Abstract
Background and Purpose—
Minimizing hematoma growth in high-risk patients is an attractive strategy to improve outcomes after intracerebral hemorrhage. We tested the hypothesis that desmopressin (DDAVP), which improves hemostasis through the release of von Willebrand factor, improves platelet activity after intracerebral hemorrhage.
Methods—
Patients with reduced platelet activity on point-of-care testing alone (5), known aspirin use alone (1), or both (8) received desmopressin 0.4 μg/kg IV. We measured Platelet Function Analyzer-epinephrine (Siemens AG, Germany) and von Willebrand factor antigen from baseline to 1 hour after infusion start and hematoma volume from the diagnostic to a follow-up computed tomographic scan.
Results—
We enrolled 14 patients with of mean age 66.8±14.6 years, 11 (85%) of whom were white and 8 (57%) were men. Mean Platelet Function Analyzer-epinephrine results shortened from 192±18 seconds pretreatment to 124±15 seconds (P=0.01) 1 hour later, indicating improved plate activity. von Willebrand factor antigen increased from 242±96% to 289±103% activity (P=0.004), indicating the expected increase in von Willebrand factor. Of 7 (50%) patients who received desmopressin within 12 hours of intracerebral hemorrhage symptom onset, changes in hematoma volume were modest, −0.5 (−1.4 to 8.4) mL and only 2 had hematoma growth. One patient had low blood pressure and another had a new fever within 6 hours of desmopressin administration.
Conclusions—
Intravenous desmopressin was well tolerated and improved platelet activity after acute intracerebral hemorrhage. Larger studies are needed to determine its potential effects on reducing hematoma growth versus platelet transfusion or placebo.
Keywords: blood platelets, cerebral hemorrhage, deamino arginine vasopressin
Background and Purpose
Hematoma growth after intracerebral hemorrhage (ICH) is associated with worse outcomes.1 Aspirin use is associated with increased mortality,2–4 detectable with point-of-care testing,5 and associated with subsequent hematoma growth and poor outcome at 3 months.6 Thus, improving platelet activity might minimize hematoma growth, improving outcomes. Platelet transfusion is potentially beneficial,7 and a clinical trial is underway.8 Platelet transfusion carries significant risks9; however, so pharmacologically increasing platelet activity is an attractive alternative.
Desmopressin (DDAVP) leads to the release of von Willebrand factor (vWF)10 and reduces clinical bleeding.11,12 Bleeding times have been replaced with point-of-care platelet activity testing. The Platelet function analyzer (Siemens AG, Germany) closure time is prolonged by aspirin, and desmopressin normalizes it for several hours.13 We tested the hypothesis that desmopressin improves platelet activity in patients with acute ICH while obtaining data on safety.
Methods
Patients
We prospectively identified patients with acute ICH confirmed with computed tomographic scan. Patients had either known aspirin use or reduced platelet activity on the VerifyNow-ASA (Accumetrics, CA) as previously described.5,6 Detailed criteria are available at the online trial registration.
All enrolled patients received desmopressin 0.4 μg/kg IV for 30 minutes and received other routine care for patients with acute ICH at our institution, including admission to the neuro–spine intensive care unit, serum chemistries, and follow-up computed tomographic scanning.
Regulatory Approval
The study was approved by the institutional review board, and written consent was obtained. Food and Drug Administration issued an exemption in response to our application for an Investigational New Drug. The study sponsor had no role in the execution of the study, analysis, or decision to submit for publication.
End Points
The primary outcome was change in the Platelet Function Analyzer-epinephrine (using epinephrine as opposed to ADP) from baseline to 1 hour after the start of desmopressin, with vWF antigen as a secondary end point. We prospectively documented acute adverse events: new fever of ≥100.4F, respiratory distress or pulmonary edema on chest radiography, or hypotension as previously reported.7 As exploratory aims, we measured hematoma volumes on the diagnostic computed tomography and a follow-up computed tomography with Mayo Clinic Analyze software (Overland Park, KS)6 and routinely obtained serum chemistries for changes in serum sodium. We measured the modified Rankin Scale (mRS) at 28 days and 3 months with a validated questionnaire.14
Statistical Analysis
Normally distributed data are presented as mean±SD, non-normally distributed data as median (Q1–Q3). To determine the change from baseline to follow-up values, we performed generalized linear models with 2 levels of repeated measures (before and after treatment) with IBM SPSS v.21 (Armonk, NY).
Results
We screened from December 2010 through December 2013. We enrolled 14 of 28 eligible patients (Figure 1). The mean age was 66.8±14.6 years, 11 (85%) were white and 8 (57%) were men. Thirteen (93%) had historical hypertension, and 5 (36%) had a history of diabetes mellitus. Desmopressin infusion started 12.25 (5.7 – 23.1) hours after ICH symptom onset.
Figure 1.
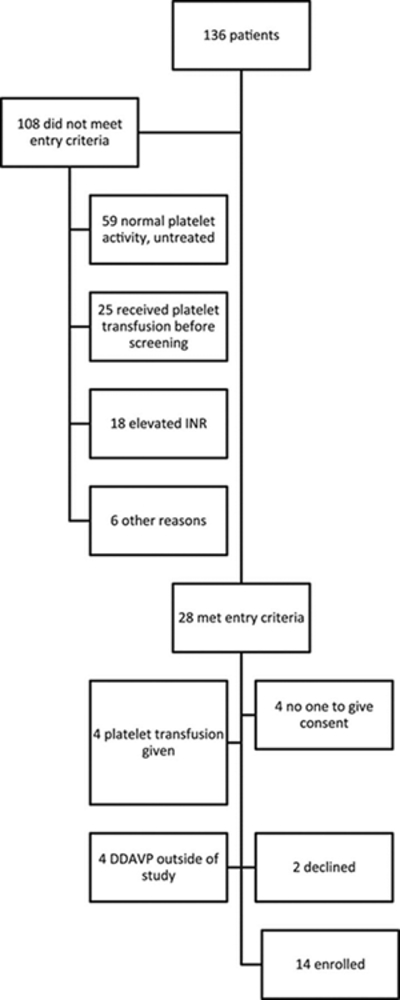
Flowchart of screening and enrollment in the study. INR indicates international normalized ratio.
Study End Points
The Platelet Function Analyzer-epinephrine decreased from 192±18 to 124±15 seconds (P=0.01), consistent with a resolution of an aspirin effect (Figure 2). One patient had a paradoxically increased Platelet Function Analyzer-epinephrine but no hematoma growth. (the machine had been calibrated that day). vWF antigen increased from 242±96% to 289±103% activity (P=0.004) and increased in every patient, indicating that desmopressin administration was associated with increased vWF levels.
Figure 2.
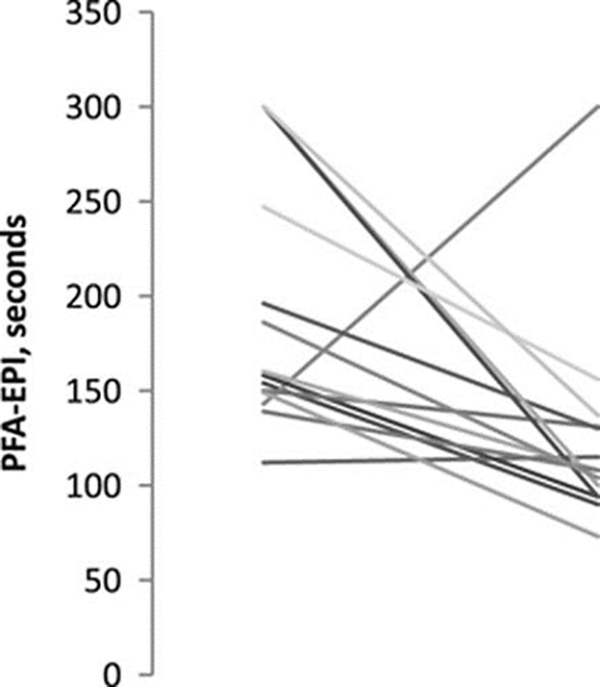
Change in the Platelet Function Analyzer (PFA)-epinephrine (EPI) from baseline to 1 hour after start of desmopressin infusion. Baseline PFA-EPI results shortened from 192±18 to 124±15 seconds (P=0.01) 1 hour later, consistent with a resolution of aspirin effect. One patient had increased PFA-EPI closure time after treatment but no hematoma growth.
Safety
One (7%) patient had hypotension and another had a new fever within 6 hours of infusion start. The mean change in serum sodium from baseline to a follow-up measure 12 to 24 hours later was 0.6 mEq/L. One patient had a decrease of serum Na from 138 to 135 mEq/L (above our laboratory’s lower limit, 134 mEq/L) in the day after desmopressin administration. Three other patients had a decrease of 2 mEq/L, and 3 had a decrease of 1 mEq/L.
Hematoma Growth
Of 7 patients who received desmopressin within 12 hours of ICH symptom onset, the median change in hematoma volume was −0.5 (−1.4 to 8.4) mL. Two had hematoma growth, one of whom was treated at 3.25 hours after ICH symptom onset and the other at 10.5 hours.
Clinical Outcomes
Three months follow-up could be obtained in 12 (86%) patients. At 3 months, 4 patients had an mRS of ≤1 (no disability), 1 had an mRS 3 (moderate disability and independent), and 3 had an mRS 4 (moderately severe disability). Four died, 2 with withdrawal of life support before hospital discharge and 2 of apparent cardiac arrest between 1 and 3 months follow-up. The other 2 patients had mRS 4 at last follow-up.
Discussion
Desmopressin infusion was well tolerated after acute ICH, associated with improved measures of platelet activity, increased vWF antigen, and low rates of hematoma growth. These preliminary results suggest that desmopressin is an attractive potential drug treatment for acute ICH.
We tested desmopressin as monotherapy, although it could be combined with platelet transfusion if platelet transfusion in cerebral hemorrhage8 is positive. If platelet transfusion in cerebral hemorrhage is negative, then desmopressin can be compared with placebo.
Other than the single-arm design, limitations include the lack of stored blood for further assays or routine performance of other specialized assays. We used the mRS as the clinical outcome but have previously noted that health-related quality of life15 may be more sensitive in survivors of ICH.
In sum, we found that desmopressin improved platelet activity, increased vWF antigen levels, and was well tolerated in patients with acute ICH. Hematoma growth was muted in the subset of patients treated within 12 hours of ICH symptom onset. Given its safety, low cost, and reduction in clinical bleeding, desmopressin is an attractive pharmacological treatment for acute ICH.
Acknowledgments
Sources of Funding
This study was supported by a grant from the Dixon Translational Research Grant Initiative and the Northwestern Memorial Foundation.
Footnotes
Disclosures
None.
References
- 1.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. ; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 2.Roquer J, Rodríguez Campello A, Gomis M, Ois A, Puente V, Munteis E. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol 2005;252:412–416. [DOI] [PubMed] [Google Scholar]
- 3.Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen ER, Hillbom M. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke. 2006;37: 129–133. [DOI] [PubMed] [Google Scholar]
- 4.Thompson BB, Béjot Y, Caso V, Castillo J, Christensen H, Flaherty ML, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology. 2010;75:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidech AM, Bernstein RA, Levasseur K, Bassin SL, Bendok BR, Batjer HH, et al. Platelet activity and outcome after intracerebral hemorrhage. Ann Neurol 2009;65:352–356. [DOI] [PubMed] [Google Scholar]
- 6.Naidech AM, Jovanovic B, Liebling S, Garg RK, Bassin SL, Bendok BR, et al. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40:2398–2401. [DOI] [PubMed] [Google Scholar]
- 7.Naidech AM, Liebling SM, Rosenberg NF, Lindholm PF, Bernstein RA, Batjer HH, et al. Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocrit Care 2012;16:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Gans K, de Haan RJ, Majoie CB, Koopman MM, Brand A, Dijkgraaf MG, et al. ; PATCH Investigators. PATCH: platelet transfusion in cerebral haemorrhage: study protocol for a multicentre, randomised, controlled trial. BMC Neurol 2010;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrow JF, Braine HG, Kickler TS, Ness PM, Dick JD, Fuller AK. Septic reactions to platelet transfusions. A persistent problem. JAMA. 1991;266:555–558. [PubMed] [Google Scholar]
- 10.Cattaneo M Desmopressin in the treatment of patients with defects of platelet function. Haematologica. 2002;87:1122–1124. [PubMed] [Google Scholar]
- 11.DiMichele DM, Hathaway WE. Use of DDAVP in inherited and acquired platelet dysfunction. Am J Hematol 1990;33:39–45. [DOI] [PubMed] [Google Scholar]
- 12.Kobrinsky NL, Israels ED, Gerrard JM, Cheang MS, Watson CM, Bishop AJ, et al. Shortening of bleeding time by 1-deamino-8-D-arginine vasopressin in various bleeding disorders. Lancet. 1984;1:1145–1148. [DOI] [PubMed] [Google Scholar]
- 13.Reiter RA, Mayr F, Blazicek H, Galehr E, Jilma-Stohlawetz P, Domanovits H, et al. Desmopressin antagonizes the in vitro platelet dysfunction induced by GPIIb/IIIa inhibitors and aspirin. Blood. 2003;102:4594–4599. [DOI] [PubMed] [Google Scholar]
- 14.Saver JL, Filip B, Hamilton S, Yanes A, Craig S, Cho M, et al. ; FAST- MAG Investigators and Coordinators. Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA). Stroke. 2010;41:992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naidech AM, Beaumont JL, Rosenberg NF, Maas MB, Kosteva AR, Ault ML, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med 2013;188:1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]


