Abstract
Background
Low levels of physical activity in older patients during hospitalization have been linked to loss of functional ability. Practical methods of measuring physical activity are needed to better understand this association and to measure the efficacy of interventions. The aims of this study were to evaluate the feasibility of using accelerometers to discriminate between lying, sitting, standing, and standing and moving and to determine the acceptability of the method from the patients' perspective.
Methods
A convenience sample of 24 inpatients was recruited. Participants wore accelerometers on their thigh and on their lower leg (just above the ankle) for 48 hours during their hospitalization. Postural changes and movement during the 48 hours were differentiated using derived pitch angles of the lower leg and thigh, and nongravity vector magnitude of the lower leg, respectively.
Results
On average, patients were lying for 61.2% of the recording time, sitting for 35.6%, standing but not moving 2.1%, and standing and moving 1.1%. All participants found the accelerometers acceptable to wear.
Conclusions
The methodology described in this study can be used to differentiate between lying, sitting, standing, and moving and is acceptable from a hospitalized older person's perspective.
1. Background
Low levels of physical activity in older hospitalized adults are well documented [1–4] and have been linked with declines in functional ability [5] and muscle strength [6]. As such, there is a need to measure physical activity when investigating hospital-associated functional decline in older patients. Accelerometers are tools used to objectively measure levels of activity in research and have been used extensively in community-dwelling adults [7, 8].
Accelerometers have been worn on a variety of anatomical positions, but most often worn on the hip or wrist, with the latter being the most common due to its high acceptability to study participants [7–9]. In community cohorts and epidemiological studies, accelerometer data are usually reported in terms of time spent at different intensities of physical activity [10]. In hospital cohorts, this information is somewhat redundant due to the severity of inactivity and low intensity of all activity. Typically, research using accelerometer-measured activity in hospital has used step count or time spent upright (standing or walking versus sitting or lying) to quantify activity [11]. However, it would be desirable to measure hospital activity in terms of time spent in specific postures (e.g., lying, sitting, and standing) and time spent walking [12–14]. This is because a more nuanced characterization of mobility patterns in older hospitalized patients may facilitate the design of specific physical interventions to try to combat hospital-associated functional decline. For instance, it has been suggested that sitting in a chair may have a therapeutic benefit in some patients [15, 16], but prolonged sitting compared to resting lying on a bed may also be detrimental [17]. To accurately differentiate between lying in bed, sitting in a chair, and standing, two sensors are required which can be positioned either on the thigh and lower leg [13, 14] or on the sternum and thigh [12].
To our knowledge, six studies have validated methods for using two accelerometers in hospitalized patients for specifically measuring posture [12–14, 18–20], including four in an acute ward setting [13, 14, 18, 20]. Four of the six studies placed accelerometers on the thigh and lower leg [13, 14, 18, 19] and two on the thigh and sternum [12, 20]. Four of these studies specified the accelerometer model used: one study used the ADXL202 [12], one used the ActivPAL [20], and two used the PAL2 device [18, 19]. Two studies reported their algorithms for defining lying, sitting, and standing positions in relation to the detected angles of the accelerometers [12, 14]. Pedersen et al. [14] attached sensors to the participants' thigh and lower leg, and Culhane et al. [12] attached sensors to the thigh and sternum. Both reported high levels of accuracy in all three positions. Two studies had a fourth category of “movement” [12, 20], one using the ActivPAL and one using ADXL202 accelerometers; Culhane et al. [12] reported high rates of movement detection accuracy, and Taraldsen et al. [20] reported low level of step count accuracy when walking speed was below 0.47 m/s.
The aims of this feasibility study were to develop a method to discriminate between lying, sitting, standing, and moving (upright), in older hospitalized patients using two accelerometers (on the thigh and lower leg), and to determine the feasibility and acceptability of the method from the patients' perspective.
2. Methods
2.1. Study Design
This is a feasibility study using a cross-sectional single center cohort design. Ethical approval was granted by the South Central Hampshire A Research Ethics Committee (reference 17/SC/0219).
2.2. Setting
The study was conducted on inpatient wards of the Department of Medicine for the Elderly (DME) at Cambridge University Hospitals (CUH) NHS Foundation Trust, England, United Kingdom. CUH is a tertiary university teaching hospital in the English National Health Service (NHS) with approximately 1000 inpatient beds. DME wards have approximately 150 beds and specialize in the provision of Consultant (i.e., senior physician)-led multidisciplinary Comprehensive Geriatric Assessment for older adults admitted to the hospital. The clinical characteristics of this setting have been described elsewhere [21, 22].
2.3. Study Period
Patients were recruited between May and October 2017.
2.4. Population
Convenience sample of patients admitted to DME wards over the age of 70, under the care of a DME Consultant (Senior Attending Physician), with an expected hospital stay of more than 72 hours. Our Patient and Public Involvement (PPI) group suggested that men and women might have different views regarding acceptability of wearing accelerometers. We therefore recruited 12 men and 12 women, using the guidance of the National Institute for Health Research (NIHR) of recruiting 12 participants per group in a feasibility study [23].
Exclusion criteria are as follows: inability to provide informed consent as assessed by the researcher taking consent; if the patient was receiving end of life care; disagreement to participation by the DME Consultant in charge of the patient's care; if the clinical team had any concerns regarding skin integrity around proposed accelerometer sites; allergy to adhesive dressings; or if the patient was already actively involved in another research study.
2.5. Procedures
Potential participants were identified by members of the clinical team who sought permission from the individuals for them to be approached by a member of the research team. If permission was given, a member of the research team (PH, a Physiotherapist with 8 years' clinical experience) would discuss the study with the participant and provide them with further written information regarding the study. Written information was produced in conjunction with the PPI group prior to the study. Participants were given 24 hours to consider their participation before being asked to provide written consent. Mental capacity was assessed at both meetings by PH and confirmed verbally with the DME Consultant.
After providing written consent, participants were fitted with two accelerometers (AX3, Axivity, Newcastle upon Tyne, UK) on the same leg, the first on the front of the thigh, proximal to the patella (a third of the way between the knee and the hip). The second sensor was placed on the side of the lower leg approximately 5cm above the lateral malleolus of the ankle (Figure 1). The lateral side of the lower leg was chosen as opposed to the anterior to minimize the risk of excessive pressure on the skin if the participants were to lie in bed with their legs crossed. The research team considered the potential risk of increased pressure on the skin if the participants were to lie on their side. However due to the pressure relieving mattresses the hospital uses, the risk was felt to be sufficiently low to proceed. In addition, the accelerometer sites were checked daily for comfort and skin integrity. The accelerometers were placed on the right leg unless there were clinical reasons prohibiting its placement (e.g., skin lesion) or the participant had a strong preference for the left leg. The thigh and lower leg sensor locations were preferred to using the thigh and sternum locations by both our PPI group and members of the clinical teams. The preference of location was based on both practical reasons (e.g., potential interference with routine clinical procedures) and the expected acceptability to patients. Following advice from our PPI group and clinical teams, the accelerometers were placed over a piece of gauze to avoid direct friction with the skin and fixed to the skin with a 10cm by 12cm transparent dressing (3M Tegaderm Film). The AX3 can be submerged in water at a 1.5m depth for 1 hour and therefore there were no restrictions on participants being able to shower or wash. The device has been used in the UK Biobank study where it was worn on the wrist by over 100,000 participants [9] and has demonstrated equivalent signal vector magnitude output on multiaxis shaking tests to the GENEActiv accelerometer [24].
Figure 1.
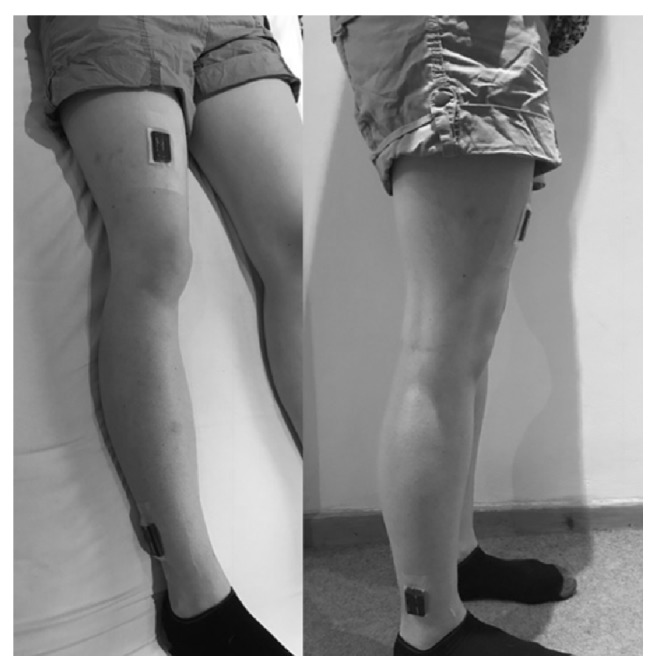
Placement of accelerometers; the accelerometers are orientated with the micro-USB port towards the feet.
Participants were asked to wear the sensors continuously over a 48-hour period. Patients were instructed to inform clinical staff if they experienced any discomfort at the accelerometer sites, in order to have them immediately removed. A member of the research team checked skin integrity (through the transparent dressing) on a daily basis. Following the 48-hour monitoring period, a member of the research team removed the accelerometers and participants were asked to complete a questionnaire regarding the acceptability of their monitoring experience (Box 1). A 48-hour wear time was felt to be a sufficient period of time to assess acceptability by both the research team and PPI group. If a patient's discharge date was changed to within the 48-hour period after study commencement, or if he/she needed to undergo an MRI scan during the monitoring period, the accelerometers were removed and the questionnaire was completed at that point.
Box 1.

Acceptability questionnaire.
2.6. Other Measurements
For descriptive purposes, we recorded the participants' age, sex, comorbidity burden, acute illness severity, degree of frailty, level of mobility, reason for hospital admission, and length of hospital stay prior to participation in the study.
Comorbidity burden was measured using the Charlson Comorbidity Index (CCI), which is based on patients' diagnoses as coded by the World Health Organization's International Classification of Diseases (10th version) [25]. Acute illness severity was measured by the National Early Warning Score [26], recorded at the points of study entry and exit.
Frailty was measured using the Clinical Frailty Scale (CFS). The scoring of the CFS is based on a global assessment of patients' comorbidity symptoms, cognition, and their level of physical activity and dependency on activities of daily living [27]. The possible scores are 1 (very fit), 2 (well), 3 (managing well), 4 (vulnerable), 5 (mildly frail), 6 (moderately frail), 7 (severely frail), 8 (very severely frail), and 9 (terminally ill). The Modified Functional Ambulatory Category (MFAC) was used to describe the participants' level of functional mobility. The MFAC is a seven-point scale with the following scores: 1: cannot ambulate and requires manual assistance to sit; 2: is able to sit for a minute without support; 3: requires continuous manual assistance of 1 person to support body weight in order to ambulate; 4: requires continuous or intermittent light manual assistance of 1 person to maintain balance when ambulating; 5: requires supervision for safety reasons when ambulating; 6: is able to ambulate independently on level surfaces; 7: is able to ambulate independently on level and nonlevel surfaces [28]. As mobility was only assessed in the hospital ward (on a level surface), for the purposes of this study participants could score a maximum of 6. Members of the direct clinical care teams described each participant's functional mobility at the beginning and end of their participation in the study to provide an MFAC score.
Acceptability was assessed by a specifically designed questionnaire (Box 1); other acceptability outcomes were as to whether the sensors had been worn for the intended time period; or if any concerns had been raised by participants, their families, or members of staff regarding skin integrity or bruising around the sensor sites.
2.7. Accelerometer Data Acquisition
The accelerometers were set up to record movement with a sampling frequency of 100 Hertz and a range of ± 4g using OMGUI software developed by Newcastle University [29]. Following removal of the devices, the same software package was used to download the recorded data.
2.8. Accelerometer Data Processing
Data processing was performed using pampro [30], an open source software package (https://github.com/Thomite/pampro). The raw acceleration data was autocalibrated to local gravity using the method described by van Hees et al. [31]. Due to the placement of the devices and the relatively short duration of measurement, calibration factors were calculated based on all data collected by each device, rather than within a single record. Machine noise was filtered out by applying a low-pass filter at 20 Hertz.
To define the participants' position or activity, three variables from the accelerometers were derived: Euclidean Norm Minus One (ENMO), thigh pitch (elevation) angle: and lower leg pitch angle. ENMO was calculated using the following formula: , where x, y and z refer to the acceleration in g detected along the x, y, and z axes, respectively. ENMO subtracts 1g from the Euclidian norm (vector magnitude) and truncates negative values to zero at sample level to remove the gravity component from the signal, thereby isolating activity-related acceleration [32]. It is normal practice to use nonwear detection procedures to identify when the accelerometers have been removed [33]. As only the research team fitted or removed the accelerometers, compliance with wear was known and therefore nonwear detection was not required.
When the accelerometer is still, the pitch angle along its primary axis x is calculated using the following formula: . The formula provides angles from +90° to -90°, where +90° or -90° represent the accelerometer's x-axis being aligned parallel to the earth's gravity vector (straight up or straight down, respectively), and 0° represents the accelerometer's x-axis angle being aligned perpendicular to the earth's gravity vector. Using our orientation of the accelerometers (micro-USB port towards the feet), 90° represented the patient being “upright” or “standing”.
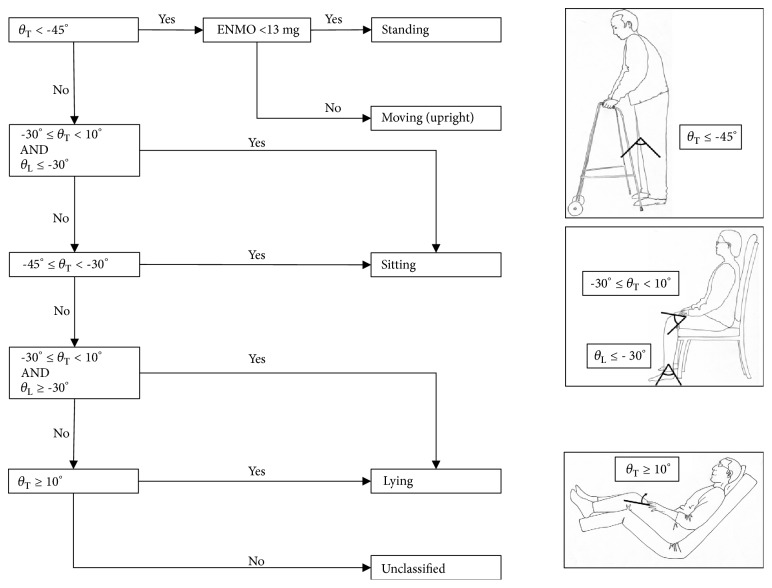
Each of the three signals was summarized from sample-level data into 5-second epochs. Before further variables were derived, a correction was made for the lower leg accelerometer pitch angle. When the accelerometer was attached to the patient, the researchers aimed to align the accelerometer with the lower leg, so that when the lower leg was perpendicular to the floor, the pitch angle would be -90°. Due to the practical constraints of positioning a patient when fitting the accelerometer, this was not always accurate. As all included participants were known to have walked during the study period it was assumed that the minimum lower leg pitch angle was -90° in all participants. All lower leg pitch recordings for each participant were therefore reduced by the difference between their minimum recorded pitch angle and -90°. Every 5-second window was classified as one of: “lying”, “sitting”, “standing”, and “moving (upright)”, based on the rules given in Figure 2. The static positions were based on the algorithm validated by Pedersen et al. [14]. The levels of correspondence between inferred position from accelerometer data and observed positions for lying, sitting, and standing were reported by Pedersen et al. [14] as 90.8–100%, 95.3–98.6%, and 89.6–96.5%, respectively. ENMO values were used from the lower leg accelerometer to define whether or not a person was moving (when already determined to be standing according to the positional criteria). Our previous work has determined usual walking speeds in this population at the point of hospital discharge as 0.33 m/s (IQR 0.21–0.50 m/s), a very low speed compared to those reported in community based samples [34]. A cut-off of ENMO value of >13 milligravity units (mg) from the accelerometer on the lower leg was used to define a participant as “moving”. This value has previously been used with wrist accelerometers to define stationary periods [9, 35]. We tested the threshold value using data from accelerometers worn by members of the research team on their lower leg when stationary, and when walking on a treadmill at 0.1 m/s. Stationary and walking at 0.1 m/s, median (IQR) ENMO values were 3.7mg (1.7–10.5mg) and 16.4mg (13.9–20.1mg), respectively.
Figure 2.
Algorithm for determining participant posture and movement (θT = pitch angle of thigh accelerometer, θL = pitch angle of lower leg accelerometer, and ENMO from lower leg accelerometer).
2.9. Data Analysis
Statistics were presented as mean and standard deviation, or as a count and percentage for categorical variables. For continuous variables with a nonnormal distribution we reported median and interquartile range (IQR) or median and range if the number of data points was less than 25.
3. Results
Participant characteristics are described in Table 1. Female participants had a median age of 81.5 years and male participants of 79.5 years. The median MFAC score when the accelerometers were first attached to the participants was 5 (requiring supervision to ambulate for safety purposes), although this improved to 6 (able to walk independently on level surfaces) by the end of the measurement period. Other clinical characteristics and outcomes are summarized in Table 1.
Table 1.
Participant characteristics.
| All | Female | Male | |
|---|---|---|---|
| Clinical characterization | (n = 24) | (n = 12) | (n = 12) |
|
| |||
| Age (years) | 80.5 (70.0–95.0) | 81.5 (71.0–89.0) | 79.5 (70.0–95.0) |
| Days since admission | 4 (3–7) | 5 (3–8) | 3 (3–5) |
| CCI | 1.5 (0–9) | 1 (0–6) | 2 (0–9) |
| CFS | 5 (1–7) | 4 (1–7) | 6 (2–7) |
| MFAC Start | 5.5 (2–6) | 5.5 (2–6) | 5 (2–6) |
| MFAC End | 6 (2–6) | 6 (2–6) | 6 (2–6) |
| NEWS Start | 1.5 (0–6) | 1 (0–4) | 2.5 (0–6) |
| NEWS End | 1 (0–5) | 1 (0–4) | 1.5 (0–5) |
|
| |||
| Accelerometer characterization | n = 21 | n = 9 | n = 12 |
|
| |||
| Average ENMO lower leg (mg) | 2.1 (±11.2) | 2.6 (±12.2) | 1.9 (±10.6) |
| Time spent lying (%) | 62.6 (±16.3) | 54.4 (±10.0) | 68.7 (±17.7) |
| Time spent sitting (%) | 34.2 (±14.7) | 41.8 (±8.9) | 28.5 (±15.9) |
| Time spent standing (%) | 2.0 (±1.9) | 2.5 (±2.4) | 1.7 (±1.5) |
| Time spent moving (upright) (%) | 1.2 (±1.1) | 1.3 (±0.9) | 1.1 (±1.2) |
Data presented as median (range) or mean(±SD).
CCI = Charlson Comorbidity Index, CFS = Clinical Frailty Scale, MFAC = Modified Functional Ambulatory Category, NEWS = National Early Warning Score, and ENMO = Euclidean Norm Minus One.
Three participants' activity data was not captured; set-up errors were made for two participants, on one or both of the accelerometers, and in one case the lower leg accelerometer fell off and the participant declined to have it refitted as they were concerned that it could get lost. In five further cases, the accelerometers were removed prematurely due to earlier than expected discharges or MRI scans, but in all those cases at least 24 hours of data was collected (Figure 3).
Figure 3.
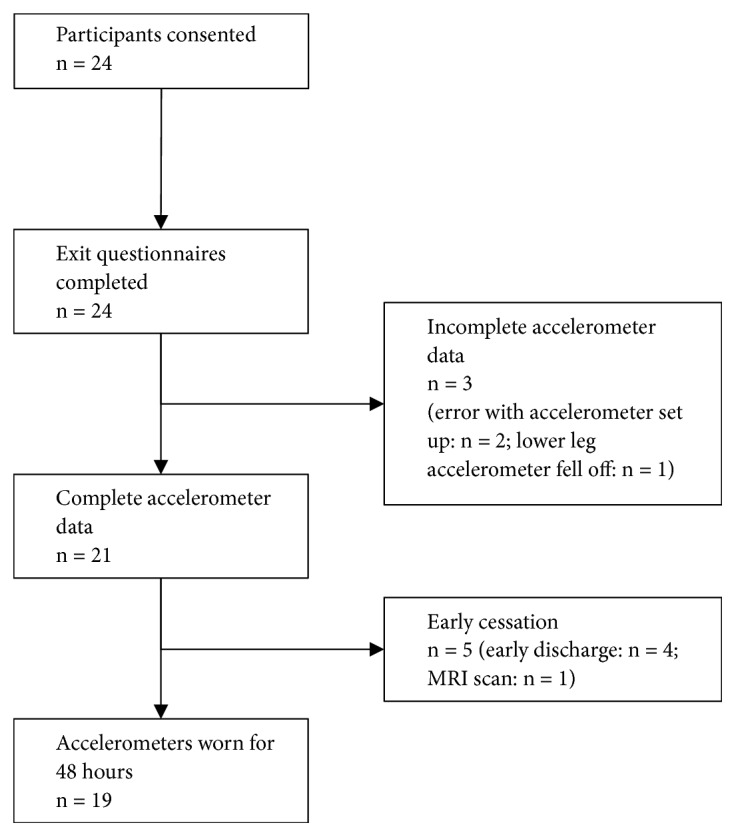
Flow of participants through study.
All participants completed the acceptability questionnaire. All participants reported ‘no discomfort', with 16 participants reporting similar comments to the effect of having forgotten that they were wearing the accelerometers. All participants reported that their sleep had not been affected by wearing the accelerometers. All but one participant reported that they would wear the accelerometers again in a future study.
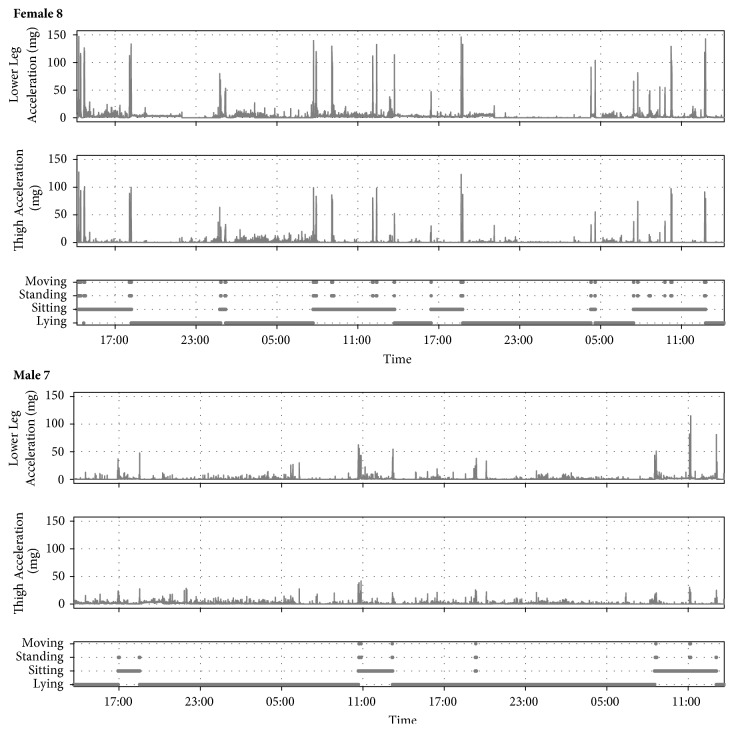
The levels of activity for each participant are summarized in Table 1 and Figure 4. On average, patients were lying for 62.6% (±16.3) of the recording time, sitting for 34.2% (±14.7), standing but not moving 2.0% (±1.9), and standing and moving 1.2% (± 1.1). Examples of participant activity over the study period are illustrated in Figure 5.
Figure 4.
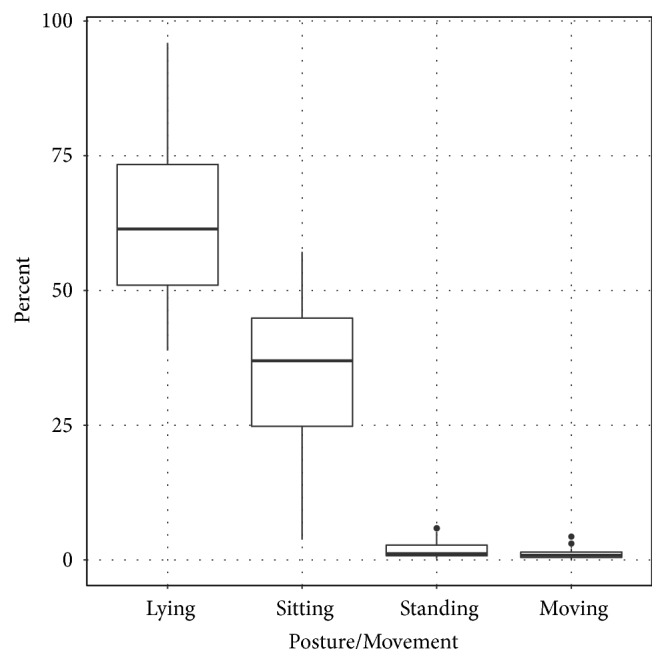
Summary of posture and movement during measurement period.
Figure 5.
Examples of participant's activity during study period.
4. Discussion
This study described a novel method of measuring modalities of physical activity in older patients during an acute hospitalization. The method was feasible and allowed differentiation between lying, sitting, standing, and moving (upright). Furthermore, this measurement was considered to be acceptable from the patients' perspective.
Our study built upon previous work using 2 accelerometers to differentiate positions of hospitalized patients. We believe this is the first study to use accelerometers on the thigh and lower leg to differentiate between lying, sitting, standing, and moving in acutely hospitalized patients.
Compared to the PAL2 device, the AX3 device and the methods we used to attach it to the participants may be more acceptable. Using the PAL2 device, Kramer et al. [18] reported two of the eight participants reported the device being uncomfortable.
The amount of time spent standing or walking was similar to that recorded by other studies of similar populations, though the amount of time spent lying was lower. Pedersen et al. [14] reported that their ambulatory patients were lying 70% of the time, sitting 21%, and standing or walking 4%; Brown et al. [1] reported participants lying 83% of the time, sitting 12.9%, and standing or walking 3.8%.
We used a convenience sample, and the duration of the study was a maximum of 48 hours; it is possible that, with a larger random sample and longer study duration, the reported acceptability of the devices may have been different. Furthermore, our participants had on average been in hospital 4 days prior to starting the study; it is possible that they would have found the accelerometers less acceptable in the first days of hospitalization. The movement threshold between standing and moving has not been validated in the patient population and is likely to be inaccurate at walking speeds of less than 0.1 m/s. This is relevant given the known slow walking speed of our population; however the significance of standing versus walking at such a slow speed is not known.
5. Conclusion
The methodology described in this study can be used to differentiate between lying, sitting, standing, and standing and moving and is very acceptable from a hospitalized patient's perspective. Work is now required to examine the significance of the derived variables on hospital outcomes.
Acknowledgments
The authors thank Antonia Smith from the Physical Activity Technical Team, MRC Epidemiology Unit, for her assistance in the processing of the physical activity data. The authors also thank all the members of the DME teams in our hospital, without which this initiative would have not been possible. The authors also thank Catherine Hartley for the illustrations. This feasibility study was part of PH's Addenbrooke's Charitable Trust/NIHR Cambridge Biomedical Research Centre (BRC) Internal Research Fellowship and completed as part of his Dunhill Medical Trust Research Training Fellowship [Grant no. RTF115/0117]. The work of Kate Westgate and Søren Brage was supported by the Medical Research Council [MC_UU_12015/3] and the NIHR Biomedical Research Centre Cambridge [IS-BRC-1215-20014]. Tom White was supported by a Ph.D. studentship from MedImmune Ltd.
Data Availability
The data used to support the findings of this study have not been made available as this was not part of our ethics application in order to protect patient confidentiality.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Brown C. J., Redden D. T., Flood K. L., Allman R. M. The underrecognized epidemic of low mobility during hospitalization of older adults. Journal of the American Geriatrics Society. 2009;57(9):1660–1665. doi: 10.1111/j.1532-5415.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 2.Villumsen M., Jorgensen M. G., Andreasen J., Rathleff M. S., Molgaard C. M. Very low levels of physical activity in older patients during hospitalization at an acute geriatric ward: A prospective cohort study. Journal of Aging and Physical Activity. 2015;23(4):542–549. doi: 10.1123/japa.2014-0115. [DOI] [PubMed] [Google Scholar]
- 3.Zisberg A., Shadmi E., Sinoff G., Gur-Yaish N., Srulovici E., Admi H. Low mobility during hospitalization and functional decline in older adults. Journal of the American Geriatrics Society. 2011;59(2):266–273. doi: 10.1111/j.1532-5415.2010.03276.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisher S. R., Goodwin J. S., Protas E. J., et al. Ambulatory activity of older adults hospitalized with acute medical illness. Journal of the American Geriatrics Society. 2011;59(1):91–95. doi: 10.1111/j.1532-5415.2010.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafont C., Gérard S., Voisin T., Pahor M., Vellas B. Reducing "iatrogenic disability" in the hospitalized frail elderly. The Journal of Nutrition, Health & Aging. 2011;15(8):645–660. doi: 10.1007/s12603-011-0335-7. [DOI] [PubMed] [Google Scholar]
- 6.Pitta F., Troosters T., Probst V. S., Spruit M. A., Decramer M., Gosselink R. Physical activity and hospitalization for exacerbation of COPD. CHEST. 2006;129(3):536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 7.Wijndaele K., Westgate K., Stephens S. K., et al. Utilization and harmonization of adult accelerometry data: review and expert consensus. Medicine & Science in Sports & Exercise. 2015;47(10):2129–2139. doi: 10.1249/mss.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strath S. J., Kaminsky L. A., Ainsworth B. E., et al. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart association. Circulation. 2013;128(20):2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 9.Doherty A., Jackson D., Hammerla N., et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0169649.e0169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward D. S., Evenson K. R., Vaughn A., Rodgers A. B., Troiano R. P. Accelerometer use in physical activity: Best practices and research recommendations. Medicine & Science in Sports & Exercise. 2005;37(11):S582–S588. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]
- 11.Ser L., Ibrahim K., Sayer A. A., Roberts H. C. Assessment of physical activity of hospitalised older adults: A systematic review. The Journal of Nutrition, Health & Aging. 2017 doi: 10.1007/s12603-017-0931-2. [DOI] [PubMed] [Google Scholar]
- 12.Culhane K. M., Lyons G. M., Hilton D., Grace P. A., Lyons D. Long-term mobility monitoring of older adults using accelerometers in a clinical environment. Clinical Rehabilitation. 2004;18(3):335–343. doi: 10.1191/0269215504cr734oa. [DOI] [PubMed] [Google Scholar]
- 13.Brown C. J., Roth D. L., Allman R. M. Validation of use of wireless monitors to measure levels of mobility during hospitalization. Journal of Rehabilitation Research and Development . 2008;45(4):551–558. doi: 10.1682/JRRD.2007.06.0086. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen M. M., Bodilsen A. C., Petersen J., et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2013;68(3):331–337. doi: 10.1093/gerona/gls165. [DOI] [PubMed] [Google Scholar]
- 15.Mundy L. M., Leet T. L., Darst K., Schnitzler M. A., Dunagan W. C. Early mobilization of patients hospitalized with community-acquired pneumonia. CHEST. 2003;124(3):883–889. doi: 10.1378/chest.124.3.883. [DOI] [PubMed] [Google Scholar]
- 16.Avert Trial Collaboration group, Bernhardt J., Langhorne P., et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. The Lancet. 2015;386(9988):46–55. doi: 10.1016/S0140-6736(15)60690-0. [DOI] [PubMed] [Google Scholar]
- 17.Bliss M. Should elderly patients be made to sit in chairs? BMJ. 2003;327(7421):p. 997. doi: 10.1136/bmj.327.7421.997. [DOI] [Google Scholar]
- 18.Kramer S. F., Cumming T., Churilov L., Bernhardt J. Measuring activity levels at an acute stroke ward: comparing observations to a device. BioMed Research International. 2013;2013:8. doi: 10.1155/2013/460482.460482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond M., Winter A., Holland A. E. Validation of an activity monitor in older inpatients undergoing slow stream rehabilitation. Journal of Physical Activity & Health. 2015;12(9):1298–1303. doi: 10.1123/jpah.2014-0347. [DOI] [PubMed] [Google Scholar]
- 20.Taraldsen K., Askim T., Sletvold O., et al. Evaluation of a body-worn sensor system to measure physical activity in older people with impaired function. Physical Therapy in Sport. 2011;91(2):277–285. doi: 10.2522/ptj.20100159. [DOI] [PubMed] [Google Scholar]
- 21.Hartley P., Adamson J., Cunningham C., Embleton G., Romero-Ortuno R. Clinical frailty and functional trajectories in hospitalized older adults: A retrospective observational study. Geriatrics & Gerontology International. 2016 doi: 10.1111/ggi.12827. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Ortuno R., Wallis S., Biram R., Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: An observational study. European Journal of Internal Medicine. 2016;35:24–34. doi: 10.1016/j.ejim.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Julious S. A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceutical Statistics. 2005;4(4):287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 24.Ladha C., Jackson D., Ladha K., Nappey T., Olivier P. Shaker table validation of OpenMovement AX3 accelerometer. Proceedings of the 3rd International Conference on Ambulatory Monitoring of Physical Activity and Movement; 2013; Amherst, Mass, USA. [Google Scholar]
- 25.Charlson M. E., Pompei P., Ales K. L., MacKenzie C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Royal College of Physicians of London. National Early Warning Score (NEWS): Standardising the Assessment of Acute-Illness Severity in the NHS—Report of a Working Party. 2012. [Google Scholar]
- 27.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosanna Chau M. W., Chan S. P., Wong Y. W., Lau M. Y. P. Reliability and validity of the Modified Functional Ambulation Classification in patients with hip fracture. Hong Kong Physiotherapy Journal. 2013;31(1):41–44. doi: 10.1016/j.hkpj.2013.01.041. [DOI] [Google Scholar]
- 29. AX3 OMGUI Configuration and Analysis Tool, v38, [computer program], GitHub, 2015.
- 30. Thomite/pampro v0.4.0, [computer program], Zenodo, 2018.
- 31.Van Hees V. T., Fang Z., Langford J., et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: An evaluation on four continents. Journal of Applied Physiology. 2014;117(7):738–744. doi: 10.1152/japplphysiol.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hees V. T., Gorzelniak L., Dean León E. C., et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061691.e61691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Hees V. T., Renström F., Wright A., et al. Estimation of daily energy expenditure in pregnant and Non-Pregnant women using a Wrist-Worn Tri-Axial accelerometer. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0022922.e22922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartley P., Keevil V. L., Romero-Ortuno R. The association between clinical frailty and walking speed in older hospitalized medical patients: A retrospective observational study. European Geriatric Medicine. 2017;8(2):130–133. doi: 10.1016/j.eurger.2017.01.005. [DOI] [Google Scholar]
- 35.White T., Westgate K., Wareham N. J., Brage S. Estimation of physical activity energy expenditure during free-living from wrist accelerometry in UK adults. PLoS ONE. 2016;11(12) doi: 10.1371/journal.pone.0167472.e0167472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study have not been made available as this was not part of our ethics application in order to protect patient confidentiality.