashi K., Ariga H. Dj-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Irrcher I., Aleyasin H., Seifert E.L., Hewitt S.J., Chhabra S., Phillips M., Lutz A.K., Rousseaux M.W.C., Bevilacqua L., Jahani-Asl A., et al. Loss of the parkinson’s disease-linked gene dj-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 130.Behrens M.I., Lendon C., Roe C.M. A common biological mechanism in cancer and alzheimer’s disease? Curr. Alzheimer Res. 2009;6:196–204. doi: 10.2174/156720509788486608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Plun-Favreau H., Lewis P.A., Hardy J., Martins L.M., Wood N.W. Cancer and neurodegeneration: Between the devil and the deep blue sea. PLoS Genet. 2010;6:e1001257. doi: 10.1371/journal.pgen.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Doshay L.J. Problem situations in the treatment of paralysis agitans. J. Am. Med. Assoc. 1954;156:680–684. doi: 10.1001/jama.1954.02950070008003. [DOI] [PubMed] [Google Scholar]
- 133.Driver J.A., Beiser A., Au R., Kreger B.E., Splansky G.L., Kurth T., Kiel D.P., Lu K.P., Seshadri S., Wolf P.A. Inverse association between cancer and alzheimer’s disease: Results from the framingham heart study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Driver J.A., Kurth T., Buring J.E., Gaziano J.M., Logroscino G. Prospective case–control study of nonfatal cancer preceding the diagnosis of parkinson’s disease. Cancer Causes Control. 2007;18:705–711. doi: 10.1007/s10552-007-9005-9. [DOI] [PubMed] [Google Scholar]
- 135.Elbaz A., Peterson B.J., Bower J.H., Yang P., Maraganore D.M., McDonnell S.K., Ahlskog J.E., Rocca W.A. Risk of cancer after the diagnosis of parkinson’s disease: A historical cohort study. Mov. Disord. 2005;20:719–725. doi: 10.1002/mds.20401. [DOI] [PubMed] [Google Scholar]
- 136.Elbaz A., Peterson B.J., Yang P., Van Gerpen J.A., Bower J.H., Maraganore D.M., McDonnell S.K., Eric Ahlskog J., Rocca W.A. Nonfatal cancer preceding parkinson’s disease: A case-control study. Epidemiology. 2002;13:157–164. doi: 10.1097/00001648-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 137.Fois A.F., Wotton C.J., Yeates D., Turner M.R., Goldacre M.J. Cancer in patients with motor neuron disease, multiple sclerosis, and parkinson’s disease: Record-linkage studies. J. Neurol. Neurosurg. Psychiatry. 2009;81:215–221. doi: 10.1136/jnnp.2009.175463. [DOI] [PubMed] [Google Scholar]
- 138.Olsen J.H., Friis S., Frederiksen K. Malignant melanoma and other types of cancer preceding parkinson disease. Epidemiology. 2006;17:582–587. doi: 10.1097/01.ede.0000229445.90471.5e. [DOI] [PubMed] [Google Scholar]
- 139.Olsen J.H., Friis S., Frederiksen K., McLaughlin J.K., Mellemkjaer L., Møller H. Atypical cancer pattern in patients with parkinson‘s disease. Br. J. Cancer. 2004;92:201. doi: 10.1038/sj.bjc.6602279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barnett K., Mercer S.W., Norbury M., Watt G., Wyke S., Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 141.Valderas J.M., Starfield B., Sibbald B., Salisbury C., Roland M. Defining comorbidity: Implications for understanding health and health services. Ann. Fam. Med. 2009;7:357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bajaj A., Driver J.A., Schernhammer E.S. Parkinson’s disease and cancer risk: A systematic review and meta-analysis. Cancer Causes Control. 2010;21:697–707. doi: 10.1007/s10552-009-9497-6. [DOI] [PubMed] [Google Scholar]
- 143.Catts V.S., Catts S.V., O’Toole B.I., Frost A.D.J. Cancer incidence in patients with schizophrenia and their first-degree relatives—A meta-analysis. Acta Psychiatr. Scand. 2008;117:323–336. doi: 10.1111/j.1600-0447.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 144.Tabarés-Seisdedos R., Rubenstein J.L. Inverse cancer comorbidity: A serendipitous opportunity to gain insight into cns disorders. Nat. Rev. Neurosci. 2013;14:293. doi: 10.1038/nrn3464. [DOI] [PubMed] [Google Scholar]
- 145.Catalá-López F., Suárez-Pinilla M., Suárez-Pinilla P., Valderas J.M., Gómez-Beneyto M., Martinez S., Balanzá-Martínez V., Climent J., Valencia A., McGrath J., et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: A meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother. Psychosom. 2014;83:89–105. doi: 10.1159/000356498. [DOI] [PubMed] [Google Scholar]
- 146.Ganesan A.K., Ho H., Bodemann B., Petersen S., Aruri J., Koshy S., Richardson Z., Le L.Q., Krasieva T., Roth M.G., et al. Genome-wide sirna-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008;4:e1000298. doi: 10.1371/journal.pgen.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Olzmann J.A., Chin L.-S. Parkin-mediated k63-linked polyubiquitination: A signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy. 2008;4:85–87. doi: 10.4161/auto.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alvarez-Erviti L., Rodriguez-Oroz M.C., Cooper J.M., Caballero C., Ferrer I., Obeso J.A., Schapira A.H.V. Chaperone-mediated autophagy markers in parkinson disease brains. Arch. Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 149.Cheung Z.H., Ip N.Y. The emerging role of autophagy in parkinson’s disease. Mol. Brain. 2009;2:29. doi: 10.1186/1756-6606-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pan T., Kondo S., Le W., Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with parkinson’s disease. Brain. 2008;131:1969–1978. doi: 10.1093/brain/awm318. [DOI] [PubMed] [Google Scholar]
- 151.Rubinsztein D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 152.Pimkina J., Murphy M.E. Arf, autophagy and tumor suppression. Autophagy. 2009;5:397–399. doi: 10.4161/auto.5.3.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miracco C., Cevenini G., Franchi A., Luzi P., Cosci E., Mourmouras V., Monciatti I., Mannucci S., Biagioli M., Toscano M., et al. Beclin 1 and lc3 autophagic gene expression in cutaneous melanocytic lesions. Hum. Pathol. 2010;41:503–512. doi: 10.1016/j.humpath.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 154.Lazova R., Klump V., Pawelek J. Autophagy in cutaneous malignant melanoma. J. Cutan. Pathol. 2010;37:256–268. doi: 10.1111/j.1600-0560.2009.01359.x. [DOI] [PubMed] [Google Scholar]
- 155.Denison S.R., Wang F., Becker N.A., Schüle B., Kock N., Phillips L.A., Klein C., Smith D.I. Alterations in the common fragile site gene parkin in ovarian and other cancers. Oncogene. 2003;22:8370. doi: 10.1038/sj.onc.1207072. [DOI] [PubMed] [Google Scholar]
- 156.Tay S.-P., Yeo C.W.S., Chai C., Chua P.-J., Tan H.-M., Ang A.X.Y., Yip D.L.H., Sung J.-X., Tan P.H., Bay B.-H., et al. Parkin enhances the expression of cyclin-dependent kinase 6 and negatively regulates the proliferation of breast cancer cells. J. Biol. Chem. 2010;285:29231–29238. doi: 10.1074/jbc.M110.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Poulogiannis G., McIntyre R.E., Dimitriadi M., Apps J.R., Wilson C.H., Ichimura K., Luo F., Cantley L.C., Wyllie A.H., Adams D.J., et al. PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc. Natl. Acad. Sci. USA. 2010;107:15145–15150. doi: 10.1073/pnas.1009941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Staropoli J.F., McDermott C., Martinat C., Schulman B., Demireva E., Abeliovich A. Parkin is a component of an scf-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron. 2003;37:735–749. doi: 10.1016/S0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 159.Ikeuchi K., Marusawa H., Fujiwara M., Matsumoto Y., Endo Y., Watanabe T., Iwai A., Sakai Y., Takahashi R., Chiba T. Attenuation of proteolysis-mediated cyclin e regulation by alternatively spliced parkin in human colorectal cancers. Int. J. Cancer. 2009;125:2029–2035. doi: 10.1002/ijc.24565. [DOI] [PubMed] [Google Scholar]
- 160.Lee S.B., Kim J.J., Nam H.-J., Gao B., Yin P., Qin B., Yi S.-Y., Ham H., Evans D., Kim S.-H., et al. Parkin regulates mitosis and genomic stability through cdc20/cdh1. Mol. Cell. 2015;60:21–34. doi: 10.1016/j.molcel.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lucas J.J., Domenico J., Gelfand E.W. Cyclin-dependent kinase 6 inhibits proliferation of human mammary epithelial cells. Mol. Cancer Res. 2004;2:105–114. [PubMed] [Google Scholar]
- 162.Wang H., Liu B., Zhang C., Peng G., Liu M., Li D., Gu F., Chen Q., Dong J.-T., Fu L., et al. Parkin regulates paclitaxel sensitivity in breast cancer via a microtubule-dependent mechanism. J. Pathol. 2009;218:76–85. doi: 10.1002/path.2512. [DOI] [PubMed] [Google Scholar]
- 163.Lee K., Lee M.H., Kang Y.W., Rhee K.-J., Kim T.U., Kim Y.S. Parkin induces apoptotic cell death in tnf-α-treated cervical cancer cells. BMB Rep. 2012;45:526–531. doi: 10.5483/BMBRep.2012.45.9.104. [DOI] [PubMed] [Google Scholar]
- 164.Gupta A., Anjomani-Virmouni S., Koundouros N., Dimitriadi M., Choo-Wing R., Valle A., Zheng Y., Chiu Y.-H., Agnihotri S., Zadeh G., et al. Park2 depletion connects energy and oxidative stress to pi3k/akt activation via pten s-nitrosylation. Mol. Cell. 2017;65:999–1013. doi: 10.1016/j.molcel.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gegg M.E., Cooper J.M., Schapira A.H.V., Taanman J.-W. Silencing of pink1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS ONE. 2009;4:e4756. doi: 10.1371/journal.pone.0004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu S., Sawada T., Lee S., Yu W., Silverio G., Alapatt P., Millan I., Shen A., Saxton W., Kanao T., et al. Parkinson’s disease–associated kinase pink1 regulates miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nakajima A., Kataoka K., Hong M., Sakaguchi M., Huh N. BRPK, a novel protein kinase showing increased expression in mouse cancer cell lines with higher metastatic potential. Cancer Lett. 2003;201:195–201. doi: 10.1016/S0304-3835(03)00443-9. [DOI] [PubMed] [Google Scholar]
- 168.Liang H., He S., Yang J., Jia X., Wang P., Chen X., Zhang Z., Zou X., McNutt M.A., Shen W.H., et al. Ptenα, a pten isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism. Cell Metab. 2014;19:836–848. doi: 10.1016/j.cmet.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Flanagan C.H., Morais V.A., Wurst W., De Strooper B., O’Neill C. The parkinson’s gene pink1 regulates cell cycle progression and promotes cancer-associated phenotypes. Oncogene. 2015;34:1363. doi: 10.1038/onc.2014.81. [DOI] [PubMed] [Google Scholar]
- 170.Agnihotri S., Golbourn B., Huang X., Remke M., Younger S., Cairns R.A., Chalil A., Smith C.A., Krumholtz S.-L., Mackenzie D., et al. Pink1 is a negative regulator of growth and the warburg effect in glioblastoma. Cancer Res. 2016;76:4708–4719. doi: 10.1158/0008-5472.CAN-15-3079. [DOI] [PubMed] [Google Scholar]
- 171.Ariga H. Common mechanisms of onset of cancer and neurodegenerative diseases. Biol. Pharm. Bull. 2015;38:795–808. doi: 10.1248/bpb.b15-00125. [DOI] [PubMed] [Google Scholar]
- 172.Kim Y.C., Kitaura H., Iguchi-Ariga S.M.M., Ariga H. Dj-1, an oncogene and causative gene for familial parkinson’s disease, is essential for sv40 transformation in mouse fibroblasts through up-regulation of c-myc. FEBS Lett. 2010;584:3891–3895. doi: 10.1016/j.febslet.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 173.Bandopadhyay R., Kingsbury A.E., Cookson M.R., Reid A.R., Evans I.M., Hope A.D., Pittman A.M., Lashley T., Canet-Aviles R., Miller D.W., et al. The expression of dj-1 (park7) in normal human cns and idiopathic parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 174.Li Y., Cui J., Zhang C., Yang D., Chen J., Zan W., Li B., Li Z., He Y. High-expression of dj-1 and loss of pten associated with tumor metastasis and correlated with poor prognosis of gastric carcinoma. Int. J. Med. Sci. 2013;10:1689–1697. doi: 10.7150/ijms.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rizzu P., Hinkle D.A., Zhukareva V., Bonifati V., Severijnen L.A., Martinez D., Ravid R., Kamphorst W., Eberwine J.H., Lee V.M.Y., et al. Dj-1 colocalizes with tau inclusions: A link between parkinsonism and dementia. Ann. Neurol. 2004;55:113–118. doi: 10.1002/ana.10782. [DOI] [PubMed] [Google Scholar]
- 176.Davidson B., Hadar R., Schlossberg A., Sternlicht T., Slipicevic A., Skrede M., Risberg B., Flørenes V.A., Kopolovic J., Reich R. Expression and clinical role of dj-1, a negative regulator of pten, in ovarian carcinoma. Hum. Pathol. 2008;39:87–95. doi: 10.1016/j.humpath.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 177.Hod Y. Differential control of apoptosis by dj-1 in prostate benign and cancer cells. J. Cell. Biochem. 2004;92:1221–1233. doi: 10.1002/jcb.20159. [DOI] [PubMed] [Google Scholar]
- 178.Kim R.H., Peters M., Jang Y., Shi W., Pintilie M., Fletcher G.C., DeLuca C., Liepa J., Zhou L., Snow B., et al. Dj-1, a novel regulator of the tumor suppressor pten. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 179.Lin J., Pan B., Li B., Li Y., Tian X., Li Z. Dj-1 is activated in medulloblastoma and is associated with cell proliferation and differentiation. World J. Surg. Oncol. 2014;12:373. doi: 10.1186/1477-7819-12-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yarden Y., Pines G. The erbb network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer. 2012;12:553. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 181.Prigent S.A., Gullick W.J. Identification of c-erbb-3 binding sites for phosphatidylinositol 3′-kinase and shc using an egf receptor/c-erbb-3 chimera. EMBO J. 1994;13:2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Soltoff S.P., Carraway K.L., Prigent S.A., Gullick W.G., Cantley L.C. Erbb3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol. Cell. Biol. 1994;14:3550–3558. doi: 10.1128/MCB.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hellyer N.J., Cheng K., Koland J.G. Erbb3 (her3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem. J. 1998;333:757–763. doi: 10.1042/bj3330757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mujoo K., Choi B.-K., Huang Z., Zhang N., An Z. Regulation of erbb3/her3 signaling in cancer. Oncotarget. 2014;5:10222–10236. doi: 10.18632/oncotarget.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vijapurkar U., Cheng K., Koland J.G. Mutation of a shc binding site tyrosine residue in erbb3/her3 blocks heregulin-dependent activation of mitogen-activated protein kinase. J. Biol. Chem. 1998;273:20996–21002. doi: 10.1074/jbc.273.33.20996. [DOI] [PubMed] [Google Scholar]
- 186.McNally R.S., Davis B.K., Clements C.M., Accavitti-Loper M.A., Mak T.W., Ting J.P.-Y. Dj-1 enhances cell survival through the binding of cezanne, a negative regulator of nf-κb. J. Biol. Chem. 2011;286:4098–4106. doi: 10.1074/jbc.M110.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bargou R.C., Emmerich F., Krappmann D., Bommert K., Mapara M.Y., Arnold W., Royer H.D., Grinstein E., Greiner A., Scheidereit C., et al. Constitutive nuclear factor-kappab-rela activation is required for proliferation and survival of hodgkin’s disease tumor cells. J. Clin. Investig. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Brown M., Cohen J., Arun P., Chen Z., Van Waes C. Nf-κb in carcinoma therapy and prevention. Expert Opin. Ther. Targets. 2008;12:1109–1122. doi: 10.1517/14728222.12.9.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Landberg G., Tan E.M. Characterization of a DNA-binding nuclear autoantigen mainly associated with s phase and g2 cells. Exp. Cell Res. 1994;212:255–261. doi: 10.1006/excr.1994.1141. [DOI] [PubMed] [Google Scholar]
- 190.Muro Y., Chan E.K.L., Landberg G., Tan E.M. A cell-cycle nuclear autoantigen containing WD-40 motifs expressed mainly in S and G2 phase cells. Biochem. Biophys. Res. Commun. 1995;207:1029–1037. doi: 10.1006/bbrc.1995.1288. [DOI] [PubMed] [Google Scholar]
- 191.Tanti G.K., Goswami S.K. Sg2na recruits dj-1 and akt into the mitochondria and membrane to protect cells from oxidative damage. Free Radic. Biol. Med. 2014;75:1–13. doi: 10.1016/j.freeradbiomed.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 192.Kawate T., Iwaya K., Koshikawa K., Moriya T., Yamasaki T., Hasegawa S., Kaise H., Fujita T., Matsuo H., Nakamura T., et al. High levels of dj-1 protein and isoelectric point 6.3 isoform in sera of breast cancer patients. Cancer Sci. 2015;106:938–943. doi: 10.1111/cas.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang G.-Q., He C., Tao L., Liu F. Role of dj-1 sirna in reverse sensitivity of breast cancer cells to chemotherapy and its possible mechanism. Inter. J. Clin. Exp. Pathol. 2015;8:6944–6951. [PMC free article] [PubMed] [Google Scholar]
- 194.Szklarczyk D., Franceschini A., Kuhn M., Simonovic M., Roth A., Minguez P., Doerks T., Stark M., Muller J., Bork P., et al. The string database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39:D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.STRING Protein-Protein Interactions Network. [(accessed on 18 July 2018)]; Available online: https://string-db.org/
- 196.Bioinformatics-and-Evolutionary-Genomics Calculate and Draw Custom Venn Diagrams. [(accessed on 18 July 2018)]; Available online: http://bioinformatics.psb.ugent.be/webtools/Venn/