Abstract
Esophageal cancer (EC) is the eighth most common and sixth leading cause of cancer-related mortality in the world. Despite breakthroughs in EC diagnosis and treatment, patients with complete pathologic response after being submitted to chemoradiotherapy are still submitted to surgery, despite its high morbidity. Single-nucleotide polymorphisms (SNPs) in miRNA, miRNA-binding sites, and in its biogenesis pathway genes can alter miRNA expression patterns, thereby influencing cancer risk and prognosis. In this review, we systematized the information available regarding the impact of these miR-SNPs in EC development and prognosis. We found 34 miR-SNPs that were associated with EC risk. Despite the promising applicability of these miR-SNPs as disease biomarkers, they still lack validation in non-Asian populations. Moreover, there should be more pathway-based approaches to evaluate the cumulative effect of multiple unfavorable genotypes and, consequently, identify miR-SNPs signatures capable of predicting EC therapy response and prognosis.
Keywords: esophageal cancer, miR-SNPs, molecular biomarkers
1. Introduction
Esophageal cancer (EC) is the eighth most common cancer and the sixth leading cause of cancer-related mortality in the world, with an estimated 400,000 deaths in 2012 [1,2]. The vast majority of ECs occur as either esophageal squamous cell carcinoma (ESCC) in the middle or upper third of the esophagus, or as esophageal adenomarcinomas (EAC) in the distal third or esophagogastric junction [3]. EC incidence is threefold higher in men than in women, and peak incidence rates occur in Southern and Eastern Africa and in Eastern Asia [2]. The global incidence of ESCC has remained more or less the same, whereas a rapid increase in the incidence of EAC has been observed in the United States and Western Europe [3,4,5]. In fact, EAC incidence is expected to rise dramatically in many Western countries in the coming years [6,7]. EAC typically arises from a metaplastic epithelium known as Barrett’s Esophagus (BE), and established risk factors for both EAC and BE include gastroesophageal reflux disease, European ancestry, male sex, obesity, and tobacco smoking [8].
Surgical resection remains the cornerstone of curative treatment for patients with locoregional EC despite high morbidity and mortality rates due to complications, especially in older patients [9,10]. Recent randomized trials have shown that neoadjuvant chemoradiotherapy significantly improved survival in patients with resectable tumors [11,12]. As such, multimodality therapy that combines neoadjuvant chemoradiotherapy followed by surgery has become the standard of care in many institutions. However, operative morbididy and mortality associated with esophagectomy remain high, and complications arise in up to 60% of patients [13]. Additionally, 25–30% of patients experience a complete pathologic response following neoadjuvant chemoradiotherapy, but are still submitted to surgery due to the lack of accurate and reliable techniques capable of determining the complete pathological response [12,13]. This fact led some authors to question if every patient should undergo esophagectomy following chemoradiotherapy, since the subgroup that presents a complete pathological response won’t benefit from surgery and, most importantly, how patients best suited to chemoradiotherapy alone should be selected. Thus, the study and development of new biomarkers capable of predicting patients’ prognosis is imperative in order to avoid surgery and related morbidity and mortality in good responders to neoadjuvant chemoradiotherapy.
The contribution of microRNAs (miRNAs) to EC development has been extensively studied, and it has become clear that they play crucial roles in the pathogenesis, diagnosis, and prognosis of this type of cancer [14]. In fact, a study published this year by Chiam and colleagues established two miRNA-ratios (miR-4521/miR-340-5p and miR-101-3p/miR-451a) that were able to predict disease-free survival following neoadjuvant chemoradiotherapy and esophagectomy in patients with EAC [15]. MiRNAs are a class of small (~22 nt) noncoding RNAs that regulate the expression of target mRNAs at a posttranscriptional level and are implicated in various biological processes, such as embryonic development, cell differentiation, proliferation, apoptosis, and cancer development [16,17]. MiRNA biogenesis consists of a primary miRNA (pri-miRNA) undergoing cleavage in subsequently regulated steps by two enzymes to form a mature miRNA that is incorporated in an RNA-induced silencing complex (RISC), ultimately guiding it to its target mRNA to perform its regulator function [18]. Briefly, the pri-miRNA is cropped into a 55 to 80 nt stem-loop precursor miRNA (pre-miRNA) by Drosha and its cofactor DiGeorge syndrome chromosomal region 8 (DGR8) in the cell nucleus. Next, the pre-miRNA is exported to the cytoplasm by nuclear export protein (XPO5) and Dicer cleaves the pre-miRNA into a mature miRNA. The mature miRNA is then incorporated into the RISC complex, which will guide it to the complementary region of its targets. This process results either in the inhibition of mRNA translation, or in mRNA degradation, depending on the degree of complementarity between the miRNA and the 3′-UTR region of its target mRNA [16]. The crucial binding location for mRNA translational regulation resides in the mature miRNA sequence and, more accurately, within nucleotides 2–7 or 2–8 from the 5′end of the miRNA, called the seed region [18,19]. It is important to note that the average size of the human 3′-UTR is about 950 nt, while an efficient miRNA-binding site consists of 6–8 nt. As such, the 3′-UTR of a specific mRNA can include tandem target sequences for a specific miRNA as for many other miRNAs [20,21]. Since the major consequence of miRNA:mRNA pairing is the loss of protein expression, resulting from either decreased transcript levels or by translational repression, alterations in miRNA expression patterns impact on the expression of oncoproteins and tumor suppressor proteins, thereby influencing cancer risk and prognosis [20].
Given the diversity of pathways that are regulated by miRNAs, genetic polymorphisms in miRNAs, miRNA-processing machinery, and miRNA target sites are implicated in carcinogenesis. MiRNA-related single-nucleotide polymorphisms (miR-SNPs) are defined as SNPs that occur in miRNA genes, at miRNA-binding sites, and in the miRNA processing machinery. This type of SNPs can ultimately affect cancer risk, treatment efficacy and, consequently, patient prognosis by modulating both miRNAs and their targets [17]. In 2016, Nariman-Saleh-Fam and colleagues used a bioinformatics approach to provide a catalog of the most potentially disruptive EC-implicated miRNA targetome polymorphisms, along with in silico insight into the pathways affected by such variations [14]. Despite the importance of these findings, validation studies are still lacking.
MiR-SNPs seem to represent an indispensable pool of novel molecular biomarkers and have recently come into focus regarding their possible role in the development of cancer. Hence, the scope of this review is to gather and systematize the information available regarding the impact of miR-SNPs in EC development and prognosis.
2. Evidence Acquisition
A literature search in PubMed was conducted using the search terms “miRNA”, “polymorphisms”, “SNPs”, and “esophageal cancer”. The articles were selected by relevance of their findings, namely, the significant association of miR-SNPs and esophageal cancer. Literature analysis includes scientific papers published in the last ten years (between 2008 and 2018). Obtained scientific papers were manually curated in order to determine associations between miR-SNPs and EC. Of the 47 papers found, 17 were excluded. The exclusion criteria for the collected papers were as follows: (1) no association between miR-SNPs and EC; (2) association with a benign tumor; and (3) individual papers that were already included in meta-analysis, collected for this study. For each study, information was extracted concerning the following characteristics: the name of the miRNA, SNP rs number, SNP effect on esophageal cancer (e.g., cancer risk, prognosis, therapy response), ethnicity, type of study (e.g., case control, association study, meta-analysis) and number of cases and controls used. Since BE is an established risk for, and the only known precursor of, EAC, we included the miR-SNPs that were common to both BE and EAC in our study.
3. Evidence Synthesis
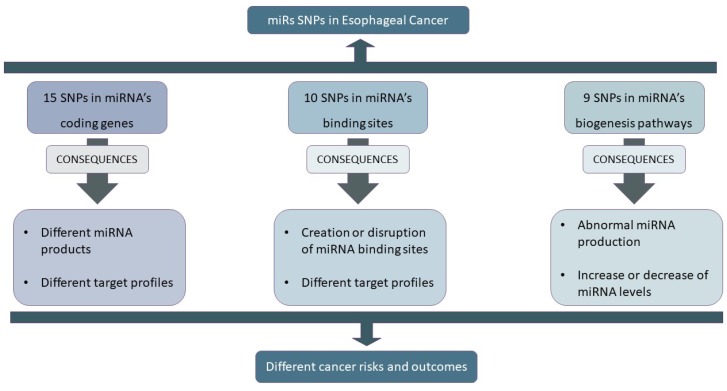
The pooled information is synthesized in Figure 1, where we divided the EC-relevant miR-SNPs into three categories: (1) SNPs in miRNA-coding genes, (2) SNPs in miRNA-binding sites, and (3) SNPs in biogenesis machinery.
Figure 1.
Overview of miRNA-related single-nucleotide polymorphisms (miR-SNPs) found and their impact on esophageal cancer (EC).
3.1. SNPs in miRNA Loci
MiRNA genes are scattered among each human chromosome. With the exception of chromosome Y, they can be encoded in independent transcription units, polycistronic clusters, or within the introns of protein-coding genes [22]. MiRNA profiling has revealed that most miRNAs are significantly downregulated in cancer [20]. Calin and colleagues mapped the chromosomal location of all known miRNA genes and discovered that many are located in regions that are frequently involved in chromosomal alterations, such as deletions or amplifications, usually found in many types of cancers [23]. Additionally, Lu and colleagues found that SNPs occur less frequently, but are more constrained in miRNAs associated with diseases when compared to the other miRNAs [24]. The majority of SNPs in miRNA-coding genes usually occur in pre-miRNAs and can be responsible for changes in stem-loop structures, consequently affecting the production of mature miRNAs [25]. Gong and colleagues performed a genome-wide scan in human pre-miRNAs, miRNA flanking regions, and target sites, and their results showed that approximately 40% of pre-miRNAs contain at least one polymorphism, and 48 SNPs were found in functionally important seed regions. The authors also observed that the SNP density of pre-miRNAs is lower than that of flanking regions, and SNP density of miRNA seed regions is significantly lower than in the pre-miRNAs and flanking regions [25]. Despite being quite rare in seed sequences, SNPs in these regions can lead to the creation or disruption of putative binding sites, consequently altering the total numbers of putative targets [26].
In this review, we found 22 studies that related a total of 13 SNPs in miRNA genes associated with an EC outcome (Table 1). The most studied SNPs were miR-423 rs6505162, miR-196a-2 rs1161491,3 and miR-146a rs2910164, and they were all located in pre-miRNA regions with impact in the respective mature miRNA production. The miR-423 rs6505162 SNP is located in pre-miR-423 and maps to 17q11.2, with a nucleotide alteration from C to A [27]. Since both miR-423-3p and miR-423-5p are produced by the pre-miRNA of miR-423, it is possible for the polymorphism of pre-miR-423 to play different roles in cancer progression, and in different types of cancer [27,28,29]. Despite the lack of studies about mature miR-423 in esophageal cancer, miRNA-423-5p expression was associated with cell proliferation and invasion in gastric cancer cells [30]. The miR-146a rs2910164 G > C SNP is located in the precursor stem-loop region, opposite to the mature miRNA-146a sequence and involves a change from a G:U pair to a C:U mismatch, with an impact on mature miRNA-146a levels [31]. MiR-146a expression was reported as dramatically decreased in ESCC tissue and it was associated with a worse prognosis [32]. The miR-196a-2 rs11614913 C > T SNP is located in the stem-loop region opposite to the mature miR-196a-2, and the nucleotide change from C to T was suggested to alter the levels of the mature 3′ passenger (3p) strand of miR-196a2 and the activity of its target mRNAs [31]. The mature miR-196a was reported as upregulated in ESCC and was suggested as a potential diagnosis biomarker and therapeutic target for this neoplasia [33,34].
Table 1.
Single Nucleotide Polymorphisms (SNPs) within miRNA-encoding genes and their association with Esophageal Cancer (EC) risk and outcome.
| Location | SNP | Population | Type of Study | Sample Size | Relevant Genotype |
Outcome | References |
|---|---|---|---|---|---|---|---|
| Pri-miR-124-1 | rs531564 | Kazach | Case controls | 239 cases/227 controls | CG+GG | ↓ ESCC risk | Wu et al. (2018) [35] |
| Pri-miR-124-1 | rs531564 | Canadian | Cohort | 368 cases | G allele | ↑ EAC OS | Faluyi et al. (2017) [36] |
| Pre-miR-423 | rs6505162 | Iranian | Case control | 200 cases/300 controls | AA | ↓ EC risk | Nariman-Saleh-Fam et al. (2016) [14] |
| Pre-miR-196a2 | rs11614913 | Chinese | Case control | 1400 cases/2185 controls | CC | ↑ ESCC risk | Shen et al. (2016) [37] |
| Pre-miR-499 | rs3746444 | C allele | |||||
|
Pre-miR-4467
Pre-miR-3117 |
rs12534337 rs7526812 |
Mixed ethnicity | Case control | 2515 EA cases 3295 BE cases 3207 controls |
A allele C allele |
↑ BE and ↑ EAC risk | Buas et al. (2015) [8] |
| Pri-miR-124-1 | rs531564 | Chinese | Meta analysis | 1738 cases/1961 controls | GG | ↓ ESCC risk | Li et al. (2015) [38] |
| Pre-miR-219-1 | rs107822 | Kazach | Case control | 248 cases/300 controls | AA/A allele | ↓ ESCC risk | Song et al. (2015) [39] |
| rs213210 | T allele | ||||||
| Pre-miR-100 | rs1834306 | Kazach | Case control | 248 cases/300 controls | CC/C allele | ↓ ESCC risk | Zhu et al. (2015) [40] |
| Pre-miR-499b | rs10061133 | Chinese | Case control | 773 cases/882 controls | GG | ↓ ESCC risk | Zhang et al. (2015) [41] |
| Pre-miR-4293 | rs12220909 | C allele | |||||
| Pre-miR-196a-2 | rs11614913 | Chinese | Case control | 381 cases/426 controls | TT | ↓ ESCC risk | Qu et al. (2014) [42] |
| Pre-miR-499 | rs3746444 | Mixed ethnicity | Meta analysis | 12799 cases/14507 controls | TC+CC | ↑ EC risk in the asian population | Chen et al. (2014) [43] |
| Pri-miR-34b/c | rs4938723 | Mixed ethnicity | Meta analysis | 7753 cases/8014 controls | CC | ↓ ESCC risk in the asian population | Li et al. (2014) [44] |
| Pre-miR-608 | rs4919510 | Taiwan | Cohort | 504 cases | GC | ↑ OS and ↑ PFS in ESCC | Yang et al. (2014) [45] |
| Pre-miR-146a | rs2910164 | Chinese | Cohort | 378 cases | CG+GG | ↑ risk of severe hematological toxicity in ESCC | Wu et al. (2014) [46] |
| Pre-miR-196a2 | rs11614913 | TT | ↓ OS ESCC | ||||
| Pre-miR-125a | rs12976445 | TT | ↓ OS ESCC | ||||
| Pre-miR-196a-2 | rs11614913 | Chinese | Case control | 597 cases/597 controls | CT+TT | ↑ ESCC risk | Wang et al. (2014) [47] |
| Pre-miR-146a | rs2910164 | Mixed ethnicity | Meta analysis | 790 cases/814 controls | GC+GG | ↑ EC risk in the asian population | Xu et al. (2014) [48] |
| Pre-miR-423 | rs6505162 | Chinese | Case control | 629 cases/686 controls | AA | ↑ ESCC risk | Yin et al. (2013) [49] |
| Pre-miR-423 | rs6505162 | Black ethnicity | Case control | 368 cases/583 controls | C allele | ↑ ESCC risk | Wang et al. (2013) [50] |
| 5 ′ -UTR miR-26a-1 | rs7372209 | Mixed ethnicity | 197 cases/420 controls | T allele | ↑ ESCC risk | ||
| Pre-miR-196a-2 | rs11614913 | Chinese | Case control | 380 cases/380 controls | CC | ↓ ESCC risk in women | Wei et al. (2013) [51] |
| Pre-miR-196a-2 | rs11614913 | Mixed ethnicity | Meta analysis | 4947 cases and 5642 controls | C allele | ↑ EC risk | Wang et al. (2013) [52] |
| Pre-miR-196a2 | rs11614913 | Indian | Meta analysis | 289 cases/309 controls | T allele | ↓ OS in ESCC | Umar et al. (2013) [53] |
| Pre-miR-146a | rs2910164 | C allele | |||||
| Pre-miR-499 | rs3746444 | C allele | |||||
| Pre-miR-423 | rs6505162 | A allele | |||||
| Pre-miR-146a | rs2910164 | Mixed ethnicity | Meta analysis | 772 cases/779 controls | C allele | ↓ EC risk in the asian population | He et al. (2012) [54] |
| Pre-miR-423 | rs6505162 | Caucasian | Case control | 346 cases/346 controls | AC+AA | ↓ EC risk | Ye et al. (2008) [55] |
EC: Esophageal Cancer; EAC: Esophageal Adenocarcinoma; ESCC: Esophageal squamous cell carcinoma; BE: Barrett Esophagus; OS: overall survival; PFS: progression-free survival; ↑: high; ↓: low.
3.2. SNPs in miRNA-Binding Sites
SNPs occurring in noncoding regions can affect transcriptional regulation or post-transcriptional gene expression, thereby affecting mRNA half life and resulting in altered protein levels trough the deregulation of miRNA–mRNA binding [56]. The most widely studied SNPs in noncoding regions are the SNPs located in the 3′-UTR of mRNAs, also known as poly-miRTSs [57]. Roughly 180,000 SNPs in the human genome that are located in the 3′-UTR region were identified, along with about 2600 mature miRNA sequences that are deposited in the mirBase (v.21), which suggests that these SNPs may introduce miRNA-binding changes [20]. Additionally, by performing a genome-wide scan, Gong and colleagues found a total of 98,008 possible functional SNPs in 3′-UTRs [25]. Despite the fact that the majority of the studies about polymorphisms in miRNA targets focus on the SNPs in 3′UTRs, it is important to note that some studies revealed that miRNAs could also bind to 5′ UTRs or coding sequences of target mRNAs, suggesting that these variants could also affect miRNA regulation [58,59]. Functional SNPs in the miRNA target regions are likely to alter gene expression via affecting miRNA targeting, namely, through the creation or disruption of miRNA-binding sites. In this review, we found eight studies relating nine different SNPs in miRNA targets (8 in 3′-UTR regions and 1 in a coding sequence) with association with EC risk and outcome (Table 2).
Table 2.
SNPs within miRNA-binding sites and their association with EC risk and outcome.
| miRNA Binding Site | SNP | Population | Type of Study | Sample Size | Relevant Genotype | Outcome | References |
|---|---|---|---|---|---|---|---|
| 3 ′ -UTR of KIAA0423 | rs1053667 | Canadian | Cohort | 368 cases | C allele | ↑ OS in EAC | Faluyi et al. (2017) [36] |
| 3 ′ -UTR of SET8 | rs16917496 | Chinese | Case control | 180 cases/142 controls | CC | ↑ OS and ↑ Post-surgery survival in ESCC | Wang et al. (2016) [60] |
| 3 ′ -UTR of BSG | rs11473 | Chinese | Case control | 624 cases/636 controls | TT/T allele | ↑ risk of ESCC | Li et al. (2016) [61] |
| 3 ′ -UTR of RDH8 | rs1644730 | Mixed ethnicity | Case control | 2515 EA cases 3295 BE cases 3207 controls |
A allele | ↓ BE and ↓ EA risk | Buas et al. (2015) [8] |
| 3 ′ -UTR of PTPRT | rs2866943 | Chinese | Case control | 790 cases/749 controls | CT/TT | ↓ risk of ESCC | Yao et al. (2015) [62] |
| rs6029959 | CC/AC | ↑ risk of ESCC | |||||
| 3 ′ -UTR of ErbB4 | rs1595066 | Chinese | Case control | 381 cases/426 controls | AA/A allele | ↓ risk of ESCC | Qu et al. (2014) [42] |
| Coding sequence of BRCA1 | rs799917 | Jinan | Case control | 540 cases/550 controls | CC | ↑ risk of ESCC | Zhang et al. (2013) [58] |
| Huaian | 588 cases/600 controls | ||||||
| 3 ′ -UTR of MDM4 | rs4245739 | Jinan | Case control | 540 cases/550 controls | AC+CC | ↓ risk of ESCC | Zhou et al. (2013) [63] |
| Huaian | 588 cases/600 controls | ||||||
| 3 ′ -UTR of RAP1A | rs6573 | Chinese | Case control | 537 cases and 608 controls | CC | ↑ risk of metastasis in ESCC | Wang et al. (2012) [64] |
In a study that evaluates the impact of SNP regulation of miRNA expression in colon-cancer risk, KIAA023 rs1053667 was found to be associated with differential expression of one of its target miRNAs, miR-19b-3p, in normal colon tissue when compared to tumor tissue [65]. Histone lysine methyltransferase (SET8) rs16917496 results in a C to T transition that might destroy the G:C bond in the miR-502 and SET8 binding site, therefore modulating SET8 expression. The C allele is associated with a perfect complementarity with miR-502, which will lead to efficient mRNA degradation and lower SET8 protein levels. In EC, lower SET8 expression was associated with a better prognosis and survival [17]. The Basigin (BSG) rs11473 consists of a C to T transition that destroys the binding site of miR-483-5p at the 3′-UTR of BSG, resulting in higher mRNA levels of this gene. In a study involving EC patients, those that carried the TT genotype expressed higher levels of BSG mRNA and protein, and consequent presents higher EC risk, compared with patients with the CC genotype carriers [61]. All-trans-retinol dehydrogenase 8 (RDH8) encodes for a short-chain dehydrogenase/reductase enzyme involved in rhodopsin regeneration in the vision pathway and its enzymatic activity has also been linked to estrogen biosynthesis. The rs1644730 is located in the 3′-UTR of RDH8 and its predicted miR-630 binding site [8]. Given the significantly higher incidence of EAC among males versus females, a potential protective effect of estrogen has been proposed, which is in agreement with the fact that patients carrying the rs1644730 A allele presented decreased EAC risk [8]. PTPRT is a tumor suppressor that plays a crucial role in regulating tumorigenesis mechanisms [62]. Two 3′-UTR SNPs, rs2866943 C>T and rs6029959 C>A, located in the binding sites of miR-218 and miR-142-5p, respectively, were studied in EC patients, but only rs2866943 was able to disrupt the inhibitory role of miR-218 on PRPT expression and act as a protective factor in ESCC risk. Patients carrying rs2866943 CT and TT genotypes presented a small tumor size as well as the low probability of metastasis [62]. The Erb-b2 receptor tyrosine kinase 4 (ErbB4) gene, also known as human epidermal growth factor receptor 4 (HER4), has been reported as overexpressed in EC tissue and is also associated with TMN stage and lymph-node metastasis [66]. ErbB4 rs1595066 creates a binding site for miR-200*, a member of miR-200 tumor-suppressor miRNAs, and is associated with a lower EC risk, probably through the downregulation of ErbB4 [42]. BRCA1 is a widely studied tumor suppressor gene and it is deregulated in several cancers. The rs799917 T>C polymorphism located in the BRCA1 coding sequence influences miR-638-mediated regulation of BRCA1 expression [58]. BRCA1 mRNA expression analyses showed that the rs799917 C allele carriers significantly decreased BRCA1 expression in both normal and cancer esophagus tissue compared with T allele carriers, suggesting that lower BRCA1 expression may lead to a higher risk of malignant transformation of esophagus cells [58]. MDM4 is an oncoprotein that negatively regulates p53 function. The rs4245739 A>C SNP, located in the MDM4 3′-UTR, creates a binding site for miR-191, resulting in decreased MDM4 expression [63]. Rs4245739 AC and CC genotype carriers significantly decreased MDM4 expression in normal esophagus tissue compared with AA genotype carriers, indicating consistent genotype–phenotype correlation [63]. The rs6573 SNP is a substitution from A to C, and disrupts the binding of miR-196a to RAP1A 3′-UTR, resulting in a higher constitutive expression of RAP1A, which is a member of the RAS oncogene family [64]. Wang and colleagues observed that RAP1A was overexpressed in ESCC tissue, and correlated with RAP1A rs6573 CC genotype and lymph-node metastasis. The authors also performed an in vitro study where they concluded that RAP1A might function as a promoter for esophageal cancer-cell migration and invasion through matrix metalloproteinase 2 [64].
3.3. SNPs in miRNA Processing Machinery
Although the role of miRNA’s biogenesis pathway genes in cancer development and its progression has been well established, the association between genetic variants of these pathway genes has been less studied. The occurrence of SNPs in the components of the miRNA biogenesis pathway can affect transcription, processing, transport and target gene identification, consequently affecting the overall expression of miRNAs. In this review, we found three studies relating SNPs in XPO5, Gem-associated protein 3 (GEMIN3), and Gem-associated protein 4 (GEMIN4) genes with EC risk outcome, and all of the SNPs reported were associated with a better prognosis (Table 3).
Table 3.
SNPs within miRNA binding sites and their association with EC risk and outcome.
| Gene | SNP | Population | Type of Study | Sample Size | Relevant Genotype | Outcome | References |
|---|---|---|---|---|---|---|---|
| XPO5 | rs11077 | Chinese | Cohort | 128 cases | AA | ↑ OS in ESCC | Wang et al. (2018) [17] |
| GEMIN 3 | rs197412 | Canadian | Cohort | 368 cases | C allele | ↑ OS EAC | Faluyi et al. (2017) [36] |
| GEMIN 4 | rs910924 | North American | Case control | 346 cases/346 controls | G allele | Haplotype associated with ↓ EC risk | Ye et al. (2008) [55] |
| rs2740348 | C allele | ||||||
| rs7813 | G allele | ||||||
| rs910925 | G allele | ||||||
| rs3744741 | C allele | ||||||
| rs1062923 | A allele | ||||||
| rs4968104 | T allele |
XPO5 is a key factor in this process, as it is responsible for the nuclear export of the pre-miRNA to the cytoplasm, where it is further processed to its final miRNA conformation in order to be loaded to RNA-induced silencing complex to exert its regulatory effect [67]. It has been postulated that XPO5 miRNA regulation can be a limiting step for miRNA development since its impairment can lead to pre-miRNA trapping in the nucleolus and therefore influence cancer risk [68]. The XPO5 rs11077 consists of an A to C transition that leads to the disruption of the miR-617 binding site and the creation of a new binding site for miR-4763-5p in XPO5 3′-UTR, with an impact on XPO5 mRNA levels [69]. In fact, patients’ carriers of rs11077 AA genotype displayed a trend for high XPO5 expression in ESCC tissues, and these high XPO5 expression levels were also associated with high survival rates [17]. GEMIN3 and GEMIN4 are members of the GEMIN protein family, and are part of the RNA-induced silencing complex (RISC) that participates in the target RNA recognition and repression by mature miRNAs. With the exception of GEMIN4 rs910924 that is located in a 5′-UTR, all the other studied GEMIN SNPs were missense variants, meaning that they resulted in different amino acid sequences that could impact GEMIN3 and GEMIN4 protein structure and consequent miRNA regulatory function [55,70].
4. Discussion
Major breakthroughs in the diagnosis and treatment of EC have been achieved during the past few decades. However, the selection criteria for operative management after chemoradiotherapy are still lacking, and patients that present a complete pathologic response are still submitted to surgery. Since miRNAs are important in carcinogenesis and are capable of regulating the expression of hundreds of target mRNAs, miR-SNPs may produce more significant functional consequences and represent an ideal candidate for disease prediction.
SNPs in miRNA and miRNA-binding sites can potentially modulate miRNA–mRNA interaction and potentially create or destroy miRNA-binding sites, while those in biogenesis pathway genes can influence miRNA transcription either through altering transcription, processing, or maturation. Due to their impact in cancer development, this new class of SNPs has been widely studied, mainly due to their potential applicability as disease biomarkers. Indeed, in this review we found a total of 34 miR-SNPs that were associated, in their majority, with EC risk and were mostly studied in Asian populations where the incidence of EC is higher. However, despite promise, the impact of these miR-SNPs requires further validation, especially in non-Asian populations where these types of studies are lacking, and the incidence of EC, especially EAC, will rise dramatically in the following years. Additionally, since EC is a type of cancer that involves multiple miR-SNPs with impact on the miRNA targetome, the candidate gene approach that considers one or few genes/SNPs at a time can give us the functional impact of that genetic variant in cancer risk or survival, but fails to relate that information with the molecular pathways involved. As such, more pathway-based approaches that evaluate the cumulative effect of multiple unfavorable genotypes on the miRNA targetome are needed to identify signatures of genetic variations capable of predicting EC therapy response and prognosis. Furthermore, it is also important to take into account that sometimes changes in a gene’s mRNA levels are not reflected in its protein levels. Hence, when studying SNPs that alter miRNA:mRNA binding capacity and, consequently, mRNA regulation, they should be accompanied by the monitorization of the protein levels. This type of approaches would shed some light on the fine regulatory mechanisms by which these variations contribute to EC pathogenesis instead of only focusing in cancer risk.
5. Conclusions
Despite the complexity of the functional effects of SNPs that occur in noncoding regions, which is the case of miR-SNPs, more attention has been paid to this recent class of genetic polymorphisms mainly due to their potential impact in cancer. In EC, the majority of the studies focuses on the association cancer risk and overall survival, but are lacking in terms of therapy response and prognosis. Despite the potential of miR-SNPs as biomarkers, more studies are needed, especially in non-Asian populations, in order to validate their application in the clinical practice.
Acknowledgments
We would like to thank to FCT—Fundação para a Ciência e Tecnologia (Portuguese Foundation for Science and Technology), Liga Portuguesa Contra o Cancro—Núcleo Regional do Norte—LPCC-NRN (Portuguese League Against Cancer-North Branch).
Author Contributions
Conceptualization, F.D.; methodology, F.D.; data curation, F.D. and M.M.; writing—original draft preparation, F.D. and M.M.; writing—review and editing, A.L.T.; Supervision, R.M.
Funding
This project was funded by the project NORTE-01-0145-FEDER-000027, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF) and F.D. is a PhD fellow from the same project. M.M. is a recipient of a research scholarship awarded by LPCC-NRN (Portuguese League Against Cancer—Northern Branch). A.L.T is a post-doctoral fellow from FCT (Portuguese Foundation for Science and Technology) (SFRH/BPD/111114/2015).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ohashi S., Miyamoto S., Kikuchi O., Goto T., Amanuma Y., Muto M. Recent Advances from Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2015;149:1700–1715. doi: 10.1053/j.gastro.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Schweigert M., Dubecz A., Stein H.J. Oesophageal cancer—An overview. Nat. Rev. Gastroenterol. Hepatol. 2013;10:230–244. doi: 10.1038/nrgastro.2012.236. [DOI] [PubMed] [Google Scholar]
- 4.Kamangar F., Dores G.M., Anderson W.F. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 5.Castro C., Bosetti C., Malvezzi M., Bertuccio P., Levi F., Negri E., La Vecchia C., Lunet N. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980–2011) and predictions to 2015. Ann. Oncol. 2014;25:283–290. doi: 10.1093/annonc/mdt486. [DOI] [PubMed] [Google Scholar]
- 6.Arnold M., Laversanne M., Brown L.M., Devesa S.S., Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am. J. Gastroenterol. 2017;112:1247–1255. doi: 10.1038/ajg.2017.155. [DOI] [PubMed] [Google Scholar]
- 7.Castro C., Peleteiro B., Bento M.J., Lunet N. Trends in gastric and esophageal cancer incidence in northern Portugal (1994-2009) by subsite and histology, and predictions for 2015. Tumori. 2017;103:155–163. doi: 10.5301/tj.5000542. [DOI] [PubMed] [Google Scholar]
- 8.Buas M.F., Onstad L., Levine D.M., Risch H.A., Chow W.-H., Liu G., Fitzgerald R.C., Bernstein L., Ye W., Bird N.C., et al. MiRNA-Related SNPs and Risk of Esophageal Adenocarcinoma and Barrett’s Esophagus: Post Genome-Wide Association Analysis in the BEACON Consortium. PLoS ONE. 2015;10:e0128617. doi: 10.1371/journal.pone.0128617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cariati A., Casano A., Campagna A., Cariati E., Pescio G. Prognostic factors influencing morbidity and mortality in esophageal carcinoma. Rev. Hosp. Clín. 2002;57:201–204. doi: 10.1590/S0041-87812002000500002. [DOI] [PubMed] [Google Scholar]
- 10.Cijs T.M., Verhoef C., Steyerberg E.W., Koppert L.B., Tran T.C.K., Wijnhoven B.P.L., Tilanus H.W., de Jonge J. Outcome of esophagectomy for cancer in elderly patients. Ann. Thorac. Surg. 2010;90:900–907. doi: 10.1016/j.athoracsur.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Liu B., Bo Y., Wang K., Liu Y., Tang X., Zhao Y., Zhao E., Yuan L. Concurrent neoadjuvant chemoradiotherapy could improve survival outcomes for patients with esophageal cancer: a meta-analysis based on random clinical trials. Oncotarget. 2017;8:20410–20417. doi: 10.18632/oncotarget.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hagen P., Hulshof M.C.C.M., van Lanschot J.J.B., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P.L., Richel D.J., Nieuwenhuijzen G.A.P., Hospers G.A.P., Bonenkamp J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 13.x2019 Sullivan K.E., Hurley E.T., Hurley J.P. Understanding Complete Pathologic Response in Oesophageal Cancer: Implications for Management and Survival. Gastroenterol. Res. Pract. 2015;2015:e9. doi: 10.1155/2015/518281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nariman-Saleh-Fam Z., Bastami M., Somi M.H., Samadi N., Abbaszadegan M.R., Behjati F., Ghaedi H., Tavakkoly-Bazzaz J., Masotti A. In silico dissection of miRNA targetome polymorphisms and their role in regulating miRNA-mediated gene expression in esophageal cancer. Cell Biochem. Biophys. 2016;74:483–497. doi: 10.1007/s12013-016-0754-5. [DOI] [PubMed] [Google Scholar]
- 15.Chiam K., Mayne G.C., Watson D.I., Woodman R.J., Bright T.F., Michael M.Z., Karapetis C.S., Irvine T., Phillips W.A., Hummel R., et al. Identification of microRNA Biomarkers of Response to Neoadjuvant Chemoradiotherapy in Esophageal Adenocarcinoma Using Next Generation Sequencing. Ann. Surg. Oncol. 2018;25:2731–2738. doi: 10.1245/s10434-018-6626-z. [DOI] [PubMed] [Google Scholar]
- 16.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 17.Wang C., Dong H., Fan H., Wu J., Wang G. Genetic polymorphisms of microRNA machinery genes predict overall survival of esophageal squamous carcinoma. J. Clin. Lab. Anal. 2018;32:e22170. doi: 10.1002/jcla.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pipan V., Zorc M., Kunej T. MicroRNA Polymorphisms in Cancer: A Literature Analysis. Cancers. 2015;7:1806–1814. doi: 10.3390/cancers7030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun G., Yan J., Noltner K., Feng J., Li H., Sarkis D.A., Sommer S.S., Rossi J.J. SNPs in human miRNA genes affect biogenesis and function. RNA. 2009;15:1640–1651. doi: 10.1261/rna.1560209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moszyńska A., Gebert M., Collawn J.F., Bartoszewski R. SNPs in microRNA target sites and their potential role in human disease. Open Biol. 2017;7:e170019. doi: 10.1098/rsob.170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood P., Krek A., Zavolan M., Macino G., Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc. Natl. Acad. Sci. USA. 2006;103:2746–2751. doi: 10.1073/pnas.0511045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez A., Griffiths-Jones S., Ashurst J.L., Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calin G.A., Sevignani C., Dumitru C.D., Hyslop T., Noch E., Yendamuri S., Shimizu M., Rattan S., Bullrich F., Negrini M., et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu M., Zhang Q., Deng M., Miao J., Guo Y., Gao W., Cui Q. An analysis of human microRNA and disease associations. PLoS ONE. 2008;3:e3420. doi: 10.1371/journal.pone.0003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong J., Tong Y., Zhang H.-M., Wang K., Hu T., Shan G., Sun J., Guo A.-Y. Genome-wide identification of SNPs in microRNA genes and the SNP effects on microRNA target binding and biogenesis. Hum. Mutat. 2012;33:254–263. doi: 10.1002/humu.21641. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y., Lee C.G.L. Single Nucleotide Polymorphisms Associated with MicroRNA Regulation. Biomolecules. 2013;3:287–302. doi: 10.3390/biom3020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia W., Zeng L., Luo S., Bai F., Zhong R., Wu L., Huang G.-L., Pu X. Association of microRNA-423 rs6505162 C>A polymorphism with susceptibility and metastasis of colorectal carcinoma. Medicine (Baltimore) 2018;97:e9846. doi: 10.1097/MD.0000000000009846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong P., Zhu X., Geng Q., Xia L., Sun X., Chen Y., Li W., Zhou Z., Zhan Y., Xu D. The microRNA-423-3p-Bim Axis Promotes Cancer Progression and Activates Oncogenic Autophagy in Gastric Cancer. Mol. Ther. 2017;25:1027–1037. doi: 10.1016/j.ymthe.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang X., Zeng X., Huang Y., Chen S., Lin F., Yang G., Yang N. miR-423-5p serves as a diagnostic indicator and inhibits the proliferation and invasion of ovarian cancer. Exp. Ther. Med. 2018;15:4723–4730. doi: 10.3892/etm.2018.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Wang X., Yang X., Liu Y., Shi Y., Ren J., Guleng B. miRNA423-5p regulates cell proliferation and invasion by targeting trefoil factor 1 in gastric cancer cells. Cancer Lett. 2014;347:98–104. doi: 10.1016/j.canlet.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Liu X., Han Z., Yang C. Associations of microRNA single nucleotide polymorphisms and disease risk and pathophysiology. Clin. Genet. 2017;92:235–242. doi: 10.1111/cge.12950. [DOI] [PubMed] [Google Scholar]
- 32.Wang C., Guan S., Liu F., Chen X., Han L., Wang D., Nesa E.U., Wang X., Bao C., Wang N., et al. Prognostic and diagnostic potential of miR-146a in oesophageal squamous cell carcinoma. Br. J. Cancer. 2016;114:e290. doi: 10.1038/bjc.2015.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fendereski M., Zia M.F., Shafiee M., Safari F., Saneie M.H., Tavassoli M. MicroRNA-196a as a Potential Diagnostic Biomarker for Esophageal Squamous Cell Carcinoma. Cancer Investig. 2017;35:78–84. doi: 10.1080/07357907.2016.1254228. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y., Wang B., Guo Y., Zhang Y., Huang S., Bao X., Bai M. Inhibition of miR-196a affects esophageal cancer cell growth in vitro. Biomed. Pharmacother. 2016;84:22–27. doi: 10.1016/j.biopha.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Wu F., Li M., You W., Ji Y., Cui X., Hu J., Chen Y., Pang L., Li S., Wei Y., et al. A Genetic Variant in miR-124 Decreased the Susceptibility to Esophageal Squamous Cell Carcinoma in a Chinese Kazakh Population. Genet. Test Mol. Biomark. 2018;22:29–34. doi: 10.1089/gtmb.2017.0115. [DOI] [PubMed] [Google Scholar]
- 36.Faluyi O.O., Eng L., Qiu X., Che J., Zhang Q., Cheng D., Ying N., Tse A., Kuang Q., Dodbiba L., et al. Validation of microRNA pathway polymorphisms in esophageal adenocarcinoma survival. Cancer Med. 2017;6:361–373. doi: 10.1002/cam4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen F., Chen J., Guo S., Zhou Y., Zheng Y., Yang Y., Zhang J., Wang X., Wang C., Zhao D., et al. Genetic variants in miR-196a2 and miR-499 are associated with susceptibility to esophageal squamous cell carcinoma in Chinese Han population. Tumor Biol. 2016;37:4777–4784. doi: 10.1007/s13277-015-4268-3. [DOI] [PubMed] [Google Scholar]
- 38.Li W.-J., Wang Y., Gong Y., Tu C., Feng T.-B., Qi C.-J. MicroRNA-124 rs531564 Polymorphism and Cancer Risk: A Meta-analysis. Asian Pac. J. Cancer Prev. 2015;16:7905–7909. doi: 10.7314/APJCP.2015.16.17.7905. [DOI] [PubMed] [Google Scholar]
- 39.Song X., You W., Zhu J., Cui X., Hu J., Chen Y., Liu W., Wang L., Li S., Wei Y., et al. A Genetic Variant in miRNA-219-1 Is Associated with Risk of Esophageal Squamous Cell Carcinoma in Chinese Kazakhs. Dis. Mark. 2015;2015:e541531. doi: 10.1155/2015/541531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J., Yang L., You W., Cui X., Chen Y., Hu J., Liu W., Li S., Song X., Wei Y., et al. Genetic variation in miR-100 rs1834306 is associated with decreased risk for esophageal squamous cell carcinoma in Kazakh patients in northwest China. Int. J. Clin. Exp. Pathol. 2015;8:7332–7340. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P., Wang J., Lu T., Wang X., Zheng Y., Guo S., Yang Y., Wang M., Kolluri V.K., Qiu L., et al. miR-449b rs10061133 and miR-4293 rs12220909 polymorphisms are associated with decreased esophageal squamous cell carcinoma in a Chinese population. Tumor Biol. 2015;36:8789–8795. doi: 10.1007/s13277-015-3422-2. [DOI] [PubMed] [Google Scholar]
- 42.Qu Y., Qu H., Luo M., Wang P., Song C., Wang K., Zhang J., Dai L. MicroRNAs related polymorphisms and genetic susceptibility to esophageal squamous cell carcinoma. Mol. Genet. Genom. 2014;289:1123–1130. doi: 10.1007/s00438-014-0873-x. [DOI] [PubMed] [Google Scholar]
- 43.Chen C., Yang S., Chaugai S., Wang Y., Wang D.W. Meta-analysis of Hsa-mir-499 polymorphism (rs3746444) for cancer risk: evidence from 31 case-control studies. BMC Med. Genet. 2014;15:e126. doi: 10.1186/s12881-014-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Wang L., Yu J., Xu J., Du J. The genetic association between pri-miR-34b/c polymorphism (rs4938723 T > C) and susceptibility to cancers: evidence from published studies. Tumor Biol. 2014;35:12525–12534. doi: 10.1007/s13277-014-2572-y. [DOI] [PubMed] [Google Scholar]
- 45.Yang P.-W., Huang Y.-C., Hsieh C.-Y., Hua K.-T., Huang Y.-T., Chiang T.-H., Chen J.-S., Huang P.-M., Hsu H.-H., Kuo S.-W., et al. Association of miRNA-related genetic polymorphisms and prognosis in patients with esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2014;21:601–609. doi: 10.1245/s10434-014-3709-3. [DOI] [PubMed] [Google Scholar]
- 46.Wu C., Li M., Hu C., Duan H. Prognostic role of microRNA polymorphisms in patients with advanced esophageal squamous cell carcinoma receiving platinum-based chemotherapy. Cancer Chemother. Pharmacol. 2014;73:335–341. doi: 10.1007/s00280-013-2364-x. [DOI] [PubMed] [Google Scholar]
- 47.Wang N., Li Y., Zhou R.-M., Wang G.-Y., Wang C.-M., Chen Z.-F., Liu W. Hsa-miR-196a2 functional SNP is associated with the risk of ESCC in individuals under 60 years old. Biomarkers. 2014;19:43–48. doi: 10.3109/1354750X.2013.866164. [DOI] [PubMed] [Google Scholar]
- 48.Xu X., Yang X., Ru G., Wu Y., Zhang S., Xing C., Wu Y., Cao J. miR-146a gene polymorphism rs2910164 and the risk of digestive tumors: A meta-analysis of 21 case-control studies. Oncol. Rep. 2014;31:472–479. doi: 10.3892/or.2013.2854. [DOI] [PubMed] [Google Scholar]
- 49.Yin J., Wang X., Zheng L., Shi Y., Wang L., Shao A., Tang W., Ding G., Liu C., Liu R., et al. Hsa-miR-34b/c rs4938723 T>C and hsa-miR-423 rs6505162 C>A polymorphisms are associated with the risk of esophageal cancer in a Chinese population. PLoS ONE. 2013;8:e80570. doi: 10.1371/journal.pone.0080570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Vogelsang M., Schafer G., Matejcic M., Parker M.I. MicroRNA polymorphisms and environmental smoke exposure as risk factors for oesophageal squamous cell carcinoma. PLoS ONE. 2013;8:e78520. doi: 10.1371/journal.pone.0078520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei J., Zheng L., Liu S., Yin J., Wang L., Wang X., Shi Y., Shao A., Tang W., Ding G., et al. MiR-196a2 rs11614913 T > C polymorphism and risk of esophageal cancer in a Chinese population. Hum. Immunol. 2013;74:1199–1205. doi: 10.1016/j.humimm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Wang F., Sun G.-P., Zou Y.-F., Fan L.-L., Song B. Quantitative assessment of the association between miR-196a2 rs11614913 polymorphism and gastrointestinal cancer risk. Mol. Biol. Rep. 2013;40:109–116. doi: 10.1007/s11033-012-2039-4. [DOI] [PubMed] [Google Scholar]
- 53.Umar M., Upadhyay R., Prakash G., Kumar S., Ghoshal U.C., Mittal B. Evaluation of common genetic variants in pre-microRNA in susceptibility and prognosis of esophageal cancer. Mol. Carcinog. 2013;52:10–18. doi: 10.1002/mc.21931. [DOI] [PubMed] [Google Scholar]
- 54.He B., Pan Y., Cho W.C., Xu Y., Gu L., Nie Z., Chen L., Song G., Gao T., Li R., et al. The association between four genetic variants in microRNAs (rs11614913, rs2910164, rs3746444, rs2292832) and cancer risk: evidence from published studies. PLoS ONE. 2012;7:e49032. doi: 10.1371/journal.pone.0049032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye Y., Wang K.K., Gu J., Yang H., Lin J., Ajani J.A., Wu X. Genetic variations in microRNA-related genes are novel susceptibility loci for esophageal cancer risk. Cancer Prev. Res. 2008;1:460–469. doi: 10.1158/1940-6207.CAPR-08-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hudson T.J. Wanted: regulatory SNPs. Nat. Genet. 2003;33:439–440. doi: 10.1038/ng0403-439. [DOI] [PubMed] [Google Scholar]
- 57.Sethupathy P., Collins F.S. MicroRNA target site polymorphisms and human disease. Trends Genet. 2008;24:489–497. doi: 10.1016/j.tig.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X., Wei J., Zhou L., Zhou C., Shi J., Yuan Q., Yang M., Lin D. A functional BRCA1 coding sequence genetic variant contributes to risk of esophageal squamous cell carcinoma. Carcinogenesis. 2013;34:2309–2313. doi: 10.1093/carcin/bgt213. [DOI] [PubMed] [Google Scholar]
- 59.Lee I., Ajay S.S., Yook J.I., Kim H.S., Hong S.H., Kim N.H., Dhanasekaran S.M., Chinnaiyan A.M., Athey B.D. New class of microRNA targets containing simultaneous 5′-UTR and 3-′UTR interaction sites. Genome Res. 2009;19:1175–1183. doi: 10.1101/gr.089367.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C., Wu J., Zhao Y., Guo Z. miR-502 medaited histone methyltransferase SET8 expression is associated with outcome of esophageal squamous cell carcinoma. Sci. Rep. 2016;6:e32921. doi: 10.1038/srep32921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H.Y., Liu Y.C., Bai Y.H., Sun M., Wang L., Zhang X.B., Cai B. SNP at miR-483-5p-binding site in the 3’-untranslated region of the BSG gene is associated with susceptibility to esophageal cancer in a Chinese population. Genet. Mol. Res. 2016;15:e15027735. doi: 10.4238/gmr.15027735. [DOI] [PubMed] [Google Scholar]
- 62.Yao Y., Shao J., Wu J., Zhang Q., Wang J., Xiao D., Huang F. The Functional Variant in the 3’UTR of PTPRT with the Risk of Esophageal Squamous Cell Carcinoma in a Chinese Population. Cell Physiol. Biochem. 2015;36:306–314. doi: 10.1159/000374073. [DOI] [PubMed] [Google Scholar]
- 63.Zhou L., Zhang X., Li Z., Zhou C., Li M., Tang X., Lu C., Li H., Yuan Q., Yang M. Association of a genetic variation in a miR-191 binding site in MDM4 with risk of esophageal squamous cell carcinoma. PLoS ONE. 2013;8:e64331. doi: 10.1371/journal.pone.0064331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang K., Li J., Guo H., Xu X., Xiong G., Guan X., Liu B., Li J., Chen X., Yang K., Bai Y. MiR-196a binding-site SNP regulates RAP1A expression contributing to esophageal squamous cell carcinoma risk and metastasis. Carcinogenesis. 2012;33:2147–2154. doi: 10.1093/carcin/bgs259. [DOI] [PubMed] [Google Scholar]
- 65.Mullany L.E., Wolff R.K., Herrick J.S., Buas M.F., Slattery M.L. SNP Regulation of microRNA Expression and Subsequent Colon Cancer Risk. PLoS ONE. 2015;10:e0143894. doi: 10.1371/journal.pone.0143894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.WeiGang G., Di G., XuGuang P., Dong X., YingYong H. Expression of HER4 in esophageal carcinoma tissues and its clinical significance. Tumor. 2009;29:673–676. [Google Scholar]
- 67.Patrao A.S., Dias F., Teixeira A.L., Maurício J., Medeiros R. XPO5 genetic polymorphisms in cancer risk and prognosis. Pharmacogenomics. 2018;19:799–808. doi: 10.2217/pgs-2018-0018. [DOI] [PubMed] [Google Scholar]
- 68.Wu K., He J., Pu W., Peng Y. The Role of Exportin-5 in MicroRNA Biogenesis and Cancer. Genom. Proteom. Bioinform. 2018;16:120–126. doi: 10.1016/j.gpb.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Larrea C.F., Navarro A., Tejero R., Tovar N., Diaz T., Cibeira M.T., Rosinol L., Ferrer G., Rovira M., Rozman M., et al. Impact of MiRSNPs on survival and progression in patients with multiple myeloma undergoing autologous stem cell transplantation. Clin. Cancer Res. 2012;18:3697–3704. doi: 10.1158/1078-0432.CCR-12-0191. [DOI] [PubMed] [Google Scholar]
- 70.Zhu W., Zhao J., He J., Qi D., Wang L., Ma X., Liu P. Genetic variants in the MicroRNA biosynthetic pathway Gemin3 and Gemin4 are associated with a risk of cancer: a meta-analysis. PeerJ. 2016;4:e1724. doi: 10.7717/peerj.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]