Abstract
The P2Y1 receptor is present in the heart, in skeletal and various smooth muscles, and in platelets, where its activation is linked to aggregation. Adenosine 3′,5′- and 2′,5′-bisphosphates have been identified as selective antagonists at the P2Y1 receptor (Boyer et al. Mol. Pharmacol. 1996, 50, 1323–1329) and have been modified structurally to increase receptor affinity (Camaioni et al. J. Med. Chem. 1998, 41, 183–190). We have extended the structure-activity relationships to a new series of deoxyadenosine bisphosphates with substitutions in the adenine base, ribose moiety, and phosphate groups. The activity of each analogue at P2Y1 receptors was determined by measuring its capacity to stimulate phospholipase C in turkey erythrocyte membranes (agonist effect) and to inhibit phospholipase C stimulation elicited by 10 nM 2-(methylthio)adenosine 5′-diphosphate (antagonist effect). 2′-Deoxyadenosine bisphosphate analogues containing halo, amino, and thioether groups at the 2-position of the adenine ring were more potent P2Y1 receptor antagonists than analogues containing various heteroatom substitutions at the 8-position. An N6-methyl-2-chloro analogue, 6, was a full antagonist and displayed an IC50 of 206 nM. Similarly, N6-methyl-2-alkylthio derivatives 10, 14, and 15 were nearly full antagonists of IC50 < 0.5 μM. On the ribose moiety, 2′-hydroxy, 4′-thio, carbocyclic, and six-membered anhydrohexitol ring modifications have been prepared and resulted in enhanced agonist properties. The 1,5-anhydrohexitol analogue 36 was a pure agonist with an EC50 of 3 μM, i.e., similar in potency to ATP. 5′-Phosphate groups have been modified in the form of triphosphate, methyl phosphate, and cyclic 3′,5′-diphosphate derivatives. The carbocyclic analogue had enhanced agonist efficacy, and the 5′-O-phosphonylmethyl modification was tolerated, suggesting that deviations from the nucleotide structure may result in improved utility as pharmacological probes. The N6-methoxy modification eliminated receptor affinity. Pyrimidine nucleoside 3′,5′-bisphosphate derivatives were inactive as agonists or antagonists at P2Y receptor subtypes.
Introduction
Extracellular adenine and uracil nucleotides act in cell signaling through two families of plasma membrane receptors: the G protein-coupled receptors and the ligand-gated cation channels, termed P2Y and P2X, respectively.1,2 Molecular biological techniques have led to the discovery of new nucleotide receptor subtypes. The P2Y1 receptor for purine nucleotides from chick brain was the first subtype of the P2Y receptor to be cloned.3 The human P2Y1 subtype4,5 has also been cloned, and the gene for the human P2Y1 receptors is localized to chromosome 3.6 Four other distinct, cloned P2Y subtypes are P2Y2 (formerly called P2U), P2Y4, P2Y6, at which uracil nucleotides are particularly active, and P2Y11, which is activated by ATP.7,8 Among the three uracil nucleotide-responsive P2Y receptors, the P2Y2 is activated equally by ATP and UTP. While the P2Y4 subtype is mainly activated by UTP, the P2Y6 is activated by UDP. Recently it was found that uracil nucleotides are released from cells in a manner similar to adenine nucleotides, and this has implications for P2Y receptor activation.9
Agonist binding at P2Y1 receptors results in activation of phospholipase C (PLC), which generates inositol phosphates and diacylglycerol from phosphatidylinositol 4,5-bisphosphate.10 The P2Y1 receptor is present in the heart, in skeletal and various smooth muscles, and in the brain. A P2Y1 receptor in platelets is involved in ADP-promoted aggregation.11,12 Thus, a selective P2Y1 receptor antagonist may have potential as an antithrombotic agent. A selective P2Y1 receptor agonist also has potential as an antihypertensive and possibly antidiabetic agent.13,14
Few potent and selective P2 receptor antagonists are known.15–18 However, adenosine 3′,5′- and 2′,5′-bisphosphates were recently shown to be selective inhibitors of P2Y1 receptors,19 and a novel series of bisphosphate adenosine analogues has been synthesized as potent and selective P2Y1 receptor antagonists.20 Among them, N6-methyl-2′-deoxyadenosine 3′,5′-bisphosphate (MRS 2179, 2; Table 1) was synthesized and is a competitive antagonist at the turkey P2Y1 receptor with a KB value of 102 nM.21 MRS 2179 was inactive at P2Y2, P2Y4, and P2Y6 subtypes, at the adenylyl cyclase-linked P2Y receptor in C6 glioma cells (J. Boyer, personal communication),22 and at a recently cloned, novel avian P2Y receptor that inhibits adenylyl cyclase.23 The antagonist MRS 2179, although competitive and selective, does not have sufficiently high affinity for use as a radioligand. Thus, further structural modifications directed at increasing affinity as well as biological stability have been carried out in the present study.
Table 1.
Structures of Deoxyadenosine 3′,5′-Bisphosphate Derivativesa
| compd | R | R′ | R′′ | X | Y | Z |
|---|---|---|---|---|---|---|
| 1 | NH2 | H | H | N | H | O |
| 2 | NH-CH3 | H | H | N | H | O |
| 3 | NH2 | H | H | N | OH | O |
| 4 | NH-CH3 | H | H | CH | H | O |
| 5 | NH2 | Cl | H | N | H | O |
| 6 | NH-CH3 | Cl | H | N | H | O |
| 7 | NH-CH3 | I | H | N | H | O |
| 8 | NH-OCH3 | H | H | N | H | O |
| 9 | NH2 | S-CH3 | H | N | H | O |
| 10 | NH-CH3 | S-CH3 | H | N | H | O |
| 11 | NH2 | S-CH2CH3 | H | N | H | O |
| 12 | NH-CH3 | S-CH2CH3 | H | N | H | O |
| 13 | NH2 | S-(CH2)2CH3 | H | N | H | O |
| 14 | NH-CH3 | S-(CH2)2CH3 | H | N | H | O |
| 15 | NH-CH3 | S-CH2CH=CH2 | H | N | H | O |
| 16 | NH-CH3 | S-(CH2)5CH3 | H | N | H | O |
| 17a | NH-CH3 | NH2 | H | N | H | O |
| 17b | OH | NH2 | H | N | H | O |
| 18 | NH2 | H | CH3 | N | H | O |
| 19 | NH2 | H | CH=CH2 | N | H | O |
| 20 | NH2 | H | O-CH3 | N | H | O |
| 21 | NH2 | H | S-CH3 | N | H | O |
| 22 | NH2 | H | NH-CH3 | N | H | O |
| 23 | NH2 | H | H | N | H | S |
| 24 | NH2 | H | H | N | H | CH2 |
| 25 | NHCH3 | NH2 | H | N | H | CH2 |
The structures of compounds 26-36 are given in Figure 2.
Results
Chemical Synthesis.
The recent discovery of N6-methyl-2′-deoxyadenosine 3′,5′-bisphosphate (2; Table 1) as a competitive antagonist at P2Y1 receptors20,21 led us to explore further structural modifications of this molecule in order to increase P2Y1 receptor affinity and selectivity. We have prepared 2′-deoxyadenosine bisphosphate analogues containing halo, amino, and thioether groups at the 2-position of the adenine ring or various heteroatom substitutions at the 8-position (Table 1). Carbocyclic, 4′-thio, and six-membered ring modifications have been prepared on the ribose moiety. 5′-Phosphate groups have been modified in the form of triphosphate, methylphosphonate, and cyclic 3′,5′-diphosphate derivatives (Figure 1). Pyrimidine 3′,5′-bisphosphate analogues also were included (Figure 1), since other subtypes of P2Y receptors are known to recognize uridine 5′-di- and triphosphates.1,7
Figure 1.
Structures of purine and pyrimidine nucleotide analogues. The corresponding ammonium salts were synthesized and tested for biological activity.
Some of the nucleosides utilized for phosphorylation were obtained commercially, leading to compounds 3, 5, 17b, and 32-35. Other nucleosides were synthesized as reported, leading to compounds 4, 18, 19, 23, 24, 30, 31, and 36,24–28 or alternatively were synthesized as shown in Schemes 1–6.
Scheme 1.
Synthesis of Nucleoside Precursors of 2-Chloro- and 2-Alkylthio-Substituted Analogues of N6-Methyladenosine 3′,5′-Bisphosphatea
a (a)Ac2O, pyridine, 25 °C, 2 h; (b) (1) IAN, CH2I2, 85 °C, 1 h, (2) 40% aq MeNH2, 25 °C, 1 h; (c) RSH, KButO, or CH3SNa, DMF, 110 °C, 4 h; (d) (1) POCl3, (MeO)3PO, Proton Sponge, 0 °C, 1 h, (2) NH4HCO3.
Scheme 6.
Synthesis of a 5′-O-Phosphonylmethyl Analogue of Adenosine 3′,5′-Bisphosphatea
a (a) KButO, DMF, 25 °C, 3 days; (b) (1) POCl3, (CH3O)3PO, Proton Sponge, 0 °C, 1 h, (2) NH4HCO3.
2-Halo groups were introduced in the structure of the lead compound 2 resulting in compounds 6 (Scheme 1) and 7 (Scheme 2). Commercially available cladribine, 37 2-chloro-2′-deoxyadenosine), was acetylated29 to give the diacetate 38 which upon diazotization-iodination followed by treatment with methylamine resulted in the N6-methyl derivative 39 in 42% yield. Compound 39 was phosphorylated to give the 2-chloro analogue 6. The 2-iodo analogue 7 was prepared in seven steps (Scheme 2). 2′-Deoxyguanosine, 45, was acetylated to the diacetate 46 in 75% yield using acetic anhydride and pyridine, as reported in the literature.29 Chlorination of 46 with POCl3 in the presence of N,N-dimethylaniline and tetraethylammonium chloride in acetonitrile at 100 °C afforded the 6-chloro derivative 47, which on treatment with methylamine resulted in 2-amino-N6-methyladenosine, 48, in 55% yield. Acetylation29 of 48 followed by diazotization-iodination and hydrolysis furnished 2-iodo-N6-methyladenosine, 50, in 48% yield (Scheme 2).
Scheme 2.
Synthesis of 2-Iodo-N6-methyladenosine and Related Nucleosidesa
a (a) Ac2O, Py, 65 °C, 4 h; (b) POCl3, NNDA, TEA Cl, CH3CN, 90 °C, 10 min; (c) 40% aq MeNH2, 25 °C, 2 h; (d) Ac2O, Py, 25 °C, 4 h; (e) (1) POCl3, (MeO)3PO, Proton Sponge, 0 °C, 1 h, (2) NH4HCO3; (f) IAN, CH2I2, 85 °C, 1 h.
2′-Deoxyadenosine nucleotides modified at the 2-position with thioether (9-16) and amino (17) groups were also synthesized. Cladribine, 37, on nucleophilic displacement with the potassium salt of methyl-, ethyl-, or propylthiol afforded the corresponding 2-thio derivatives 51-53, in 70–80% yield (Scheme 3), leading to phosphorylated 2-alkylthio analogues 9, 11, and 13. To obtain the corresponding N6-methyl derivatives, 2-chloroN6-methyl-2′-deoxyadenosine (39; (Scheme 1) was converted to various 2-thioethers by displacement of chlorine at the 2-position with the corresponding alkanethiol potassium salts in 70–80% yield, leading to compounds 10, 12, and 14-16.
Scheme 3.
Synthesis of Nucleoside Precursors of 2-Alkylthio-Substituted Analogues of Adenosine 3′,5′-Bisphosphatea
a (a) RSH, KButO, or CH3SNa, DMF, 110 °C, 4 h; (b) (1) POCl3, (CH3O)3PO, Proton Sponge, 0 °C, 1 h, (2) NH4HCO3.
The synthetic approach for the modification at the 8-position utilized commercially available 2′-deoxyadenosine, 54, as the starting compound (Scheme 4). Bromination of 54 using NBS yielded the 8-bromo derivative, 55, in 40% yield. Displacement of bromine on 53 with various nucleophiles, sodium methoxide, methylamine, or sodium thiomethoxide furnished the corresponding substituted products 56-58 in ~70–90% yield. Phosphorylation of 56-58 and 8-methyl- or 8-vinyl-substituted 2′-deoxyadenosine derivatives30 using the general phosphorylation conditions resulted in the corresponding bisphosphates 18-22 in low to moderate yields.
Scheme 4.
Synthesis of Nucleoside Precursors of 8-Substituted Analogues of Adenosine 3′,5′-Bisphosphatea
a (a) NBS, DMF, 25 °C, 12 h; (b) NaOCH3, DMF, 110 °C, 6 h, or NaSCH3, DMF 110 °C, 4 h, or MeNH2, MeOH, 25 °C, 3 h; (c) (1) POCl3, (CH3O)3PO, Proton Sponge, 0 °C, 1 h, (2) NH4HCO3.
Compound 8, i.e., the methoxy analogue of 2, was prepared. The nucleoside precursor to be phosphorylated, 59, was synthesized by reaction of methoxyamine and 6-chloro-2′-deoxypurine riboside using the general method previously described.20
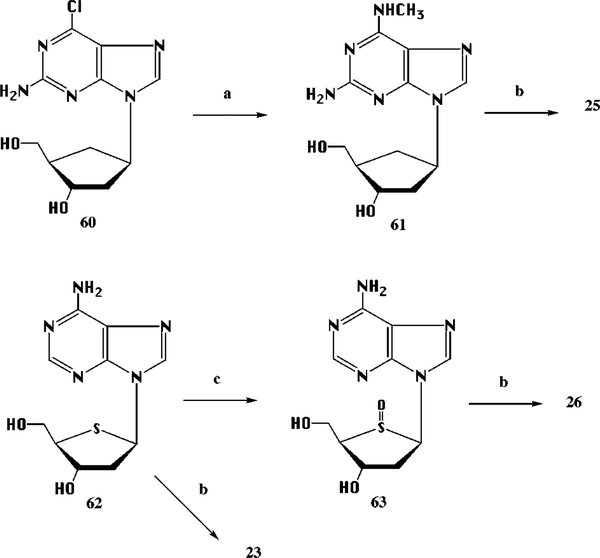
We prepared the bisphosphates of 1,5-anhydro-2-(adenin-9-yl)-2,3-dideoxy-D-arabino-hexitol,25 4′-thioadenosine,26,31 23, and carbocyclic32 analogues, 24 and 25, of 2′-deoxyadenosine 3′,5′-bisphosphate (Table 1). Although difficulties in phosphorylation of 4′-thiopyrimidines were previously reported,37 we have isolated the bisphosphate derivative of 4′-thioadenosine by HPLC of a mixture in low yield. The optimal reaction conditions were a brief reaction time 20 min) at 0 °C. Carbocyclic 2-amino-6-chloropurine 2′-deoxyriboside27 was phosphorylated to provide 24. For inclusion of 2-position substitution, carbocyclic 2-amino-6-chloropurine 2′-deoxyriboside was treated with methylamine and phosphorylated (Scheme 5). 2′-Deoxy-4′-thioadenosine, 62, was also oxidized to the corresponding sulfoxide 63, which upon phosphorylation provided only the 5′-monophosphate 26 (Scheme 5).
Scheme 5.
Synthesis of Precursors of Various Ribose-Modified Analogues (carbocyclic and 4′-thio) of Adenosine 3′,5′-Bisphosphatea
a (a) 40% aq CH3NH2, 25 °C, 3 h; (b) (1) POCl3, (MeO)3PO, Proton Sponge, 0 °C, 1 h, (2) NH4HCO3; (c) NaIO4, 25 °C, 1 h.
The effect of various substitutions and hence various charges on the phosphates also was examined. An O-phosphonylmethyl group was introduced selectively at the 5′-position followed by phosphorylation at the 3′position (Scheme 6) resulting in compound 27. Reaction of 2′-deoxyadenosine, 54, with diethyl tosyl methylphosphonate in the presence of potassium tert-butoxide in DMF for 3 days yielded the 5′-diethyl phosphonate of 2′-deoxyadenosine 64 in 32% yield, which was phosphorylated by a general procedure to provide compound 27. Alternatively, phosphorylation of 54 using the general procedure with methyl dichlorophosphate yielded the corresponding bis(methyl phosphate) 28 in good yield. Attempted phosphorylation of the 3′,5′-bisphosphate 2 to prepare corresponding 5′-di- and triphosphates resulted instead in isolation of 3′,5′-cyclic di- and tetraphosphates 29a and 29b (Scheme 7). The structures of the 3′,5′-cyclic phosphate derivatives were assigned by 2D phosphorus and proton-phosphorus decoupling NMR analysis.
Scheme 7.
Synthesis of 3′,5′-Cyclic Di- and Tetraphosphate Derivativesa
a (a) (1) Carbonyldiimidazole, DMF, 25 °C, 12 h, (2) bis(tributylammonium)pyrophosphate, 25 °C, 42 h, (3) NH4HCO3.
Biological Activity.
The deoxyadenosine bisphosphate nucleotide analogues prepared in the present study were tested separately for agonist and antagonist activity in the PLC assay at the P2Y1 receptor in turkey erythrocyte membranes,21,39 and the results are reported in Table 2. Concentration-response curves were obtained for each compound alone and in combination with 2-(methylthio)adenosine 5′-diphosphate 2-MeSADP), which itself resulted in a marked and concentrationdependent activation of the turkey erythrocyte phospholipase C.21 2-MeSADP was used at a concentration of 10 nM (approximately ) EC50). The activities of all the newly synthesized analogues were compared to that of 2, which we previously identified as the highest potency P2Y1 receptor antagonist thus far identified.20,21 Concentration-response curves for representative compounds are shown in Figure 2.
Table 2.
In Vitro Pharmacological Data for Stimulation of PLC at Turkey Erythrocyte P2Y1 Receptors (agonist effect) and Inhibition of PLC Stimulation Elicited by 10 NM 2-MeSADP (antagonist effect), for at Least Three Separate Determinations
| compd | agonist effect, % of maximal increasea | EC50, μMa | antagonist effect, % of maximal inhibitionb | IC50, μMb (n) |
|---|---|---|---|---|
| 1 | 12±3 | 6.29 ± 2.54 | 87 ± 4 | 5.76±0.68 |
| 2e | NE | 99 ±1 | 0.331 ±0.059 (5) | |
| 3 | 44 ± 5 | 2.34±0.50 | 56 ± 6 | 14.9 ± 1.0 (4) |
| 4 | NE | 96 ± 2 | 0.904 ± 0.239 | |
| 5 | 19±3 | 0.651 ± 0.160 | 80 ± 3 | 2.01 ± 0.83 |
| 6e | NE | 95±1 | 0.206 ± 0.053 | |
| 7 | 13 ± 3 (n = 2) | 4.44 | 87 ±3 | 0.891 ± 0.233 |
| 8 | NE | low potency/NE | >100 (4) | |
| 9 | 22 ± 2 | 0.550 ± 0.117 | 78 ± 2 | 1.89 ±1.07 |
| 10 | 6 ±2 | d | 94 ± 2 | 0.362 ± 0.119 |
| 11 | 16 ± 1 | 1.98 ± 0.42 | 84 ± 1 | 4.59 ± 1.61 |
| 12 | NE | 94 ± 1 | 0.98 ± 0.27 | |
| 13e | 53 ± 7 | 1.71 ± 0.78 | 46 ±7 | c (4) |
| 14 | 7 ± 2 | d | 94 ± 3 | 0.449 ± 0.190 |
| 15 | NE | 97 ± 3 | 0.475 ± 0.206 | |
| 16 | NE | 100 | 3.37 ± 0.91 | |
| 17a | 4 | d | 96 ±2 | 1.85±0.74 |
| 17b | NE | 93 ± 4 | 14.9 ±5.1 | |
| 18 | NE | 53 ± 10 | 75.1 ±14.5 | |
| 19 | NE | 59 ± 10 | 30.7 ±14.8 | |
| 20 | NE | NE | ||
| 21 | NE | low potency/NE | ||
| 22 | NE | low potency/NE | (2) | |
| 23 | 56 ± 7 | 12.2±3.9 | 38 | c |
| 24 | 27±11 | 7.21 ± 4.40 | 73 ± 11 | 2.53 ± 0.57 |
| 25 | 26 ± 3 | 6.49 ± 2.76 | 74±3 | 5.42 ± 2.13 |
| 26 | NE | NE | ||
| 27 | 22 ± 5 | 27.7 ± 16.6 | 78±5 | 19.6 ± 6.1 (4) |
| 28 | NE | NE | ||
| 29a | NE | low potency/NE | ||
| 29b | 14±2 | 0.02 | 87 ± 4 | 0.158 ± 0.064 |
| 30 | 46 ± 10 at 100 μM | low-potency agonist | NE | |
| 31 | 49 ±7 at 100 μM | low-potency agonist | low potency/NE | |
| 32 | NE | NE | ||
| 33 | NE | NE | ||
| 34 | NE | NE | ||
| 35 | NE | NE | ||
| 36e | 100 | 2.99 ± 0.35 | NE | (2) |
Agonist potencies were calculated using a four-parameter logistic equation and the GraphPad softaware package (GraphPad, San Diego, CA). EC50 values (mean ( standard error) represent the concentration at which 50% of the maximal effect is achieved. Relative efficacies (%) were determined by comparison with the effect produced by a maximal effective concentration of 2-MeSADP in the same experiment. Small increase refers to <10% at 100 μM.
Antagonist IC50 values (mean ( standard error) represent the concentration needed to inhibit by 50% the effect elicited by 10 nM 2-MeSADP. The percent of maximal inhibition is equal to 100 minus the residual fraction of stimulation at the highest antagonist concentration; n = 3, unless otherwise indicated in parentheses.
The IC50 of partial agonists with high intrinsic agonist efficacy (>60% of the maximal response) cannot be accurately estimated under these assay conditions due to the small difference between the submaximal response of the full agonist (2-MeSADP) and that produced by the partial agonist.
EC50 was not calculated for increases of )10% at 100 μM.
Figure 2.
Effects of deoxyadenosine bisphosphate derivatives on phospholipase C in turkey erythrocyte membranes: both concentration-dependent stimulation of inositol phosphate formation by 2-MeSADP (●), compound 9 (◻), and compound 36 (b) and its inhibition in the presence of 10 nM 2-MeSADP by compound 6 (▲) and compound 9 (■). Membranes from [3H]-inositol-labeled erythrocytes were incubated for 5 min at 30 °C in the presence of the indicated concentrations of 2-MeSADP or of test compound, either alone or in combination with 10 nM 2-MeSADP. The data shown are typical curves for at least three experiments carried out in duplicate using different membrane preparations.
The presence of the 2′-hydroxyl group in the arabinose derivative 3 compared to the corresponding 2′-deoxy compound 1 resulted in a 3-fold loss of antagonist potency and an increase in the agonist potency and efficacy. The 1-deaza modification in 4 was tolerated at P2Y1 receptors, resulting in a 3-fold loss of antagonist potency versus the corresponding aza analogue 2. The combination of 2-halo and N6-methyl groups resulted in higher antagonist potency for the chloro derivative 6 (IC50 0.20 μM, Figure 2) and a lower potency for the iodo derivative 7 in comparison with 2. Both 6 and 7 were nearly pure antagonists. The 6-(methoxyamino)purine analogue 8 was nearly inactive at P2Y1 receptors, which is consistent with the previously observed critical requirement for a 6-amino or small alkylamino group.
Substitution at the adenine 2-position was generally well-tolerated at P2Y1 receptors and was compatible with the antagonist potency-enhancing effects of an N6methyl group. For example, the N6-methyl-2-methylthio analogue 10 was nearly a full antagonist and equipotent to the corresponding 2-H analogue 2. Compound 10 was 5-fold more potent as an antagonist than the corresponding 6-NH2 analogue 9 (Figure 2), which also displayed agonist activity. The 2-ethylthio substitution curiously resulted in ~3-fold lower potency than 2-methylthio, either as an agonist (in the case of the 6-aminopurine, 11) or as an antagonist (with N6-methyl substitution, 12). The N6-methyl-2-propylthio analogue 14 was an antagonist, while the corresponding 6-NH2 analogue 13 was a mixed agonist/antagonist of equal proportions. The 2-allylthio and 2-n-hexylthio analogues containing the N6-methyl substitution, 15 and 16, respectively, were full antagonists. The allylthio and n-propylthio analogues were equipotent 0.4–0.5 μM IC50), while the n-hexylthio analogue was 7-fold less potent as an antagonist. Thus, as for P2Y1 receptor agonists,33,34 the 2-position can accommodate steric bulk without substantial loss of potency as a P2Y1 receptor antagonist. Substitutions at the 2-position other than thioethers were also studied. The 2-amino-N6-methyl analogue 17a was an antagonist of moderate potency. The corresponding 6-hydroxyl (deoxyguanosine) analogue 17b was an 8-fold weaker antagonist than 17a.
8-Position substitution in the methyl, vinyl, methoxy, methylthio, and methylamino analogues 18-22 resulted in only weak P2Y1 receptor antagonist properties, similar to the activity previously reported for the 8-bromo analogue. Within this group, carbon substituents, such as the 8-vinyl group, in 19, were best tolerated at P2Y1 receptors, while the 8-methyloxy substituent, in 20, resulted in a complete loss of activity.
Ribose ring substitutions tended to enhance the agonist properties of the derivatives. The 4′-thio analogue 23 and the carbocyclic analogue 24 were mixed agonist/antagonists, with the 4′-thio being more efficacious in receptor activation. The N6-methyl-2-amino carbocyclic analogue 25 was also a mixed agonist/ antagonist but was 2-fold less potent than 24 as an antagonist. Compound 26, a sulfoxide 5-monophosphate, was inactive at P2Y1 receptors.
Phosphate group modifications were made. The 5′phosphonate analogue 27 was a mixed agonist/antagonist. While producing a 78% maximal inhibition, 27 had antagonist potency that was only 3.5-fold lower than for the corresponding 5′-phosphate 1. The dimethyl phosphate 28 and the 3′,5′-cyclic diphosphate 29a were essentially inactive at P2Y1 receptors. The 3′,5′-cyclic tetraphosphate 29b displayed potent antagonist activity; however the possibility of hydrolysis of the unusual cyclic structure during the assay was not explored. The effect of lengthening the 5′-substituent from a monophosphate to a triphosphate was examined in 3′-deoxy-3′-azidoxylose derivatives, compounds 30 and 31. The triphosphate was a weak agonist, while the monophosphate was nearly inactive, suggesting that the triphosphate group may tend to enhance agonist potency versus the 5′-monophosphate, as was observed for nucleotides phosphorylated solely at the 5′-position.33
Introduction of a variety of pyrimidine bases in compounds 32-35 completely abolished both agonist and antagonist activity at P2Y1 receptors. In addition, the 2′-deoxyuridine derivative 32 at 100 μM was shown to be inactive as either an agonist or antagonist at P2Y2, P2Y4, or P2Y6 receptors (all of which are activated by uracil nucleotides) and at the C6 glioma P2 receptor22 linked to inhibition of adenylyl cyclase.
The ribose was also replaced with a six-membered ring in which the glycosidic bond was absent. Thus, the enlargement of the ribose ring in the anhydrohexitoladenine25 derivative 36 resulted in a pure P2Y1 receptor agonist activity, with an EC50 of 3 μM (Figure 2).
Discussion
The aim of the present study was to synthesize novel analogues and isosteres of 2 in order to increase the affinity and/or alter the efficacy of these nucleotides as antagonists, mixed agonists, or pure agonists at turkey P2Y1 receptors. The major conclusions are that 2-position substituents are compatible with the 2′-deoxy modification and in fact may enhance affinity, as in the 2-chloro analogue 6, and that substantial modification of the ribose moiety is possible. However, the agonist component of the biological activity of the bisphosphate analogues tends to increase upon 2-alkylthio substitution. This was particularly pronounced in the absence of the N6-methyl group. The effect of length of 2-alkylthio ethers on antagonist activity does not strictly follow the trend previously observed for potency of 5′-monophosphate agonists, e.g., 2-hexylthio > 2-methylthio. In the present series the most potent antagonists among the 2-thioethers were the S-methyl, propyl, and allyl analogues 10, 14, and 15, respectively, which also contained an N6-methyl group. In the 2-alkylthioether series, the presence of the N6-methyl group greatly decreased agonist efficacy. Amino substitution at the 2-position also was found to be tolerated in P2Y1 receptor antagonists.
Among intended isosteres 2, we observed that the carbocyclic analogue 24 was active as a mixed agonist/antagonist at P2Y1 receptors. The carbocyclic modification is expected to increase the in vivo stability of the compounds since these are N-cycloalkyl analogues, and the equivalent of deglycosylation is not expected to readily occur. The carbocyclic modification also may increase the selectivity of the compounds for P2 receptors, since these are structurally distinct from natural nucleotides.
The balance of agonist versus antagonist properties was highly dependent on ribose modifications, particularly at the 2′- and 4′-positions. Substitution at the ribose 2′-position generally appeared to increase the degree of agonism observed, as is evident from the present and previous20 sets of analogues (Figure 3). The presence of a 2′-hydroxyl, in either configuration, or a 2′-methoxy group increased the agonist efficacy. Perhaps the most unexpected result was that an expansion of the ribose ring in the 1,5-anhydrohexitol-adenine derivative, 36, results in pure agonism at the turkey P2Y1 receptor. As for the five-membered carbocyclic analogue 24, compound 36 would be resistant to deglycosylation by the usual mechanisms.
Figure 3.
Effects of 2′-position substitution of adenosine 3′,5′bisphosphate derivatives17 on agonist versus antagonist properties at turkey erythrocyte P2Y1 receptors. IC50 and EC50 values are given in μM.
In general, for pharmacological studies of receptor subtypes, highly selective antagonists are preferable. However, for some therapeutic applications partial agonists may provide low intrinsic activities that might avoid side effects.35 For example, pindolol is such a partial agonist at β1-adrenergic receptors and displays diminished bradycardiac side effects.36 Thus, the partial agonists in this series of nucleotide derivatives may potentially be therapeutically useful in modulating the activity of P2Y1 receptors. The effects of partial P2Y1 receptor agonists on platelet aggregation or on insulin release have not yet been explored.
A preliminary report37 indicates that 2 is a relatively potent antagonist at rat P2X1 receptors with an IC50 value of 1.2 μM, as determined in a study of ATPinduced ion flux in Xenopus oocytes transfected with the recombinant rat P2X1 receptor. In similar experiments using rat P2X2 and P2X4 receptors, no antagonist activity was observed, and at rat P2X3 receptors only weak antagonism was observed. The finding of affinity of 2, an N6-methyl derivative, at P2X1 receptors is in contrast with findings previously observed for adenosine 5′-triphosphate derivatives as agonists,33, 34 in which the presence of the N6-methyl group precluded activity at guinea pig vas deferens or urinary bladder P2X receptors. Thus, 2 is a selective antagonist for P2Y1 receptors within the family of metabotropic P2 receptors and a selective antagonist for P2X1 receptors within the family of ionotropic P2 receptors. The effects on activity at P2X receptors of the structural modifications of 2 reported in the present study have yet to be determined.
In platelets, three subtypes11 of P2 receptors are present: P2X1, P2Y1, and a yet uncloned P2T receptor, which inhibits adenylate cyclase. A P2Y1 receptor antagonist may prove to inhibit platelet aggregation,12 as do adenosine 5′-triphosphate analogues. There has been some discussion of whether ATP acts as a pure antagonist or as a partial agonist in platelets. It has been proposed that ATP itself has lower efficacy than ADP at a platelet P2Y receptor, and that may be why it apparently antagonizes the effects of ADP.15 Furthermore, in platelets, the presence of P2X1 antagonist properties, as has been demonstrated for 2,37 may increase the potential utility as antiaggregatory agents through action at two receptors having the same net pharmacological effect, i.e. platelet aggregation. Other members of the present series may also prove to be dual antagonists.
We have used mutagenesis and molecular modeling of human P2Y1 receptors to define a hypothetical nucleotide binding site, defined by TM3, TM6, and TM7 domains, for both the agonist 2-MeSADP and the antagonist 2. In the present study we have determined structural features which dramatically influence the degree of efficacy of the compound, such that nearly pure agonists and pure antagonists are present among closely related derivatives.
The pyrimidine nucleotide, 2′-deoxyuridine 3′,5′-bisphosphate, 32, was found to be inactive at subtypes of P2Y receptors known to be activated by uracil nucleotides, e.g., P2Y2, P2Y4, and P2Y6. At the P2Y2 receptor this was not surprising, since in the SAR of nucleotide agonists at this subtype a 5′-triphosphate group is required for activation. However, even P2Y6 receptors, which are activated by UDP, would not accept as ligand the 2′-deoxy-3′,5′-bisphosphate analogue 32. Thus, the search for effective antagonists at these subtypes continues.
In conclusion, the present study has identified new pharmacological probes of P2Y1 receptors. The SAR of 2 indicates that 2-position substitution is favorable for affinity at P2Y1 receptors, and nearly pure antagonism is maintained provided that the N6-methyl group is present. An N6-methyl-2-chloro analogue 6 was a full antagonist and displayed an IC50 of 206 nM. Similarly, N6-methyl-2-alkylthio derivatives 10, 14, and 15 were nearly full antagonists of IC50 < 0.5 μM. Thus, the affinity of 2-substituted N6-methyl analogues decreases in the order: Cl > H, SCH3 > S-propyl, S-allyl > NH2, S(CH2)5CH3. The 2-(propylthio)-6-aminopurine analogue 13 is the most potent mixed agonist/antagonist, in which the two components are roughly equal. Among nucleotides with mixed agonist and antagonist properties, but in which the antagonist component predominates, compounds 5 and 9, 2-chloro- and 2-(methylthio)-6-aminopurine analogues,20 respectively, are the most potent. The 1,5-anhydrohexitol-adenine derivative 36 is a pure agonist of potency similar to ATP.33
Experimental Section
Chemical Synthesis.
Nucleosides and synthetic reagents were purchased from Sigma Chemical Co. (St. Louis, MO) and Aldrich (St. Louis, MO). 6-Chloro-2′-deoxypurine riboside was obtained from Sigma. MRS 2179 was synthesized in our laboratory as described.20 Several 2′-deoxynucleosides, including 1-deaza-2′-deoxyadenosine,24 anhydrohexitol-adenine nucleoside,25 2′-deoxy-4′-thioadenosine,26 2′-deoxyaristeromycin,27 8-alkyl-2′-deoxyadenosine,30 and 3′-deoxy-3′-azidoxylose,28 were synthesized as reported.
1H NMR spectra were obtained with a Varian Gemini-300 spectrometer using D2O as a solvent. 31P NMR spectra were recorded at room temperature by use of a Varian XL-300 spectrometer (121.42 MHz); orthophosphoric acid (85%) was used as an external standard.
Purity of compounds was checked by a Hewlett-Packard 1090 HPLC apparatus using SMT OD-5–60 RP-C18 250 × 4.6 mm; Separation Methods Technologies, Inc., Newark, DE) as an analytical column in two solvent systems. The flow rate was 1 mL/min, with a typical pressure of 100 bar. System A: linear gradient solvent system, 0.1 M TEAA/CH3CN from 95/5 to 40/60 in 20 min. System B: linear gradient solvent system, 5 mM TBAP/CH3CN from 80/20 to 40/60 in 20 min. Peaks were detected by UV absorption using a diode array detector. All derivatives showed more than 95% purity in HPLC system.
Low-resolution CI-NH3 (chemical ionization) mass spectra were carried out with a Finnigan 4600 mass spectrometer and high-resolution EI (electron impact) mass spectra with a VG7070F mass spectrometry at 6 kV. High-resolution FAB (fast atom bombardment) mass spectrometry was performed with a JEOL SX102 spectrometer using 6-kV Xe atoms following desorbtion from a glycerol matrix.
Purification of most of the nucleotide analogues was carried out on DEAE-A25 Sephadex columns as described below. However, compounds 17a, 18, and 22 (system A) and compounds 26 and 36 (system B followed by Sephadex column) required HPLC purification (semipreparative C18 column) of the reaction mixtures.
General Procedure of Phosphorylation.
Nucleoside 0.1 mmol) and Proton Sponge 107 mg, 0.5 mmol) were dried for several hours in high vacuum at room temperature and then suspended in 2 mL of trimethyl phosphate. Phosphorus oxychloride 37 μL, 0.4 mmol) was added, and the mixture was stirred for 1 h at 0 °C. The reaction was monitored by analytical HPLC (eluting with gradient from buffer, CH3CN ) 95:5 to 40:60; buffer, 0.1 M triethylammonium acetate (TEAA); elution time, 20 min; flow rate, 1 mL/min; column, SMT OD-5–60 RP-C18; detector, UV, Emax ˜ 260–300 nm). The reaction was quenched by adding 2 mL of triethylammonium bicarbonate buffer and 3 mL of water. The mixture was subsequently frozen and lyophilized. Purification was performed on an ion-exchange column packed with Sephadex-DEAE A-25 resin, linear gradient 0.01–0.5 M) of 0.5 M ammonium bicarbonate was applied as the mobile phase, and UV and HPLC were used to monitor the elution. All nucleotides were collected, frozen, and lyophilized as the ammonium salts. All synthesized compounds gave correct molecular masses and showed more than 95% purity (high-resolution FAB, purity, and HPLC retention times are reported in Table 3).
Table 3.
Synthetic Data for Nucleotide Derivatives, Including Structural Verification Using HRMS and Purity Verification Using HPLC
| FAB (M - H+) |
HPLC tR, mina |
|||||
|---|---|---|---|---|---|---|
| compd | formula | calcd | found | system A | system B | yields, %b |
| 1 | C10H15O9N5P2 | 410.0267 | 410.0249 | 5.98 | 11.51 | 44 |
| 2 | C11H17O9N5P2 | 424.0423 | 424.0404 | 6.93 | 12.32 | 41 |
| 3 | C10H15O10N5P2 | 426.0216 | 426.0226 | 6.12 | 10.51 | 15 |
| 4 | C12H18O9N4P2 | 423.0471 | 423.0479 | 8.49 | 10.64 | 19 |
| 5 | C10H14O9N5P2Q | 443.9877 | 443.9872 | 7.20 | 12.14 | 58 |
| 6 | CUH16O9N5P2Cl | 458.0034 | 458.0035 | 8.51 | 11.42 | 17 |
| 7 | C11H16O9N5P2I | 549.9390 | 549.9396 | 7.84 | 8.40 | 16 |
| 8 | C11H17O10N5P2 | 440.0372 | 440.0358 | 6.32 | 10.13 | 23 |
| 9 | C11H17O9N5P2S | 456.0144 | 456.0122 | 8.14 | 12.98 | 48 |
| 10 | C12H19O9N5P2S | 470.0301 | 470.0307 | 9.99 | 12.07 | 17 |
| 11 | C12H19O9N5P2S | 470.0301 | 470.0307 | 8.94 | 9.84 | 23 |
| 12 | C13H21O9N5P2S | 484.0457 | 484.0453 | 10.49 | 11.81 | 23 |
| 13 | C13H21O9N5P2S | 484.0457 | 484.0452 | 7.77 | 11.65 | 21 |
| 14 | C14H23O9N5P2S | 498.0614 | 498.0605 | 9.75 | 12.40 | 10 |
| 15 | C14H21O9N5P2S | 496.0457 | 496.0445 | 9.20 | 12.35 | 13 |
| 16 | C17H29O9N5P2S | 540.1083 | 540.1057 | 12.87 | 15.11 | 25 |
| 17a | C11H18O9N6P2 | 439.0532 | 439.0535 | 6.48 | 7.15 | 19 |
| 17b | C10H15O10N5P2 | 426.0216 | 426.0216 | 9.28 | 9.69 | 39 |
| 18 | C11H17O9N5P2 | 424.0423 | 424.0412 | 6.07 | 8.72 | 9 |
| 19 | C12H17O9N5P2 | 436.0423 | 436.0429 | 6.43 | 9.00 | 13 |
| 20 | C11H17O10N5P2 | 440.0372 | 440.0380 | 4.14 | 7.28 | 17 |
| 21 | C11H17O9N5P2S | 456.0144 | 456.0160 | 6.89 | 8.23 | 37 |
| 22 | C11H18O9N6P2 | 439.0532 | 439.0497 | 6.67 | 9.20 | 5 |
| 23 | C10H15O8N5P2S | 426.0038 | 426.0051 | 6.75 | 8.92 | 13 |
| 24 | C11H17O8N5P2 | 408.0474 | 408.0474 | 5.97 | 7.04 | 24 |
| 25 | C12H20O8N6P2 | 437.0740 | 437.0759 | 3.70 | 5.21 | 10 |
| 26 | C10H14O6N5PS | 362.0324 | 362.0319 | 2.76 | 7.63 | 2 |
| 27 | C11H17O9N5P2 | 424.0423 | 424.0432 | 7.04 | 7.37 | 10 |
| 28 | C12H19O9N5P2 | 438.0580 | 438.0580 | 3.06 | 4.48 | 22 |
| 29a | C11H15O8N5P2 | 406.0318 | 406.0336 | 7.60 | 7.47 | 20 |
| 29b | C11H17O14N5P4 | 565.9644 | 565.9664 | 6.91 | 12.11 | 5 |
| 30 | C10H14O9N8P2 | 451.0281 | 451.0288 | 3.70 | 5.78 | 13 |
| 31 | C10H16O15N8P4 | 610.9607 | 610.9602 | 3.81 | 5.99 | 3 |
| 32 | C9H14O11N2P2 | 386.9995 | 386.9977 | 4.20 | 9.85 | 39 |
| 33 | C10H16O11N2P2 | 401.0151 | 401.0160 | 4.63 | 9.73 | 39 |
| 34 | C9H13O11N2P2F | 404.9889 | 404.9901 | 3.66 | 7.83 | 27 |
| 35 | C9H15O10N3P2 | 386.0154 | 386.0136 | 2.10 | 3.34 | 36 |
| 36 | C11H17O9N5P2 | 424.0423 | 424.0423 | 4.31 | 7.56 | 1 |
Purity of each derivative was 95%, as determined using HPLC (Hewlett-Packard 1090) with two different mobile phases, using an SMT OD-5–60 RP-C18 column (250 × 4.6 mm; Separation Methods Technologies, Inc., Newark, DE). The flow rate was 1 mL/min, with a typical pressure of 100 bar. System A: linear gradient solvent system, 0.1 M TEAA/CH3CN from 95/5 to 40/60 in 20 min. System B: linear gradient solvent system, 5 mM TBAP/CH3CN from 80/20 to 40/60 in 20 min.
The percent yields refer to phosphorylation reactions
Adenine Arabinofuranoside 3′,5′-Bis(diammonium phosphate) (3).
Starting from 40 mg 0.15 mmol) of adenine arabinofuranoside and following the general procedure, using an additional 2 mL of acetonitrile to dissolve starting material, we obtained 11.2 mg 0.023 mmol, 15% yield) of 3: 1H NMR (D2O) δ 4.09 2H, m, CH2-5′), 4.33 1H, bs, H-4′), 4.56 1H, m, H-2′), 4.65 1H, m, H-3′), 6.43 1H, d, J = 3.9 Hz, H-1′), 8.18 1H, s, H-2), 8.50 1H, s, H-8); 31P NMR (D2O) δ 3.49 (s, 1-P), 3.70 (d, J = 6.0 Hz, 1-P).
2′-Deoxy-1-deaza-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (4).
Starting from 14 mg 0.053 mmol) of 2′-deoxy-1-deaza-N6-methyladenosine and following the general procedure, we obtained 4.2 mg 0.009 mmol, 17% yield) of 4: 1H NMR (D2O) δ 2.76 (2H, m, CH2-2′), 3.06 (3H, s, NHCH3), 4.11 (2H, m, CH2-5′), 4.49 (1H, bs, H-4′), 4.97 (1H, m, H-3′), 6.45 (1H, t, J = 6.8 Hz, H-1′), 6.59 (1H, d, J = 6.8 Hz, H-1′), 8.02 (1H, d, J = 6.8 Hz, H-2), 8.32 (1H, s, H-8); 31P NMR (D2O) δ 0.56 (d, J = 16.6 Hz, 3′-P), 1.43 (bs, 5′-P).
2-Chloro-2′-deoxy-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (6).
Starting from 25 mg (0.083 mmol) of 2-chloro-2′-deoxy-N 6-methyladenosine, 39, and following the general procedure, we obtained 7.5 mg (0.014 mmol, 17% yield) of 6: 1H NMR (D2O) δ 2.76 (2H, m, CH2-2′), 2.95 (3H, s, NHCH3), 4.06 (2H, m, CH2-5′), 4.43 (1H, bs, H-4′), 4.96 (1H, m, H-3′), 6.32 (1H, t, J = 6.9 Hz, H-1′), 8.31 (1H, s, H-8); 31P NMR (D2O) δ 0.06 (d, J = 9.0 Hz, 3′-P), 0.73 (bs, 5′-P).
2′-Deoxy-2-iodo-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (7).
Starting from 28 mg (0.072 mmol) of 2′-deoxy-2-iodo-N6-methyladenosine, 50, and following the general procedure, we obtained 6.6 mg (0.012 mmol, 17% yield) of 7: 1H NMR (D2O) δ 2.31 (1H, m, CH2-2′), 2.97 (1H, m, CH2-2′), 3.13 (3H, s, NHCH3), 4.11 (2H, m, CH2-5′), 4.46 (1H, m, H-4′), 5.06 (1H, m, H-3′), 6.65 (1H, t, J = 5.9 Hz, H-1′), 8.65 (1H, s, H-8); 31P NMR (D2O) δ 0.56 (bs, 5′P), −0.19 (bs, 3′P).
2′-Deoxy-N6-methoxyadenosine 3′,5′-Bis(diammonium phosphate) (8).
Starting from 12 mg (0.043 mmol) of 2′-deoxyN6-methoxyladenosine, 59, and following the general procedure, we obtained 5.1 mg (0.010 mmol, 23% yield) of 8: 1H NMR (D2O) δ 2.75 (2H, m, CH2-2′), 3.84 (3H, s, OCH3), 4.04 (2H, m, CH2-5′), 4.43 (1H, bs, H-4′), 4.97 (1H, m, H-3′), 6.45 (1H, t, J = 6.9 Hz, H-1′), 7.95 (1H, s, H-2), 8.30 (1H, s, H-8); 31P NMR (D2O) δ 0.60 (d, J = 7.5 Hz, 3′-P), 1.13 (bs, 5′-P).
2′-Deoxy-2-(methylthio)-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (10).
Starting from 25 mg (0.083 mmol) of 2′-deoxy-2-(methylthio)-N6-methyladenosine, 40, and following the general procedure, we obtained 7.5 mg (0.014 mmol, 17% yield) of 10: 1H NMR (D2O) δ 2.55 (3H, s, SCH3), 2.67–286 (2H, m, CH2-2′), 3.02 (3H, s, NHCH3), 3.95 (2H, m, CH2-5′), 4.37 (1H, bs, H-4′), 4.88 (1H, m, H-3′), 6.47 (1H, t, J = 6.9 Hz, H-1′), 8.29 (1H, s, H-8); 31P NMR (D2O) δ 0.06 (d, J = 9.0 Hz, 3′-P), 0.73 (bs, 5′-P).
2′-Deoxy-2-(ethylthio)adenosine 3′,5′-Bis(diammonium phosphate) (11).
Starting from 22 mg (0.071 mmol) of 2′-deoxy-2-(ethylthio)adenosine, 52, and following the general procedure, we obtained 7.6 mg (0.016 mmol, 23% yield) of 11: 1H NMR (D2O) δ 1.50 (3H, t, J = 7.3 Hz, CH3CH2S), 2.85 (1H, m, CH2-2′), 3.05 (1H, m, CH2-2′), 3.32 (2H, q, J = 7.81 Hz, CH3CH2S), 4.18 (2H, m, CH2-5′), 4.58 (1H, m, H-4′), 5.10 (1H, m, H-3′), 6.72 (1H, t, J = 6.5 Hz, H-1′), 8.49 (1H, s, H-8); 31P NMR (D2O) δ 1.48 (d, J = 9.1 Hz, 5′-P), 1.02 (d, J = 14.1 Hz, 3′-P).
2′-Deoxy-2-(ethylthio)-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (12).
Starting from 20 mg (0.061 mmol) of 2′-deoxy-2-(ethylthio)-N6-methyladenosine, 41, and following the general procedure, we obtained 6.8 mg (0.014 mmol, 23% yield) of 12: 1H NMR (D2O) δ 1.49 (3H, t, J = 7.7 Hz, CH3CH2S), 2.85–3.01 (2H, m, CH2-2′), 3.22 (3H, s, NHCH3), 3.32 (2H, q, J = 7.6 Hz, CH3CH2S), 4.06 (2H, m, CH25′), 4.56 (1H, m, H-4′), 5.12 (1H, m, H-3′), 6.69 (1H, t, J = 6.8 Hz, H-1′), 8.55 (1H, s, H-8); 31P NMR (D2O) δ 3.97 (s, 5′-P), 3.43 (s, 3′-P).
2′-Deoxy-2-(propylthio)adenosine 3′,5′-Bis(diammonium phosphate) (13).
Starting from 39.6 mg (0.122 mmol) of 2-(propylthio)-2′-deoxyadenosine, 53, and following the general procedure, we obtained 14.2 mg (0.026 mmol, 21.3% yield) of 13: 1H NMR(D2O) δ 0.98 (3H, t, J = 6.8 Hz, CH3CH2CH2S), 1.66–1.78 (2H, m, CH3CH2CH2S) 2.69–2.75 (1H, m, CH2-2′), 2.81–2.93 (1H, m, CH2-2′), 3.16 (2H, t, J = 6.8 Hz, CH3CH2CH2S), 4.06 (2H, m, CH2-5′), 4.43 (1H, bs, H-4′), 4.97(1H, bs, H-3′), 6.51 (1H, t, J = 6.8 Hz, H-1′), 8.32 (1H, s, H-8); 31P NMR (D2O) 0.54 (s, 5′-P), −0.22 (d, J = 1.8 Hz, 3′-P).
2′-Deoxy-2-(propylthio)-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (14).
Starting from 31.0 mg (0.091 mmol) of N 6-methyl-2-(propylthio)-2′-deoxyadenosine, 42, and following the general procedure, we obtained 5.0 mg (0.009 mmol, 10% yield) of 14: 1H NMR (D2O) δ 0.96 (3H, t, J = 6.8 Hz, CH3CH2CH2S), 1.64–1.71 (2H, m, CH3CH2CH2S) 2.16–2.73 (1H, m, CH2-2′), 2.78–2.87 (1H, m, CH2-2′), 2.98 (3H, s, NHCH3), 3.07 (2H, t, J = 6.8 Hz, CH3CH2CH2S), 4.03 (2H, m, CH2-5′), 4.40 (1H, bs, H-4′), 4.94 (1H, bs, H-3′) 6.40 (1H, t, J = 5.8 Hz, H-1′), 8.20 (1H, s, H-8); 31P NMR (D2O) δ 0.86 (bs, 5′-P), 0.18 (bs, 3′-P).
2-(Allylthio)-2′-deoxy-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (15).
Starting from 20 mg (0.084 mmol) of 2-(allylthio)-2′-deoxy-N6-methyladenosine, 43, and following the general procedure, we obtained 5.8 mg (0.117 mmol, 13% yield) of 15: 1H NMR (D2O) δ 2.89 (2H, m, CH2-2′), 3.17 (3H, s, NHCH3), 3.97 (2H, m, CH2=CHCH2S), 4.17 (2H, m, CH2-5′), 4.55 (1H, m, H-4′), 5.09 (1H, m, H-3′), 5.27 (1H, d, J = 10.0 Hz, CH2=CHCH2S), 5.51 (1H, d, J = 12.0 Hz, CH2=CHCH2S), 6.20 (1H, m, CH2=CHCH2S), 6.60 (1H, t, J = 6.0 Hz, H-1′), 8.39 (1H, s, H-8); 31P NMR (D2O) δ 1.31 (bs, 5′- P), 0.78 (bs, 3′-P).
2′-Deoxy-2-(hexylthio)-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (16).
Starting from 28 mg (0.073 mmol) of 2′-deoxy-2-(hexylthio)-N6-methyladenosine, 44, and following the general procedure, we obtained 10.8 mg (0.018 mmol, 25% yield) of 16: 1H NMR (D2O) δ 0.82 (3H, m, CH3-CH2), 1.26 (4H, m, CH2CH2), 1.40 (2H, m, CH2), 1.72 (2H, m, CH2CH2S), 2.68–2.88 (2H, m, CH2-2′), 3.05 (3H, bs, NHCH3), 3.15 (2H, m, CH2S), 4.03 (2H, m, CH2-5′), 4.40 (1H, bs, H-4′), 4.94 (1H, m, H-3′), 6.47 (1H, t, J = 6.8 Hz, H-1′), 8.30 (1H, s, H-8); 31P NMR (D2O) δ 1.44 (s, 3′-P); 1.90, (s, 5′-P).
2-Amino-2′-deoxy-N6-methyladenosine 3′,5′-Bis(diammonium phosphate) (17a).
Starting from 15 mg (0.053 mmol) of 2-amino-2′-deoxy-N6-methyladenosine, 48, and following the general procedure, we obtained 4.4 mg (0.01 mmol, 19% yield) of 17a: 1H NMR (D2O) δ 2.50 (1H, m, CH2-2′), 2.67 (1H, m, CH2-2′), 2.99 (1H, s, NHCH3), 3.96 (2H, m, CH2-5′), 4.21 (1H, m, H-4′), 4.85 (1H, m, H-3′), 6.27 (1H, t, J = 6.6 Hz, H-1′), 8.06 (1H, s, H-8); 31P NMR (D2O) δ 0.99 (s, 5′-P), 0.49 (bs, 3′-P).
2′-Deoxyguanosine 3′,5′-Bis(diammonium phosphate) (17b).
Starting from 50 mg (0.22 mmol) of 2′-deoxyguanosine and following the general procedure, we obtained 39.4 mg (0.086 mmol, 39% yield) of 17b: 1H NMR (D2O) δ 2.40 (1H, m, CH2-2′), 2.55 (1H, m, CH2-2′), 4.08 (2H, m, CH2-5′), 4.36 (1H, bs, H-4′), 4.80 (H-3′ under D2O peak), 5.93 (1H, d, J = 8.8 Hz, H-5), 6.37 (1H, t, J = 6.8 Hz, H-1′), 7.98 (1H, d, J = 8.8 Hz, H-6); 31P NMR (D2O) δ 0.89, (s, 5′-P); 0.31 (s, 3′-P).
2′-Deoxy-8-methyladenosine 3′,5′-Bis(diammonium phosphate) (18).
Starting from 12.0 mg (0.045 mmol) of 8-methyl-2′-deoxyadenosine(30 and following the general procedure, we obtained 2.0 mg (0.004 mmol, 8.9% yield) of 18: 1H NMR (D2O) δ 2.50–2.59 (1H, m, CH2-2′), 3.02–3.20 (1H, m, CH2-2′), 2.68 (3H, s, 8-CH3), 4.06–4.10 (2H, m, CH2-5′), 4.30 (1H, bs, H-4′), 4.90 (1H, m, H-3′), 6.49 (1H, t, J = 6.8 Hz, H-1′), 8.20 (1H, s, H-2); 31P NMR (D2O) δ 1.88 (bs, 5′-P), 1.62 (bs, 3′-P).
2′-Deoxy-8-vinyladenosine 3′,5′-Bis(diammonium phosphate) (19).
Starting from 15.0 mg (0.054 mmol) of 8-vinyl2′-deoxyadenosine(30 and following the general procedure, we obtained 3.7 mg (0.0073 mmol, 13.6% yield) of 19: 1H NMR(D2O) δ 2.50–2.55 (1H, m, CH2-2′), 2.95–3.10 (1H, m, CH2-2′), 4.01–4.05 (2H, m, CH2-5′), 4.30 (1H, bs, H-4′), 4.91 (1H, m, H-3′), 5.84 (1H, d, J = 10.7 Hz, vinyl), 6.37 (1H, d, J = 16.6 Hz, vinyl), 6.56 (1H, t, J = 5.8 Hz, H-1′), 7.20 (1H, dd, J = 16.6, 10.7 Hz, vinyl), 8.20 (1H, s, H-2); 31P NMR (D2O) δ 3.85 (bs, 5′-P), 3.36 (bs, 3′-P).
2′-Deoxy-8-O-methyladenosine 3′,5′-Bis(diammonium phosphate) (20).
Starting from 24 mg (0.085 mmol) of 2′-deoxy-8-O-methyl-adenosine, 56, and following the general procedure, we obtained 4.2 mg (0.009 mmol, 17% yield) of 20: 1H NMR (D2O) δ 2.42 (1H, m, CH2-2′), 3.20 (1H, m, CH2-2′), 3.89 (3H, s, OCH3), 4.0–4.35 (3H, m, CH2-5′ and H-4′), 5.1 (1H, m, H-3′), 6.61 (1H, t, J = 6.4 Hz, H-1′), 8.10 (1H, s, H-2); 31P NMR (D2O) δ 0.78 (s, 5′-P), 0.25 (bs, 3′-P).
2′-Deoxy-8-(methylthio)adenosine 3′,5′-Bis(diammonium phosphate) (21).
Starting from 25 mg (0.084 mmol) of 2′-deoxy-8-(methylthio)adenosine, 57, and following the general procedure, we obtained 14.2 mg (0.031 mmol, 37% yield) of 21: 1H NMR (D2O) δ 2.45 (1H, m, CH2-2′), 2.72 (3H, s, SCH3), 3.21 (1H, m, CH2-2′), 4.08 (2H, m, CH2-5′), 4.25 (1H, m, H-4′), 5.0 (1H, m, H-3′), 6.5 (1H, t, J ) 6.5 Hz, H-1′), 8.15 (1H, s, H-2); 31P NMR (D2O) δ 0.98 (bs, 5′-P), 0.31 (bs, 3′-P).
2′-Deoxy-8-(methylamino)adenosine 3′,5′-Bis(diammonium phosphate) (22).
Starting from 28.1 mg (0.1 mmol) of 2′-deoxy-8-(methylamino)adenosine, 58, and following the general procedure, we obtained 2.4 mg (0.0055 mmol, 5.5% yield) of 22: 1H NMR (D2O) δ 2.53 (1H, m, CH2-2′), 3.09 (3H, s, NHCH3), 3.24 (1H, m, CH2-2′), 4.12 (2H, m, CH2-5′), 4.30 (1H, m, H-4′), 4.99 (1H, m, H-3′), 6.47 (1H, t, J = 7.4 Hz, H-1′), 8.15 (1H, s, H-2); 31P NMR (D2O) δ 0.91 (s, 5′-P), 0.25 (bs, 3′-P).
2′-Deoxy-4′-thioadenosine 3′,5′-Bis(diammonium phosphate) (23).
Starting from 12 mg (0.045 mmol) of 2′-deoxy-4′-thioadenosine, 62, and following the general procedure, we obtained 2.4 mg (0.0056 mmol, 13% yield) of 23: 1H NMR (D2O) δ 2.59 (2H, m, CH2-2′), 3.97 (2H, m, CH2-5′), 4.32 (1H, m, H-4′), 4.95 (1H, m, H-3′), 6.30 (1H, t, J = 7.0 Hz, H-1′), 8.06 (1H, s, H-2), 8.18 (1H, s, H-8); 31P NMR (D2O) δ 0.55 (s, 5′-P), −0.20 (d, J = 2.4 Hz, 3′-P).
(1R,2R,4R)-4-(9H-Adenin-9-yl)-2-(phosphatomethyl)cyclopentan-1-yl Phosphate (24).
Starting from 6.0 mg (0.024 mmol) of carbocyclic 2′-deoxyadenosine, 56,(27 and following the general procedure, we obtained 2.4 mg (0.0059 mmol, 24.5% yield) of 24, the carbocyclic equivalent of 2′-deoxyadenosine 3′,5′-bis(diammonium phosphate): 1H NMR (D2O) δ 1.98 (1H, m, CH2-2′), 2.5 (3H, m, CH2-2′ and CH2-6′), 4.03 (2H, m, CH2-5′), 4.68 (1H, m, H-4′), 5.12 (1H, m, H-3′), 5.25 (1H, m, J = 5.5 Hz, H-1′), 8.28 (1H, s, H-2), 8.34 (1H, s, H-8); 31P NMR (D2O) δ 0.94 (s, 5′-P), −0.07 (s, 3′-P).
(1R,2R,4R)-4-(9H-2-Amino-N6-methyladenin-9-yl)-2(phosphatomethyl)cyclopentan-1-yl Phosphate (25).
Starting from 15 mg (0.054 mmol) of the carbocyclic equivalent of 2-amino-2′-deoxy-N6-methyladenosine, 61, and following the general procedure, we obtained 2.4 mg (0.0055 mmol, 10% yield) of 25, the carbocyclic equivalent of 2-amino-2′-deoxy-N6-methyladenosine 3′,5′-bis(diammonium phosphate): 1H NMR (D2O) δ 1.87 (1H, m, CH2-2′), 2.29 (2H, m, CH2-6′), 2.54 (1H, m, CH2-2′), 3.13 (3H, s, NHCH3), 3.99 (2H, m, CH2-5′), 4.40 (1H, m, H-4′), 4.96 (2H, m, H-3′ and H-1′), 8.00 (1H, s, H-8); 31P NMR (D2O) δ 0.92 (bs, 5′-P), 0.42 (s, 3′-P).
2′-Deoxy-4′-thioadenosine S-Oxide 5′-Mono(diammonium phosphate) (26).
\Starting from 14.0 mg (0.049 mmol) of 2′-deoxy-4′-thioadenosine S-oxide, 63, and following the general procedure, we obtained 0.50 mg (0.001 mmol, 2.0% yield) of 26: 1H NMR (D2O) δ 2.79–2.95 (1H, m, H-2′), 3.11–3.27 (1H, m, H-2′), 3.51–3.66 (2H, m, H-5′), 3.72–3.77 (1H, m, H-4′), 4.29–4.34 (1H, m, H- 3′) 6.18–6.38 (1H, m, H-1′), 8.30 (1H, s, H-2) 8.37 (1H, s, H-8); 31P NMR (D2O) δ 0.46, 0.34 (2s, mixture of diastereomers).
2′-Deoxy-5′-O-phosphonylmethyladenosine 3′-Phosphate (tetrammonium salt) (27).
Starting from 15 mg (0.043 mmol) of 2′-deoxy-5′-[diethyl(methylphosphonyl)]adenosine, 64, and following the general procedure, we obtained 1.8 mg (0.0042 mmol, 9.8% yield) of 27. Cleavage of the phosphonate esters was verified by NMR and MS: 1H NMR (D2O) δ 2.83 (2H, m, CH2-2′), 4.10 (4H, m, CH2-5′ and CH2-P), 4.47 (1H, m, H-4′), 5.10 (1H, m, H-3′), 6.58 (1H, t, J = 7.9 Hz, H-1′), 8.28 (1H, s, H-2), 8.51 (1H, s, H-8); 31P NMR (D2O) δ 27.49 (s, 5′-CH2P), 3.31 (s, 3′-P).
2′-Deoxyadenosine 3′,5′-Bis(ammonium methyl phosphate) (28).
Starting from 25 mg (0.1 mmol) of 2′-deoxyadenosine and following the general procedure using methyl dichlorophosphate (59.6 mg, 41.13 μL, 0.4 mmol), we obtained 9.8 mg (0.018 mmol, 22% yield) of 28: 1H NMR (D2O) δ 2.64 (1H, m, CH2-2′), 2.86 (1H, m, CH2-2′), 3.42 (3H, d, J = 10.8 Hz, CH3OP), 3.58 (3H, d, J = 10.8 Hz, CH3OP), 4.02 (2H, m,nCH2-5′), 4.28 (1H, m, H-4′), 4.53 (1H, m, H-3′), 6.46 (1H, t, J = 6.9 Hz, H-1′), 8.18 (1H, s, H-2), 8.37 (1H, s, H-8); 31P NMR (D2O) δ 3.30 (bs, 5′-P), 1.79 (d, J = 2.4 Hz, 3′-P).
2′-Deoxy-N6-methyladenosine 3′,5′-Cyclic-diphosphate (diammonium salt) (29a) and 2′-Deoxy-N6-methyladenosine 3′,5′-Cyclic-tetraphosphate (tetraammonium salt) (29b).
A solution of 20 mg (0.04 mmol) of 2 in anhydrous DMF was evaporated under reduced pressure below 30 °C, and this drying procedure was repeated three times. The residue was resuspended in 2 mL of anhydrous DMF under nitrogen atmosphere, and 38 μL (0.16 mmol) of tributylamine was added at room temperature. The mixture was stirred for 30 min, and a solution of 33 mg (0.2 mmol) of carbonyldiimidazole was added. The mixture was stirred for 12 h at room temperature before the excess carbonyldiimidazole was quenched by addition of 0.05 mL of methanol, and the mixture was stirred for 30 min. A solution of 0.091 g of bis(tributylammonium)-pyrophosphate (0.2 mmol) in 2 mL of anhydrous DMF was added at room temperature, and the mixture was stirred for 42 h. The reaction mixture was evaporated under reduced pressure below 30 °C, and two products were purified by the ion-exchange column chromatography in the general procedure and semipreparative HPLC using SMT OD-5–60 C18 column with a linear gradient elution of 1.0 M triethylammonium acetate solution and CH3CN (95/5–40/60 for 30 min). After evaporation of the pure fraction under reduced pressure, the products were redissolved in 0.5 M ammonium bicarbonate solution, and repeated lyophilization with water gave 3.5 mg of 29a (yield 20%) and 1.2 mg of 29b (yield 5%).
29a:
1H NMR (D2O) δ 2.74–2.84 (1H, m, CH2-2′), 2.97–3.02 (1H, m, CH2-2′), 3.04 (3H, s, NHCH3), 3.83–3.96 (1H, m, H-4′), 4.21–4.39 (2H, m, H-5′), 5.19 (1H, pen, J = 7.8 Hz, H-3′), 6.39–6.42 (1H, m, H-1′), 8.20 (1H, s, H-2), 8.23 (1H, s, H-8); 31P NMR (D2O) δ−12.45 (dd, J = 8.5, 24.5 Hz, 3′-CHOP-), −11.71 (2, dd, J = 10.3, 18.6, 24.4 Hz, 5′-CH2OP-), with proton decoupling off mode.
29b:
1H NMR (D2O) δ 2.75–2.84 (1H, m, CH2-2′), 2.90–2.99 (1H, m, CH2-2′), 3.09 (3H, s, NHCH3), 4.10–4.16 (1H, m, H-4′), 4.29–4.40 (2H, m, H-5′), 5.27 (1H, pen, J = 6.8 Hz, H-3′), 6.50 (1H, t, J = 5.8 Hz, H-1′), 8.26 (1H, s, H-2), 8.42 (1H, s, H-8); 31P NMR (D2O) δ−23.38 to −23.22 (2P, m), −12.01 to −11.80 (m), −11.05 to −10.95 (m), with proton decoupling off mode; Negative API-ES Mass 566 (M - H).
3′-Azido-3′-deoxyxyloadenosine 2′,5′-Bis(diammonium phosphate) (30).
Starting from 25 mg (0.085 mmol) of 3′-azido-3′-deoxyxyloadenosine and following the general procedure, we obtained 4.9 mg (0.011 mmol, 13% yield) of 30: 1H NMR (D2O) δ 3.1 (1H, m, H-2′), 3.64 (1H, m, H-3′), 4.30 (2H, m, CH2-5′), 4.53 (1H, m, H-4′), 6.12 (1H, bs, H-1′), 8.25 (1H, s, H-2), 8.48 (1H, s, H-8); 31P NMR (D2O) δ 1.37 (bs, 5′-P), 0.55 (bs, 3′-P).
3′-Azido-3′-deoxyxyloadenosine 2′-Monophosphate 5′Triphosphate (hexaammonium salt) (31).
Starting from 25 mg (0.085 mmol) of 3′-azido-3′-deoxyxyloadenosine and following the general procedure, we obtained 1.7 mg (0.0028 mmol, 3.0% yield) of 31: 1H NMR (D2O) δ 3.14 (1H, m, H-2′), 3.65 (1H, m, H-3′), 4.35 (2H, m, CH2-5′), 4.56 (1H, M, H-4′), 6.13 (1H, bs, H-1′), 8.28 (1H, s, H-2), 8.51 (1H, s, H-8); 31P NMR (D2O) δ 0.43 (s, 3′-P), −10.43 (d, J = 19.5 Hz, 5′-Pγ), −11.11, −11.27 (2t, J = 6.1 Hz, 5′-Pβ), −22.76 (t, J = 19.7 Hz, 5′-PR).
2′-Deoxyuridine 3′,5′-Bis(diammonium phosphate) (32).
Starting from 50 mg (0.22 mmol) of 2′-deoxyuridine and following the general procedure, we obtained 39.4 mg (0.086 mmol, 39% yield) of 32: 1H NMR (D2O) δ 2.40 (1H, m, CH22′), 2.55 (1H, m, CH2-2′), 4.08 (2H, m, CH2-5′), 4.36 (1H, bs, H-4′), 4.80 (H3′ under D2O peak), 5.93 (1H, d, J = 8.8 Hz, H-5), 6.37 (1H, t, J = 6.8 Hz, H-1′), 7.98 (1H, d, J = 8.8 Hz, H-6); 31P NMR (D2O) δ 0.89 (s, 5′-P), 0.31 (s, 3′-P).
Thymidine 3′,5′-Bis(diammonium phosphate) (33).
Starting from 50 mg (0.22 mmol) of thymidine and following the general procedure, we obtained 39.4 mg (0.086 mmol, 39% yield) of 33: 1H NMR (D2O) δ 2.40 (1H, m, CH2-2′), 2.55 (1H, m, CH2-2′), 4.08 (2H, m, CH2-5′), 4.36 (1H, bs, H4′), 4.80 (H3′ under D2O peak), 5.93 (1H, d, J = 8.8 Hz, H5), 6.37 (1H, t, J = 6.8 Hz, H1′), 7.98 (1H, d, J = 8.8 Hz, H6); 31P NMR (D2O) δ 1.04 (s, 5′-P), 0.75 (s, 3′-P).
5-Fluoro-2′-deoxyuridine 3′,5′-Bis(diammonium phosphate) (34).
Starting from 20 mg (0.081 mmol) of 5-fluoro-2′-deoxyuridine and following the general procedure, we obtained 8.9 mg (0.022 mmol, 27% yield) of 34: 1H NMR (D2O) δ 2.38 (1H, m, CH2-2′), 2.55 (1H, m, CH2-2′), 4.08 (2H, m, CH2-5′), 4.35 (1H, m, H-4′), 4.94 (1H, m, H-3′), 6.35 (1H, bs, H-1′), 8.17 (1H, s, H-5); 31P NMR (D2O) δ 1.52 (s, 5′-P), 1.19 (s, 3′-P).
2′-Deoxycytidine 3,5′-Bis(diammonium phosphate) (35).
Starting from 20 mg (0.087 mmol) of 2′-deoxycytidine and following the general procedure, we obtained 12 mg (0.031 mmol, 36% yield) of 35: 1H NMR (D2O) δ 2.41 (1H, m, CH2-2′), 2.65 (1H, m, CH2-2′), 4.11 (2H, m, CH2-5′), 4.42 (1H, bs, H-4′), 4.85 (1H, m, H-3′), 6.37 (1H, t, J = 6.2 Hz, H-1′), 8.07 (1H, d, J = 8.0 Hz, 1H), 8.18 (d, J = 7.5 Hz, 1H); 31P NMR (D2O) δ 0.51 (s, 5′-P), −0.13 (s, 3′-P).
1′,5′-Anhydro-2′-(adenin-9-yl)-2′,3′-dideoxy-D-arabino-hexitol 4′,6′-Bis(diammonium phosphate) (36).
Starting from 19.2 mg (0.072 mmol) of 1′,5′-anhydro-2′-(adenin-9-yl)-2′,3′-dideoxy-D-arabino-hexitol(25 and following the general phosphorylation procedure, we obtained 0.50 mg (0.001 mmol, 1.4% yield) of 36: 1H NMR (D2O) δ 2.04–2.74 (2H, m, CH2-3′), 3.57–3.60 (1H, m, H-5′), 3.69–3.72 (1H, m, H-4′), 4.09 (2H, bs, CH2-6′), 4.11–4.33 (2H, m, CH2-1′), 4.93 (1H, bs, H-2′), 8.23 (1H, s, H-2), 8.51 (1H, s, H-8); 31P NMR (D2O) δ 3.28, 1.75 (2s).
2-Chloro-2′-deoxy-3′,5′-di-O-acetyladenosine (38).
A mixture of commercial 2-chloro-2′-deoxyadenosine (37; 285 mg, 1 mmol), pyridine (3 mL), and acetic anhydride (2 mL) was stirred at room temperature for 2 h under nitrogen atmosphere. The reaction mixture was evaporated to dryness and chromatographed by pTLC (CHCl3/MeOH, 95/5) to obtain 38 as a white solid (300 mg, 0.81 mmol, 81% yield): MS (CI-NH3) 370 (M++ 1), 387 (M + NH4+); 1H NMR (CDCl3) δ 2.11 (3H, s, CH3CO), 2.14 (3H, s, CH3CO), 2.61–2.69 (1H, m, CH2-2′), 2.76–2.86 (1H, m, CH2-2′), 4.38 (3H, m, CH2-5′ and H-4′), 5.39 (1H, m, H-3′), 6.04 (2H, s, NH2), 6.45 (1H, t, J = 6.8 Hz, H-1′), 7.99 (1H, s, H-8).
2-Chloro-N6-methyl-2′-deoxyadenosine (39).
A flame dried 25-mL round-bottom flask fitted with a reflux condenser was charged with 38 (250 mg, 0.68 mmol), isoamyl nitrite (2 mL), and diiodomethane (2 mL) under nitrogen atmosphere. The mixture was stirred at 85 °C for 1 h. The solvent was removed with nitrogen purging, and the crude residue was stirred with aqueous methylamine (5 mL, 40% solution) for 1 h. The water was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to furnish 39 as a colorless solid (85.4 mg, 0.29 mmol, 42% yield): MS (CI-NH3) 300 (M++ 1); 1H NMR (CD3OD) δ 2.35–2.43 (1H, m, CH2-2′), 2.68, 2.68–2.80 (1H, m, CH2-2′), 3.06 (3H, bs, NHCH3), 3.10 (2H, m, CH2S), 3.79 (2H, m, CH2-5′), 4.04 (1H, m, H-4′), 4.57 (1H, m, H-3′), 6.34 (1H, t, J = 6.8 Hz, H-1′), 8.20 (1H, s, H-8).
2-(Methylthio)-N6-methyl-2′-deoxyadenosine (40).
To a solution of 2-chloro-2′-deoxy-N 6-methyladenosine (39; 50 mg, 0.17 mmol) in dry DMF (3 mL) was added sodium thiomethoxide (35 mg, 0.5 mmol), and the reaction mixture was stirred and heated in a sealed tube at 110 °C for 4 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to give 40 as a colorless solid (40 mg, 0.13 mmol, 76% yield): MS (CI-NH3) 312 (M++ 1); 1H NMR (CD3OD) δ 2.35–2.42 (1H, m, CH2-2′), 2.65 (3H, s, CH3S), 2.78–2.88 (1H, m, CH2-2′), 3.09 (3H, bs, NHCH3), 3.71–3.84 (2H, m, CH2-5′), 4.01–4.03 (1H, m, H-4′), 4.56–4.58 (1H, m, H-3′), 6.36 (1H, t, J = 6.8 Hz, H-1′), 8.09 (1H, s, H-8).
2-(Ethylthio)-N6-methyl-2′-deoxyadenosine (41).
To a suspension of potassium tert-butoxide (112 mg, 1 mmol) in dry DMF (3 mL) in a sealed tube was added ethanethiol (60 mg, 71 μL, 1 mmol), and the reaction mixture was stirred at room temperature for 30 min. 2-Chloro-2′-deoxy-N 6-methyladenosine (39; 50 mg, 0.17 mmol) in dry DMF (1 mL) was added and heated at 110 °C for 4 h while stirring. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to provide 41 as a colorless solid (39 mg, 0.12 mmol, 70% yield): MS (CI-NH3) 326 (M++ 1); 1H NMR (CD3OD) δ 1.45 (3H, t, J = 6.9 Hz, CH3CH2S), 2.45–2.52 (1H, m, CH2-2′), 2.88–297 (1H, m, CH2-2′), 3.10 (3H, s, N-CH3), 3.22 (2H, q, J = 6.4 Hz, CH3CH2S), 3.77–3.90 (2H, m, CH2-5′), 4.09 (1H, bs, H-4′), 4.64 (1H, bs, H-3′), 6.44 (1H, t, J = 5.9 Hz, H-1′), 8.03 (2H, bs, NH2), 8.23 (1H, s, H-8).
2-(Propylthio)-N6-methyl-2′-deoxyadenosine (42).
To a suspension of potassium tert-butoxide (112 mg, 1 mmol) in dry DMF (3 mL) in a sealed tube was added propanethiol (72 mg, 86 μL, 1 mmol), and the reaction mixture was stirred at room temperature for 30 min. 2-Chloro-2′-deoxy-N 6-methyladenosine (39; 50 mg, 0.17 mmol) in dry DMF (1 mL) was added and heated at 110 °C for 4 h while stirring. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to provide 42 as a colorless solid (46 mg, 0.14 mmol, 80% yield): MS (CINH3) 340 (M++ 1); 1H NMR (CD3OD) δ 1.13 (3H, t, J = 6.8 Hz, CH3CH2CH2S), 1.83–1.87 (2H, m, CH3CH2CH2S), 2.39–2.42 (1H, m, CH2-2′), 2.84–2.92 (1H, m, CH2-2′), 3.17 (3H, s, N-CH3), 3.19 (2H, bs, CH3CH2CH2S), 3.80–3.92 (2H, m, CH25′), 4.12 (1H, bs, H-4′), 4.62 (1H, bs, H-3′), 6.42 (1H, t, J = 6.4 Hz, H-1′), 8.17 (1H, s, H-8).
2-(Allylthio)-N6-methyl-2′-deoxyadenosine (43).
To a suspension of potassium tert-butoxide (112 mg, 1 mmol) in dry DMF (3 mL) in a sealed tube was added allylthiol (70 mg, 1 mmol), and the reaction mixture was stirred at room temperature for 30 min. 2-Chloro-2′-deoxy-N6-methyladenosine (39; 50 mg, 0.17 mmol) in dry DMF (1 mL) was added and heated at 110 °C for 4 h while stirring. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to provide 43 as a colorless solid (41 mg, 0.12 mmol, 72% yield): MS (CI-NH3) 338 (M+ + 1); 1H NMR (CD3OD) δ 2.32–2.37 (1H, m, CH2-2′), 2.72–2.80 (1H, m, CH2-2′), 3.09 (3H, s, NCH3), 3.68–3.72 (2H, m, CH2d CHCH2S), 4.01 (1H, bs, H-4′), 4.55 (1H, m, H-3′), 5.05 (1H, d, J = 10.5 Hz, CH2dCHCH2S), 5.31 (1H, d, J = 12.6 Hz, CH2d CHCH2S), 5.80 (1H, m, CH2)CHCH2S), 6.35 (1H, t, J = 5.6 Hz, H-1′), 8.11 (1H, s, H-8).
2-(Hexylthio)-N6-methyl-2′-deoxyadenosine (44).
To a suspension of potassium tert-butoxide (146 mg, 1.3 mmol) in dry DMF (3 mL) in a sealed tube was added hexanethiol (183 μL, 1.3 mmol), and the reaction mixture was stirred at room temperature for 30 min. To the reaction mixture was added a solution of 2-chloro-2′-deoxy-N 6-methyladenosine (39; 38 mg, 0.13 mmol) in dry DMF (2 mL), and the mixture was stirred and heated at 110 °C for 4 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to obtain the desired compound 2′deoxy-2-(hexylthio)-N6-methyladenosine (44) as a colorless solid (35 mg, 0.091 mmol, 70% yield): MS (CI-NH3) 382 (M+ + 1); 1H NMR (CD3OD) δ 0.87 (3H, m, CH3CH2), 1.29 (4H, m, CH2CH2), 1.38 (2H, m, CH2), 1.67 (2H, m, CH2CH2S), 2.29–2.37 (1H, m, CH2-2′), 2.68–2.78 (1H, m, CH2-2′), 3.05 (3H, bs, NHCH3), 3.10 (2H, m, CH2S), 3.76 (2H, m, CH2-5′), 4.00 (1H, m, H-4′), 4.51 (1H, m, H-3′), 6.31 (1H, t, J = 6.8 Hz, H-1′), 8.05 (1H, s, H-8).
2′-Deoxy-3′,5′-di-O-acetylguanosine (46).
A mixture of commercial 2′-deoxyguanosine (267 mg, 1 mmol), pyridine (4 mL), and acetic anhydride (2 mL) was heated while stirring at 65 °C for 4 h under nitrogen atmosphere. The reaction mixture was cooled, quenched with methanol, and evaporated to dryness. The crude residue was purified by flash column chromatography (CHCl3/MeOH, 95/5) to obtain 46 as a white solid (263 mg, 0.72 mmol, 75% yield): MS (CI-NH3) 352 (M+ + 1), 369 (M + NH4+); 1H NMR (CD3OD) δ 2.06 (3H, s, CH3-CO), 2.11 (3H, s, CH3CO), 2.53–2.60 (1H, m, CH2-2′), 2.96–3.01 (1H, m, CH2-2′), 3.32 (1H, bs, H-4′), 4.25–4.36 (2H, m, CH2-5′), 5.44 (1H, bs, H-3′), 6.27 (1H, t, J = 6.4 Hz, H-1′), 7.87 (1H, s, H-8).
6-Chloro-9-(2-deoxy-3,5-di-O-acetyl-β-D-erythropentofuranosyl)guanine (47).
A flame-dried 50-mL round-bottom flask fitted with a reflux condenser was charged with 46 (351 mg, 1 mmol), predried tetraethylammonium chloride (183 mg, 1.1 mmol), freshly distilled N,N-dimethylaniline (122 mg, 128 μL, 1 mmol), and dry acetonitrile (5 mL) under nitrogen atmosphere while stirring followed by cooling the mixture in an ice bath. Phosphorus oxychloride (306 mg, 186 μL, 2 mmol) was added dropwise, and stirring was continued for 10 min at room temperature. The reaction mixture was then refluxed for 10 min. The solvent and volatile materials were evaporated under reduced pressure, and the resultant yellow oil was stirred with crushed ice for 15 min. The yellow oil was extracted with chloroform (5 × 15 mL), washed with saturated NaHCO3 solution and cold water, dried over anhydrous Na2SO4, filtered, and evaporated to give a light-yellow syrup which was purified by flash column chromatography (CHCl3/MeOH, 95/5) to furnish 47 as a light-yellow solid (164 mg, 0.44 mmol, 44% yield): MS (CI-NH3) 370 (M+ + 1); 1H NMR (CDCl3) δ 2.11 (3H, s, CH3CO), 2.16 (3H, s, CH3CO), 2.67–2.74 (1H, m, CH2-2′), 2.79–2.89 (1H, m, CH2-2′), 4.39 (3H, bs, CH2-5′ and H-4′), 5.40 (1H, m, H-3′), 6.45 (1H, t, J = 6.9 Hz, H-1′), 8.34 (1H, s, H-8).
2-Amino-N6-methyl-2′-deoxyadenosine (48).
A mixture of 2-chloro-2′-deoxy-3′,5′-di-O-acetyl-6-chloropurine riboside (47; 230 mg, 0.62 mmol) and 40% aqueous methylamine (5 mL) was stirred at room temperature for 2 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 95/5) to obtain the desired compound 2-chloro-2′-deoxy-N 6-methyladenosine (48) as a white solid (113 mg, 0.40 mmol, 55% yield): MS (CI-NH3) 281 (M++ 1); 1H NMR (CD3OD) δ 2.28–2.33 (1H, m, CH2-2′), 2.77–2.82 (1H, m, CH2-2′), 3.04 (3H, s, N-CH3), 3.71–3.88 (2H, m, CH2-5′), 4.06 (1H, bs, H-4′), 4.56 (1H, bs, H-3′), 6.28 (1H, t, J = 5.7 Hz, H-1′), 7.87 (1H, s, H-8).
2-Amino-N6-methyl-2′-deoxy-3′,5′-di-O-acetyladenosine (49).
A mixture of 2-amino-N 6-methyl-2′-deoxyadenosine (48, 140 mg, 0.5 mmol), pyridine (2 mL), and acetic anhydride (1.5 mL) was stirred at room temperature for 4 h. The mixture was evaporated to dryness and chromatographed by pTLC (CHCl3/MeOH, 95/5) to obtain 49 as a colorless solid (142 mg, 0.39 mmol, 78% yield): MS (CI-NH3) 365 (M++ 1); 1H NMR (CDCl3) δ 2.09 (3H, s, CH3CO), 2.14 (3H, s. CH3CO), 2.59–2.67 (1H, m, CH2-2′), 2.89–2.99 (1H, m, CH2-2′), 4.34–4.39 (3H, m, CH2-5′ and H-4′), 5.42–5.45 (1H, m, H-3′), 6.02 (2H, bs, NH2), 6.44 (1H, t, J = 5.9 Hz, H-1′), 8.24 (1H, s, H-8).
2-Iodo-N6-methyl-2′-deoxyadenosine (50).
A flame-dried 25-mL round-bottom flask fitted with a reflux condenser was charged with compound 49 (140 mg, 0.38 mmol), isoamyl nitrite (1.5 mL) and diiodomethane (1.5 mL), under nitrogen atmosphere. The mixture was stirred at 85 °C for 1 h. The solvent was removed with nitrogen purging, and the crude residue was stirred with aqueous methylamine (5 mL, 40% solution) for 1 h. The water was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to furnish compound 50 as a colorless solid (71 mg, 0.18 mmol, 48% yield): MS (CI-NH3) 392 (M++ 1); 1H NMR (CD3OD) δ 2.35–2.43 (1H, m, CH2–2′), 2.69–2.80 (1H, m, CH2–2′), 3.06 (3H, s, NCH3), 3.71–3.82 (2H, m, CH2–5′), 4.02–4.05 (1H, m, H-4′), 4.54–4.58 (1H, m, H-3′), 6.34 (1H, t, J = 5.95 Hz, H-1′), 8.20 (1H, s, H-8).
2-(Methylthio)-2′-deoxyadenosine (51).
To a solution of 2-chloro-2′-deoxyadenosine (37; 31 mg, 0.1 mmol) in dry DMF (2 mL) was added sodium thiomethoxide (70 mg, 1 mmol), and the mixture was stirred and heated in a sealed tube at 110 °C for 4 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 93/7) to afford 51 as a white solid (21 mg, 0.07 mmol, 70% yield): MS (CI-NH3) 298 (M++ 1); 1H NMR (CD3OD) δ 2.28–2.30 (1H, m, CH2-2′); 2.76–2.85 (1H, m, CH2-2′), 3.39 (3H, s, CH3S), 3.52–3.68 (2H, m, CH2-5′), 3.91 (1H, m, H-4′), 4.47 (1H, m, H-3′), 4.99 (1H, bs, OH), 5,38 (1H, bs, OH), 6.35 (1H, t, J = 5.7 Hz, H-1′), 7.41 (2H, bs, NH2), 8.27 (1H, s, H-8).
2-(Ethylthio)-2′-deoxyadenosine (52).
To a suspension of potassium tert-butoxide (112 mg, 1 mmol) in dry DMF (2 mL) in a sealed tube was added ethanethiol (62 mg, 74 μL, 1 mmol), and the reaction mixture was stirred at room temperature for 30 min. A solution of 2-chloro-2′-deoxy-N 6-methyladenosine (37; 31 mg, 0.1 mmol) in dry DMF (1 mL) was added to the above mixture, and the reaction was stirred and heated at 110 °C for 4 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to afford 52 as a colorless solid (25 mg, 0.08 mmol, 80% yield): MS (CI-NH3) 312 (M++ 1); 1H NMR (CD3OD) δ 1.44 (3H, t, J = 7.8 Hz, CH3CH2S), 2.44–2.51 (1H, m, CH2-2′), 2.90–2.98 (1H, m, CH2-2′), 3.24 (2H, q, J = 6.86 Hz, CH3CH2S), 3.77–3.91 (2H, m, CH2-5′), 4.09 (1H, bs, H-4′), 4.62–4.64 (1H, m, H-3′), 6.46 (t, J = 7.0 Hz, H-1′), 8.18 (1H, s, H-8).
2-(Propylthio)-2′-deoxyadenosine (53).
To a suspension of potassium tert-butoxide (112 mg, 1 mmol) in dry DMF (2 mL) in a sealed tube was added propanethiol (75 mg, 89 μL, 1 mmol), and the reaction mixture was stirred at room temperature for 30 min. A solution of 2-chloro-2′-deoxy-N 6-methyladenosine (37; 31 mg, 0.1 mmol) in dry DMF (1 mL) was added to the above mixture, and the reaction was stirred and heated at 110 °C for 4 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to afford 53 as a colorless solid (25 mg, 0.08 mmol, 76% yield): MS (CI-NH3) 326 (M++ 1); 1H NMR (CD3OD) δ 1.12 (3H, t, J = 7.8 Hz, CH3CH2CH2S), 1.76–1.84 (2H, m, CH3CH2CH2S), 2.45–2.51 (1H, m, CH2-2′), 2.88–2.94 (1H, m, CH2-2′), 3.03 (2H, bs, 2OH), 3.18 (2H, q, J = 5.9 Hz, CH3CH2CH2S), 3.78–3.90 (2H, m, CH2-5′), 4.08–4.11 (1H, m, H-4′), 4.62–4.65 (1H, m, H-3′), 6.43 (1H, t, J = 6.8 Hz, H-1′), 8.03 (2H, bs, NH2), 8.22 (1H, s, H-8).
8-Bromo-2′-deoxyadenosine (55).
A mixture of 2′-deoxyadenosine (54; 270 mg, 1 mmol) and N-bromosuccinimide (267 mg, 1.5 mmol) in dry DMF (6 mL) was stirred at room temperature for 12 h under nitrogen atmosphere. The solvent was removed under reduced pressure, and the residue was purified using pTLC (CHCl3/MeOH, 9/1) to give 55 as a colorless solid (132 mg, 0.40 mmol, 40% yield): MS (CI-NH3) 330 (M++ 1); 1H NMR (CD3OD) δ 2.18–2.22 (1H, m, CH2-2′), 3.23–3.30 (1H, m, CH2-2′), 3.48–3.54 (1H, m, CH2-5′), 3.66–3.70 (1H, m, CH2-5′), 3.92 (1H, bs, H-4′), 4.52 (1H, bs, H-3′), 5.31–5.38 (2H, bs, 2 OH), 6.32 (1H, t, J = 6.9 Hz, H-1′), 7.55 (2H, bs, NH2), 8.14 (1H, s, H-2).
8-Methoxy-2′-deoxyadenosine (56).
To a solution of 8-bromo-2′-deoxyadenosine (55; 50 mg, 0.15 mmol) in dry DMF (2 mL) was added sodium methoxide (54 mg, 1 mmol), and the reaction mixture was stirred and heated in a sealed tube at 110 °C for 6 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to give 56 as a light-yellow solid (30 mg, 0.11 mmol, 72% yield): MS (CI-NH3) 282 (M++ 1); 1H NMR (CD3OD) δ 2.20–2.27 (1H, m, CH2-2′), 2.48–2.62 (1H, m, CH2-2′), 3.66 (3H, s, OCH3), 3.71–3.90 (2H, m, CH2-5′), 4.09 (1H, bs, H-4′), 4.60 (1H, bs, H-3′), 6.34 (1H, t, J = 6.0 Hz, H-1′), 8.06 (1H, s, H-2).
8-(Methylthio)-2′-deoxyadenosine (57).
To a solution of 8-bromo-2′-deoxyadenosine (55, 50 mg, 0.15 mmol) in dry DMF (2 mL) was added sodium thiomethoxide (70 mg, 1 mmol), and the reaction mixture was stirred and heated in a sealed tube at 110 °C for 4 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to give the desired compound 8-(methylthio)-2′-deoxyadenosine (57) as a light-yellow solid (37.8 mg, 0.13 mmol, 85% yield): MS (EI) 297 (M+); 1H NMR (CD3OD) δ 2.20–2.28 (m, 1H, CH2-2′), 2.47–2.61 (1H, m, CH2-2′), 2.76 (3H, s, SCH3), 3.68–3.91 (2H, m, CH2-5′), 4.01 (1H, bs, H-4′), 4.60 (1H, m, H-3′), 6.36 (1H, t, J = 5.9 Hz, H-1′), 8.10 (1H, s, H-8).
8-(Methylamino)-2′-deoxyadenosine (58).
A 40% aqueous solution of methylamine (5 mL) was added to 8-bromo-2′deoxyadenosine (55, 50 mg, 0.15 mmol), and the reaction mixture was stirred at room temperature for 3 h. The solvent was removed under nitrogen flow, and the residue was purified using pTLC (CHCl3/MeOH, 9/1) to afford 58 as a white solid (37.8 mg, 0.14 mmol, 90% yield): MS (CI-NH3) 281 (M++ 1); 1H NMR (CD3OD) δ 2.11–2.52 (2H, m, CH2-2′), 3.31 (3H, s, NCH3), 3.81–3.84 (2H, m, CH2-5′), 4.06 (1H, m, H-4′), 4.58 (1H, m, H-3′), 6.44 (1H, t. J = 5.8 Hz, H1′), 7.99 (1H, s, H-8).
2′-Deoxy-N6-methoxyladenosine (59).
To a mixture of 6-chloro-2′-deoxypurine riboside (25 mg, 0.092 mmol) and methoxyamine hydrochloride (100 mg, 1.19 mmol) in DMF (2 mL) was added 1 M NaOH (1.19 mL). The reaction mixture was stirred at room temperature for 72 h and then neutralized with 0.1 M HCl. The mixture was evaporated to dryness and chromatographed by pTLC (CHCl3/MeOH, 90/10) to obtain the desired compound 59 (14 mg, 0.050 mmol, 54% yield): MS (CINH3) 282 (M++ 1), 299 (M + NH4+); 1H NMR (CD3OD) δ 2.52–2.58 (1H, m, CH2-2′), 2.74–2.83 (1H, m, CH2-2′), 3.80 (2H, m, CH2-5′), 3.85 (3H, s, OCH3), 4.14 (1H, bs, H-4′), 4.61 (1H, m, H-3′), 6.39 (1H, t, J = 6.9 Hz, H-1′), 7.93 (1H, s, H-2), 8.14 (1H, s, H-8).
Carbocyclic 2-Amino-N6-methyl-2′-deoxyadenosine (61).
A solution of carbocyclic 2-amino-6-chloro-2′-deoxyadenosine (60; 28 mg, 0.1 mmol) and 40% aqueous methylamine (4 mL) was stirred at room temperature for 3 h. The solvent was removed under reduced pressure, and the residue was chromatographed using pTLC (CHCl3/MeOH, 9/1) to provide 61 as colorless solid (26 mg, 0.09 mmol, 92% yield): MS (CI-NH3) 279 (M++ 1); 1H NMR (CD3OD) δ 1.72–1.79 (1H, m, CH2-2′), 2.01–2.19 (2H, m, CH2-6′), 2.20–2.29 (1H, m, CH2-2′), 2.32–2.43 (1H, m, H-1′), 2.96 (3H, s, N-CH3), 3.58–3.69 (2H, m, CH2-5′), 4.20 (1H, bs, H-3′), 7.75 (1H, s, H-8).
2′-Deoxy-4′-thioadenosine S-Oxide (63).
A solution of sodium metaperiodate (22.5 mg, 0.105 mmol) in water (0.4 mL) was added dropwise to a vigorously stirred ice-cold solution of 2′-deoxy-4′-thioadenosine (62; 13.3 mg, 0.050 mmol) in methanol (1 mL). The reaction mixture was stirred for 48 h at room temperature. The solvent was removed under nitrogen purging, and the residue was chromatographed using pTLC (CHCl3/MeOH, 10/1) to obtain the desired 2′-deoxy-4′-thioadenosine S-oxide, 63 (mixture of diastereomers), as a colorless solid (14.0 mg, 0.049 mmol, 98% yield): MS (CI-NH3) 284 (M+ + 1); 1H NMR (CD3OD) δ 2.70–3.19 (2H, m, CH2-2′), 3.44–3.48 (1H, m, H-4′), 4.01–4.07 (2H, m, CH2-5′), 4.55–4.66 (1H, 2m, H-3′), 6.08–6.28 (1H, 2m, H-1′), 8.20, 8.25 (1H, 2s, H-2), 8.26, 8.29 (1H, 2s, H-8).
2′-Deoxy-5′-[diethyl(O-phosphonylmethyl)]adenosine (64).
A flame-dried 50-mL round-bottom flask was charged with 2′-deoxyadenosine (51; 270 mg, 1 mmol) and potassium tert-butoxide (146 mg, 1.3 mmol) in dry DMF (5 mL) under argon atmosphere, and the reaction mixture was stirred at room temperature for 2 h. A solution of diethyl tosylmethanephosphonate (320 mg, 1.1 mmol) in dry DMF (1 mL) was added dropwise, and the reaction mixture was allowed to stir for 3 days at room temperature. The solvent was removed by nitrogen purging, and the crude product was purified by pTLC (CHCl3/MeOH, 85/15) to afford 64 as a light-yellow solid (128 mg, 0.32 mmol, 32% yield): MS (CI-NH3) 402 (M+ + 1); 1H NMR (CD3OD) δ 1.21–1.25 (6H, m, OCH2CH3); 2.34–2.42 (1H, m, CH2-2′), 2.74–2.85 (1H, m, CH2-2′), 3.44 (2H, d, J = 10.5 Hz, -CH2-P), 3.71–4.13 (6H, m, CH2-5′ and P-CH2CH3), 4.19 (1H, m, H-4′), 4.60 (1H, m, H-3′), 6.42 (1H, t, J = 6.5 Hz, H-1′), 7.97 (2H, bs, NH2), 8.18 (1H, s, H-2), 8.26 (1H, s, H-8).
Pharmacological Analyses.
P2Y1 receptor-promoted stimulation of inositol phosphate formation by adenine nucleotide analogues was measured in turkey erythrocyte membranes as previously described.10,39 The EC50 values were averaged from 3–8 independently determined concentration-effect curves for each compound. Briefly, 1 mL of washed turkey erythrocytes was incubated in inositol-free medium (DMEM; Gibco, Gaithersburg, MD) with 0.5 mCi of 2-[3H]myo-inositol (20 Ci/mmol; American Radiolabeled Chemicals, Inc., St. Louis, MO) for 18–24 h in a humidified atmosphere of 95% air/5% CO2 at 37 °C. Erythrocyte ghosts were prepared by rapid lysis in hypotonic buffer (5 mM sodium phosphate, pH 7.4, 5 mM MgCl2, 1 mM EGTA) as described.(39 Phospholipase C activity was measured in 25 μL of [3H]inositol-labeled ghosts (approximately 175 μg of protein, 200–500 000 cpm/assay) in a medium containing 424 μM CaCl2, 0.91 mM MgSO4, 2 mM EGTA, 115 mM KCl, 5 mM KH2PO4, and 10 mM Hepes, pH 7.0. Assays (200 μL final volume) contained 1 μM GTPγS and the indicated concentrations of nucleotide analogues. Ghosts were incubated at 30 °C for 5 min, and total [3H]inositol phosphates were quantitated by anion-exchange chromatography as previously described.10,39 Stimulation of inositol phosphate formation in 1321N1 human astrocytoma cells stably expressing recombinant human P2Y2 receptors (activated by 100 nM UTP), recombinant human P2Y4 receptors (activated by 100 nM UTP), and recombinant rat P2Y6 receptors (activated by 100 nM UDP) was measured in a similar fashion.21,38
Data Analysis.
Agonist potencies were calculated using a four-parameter logistic equation and the GraphPad software package (GraphPad, San Diego, CA). EC50 values (mean ± standard error) represent the concentration at which 50% of the maximal effect is achieved. Relative efficacies (%) were determined by comparison with the effect produced by a maximal effective concentration of 2-MeSADP in the same experiment.
Antagonist IC50 values (mean ± standard error) represent the concentration needed to inhibit by 50% the effect elicited by 10 nM 2-MeSADP. The percent of maximal inhibition is equal to 100 minus the residual fraction of stimulation at the highest antagonist concentration.
All concentration-effect curves were repeated in at least three separate experiments carried out with different membrane preparations using duplicate or triplicate assays.
Acknowledgment.
We thank Gilead Sciences (Foster City, CA) for financial support to E.N. and Dr. Stefano Moro (NIDDK) for helpful discussions. We also are grateful to Dr. Lewis Pannell, Noel Whittaker, Mary Furr, and Wesley White for technical assistance. This work was supported by USPHS Grants GM38213 and HL54889.
Abbreviations:
- ATP
adenosine 5′-triphosphate
- DEAE
diethylaminoethyl
- DMF
dimethylformamide
- DMSO
dimethyl sulfoxide
- FAB
fast atom bombardment (mass spectroscopy)
- HPLC
high-pressure liquid chromatography
- MS
mass spectroscopy
- HRMS
high-resolution mass spectroscopy
- IAN
isoamyl nitrite
- 2-MeSADP
2-(methylthio)adenosine 5′-diphosphate
- NBS
N-bromosuccinimide
- NNDA
N,N-dimethylaniline
- Py
pyridine
- TBAP
tetrabutylammonium phosphate
- TEAA
triethylammonium acetate
- pTLC
preparative thinlayer chromatography
References
- (1).Fredholm BB; Abbracchio MP; Burnstock G; Dubyak GR; Harden TK; Jacobson KA; Schwabe U; Williams M Toward a revised nomenclature for P1 and P2 receptors. Trends Pharm. Sci 1997, 18, 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).North RA; Barnard EA Nucleotide receptors. Curr. Opin. NeuroBiol 1997, 7, 346–357. [DOI] [PubMed] [Google Scholar]
- (3).Webb TE; Simon J; Krishek BJ; Bateson AN; Smart TG; King BF; Burnstock G; Barnard EA Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993, 324, 219–225. [DOI] [PubMed] [Google Scholar]
- (4).Schachter JB; Li Q; Boyer JL; Nicholas RA; Harden TK 2nd messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br. J. Pharmacol. 1996, 118, 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Janssens R; Communi D; Pirotton S; Samson M; Parmentier M; Boeynaems J-M Cloning and tissue distribution of the human P2Y1 receptor. Biochem. Biophys. Res. Commun. 1996, 221, 588–593. [DOI] [PubMed] [Google Scholar]
- (6).Ayyanathan K; Webb TE; Sandhu AK; Athwal RS; Barnard EA; Kunapuli SP Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem. Biophys. Res. Commun. 1996, 218, 783–788. [DOI] [PubMed] [Google Scholar]
- (7).Burnstock G; King BF Numbering of cloned P2 purinoceptors. Drug Dev. Res. 1996, 38, 67–71. [Google Scholar]
- (8).Communi D; Govaerts C; Parmentier M; Boeynaems JM Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J. Biol. Chem. 1997, 272, 31969–31973. [DOI] [PubMed] [Google Scholar]
- (9).Lazarowski ER; Homolya L; Boucher RC; Harden TK Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J. Biol. Chem. 1997, 272, 24348–24354. [DOI] [PubMed] [Google Scholar]
- (10).Harden TK; Hawkins PT; Stephens L; Boyer JL; Downes P Phosphoinositide hydrolysis by guanosine 5′-(gammathio]triphosphate-activated phospholipase C of turkey erythrocyte membranes. Biochem. J. 1988, 252, 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Jin J; Daniel JL; Kunapuli SP Molecular basis for ADPinduced platelet activation. II. The P2Y1 receptor mediates ADPinduced intracellular calcium mobilization and shape change in platelets. J. Biol. Chem. 1998, 273, 2030–2034. [DOI] [PubMed] [Google Scholar]
- (12).Hechler B; Leon C; Vial C; Vigne P; Frelin C; Cazenave JP; Gachet C The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood 1998, 92, 152–159. [PubMed] [Google Scholar]
- (13).Crowley MR Oxygen-induced pulmonary vasodilation is mediated by adenosine triphosphate in newborn lambs. J. Cardiovasc. Pharmacol. 1997, 30, 102–109. [DOI] [PubMed] [Google Scholar]
- (14).Loubatières-Mariani M-M; Hillaire-Buys D; Chapal J; Bertrand G; Petit P P2 purinoceptor agonists: New insulin secretagogues potentially useful in the treatment of noninsulin-dependent diabetes mellitus In Purinergic Approaches in Experimental Therapeutics; Jacobson KA, Jarvis MF, Eds.; Wiley: New York, 1997; pp 253–260. [Google Scholar]
- (15).Fagura MS; Dainty IA; McKay GD; Kirk IP; Humphries RG; Robertson MJ; Dougall IG; Leff P P2Y1-receptors in human platelets which are pharmacologically distinct from P2Y(ADP)-receptors. Br. J. Pharmacol. 1998, 124, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Virginio C; Robertson G; Surprenant A; North RA Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol. Pharmacol. 1998, 53, 969–973. [PubMed] [Google Scholar]
- (17).Humphreys BD; Virginio C; Surprenant A; Rice J; Dubyak GR Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol. Pharmacol. 1998, 54, 22–32. [DOI] [PubMed] [Google Scholar]
- (18).Kim Y-C; Camaioni E; Ziganshin AU; Ji X-J; King BF; Wildman SS; Rychkov A; Yoburn J; Kim H; Mohanram A; Harden TK; Boyer JL; Burnstock G; Jacobson KA Synthesis and structure activity relationships of pyridoxal-6-azoaryl-5′-phosphate and phosphonate derivatives as P2 receptor antagonists. Drug Dev. Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Boyer JL; Romero-Avila T; Schachter JB; Harden TK Identification of competitive antagonists of the P2Y1 receptor. Mol. Pharmacol. 1996, 50, 1323–1329. [PubMed] [Google Scholar]
- (20).Camaioni E; Boyer JL; Mohanram A; Harden TK; Jacobson KA Deoxyadenosine-bisphosphate derivatives as potent antagonists at P2Y1 receptors. J. Med. Chem. 1998, 41, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Boyer JL; Mohanram A; Camaioni E; Jacobson KA; Harden TK Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Brit. J. Pharmacol. 1998, 124, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Boyer JL; Lazarowski E; Chen X-H; Harden TK Identification of a P2Y-purinergic receptor that inhibits adenylyl cyclase but does not activate phospholipase C. J. Pharmacol. Exp. Ther. 1993, 267, 1140–1146. [PubMed] [Google Scholar]
- (23).Boyer JL; Mohanram A; Deleney S; Waldo G; Harden TK Signaling mechanism and pharmacological selectivity of an avian P2 receptor Nucleotides and their receptors in the nervous system; Leipzig, Germany, Aug 1, 1998; Abstact A03. [Google Scholar]
- (24).Cristalli G; Vittori S; Eleuteri A; Volpini R; Camaioni E; Lupidi G Synthesis of 2′-deoxynucleoside derivatives of 1-deazapurine. Nucleosides Nucleotides 1994, 13, 835–848. [Google Scholar]
- (25).Verheggen I; van Aerschot A; Toppet S; Snoeck R; Janssen G; Balzarini J; de Clercq E; Herdewijn P Synthesis and antiherpes virus activity of 1,5-anhydrohexitol nucleosides. J. Med. Chem. 1993, 36, 2033–2040. [DOI] [PubMed] [Google Scholar]
- (26).Messini L; Tiwari KN; Montgomery JA; Secrist JA III. Synthesis and Biological Activity of 4′-Thio-2′-deoxy Purine Nucleosides; 13th International Round Table on Nucleosides, Nucleotides and their Biological Applications; Sept. 6–10, 1998. [DOI] [PubMed] [Google Scholar]
- (27).Shealy YF; O’Dell CA Carbocyclic Analogues of 2′-Deoxyadenosine. Tetrahedron Lett. 1969, 2231–2234. [Google Scholar]
- (28).Robins MJ; Fouron Y; Mengel R Adenosine 2′,3′-riboepoxide. Synthesis, intramolecular degradation, and transformation into 3′-substituted xylofuranosyl nucleosides and the lyxoepoxide. J. Org. Chem. 1974, 39, 1564–1570. [DOI] [PubMed] [Google Scholar]
- (29).Robins MJ; Uza’nski B Nucleic acid related compounds. 33. Conversions of adenosine and guanosine to 2,6-dichloro, 2-amino-6-chloro, and derived nucleosides. Can. J. Chem. 1981, 59, 2601–2607. [Google Scholar]
- (30).van Aerschot A; Mamos P; Weyns N; Ikeda S; de Clercq E; Herdewijn P Antiviral activity of C-alkylated purine nucleosides obtained by cross-coupling with tetraalkyltin reagents. J. Med. Chem. 1993, 36, 2938–2942. [DOI] [PubMed] [Google Scholar]
- (31).Hoffman DJ; Whistler RL Synthesis and properties of nucleotides containing 4-thio-D-ribofuranoside. Biochemistry 1970, 9, 2367–2372. [DOI] [PubMed] [Google Scholar]
- (32).Hancox EL; Walker RT Some reactions of 4′-thionucleosides and their sulfones. Nucleosides Nucleotides 1996, 15, 1–3. [Google Scholar]
- (33).Fischer B; Boyer JL; Hoyle CHV; Ziganshin AU; Brizzolara AL; Knight GE; Zimmet J; Burnstock G; Harden TK; Jacobson KA Identification of potent, selective P2Y-purinoceptor agonists - structure-activity-relationships for 2-thioether derivatives of adenosine 5′-triphosphate. J. Med. Chem. 1993, 36, 3937–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Boyer JL; Siddiqi S; Fischer B; Romero-Avila T; Jacobson KA; Harden TK Identification of potent P2Y-purinoceptor agonists that are derivatives of adenosine 5′-monophosphate. Br. J. Pharmacol. 1996, 118, 1959–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).van der Wenden EM; Carnielli M; Roelen HC; Lorenzen A; von Frijtag Drabbe Künzel JK; IJzerman AP 5′-substituted adenosine analogues as new high-affinity partial agonists for the adenosine A1 receptor. J. Med. Chem. 1998, 41, 102–108. [DOI] [PubMed] [Google Scholar]
- (36).Jasper JR; Insel PA Evolving concepts of partial agonism. The beta-adrenergic receptor as a paradigm. Biochem. Pharmacol. 1992, 43, 119–130. [DOI] [PubMed] [Google Scholar]
- (37).Jacobson KA; Hoffmann C; Kim Y-C; Camaioni E; Nandanan E; Jang SY; Guo D-P; Ji X.-d.; von Kügelgen I; Moro S; Ziganshin AU; Rychkov A; King BF; Brown S; Wildman SS; Burnstock G; Boyer JL; Mohanram A; Harden TK Molecular recognition in P2 receptors: Ligand development aided by molecular modeling and mutagenesis. Prog. Brain Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Jacobson KA; Kim Y-C; Wildman SS; Mohanram A; Harden TK; Boyer JL; King BF; Burnstock G A pyridoxine cyclic-phosphate and its 6-arylazo-derivative selectively potentiate and antagonize activation of P2X1 receptors. J. Med. Chem. 1998, 41, 2201–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Boyer JL; Downes CP; Harden TK Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J. Biol. Chem. 1989, 264, 884–890. [PubMed] [Google Scholar]