Abstract
In this study, the performance of a laboratory scale upflow anaerobic sludge blanket (UASB) reactor operating at mesophilic temperature (35 °C) was examined. Cattle slaughterhouse wastewater (CSWW) was used as the main substrate. The total and effective volumes of the reactor were 8 L and 6 L, respectively. Twelve different organic loading rates (OLR) were applied and the performance was evaluated. The chemical oxygen demand (COD) removal efficiency was more than 90% during batch study. In the continuous study, COD removal was also approximately 90% at OLR 0.4 g/L d−1 which subsequently dropped to below 50% when the loading rate increased to 15 g/L d−1. Approximately 5 L/d of biogas was obtained with high methane concentration at stages VI and XI corresponding to OLR of 2 and 10 g/L d−1, respectively. It was observed that the concentration of volatile fatty acids was low and that the alkalinity of the wastewater was sufficient to avoid acidification. Specific methane yields of 0.36 and 0.38 LCH4/g COD added were achieved at OLR 7 and 10 g/L d−1. A hydraulic retention time (HRT) of 1 day was sufficient to remove greater than 70% of COD which correspond to 89% methane concentration. Parameters like soluble COD, NH3-N, pH, alkalinity, total suspended solid (TSS), fats, oil, and grease were also investigated. The results show that the UASB reactor could serve as a good alternative for anaerobic treatment of CSWW and methane production.
Keywords: UASB reactor, cattle slaughterhouse wastewater, biogas production, methane yield, COD removal
1. Introduction
Recently, the use of biodegradable waste materials to generate energy in the form of biogas has gained more popularity due to climate change [1,2]. Also, laws and regulations imposed on industrial activities regarding effluent quality standard discharge limits are now more stringent than ever before. Cattle slaughterhouses are among the many food industries that consume a considerable amount of freshwater and generate large volumes of wastewater rich in organic contaminants and nutrients [3]. The water produced during and after the operation is considered as high-strength wastewater, because of the presence of protein, fats, oil, and grease (FOG) with a high tendency for rapid acidification. The chemical oxygen demand (COD) and biological oxygen demand (BOD) are normally found to be tens of thousands mg/L [4]. This range varies from 18,000 mg/L to 43,000 mg/L [5], but the strength differs from one industry to another depending on the number of animals slaughtered. The chemicals and detergents used for cleaning of the abattoir facilities also influence the concentration of COD. The presence of FOGs in CSWW is recognized as one major component that largely contributes to the BOD and COD which portrays waste as a potential source of biogas production [6]. Also, its recalcitrant nature could result in numerous problems such as foul odor, clogging of pipes, bacterial cell surface adhesion, and scum layer formation. These challenges could affect smooth biogas transition within the reactor [7].
Discharge of wastewater without proper treatment constitutes an environmental problem to both flora and fauna. The United State Environmental Protection Agency (US EPA, 2004) [8] has classified slaughterhouse wastewater as one of the most harmful waste substances to be released to the environment. Furthermore, untreated discharge of wastewater to the receiving water body could inhibit light penetration to aquatic animals and encourage eutrophication as well as unpleasant aesthetic value [9]. Table 1 presents the prominent characteristics of meat processing wastewater effluent and its limits from various jurisdictions worldwide.
Table 1.
Comparison of standard limits for slaughterhouse wastewater discharge from different jurisdictions worldwide [10].
| Parameter | World Bank | EU | USA | Canada | Colombia | China | India | Australia |
|---|---|---|---|---|---|---|---|---|
| BOD5 (mg/L) | 30 | 25 | 16–26 | 5–30 | 50 | 20–100 | 30–100 | 5–20 |
| COD (mg/L) | 125 | 125 | n.a. | n.a. | 150 | 100–300 | 250 | 40 |
| TN (mg/L) | 10 | 10–15 | 4–8 | 1.25 | 10 | 15–20 | 10–50 | 10–20 |
| TOC (mg/L) | n.a. | n.a. | n.a. | n.a. | n.a. | 20–60 | n.a. | 10 |
| TP (mg/L) | 2 | 1–2 | n.a. | 1.00 | n.a. | 0.1–1.0 | 5 F | 2 |
| TSS (mg/L) | 50 | 35–36 | 20–30 | 5–30 | 50 | 20–30 | 100 | 5–20 |
| pH | 6–9 | n.a. | 6–9 | 6–9 | 6–9 | 6–9 | 5.5–9 | 5–9 |
| Temperature (°C change) | n.a. | n.a. | n.a. | <1 °C | n.a. | n.a. | <5 °C | <2 °C |
Conventional treatment of slaughterhouse wastewater include physicochemical (primary) which is the separation of floating and settleable solids typically by sedimentation or coagulation/flocculation. Secondly, the biological treatment (secondary) is system where lagoons and activated sludge are commonly utilized. Lastly, the advanced oxidation ditches (tertiary) are systems that require high aeration (oxygen) to remove nitrogen (N2) and phosphorus (P) [11,12]. These methods are used to produce excellent effluent quality; however, the general drawbacks include large space requirement, high cost of aeration, huge volume of sludge generation with offensive odor, and capital intensive [13]. Moreover, they lack focus on energy recovery, instead these processes form part of the pathways where potential greenhouse gases such as methane (CH4) and carbon dioxide (CO2) escape to the atmosphere [14].
Therefore, a reliable, cost-effective and efficient treatment process is required to remove wide range of pollutants and recover energy. Water ready for discharge or reuse into the environment should not only be free from organic or mineral contaminants, but should also take into consideration the presence of bacteria, pathogens, and viruses. Otherwise, the standards set by the various environmental regulatory bodies could be violated. In an attempt to meet the stringent water quality regulations, several techniques including physical, chemical, and biological methods of wastewater treatments have been used over time. But, the biological method through the anaerobic digestion process appears to be a promising technique owing to less sludge production, better energy recovery and the overall low cost of application [13,14,15]. Basically, there are four diverse groups of microorganism responsible for the degradation process. All of the four metabolic reaction stages usually occur in the absence of oxygen. These include hydrolysis, acidogenesis, acetogenesis, and methanogenisis [16]. Besides biogas production, other byproducts like liquid or solid residue are also produced, which are applied to the soil as fertilizer or amendment [17]. Under strict anaerobic condition, a typical biogas consists of methane (CH4) and carbon dioxide (CO2). It may also contain significant amount of undesirable traces of compounds like hydrogen sulfide (H2S) and ammonia (NH3) [18]. Most of the organic fraction of slaughterhouse contained large amount of lipids that consist of triglycerides and long chain fatty acids (LCFA). However, compounds such as triglyceride easily hydrolyze to form LCFA and glycerol. Inhibition of the anaerobic digestion process may be due to high accumulation of LCFA. It could affect the normal function of acetogenic and methanogenic bacteria as a result of its toxicity [19]. Moreover, adsorption of the surface active acids on to the cell wall may also be part of the mechanisms responsible for anaerobic digestion inhibition during transportation and protection processes [20].
Wastewater produced from meat processing activities is highly concentrated in terms of COD, BOD, and FOG. Also, treatment of CSWW can be as energy intensive as municipal wastewater [21]. Therefore, the goal of this work is to evaluate the efficiency of an upflow anaerobic sludge blanket reactor for treatment of CSWW and biogas production at different organic loading rates.
2. Materials and Methods
2.1. Substrate
The cattle slaughterhouse wastewater sample was collected directly from the discharge point of the abattoir in Selangor, Malaysia. This slaughter house is the biggest in the state of Selangor. Specimens were collected in three containers, each of 25 L volume. They were immediately transported to a public health laboratory situated at the Faculty of Engineering, Universiti Putra Malaysia. Prior to use, the content of the three containers were mixed homogeneously and screened to remove particles larger than 5 mm. Tests such as pH, COD, BOD5, and ammonia nitrogen were immediately carried out after mixing. The sample was then stored in a refrigerator at 4 °C for further use. Table 2 shows the characteristics of the sample used for the experiment.
Table 2.
Characterization of raw cattle slaughterhouse wastewater (CSWW) used in this study.
| Parameter | Unit | Average Value |
|---|---|---|
| BOD5 | mg/L | 6345 ± 86 |
| COD | mg/L | 14,720 ± 312 |
| SCOD | mg/L | 767 ± 121 |
| TS | mg/L | 7800 ± 86 |
| TSS | mg/L | 1955 ± 65 |
| TDS | mg/L | 635 ± 23 |
| VSS | mg/L | 1320 ± 32 |
| pH | mg/L | 6.66 ± 1 |
| FOG | mg/L | 205.6 ± 12 |
| NH3-N | mg/L | 69 ± 2 |
| TN | mg/L | 780 ± 16 |
| Color | Pt Co | 500 ± 5 |
| Turbidity | FAU | 576 ± 11 |
| Temperature | °C | 27.2 ± 2 |
| Alkalinity | mg/L as CaCO3 | 485 ± 14 |
2.2. Inoculum
The seed sludge used in the UASB reactor was collected from the Faculty of Engineering wastewater treatment plant at Universiti Putra Malaysia (UPM). It contained a black mixture of fine particles with relatively small size granules. After three replicates, the total chemical oxygen demand (COD), soluble chemical oxygen demand (SCOD), total suspended solids (TSS), and volatile suspended solid (VSS) achieved were 0.24, 0.158, 11.24, and 9.87 g/L, respectively. These parameters were determined based on the procedures given in the standard methods for the examination of water and wastewater of the American Public Health Association Manual [22].
2.3. Analytical Methods
The performance evaluation of the UASB reactor was carried out regularly at an interval of 2 days. Parameters such as total COD, alkalinity, total solid (TS), total, FOG, and volatile suspended solid (VSS) were determined using standard methods for the examination of water and wastewater by the American Public Health Association (APHA) [22]. Other parameters such as TSS and SCOD were carried out after filtration using a 0.47 μm filter on the vacuum pump. Other parameters like ammonia nitrogen (NH3-N), color, and turbidity concentrations were measured using multiparameter portable calorimeter HACH system (DR 900). The methods used for each of these parameters were; Spectrophotometer (Salicylate powder pillow method 8155) for NH3-N, Platinum-Cobalt Standard Method 8025 for color, while the Absorptometric method 8237 was used for the turbidity measurement. A pH meter (PHS-3C, INESA, Shanghai, China) was used to determine the pH of the system. The volume of biogas produced was measured by the water displacement method. In addition, the biogas composition analyses were examined using a gas chromatograph (HP 6890 N) (Agilent, Santa Clara, CA 95051, USA) equipped with a thermal conductivity detector (TCD) (Agilent, Santa Clara, CA 95051, USA). The column was HP Molesieve (Agilent Technologies, Santa Clara, CA, USA) of 30 m length × 0.5 mm ID × 40 μm film thickness capillary column. The split less inlet, oven, and TCD detector temperatures were all kept at 60 °C, 70 °C, and 200 °C, respectively. Argon functioned as the carrier gas while nitrogen was used as the makeup gas. The peaks of samples were identified and the concentration was calculated based on the retention time and peak area of known standards (Sigma Chemical, St. Louis, MO, USA).
2.4. Biochemical Methane Potential Test (BMP)
In order to determine the maximum volume of methane that can be produced from a specific substrate a biochemical methane potential test was performed following the procedure reported by Angelidaki et al. [23]. However, BMP may also be used to estimate the fraction of the substrate that can be converted to methane. The tests were carried out in (duplicates) to achieve reliable results. Two scotch bottles of 1000 mL containing 450 mL inoculum (sludge) and substrate of equal volume of inoculum were filled up to 900 mL, leaving 100 mL for biogas head space. The substrate biomass to inoculum ratio was kept at ratio of 1:1. Additionally, two blank bottles containing 450 mL inoculum topped up to 900 mL with tap water were used as controls. All bottles were flushed with 100% nitrogen gas (N2) for 1 min to avoid the presence of air in the head space before closing with the rubber stopper. The digester was then incubated in a water bath at 35 °C with the water level at the bottle neck. Each digester was shaken every day for 1 min in the water bath until the biogas production cease after eleven (11) days. The biogas production was observed by the water displacement method using a graduated measuring cylinder. The methane content was analyzed by gas chromatography and calculated by subtracting the corresponding values for control and substrate runs. Other physicochemical parameters such COD, TSS, VSS, and NH3-N were also examined before and after the BMP test.
2.5. Experimental Process Flow
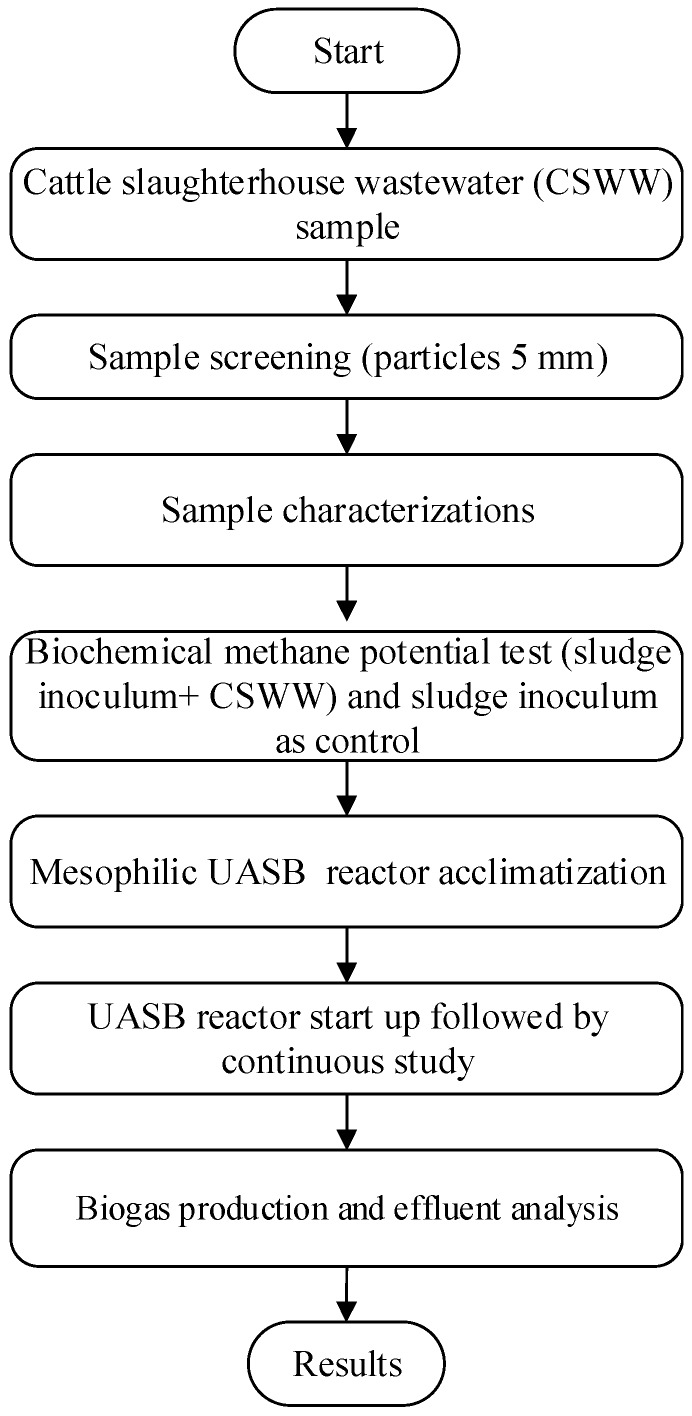
Figure 1 shows the process flowchart of the experimental procedure of the anaerobic digestion of CSWW conducted following steps described in Guimarães et al. [24]. An effluent characteristic for both batch and continuous studies was evaluated in triplicates and average values were considered.
Figure 1.
Flowchart of the experimental phases that was used in the study.
2.6. UASB Reactor Set-Up
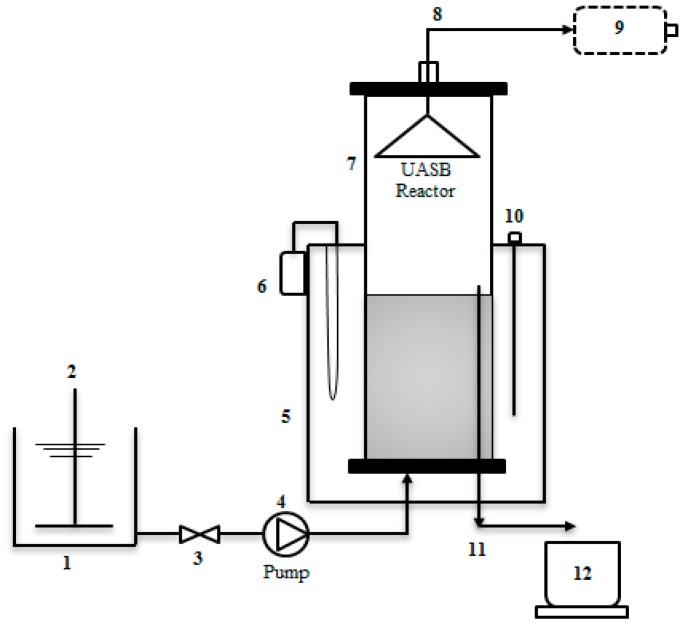
Figure 2 shows the laboratory scale of a 15 cm diameter and 60 cm height PVC column UASB reactor setup with total and effective volumes of 8 L and 6 L, respectively. The effective volume occupies 75% of the volume from the bottom. The sludge granular formations at the bottom have better settling properties and do not wash out easily. In addition, this allows for a higher OLR potential of the reactor. The formation of well-stabilized granular properties for a long period of time enhances the biomass retention within the reactor [25]. Feeding of the reactor was done from the bottom of the system through a blanket of biologically activated sludge, which is largely in the form of granular aggregates. This was done to provide sufficient contact between the CSWW (influent) and sludge blanket-containing microbes. The upflow velocity exhibits less effect on the movement of microbes, as the feeding was done at an interval of time over 24 h. This strategy would drastically increase biogas flow and reduce the chances of scum formation that usually prevents the steady flow of biogas out of the reactor.
Figure 2.
Schematic diagram of upflow anaerobic sludge blanket (UASB) reactor used in the study. Legend: 1—Influent; 2—Stirrer; 3—open and close Valve; 4—Peristaltic pump; 5—Water bath; 6—Heating element; 7—PVC column reactor; 8—Biogas outlet; 9—Tedlar bag; 10—Thermometer; 11—Effluent outlet; 12—Collection tank.
The reactor start-up commenced by acclimatizing the environment (UASB reactor) using synthetic wastewater with a composition as shown in Table 3. The procedure follows a synthetic wastewater prepared according to Idrus et al. [26]. The COD concentration was 203,000 mg/L and the acclimatization period lasted for three weeks. The plant where inoculum was collected operates under mesophilic condition and as such, the temperature shift was needless. To avoid any change of state of microbial functions, the UASB reactor was also maintained at 35 °C by heating the water bath jacket throughout the experiment. However, the reactor was purged with 100% N2 gas prior to startup. Table 4 depicts the summary of the strategy employed during the UASB reactor startup. Acclimatization of the environment was immediately followed with Batch study (same as in the case of BMP test) using raw slaughterhouse wastewater at an organic loading rate of 0.2 g/L d−1. The raw CSWW was fed once until biogas production ceased on the eleventh day (HRT = 11 day). During the batch study, the reactor performance had greatly improved in terms of total COD and soluble COD removal. On the twelve day, the continuous study (Run II) was achieved by daily effluent discharge followed by feeding the same amount of CSWW using a peristaltic pump at a flow rate of 0.6 L/m, as shown in Figure 2. Exclusive feeding of the CSWW was achieved by increasing OLR after each run. HRT of day 1 was also maintained by reducing the dilution factor of the substrate until the reactor show signs of decreased performance. The loading rates were 0.4, 0.7,1, 1.5, 2, 2.5, 3, 5, 7, 10, and 15 g/L d−1.
Table 3.
Synthetic wastewater composition.
| Material | Quantity | Unit |
|---|---|---|
| Yeast (granular form) | 23 | g |
| Urea | 2 | g |
| Sugar | 11.5 | g |
| Ammonium phosphate (NH4)2HPO4 | 3.4 | g |
| Full cream milk | 144 | mL |
| Raw blood from cattle slaughter point | 5.75 | mL |
| Tap water | Full to 1 L |
Table 4.
Start-up of the UASB reactor.
| Fixed Parameter | Units | Value | ||||
|---|---|---|---|---|---|---|
| Temperature | °C | 35 ± 1 | ||||
| HRT | hr. | 24 | ||||
| pH | ‒ | 6.5 | ||||
| Experimental Run | Day | Influent COD concentration (g/L) | Feed flow rate (L/d) | Maximum COD removal efficiency | OLR (g/L d−1) | Average effluent pH |
| (%) | ||||||
| Batch feed | ||||||
| Stage I | 1–11 | 0.4 | 3 | 98.6 | 0.2 | 6.7 |
| Continuous feed | ||||||
| Stage II | 11–34 | 0.8 | 3 | 90.3 | 0.4 | 6.95 |
| Stage III | 34–49 | 1.4 | 3 | 80.4 | 0.7 | 6.87 |
| Stage IV | 49–67 | 2 | 3 | 73.6 | 1.0 | 6.63 |
| Stage V | 67–74 | 3 | 3 | 71.0 | 1.5 | 6.72 |
| Stage VI | 74–86 | 4 | 3 | 70.2 | 2.0 | 6.85 |
| Stage VII | 86–95 | 5 | 3 | 73.0 | 2.5 | 6.77 |
| Stage VIII | 95–100 | 6 | 3 | 70.0 | 3.0 | 6.60 |
| Stage IX | 100–106 | 10 | 3 | 72.5 | 5.0 | 6.7 |
| Stage X | 106–113 | 14 | 3 | 72.1 | 7.0 | 6.8 |
| Stage XI | 113–120 | 20 | 3 | 65.0 | 10.0 | 7.1 |
| Stage XII | 120–127 | 30 | 3 | 42.0 | 15.0 | 7.3 |
Previous research on anaerobic digestion revealed that, during reactor startup, an appreciable level of biomass degradation could be achieved by increasing influent substrate concentration at constant HRT [27]. For instance, the studies of Najafpour et al. [28] and Zhang et al. [29] have shown that high COD removal efficiency can be achieved by increasing organic loading rate and maintaining constant HRT in the treatment of substrate with high lipids. Furthermore, Ghaniyari et al. [30] and Sowmeyan et al. [31] revealed that anaerobic degradation of wastewater in a bioreactor could startup by increasing OLR and reducing HRT without changing influent COD concentration. Therefore, the reactor in this work was maintained at constant HRT throughout the experimental period. Also, the pH of the system was closely monitored on daily basis to avoid pH drop to below 6.5 which could cause system failure.
2.7. Mesophilic UASB Reactor Performance Study on CSWW
The performance of the UASB reactor in the treatment of CSWW was first investigated in batch mode. The organic loading rate, OLR, and hydraulic retention time, HRT, remained unchanged. To assess the performance treatment of the system, HRT, feed, and biomass concentration were all constant. Subsequently, the effect of increasing loading rate on the treatment efficiency was evaluated by varying the feed COD concentration whilst operating at a constant HRT. The influent substrate, effluent discharge flow rates, biogas production, and pH where observed on daily basis.
2.8. Scanning Electron Microscopy Analysis
Surface morphology of the sludge (inoculum) before and after contact with CSWW was conducted using a scanning electron microscope (S-3400N SEM HITACHI, Milpitas, CA, USA). The samples were initially coated with gold to induce conductivity. The surfaces of the samples were viewed at 300× magnification with the objective of obtaining some information on the behavior of the materials.
3. Results and Discussion
3.1. Biochemical Methane Potential of CSWW
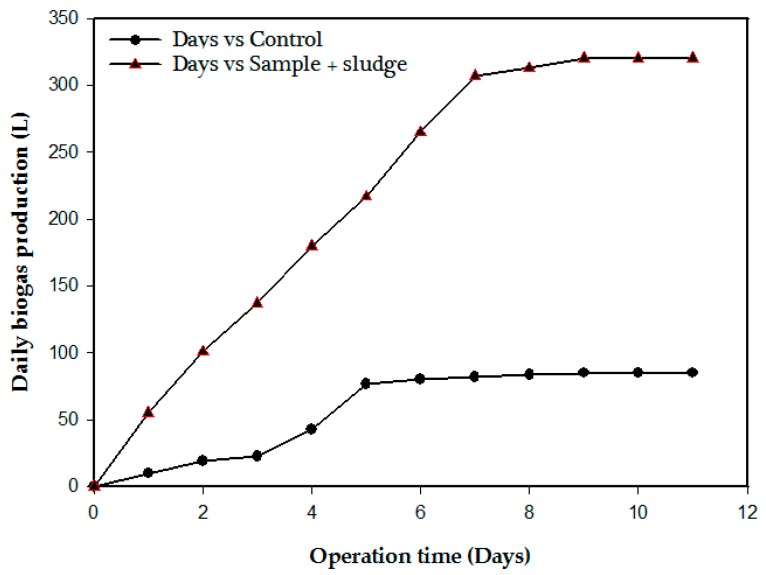
Figure 3 shows the biochemical methane potential test carried out to determine the degradability of CSWW. The OLR of the feed introduced to the scotch bottles was 0.2 g/L which correspond to 0.4 g/L COD. An average total cumulative biogas produced in the mixture of feed to inoculum (F/I) ratio was 320 mL and 85 mL in the blank bottles. The composition of the biogas in the (feed/inoculum) ratio was 0.80% oxygen, 18.45% nitrogen, 75.80% methane, and 4.92% carbon dioxide. After deducting the amount of biogas produced in the average of blank bottles, the resulting biogas produced by the feed was 235 mL. Based on the percentage of methane gas present in the biogas (75.80%), the methane yield corresponds to 178 mL. Therefore, 0.18 gCOD/L is the actual amount that produced the 178 mL of methane. Hence, the methane produced by the CSWW used as feed was 31 mL CH4/gCODadded. The COD, SCOD, and TSS removal efficiencies were 95%, 98%, and 98.5%, respectively. Ammonia nitrogen concentration (NH3-N) and alkalinity ratio (IA/PA) were 38 mg/L and 0.2, respectively.
Figure 3.
Result of biochemical methane potential tests.
3.2. Effect of Organic Loading Rate on COD and SCOD Removal Efficiency
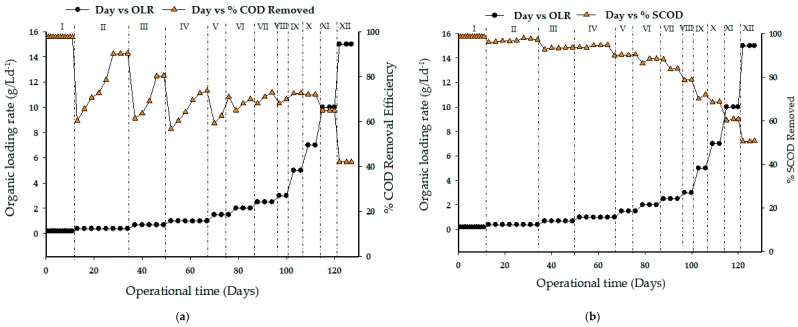
Figure 4 shows the changes in OLR corresponding to COD removal efficiency in the UASB reactor during the period of the operation. In the beginning, 0.2 g/L d−1 was fed to the reactor for the batch studies (Run I). It took eleven days for the reactor to completely stabilize the biomass. The HRT was observed to take too long due to the fluctuation in daily biogas production. Also, it was observed that at the lowest organic loading rate of more than 98% COD removal was realized. After batch feeding attained a stable state, the reactor was then fed continuously. Prior to subjecting the reactor to higher OLR, continuous study was start-up with 0.4 g/L d−1 (Run II). During this period, biodegradation took a longer period before final stabilization. It was observed that the COD removal efficiency was 90% for three consecutive days. However, the period the reactor took before stabilizing was probably due to the shock received during the change of state of the OLR. The influent COD was further increased to 0.7 g/L d−1, which corresponds to an organic loading rate of 1.4 g/L−1. A continuous increase in OLR with fat rich wastewater was maintained until the COD removal efficiency declined to 42% at 15 g/L d−1. The soluble COD removal efficiency of the UASB reactor, in terms of OLR, is shown in Figure 3b. At the initial state of the study, the loading rate was maintained at 0.2 g/L d−1 and subsequently increased from 0.4 to 15 g/L d−1 during continuous studies. SCOD removal efficiencies showed an increasing trend from a maximum of 92% at 1.5 g/L d−1 OLR to approximately 80 to 70% between run VI and IX. However, at the beginning of each phase where OLR increased, there existed a corresponding decrease in removal efficiency; however, the system recovered shortly and adapted to the new condition with time. It can be observe that at an OLR of 7 g/L d−1, the COD and SCOD removal were approximately 70% and 85% and this was regarded as the upper limit for satisfactory performance of this type of wastewater under the conditions of this study.
Figure 4.
Effect of organic loading rate on (a) chemical oxygen demand (COD) removal efficiency and (b) soluble COD removal.
Comparatively, the results obtained in this work showed a better performance compared to the values obtained in a past paper [32]. The COD and SCOD removal efficiencies were (97% ± 2%) and (95% ± 3%) at OLR 4.5 kg TCOD m−3 d−1. This is because a large amount of energy was expended during the aeration process and the membrane technology was further applied to achieve better efficiency in the treatment of wastewater. Furthermore, the concentration of raw COD in this study was much higher compared to the raw COD of 2711 ± 487 achieved in the aforementioned study. Similar results of COD and SCOD removals were also obtained in the research of Rajakumar et al. [33], but the data showed lower performance of this study, especially when HRT, OLR, and initial COD concentrations of the two substrates were compared. Moreover, these concepts have been positively reinforced by the findings of Nachev et al. [34] which presented high COD removal efficiency.
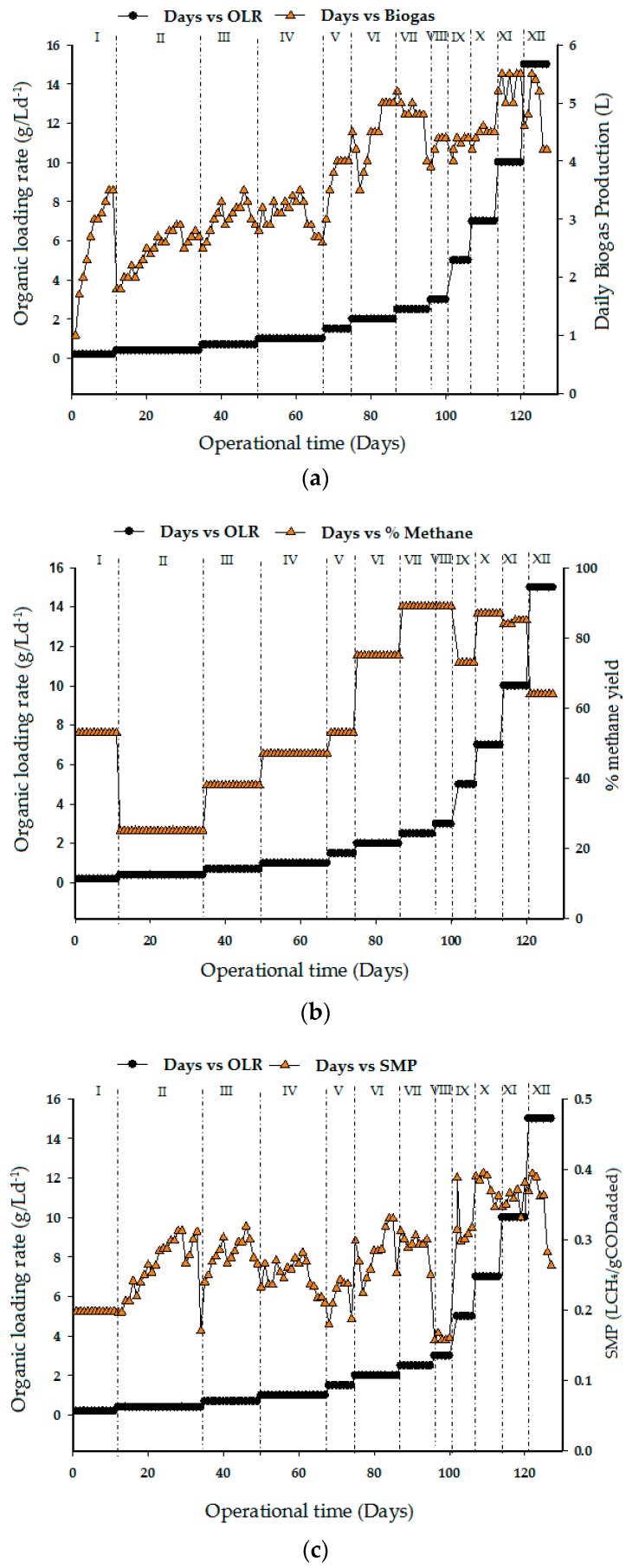
3.3. Biogas Production, Percentage of Methane Yield, and Specific Methane Production
Figure 5a reveals the total amount of biogas produced during the period of UASB reactor operation. After acclimatization and subsequent batch studies that lasted for 11 days, the initial OLR 0.4 g/L d−1 was gradually elevated to 15 g/L d−1 until biogas production reduced to 4.2 L in the last phase. Biogas production increased tremendously at OLR 2.5 and 10 g/L d−1, which corresponds to 4 and 5.5 L biogas, respectively. The increasing OLR by feeding the CSWW in run VII and XI with COD concentrations of 5 and 20 g/L d−1, respectively, yields methane concentrations of 89% and 85%, as shown in Figure 5b. Subsequently the OLR was then increased to 15 g/L d−1 in (run XII), after which the COD and methane concentration in run XI remained at 64%. It is clear that a decrease in methane concentration of biogas decreases with the increase in OLR at some point. However, the specific methane yield is still higher within these phases as shown in Figure 5c. A slight decrease of methane yield was experience in run VIII. This was attributed to the shock received by the system due to changes in the OLR that affect the methanogenic activities. Therefore, counter measures are required to avoid continuity of the process. From the initial phase (run I), variation in CH4 content was observed from a minimum of 0.1981 LCH4/gCOD added (day 1) to a maximum of 0.3950 LCH4/gCOD added at OLR 7 g/L d−1. A similar trend was recorded in runs XI and XII until the specific methane remained quite constant and close to the average value of 0.2631 LCH4/gCOD added.
Figure 5.
Effect of organic loading rate on (a) biogas production, (b) percentage of methane yield, and (c) specific methane production.
In general, Figure 5a–c depicts the biogas production, percentage of methane, and the specific CH4 production rates with respect to the increasing OLR values. Conversely, the biogas and the CH4 production rates decreased markedly with increasing COD loading rates in the last phase (Run XII). Interestingly, when the UASB system was operated at COD loading rates below 3 g/L d−1, the process performance in terms of COD removal efficiency and biogas production rates was observed to be higher, because the system was under a low range of organic loads. The trend in Figure 5a,b proved that an increased COD loading rate causes an increased level of organic substrates that are available for conversion to biogas resulting in CH4 production. However, at some points of the trend of biogas and methane production rates, an increase in organic loading rate also caused a reduction in the biogas and CH4 production rates. But most importantly, no increases in toxicity or volatile fatty acid (VFA) accumulation in the reactor were noticed. Hence, the decrease in the overall yield at 15 g/L d−1 OLR could be due low hydraulic retention time for microbe to consume the substrate.
The investigation of Marcos et al. [35] also supported the findings of the present work. However, they reported a biogas production yield of 18 L after mixing with serum slaughterhouse waste and approximately 10 L of biogas for purely slaughterhouse waste on daily productions. But, their system used a continuous stirrer tank reactor (CSTR) where mixing was provided with the use of an electric motor that led to sufficient contact between the microbes and the biomass. A comparison of these results with others investigations also obtained from UASB reactor working under similar conditions reveal quite a satisfactory result. For instance, Kwarciak-Kozłowska et al. [36] revealed maximum COD and BOD5 removal efficiencies of 85% and 82%, respectively, at HRT of 6 days. The removal of organic pollutants decreased with the shortened HRT and the daily biogas production decreased with an increase in the HRT. The fermentation process of the wastewater was highly characterized by high methane content (75%). However, from an economic point of view, HRT is too long compared to the present study.
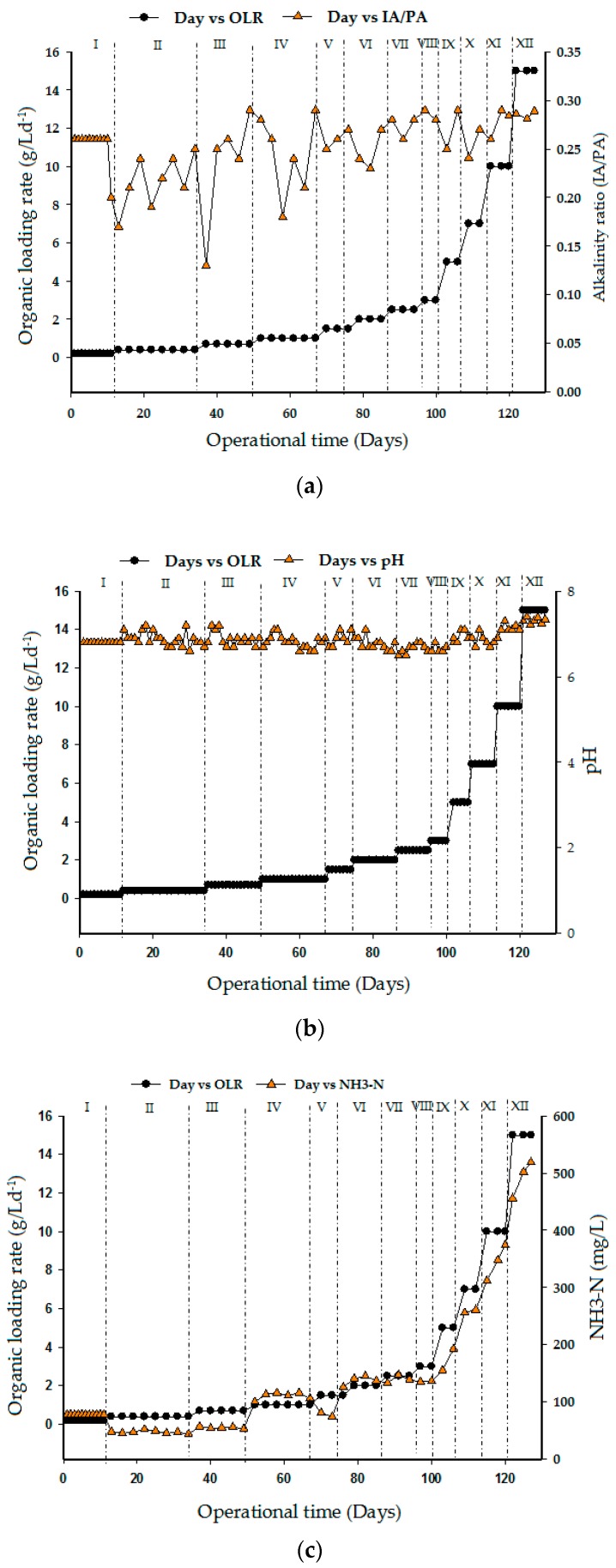
3.4. Effect of Alkalinity, pH, and Ammonia Nitrogen
The high protein and lipid content of slaughterhouse wastewater makes it a challenging material for anaerobic digestion due to the inhibition caused during degradation. Functional anaerobic digestion system depends on the buffering capacity and the degree of adaptation of the microorganisms [37]. Alkalinity is an important parameter in anaerobic digestion that shows the capability of a solution to withstand a drop in pH produced by the release of organic acids. It is also termed as the system buffer capacity [38]. The ability of methanogenic bacteria to resist higher VFA accumulation is highly dependent on the alkalinity value of the system as well its buffering capacity. Thus, methane production processes in a bioreactor are basically a function of alkalinity and pH stability [39]. In anaerobic digestion, microorganisms have a working range of pH. Methanogens are sensitive to pH between 6.5 and 7.5 and have optimal pH of between 7.0 and 7.2 [40]. For efficient methane production, the anaerobic digestion process normally operates at the aforementioned optimal pH range. But, the formation of degradable intermediates (VFA) tends to lower the pH of the bioreactor during operation. Changes in pH of the bioreactor during operation are easily detected through alkalinity (gCaCO3/L) which is the main indicator of buffering capacity of the system. According to Mata-Alvarez et al. [41], desirable alkalinity in bioreactor is between 2000 and 4000 mg CaCO3/L and the VFA/alkalinity ratio should be less than 0.3.
Figure 6a–c shows the alkalinity, pH, and ammonia nitrogen profiles at various OLR loading rates. The maximum alkalinity ratio attained throughout the period of operation was 0.29 which depicts a stable operating performance. Likewise, the VFA concentration of the effluent was less than 0.60 g/L with no major fluctuations detected from other parameters (pH and CH4 concentration). Furthermore, the pH values were consistent with the increased levels of OLR biogas production and COD loading rates as shown in Figure 6b. Hence, the effluent from this reactor exhibited high buffering capacity and, as such, does not require pH correction. In addition, ammonia/nitrogen was measured at one day intervals during fermentation and methanation phases shown in Figure 6c. The ammonia nitrogen NH3-N level in the effluent was observed to keep increasing in values corresponding to the increase in COD levels and organic loading rates. Interestingly, the level of ammonia increase in the effluent at 35 °C neither inhibits nor caused any significant changes in the reactor operation. It is evident, that process instability due to ammonia often results in the accumulation of volatile fatty acids (VFAs). Again, this leads to a decrease in pH and thereby reduces the concentration of fatty acids. The process was carried out without inhibition even when the concentration of NH3-N increased from 78 mg/L to a value above 500 mg/L. The absence of inhibition in the system may be attributed to the higher activity of mesophiles, which were primarily responsible for converting the proteins to CH4 gas during the fermentation process. The quantity of ammonia generated from an anaerobic biodegradation of organic substrate can be estimated using stoichiometric [42].
Figure 6.
Effect of organic loading rate on (a) alkalinity ratio, (b) pH, and (c) ammonia/nitrogen.
| CaHbOcNd + ((4a − b − 2c + 3d)/4) H2O → ((4a + b − 2c − 3d)/8) CH4 + ((4a − b + 2c + 3d)/8) CO2 + dNH3 | (1) |
In order to counteract the effect of high ammonia nitrogen accumulation in the anaerobic digestion process frequent maintenance, a change in the intracellular pH of methanogens, and the inhibition of specific enzyme reactions are some of the numerous suggested pathways for overcoming ammonia inhibition.
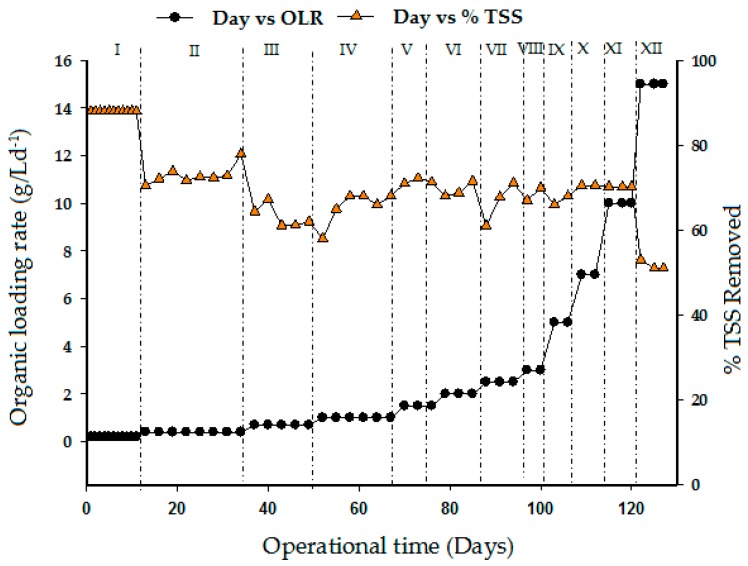
3.5. Total Suspended Solid and Oil and Grease Removal Profile
The TSS concentration in the effluent was measured for the purpose of knowing the insoluble organic present in the treated cattle slaughterhouse wastewater. TSS was also used in this study to measure the performance of the UASB reactor at an HRT of 1 day with different organic loading rates. As a record of performance, Figure 7 shows the TSS concentration in the effluent of treated CSWW after COD removal. The raw substrate TSS concentration of 1955 mg/L was reduced to 47–158 mg/L after treatment at OLR 15 g/L d−1. The TSS removal during the continuous study was between 85 and 72%. The minimum TSS removal percentage was 72%, obtained at OLR 10 g/L d−1 and HRT of 24 h. A sudden decrease in TSS removal efficiency to 50% was observed in stage XII. However, the TSS removal efficiency of the UASB reactor does not only depend on the anaerobic digestion process, but also on the physicochemical reactions within the effluent biomass which in turn facilitates its detainment within the reactor. This phenomenon is similar to experiment reported in a previous study by Tawfik et al. [43]. In their studies, the UASB reactor was operated at a constant HRT of 24 h throughout the study, while OLRs varied from 1.9 to 4.4 kgCOD total/m3.d due to changes in influent composition. The results clearly demonstrate that the UASB reactor achieved substantial reduction of TSS removal of 72%. But, the treatment performance was achieved by applying the activated sludge (AS) system coupled with the UASB reactor.
Figure 7.
Effect of organic loading rate on total suspended solid removal efficiency.
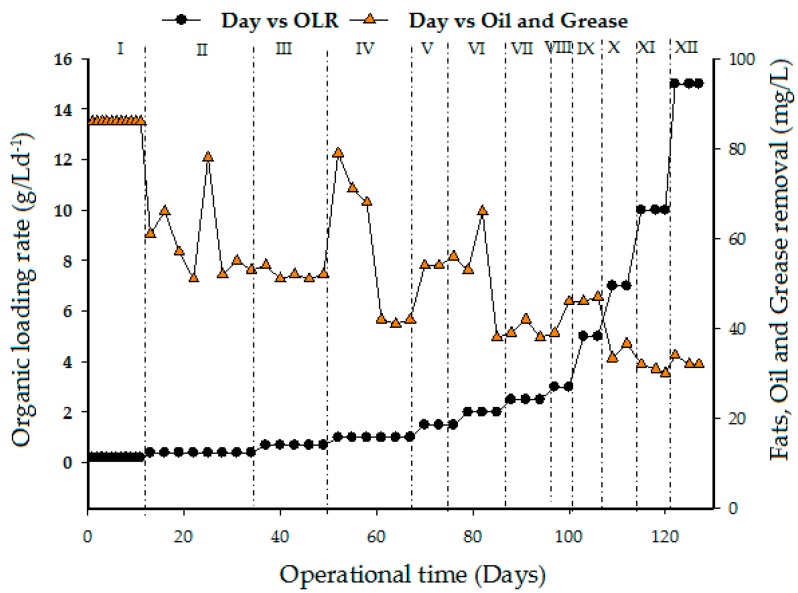
Fats, oil, and grease (FOG) mainly consists of long chain fatty acids (LCFA) that are bonded to glycerol, esters, waxes, phospholipids, sterols, and sterol esters [44]. Figure 8 demonstrates the performance of the UASB reactor in removing oil and grease. The concentration of oil and grease removal decreases with an increase in oil OLR. However, a serious decrease in removal (below 40 mg/L) was recorded in the last three phases (X, XI, and XII). Hence, anaerobically treated effluents usually require additional post-treatment to remove organic matter and other constituents like total nitrogen, phosphorus, and pathogenic organisms to acceptable limits. Based on the results obtained, the quality of the effluent did not comply with the set standard A and B of the department of environment Malaysia [45].
Figure 8.
Effect of organic loading rate on fats, oil, and grease removal.
3.6. Scanning Electron Microscopy
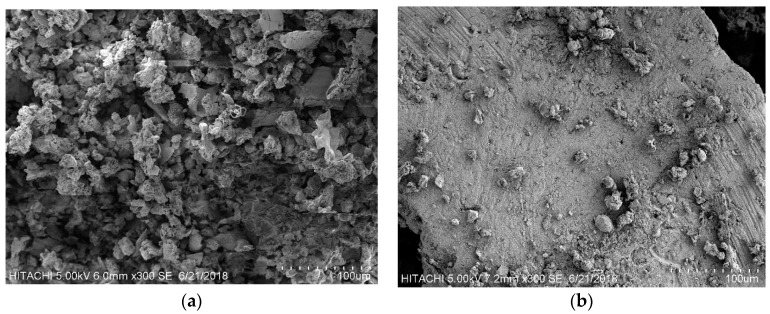
Scanning electron microscopy was used to describe the surface morphology of the mesophilic sludge before and after contact with CSWW as shown in Figure 9. It can be observed that after 127 days of operation, the activated sludge becomes finer with compacted granules. Thus, no sludge washes out and a large proportion of the microbial community was retained which led to high COD removal efficiency and biogas production. This finding is consistent with the result found in Wu et al. [46]. Finer granulation of sludge in UASB is one important factor that usually contributes to high COD removal efficiency [47]. However, the granulation of the sludge in this study has not significantly affect COD removal efficiency, especially in the last run (run XII) where the COD removal was less than 50%. This could be attributed to high color and turbidity and TSS of the influent substrate.
Figure 9.
SEM image of the sludge inoculum (a) before contact with CSWW and (b) after contact with CSWW.
4. Conclusions
The organic loading rate (OLR) is an important parameter that significantly affects microbial ecology and characteristics of upflow anaerobic sludge blanket (UASB) reactor. In this work, a UASB reactor was operated at mesophilic temperature (35 °C) for the treatment of slaughterhouse wastewater. The COD and SCOD removal efficiencies were 73% and 85%, respectively, at an optimum OLR of 7 g/L d−1. Generally, daily biogas production increases with increasing organic loading rate. Likewise the yield coefficient of methane production also increases with the load rise, reaching 0.38 LCH4/gCODadded at OLR 10 g/L d−1 with an influent COD concentration of 6 g/L d−1. The maximum alkalinity ratio attained was 0.29 and this demonstrates a stable operating condition that is enough to prevent drop in pH to below 6.5. Ammonia/nitrogen serves as a nutrient for bacteria. However, its presence in high concentration could inhibit the reactor’s performance.
Therefore, the ammonia/nitrogen concentration in the reactor effluent did not affect the performance since it does not exceed the permissible limit. The influent TSS was 1955 mg/L and more than 70% was removed at 10 g/L d−1. It was observed that 1 day of HRT is sufficient to achieve organic matter removals. Also, the comparison with literature studies for similar wastewater indicates that this study attained higher treatment efficiencies compared with others at higher sustainable loading rates. The overall quality of the effluent from the UASB reactor did not comply with the standard (B) for water quality discharge parameters like (COD = 200 mg/L, Oil and grease = 10 mg/L, and NH3-N = 20 mg/L) set by the Department of Environment (DOE) Malaysia. Therefore, post-treatment of the effluent is required; if possible the use of renewable raw materials (waste from biomass) should be encouraged rather than applying chemicals or other energy demanding resources. The presence of high protein, fats, oil, and grease in the wastewater portray the waste as a strong source of energy in the slaughterhouse environment. Also, the availability of the waste is an additional advantage to scale up energy production to a commercial scale.
Acknowledgments
The authors acknowledged the support received from Universiti Putra Malaysia, from the preparation, execution and writing of this research article.
Abbreviations
| BOD | Biochemical oxygen demand |
| BMP | Biochemical methane potential |
| COD | Chemical oxygen demand |
| CSWW | Cattle slaughterhouse wastewater |
| FOG | Fats, oil, and grease |
| HRT | Hydraulic retention time |
| OLR | Organic loading rat |
| SCOD | Soluble chemical oxygen demand |
| TSS | Total suspended solid |
| UASB | Upflow anaerobic sludge blanket |
| VSS | Volatile suspended solid |
Author Contributions
Conceptualization, M.A.M. and S.I.; Methodology, S.I., C.M.H. and N.N.N.D.; Validation, S.I., C.M.H. and N.N.N.D.; Formal Analysis, M.A.M. and S.I.; Investigation, M.A.M.; Data verification, C.M.H. and N.N.N.D.; Writing—Original Draft Preparation, M.A.M.; Review & Editing, S.I., C.M.H. and N.N.N.D. All authors have read and approved this article.
Funding
This research was financially supported by Universiti Putra Malaysia (UPM) on baseline grant No. GP-IPS/9633400.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Mattioli A., Boscaro D., Venezia F.D., Santacroce F.C., Pezzuolo A., Sartori L., Bolzonella D. Biogas from Residual Grass: A Territorial Approach for Sustainable Bioenergy Production. Waste Biomass Valorization. 2017;8:47–56. doi: 10.1007/s12649-017-0006-y. [DOI] [Google Scholar]
- 2.Boscaro D., Andrea P., Luigi S., Francesco M., Andrea M., David B., Stefano G. Evaluation of the Energy and Greenhouse Gases Impacts of Grass Harvested on Riverbanks for Feeding Anaerobic Digestion Plants. J. Clean. Prod. 2018;172:4099–4109. doi: 10.1016/j.jclepro.2017.02.060. [DOI] [Google Scholar]
- 3.Jensen P.D., Yap S.D., Boyle-Gotla A., Janoschka J., Carney C., Pidou M., Batstone D.J. Anaerobic membrane bioreactors enable high rate treatment of slaughterhouse wastewater. Biochem. Eng. J. 2015;7:132–141. doi: 10.1016/j.bej.2015.02.009. [DOI] [Google Scholar]
- 4.Aziz H., Puat N., Alazaiza M., Hung Y.T. Poultry Slaughterhouse Wastewater Treatment Using Submerged Fibers in an Attached Growth Sequential Batch Reactor. Int. J. Environ. Res. Public Health. 2018;15:1734. doi: 10.3390/ijerph15081734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad M.I., Ejaz O., Ali A., Durrani M.A.Q.J., Khan I.A. Anaerobic digestion of waste from a slaughterhouse. J. Environ. Chem. Eng. 2014;2:1317–1320. doi: 10.1016/j.jece.2014.04.001. [DOI] [Google Scholar]
- 6.Luostarinen S., Luste S., Sillanpää M. Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour. Technol. 2014;100:79–85. doi: 10.1016/j.biortech.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Cirne D., Paloumet X., Björnsson L., Alves M.M., Mattiasson B. Anaerobic digestion of lipid-rich waste-Effects of lipid concentration. Renew. Energy. 2007;32:965–975. doi: 10.1016/j.renene.2006.04.003. [DOI] [Google Scholar]
- 8.Technical Development Document for the Final Effluent Limitations Guidelines and Standards for the Meat and Poultry Products Point Source Category (40 CFR 432) United States Environmental Protection Agency; Washington, DC, USA: 2004. [Google Scholar]
- 9.Kist L.T., El Moutaqi S., Machado E.L. Cleaner production in the management of water use at a poultry slaughterhouse of Vale do Taquari. Brazil: A case study. J. Clean. Prod. 2009;17:1200–1205. doi: 10.1016/j.jclepro.2009.04.006. [DOI] [Google Scholar]
- 10.Ciro B.-L., Mehrab M. Slaughterhouse Wastewater: Treatment, Management and Resource Recovery. Phys.-Chem. Wastewater Treat. Resour. Recover. 2017:154–173. doi: 10.5772/65499.. [DOI] [Google Scholar]
- 11.Jensen P.D., Mehta C.M., Carney C., Batstone D. Recovery of energy and nutrient resources from cattle paunch waste using temperature phased anaerobic digestion. Waste Manag. 2016;51:72–80. doi: 10.1016/j.wasman.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Mittal G. Treatment of wastewater from abattoirs before land application—A review. Bioresour. Technol. 2006;97:1119–1135. doi: 10.1016/j.biortech.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Bustillo-Lecompte C., Mehrvar M. Treatment of an actual slaughterhouse wastewater by integration of biological and advanced oxidation processes: Modeling, optimization, and cost-effectiveness analysis. J. Environ. Manag. 2016;182:651–666. doi: 10.1016/j.jenvman.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 14.Bugallo P.M., Andrade L.C., de la Torre M.A., López R.T. Analysis of the slaughterhouses in Galicia (NW Spain) Sci. Total Environ. 2014;481:656–661. doi: 10.1016/j.scitotenv.2013.11.079. [DOI] [PubMed] [Google Scholar]
- 15.Gebreeyessus G.D., Jenicek P. Thermophilic versus Mesophilic Anaerobic Digestion of Sewage Sludge: A Comparative Review. Bioengineering. 2016;3:15. doi: 10.3390/bioengineering3020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C., Guo W., Ngo H.H., Lee D.-J., Tung K.-L., Jin P., Wang J., Wu Y. Challenges in biogas production from anaerobic membrane bioreactors. Renew. Energy. 2016;98:120–134. doi: 10.1016/j.renene.2016.03.095. [DOI] [Google Scholar]
- 17.Braguglia C.M., Gallipoli A., Gianico A., Pagliaccia P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2017 doi: 10.1016/j.biortech.2017.06.145. [DOI] [PubMed] [Google Scholar]
- 18.Aryal N., Kvist T. Alternative of Biogas Injection into the Danish Gas Grid System—A Study from Demand Perspective. Chem. Eng. 2018;2:43. doi: 10.3390/chemengineering2030043. [DOI] [Google Scholar]
- 19.Salminen E.A., Rintala J.A. Semi-continuous anaerobic digestion of solid poultry slaughterhouse waste: Effect of hydraulic retention time and loading Semi-continuous anaerobic digestion of solid poultry slaughterhouse waste: Effect of hydraulic retention time and loading. Water Res. 2002;36:3175–3182. doi: 10.1016/S0043-1354(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 20.Masse L., Massé D.I., Kennedy K.J., Chou S.P. Neutral fat hydrolysis and long-chain fatty acid oxidation during anaerobic digestion of slaughterhouse wastewater. Biotechnol. Bioeng. 2002;79:43–52. doi: 10.1002/bit.10284. [DOI] [PubMed] [Google Scholar]
- 21.Hamawand I., Ghadouani A., Bundschuh J., Hamawand S., Al Juboori R.A., Chakrabarty S., Yusaf T. A Critical Review on Processes and Energy Profile of the Australian Meat Processing Industry. Energies. 2017;10:731. doi: 10.3390/en10050731. [DOI] [Google Scholar]
- 22.Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Water Environment Federation; Washington, DC, USA: 2005. [Google Scholar]
- 23.Angelidaki I., Alves M., Bolzonella D., Borzacconi L., Campos J.L., Guwy A.J., Kalyuzhnyi S., Jenicek P., van Lier J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009;59:927–934. doi: 10.2166/wst.2009.040. [DOI] [PubMed] [Google Scholar]
- 24.De Souza Guimarães C., da Silva Maia D.R., Serra E.G. Construction of biodigesters to optimize the production of biogas from anaerobic co-digestion of food waste and sewage. Energies. 2018;11:870. doi: 10.3390/en11040870. [DOI] [Google Scholar]
- 25.Lettinga G. Anaerobic digestion and wastewater treatment systems. J. Microbiol. 1995;1964:3–28. doi: 10.1007/BF00872193. [DOI] [PubMed] [Google Scholar]
- 26.Idrus S., Banks C.J., Heaven S. Assessment of the potential for biogas production from wheat straw leachate in upflow anaerobic sludge blanket digesters. Water Sci. Technol. 2012;66:2737–2744. doi: 10.2166/wst.2012.511. [DOI] [PubMed] [Google Scholar]
- 27.Michaud S., Bernet N., Buffière P., Roustan M., Moletta R. Methane yield as a monitoring parameter for the start-up of anaerobic fixed film reactors. Water Res. 2002;36:1385–1391. doi: 10.1016/S0043-1354(01)00338-4. [DOI] [PubMed] [Google Scholar]
- 28.Najafpour G.D., Zinatizadeh A.A.L., Mohamed A.R., Hasain Isa M., Nasrollahzadeh H. High-rate anaerobic digestion of palm oil mill effluent in an upflow anaerobic sludge-fixed film bioreactor. Process Biochem. 2006;41:370–379. doi: 10.1016/j.procbio.2005.06.031. [DOI] [Google Scholar]
- 29.Zhang Y., Yan L., Chi L., Long X., Mei Z., Zhang Z. Startup and operation of anaerobic reactor treating palm oil mill effluent. J. Environ. Sci. 2008;20:658–663. doi: 10.1016/S1001-0742(08)62109-9. [DOI] [PubMed] [Google Scholar]
- 30.Ghaniyari-Benis S., Borja R., Monemian S.A., Goodarzi V. Anaerobic treatment of synthetic medium-strength wastewater using a multistage biofilm reactor. Bioresour. Technol. 2009;100:1740–1745. doi: 10.1016/j.biortech.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 31.Sowmeyan R., Swaminathan G. Evaluation of inverse anaerobic fluidized bed reactor for treating high strength organic wastewater. Bioresour. Technol. 2008;99:3877–3880. doi: 10.1016/j.biortech.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Rajab A.R., Salim M.R., Sohaili J., Anuar A.N., Salmiati, Lakkaboyana S.K. Performance of integrated anaerobic/aerobic sequencing batch reactor treating poultry slaughterhouse wastewater. Chem. Eng. J. 2017;313:967–974. doi: 10.1016/j.cej.2016.10.144. [DOI] [Google Scholar]
- 33.Rajakumar R., Meenambal T. Comparative study on start-up performance of HUASB and AF reactors treating poultry slaughterhouse wastewater. Int. J. Environ. Res. 2008;2:401–410. [Google Scholar]
- 34.Mijalova Nacheva P., Reyes Pantoja M., Lomelí Serrano E.A. Treatment of slaughterhouse wastewater in upflow anaerobic sludge blanket reactor. Water Sci. Technol. 2011;63:877–884. doi: 10.2166/wst.2011.265. [DOI] [PubMed] [Google Scholar]
- 35.Marcos A., Al-Kassir A., Cuadros F., Yusaf T. Treatment of slaughterhouse waste water mixed with serum from lacteal industry of extremadura in Spain to produce clean energy. Energies. 2017;10:765. doi: 10.3390/en10060765. [DOI] [Google Scholar]
- 36.Kwarciak-kozłowska A., Bohdziewicz J., Mielczarek K., Krzywicka A. The Application of Uasb Reactor in Meat Industry Wastewater Treatment. Civ. Environ. Eng. Rep. 2011;7:119–128. [Google Scholar]
- 37.Buendía I., Fernández F.J., Villaseñor J., Rodríguez L. Biodegradability of meat industry wastes under anaerobic and aerobic conditions. Water Res. 2008;42:3767–3774. doi: 10.1016/j.watres.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 38.Sakar S., Yetilmezsoy K., Kocak E. Anaerobic digestion technology in poultry and livestock waste treatment—A literature review. Waste Manag. Res. 2009;27:3–18. doi: 10.1177/0734242X07079060. [DOI] [PubMed] [Google Scholar]
- 39.Tangkathitipong P., Intanoo P., Butpan J., Chavadej S. Separate production of hydrogen and methane from biodiesel wastewater with added glycerin by two-stage anaerobic sequencing batch reactors (ASBR) Renew. Energy. 2017;113:1077–1085. doi: 10.1016/j.renene.2017.06.056. [DOI] [Google Scholar]
- 40.Bitton G. Wastewater Microbiology. 2nd ed. John Wiley & Sons; New York, NY, USA: 2005. [Google Scholar]
- 41.Mata-Alvarez J. Biomethanization of the Organic Fraction of Municipal Solid Wastes. IWA; London, UK: 2003. [Google Scholar]
- 42.Musa M.A., Idrus S., Man H.C., Daud N.N.N. Wastewater Treatment and Biogas Recovery Using Anaerobic Membrane Bioreactors (AnMBRs): Strategies and Achievements. Energies. 2018;11:1675. doi: 10.3390/en11071675. [DOI] [Google Scholar]
- 43.Tawfik A., Sobhey M., Badawy M. Treatment of a combined dairy and domestic wastewater in an up-flow anaerobic sludge blanket (UASB) reactor followed by activated sludge (AS system) Desalination. 2008;227:167–177. doi: 10.1016/j.desal.2007.06.023. [DOI] [Google Scholar]
- 44.Klaucans E., Karlis S. Problems with Fat, Oil, and Grease (FOG) in Food Industry Wastewaters and Recovered FOG Recycling Methods Using Anaerobic Co-Digestion: A Short Review. Key Eng. Mater. 2018;762:61–68. doi: 10.4028/www.scientific.net/KEM.762.61. [DOI] [Google Scholar]
- 45.A Guide for Investors. Federal Government Administrative Centre; Putra Jaya, Malaysia: 2010. Environmental Requirements: Department of Environment Ministry of Natural Resources and Environment Wisma Sumber Asli, Precinct 4. [Google Scholar]
- 46.Wu J., Afridi Z.U.R., Cao Z.P., Zhang Z.L., Poncin S., Li H.Z., Zuo J.E., Wang K.J. Size effect of anaerobic granular sludge on biogas production: A micro scale study. Bioresour. Technol. 2016;202:165–171. doi: 10.1016/j.biortech.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Rosli N.S., Idrus S., Daud N.N., Ahsan A. Assessment of potential biogas production from rice straw leachate in upflow anaerobic sludge blanket reactor (UASB) Int. J. Smart Grid Clean Energy. 2016;5:135–143. doi: 10.12720/sgce.5.3.135-143. [DOI] [Google Scholar]