Abstract
Mucosal healing in Crohn’s disease (CD) can be evaluated by capsule endoscopy (CE). However, only a few studies have utilized CE to demonstrate the therapeutic effect of medical treatment. We sought to evaluate the validity of using CE to monitor the effect of medical treatment in patients with CD. One hundred (n = 100) patients with CD were enrolled. All patients had a gastrointestinal (GI) tract patency check prior to CE. Patients with baseline CE Lewis score (LS) ≤ 135 were included in the non-active CD group and ended the study. In those with LS > 135 (active CD group), additional treatment was administered, regardless of symptoms, as per the treating clinician’s advice. Patients of the active CD group underwent follow-up CE assessment 6 months later. Out of 92 patients with confirmed GI patency who underwent CE, 40 (43.4%) had CE findings of active inflammation. Of 29 patients with LS > 135 who received additional medications and underwent follow-up CE, improvement of the LS was noted in 23 (79.3%) patients. Eleven patients were asymptomatic but received additional medications; 8 (72.7%) had improvement of the LS. This study demonstrated that additional treatment even for patients with CD in clinical remission and active small-bowel inflammation on CE can reduce mucosal damage.
Keywords: capsule endoscopy, Crohn’s disease, mucosal healing, small bowel
1. Introduction
The main goal in the treatment for Crohn’s disease (CD) is mucosal healing (MH). MH is predictive of reduced subsequent disease activity and clinical upset, and decreased need for further active treatment [1,2,3]. Several modalities are used in assessing overall disease activity and MH in CD. For instance, faecal calprotectin (FC) is a simple, non-invasive, and readily available tool; however, its accuracy in evaluating active small-bowel (SB) mucosal lesions in CD has often been debated [4]. Although cross-sectional imaging has been traditionally used in the evaluation of SB CD [5,6], endoscopy remains the ‘gold standard’ for assessing SB MH because it provides direct and clear observation of the SB mucosa.
Capsule endoscopy (CE) enables physicians to visualize the SB in a non-invasive manner. Hence, CE allows detection of SB mucosal lesions, as well as linear ulceration and luminal stenosis [7]. Recently, Esaki et al. [8] reported that CE enables the identification of SB damage in 88% of patients with established SB CD. Capsule retention is a potentially serious complication of CE; however, the rate of this complication decreases substantially if gastrointestinal (GI) patency is assessed prior to performing regular CE [9,10]. Consequently, CE is recommended as the initial diagnostic modality for SB assessment in patients with suspected CD and negative ileocolonoscopy [11].
To quantify/categorize SB inflammation, Gralnek et al. [12] developed a CE index, the Lewis score (LS), comprising 3 parameters: villous oedema, mucosal ulcer(s), and luminal stenosis. A LS < 135 indicates normal or insignificant mucosal inflammation, LS 135–790 indicates mild mucosal inflammation, and LS ≥ 790 indicates moderate-to-severe inflammation. LS has been validated in the diagnosis and follow-up of established CD [13]. Stratifying SB inflammatory activity at the time of diagnosis has relevant prognostic value in patients with isolated SB CD [14]. A CE-based assessment of SB MH is reported to be useful for predicting long-term clinical remission in CD [15]. Moreover, several retrospective studies have highlighted the potential effect of CE on the therapeutic management of patients with established CD [16,17,18].
However, a high percentage of patients in clinical remission have findings suggestive of on-going inflammatory activity, as evidenced by C-reactive protein (CRP) and FC levels, CE, and cross-sectional imaging [19]. Kopylov et al. [20] demonstrated that a positive CRP level was found in 30.8% of patients with CD in clinical remission, while a high LS on CE was found in 84.6%. Nevertheless, patients with CD who are clinically asymptomatic may not wish to receive additional treatment, and/or physicians may be reluctant to recommend additional treatment in such situations.
Therefore, the aim of this prospective study was to evaluate using CE additional treatment in patients with inflammatory activity, regardless of the presence of clinical symptoms.
2. Patients & Methods
2.1. Patients
This prospective, multicenter study was conducted in hospitals affiliated with the Department of Gastroenterology & Hepatology at Nagoya University Graduate School of Medicine. Key inclusion criteria were patients with established CD who were >10-years-old and scheduled to receive a PillCam patency capsule (PPC), and thereafter CE. For patients to be eligible for inclusion, the colon had to be clear of any inflammation, as confirmed using a conventional colonoscopy. Key exclusion criteria were the presence of any contraindications to anti-tumor necrosis factor (anti-TNF) agents and/or those who were considered non-appropriate candidates by their physician.
2.2. Study Protocol
The study protocol involved two rounds of CE. Patients with CD referred for CE evaluation were informed of the study, aims, and its protocol. Once consent was obtained, patients who accepted to participate underwent the baseline CE at their local hospital. For every patient who was considered for study inclusion, the absence of colonic inflammation, confirmed by a conventional colonoscopy, was necessary. PPC was used before CE, including follow-ups, in all patients who agreed to participate. The PPC consists of lactose and 10% barium, which dissolves when intestinal fluids come into contact with them through a window at the edges of the PPC. PPC is similar to the second-generation Agile patency capsule, with the only difference being that the radiofrequency identification tag has been removed [10] (Figure 1). GI patency was evaluated either by confirming excretion of an intact PPC or by obtaining a plain abdominal X-ray and/or computed tomography (CT), generally between 30 and 33 h after PPC ingestion. The capsules used for CE were PillCam®SB2 or SB3 (manufactured by Medtronic, Minneapolis, MN, USA), which measure 26 × 11 mm and are propelled by peristalsis. All subjects whose GI patency was confirmed underwent CE, at their earliest convenience, following an overnight fast without prokinetics or prior laxative bowel preparation. Patients whose GI patency was not confirmed were excluded from further participation in this study. Following anonymization, the CE videos were sent to Nagoya University Hospital via the Nagoya network system, as described in References [21,22]. Two expert CE readers (MN, TY) independently reviewed each CE and calculated the LS. They also read the second CE videos and remained blinded to the treatment provided following the baseline CE reports. SB cleansing was evaluated in four grades according to previous literature [23,24]. In cases of any discordance in LS results, the experts discussed all relevant CE images and provided final LS by consensus agreement. The report of each CE was then sent back to the local hospital and further clinical management was left with the treating physician/team. Clinical data related to PPC and CE, including adverse events, were collected.
Figure 1.
PillCam patency capsule.
Clinical remission was defined as a CD activity index (CDAI) score < 150. Since a LS < 135 can occasionally be associated with the presence of aphtha(e) or villous/fold oedema on CE, only a LS =0 was defined as complete MH. If LS ≤ 135 on baseline CE, suggestive of normal or clinically insignificant mucosal inflammatory change [25], the patient finished the study (non-active CD group). In the case of LS > 135, the treating teams could proceed with additional treatment, regardless of the presence of any clinical symptoms (active CD group). For patients of the active group, the follow-up CE was scheduled 6 months later, and it was performed with the same protocol as the baseline one.
2.3. Evaluations
Comparison was made between the clinical background and the baseline CE findings between the active and non-active groups. Additional therapeutic effects were evaluated in the active group, especially in asymptomatic patients, who underwent follow-up CEs. Primary outcome was any reported change in CE findings between the two CE procedures. Key secondary outcomes were changes in the CDAI and CRP level at the time of follow-up CE (for the active group), as well as any adverse events of PPC and CE procedures.
2.4. Statistical Analysis
The statistical software package SPSS for Windows (SPSS Inc., Chicago, IL, USA) was used for data analysis. The Wilcoxon signed-rank test was used to compare the LS based on CE and changes in each marker before and after any additional treatment. Patients’ demographic data at the baseline examination was compared between the active and non-active groups using the Mann-Whitney U test or χ2 test. In all analyses, a p value < 0.05 was considered statistically significant.
2.5. Ethical Considerations
This study was approved by the ethics Committee of Nagoya University Hospital. This study was registered in the University Hospital Medical Information Network, a clinical trials registry (UMIN000008486). Written informed consent was obtained from all participants.
3. Results
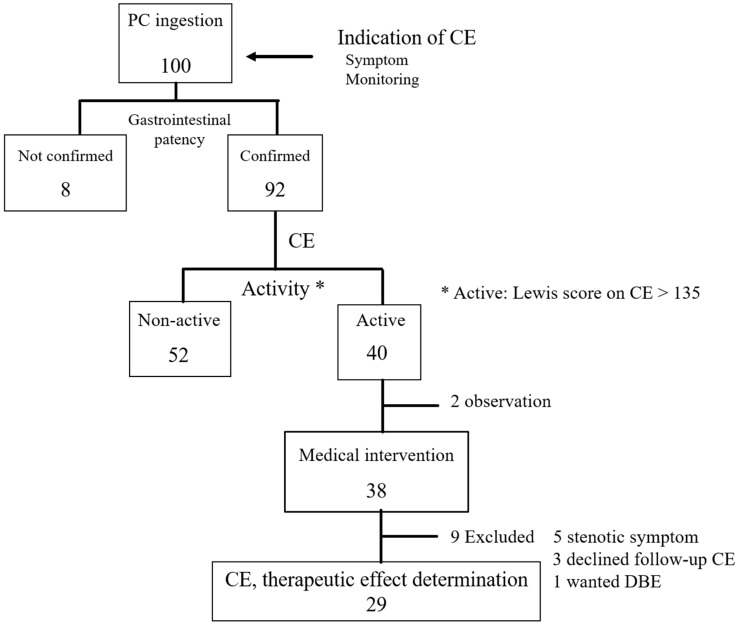
Between September 2012 and April 2016, 100 patients were referred for CE and approached for inclusion in this study at the Nagoya University Hospital and its 5 affiliated hospitals, as shown in Figure 2. Of these patients, 92 had confirmed GI patency and were eventually included in this study. Absence of colonic inflammation was confirmed, prior to study entry, with a conventional colonoscopy which was performed at a median of 14 (range 1–29) months prior to inclusion in the study.
Figure 2.
Flow chart of present study. Abbreviations PC, patency capsule; CE, capsule endoscopy; DBE, double balloon enteroscopy.
In the 92 baseline CEs, the grades of SB cleansing (for the whole SB) were excellent, good, fair, and poor in 19, 59, 12, and 2 patients, respectively. Of 92 patients, 40 (43.4%) had findings of active CD (active CD group); clinically, 28/40 patients were symptomatic. Their symptoms were diarrhea (n = 20), abdominal pain (n = 4), bloody stool (n = 3), and abdominal fullness (n = 1). The remainder (n = 52), with LS < 135 on baseline CE, comprised the non-active CD group. CDAI scores were not significantly different between the two groups; however, LS was significantly higher in the active group than in the non-active CD group. Hemoglobin and serum albumin levels were significantly lower in the active group than in the non-active CD group (Table 1).
Table 1.
Demographic data of the patients at the point of study inclusion.
| Total | Active Group | Non Active Group | p Value | |
|---|---|---|---|---|
| N | 92 | 40 | 52 | |
| Age, mean ± SD, years old | 37.2 ± 12.3 | 37.5 ± 12.3 | 37.1 ± 12.9 | 0.8624 |
| Gender, M/F | 68/24 | 29/11 | 39/13 | 0.9751 |
| BMI | 21.3 ± 3.2 | 21.5 ± 3.6 | 21.1 ± 2.9 | 0.603 |
| Duration of disease, mean ± SD, months | 117.1 ± 96.7 | 93.9 ± 73.3 | 135 ± 108.8 | 0.093 |
| Montreal classification | ||||
| Age at diagnosis | ||||
| <17 years | 7 | 3 | 4 | |
| 17–40 years | 70 | 30 | 40 | |
| >40 years | 15 | 7 | 8 | |
| Location | ||||
| L1—ileal | 38 | 17 | 21 | |
| L2—colonic | 0 | |||
| L3—ileocolonic | 54 | 23 | 31 | |
| Behavior | ||||
| B1—Non-stricturing, non-penetrating | 72 | 28 | 44 | |
| B2—Stricturing | 15 | 9 | 6 | |
| B3—Penetrating | 5 | 3 | 2 | |
| p-perianal disease | 9 | 6 | 3 | |
| History of GI surgery | 53/92 | 25/40 | 28/52 | 0.5353 |
| Ileo-colonic resection | 26 | 12 | 14 | |
| Ileal resection | 21 | 9 | 12 | |
| Ileo-colonic resection plus Ileal resection | 4 | 3 | 1 | |
| Colonic resection | 2 | 1 | 1 | |
| Any symptom | 28/92 | 19/40 | 9/52 | 0.0038 |
| CDAI | 104 ± 56 | 116 ± 72 | 95 ± 39 | 0.277 |
| Laboratory data | ||||
| CRP (mg/dL), mean ± SD | 0.36 ± 0.62 | 0.53 ± 0.75 | 0.24 ± 0.47 | 0.0761 |
| Hb (g/dL), mean ± SD | 13.3 ± 2.1 | 12.7 ± 2.5 | 13.8 ± 1.6 | 0.0201 |
| Albumin (g/dL), mean ± SD | 4.0 ± 0.5 | 3.9 ± 0.5 | 4.2 ± 0.4 | 0.0014 |
| Indication of CE | ||||
| Symptom(s) | 28 | 19 | 9 | |
| diarrhea | 20 | 15 | 5 | |
| abdominal pain | 4 | 2 | 2 | |
| bloody stools | 3 | 2 | 1 | |
| abdominal fullness | 1 | 0 | 1 | |
| Monitoring | 64 | 21 | 43 | |
| PPC and CE | ||||
| PPC intact body excretion | 62/92 | 26/40 | 36/52 | 0.8377 |
| Gastric transit time (min.) | 45.5 ± 42.4 | 45.6 ± 40.3 | 47.2 ± 44.3 | 0.9904 |
| SBTT (min.) | 248.8 ± 128.8 | 270.7 ± 149.5 | 231.7 ± 108.5 | 0.3042 |
| Lewis score, mean ± SD | 396 ± 706 | 844 ± 892 | 52.1 ± 66.1 | <0.0001 |
| Treatment | ||||
| Anti TNF-α agent | 54/92 | 23/40 | 31/52 | |
| 5-ASA | 78/92 | 35/40 | 43/52 | |
| Immunomodulator | 14/92 | 5/40 | 9/52 | |
| Elemental diet | 57/92 | 23/40 | 34/52 | |
We defined as regular use of non-steroidal anti-inflammatory drugs (NSAIDs), the use of this class of medications for more than 6 months irrespective of type and dose. This was clarified by review of the medical charts and patient interview. None of the patients regularly had used NSAIDs before and/or after CEs. Of 38/40 patients of the active CD group who received additional anti-inflammatory treatment(s) (infliximab, n = 8; 5-aminosalicylic acid, n = 7; azathioprine, n = 6; adalimumab, n = 4; elemental diet, n = 2; prednisolone, n = 1; and mercaptopurine, n = 1), 29/40 (72%) underwent follow-up CEs to assess the therapeutic effect on MH.
Following additional treatment for 6 months, the mean LS improved from 691 ± 126 to 394 ± 99. Villous oedema before and after treatment was detected in 20 and 11 patients, respectively. Of all the 29 patients who had ulcers at baseline CE, 19 improved and 4 no longer had an ulcer. The LS of 4 patients who had a baseline LS > 1000 decreased; however, only 2/29 patients achieved MH (Table 2). The mean CDAI score and LS significantly improved 6 months after the start of additional treatment (Table 3), although the mean CRP level did not improve dramatically.
Table 2.
29 cases where intervention.
| Case | Medicine | Intervention | Lewis Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Score | Villous Edema | Ulcer | Stenosis | |||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||
| 1 | Infliximab | dose up 5 ⇒ 10 mg/kg | 2914 | 2824 | 112 | 112 | 450 | 360 | 2352 | 2352 |
| 2 | Infliximab | introduction 5 mg/kg | 2585 | 280 | 8 | 0 | 225 | 0 | 2352 | 280 |
| 3 | Adalimumab | introduction 160 mg | 2140 | 600 | 340 | 0 | 1800 | 600 | 0 | 0 |
| 4 | Azathiopurin | introduction 50 mg | 1012 | 337 | 112 | 112 | 900 | 225 | 0 | 0 |
| 5 | mercaptopurine | introduction 20 mg | 900 | 1368 | 0 | 168 | 900 | 1200 | 0 | 0 |
| 6 | 5-ASA | dose up 3000 ⇒ 4000 mg | 712 | 712 | 112 | 112 | 600 | 600 | 0 | 0 |
| 7 | Infliximab | introduction 5 mg/kg | 712 | 421 | 112 | 0 | 600 | 225 | 0 | 196 |
| 8 | Adalimumab | introduction 160 mg | 654 | 0 | 204 | 0 | 450 | 0 | 0 | 0 |
| 9 | Adalimumab | introduction 160 mg | 600 | 0 | 0 | 0 | 600 | 0 | 0 | 0 |
| 10 | Infliximab | dose up 5 ⇒ 10 mg/kg | 562 | 196 | 112 | 0 | 450 | 0 | 0 | 196 |
| 11 | 5-ASA | dose up 1500 ⇒ 2000 mg | 562 | 225 | 112 | 0 | 450 | 225 | 0 | 0 |
| 12 | 5-ASA | introduction 3000 mg | 562 | 337 | 112 | 112 | 450 | 225 | 0 | 0 |
| 13 | Infliximab | introduction 5 mg/kg | 562 | 233 | 112 | 8 | 450 | 225 | 0 | 0 |
| 14 | Azathiopurin | introduction 50 mg | 504 | 180 | 204 | 0 | 300 | 180 | 0 | 0 |
| 15 | Prednisolone | introduction 20 mg | 458 | 450 | 8 | 0 | 450 | 450 | 0 | 0 |
| 16 | 5-ASA | dose up 2000 ⇒ 3000 mg | 458 | 147 | 8 | 12 | 450 | 135 | 0 | 0 |
| 17 | Azathiopurin | introduction 50 mg | 450 | 458 | 0 | 8 | 450 | 450 | 0 | 0 |
| 18 | Adalimumab | introduction 160 mg | 436 | 225 | 136 | 0 | 300 | 225 | 0 | 0 |
| 19 | Elemental diet | dose up | 429 | 225 | 204 | 0 | 225 | 225 | 0 | 0 |
| 20 | Elemental diet | dose up | 412 | 225 | 112 | 0 | 300 | 225 | 0 | 0 |
| 21 | Infliximab | dose up 5 ⇒ 10 mg/kg | 412 | 225 | 112 | 0 | 300 | 225 | 0 | 0 |
| 22 | Infliximab | introduction 5 mg/kg | 337 | 135 | 112 | 0 | 225 | 135 | 0 | 0 |
| 23 | Azathiopurin | introduction 50 mg | 300 | 278 | 0 | 8 | 300 | 270 | 0 | 0 |
| 24 | Azathiopurin | dose up 50 ⇒ 75 mg | 300 | 180 | 0 | 0 | 300 | 180 | 0 | 0 |
| 25 | Azathiopurin | introduction 50 mg | 233 | 143 | 8 | 8 | 225 | 135 | 0 | 0 |
| 26 | Infliximab | dose up 5 ⇒ 10 mg/kg | 225 | 233 | 0 | 8 | 225 | 225 | 0 | 0 |
| 27 | 5-ASA | dose up 2000 ⇒ 3000 mg | 225 | 225 | 0 | 0 | 225 | 225 | 0 | 0 |
| 28 | 5-ASA | dose up 1500 ⇒ 3000 mg | 225 | 450 | 0 | 0 | 225 | 450 | 0 | 0 |
| 29 | 5-ASA | dose up 2000 ⇒ 3000 mg | 180 | 135 | 0 | 0 | 180 | 135 | 0 | 0 |
Table 3.
Biomarker levels at baselines and 6 months later.
| Pre-Treatment | Post-Treatment | p Value | |
|---|---|---|---|
| CDAI | 102 (5–253) | 68 (0–231) | 0.0057 |
| Lewis score | 458 (180–2914) | 233 (0–2824) | 0.0004 |
| CRP level (mg/dL) | 0.55 ± 0.80 | 0.30 ± 0.51 | 0.0652 |
| WBC count (/µL) | 6822 ± 2602 | 5920 ± 1807 | 0.1663 |
| Hb level (g/dL) | 12.7 ± 2.5 | 13.2 ± 1.9 | 0.7843 |
| Plt count(×1000/mm3) | 26.6 ± 6.1 | 26.7 ± 7.0 | 0.5014 |
| Albumin level (g/dL) | 3.9 ± 0.6 | 4.1 ± 0.5 | 0.1297 |
Data are presented as a mean ± standard deviation. CDAI, Crohn’s disease activity index; CRP, C-reactive protein; WBC, white blood cell; Hb, hemoglobin; Plt, platelet.
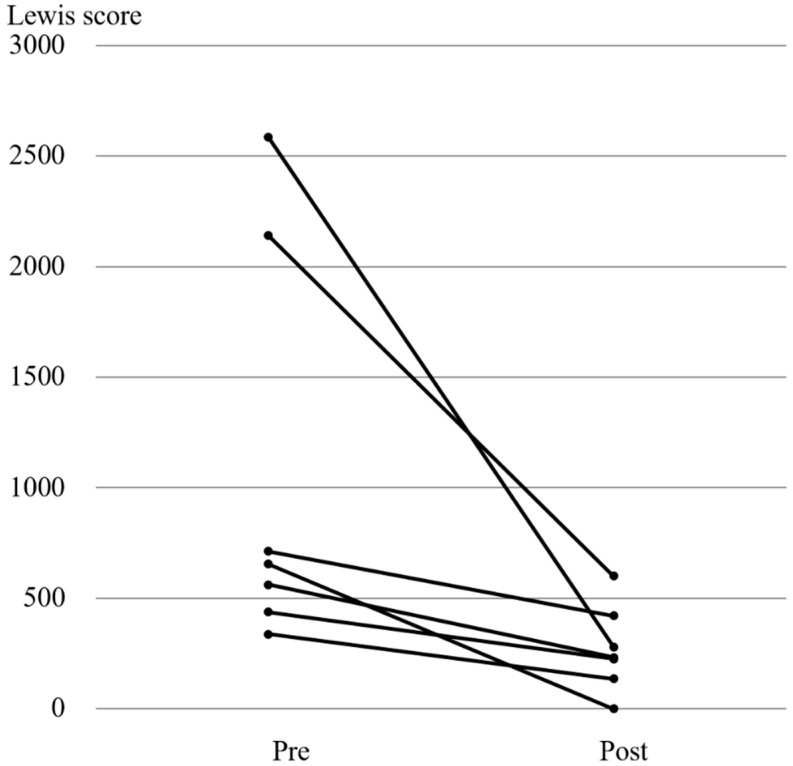
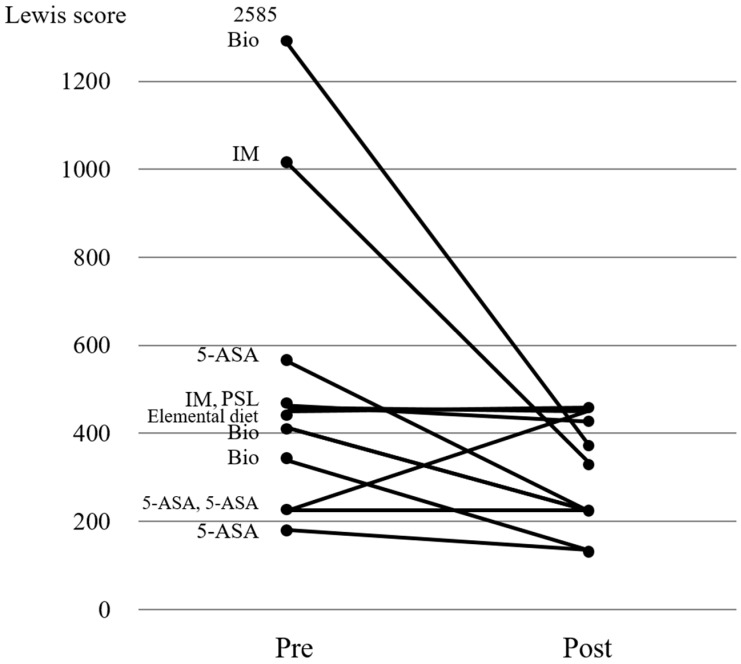
All 7 patients who received additional treatment with biologics had improvement of LS on repeat CE (Figure 3). Of the 11 patients who were asymptomatic, 4 received 5-ASA, 3 received biologics, 2 received immunomodulators, 1 received prednisolone, and 1 received elemental diet as additional treatment (Figure 4). LS improvement was noted in 8/11 (72.7%) patients; however, none of them achieved MH. No adverse events occurred throughout the study, as no retention of PPC or capsule was noted. Of 29 patients who received additional treatment and underwent follow-up CE, 23 patients’ clinical course could be confirmed post-study in November 2017. During the median follow-up 36 months, 12 patients did not undergo change in treatment, although 10 patients were given additional treatment and 1 underwent surgery.
Figure 3.
Changes in the Lewis scores in patients who received biologics as additional treatment. Abbreviations Pre, pre-treatment; post, post-treatment.
Figure 4.
Changes in the Lewis scores in asymptomatic patients before and after treatment. Bio, biologic; IM, infliximab; 5-ASA, 5-aminosalicylic acid; PSL, prednisolone; pre, pre-treatment; post, post-treatment.
4. Discussion
Our study confirms LS improvement in 23/29 patients (79.3%), irrespective of symptoms status, who received additional treatment for active CD based on the findings of baseline CE. Interestingly, 8/11 asymptomatic CD patients (72.7%) had improvement in their LS, regardless of the type of additional treatment provided. These results underline a couple of key points. First, asymptomatic CD patients may require additional treatment or simply increasing the dose of their existing therapeutic regimen to achieve MH. Additional medication had a positive effect in both asymptomatic and symptomatic CD patients, as shown in Figure 2, Figure 3 and Figure 4. Second, CE is a valid tool in evaluating both therapeutic effect, as well as confirming MH in patients with asymptomatic (active) CD.
In CD, it remains controversial whether the physician should set the therapeutic target at MH or clinical remission. If the therapeutic goal is MH rather than clinical (symptoms) remission, this can only be evaluated by endoscopy. The POCER study suggested that monitoring using early colonoscopy and treatment step-up was better in preventing postoperative CD recurrence, than conventional drug therapy alone [26]. On the other hand, Kim et al. reported that endoscopic monitoring did not significantly contribute to the non-hospitalization rate associated with CD, compared with ulcerative colitis [27]. However, the definition of MH remains unclear. Total disappearance of mucosal ulcerations has the advantage of providing irrefutable evidence of MH, but this strict ‘black-and-white’ goal may be difficult to achieve [28]. For instance, a patient with numerous deep mucosal ulcerations in whom treatment leads to healing of all but a superficial mucosal ulceration will be classified as a ‘non-responder’.
To date, only few studies have shown the necessity and effectiveness of additional treatment for CD patients in clinical remission and endoscopically-confirmed active lesions, although the significance of monitoring has been widely recognized. Once the therapeutic effects for such patients have been clarified, physicians may recommend a change of the medication regimen. Under the notion of treat-to-target, Ungar et al. suggested a significant association between serum levels of anti-TNF agents and the level of mucosal healing as the therapeutic goal [29]. Physicians may modify the dose of biologic treatment with reference to the serum levels even if patients have no symptoms. The CALM study demonstrated the significance of tight control, including timely escalation with anti-TNF therapy. Combining clinical symptoms with biomarkers in patients with early CD results in better clinical and endoscopic outcomes than symptom-driven decisions alone [30]. This study also supported the importance of tight control in CD by evaluating treatment outcomes in asymptomatic patients.
The development and introduction of a new, more effective drug will provide better CD monitoring. Endoscopic findings may become the best way to evaluate MH in CD because endoscopy provides direct visualization of the intestinal mucosa and enables physicians to detect even subtle mucosal lesions. However, it is unclear whether small lesions affect the long-term clinical outcome in CD. Moreover, it is questionable whether physicians can determine MH in patients with small lesions. LS calculation does not include the evaluation of such lesions. Therefore, MH according to LS means that there is no mucosal ulcer, but this does not preclude the presence of erosions or mucosal aphtha(e). Niv score or CECDAI, on the other hand, classifies erosion and small ulcers <5 mm under the same category [31,32]. It may be necessary to evaluate the outcome of patients with and without erosions.
If physicians can ignore small lesions to consider the long-term outcome of CD, abdominal ultrasonography and magnetic resonance enterography (MRE) will also be excellent modalities to evaluate MH. Koulaouzidis et al. reported that the LS appears to have only a fair correlation with the FC level, as well as other serological markers of inflammation [33]. FC level does not seem to be a reliable biomarker for significant SB inflammation. Nevertheless, an FC level ≥ 76 µg/g may be associated with appreciable visual inflammation on SB CE in patients with a prior negative diagnostic workup, and it may become the surrogate marker of CD.
This study has few limitations. The overall sample size of this study was small. This study focused on the Lewis score for next treatment, and laboratory data or calprotectin was not collected on follow-up. Of the patients with active CD based on CE, the percentage of patients who received an intervention was low because five patients had developed small-bowel stenosis, as demonstrated on a CT scan. The others did not wish to receive additional treatment. The kind of intervention depended on the physician and patients according to the protocol. Therefore, the balance between the degree of CD activity and kind of medication may not always be suitable. In conclusion, this prospective, multi-center study demonstrated that additional treatment regardless of clinical remission reduces mucosal inflammation in CD.
Acknowledgments
For Data Availability, The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
5-aminosalicylic acid (5-ASA), C-reactive protein (CRP), Crohn disease (CD), Crohn’s disease activity index (CDAI), capsule endoscopy (CE), double-balloon endoscopy (DBE), faecal calprotectin (FC), gastrointestinal (GI), Lewis score (LS), mucosal healing (MH), non-steroidal anti-inflammatory drugs (NSAIDs), PillCam patency capsule (PPC).
Author Contributions
Conceptualization, M.N., T.S. and Y.M.; Data curation, T.Y., K.M., T.S., K.F. and Nagoya University Crohn’s Disease Study Group; Formal analysis, M.N., T.I., E.O. and H.K.; Investigation, M.N.; Methodology, M.N. and T.Y.; Critical review of the manuscript versions, A.K.; Validation, R.M.; Visualization, Y.M.; Writing—original draft, M.N.; Writing—review & editing, A.K.; Approval of this study, Y.H.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Baert F., Moortgat L., Van Assche G., Caenepeel P., Vergauwe P., De Vos M., Stokkers P., Hommes D., Rutgeerts P., Vermeire S., et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L., Ferrante M., Magro F., Campbell S., Franchimont D., Fidder H., Strid H., Ardizzone S., Veereman-Wauters G., Chevaux J.B., et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J. Crohns Colitis. 2011;5:477–483. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Frøslie K.F., Jahnsen J., Moum B.A., Vatn M.H., IBSEN Group Mucosal healing in inflammatory bowel disease: Results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 4.Sipponen T., Savilahti E., Kolho K.L., Nuutinen H., Turunen U., Farkkila M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn’s disease activity index and endoscopic findings. Inflamm. Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 5.Kopylov U., Klang E., Yablecovitch D., Lahat A., Avidan B., Neuman S., Levhar N., Greener T., Rozendorn N., Beytelman A., et al. Magnetic resonance enterography versus capsule endoscopy activity indices for quantification of small bowel inflammation in Crohn’s disease. Ther. Adv. Gastroenterol. 2016;9:655–663. doi: 10.1177/1756283X16649143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moy M.P., Kaplan J.L., Moran C.J., Winter H.S., Gee M.S. MR enterographic findings as biomarkers of mucosal healing in young patients with Crohn disease. Am. J. Roentgenol. 2016;207:896–902. doi: 10.2214/AJR.16.16079. [DOI] [PubMed] [Google Scholar]
- 7.Kopylov U., Koulaouzidis A., Klang E., Carter D., Ben-Horin S., Eliakim R. Monitoring of small bowel Crohn’s disease. Expert Rev. Gastroenterol. Hepatol. 2017;11:1014–1058. doi: 10.1080/17474124.2017.1359541. [DOI] [PubMed] [Google Scholar]
- 8.Esaki M., Matsumoto T., Watanabe K., Arakawa T., Naito Y., Matsuura M., Nakase H., Hibi T., Matsumoto T., Nouda S., et al. Use of capsule endoscopy in patients with Crohn’s disease in Japan: A multicenter survey. J. Gastroenterol. Hepatol. 2014;29:96–101. doi: 10.1111/jgh.12411. [DOI] [PubMed] [Google Scholar]
- 9.Nemeth A., Kopylov U., Koulaouzidis A., Wurm Johansson G., Thorlacius H., Amre D., Eliakim R., Seidman E.G., Toth E. Use of patency capsule in patients with established Crohn’s disease. Endoscopy. 2016;48:373–379. doi: 10.1055/s-0034-1393560. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura M., Hirooka Y., Yamamura T., Miyahara R., Watanabe O., Ando T., Ohmiya N., Goto H. Clinical usefulness of novel tag-less Agile patency capsule prior to capsule endoscopy for patients with suspected small bowel stenosis. Dig. Endosc. 2015;27:61–66. doi: 10.1111/den.12306. [DOI] [PubMed] [Google Scholar]
- 11.Pennazio M., Spada C., Eliakim R., Keuchel M., May A., Mulder C.J., Rondonotti E., Adler S.N., Albert J., Baltes P., et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352–376. doi: 10.1055/s-0034-1391855. [DOI] [PubMed] [Google Scholar]
- 12.Gralnek I.M., Defranchis R., Seidman E., Leighton J.A., Legnani P., Lewis B.S. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment. Pharmacol. Ther. 2008;27:146–154. doi: 10.1111/j.1365-2036.2007.03556.x. [DOI] [PubMed] [Google Scholar]
- 13.Koulaouzidis A., Douglas S., Plevris J.N. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn’s Disease Activity Index. Dig. Dis. Sci. 2012;57:987–993. doi: 10.1007/s10620-011-1956-8. [DOI] [PubMed] [Google Scholar]
- 14.Dias de Castro F., Boal Carvalho P., Monteiro S., Rosa B., Firmino-Machado J., Moreira M.J., Cotter J. Lewis score—Prognostic value in patients with isolated small bowel Crohn’s disease. J. Crohns Colitis. 2015;9:1146–1151. doi: 10.1093/ecco-jcc/jjv166. [DOI] [PubMed] [Google Scholar]
- 15.Niv Y. Small-bowel mucosal healing assessment by capsule endoscopy as a predictor of long-term clinical remission in patients with Crohn’s disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017;29:844–848. doi: 10.1097/MEG.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 16.Long M.D., Barnes E., Isaacs K., Morgan D., Herfarth H.H. Impact of capsule endoscopy on management of inflammatory bowel disease: A single tertiary care center experience. Inflamm. Bowel Dis. 2011;17:1855–1862. doi: 10.1002/ibd.21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenzo-Zúñiga V., de Vega V.M., Domènech E., Cabré E., Mañosa M., Boix J. Impact of capsule endoscopy findings in the management of Crohn’s Disease. Dig. Dis. Sci. 2010;55:411–414. doi: 10.1007/s10620-009-0758-8. [DOI] [PubMed] [Google Scholar]
- 18.Sidhu R., McAlindon M.E., Drew K., Hardcastle S., Cameron I.C., Sanders D.S. Evaluating the role of small-bowel endoscopy in clinical practice: The largest single-centre experience. Eur. J. Gastroenterol. Hepatol. 2012;24:513–519. doi: 10.1097/MEG.0b013e328350fb05. [DOI] [PubMed] [Google Scholar]
- 19.Annese V., Daperno M., Rutter M.D., Amiot A., Bossuyt P., East J., Ferrante M., Götz M., Katsanos K.H., Kießlich R., et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Kopylov U., Yablecovitch D., Lahat A., Neuman S., Levhar N., Greener T., Klang E., Rozendorn N., Amitai M.M., Ben-Horin S., et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am. J. Gastroenterol. 2015;110:1316–1323. doi: 10.1038/ajg.2015.221. [DOI] [PubMed] [Google Scholar]
- 21.Goto H., Nakamura M., Ohmiya N., Hirooka Y., Itoh A. Establishment of an interpretation system for video capsule endoscopy for obscure gastrointestinal bleeding. J. Gastroenterol. 2010;45:468–469. doi: 10.1007/s00535-010-0208-6. [DOI] [PubMed] [Google Scholar]
- 22.Goto H., Nakamura M., Ohmiya N. Advanced network system for reading capsule endoscopy images. Dig. Endosc. 2013;25:91. doi: 10.1111/j.1443-1661.2012.01389.x. [DOI] [PubMed] [Google Scholar]
- 23.Brotz C., Nandi N., Conn M., Daskalakis C., DiMarino M., Infantolino A., Katz L.C., Schroeder T., Kastenberg D. A validation study of 3 grading systems to evaluate small-bowel cleansing for wireless capsule endoscopy: A quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest. Endosc. 2009;69:262–270. doi: 10.1016/j.gie.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Yung D.E., Rondonotti E., Sykes C., Pennazio M., Plevris J.N., Koulaouzidis A. Systematic review and meta-analysis: Is bowel preparation still necessary in small bowel capsule endoscopy? Expert Rev. Gastroenterol. Hepatol. 2017;11:979–993. doi: 10.1080/17474124.2017.1359540. [DOI] [PubMed] [Google Scholar]
- 25.Cotter J., Dias de Castro F., Magalhães J., Moreira M.J., Rosa B. Validation of the Lewis score for the evaluation of small-bowel Crohn’s disease activity. Endoscopy. 2015;47:330–335. doi: 10.1055/s-0034-1391621. [DOI] [PubMed] [Google Scholar]
- 26.De Cruz P., Kamm M.A., Hamilton A.L., Ritchie K.J., Krejany E.O., Gorelik A., Liew D., Prideaux L., Lawrance I.C., Andrews J.M., et al. Crohn’s disease management after intestinal resection: A randomised trial. Lancet. 2015;385:1406–1417. doi: 10.1016/S0140-6736(14)61908-5. [DOI] [PubMed] [Google Scholar]
- 27.Kim D.H., Park S.J., Park J.J., Yun Y.H., Hong S.P., Kim T.I., Kim W.H., Cheon J.H. Effect of follow-up endoscopy on the outcomes of patients with inflammatory bowel disease. Dig. Dis. Sci. 2014;59:2514–2522. doi: 10.1007/s10620-014-3197-0. [DOI] [PubMed] [Google Scholar]
- 28.Pineton de Chambrun G., Peyrin-Biroulet L., Lémann M., Colombel J.F. Clinical implications of mucosal healing for the management of IBD. Nat. Rev. Gastroenterol. Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 29.Ungar B., Levy I., Yavne Y., Yavzori M., Picard O., Fudim E., Loebstein R., Chowers Y., Eliakim R., Kopylov U., et al. Optimizing anti-TNF-α therapy: Serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2016;14:550–557. doi: 10.1016/j.cgh.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Colombel J.F., Panaccione R., Bossuyt P., Lukas M., Baert F., Vaňásek T., Danalioglu A., Novacek G., Armuzzi A., Hébuterne X., et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet. 2017;17:32641–32647. doi: 10.1016/S0140-6736(17)32641-7. [DOI] [PubMed] [Google Scholar]
- 31.Gal E., Geller A., Fraser G., Levi Z., Niv Y. Assessment and validation of the new capsule endoscopy Crohn’s disease activity index (CECDAI) Dig. Dis. Sci. 2008;53:1933–1937. doi: 10.1007/s10620-007-0084-y. [DOI] [PubMed] [Google Scholar]
- 32.Niv Y., Ilani S., Levi Z., Hershkowitz M., Niv E., Fireman Z., O’Donnel S., O’Morain C., Eliakim R., Scapa E., et al. Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): A multicenter prospective study. Endoscopy. 2012;44:21–26. doi: 10.1055/s-0031-1291385. [DOI] [PubMed] [Google Scholar]
- 33.Koulaouzidis A., Sipponen T., Nemeth A., Makins R., Kopylov U., Nadler M., Giannakou A., Yung D.E., Johansson G.W., Bartzis L., et al. Association between fecal calprotectin levels and small-bowel inflammation score in capsule endoscopy: A multicenter retrospective study. Dig. Dis. Sci. 2016;61:2033–2040. doi: 10.1007/s10620-016-4104-7. [DOI] [PubMed] [Google Scholar]