Abstract
The carrageenophyte Kappaphycus alvarezii (Rhodophyta) has neurotrophic activity in primary hippocampal neurons. This seaweed is abundant and easily cultivated in tropical coastal areas. To determine the best growth conditions for neurotrophic activity, thalli were grown at different depths and for different periods in various areas of Indonesia. Neurotrophic activity was measured based on the number of primary neurites, the total length of the primary neurites, and the length of the longest neurite. K. alvarezii had higher neurotrophic activity than carrageenophytes K. striatum and Eucheuma denticulatum cultured under the same conditions. K. alvarezii grown at the surface for 45 days had higher (1.4- to 1.8-fold) neurotrophic activity than thalli grown at depth (2 m) or harvested sooner (15 days) (P < 0.05). Relatively high activities were detected in thalli cultured at Ternate and Garut, Indonesia. Therefore, from a commercial perspective, the culture conditions at the surface for 45 days were optimal for the production of both neurotrophic compounds and carrageenan. K. alvarezii produced neurotrophic compounds under various environmental conditions, although some conditions were optimal.
1. Introduction
Neurodegenerative disorders are a crucial threat to human health, especially in the elderly. These disorders are characterized by gradual degeneration of the structure and function of the central or peripheral nervous system [1]. The most common neurodegenerative disorder is Alzheimer's disease. People with Alzheimer's disease have memory dysfunction and hippocampal atrophy [2, 3]. Neurotrophic activity can prevent cell death in neurodegenerative processes and play key roles in the survival, differentiation, synaptogenesis, and maturation of affected neurons in Alzheimer's disease [4].
Macroalgae are used in health foods, diet supplements, and other useful compounds. The edible rhodophyte Kappaphycus alvarezii produces a hydrocolloid carrageenan that is used as a food additive and a gelling, emulsifying, and stabilizing agent in nutraceutical and pharmaceutical products [5]. Recently, this seaweed was reported to have beneficial effects in preventing diet-induced metabolic syndrome [6]. Other research has shown that K. alvarezii is cardioprotective [7] and has wound healing [8], antioxidant [9], antimicrobial [10], and anti-inflammatory [11] properties. Pangestuti and Kim [12] reported that K. alvarezii has antioxidant, anti-neuroinflammatory, and anti-cholinesterase activities. Previously, we showed that K. alvarezii exerts its neurotrophic activity by accelerating the initial neuronal maturation and stimulating axodendritic arborization in primary cultures of hippocampal neurons [13, 14].
In Indonesia, K. alvarezii is abundant and is cultivated in many localities. Generally, the bioactive compound content of macroalgae depends on physical and biological factors, such as climate, water quality, reproductive state, blade age, thallus section, locality, and seasonality [15]. The biochemical composition of K. alvarezii can vary with the cultivation period and planting density [16], depth [17], season [18], and spatiotemporal conditions [19]. It is important to evaluate the growth conditions and bioactivity when macroalgae are exploited commercially. Studies of the depth, growth period, and locality are essential when characterizing the bioactivities of algae.
Therefore, we cultivated K. alvarezii thalli at different depths and for different growth periods in various areas of Indonesia and compared the neurite outgrowth-promoting activity on hippocampal neuron cells.
2. Materials and Methods
2.1. Macroalgal Materials and Aquaculture
The floating bamboo method was used for aquaculture, with the cages placed 200 meters from the shore. The bamboo frames (5 × 5 m2) were equipped with polyethylene plastic buoys for floatation and sandbags for anchors to prevent the influence of tides (Figure 1). Nylon lines were connected to the bamboo frame vertically and horizontally. The depth of nylon culture nets was adjusted to 0, 1, or 2 meters. At each depth, a culture net was used for macroalgae cultivation. Approximately 200 g (wet weight) of fresh K. alvarezii thalli was tied to the culture ropes with plastic twine. Tissue samples were collected after 0, 15, 30, or 45 days, rinsed twice with seawater to remove debris, and rinsed again with freshwater to remove salt. Then, the tissues were dried in the shade for 2~3 days. The dried samples were pulverized into a powder in a grinder (HMF-340, Hanil, Seoul, Korea) and kept in the dark at –20°C until used.
Figure 1.
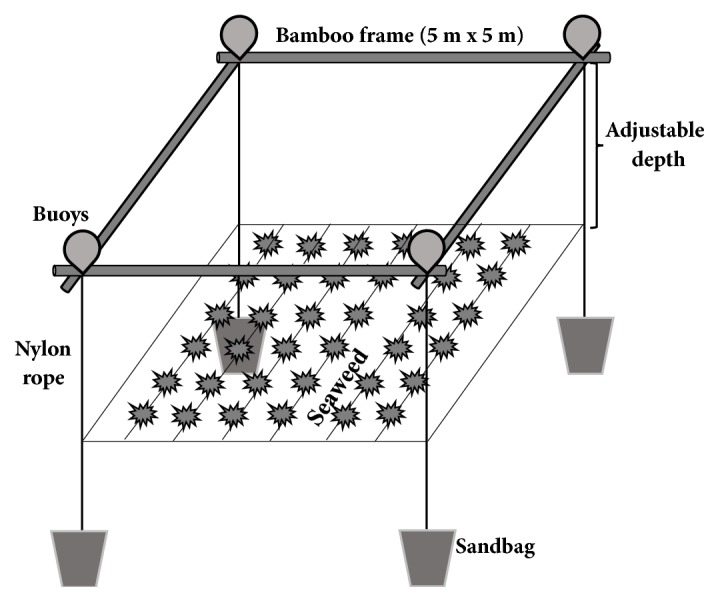
The floating bamboo frame system used in this study. The bamboo frame measured 5 m × 5 m. Six culture nets were fixed to the frame. The depth of the culture nets was adjusted to 0, 1, or 2 m. Six algal thalli were grown on each culture line (5 m).
Thalli of the carrageenophytes K. alvarezii, K. striatum, and Eucheuma denticulatum were cultured in Buleleng Regency (8°11′01.5′′ S, 114°48′39.2′′ E), western Bali, Indonesia, during the dry seasons (April–June) from 2015 to 2017. The three carrageenophytes were cultured on nets at the surface (0 m depth) for 45 days. For the experiments at different depths and for growing periods, K. alvarezii was cultivated at 0, 1, and 2 m for 0, 15, 30, and 45 days at the same site in Buleleng Regency. For the experiments comparing location, K. alvarezii thalli were cultured at the surface (0 m depth) in cages of the same size for 45 days at 14 localities in Indonesia: Bali, Banten, Batam, Garut, Kalimantan, Karimunjawa, Kendari, Lampung, Lombok, Morotai, Papua, Sumbawa, Takalar, and Ternate.
2.2. Culture and Treatment of Primary Hippocampal Neurons
Cultures of hippocampal neurons were prepared in 24-well polystyrene plates. All cell culture reagents were purchased from Invitrogen (Carlsbad, CA, USA) unless otherwise stated. All animal care and use were in agreement with institutional guidelines and approved by the Institutional Animal Care and Use Committee of the College of Medicine, Dongguk University, Korea. Primary hippocampal neurons were prepared as described previously [20, 21]. Briefly, Sprague-Dawley rats on day 19 of pregnancy were euthanized with isoflurane, and the fetuses were collected. The neuronal cells dissociated from the fetal hippocampi were counted with a hemocytometer and plated at a density of approximately 1–2 × 104 cells/cm2 onto poly-DL-lysine-coated glass coverslips in 24-well culture plates. Cultures were maintained in serum-free neurobasal media supplemented with B27 and incubated at 37°C under 5% CO2 and 95% air for 2 days in vitro (DIV 2).
Fine powder of K. alvarezii was processed to obtain an extract using 95% ethanol according to Tirtawijaya et al. [13]. The ethanol extract was dissolved in dimethyl sulfoxide (DMSO) to 8 mg/ml. The extract with an optimal concentration of 1 μg/ml or vehicle control (DMSO, final concentration ≤ 0.5%) was added to the culture media prior to cell plating.
2.3. Image Acquisition
Images (1,388 × 1,039 pixels) were captured using a Leica DM IRE2 research microscope equipped with I3 S, N2.1S, and Y5 filter systems (Leica Microsystems AG, Wetzlar, Germany) and a high-resolution CoolSNAP™ CCD camera (Photometrics, Munich, Germany) under the control of a computer using the Leica FW4000 program. The digital images were processed using Adobe Photoshop 7.0 software (Adobe, San Jose, CA, USA).
2.4. Image Analysis and Quantification
Morphometric analyses and quantification were performed using ImageJ software, version 1.48 (National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij) with the simple neurite tracer plug-in. Morphometric parameters such as the number of primary neurites (NPN; neurites that originated directly from the soma), the total length of primary neurites (TLPN; the sum of primary neurite lengths), and the length of the longest neurite (LLN) were measured. Neurons (a minimum of 50 cells) that were not intermingled with the processes of adjacent neurons were selected for analysis. We always compared the extract-treated cultures with the vehicle control (cultures with DMSO).
2.5. Statistical Analysis
Results are shown as the means ± standard error (SE) of at least three independent experiments. Data were checked for the normality of the distribution by the Kolmogorov-Smirnov test, and normally distributed data were used for further analysis. We conducted a two-way analysis of variance (ANOVA) on data of different depths and culture times, while data from different species and localities were analyzed by a one-way ANOVA. Duncan's post hoc multiple comparison was used to determine significant (P < 0.05) differences among ≥ 3 treatment means. Correlations between cultivation conditions and NPN, TLPN, and LLN were examined using Pearson's correlation coefficients with α < 0.05. All calculations were carried out using SPSS statistical software for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results
Neurotrophic activity was quantified based on morphometric analyses of hippocampal neurons using NPN, TLPN, and LLN. Adding the K. alvarezii extract to the neuron cultures enhanced the growth of neurites. First, we compared three abundant carrageenophytes (K. alvarezii, K. striatum, and E. denticulatum) that are aquaculturable in Indonesia to select the one with the highest neurotrophic activity. We grew the plants at the surface (0 m depth) for 45 days on bamboo frame nets in Buleleng Regency and compared their neurotrophic activities (Figure 2). The three species did not differ significantly in terms of NPN, but they differed significantly in terms of TLPN and LLN (P < 0.05). K. alvarezii had higher neurotrophic activity in terms of TLPN and LLN than K. striatum or E. denticulatum, so K. alvarezii was selected as the best species for further neurotrophic experiments.
Figure 2.
Neurotrophic activity of Kappaphycus alvarezii (A), Kappaphycus striatum (B), and Eucheuma denticulatum (C) on primary hippocampal neurons at day in vitro (DIV) 2. The seaweeds were grown at the surface for 45 days in west Bali. The morphometry of the neurons (≥ 50 cells) was measured based on (a) the number of primary neurites (NPN), (b) the total length of the primary neurites (TLPN), and (c) the length of the longest neurite (LLN). Activity (%) is expressed as the mean ± SE (n ≥ 3) relative to the control. Extracts of 1 μg/ml (black) and 10 μg/ml (grey) were added to the neuron cultures. Different letters at the 1 μg/ml (a, b) and 10 μg/ml (x, y) concentrations indicate significant differences (P < 0.05).
Next, to determine the effective growth conditions to promote the neurotrophic activity of K. alvarezii, thalli were grown at different depths and for different periods. The K. alvarezii grown at 0 m for 45 days and at 1 m for 30 days had a significantly higher NPN, increasing by approximately 1.4- and 1.4-fold, respectively, compared with the vehicle control (Table 1). The initial seeds at 0 d, harvested from thalli grown for 45 days, had an NPN 1.2-fold higher than the control. After growth for 15 days at all depths, i.e., the young growing stage, neurotrophic activity was similar to that of the controls; i.e., there was no neurotrophic activity. NPN tended to be lower in cultures at a depth of 2 m for all harvest times.
Table 1.
Comparison of the number of primary neurites (NPN) in hippocampal neurons exposed to extracts of K. alvarezii grown at different depths and for different culture periods.
| Depth (m) | Culture period (days) | |||
|---|---|---|---|---|
| 0 | 15 | 30 | 45 | |
| 0 | 121 ± 3 abx | 107 ± 4 ax | 126 ± 5 bxy | 135 ± 9 bx |
| 1 | 121 ± 3 abx | 105 ± 2 bx | 140 ± 2 cx | 122 ± 8 ax |
| 2 | 121 ± 3 abx | 110 ± 3 ax | 122 ± 5 by | 118 ± 3 abx |
Activity (%) is expressed as the mean ± SE (n ≥ 3) relative to the control. Day 0: initial seeding day. Means within each column with different letters (x, y) differ significantly (P < 0.05). Means within each row with different letters (a–c) differ significantly (P < 0.05).
In terms of TLPN, the seaweed thalli grown at 0 m for 45 days and at 1 m for 30 days had significantly higher activity than the control, increasing by approximately 1.8- and 1.8-fold, respectively (Table 2). Generally, thalli grown at less than 1 m and for longer than 30 days had high activity. The initial seeds at 0 day, from seaweed previously grown for 45 days, also had a high TLPN. No promotional activity was seen after growth for 15 days at all depths. TLPN tended to be lower for all cultures at a depth of 2 m for all harvest times.
Table 2.
Comparison of the total length of primary neurites (TLPN) in hippocampal neurons exposed to extracts of K. alvarezii grown at different depths and for different culture periods.
| Depth (m) | Culture period (days) | |||
|---|---|---|---|---|
| 0 | 15 | 30 | 45 | |
| 0 | 158 ± 5 ax | 115 ± 6 bx | 160 ± 5 ax | 180 ± 10 ax |
| 1 | 158 ± 5 ax | 100 ± 1 bx | 177 ± 6 ax | 164 ± 17 axy |
| 2 | 158 ± 5 ax | 100 ± 7 bx | 123 ± 6 cy | 131 ± 2 cy |
Activity (%) is expressed as the mean ± SE (n ≥ 3) relative to the control. Day 0: initial seeding day. Means within each column with different letters (x, y) differ significantly (P < 0.05). Means within each row with different letters (a–c) differ significantly (P < 0.05).
With regard to the promotion of LLN, thalli grown at depth 0 m for 45 days had the highest activity, increasing by approximately 1.7-fold higher than the control (Table 3). Generally, thalli grown at less than 1 m and for longer than 30 days had high activity. The initial seeds at 0 day also had a high LLN. At a culture time of 15 days at all depths, no promotional activities were observed. LLN tended to decrease at a depth of 2 m for all harvest times.
Table 3.
Comparison of the length of the longest neurites (LLN) in hippocampal neurons exposed to extracts of K. alvarezii grown at different depths and for different culture periods.
| Depth (m) | Culture period (days) | |||
|---|---|---|---|---|
| 0 | 15 | 30 | 45 | |
| 0 | 153 ± 7 ax | 105 ± 7 bxy | 154 ± 5 ax | 172 ± 18 ax |
| 1 | 153 ± 7 ax | 101 ± 5 bxy | 160 ± 5 ax | 155 ± 12 ax |
| 2 | 153 ± 7 ax | 93 ± 5 by | 106 ± 6 bcy | 122 ± 2 cy |
Activity (%) is expressed as the mean ± SE (n ≥ 3) relative to the control. Day 0: initial seeding day. Means within each column with different letters (x, y) differ significantly (P < 0.05). Means within each row with different letters (a–c) differ significantly (P < 0.05).
To investigate the influence of location, thalli of K. alvarezii were cultured at the surface for 45 days in 14 localities in Indonesia. Extracts were prepared from the thalli from the 14 localities and their neurotrophic activities were tested. The NPN, TLPN, and LLN activities in extracts from different areas differed to varying degrees (Table 4). Relatively high activities were detected in thalli cultured at Ternate, North Maluku, and Garut, West Java. Thalli from Ternate promoted NPN, TLPN, and LLN by 1.8-, 2.1-, and 1.9-fold, respectively, compared with the control, while thalli from Garut promoted them by 1.7-, 2.0-, and 2.0-fold. The highest NPN was obtained with the extract from Ternate and the lowest with that from Takalar (1.1-fold the control). The highest TLPN was obtained with the extract from Ternate and the lowest from Batam (1.2-fold). The highest LLN was obtained from Lombok (2.0-fold) and the lowest from Batam (1.1-fold). The neurotrophic activity of the K. alvarezii extracts varied significantly with location (P < 0.05). These results confirmed that different environmental conditions affect the production of neurotrophic compounds in K. alvarezii tissues. Figure 3 shows representative neurons grown in vehicle and extracts of K. alvarezii cultivated in Ternate and Garut on DIV 2.
Table 4.
Neurotrophic activities of extracts of K. alvarezii thalli grown in various areas in Indonesia.
| Culture area | Activity (%) | ||
|---|---|---|---|
| NPN | TLPN | LLN | |
| West Bali, Bali | 135 ± 9 ab | 180 ± 10 a | 172 ± 18 ab |
| Banten, Banten | 149 ± 4 bc | 200 ± 11 ab | 184 ± 4 ab |
| Batam, Riau Islands | 135 ± 8 ab | 118 ± 7 c | 106 ± 6 c |
| Garut, West Java | 169 ± 3 cd | 196 ± 10 ab | 198 ± 12 b |
| Bunyu Island, North Kalimantan | 164 ± 4 cd | 183 ± 3 ab | 189 ± 10 ab |
| Karimunjawa, Central Java | 138 ± 2 ab | 144 ± 10 cde | 141 ± 12 de |
| Kendari, Southeast Sulawesi | 152 ± 12 bc | 186 ± 6 ab | 162 ± 9 ae |
| Lampung, Lampung | 149 ± 9 bc | 175 ± 10 a | 177 ± 7 ab |
| Lombok, West Nusa Tenggara | 154 ± 4 bc | 187 ± 4 ab | 199 ± 8 b |
| Morotai, North Maluku | 171 ± 9 cd | 185 ± 5 e | 152 ± 13 ab |
| Papua, Papua | 120 ± 1 ae | 132 ± 5 cde | 118 ± 5 cd |
| Sumbawa, West Nusa Tenggara | 135 ± 4 ab | 148 ± 9 de | 140 ± 6 de |
| Takalar, South Sulawesi | 111 ± 2 e | 121 ± 2 cd | 136 ± 7 de |
| Ternate, North Maluku | 181 ± 13 d | 209 ± 11 b | 189 ± 11 ab |
Thalli were cultured at the surface for 45 days in 14 different locations. Activity (%) is expressed as the mean ± SE (n ≥ 3) relative to the control. Means within each column with different letters (a–e) differ significantly (P < 0.05).
Figure 3.
Representative images of neurons grown in vehicle (a) and extracts of Kappaphycus alvarezii grown at Ternate (b) and Garut (c). Primary neurites (arrows) and the longest neurite (arrowheads) were observed using ImageJ software to determine the NPN, TLPN, and LLN.
4. Discussion
Neurotrophic factors are molecules that are critical for neurite outgrowth and affect neuron survival, synaptic plasticity, and the formation of long-lasting memories [22]. Previously, we found that the rhodophyte K. alvarezii was the best candidate for the production of neurotrophic factors from among 34 common Indonesian macroalgae tested [13]. This macroalga contains several lipophilic compounds that contribute to its neurotrophic activity [14]. There is high demand for K. alvarezii aquaculture in Indonesia for the production of hydrocolloid carrageenan. K. striatum and E. denticulatum, two other carrageenan producers, are also widely cultivated in Indonesia. The genus Kappaphycus produces kappa-carrageenan, which has greater gel strength and 3,6-anhydrogalactose levels and fewer sulfate radicals than iota-carrageenan [23]. The genus Eucheuma produces iota-carrageenan. Comparison of these three common carrageenophytes revealed that K. alvarezii had greater neurotrophic activity than the others. The high neurotrophic activity of K. alvarezii did not appear to be related to the gel strength of carrageenan or the amount of sulfate or anhydrogalactose moieties in carrageenan. Some research has indicated that the antioxidant activity and total phenol and carotene contents differ between E. cottonii (now known as K. alvarezii) and E. spinosum (now known as E. denticulatum) [24], but Rosni et al. [25] reported that several varieties of K. alvarezii, K. striatum, and E. denticulatum had insignificant protein and total phenolic contents. Therefore, the neurotrophic activity is not directly related to the levels of antioxidants, phenolics, carotene, or proteins.
Two common methods are used for carrageenophytes aquaculture: longlines and floating cages. A floating cage results in higher biomass production than a longline because the macroalgae can be cultivated vertically in more space and protected from herbivores [26]. Given that light decreases exponentially as depth increases, the chlorophyll and carotenoid pigments increase with depth [17]. We found that the neurotrophic activity was highest in K. alvarezii grown at the surface. Therefore, the neurotrophic activity is also not related to pigment production. The culture conditions that yield the best carrageenan from a commercial perspective were 45 days growth at the surface [16]. These culture conditions were optimal for the production of both carrageenan and neurotrophic compounds in K. alvarezii. When grown for longer than 45 days, thalli are prone to breaking and are lost [16].
The values of NPN, TLPN, and LLN varied from 111.19 to 181.09%, 117.51 to 208.49%, and 105.80 to 199.06%, respectively, at all sites studied. Significant spatial differences were observed in NPN, TLPN, and LLN (one-way ANOVA, all P ≤ 0.05), indicating that different environmental conditions affected the neurotrophic activity. The concentrations of secondary metabolites in macroalgae may vary with changes in environmental conditions [15, 27]. Overall, the neurotrophic activity of K. alvarezii from all localities studied was better than the vehicle control. Therefore, the carrageenophyte K. alvarezii produces neurotrophic compounds under various environmental conditions, although some conditions are more optimal. The macroalgae studied came from all regions of Indonesia, suggesting that K. alvarezii can be cultivated in various localities. Future work should examine the relationship between culture conditions in different localities and the composition of neurotrophic compounds in K. alvarezii tissues.
5. Conclusions
Neurotrophic activities from the carrageenophyte K. alvarezii (Rhodophyta) were measured in primary cultures of rat hippocampal neurons. This seaweed had higher neurotrophic activity than the other carrageenophytes, K. striatum and Eucheuma denticulatum, cultured under the same conditions. K. alvarezii grown at the surface for 45 days had higher neurotrophic activity than thalli grown at a depth of 2 m or harvested sooner (15 days). Relatively high activity levels were detected in thalli cultured at Ternate and Garut, Indonesia. Thus, from a commercial perspective, the culture conditions at the surface for 45 days were optimal for the production of both neurotrophic compounds and carrageenan.
Acknowledgments
We thank Mr. Gigih Nur Akhmad and other farmers who worked for the seaweed aquaculture. This research was supported by the International Research Collaboration and International Publication Grant, Ministry of Research, Technology, and Higher Education, Republic of Indonesia.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Gabriel Tirtawijaya and Maria Dyah Nur Meinita contributed equally to this work.
References
- 1.Gao H. M., Hong J. S. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends in Immunology. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes J., Bartlett J. W., van de Pol L. A., et al. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiology of Aging. 2009;30(11):1711–1723. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahn H. Memory loss in alzheimer's disease. Dialogues in Clinical Neuroscience. 2013;15(4):445–454. doi: 10.31887/DCNS.2013.15.4/hjahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampaio T. B., Savall A. S., Gutierrez M. E. Z., Pinton S. Neurotrophic factors in Alzheimer’s and parkinson’s diseases: Implications for pathogenesis and therapy. Neural Regeneration Research. 2017;12(4):549–557. doi: 10.4103/1673-5374.205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickering T. D., Skelton P., Sulu R. J. Intentional introductions of commercially harvested alien seaweeds. Botanica Marina. 2007;50(5):338–350. [Google Scholar]
- 6.Wanyonyi S., Du Preez R., Brown L., Paul N. A., Panchal S. K. Kappaphycus alvarezii as a food supplement prevents diet‐induced metabolic syndrome in rats. Nutrients. 2017;9(11):1261–1276. doi: 10.3390/nu9111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matanjun P., Mohamed S., Muhammad K., Mustapha N. M. Comparison of cardiovascular protective effects of tropical seaweeds, kappaphycus alvarezii, caulerpa lentillifera, and sargassum polycystum, on high-cholesterol/high-fat diet in rats. Journal of Medicinal Food. 2010;13(4):792–800. doi: 10.1089/jmf.2008.1212. [DOI] [PubMed] [Google Scholar]
- 8.Fard S. G., Tan R. T. R., Mohammed A. A., et al. Wound healing properties of Eucheuma cottonii extracts in Sprague-Dawley rats. Journal of Medicinal Plant Research. 2011;5(27):6373–6380. [Google Scholar]
- 9.Nagarani N., Kumaraguru A. K. Investigation of the effect of K. alvarezii on antioxidant enzymes, cell viability and DNA damage in male rats. Frontiers in Life Science. 2012;6(3-4):97–105. doi: 10.1080/21553769.2013.811123. [DOI] [Google Scholar]
- 10.Prabha V., Prakash D. J., Sudha P. N. Analysis of bioactive compounds and antimicrobial activity of marine alga Kappaphycus alvarezii using three solvent extracts. International Journal of Pharmaceutical Sciences and Research. 2013;4(1):306–310. [Google Scholar]
- 11.Ranganayaki P., Susmitha S., Vijayaraghavan R. Study on metabolic compounds of Kappaphycus alvarezii and its in vitro analysis of anti-inflammatory activity. International Journal of Current Research and Academy Review. 2014;2(10):157–166. [Google Scholar]
- 12.Pangestuti R., Kim S.-K. Neuroprotective effects of marine algae. Marine Drugs. 2011;9(5):803–818. doi: 10.3390/md9050803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirtawijaya G., Mohibbullah M., Meinita M. D. N., Moon I. S., Hong Y.-K. The ethanol extract of the rhodophyte Kappaphycus alvarezii promotes neurite outgrowth in hippocampal neurons. Journal of Applied Phycology. 2016;28(4):2515–2522. doi: 10.1007/s10811-016-0795-6. [DOI] [Google Scholar]
- 14.Tirtawijaya G., Mohibbullah M., Meinita M. D. N., Moon I. S., Hong Y.-K. The tropical carrageenophyte Kappaphycus alvarezii extract promotes axodendritic maturation of hippocampal neurons in primary culture. Journal of Applied Phycology. 2018:1–9. doi: 10.1007/s10811-018-1448-8. [DOI] [Google Scholar]
- 15.Hafting J. T., Craigie J. S., Stengel D. B., et al. Prospects and challenges for industrial production of seaweed bioactives. Journal of Phycology. 2015;51(5):821–837. doi: 10.1111/jpy.12326. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi L., Oliveira E. C., Bleicher-Lhonneur G., et al. The effects of selected cultivation conditions on the carrageenan characteristics of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in Ubatuba Bay, São Paulo, Brazil. Journal of Applied Phycology. 2007;19(5):505–511. doi: 10.1007/s10811-007-9163-x. [DOI] [Google Scholar]
- 17.Indriatmoko, Heriyanto, Limantara L., Brotosudarmo T. H. P. Composition of photosynthetic pigments in a red alga Kappaphycus alvarezii cultivated in different depths. Procedia Chemistry. 2015;14:193–201. [Google Scholar]
- 18.Suresh Kumar K., Ganesan K., Subba Rao P. V. Seasonal variation in nutritional composition of Kappaphycus alvarezii (Doty) Doty - an edible seaweed. Journal of Food Science and Technology. 2015;52(5):2751–2760. doi: 10.1007/s13197-014-1372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Periyasamy C., Rao P. V. S., Anantharaman P. Spatial and temporal variation in carrageenan yield and gel strength of cultivated Kappaphycus alvarezii (Doty) Doty in relation to environmental parameters in Palk Bay waters, Tamil Nadu, Southeast coast of India. Journal of Applied Phycology. 2016;28(1):525–532. doi: 10.1007/s10811-015-0536-2. [DOI] [Google Scholar]
- 20.Goslin K., Asmussen H., Banker G. Rat hippocampal neurons in low-density culture. In: Banker G., Goslin K., editors. Culturing Nerve Cells. 2nd. Vol. 58. Massachusetts, MA, USA: MIT Press; 1998. pp. 339–370. [DOI] [Google Scholar]
- 21.Hannan M. A., Kang J.-Y., Hong Y.-K., et al. The marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytotherapy Research. 2013;27(1):21–29. doi: 10.1002/ptr.4684. [DOI] [PubMed] [Google Scholar]
- 22.Deister C., Schmidt C. E. Optimizing neurotrophic factor combinations for neurite outgrowth. Journal of Neural Engineering. 2006;3(2):172–179. doi: 10.1088/1741-2560/3/2/011. [DOI] [PubMed] [Google Scholar]
- 23.Santos G. A. Carrageenans of species of Eucheuma J. Agardh and Kappaphycus Doty (Solieriaceae, Rhodophyta) Aquatic Botany. 1989;36(1):55–67. doi: 10.1016/0304-3770(89)90091-0. [DOI] [Google Scholar]
- 24.Damongilala L. J., Widjanarko S. B., Zubaidah E., Runtuwene M. R. J. Antioxidant activity against methanol extraction of Eucheuma cottonii and E. spinosum collected from North Sulawesi Waters, Indonesia. Food Science and Quality Management. 2013;17:7–13. [Google Scholar]
- 25.Mohd Rosni S., Fisal A., Azwan A., Chye F. Y., Matanjun P. Crude proteins, total soluble proteins, total phenolic contents and SDS-PAGE profile of fifteen varieties of seaweed from Semporna, Sabah, Malaysia. International Food Research Journal. 2015;22(4):1483–1493. [Google Scholar]
- 26.Kasim M., Mustafa A. Comparison growth of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) cultivation in floating cage and longline in Indonesia. Aquaculture Reports. 2017;6(1):49–55. doi: 10.1016/j.aqrep.2017.03.004. [DOI] [Google Scholar]
- 27.Shalaby E. A. Influence of abiotic stress on biosynthesis of alga-chemicals and its relation to biological activities. Indian Journal of Geo Marine Sciences. 2017;46(1):23–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




