SUMMARY
Gliomas comprise heterogeneous malignant glial and stromal cells. While blood vessel co-option is a potential mechanism to escape anti-angiogenic therapy, the relevance of glial phenotype in this process is unclear. We show that Olig2+ oligodendrocyte precursor-like glioma cells invade by single-cell vessel co-option and preserve the blood-brain barrier (BBB). Conversely, Olig2-negative glioma cells form dense perivascular collections and promote angiogenesis and BBB breakdown, leading to innate immune cell activation. Experimentally, Olig2 promotes Wnt7b expression, a finding that correlates in human glioma profiling. Targeted Wnt7a/7b deletion or pharmacologic Wnt inhibition blocks Olig2+ glioma single-cell vessel co-option and enhances responses to temozolomide. Finally, Olig2 and Wnt7 become upregulated after anti-VEGF treatment in preclinical models and patients. Thus, glial-encoded pathways regulate distinct glioma-vascular microenvironmental interactions.
In Brief
Griveau et al. show that Olig2+ glioma cells invade by single-cell vessel co-option, whereas Olig2− glioma cells promote angiogenesis and that anti-VEGF treatment selects for the Olig2+/Wnt7+ phenotype. Wnt7 is necessary for vessel co-option, and Wnt inhibition enhances the response to temozolomide treatment.
INTRODUCTION
About 25,000 individuals/year in the US are diagnosed with glioblastoma (GBM), a leading cause of cancer-related death (Ostrom et al., 2015). Gliomas typically escape microscopic surgical resection and recur because of their ability to invade diffusely into brain parenchyma (Olar and Aldape, 2014; Prados et al., 2015). GBMs have high metabolic requirements and use multiple mechanisms to ensure adequate access to the vasculature, including angiogenesis, vasculogenesis, and trans-differentiation into endothelial cells (Boer et al., 2014; Carmeliet and Jain, 2011; Hu et al., 2016). In the distinct process of vessel “co-option,” glioma cells invade the brain along the pre-existing vasculature (Jain, 2014). Although inhibitors of vascular endothelial growth factor (VEGF) have been proven to control edema and prolong progression-free survival in glioma patients (Chinot et al., 2014; Gilbert et al., 2014; Wick et al., 2017), these tumors become resistant to anti-VEGF treatment (Lu-Emerson et al., 2015) by deploying alternative pathways and growth patterns. Indeed, both newly diagnosed and recurrent gliomas appear to exploit vessel co-option as a mechanism of escape from anti-VEGF/R2 treatment (di Tomaso et al., 2011; Keunen et al., 2011; Rubenstein et al., 2000).
In common with other cancers, gliomas can migrate either as single cells along blood vessels or collectively as perivascular groups of cells (Te Boekhorst and Friedl, 2016), and this has implications for invasion of the brain and maintenance of the blood-brain barrier (BBB) (Watkins et al., 2014). However, the cellular and molecular mechanisms that regulate glioma co-option are poorly understood. One possibility is that glioma cell plasticity enables use of different vascular strategies depending on micro-environmental or treatment circumstances. Indeed, gliomas are highly heterogeneous tumors that show features of stem cells, oligodendrocyte precursors, astrocytes, and oligodendrocytes (Patel et al., 2014). Olig2 (expressed in almost all glioma sub-types; Ligon et al., 2004) has multiple functions, including regulation of stem cell identity (Suva et al., 2014), tumor cell proliferation (Ligon et al., 2007), and oligodendrocyte versus astrocyte phenotype (Mehta et al., 2011). Moreover, these roles depend on the genetic context, as a critical function of Olig2 is antagonism of p53 activity (Sun et al., 2011). While Olig2 status may not be useful in determining clinical prognosis, it has been proposed as a direct therapeutic target (Mehta et al., 2011) through inhibitors that prevent phosphorylation needed for its pro-tumorigenic activities (Zhou et al., 2017).
Oligodendrocyte precursors (OPCs), expressing Olig2, Nkx2.2, PDGFRα, NG2, and other markers (Gallo and Deneen, 2014), can serve as tumor progenitors in adult high-grade glioma and oligodendroglioma (OD) (Liu et al., 2011; Persson et al., 2010). OPC-encoded Wnt7 signaling instructs white matter vascularization (Yuen et al., 2014), and Wnt-CXCR4 signaling regulates extensive OPC migration along the embryonic CNS vasculature (Tsai et al., 2016). In contrast, astrocytes migrate in a pattern restricted by the trajectory of their radial glial precursors (Tsai et al., 2012). Astrocytes have reduced proliferative potential compared with OPCs but carry out other important roles such as regulation of vascular flow and maintenance of the BBB through tight junctions with endothelial cells (Zhao et al., 2015). Although glial cells encode distinct regulatory pathways to achieve normal vascular function in the developing brain, a systematic assessment of glial subtype roles in glioma has not been carried out. Here we addressed this question with a focus on tumor-stromal and vascular regulation.
RESULTS
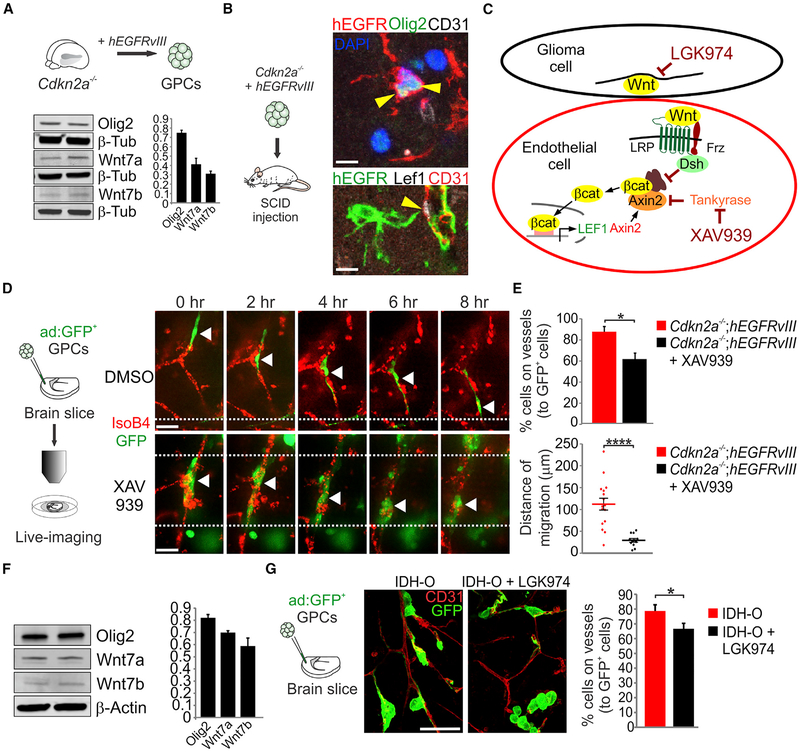
Olig2+ Glioma Cells Invade the Brain by Single-Cell Vessel Co-option
To determine vessel regulatory functions of OPC-like (OPCL) cells in glioma, we used an EGFRvIII-driven murine model that lacks p53 function (Figure 1A) and allows for variation in Olig2 functional status (Mehta et al., 2011). Olig2cre/+;Trp53fl/fl;hEGFRvIII (Olig2+) gliomas showed prevalent OPCL cells that expressed Olig2, PDGFRα, and NG2 (Figures S1A–S1C), whereas Olig2cre/cre;Trp53fl/fl;hEGFRvIII (Olig2−) tumors expressed astrocyte markers, such as glial fibrillary acidic protein (GFAP). Although Olig2+ tumors developed more quickly than Olig2− tumors (Mehta et al., 2011), no preference in the tumor location (e.g., ventrally or dorsally) or size was observed, and the proliferative index was not significantly different between the two types (Figure S1D); unsupervised hierarchical clustering analysis of Olig2+ versus Olig2− tumors demonstrated clear differences between the two types (Figure S1E). A total of 2,551 genes were differentially expressed, including genes involved in glial differentiation (Table S1), and Olig2+ tumors showed an enrichment of OPCs versus astrocytic genes (Figure 1B and Table S2) (Zhang et al., 2014). We compared gene expression in Olig2+ and Olig2− mouse tumors with human OD and subtypes of GBM (Tirosh et al., 2016; Verhaak et al., 2010; Wang et al., 2017). As shown (Figures S1F, 1G, and Table S2), clustering analysis of differentially upregulated genes in Olig2+ (versus Olig2−) tumors indicated strongest association with human OD and proneural GBM gene profiles.
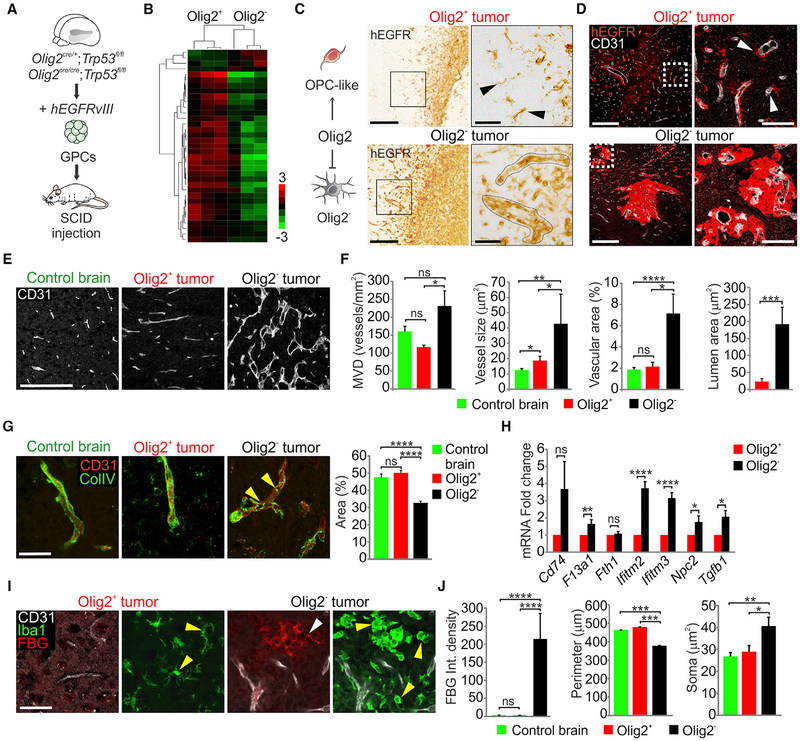
Figure 1. Olig2 Function Regulates Vessel Co-option and the Tumor Microenvironment.
(A) Schematic of Olig2+ and Olig2− tumors generation from glioma progenitor cells (GPCs). SCID, severe combined immunodeficiency.
(B) Heatmap (log2 fold change) of genes upregulated in Olig2+ versus Olig2− tumors (FDR adjusted p < 0.05) comparing murine oligodendrocyte precursor cells with astrocytes (Zhang et al., 2014).
(C) Immunostaining for hEGFR at tumor borders in dorsal cortex. Right panels represent high magnifications of the boxed areas in left panels. Black arrowheads show single-cell migration (n = 6 per genotype). Scale bars, 200 μm left and 50 μm right.
(D) Immunostaining for hEGFR and CD31 in Olig2+ and Olig2− tumors 1 month post-transplantation. Right panels represent high magnifications of the boxed areas in left panels. White arrowheads show single-cell vessel co-option (n = 3 per genotype). Scale bars, 200 μm left and 50 μm right.
(E and F) Immunostaining (E) and quantification (F) for CD31 and analysis of MVD, vessel size, percentage of vascular area and lumen area in normal brain, Olig2+ and Olig2− tumors. Data are means ± SEM (n = 4–6 per genotype; ns, non-significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Scale bar, 200 μm.
(G) Collagen IV (ColIV) and CD31 immunostaining in Olig2+ versus Olig2− tumors. Yellow arrowheads indicate absence of vascular basement membrane coverage. Data are means ± SEM (n = 3 per genotype; ns, non-significant; ****p < 0.0001). Scale bar, 50 μm.
(H) Fold change in macrophage markers expression using microarrays comparing Olig2+ versus Olig2− tumors. Data are means ± SEM (n = 3 per genotype; ns, non-significant; *p < 0.05, **p < 0.01, ****p < 0.0001).
(I) FBG, Iba1, and CD31 immunostaining in Olig2+ and Olig2− tumors. White and yellow arrowheads indicate FBG leakage and microglial cells, respectively (n = 3 per genotype). Scale bar, 25 μm.
(J) FBG integrated density of fluorescence in normal brains, Olig2+ and Olig2− tumors. Quantifications of Iba1+ cells perimeter and soma size in normal brains, Olig2+ and Olig2− tumors. Data are means ± SEM (n = 3 per genotype; ns, non-significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). See also Figure S1 and Tables S1 and S2
We next investigated the vascular relationships of Olig2+ versus Olig2− gliomas. As shown by tumor-specific hEGFR labeling (Figures 1C and 1D), Olig2+ gliomas at the leading edge showed discrete single glioma cells extending from the tumor mass. In contrast, Olig2− tumor cells extended as dense perivascular collections. While Olig2+ tumors showed nearly normal vasculature, the microvessel density (MVD), vessel size, and the vascular and lumen areas within Olig2− gliomas were significantly increased (Figures 1E and 1F), which correlated with increased endothelial Slc2a1 expression (Figure S1H). Additionally, Olig2− glioma had defects in the vascular basement membrane, as shown by the decreased vascular collagen IV expression (Figure 1G). We did not see evidence for trans-differentiation of hEGFRvIII+ tumor cells into endothelial cells regardless of Olig2 status (Figure S1I). These findings indicate that OPCL cells in glioma invade the brain by single-cell vessel co-option that minimally affects structure of the underlying stromal vasculature.
Loss of BBB Integrity, Microglial Activation, and Macrophage Infiltration in Olig2-Null Gliomas
We further characterized BBB integrity in Olig2+ and Olig2− gliomas. BBB leakage causes macrophage infiltration (Bardehle et al., 2015) and migration and activation of resident microglia, which retract their processes and have an ameboid appearance with an enlarged soma (Kozlowski and Weimer, 2012). Fibrinogen (FBG) leakage into brain parenchyma is a potent inflamma-tory stimulant of resident microglia (Bardehle et al., 2015; Petersen et al., 2017). As shown (Figures 1I and 1J), Olig2− gliomas showed marked FBG leakage from blood vessels as well as increased expression of Plasmalemma Vesicle Associated Protein (Plvap), confirming loss of BBB integrity (Figure S1J). While we found increased numbers of Iba1+ microglial cells in both Olig2+ and Olig2− tumors versus normal brain, Olig2− tumors contained significantly higher numbers of ameboid microglia (Figures 1I, 1J, and S1K). Expression of macrophage-specific genes (Bennett et al., 2016; Lavin et al., 2014; Venteicher et al., 2017) indicated significant infiltration of macrophages expressing F13a1, Ifitm2/3, Npc2, and Tgfb1 in Olig2− relative to Olig2+ gliomas (Figure 1H). Since these markers distinguish infiltrating macrophages from brain-resident microglia, this result also confirms BBB disruption in Olig2− glioma. We conclude that Olig2+ glioma cells migrate as single cells along a normal-appearing vasculature; in contrast Olig2− glioma cells grow as dense perivascular collections and disrupt astrocyte endfeet (Watkins et al., 2014), resulting in BBB leakage and neuroinflammation.
Wnt7a/7b Function Is Essential for Olig2+ Glioma Single-Cell Co-option
The increased vessel density in Olig2− gliomas indicated that these tumors were relatively angiogenic. To further investigate distinct vascular features of Olig2+ versus Olig2− tumors, we compared the expression of a panel of known regulatory factors. As shown (Figure 2A), Olig2+ glioma showed higher levels of Wnt7a, Wnt7b, and Lef1. OPCL tumor cells (hEGFR+, Nkx2.2+) expressed Wnt7 proteins and abutted Lef1+, β-catenin+ blood vessels (Figures S2A and S2B), indicating a Wnt-active state in Olig2+ gliomas. In contrast, Olig2− tumors expressed higher levels of Vegfc and Vegfr1/2/3. Gene ontology analysis (Table S1) revealed that angiogenesis was the second most upregulated process in Olig2− tumors (Figure S2C) and such tumors showed increase in Ki67+ proliferating endothelial cells (Figure 2B). We did not observe significant differences in markers for lymphatics within the tumor (Figures S2D and S2E).
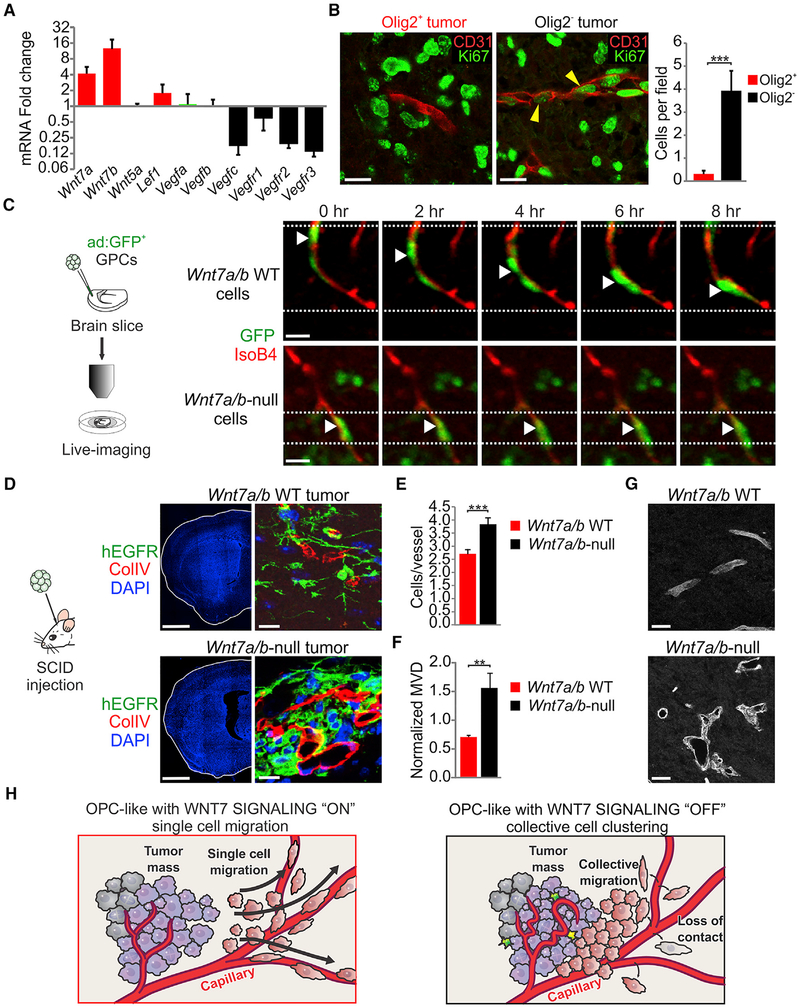
Figure 2. Wnt7 Signaling Regulates Single-Cell Vessel Co-option.
(A) Quantitative RT-PCR for vascular regulatory factors in Olig2+ versus Olig2− tumors. Rn18s and Actb were used for expression normalization. Data are means ± SEM (n = 3 experiments).
(B) Ki67 and CD31 immunostaining and quantifications in Olig2+ and Olig2− tumors. Yellow arrowheads show Ki67+ CD31+ cells. Data are means ± SEM (n = 3 per genotype, ***p < 0.001). Scale bars, 20 μm.
(C) Confocal live-imaging of Wnt7a/b WT and Wnt7a/b-null GPCs. White arrowheads indicate the position of the same GFP+ cell at different time points. Scale bars, 20 μm.
(D) Immunostaining for hEGFR, collagen IV, and DAPI in Wnt7a/b WT and Wnt7a/b-null tumors (n = 3 per genotype). Scale bars, 250 μm (left) and 20 μm (right).
(E) Quantification of the number of hEGFR per vessel in Wnt7a/b WT and Wnt7a/b-null tumors 1 month after transplantation. Data are means ± SEM (n = 3 per genotype, ***p < 0.001).
(F) Quantification of the microvessel density in Wnt7a/b WT and Wnt7a/b-null tumors at the time of neurological symptoms appearance. Data were normalized to microvessel density of the normal brain. Data are means ± SEM (n = 3 per genotype, **p < 0.01).
(G) CD31 immunostaining in Wnt7a/b WT and Wnt7a/b-null tumors (n = 3 per genotype). Scale bars, 20 μm.
(H) Schematic summarizing the effects of genetic Wnt signaling inhibition on tumor cell migration and association to the vasculature. See also Figure S2 and Video S1
To establish cell-intrinsic roles for Wnt7 function in Olig2+ glioma, we first analyzed single-cell co-option in organotypic cortical explant co-cultures with glioma progenitors that carried the same hEGFRvIII and Trp53 mutations as above, in addition to knockout of Wnt7a and conditional deletion of Wnt7b. As shown (Figure 2C), Olig2+ cells with intact Wnt7 function employed single-cell co-option of blood vessels; video microscopy showed cells tugging the endothelium at their trailing edge, indicating direct contact (Video S1 and Figure S2F). In contrast, Wnt7a/7b-null glioma cells failed to directly migrate on vessels. We also observed dramatic in vivo differences in which Wnt7a/7b-null cells migrated as perivascular collections rather than discrete/individual cells (Figures 2D and 2E). Similar to Olig2− tumors (Figure 1E) Olig2+, Wnt7a/7b-null gliomas showed markedly abnormal vasculature (Figures 2F and 2G). These findings indicate a critical role for cell-intrinsic Wnt7 signaling in regulating single-cell versus collective migration in Olig2+ glioma cells (Figure 2H).
Olig2 Regulates Wnt7 Expression in a p53-Dependent Manner
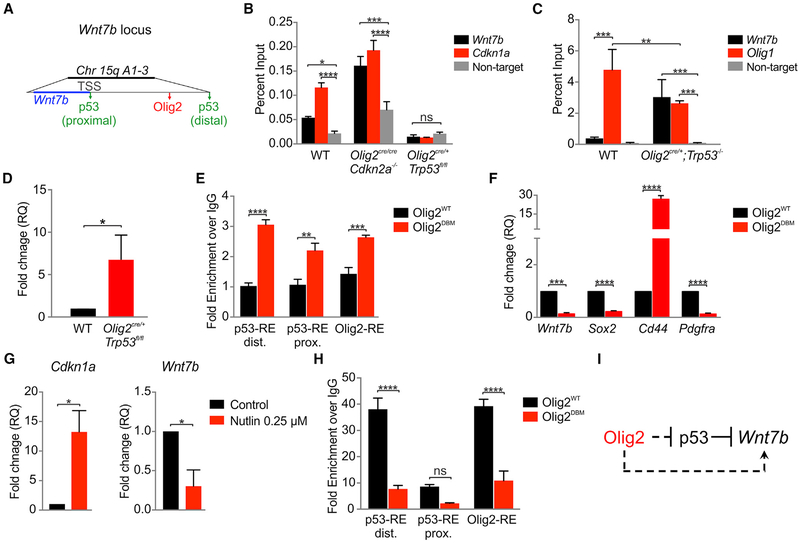
We investigated direct Olig2 regulation of Wnt7 expression by chromatin immunoprecipitation (ChIP) and biochemical approaches. Based on previously published ChIP sequencing analysis (Mateo et al., 2015; Mehta et al., 2011; Tonelli et al., 2015), we identified two putative p53 binding sites at the Wnt7b locus near the transcription start site and ~50 kb upstream, and one Olig2 binding site ~30 kb upstream of the Wnt7b locus (Figure 3A). Loss of Olig2 function resulted in significantly higher p53 occupancy at the Wnt7b locus; conversely, in Trp53−/− cells, Olig2 binding was enriched (Figures 3B and 3C). Loss of Trp53 resulted in a 6-fold increased Wnt7b transcript level, suggesting that Olig2-driven upregulation of Wnt7b is inhibited by p53 (Figure 3D). In order to confirm this antagonism, we showed that using DNA-binding mutant of Olig2 (Olig2DBM) (Mehta et al., 2011; Meijer et al., 2014) p53 binding on Wnt7b was increased (Figure 3E). In addition, we demonstrated that the binding site of Olig2 within Wnt7b is functional as DBM failed to regulate Wnt7b expression, as well as Sox2 and Pdgfra (Figure 3F). To confirm these findings, we treated Cdkn2a−/−;hEGFRvIII cells with 0.25 μM Nutlin, which induces p53 stabilization. Nutlin treatment resulted in an increase in Cdkn1a expression, as expected (Mehta et al., 2011; Vassilev et al., 2004), and a decrease in the expression of Wnt7b (Figure 3G), confirming that p53 represses Wnt7b expression. Olig2 can recruit chromatin-remodeling factors at enhancers and shift them from a latent inactive state to an active state (Yu et al., 2013). Indeed, in Trp53−/− cells we found strong enrichment of the active enhancer mark H3K27ac at the Wnt7b locus (Figure 3H). These findings indicate that p53 can directly repress Wnt7b, whereas Olig2 promotes Wnt7b expression especially in gliomas that are Trp53−/− (Figure 3I).
Figure 3. Olig2 Function Promotes Wnt7b Expression in Trp53−/− Glioma.
(A) Schematic of Olig2 and p53 binding sites within the Wnt7b locus. TSS, transcription start site.
(B) ChIP experiments showing p53 binding at the Wnt7b locus. Cdkn1a was used as a known target for p53. Data are means ± SEM (n = 3 per genotype; ns, nonsignificant; *p < 0.05, ***p < 0.001, ****p < 0.0001).
(C) ChIP experiments showing Olig2 binding at the Wnt7b locus. Olig1 (+36 kb) was used as a known target for p53. Data are means ± SEM (n = 3 per genotype, **p < 0.01, ***p < 0.001).
(D) Quantitative RT-PCR for Wnt7b. Data are means ± SEM (n = 3 per genotype, *p < 0.05). Rn18s and Actb were used for expression normalization. RQ, relative quantification.
(E) ChIP assay for p53 distal and proximal responsive elements (RE) and Olig2 RE at the Wnt7b locus using Olig2cre/cre cells transfected with WT (OligWT) and DNA-binding mutant (OligDBM). Data are means ± SEM (n = 3 per genotype, **p < 0.01, ***p < 0.001, ****p < 0.0001).
(F) Quantitative RT-PCR using Olig2cre/cre cells transfected with WT and DBM. Data are means ± SEM (n = 3 per genotype, ***p < 0.001, ****p < 0.0001). Rn18s and Actb were used for expression normalization.
(G) Quantitative RT-PCR analysis of Cdkn2a−/−;hEGFRvIII cells treated with DMSO (control) or 0.25 μM Nutlin for 16 hr. Data are means ± SEM (n = 3 per genotype, *p < 0.05). Rn18s and Actb were used for expression normalization.
(H) ChIP assay for H3K27ac at Wnt7b promoter region using Olig2cre/cre cells transfected with WT and DBM. Data are means ± SEM (n = 3 per genotype; ns, nonsignificant; ****p < 0.0001); dist., distal; prox., proximal.
(I) Regulatory model: p53 directly represses Wnt7b, whereas Olig2 indirectly promotes its expression.
WNT7 Expression Correlates with OPC Markers in Human Glioma
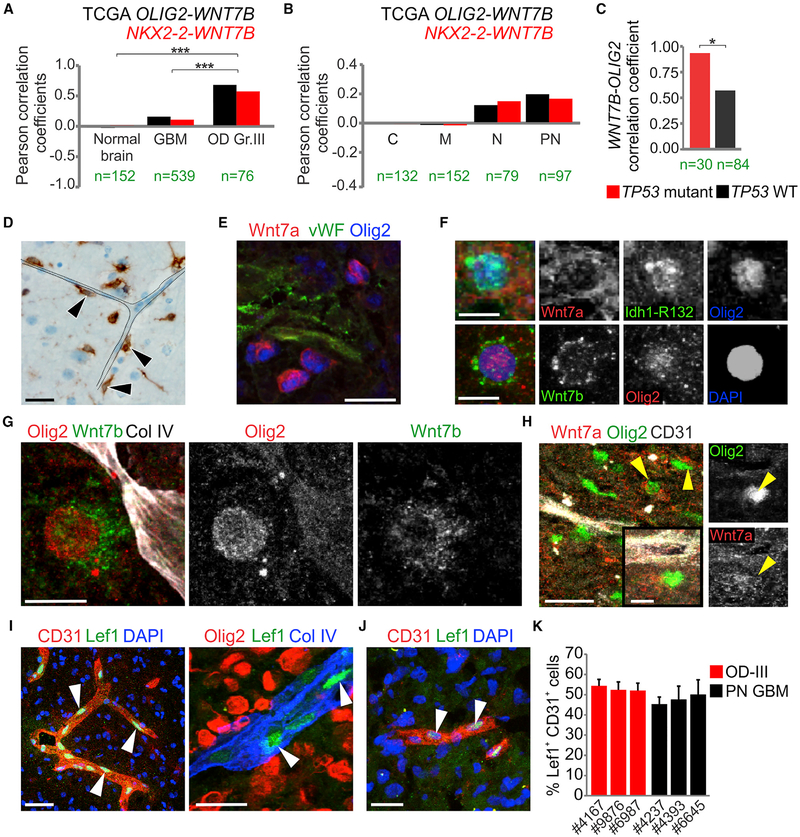
To confirm the relevance of Wnt7 signaling in human glioma we investigated the relationship of OPC markers and WNT7 expression in The Cancer Genome Atlas (TCGA) database (Brennan et al., 2013). As shown (Figures 4A and 4B, S3A, and Table S3), we observed a strong positive correlation of OPC markers (OLIG2, NKX2–2, PDGFRA) with WNT7B mRNA levels in high-grade OD and proneural GBM. Consistent with our molecular model (Figure 3I), the correlation between OLIG2 levels and WNT7B was significantly stronger in TP53 mutant gliomas versus TP53 wild-type (WT) glioma (Figure 4C). In contrast, WNT5A and all other Wnt genes lacked positive correlation (Figure S3A). We also investigated stem cell markers (e.g., CD133, ABCG2, and ITGA6), but we only found a link of ABCG2 with OLIG2 specifically in OD, and no correlation with WNT7B (Figure S3B). To investigate gene expression in glioma cells per se (versus stromal tissue), we took advantage of single-cell transcriptomic profiling (Venteicher et al., 2017) of IDH mutant OD (IDH-O) and astrocytoma (IDH-A). This analysis showed that WNT7A expression was detected only in a minority (3.7%) of glioma cells, whereas WNT7B was expressed by 59% of glioma cells. To confirm Wnt7 expression in human gliomas, we used western blot, qPCR (Figures S3C and S3D), and immunohisto-chemistry (Figures 4D–4H, S3E, and S3F). Analysis of IDH-O revealed that Olig2+ tumor cells abutted blood vessels (Figures 4D–4F) and we confirmed Olig2/Wnt7+ cells adjacent to blood vessels in several proneural GBM cases (Figures 4G, 4H, and S3F). Stromal vessels within high-grade OD and proneural GBMs showed endothelial expression of Lef1 and Tcf4, suggesting a Wnt-activated state (Figures 4I–4K, and S3G).
Figure 4. WNT7 Expression Strongly Correlates with OPC Markers in Human Glioma.
(A and B) Pearson correlation coefficients of OLIG2 and NKX2–2 with WNT7B mRNA levels in normal brain, glioblastoma (GBM), grade III oligodendroglioma (OD Gr.III) (A) and in GBM subtypes: classical (C), mesenchymal (M), neural (N), and proneural (PN) (B). n is indicated for each group (***p < 0.001).
(C) Pearson correlation coefficients of OLIG2 with WNT7B mRNA levels in p53 WT and mutant grade III OD (OD). *p < 0.05.
(D–F) Immunostaining for Idh1-R132 (D), Wnt7a, vWF, Olig2 (E) and Idh1-R132, Wnt7a, Wnt7b, Olig2 (F) on IDH1R132H-mutant grade III OD. Black arrowheads indicate tumor cells (n = 4 cases). Scale bars, 20 μm (D and E) and 10 μm (F).
(G and H) Immunostaining for Olig2, Wnt7b, collagen IV (G) and Wnt7a, Olig2, CD31 (H) on proneural glioblastoma. Yellow arrowheads indicate Olig2+ Wnt7a+ cells (n = 3 cases). Scale bars, 50 μm (H, left) and 10 μm (G), inset in (H).
(I) Immunostaining for CD31, Lef1, Olig2, and collagen IV on IDH1R132H-mutant grade III OD. White arrowheads indicate Lef1+ cells (n = 3 cases). Scale bars, 50 μm (left) and 20 μm (right). (J) Immunostaining for CD31 and Lef1 on proneural glioblastoma. White arrowheads indicate Lef1+ cells (n = 3 cases). Scale bar, 20 μm. (K) Quantifications of the percentage of Lef1+ CD31+ cells in grade III OD (OD-III) and proneural glioblastoma (PN GBM). Data are means ± SEM (n = 3 cases per group). See also Figure S3 and Table S3
Inhibition of Canonical Wnt Signaling Prevents Glioma Single-Cell Co-option
We next investigated a genetically faithful mouse model of high-grade GBM, which carries hEGFRvIII and deletion of Cdkn2a (Bachoo et al., 2002; Ligon et al., 2007). Such tumor cells expressed Wnt7a/b and contained abundant Olig2+ cells that showed single-cell co-option in vivo (Figures 5A and 5B). Similar to the above findings (Figure S2B), Lef1, in this model, marks the endothelium but not the tumor itself, so we used the canonical Wnt inhibitor XAV939 (Fancy et al., 2011) to inhibit the endothelial response (Figure 5C). Time-lapse video confocal imaging revealed that untreated GFP-labeled glioma cells used single-cell migration along the vasculature (Figures 5D and 5E and Videos S2 and S3). In contrast, XAV939-treated cells lost contact with vessels and formed clusters. We next investigated Wnt inhibition in a hypermutated recurrent IDH-O cell line expressing Wnt7a/b (Figure 5F). IDH-O cells were transplanted on slices and cultured in the presence of DMSO or the porcupine inhibitor LGK974 (Liu et al., 2013). Analysis of the percentage of cells contacting the vessels indicated a significant decrease after LGK974 treatment (Figure 5G). These findings indicate that both canonical Wnt and porcupine inhibitors prevent glioma single-cell vessel migration.
Figure 5. Targeting Canonical Wnt Signaling Prevents Single-Cell Glioma Co-option.
(A) Western blot and densitometry analysis using protein lysates from Cdkn2a−/−;hEGFRvIII cells. Data are means ± SEM (n = 2 lines with replicates).
(B) Immunostaining for Olig2, hEGFR, CD31, and Lef1 using tumors 3 months post-transplantation with Cdkn2a−/−;hEGFRvIII cells (n = 3 per genotype). Yellow arrowheads show hEGFR+ Olig2+ (top) and Lef1+ CD31+ (bottom) cells. Scale bars, 10 μm.
(C) Schematic of Wnt signaling inhibition.
(D) Confocal live-imaging of vehicle- and XAV939-treated Cdkn2a−/−;hEGFRvIII GPCs. White arrowheads indicate the position of the same GFP+ cell at different time points. Scale bars, 20 μm.
(E) Quantifications of the number of cells contacting vessels relative to the total number of GFP+ cells (top) and of the distance traveled by the individual cells on the vessels (μm). Data are means ± SEM (n = 2 experiments with four fields quantified, *p < 0.05, ****p < 0.0001).
(F) Western blot and densitometry analysis using IDH1R132H-mutated oligodendroglioma cell line (SF10417) under proliferation conditions at two different passages. Data are means ± SEM (triplicates).
(G) Slice culture experiments using IDH1R132H-mutated oligodendroglioma cell line (SF10417) in the absence (DMSO) or in the presence of LGK974 (1 μM) for 24 hr. Quantifications of the number of cells contacting the vessels relative to the total number of GFP+ cells. Data are means ± SEM (n = 3 experiments, *p < 0.05). Scale bars, 20 μm. See also Videos S2 and S3
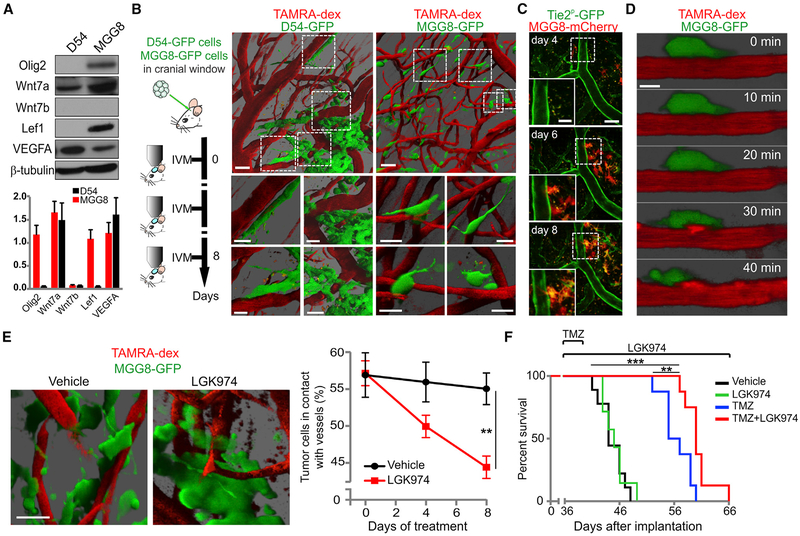
Systemic Wnt Inhibition Prevents Single-Cell Co-option and Improves Survival With Temozolomide Treatment in a Patient-Derived Proneural GBM Model
We screened various glioma cell lines on the basis of Olig2 and Wnt7 expression. As shown (Figures 6A, S4A, and S4B), the proneural patient-derived cell line MGG8 (Suva et al., 2014; Wakimoto et al., 2012) expressed both Olig2 and Wnt7a proteins, in contrast to D54 cells that expressed Wnt7a but lacked Olig2. Using multiphoton intravital microscopy, we observed that both cell lines employed vessel co-option. However, D54 used collective migration, while MGG8 predominantly employed single-cell migration. Based on these findings, we focused our analyses on MGG8. As shown (Figures 6B and S4C–S4E), 55% ± 2% of MGG8 cells contacted blood vessels, and longitudinal imaging demonstrated that cells moved toward blood vessels (Figure S4C) forming the Tie2P-GFP endothelium (Figure 6C). High-resolution imaging of MGG8 showed the well-described steps of 2D migration: extension, adhesion, translocation, and de-adhesion (Figure 6D). Thus, MGG8 cells represent a suitable model of glioma single-cell vessel co-option, and this is consistent with previous findings where the vasculature of MGG8 tumors was shown to be similar to normal brain (Kloepper et al., 2016).
Figure 6. Systemic Wnt Inhibition Reduces Vessel Co-option and Enhances Temozolomide Effect in Patient-Derived Proneural GBM Model.
(A) Western blot and densitometry analysis from cultured patient-derived cell lines (D54 and MGG8). Data are means ± SEM (duplicates).
(B) Schematic of intravital imaging (IVM) procedure and 3D renderings of 20-μm-deep z-stacks of D54-GFP and MGG8-GFP cells by IVM. Vessels were visualized using TAMRA (tetramethylrhodamine)-dextran. Scale bars, 50 μm and 25 μm (insets).
(C) Intravital imaging of MGG8-mCherry cells implanted in Tie2P-GFP;Rag1−/− mice. Days in the legend are from intracranial transplantation. Insets are magnifications of the white dashed squares. Mean intensity projections of 24-μm-deep z-stacks. Scale bars, 100 μm and 50 μm (inserts).
(D) Time-lapse IVM of a single cell at the invasion front of MGG8 tumors 6 days after implantation. Vessels were visualized using TAMRA-dextran. Mean intensity projections of 6-μm-deep z-stacks. Scale bar, 10 μm.
(E) 3D rendering of IVM images of tumors from vehicle- and LGK974-treated MGG8-bearing mice and quantification of the percentage of cells in contact with vessels. Data are means ± SEM (n = 2 experiments, n = 9 mice for vehicle and n = 7 mice for LGK974, **p < 0.01). Scale bar, 25 μm.
(F) Kaplan-Meier analysis of MGG8-bearing mice treated with vehicle, TMZ, LGK974, and TMZ + LGK974. LGK974 + TMZ (median survival = 24 days of treatment, n = 8) compared with TMZ (median survival = 20 days of treatment; hazard ratio [HR], 6.19; 95% confidence interval [CI], 1.62–23.63; **p = 0.008; n = 8), and vehicle (HR, 18.45; 95% CI, 4.3–78.61; ***p = 0.0004; n = 9). See also Figures S4 and S5
To investigate Wnt signaling in vivo, we treated MGG8-bearing mice with LGK974 (Liu et al., 2013). Pharmacokinetic and IHC analyses showed that LGK974 crossed the BBB and reduced Wnt7a expression in vivo (Figures S5A and S5B). Systemic LGK974 treatment (5 mg/kg twice a day) significantly reduced the percentage of MGG8 cells in contact with blood vessels (Figure 6E), indicating that glioma Wnt signaling is required for single-cell vessel co-option in vivo. Treatment of MGG8-GFP-GLuc-bearing mice at early stages of tumor development (around 4 mm3) with 5 mg/kg of LGK974 once a day was well tolerated (Figures S5C and S5D) and showed a trend of improved survival (Figure S5E, p < 0.06). To better model the standard of care in glioma patients, we treated MGG8-GFP-GLuc tumor-bearing mice with LGK974 alone or in combination with temozolomide (TMZ) at a late stage of the disease. Using the serum GLuc (Kloepper et al., 2016), we started treatment when the tumor reached an approximate volume of about 9–10 mm3. LGK974 significantly improved survival when combined with TMZ of 20% in comparison with TMZ alone (Figure 6F). Thus, pharmacologic Wnt inhibition blocks Olig2+ glioma single-cell vessel co-option and enhances TMZ efficacy.
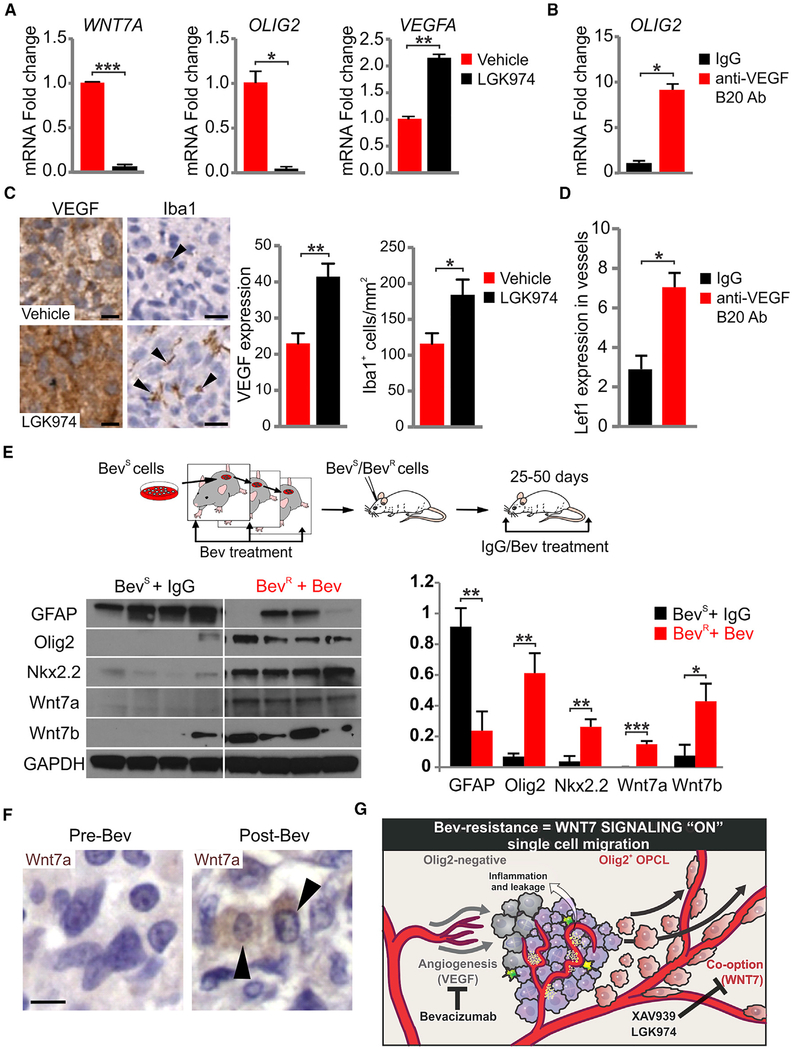
Anti-VEGF or -Wnt Treatments Select for Distinct Glial Phenotypes and Vascular Regulatory Pathways in Glioma
Clinical and experimental studies indicate that co-option in glioma is promoted by prolonged treatment with VEGF inhibitors (di Tomaso et al., 2011). We next investigated Olig2 expression, and VEGF and Wnt signaling under conditions of inhibitor treatment. As shown (Figures 7A and S6A), addition of LGK974 to cultured MGG8 and MGG6 (another patient-derived proneural cell line) (Wakimoto et al., 2012) cells resulted in a downregulation of OLIG2 and WNT7A and an upregulation of VEGFA. Conversely, the VEGF inhibitor B20 treatment increased OLIG2 expression (Figures 7B and S6B). In vivo, MGG8 tumor-bearing mice treated with LGK974 showed increased VEGF expression and microglial cell number (Figure 7C), whereas B20 antibody treatment showed a significant increase in Lef1 expression within the endothelial compartment of the tumors, indicating an increase in Wnt signaling (Figure 7D). These findings further support a model where Wnt and VEGF signaling are coupled to glial phenotype.
Figure 7. Wnt7 Signaling Is Upregulated by Anti-VEGF Therapy.
(A) Quantitative RT-PCR using vehicle and LGK974-treated MGG8 cells (100 mM LGK974 for 3 days). GAPDH was used for expression normalization. Data are means ± SEM (n = 2 experiments with technical triplicates, *p < 0.05, **p < 0.01, ***p < 0.001).
(B) Quantitative RT-PCR using IgG- and B20 (anti-VEGF)-treated MGG8 cells. GAPDH was used for expression normalization. Data are means ± SEM (n = 2 experiments with technical triplicates, *p < 0.05).
(C) VEGF and Iba1 immunostaining and quantification of 7 days of vehicle and LGK974-treated MGG8 tumors. Black arrowheads indicate Iba1+ cells. Data are means ± SEM (n = 3 mice per group, *p < 0.05, **p < 0.01). Scale bars, 10 μm.
(D) B20 administration in MGG8-transplanted animals for 10 days (10 mg/kg once a day intraperitoneally). Lef1+ cells per vessels were quantified in IgG and B20 animals. Data are means ± SEM (n = 6 mice for IgG cohort and n = 16 mice for B20 cohort, *p < 0.05).
(E) Model of progressive U87 glioma cell resistance. Western blot and densitometry analysis using lysates from IgG-treated Bev-sensitive (BevS) and Bev-treated BevR tumors. Data are means ± SEM (n = 4 mice per genotype, *p < 0.05, **p < 0.01, ***p < 0.001).
(F) Human GBM paired samples taken at biopsy pre-treatment and postmortem/post-treatment with Bev and stained for Wnt7a and H&E. Black arrowheads indicate Wnt7a+ cells. Scale bar, 10 μm.
(G) Schematic summarizing the effects of glial subtypes on vessel co-option and the tumor microenvironment, and their modulation upon VEGF and Wnt signaling inhibition. See also Figure S6
To further test this idea, we chose the U87 cell line, which is Olig2− and grows as a mass reliant on angiogenesis and VEGF signaling (Peterson et al., 2016). We used a multi-generational model with increasing resistance to bevacizumab (Bev) (Figure 7E), which has been shown to promote glioma vessel co-option (Jahangiri et al., 2013). As shown (Figures 7E and S6C), we found that both Wnt7a and Wnt7b were upregulated in Bev-resistant (BevR) U87 compared with Bev naive/sensitive glioma cells. Moreover, this was associated with dramatically increased levels of Olig2 and Nkx2.2. Thus, even the entrenched astroglial U87 glioma line can select for an OPC phenotype in the context of multi-generational Bev treatment.
To determine relevance of these findings in humans, we took advantage of a unique collection of five GBM paired samples taken at biopsy pre-treatment and postmortem/post-treatment with Bev. We found increased Wnt7a expression in post-Bev-treatment tumor tissue in three of five samples (Figure 7F and Table S4); greater staining was noted at the infiltrative tumor edge compared with the tumor core in one additional sample. Although further cases of Bev-treated GBM are needed to confirm this finding in humans, these results combined with the others above collectively suggest that OPC features and Wnt7 signaling are selected for by anti-VEGF therapy (Figure 7G).
DISCUSSION
Glioma cellular heterogeneity is used to adapt to environmental and genotoxic stress, enhance survival, and promote invasiveness (Carmeliet and Jain, 2011). Since Olig2 is a master regulator of glial cell fate in development and glioma (Lu et al., 2002; Mehta et al., 2011), we reasoned that Olig2+ OPCL and Olig2-negative “astrocyte-like” cells could serve different functions in tumor growth and survival. We developed archetypal Olig2+ OPCL and Olig2-null glioma models and used expression profiling as a means of identifying the glial subtype-encoded molecular pathways relevant for vascular regulation. We found that OPCL cells in human glioma expressed Wnt7 and invaded the brain via single-cell vessel co-option. Gene targeting of Wnt7a/7b or pharmacological inhibition of Wnt prevented vessel contact of OPCL glioma cells. In contrast, Olig2− cells showed collective perivascular migration and enhanced VEGF signaling; this led to increased and distorted vasculature within the tumor and BBB leakage, in turn leading to microglial activation and macrophage infiltration. Interestingly, normal embryonic OPCs also express Wnt7a/7b and migrate along blood vessels (Tsai et al., 2016), suggesting that glioma OPCL co-option uses mechanisms analogous to that for developmental spread of OPCs and possibly downstream Wnt signaling in the endothelium (Posokhova et al., 2015; Zhou and Nathans, 2014). Functions of glioma Wnt7a/7b revealed in our study are distinct from reported roles for other Wnts in endothelial trans-differentiation or angio-genesis (Hu et al., 2016; Reis et al., 2012), as we found no evidence for trans-differentiation in mouse or human IDH mutant tumors by histology or gene expression (Venteicher et al., 2017).
Mouse modeling of IDH mutation has an impact on the micro-environment (Amankulor et al., 2017), and single-cell transcriptomic analysis of human tumors indicates that IDH-A (versus IDH-O) tumors show significantly more contribution of activated microglia and macrophage markers (Venteicher et al., 2017). This fits with our findings showing that Olig2-null (astrocyte-like) gliomas have BBB breakdown, and microglial and macrophage activation and infiltration. Moreover, this is consistent with a prior study that showed (Olig2−) D54 cells displacing stromal astrocyte endfeet leading to BBB leakage (Watkins et al., 2014). In contrast, Wnt7-expressing OPCL gliomas showed little microglial activation, these findings suggest that the tumor inflammatory microenvironment is regulated, at least in part, by glial phenotype. However, further studies are needed to understand the mechanisms involved in the immune response (e.g., cytokines expression) as well as structural and/or cell-cell interactions that account for clinically relevant BBB properties in glioma. For example, magnetic resonance and fluid-attenuated inversion recovery imaging can reveal tumor margins by BBB leakage. Our findings suggest that OPCL glioma cells could invade the brain without any detectable MRI signature, and as such could lie beyond margins of surgical resection or a radio-therapy field. If so, interventions to visualize OPCL populations would be important to better define glioma therapeutic boundaries. Although OPCL glioma cells might use other signaling pathways, CNS-specific knockout of Wnt7a/b or endothelial cell-specific deletion of Ctnnb1 causes severe leak and vessel breakdown (Daneman et al., 2009; Stenman et al., 2008; Tran et al., 2016) and as such would be reflected by MRI.
Previous studies have suggested that Bev reduces edema and prolongs progression-free but not overall survival in newly diagnosed GBMs (Chinot et al., 2014; Gilbert et al., 2014) and progressive GBMs (Wick et al., 2017). Analysis of AVAglio, a randomized phase III clinical study that investigated the addition of Bev to radiotherapy/TMZ, suggests that IDH1 WT proneural versus mesenchymal GBMs may benefit (Sandmann et al., 2015). In contrast, the BELOB trial with recurrent GBMs showed that the classical subtype was most responsive to Bev in combination with lomustine (Erdem-Eraslan et al., 2016). Thus, the GBM molecular subgroup may not be an adequate predictor of response to anti-angiogenic therapy and further criteria are needed.
Vessel co-option has been proposed as an intrinsic or acquired resistance mechanism to anti-angiogenesis (Carmeliet and Jain, 2011; di Tomaso et al., 2011; Emblem and Jain, 2016; Frentzas et al., 2016; Pezzella and Gatter, 2015). Our findings indicate a “glial switch” and Wnt7 signaling as targets in this regard because prolonged anti-VEGF therapy in a mouse model selected for strong upregulation of Olig2 and Wnt7. Our results indicate that glial phenotype is critical to provide the cellular context for Wnt7 activity in co-option (or VEGF in angiogenesis). For example, Olig2− glioma also expressed Wnt7a/7b, albeit at significantly lower levels, and show less single-cell co-option. Similarly, we show that D54 and U87 lines expressed Wnt7a or 7b but did not show single-cell co-option owing to lack of OPCL (Olig2) character. Glial lineage regulators (e.g., Olig2, Sox10, NF1A) are expressed in glioma (Glasgow et al., 2014) and our study suggests that they are recruited to determine cell fate and, consequently, co-option and other modes of invasion (Osswald et al., 2015), as well as angiogenesis and regulation of the tumor microenvironment.
Finally, considering therapeutic implications, we show feasibility of LGK974 treatment to inhibit single-cell co-option of a proneural GBM patient-derived line in vivo and improve survival when combined with TMZ. LGK974, a porcupine inhibitor, crossed the BBB and prevented the secretion of Wnt7. These and other studies suggest targeting glioma WNT signaling might be clinically useful to promote BBB permeability and delivery of chemotherapy. For example, Phoenix et al. (2016) showed that medulloblastoma xenografts that express Wnt inhibitors have a leaky BBB, enhanced penetration of vincristine, and a better therapeutic response. However, further investigation is needed to understand the potential benefits of anti-Wnt signaling approaches in adjuvant therapy in other classes of patient-derived GBMs. In sum, our study provides mechanistic insights into the plasticity of glioma glial cells and the molecular strategies they encode to exploit their environment, especially in response to anti-angiogenic therapy.
STAR⋆METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-b actin | Sigma-Aldrich | Cat# A5316; RRID: AB_476743 |
| Mouse monoclonal anti-b catenin | Millipore | Cat# 05-665; RRID: AB_309887 |
| Mouse monoclonal anti-alpha tubulin | Sigma-Aldrich | Cat# T5168; RRID: AB_477579 |
| Mouse monoclonal anti-b tubulin | Sigma-Aldrich | Cat# T4026; RRID: AB_477577 |
| Rabbit polyclonal anti-CD31 | Abcam | Cat# ab28365; RRID: AB_726365 |
| Rat monoclonal anti-CD31 | BD Biosciences | Cat# 550274; RRID: AB_393571 |
| Goat polyclonal anti-Collagen IV | Millipore | Cat# ab769; RRID: AB_92262 |
| Mouse monoclonal anti-human EGFR | Dako | Cat# M7298; RRID: AB_2286187 |
| Sheep polyclonal anti-fibrinogen | Abcam | Cat# ab118533; RRID: AB_10900171 |
| Mouse monoclonal anti-GADPH | Abcam | Cat# ab8245; RRID: AB_2107448 |
| Chicken polyclonal anti-GFP | Aves Lab | GFP-1020; RRID: AB_10000240 |
| Rabbit polyclonal anti-GFAP | Dako | Cat# Z0334; RRID: AB_10013382 |
| Rabbit polyclonal anti-Histone H3 (acetyl K27) | Abcam | Cat# ab4729; RRID: AB_2118291 |
| Mouse monoclonal anti-HLA Class 1 ABC | Abcam | Cat# ab70328; RRID: AB_1269092 |
| Rabbit polyclonal anti-Iba1 | Wako | Cat# 019-19741; RRID: AB_839504 |
| Mouse monoclonal anti-Idh1-R132H | Dianova | Cat# DIA-H09; RRID: AB_2335716 |
| Rabbit polyclonal anti-Ki67 | Thermo Scientific | Cat# RM-9106; RRID: AB_2341197 |
| Rabbit monoclonal anti-Lef1 | Cell Signaling | Cat# 2230; RRID: AB_823558 |
| Rabbit polyclonal anti-LYVE1 | Novus Biologicals | Cat# NB100-725; RRID: AB_10003273 |
| Rabbit polyclonal anti-NG2 | Millipore | Cat# AB5320; RRID: AB_91789 |
| Mouse monoclonal anti-Nkx2.2 | DSHB | Cat# 74.5A5; RRID: AB_531794 |
| Mouse monoclonal anti-Olig2 | Charles D. Stiles, Harvard Medical School, Cambridge, Massachusetts | RRID: AB_10807410 |
| Rabbit polyclonal anti-Olig2 | Millipore | Cat# AB9610; RRID: AB_570666 |
| Rabbit polyclonal anti-Olig2 | Charles D. Stiles, Harvard Medical School, Cambridge, Massachusetts | DF308; RRID: AB_2336877 |
| Rabbit polyclonal anti-p53 | Santa Cruz | Cat# FL-393; RRID: AB_653753 |
| Rat monoclonal anti-CD140a | BD Biosciences | Cat# 558774; RRID: AB_397117 |
| Rabbit monoclonal anti-Tcf4 | Cell Signaling | Cat# 2569S; RRID: AB_2199816 |
| Mouse monoclonal anti-VEGF | Thermo Fisher | Cat# MS-1467; RRID: AB_144688 |
| Rabbit polyclonal anti-vWF | Abcam | Cat# ab6994; RRID: AB_305689 |
| Goat polyclonal anti-Wnt7a | Santa Cruz | Cat# sc-26361; RRID: AB_2215743 |
| Rabbit polyclonal anti-Wnt7a | Abcam | Cat# ab100792; RRID: AB_10858110 |
| Rabbit polyclonal anti-Wnt7b | Abcam | Cat# ab94915; RRID: AB_10675749 |
| Bacterial and Virus Strains | ||
| eGFP adenovirus | Vector Biolabs | Cat# 1060 |
| GFP-GLuc lentivirus | Wurdinger et al., 2008 | |
| mCherry lentivirus | Massachusetts General Hospital Viral Vector Core Facility | |
| Biological Samples | ||
| Human grade III oligodendroglioma and proneural GBMs | The Brain Tumor SPORE Biospecimen/Pathology Core, University of California San Francisco | http://cancer.ucsf.edu/research/spores/brain-spore/cores#one |
| Paired pre-Bev (biopsies) and post-Bev (autopsies) GBMs | Tissue Bank Core, Mass General Hospital | |
| Human primary GBMs flash-frozen tissue | Tissue Bank Core, Mass General Hospital | |
| Chemicals, Peptides, and Recombinant Proteins | ||
| XAV-939 | Tocris Bioscience | Cat# 3748; CAS: 284028-89-3 |
| LGK-974 | Selleckchem | Cat# S7143; CAS: 1243244-14-5 |
| B20-4.1 | Genentech/Hoffmann La-Roche | |
| Bevacizumab | Genentech | CAS: 216974-75-3 |
| Temolozomide | Tocris Bioscience | CAS: 85622-93-1 |
| Deposited Data | ||
| Raw and analyzed data | This paper | GEO: GSE110052 |
| TCGA data portal | TCGA | https://tcga-data.nci.nih.gov/tcga/ |
| Allen Brain Institute data portal | ABI | http://human.brain-map.org/static/download |
| Experimental Models: Cell Lines | ||
| Human: MGG8 cells | Wakimoto et al., 2012 | |
| Human: MGG6 cells | Wakimoto et al., 2012 | |
| Human: D-54 cells | Bigner et al., 1981 | RRID:CVCL_7185 |
| Human: U-87MG ATCC cells | ATCC | Cat# 300367/p658_U-87_MG, RRID:CVCL_0022 |
| Human: SF10417 cells | Joseph Costello, University of California San Francisco | |
| Experimental Models: Organisms/Strains | ||
| Mouse: Olig2tm2(TVA,cre)Rth/J | The Jackson Laboratory | RRID:IMSR_JAX:011103 |
| Mouse: FVB.129-Trp53tm1Brn/Nci | NCI | RRID:IMSR_NCIMR:01XC2 |
| Mouse: B6;129S1-Wnt7atm1Amc/J | The Jackson Laboratory | RRID:IMSR_JAX:004715 |
| Mouse: B6;129X1-Wnt7btm2Amc/J | The Jackson Laboratory | RRID:IMSR_JAX:008467 |
| Mouse: B6.129-Cdkn2atm1Rdp/Nci | NCI | RRID:IMSR_NCIMR:01XB1 |
| Mouse: TieP-GFP Rag1−/− | Cheng et al., 2011 | |
| Mouse: IcrTac:ICR-Prkdcscid | Taconic | RRID:IMSR_TAC:icrsc |
| Mouse: NOD.CB17-Prkdcscid/J | The Jackson Laboratory | RRID:IMSR_JAX:001303 |
| FVB/NCrl | Charles River | RRID:IMSR_CRL:207 |
| Oligonucleotides | ||
| Primers for mouse Lef1, LYVE-1, Plvap, Slc2a1, Prox1, Tbx1, VEGFR1/2/3, Wnt5a, Wnt7a/b, Vegfa/b/c, see Table S5 | This paper | N/A |
| Primers for mouse Lef1, LYVE-1, Plvap, Slc2a1, Prox1, Tbx1, VEGFR1/2/3, Wnt5a, Wnt7a/b, Vegfa/b/c, see Table S5 | This paper | N/A |
| Software and Algorithms | ||
| R package | http://cran.us.r-project.org | |
| Gene Cluster 3.0 | de Hoon et al., 2004 | RRID:SCR_002250 |
| Imaris v7.6-8.2 | Bitplane | RRID:SCR_007370 |
| Java Treeview | Saldanha, 2004 | RRID:SCR_013503 |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, David H. Rowitch (dhr25@medschl.cam.ac.uk).
EXPERIMENTAL MODELS AND HUMAN TISSUE DETAILS
Mice
All animal protocols were approved by and in accordance with the guidelines established by the Institutional Animal Care and Use Committee (IACUC), the Laboratory Animal Resource Center (LARC) at the University of California San Francisco (UCSF) and the subcommittee on Research Animal Care at Massachusetts General Hospital (MGH). Mouse colonies were maintained in accordance with National Institutes of Health (NIH), UCSF and MGH guidelines. Olig2cre/+, Trp53fl/fl (Mehta et al., 2011), Wnt7a+/− and Wnt7bfl/fl (Stenman et al., 2008) mouse lines were intercrossed to generate Olig2cre/+;Trp53fl/fl, Olig2cre/cre;Trp53fl/fl and Olig2cre/+;Trp53fl/fl; Wnt7a−/−;Wnt7bfl/fl animals. Cdkn2a−/− mice were obtained from the National Cancer Institute (NCI) mouse repository. SCID and nude mice were purchased from Taconic and Jackson laboratory, and FVB mice from Charles River (Willmingston, MA).
Human Samples
De-identified human brain tumor tissue was acquired via the UCSF Brain Tumor SPORE Biospecimen/Pathology Core under the protocol IRB #10-01318 approved by the UCSF Human Research Protection Program Committee on Human Research, and via MGH under the protocol #2011P002486 approved by the Partners Human Research Committee and MGH Institutional Review Board. Informed consent was obtained from all subjects under the existing consent for the UCSF Brain Tumor SPORE Biospecimen/Pathology Core and MGH Tissue Bank Core. Upon excision, the tissue samples were immediately snap frozen in liquid nitrogen and stored at −80°C or fixed in 10% buffered formalin, dehydrated through graded ethanol, and embedded in Paraplast Plus wax (McCormick Scientific) using standardized techniques for pathological analysis.
METHOD DETAILS
Cell Culture
Glioma neurosphere cultures were established from ganglionic eminences of E14.5 Olig2cre/+;Trp53fl/fl, Olig2cre/cre;Trp53fl/fl and Olig2cre/+;Trp53fl/fl;Wnt7a−/−;Wnt7bfl/fl embryos and from dorsal subventricular zone of P5 Cdkn2a−/− pups. Cells were grown in DMEM/F12 (Invitrogen) supplemented with penicillin/streptavidin, apotransferrin, progesterone, sodium selenite, putrescine, insulin and D-Glucose and passaged once a week. Cultures were grown in the presence of EGF and bFGF (20 ng/mL each). Cells were infected with retroviral human EGFRvIII, as previously described (Mehta et al., 2011). MGG8 and MGG6, cell lines derived from GBM patients, were previously established in the Department of Neurosurgery at Massachusetts General Hospital (MGH) (Wakimoto et al., 2012). MGG8 and MGG6 cells were grown in serum-free conditions using the NeuroCult NS-A proliferation kit (STEMCELL Technologies) and were negative for mycoplasma using the Mycoalert Plus Mycoplasma Detection Kit (Lonza). D54 (aka H54), a cell line derived from a GBM patient, was previously established at Duke University Medical Center (Bigner et al., 1981). D54 cells were grown in Improved MEM supplemented with Hepes, NaHCO3 and 10% FCS. Human oligodendroglioma cells (SF10417) were isolated by Lindsey Jones (Joseph Costello’s, lab, UCSF) from a recurrent hypermutated oligodendroglioma, 1p/19q codeleted and IDH1R132H mutant (the patient was treated with TMZ). Cells were grown in serum-free conditions using the NeuroCult NS-A media (STEMCELL Technologies) supplemented with L-Glutamine, B27, N2, Sodium Pyruvate and Pen/Strep in the presence of growth factors (bFGF, EGF, PDGFAA).
Western Blot
Total cell lysate was collected from cells in RIPA buffer supplemented with proteinase and phosphatase inhibitor cocktails (Roche), resolved by SDS-PAGE, and then immunoblotted using standard techniques. Primary antibodies are listed in the Key Resources Table.
Quantitative RT-PCR
Total RNA was isolated from Olig2cre/+;Trp53fl/fl;hEGFRvIII and Olig2cre/cre;Trp53fl/fl;hEGFRvIII tumors at the time of neurological symptoms appearance (n=3 per genotype) and cells in culture using Trizol reagent (Invitrogen). RNA was cleaned up with an RNeasy Kit (Qiagen) and DNAse-digested to remove DNA contamination. 100–500 ng of purified RNA was retro-transcribed using the High Capacity RNA to cDNA Master Mix (Applied Biosystems). qPCR was performed by using SYBR Green master mix (Roche) in Light-cycler 480 (Roche) with specific primers designed for amplicons of 75–150 bp using Primer 3. Primer sequences are listed in Table S5. Rn18s, GADPH and Actb were used as reference genes and experiments were performed in duplicates for each sample.
In Vitro Treatment with LGK974 or B20
MGG8 or MGG6 patient-derived cells were cultured in NeuroCult NS-A proliferation kit (STEMCELL Technologies) with 10 μM of LGK974 (Selleckchem, S7143) diluted in DMSO (Wickstrom et al., 2015) or 50 mg/ml of the anti-VEGF B20 (Lu et al., 2012) for 72 hr. Then mRNA was extracted and RT-PCR performed as described above.
Microarray Samples Preparation
Total RNA from eight tumor samples (4 Olig2cre/+;Trp53fl/fl;hEGFRvIII and 4 Olig2cre/cre;Trp53fl/fl;hEGFRvIII tumors) were shipped on dry ice to the University of California, Los Angeles (UCLA) Neurogenomics Core facility (Los Angeles, CA) for analysis using the Illumina MouseRef-8 v2.0 expression BeadChip microarrays (25 697 probes). Amplification was performed using the Ambion TotalPrep RNA amplification kit (Life Technologies Inc, Carlsbad, CA). Raw bead-level data were minimally processed by the UCLA Neurogenomics Core facility (no normalization or background correction) using BeadStudio software (Illumina, San Diego, CA).
Chromatin Immunoprecipitation Assays
Neurosphere cultures (~107 cells) were fixed with 1% formaldehyde in phosphate-buffered saline and then lysed, sonicated, and immunoprecipitated as previously described (Schmidt et al., 2009). Immunoprecipitations were performed using antibodies against Olig2 (DF308, Stiles laboratory), p53 (Santa Cruz, FL393) and H3K27ac (Abcam, ab4729). Precipitated DNA was analyzed using real-time quantitative PCR using specific primers for Olig2 and p53 binding sites at the Wnt7b locus. Primer sequences are available upon request. Motif analysis of p53 and Olig2 binding sites (related to Figure 3): p53 consensus binding site: RRRC(A/T) (A/T) GYYY (0–13 bp); p53 binding site upstream of Wnt7b: GGGCAAGGCTCCGCCTCTAGACA (~50 kb upstream); Olig2 binding site upstream of Wnt7b: CAGCTG, CAAATG and CATCTG (~30 kb upstream).
Organotypic Explants and Time-Lapse Imaging
Brains from P20-P35 FVB mice were dissected and put in cold aCSF. 15 μl of isolectin GS-IB4 (Molecular Probes) was added to each well used for live-imaging. Brains were cut into slices using vibratome (at the border between rostral hippocampus and cortex caudally, and rostrally at ~1 mm caudal of olfactory bulbs). After sectioning, slices were washed once in aCSF and once in cell culture media (Hansen et al., 2010) and then transferred to the membranes. Olig2cre/+;Trp53fl/fl;hEGFRvIII, Olig2cre/+;Trp53fl/fl;Wnt7a−/−; Wnt7bfl/fl;hEGFRvIII, Cdkn2a−/−;hEGFRvIII and patient-derived SF10417 cells, infected with adeno-GFP virus (Vector Labs, 1060) 48 hr before transplantation, were injected into the slices using a Hamilton syringe (one injection of 2–3 μl cell suspension/striatum) and plates were incubated for 2 hr at 37°C. GPCs were infected with adeno-GFP virus (ad:GFP) 48 hr before organotypic transplantation and live-imaging. To label the vasculature, Isolectin B4 (IsoB4) was added in the slice culture media. For Wnt inhibition experiments, DMSO, 0.1 μM XAV939 (Tocris Bioscience, 284028-89-3) or 1 μM LGK974 (Selleckchem, S7143) was added to the cells before transplantation. Of note, we treated with 0.1 μM XAV939, which modulates Wnt in normal OPCs but does not cause cell death (Fancy et al., 2011). Z-stack images were taken every 15–30 min over a time period of 12–24 hr. After 8–12 hr, z positions were readjusted to compensate for tissue movements. At the end of the recording period, sections were processed for immunohistochemistry. Imaging files from individual acquisitions were compiled and analyzed using Imaris Image Analysis software (v7.6–8.2, Bitplane) and annotated using Imaris and ImageJ (U.S. National Institutes of Health, Bethesda, Maryland) software.
Longitudinal Intravital Microscopy (IVM)
In vivo multiphoton laser scanning microscopy (MPLSM) analysis of vessels and MGG8 or D54 cells was performed on chronic cranial window-bearing three to four month-old male nude mice or Tie2P-GFP Rag1−/− mice. To implant transparent cranial windows, a 6 mm circle was drawn over the frontal and parietal regions of the skull bilaterally. Using a high-speed air-turbine drill with a burr-tip 0.5 mm in diameter, a groove was made on the margin of the drawn circle, until the bone flap becomes loose. The bone flap was separated from the dura mater underneath. The dura and arachnoid membranes were cut completely from the surface of both hemispheres, avoiding any damage to the sagittal sinus. The window was sealed with a 7 mm cover glass, glued to the bone with histocompatible cyanoacrylate glue. Ten days after, the cover glass was removed and 1 μl of 20 000 MGG8-GFP-GLuc or D54-GFP-GLuc cells were stereotactically and slowly injected with a 28-gauge micro-syringe and a new cover glass was glued. For intravital microscopy, the animal were anesthetized with isoflurane and fixed with metal ring upper frontal tooth holder and a bilateral plastic ear holder. The MPLSM consisted of a MilleniaX pumped Tsunami Ti:sapphire laser (Spectra-Physics). Two-photon excitation of TAMRA, GFP and second-harmonic generation (SHG) beam was achieved using 810 nm light, while in the Tie2P-GFP/MGG8-mCherry setup we performed sequential imaging using 730 nm and 930 nm lights. Power at the sample was estimated to be 1–3 mW. MPLSM microscope consisted of an Olympus Fluoview FV300 system customized for multiphoton imaging. We performed vessel angiography after retro-orbital injection of 0.1 ml of 10 mg/ml dextran-TAMRA (500 kDa; in-house conjugated). The images shown are 3D rendering of high-resolution z-stacks: 706×706 μm (xy-voxel of 0.69) and at least 60 μm of z-stack (z-steps 2 μm). We segmented and rendered vessels and tumor cells using a semi-automated algorithm (Bitplane Imaris Image Analysis software). The longitudinal studies were performed every 3–4 days.
LGK974 In Vivo Measurement
Three to four months old male nude mice were treated by gavage with 5 mg/kg of LGK974 (Selleckchem, S7143) dissolved in 0.5% Tween-80 0.5% methylcellulose 3, 6, 9, 12 hr before euthanasia. At described times, we first collected plasma and then harvested brain and liver after heart-perfusion with PBS, to avoid traces of the drug in the remaining blood. LGK974 was quantified by HPLC analysis (3 mice per time-point).
Systemic LGK974 Treatment Protocol for Intravital Microscopy
Three to four months old male nude mice bearing cranial window were transplanted with 20 000 MGG8-GFP-GLuc cells. One day after implantation, we performed IVM, randomized mice and started treatments (day 0). Mice were treated by gavage with 5 mg/kg of LGK974 (Selleckchem, S7143) in 0.5% Tween-80 0.5% methylcellulose or vehicle twice a day, for a total of 15 injections when mice actually started showing rapid body weight loss. Administration of 5 mg/kg by gavage twice a day was toxic for long-period treatments, causing massive body weight loss and diarrhea in 80% of the treated mice after 10 days. IVM was also performed at day 4 and day 8 from the beginning of the treatment. The quantification of MGG8-GFP-GLuc cells in contact with the vessels was performed on 3D rendering of high-resolution z-stacks – 706×706 μm (xy-voxel of 0.69) and at least 60 μm of z-stack (z-steps 2 μm) – of regions of interests at the border of the tumor masses. We did not consider big masses (more than 100 μm in diameter) of tumor cells. In order to quantify only the tumor cells integrated in the brain and avoid the superficial cells, we used second-harmonic generation (SHG) beam – present at the border of the brain – as a reference. The presented result is based on n = 2 experiments, in total n = 9 mice for vehicle cohort and n = 7 mice for LGK974 cohort, at least 3 regions of interest per mouse, cells manually counted in a double blinded manner (n = 13 994 in total).
Toxicity Studies for LGK974 Dosage
In order to investigate toxicity of LGK974 treatment, we first examined the effect of different doses in nude mice. Mice were treated by gavage with LGK974 (Selleckchem, S7143) in 0.5% Tween-80 0.5% methylcellulose or vehicle. We found that 5 mg/kg twice a day (dosage used for the intravital imaging study) was toxic if given long-term, inducing body weight loss and diarrhea. In contrast, 5 mg/kg once a day was tolerated for up to 28 days in 80% of mice (dosage then used for the survival studies).
Systemic LGK974 Treatment Protocol for Survival Studies
Treatment for survival studies was performed with a 5 mg/kg by gavage once a day of LGK974. In the case of LGK974 alone (Figure S5E), treatment was initiated at day 12 after orthotopic implantation of 20 000 MGG8-GFP-GLuc cells, corresponding to a median blood Gluc activity of 7 400 ± 4 900 RLU/s, equivalent to MGG8 tumor volumes of around 4 mm3 (Kloepper et al., 2016). In case of combinatorial survival study (Figure 6F), treatment was initiated at day 36 after orthotopic implantation of 200 000 MGG8 cells, corresponding to a median blood Gluc activity of 435 000 ± 29 000 RLU/s, equivalent to MGG8 tumor volumes of around 9–10 mm3 (Kloepper et al., 2016). In both studies, mice were randomized by pre-treatment GLuc activity and body weight. MGG8-bearing nude mice were treated with vehicle (0.5% Tween-80 and 0.5% Methylcellulose; Sigma) and PBS; vehicle + TMZ (2.5 mg/kg) in PBS; PBS + LGK974 (5 mg/kg) in 0.5% Tween-80 and 0.5% Methylcellulose; or TMZ + LGK974. LGK974 or vehicle was administered by gavage daily. TMZ or PBS were administered i.p daily for 5 consecutive days. Animals were treated until they were killed because of the occurrence of lethargy, body weight loss of >20%, or impairing neurological symptoms.
In Vivo Primary Mouse Cells Transplants
Olig2cre/+;Trp53fl/fl;hEGFRvIII (Olig2+ tumors), Olig2cre/cre;Trp53fl/fl;hEGFRvIII (Olig2− tumors), Olig2cre/+;Trp53fl/fl;Wnt7a−/−;Wnt7bfl/fl; hEGFRvIII (Wnt7a/b-null tumors) and Cdkn2a−/−;hEGFRvIII tumor cells were grown in culture and transplanted into the brains of SCID mice as previously described (Mehta et al., 2011), at the following coordinates, according to Bregma: 1 mm (anterior), 2 mm (lateral), 2.5 mm (deep). 2 × 105 cells in Hanks’ Balanced Salt Solution without Ca2+ and Mg2+ (HBSS) were manually injected into the striatum. Animals were sacrificed upon development of neurological deficits for all experiments except invasion experiments where animals were sacrificed at one-month post-transplantation (Figures 1D, 2D (right panel), S1C, and S1I).
Bevacizumab-Resistant Xenograft Models
As described in (Jahangiri et al., 2013), 500 000 U87 cells were implanted subcutaneously in combination with 1:1 matrigel in 20 nude mice. 15 of the animals were treated with Bevacizumab at 10 mg/kg twice a week while the control cohort of 5 mice was treated with equal concentrations of humanized IgG. The first tumor from each treatment arm to reach 2 cm in any diameter was resected, dissociated and re-implanted in a new generation of 5 mice. This was repeated for nine generations creating the Bev-Resistant and Bev-Sensitive cell lines. For the intracranial studies, the largest tumor of the 9th generation were then taken, and implanted intracranially at a concentration of 250 000 cells. Similarly, these animals received the same concentration of treatment as their subcutaneous donors. Animals were sacrificed upon development of neurological deficits or signs of decompensation or cachexia. Each mouse was perfused transcardially with PBS and the tumor was dissected under a surgical microscope, lysed and prepared for immunoblotting.
Immunohistochemistry
For human tissue analysis, sections were fixed 5 min in 4% paraformaldehyde, rinsed in PBS 0.1% Triton-5% Horse serum and incubated overnight at 4°C with primary antibodies in PBS 0.1% Triton-5% Horse serum. Sections were then rinsed several times in PBS 0.1% Triton-5% Horse serum and incubated with the secondary antibodies in PBS 0.1% Triton-5% Horse serum for 30 min. Sections were rinsed and mounted using DAPI Fluoromount-G. Mouse brains were perfused with 4% paraformaldehyde, rinsed in phosphate buffer saline (PBS) pH 7.2 for 1 hr. Brains were cryoprotected in 30% sucrose and embedded in OCT (TissueTek, Sakura). 14 μm cryostat sections were used for immunohistochemical staining. Sections were incubated overnight at 4°C with the primary antibodies in PBS 0.1% Triton-1% Horse serum. Sections were then rinsed several times in PBS 0.1% Triton-1% Horse serum and incubated with the secondary antibodies in PBS 0.1% Triton-1% Horse serum for 30 min. Sections were rinsed and mounted using DAPI Fluoromount-G. Primary antibodies are listed in the Key Resources Table.
Image Acquisition
Confocal images were obtained using a Leica SP5 AOBS Upright Microscope. Brightfield and fluorescence acquisition was realized using a Zeiss Axioskop2 microscope coupled to an AxioCam HRc camera. Images in Figure S4 were acquired with a Zeiss AxioImager 2 upright epifluorescence microscope with a motorized Ludl stage (Zeiss). Mosaic TIFF images of stained tissue sections were generated using the TissueFAXS software (TissueGnostics, Vienna, Austria).
QUANTIFICATION AND STATISTICAL ANALYSIS
Microarray Data Processing
Preprocessing of data was performed within the R statistical computing environment. The SampleNetwork (Oldham et al., 2012) R function was used to determine outlying samples, assess technical batch effects, and perform data normalization. Two mice died before the end of the time course. These samples were removed, leaving three samples in each group. Following removal of outlying samples and quantile normalization (Bolstad et al., 2003), differential expression analysis was performed to identify genes that were significantly up- or down-regulated in Olig2− tumors relative to Olig2+ tumors (three samples per group). Probes on the microarray that were detected above background levels in at least one sample (n=17 078) were included in the analysis. Differential expression analysis was performed on expression data using the bayes.t.test R function. The p values were adjusted to account for multiple testing using the false discovery rate (FDR) approach, and probes with FDR p < 0.05 were considered to be differentially expressed. Genes up-regulated in Olig2+ tumors were compared to murine OPCs (versus astrocytic), human oligodendroglioma and GBM subtypes using published dataset (Tirosh et al., 2016; Verhaak et al., 2010; Wang et al., 2017; Zhang et al., 2014). Hierarchical clustering was performed using Gene Cluster 3.0 (de Hoon et al., 2004) and Treeview (Saldanha, 2004).
The Cancer Genome Atlas Data Processing
Normalized (level 3) data were obtained for GBM (microarray) and oligodendroglioma (RNAseq) groups through the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/). In some cases multiple samples were associated with a single patient ID. Since replicate samples from a single patient might bias pairwise correlations, patient IDs with multiple associated samples were removed from the analysis. Technical batch effects were assessed using the SampleNetwork R function (Oldham et al., 2012). Technical batch effects associated with “bcr_batch” and “A260:A280” were identified in the GBM data and corrected with sequential rounds of the ComBat (Johnson et al., 2007) function from the sva R package. No other technical batch effects were identified and no additional normalization was performed. Tumors carrying mutations in TP53 were identified using the curated .maf file from the oligodendroglioma exome sequencing data. Pairwise Pearson correlation coefficients (r) were calculated between genes within each group using log2-transformed expression values. Barplots of pairwise correlations were produced using the ggplot2 R package (Figures 4A–4C and S3) and heatmaps were produced using the labeledHeatmap function from the WGCNA R package (Langfelder and Horvath, 2008) (Figure S3).
Allen Brain Institute Data Processing
RNAseq data from two human adult brains (H0351.2001 and H0351.2002) were obtained through the Allen Brain Institute (ABI) data portal. The raw reads were taken as input for the SampleNetwork R function (Oldham et al., 2012) and the data were quantile normalized (Bolstad et al., 2003). Technical batch effects were assessed and a batch effect associated with “rnaseq_run_id” was identified and corrected with the ComBat function in the sva R package. After correcting for rnaseq_run_id six outlying samples (S020722, S020656, S010416, S010536, S020697, S010508) with standardized sample connectivities < −3 were identified and removed (Oldham et al., 2012). A batch effect associated with “RIN” was revealed and corrected with the ComBat function as before. No further normalization was performed. Pairwise Pearson correlation coefficients were calculated between genes using log2-transformed data across cortical samples from the frontal, parietal and occipital lobes (n = 152).
Statistical Analysis of Pairwise Correlations
All statistical analysis was performed within the R statistical computing environment. To assess whether pairwise Pearson correlation coefficients (r) between genes showed a significant difference between groups they were first rescaled using Fisher’s r-to-z transformation:
where k indexes the groups being compared. The intergroup difference between the resulting z-scores (zdiff) (e.g. for groups 1 and 2) was divided by the joint standard error:
where n1 and n2 denote the number of samples in group 1 and 2, respectively. The significance level of zdiff (p value) was then calculated based on the standard normal distribution (Oldham et al., 2012).
Vasculature and Imaging Metrics Analysis
Quantifications of density (MVD, vessel/mm2), size, area (percentage of vascular area) and lumen of CD31 (endothelial cells) positive vessels, and basement membrane coverage (Collagen IV) were performed using in-house segmentation ImageJ algorithms (U.S. National Institutes of Health, Bethesda, Maryland) on 40X normal and tumor fields (five to eight per animal) from Olig2cre/+;Trp53fl/fl;hEGFRvIII (Olig2+ tumors), Olig2cre/cre;Trp53fl/fl;hEGFRvIII (Olig2− tumors), Olig2cre/+;Trp53fl/fl;hEGFRvIII (Wnt7a/b WT tumors) and Olig2cre/+;Trp53fl/fl;Wnt7a−/−;Wnt7bfl/fl;hEGFRvIII (Wnt7a/b-null tumors) transplanted animals (n = 3–4 per genotype). Quantification of fibrinogen staining was performed using the Integrated Density function from ImageJ (U.S. National Institutes of Health, Bethesda, Maryland) on 20X normal and tumor fields (n = 3 animals per genotype). Quantifications of Iba1+ microglia numbers, cell perimeter (μm) and soma cell size (area, μm2) were performed using ImageJ (U.S. National Institutes of Health, Bethesda, Maryland) on 20X normal and tumor fields (n = 3 animals per genotype). Quantifications of the proliferative index (Ki67+ tumor cells) were performed on 10X fields from Olig2cre/+;Trp53fl/fl;hEGFRvIII (Olig2+ tumors) and Olig2cre/cre;Trp53fl/fl;hEGFRvIII (Olig2− tumors) transplanted animals (n = 3 animals per genotype). Quantifications of Ki67+ CD31+ cells were performed on 20X fields from Olig2cre/+;Trp53fl/fl; hEGFRvIII (Olig2+ tumors) and Olig2cre/cre;Trp53fl/fl;hEGFRvIII (Olig2− tumors) transplanted animals (n = 3 animals per genotype). Quantifications of Lef1+ CD31+ cells were performed on 20X normal and tumor fields from Olig2cre/+;Trp53fl/fl;hEGFRvIII (Olig2+ tumors) and Olig2cre/cre;Trp53fl/fl;hEGFRvIII (Olig2− tumors) transplanted animals (n = 3 per genotype) and human oligodendroglioma and glioblastoma samples (n = 3 cases each). Quantifications of hEGFR+ DAPI+ cells per 100 μm vessel were performed on 40X tumor fields at the invasion border from Olig2cre/+;Trp53fl/fl;hEGFRvIII (Wnt7a/b WT tumors) and Olig2cre/+;Trp53fl/fl;Wnt7a−/−;Wnt7bfl/fl; hEGFRvIII (Wnt7a/b-null tumors) transplanted animals (n = 3 per genotype). Olig2, Lef1 and Wnt7 stained areas were normalized with DAPI or GFP areas to quantify the percentage of stained areas within the MGG8 tumors (n = 4 mice). VEGF in LGK974-treated tumors was analyzed by quantifying the percentage of area with VEGF staining within the whole tumor (n = 3 per treatment, size- and time-matched tumors). Iba1+ cells were counted within the whole are of the tumor in LGK974 or vehicle-treated mice (n = 3 per treatment, size- and time-matched tumors).
Statistical Analysis
Unless otherwise specified, data are presented as mean ± s.e.m. Student’s two-tailed t test based on Gaussian distributions were used to assess the significance. Blinding during analysis was used for all the in vivo experiments. Statistics were performed using Graphpad Prism and Microsoft Excel software.
DATA AND SOFTWARE AVAILABILITY
Accession Codes
Microarray data are deposited in the NCBI Gene Expression Omnibus (GEO). The accession number is GSE110052.
Supplementary Material
Significance.
Although malignant glioma employs angiogenesis, anti-vascular endothelial growth factor (VEGF) inhibitors have failed to improve glioblastoma patient survival. We investigated whether a “glial code” underlies glioma-vascular interactions. We find that Olig2+ oligodendrocyte precursor-like (OPCL) cells invade the parenchyma by single-cell vessel co-option and do not affect the underlying vasculature. In distinction, Olig2− gliomas grow as perivascular clusters, leading to disruption of the blood-brain barrier and innate immune cell activation. We show that Wnt7 expression in OPCL cells is needed for vessel co-option and that Wnt inhibition enhances the response to temozolomide therapy. Finally, anti-VEGF treatment selects for the Olig2/Wnt7+ glial phenotype, providing insight into potential mechanisms underlying glioma escape from anti-angiogenic therapy.
Highlights.
Glial phenotype regulates glioma co-option or angiogenesis
Glioma oligodendrocyte-like (OPCL) cells express Wnt7 necessary for co-option
Wnt inhibitors significantly improve survival with temozolomide
Anti-VEGF-treatment of glioma selects for Olig2/Wnt7+ cells
ACKNOWLEDGMENTS
We are grateful to Sandra Chang, Sylvie Roberge, Huang Peigen, Dai Fukumura, and Khalida Sabeur for expert technical help; UCLA Neuroscience Genomics Core, Timothy Phoenix, and Richard Gilbertson for helpful discussions; and Ken Probst for illustrations. Lindsey Jones and Joseph Costello provided the oligodendroglioma cell line, Hiroaki Wakimoto provided MGG8 cells, and Darell Bigner provided D54 cells. Postdoctoral fellowship support is gratefully acknowledged from the ABTA (A.G., A.-C.T. and E.H.), AACR Anna D. Barker Fellowship (A.G.), Susan Komen Fellowship (G.S.), NCI (J.K.S., T32CA151022), and Nuovo-Soldati Foundation (L.L.). The studies were supported by the UCSF Brain Tumor SPORE Tissue Core (P50CA097257) and MGH (supported by NFCR), and were made possible by grants from the Bryan’s Dream Foundation, Pediatric Brain Tumor Foundation (W.A.W. and D.H.R.), Loglio Foundation (D.H.R.), HHMI (D.H.R.), Wellcome Trust (D.H.R.), NCI (R35CA197743, P01CA080124, P50CA165962 to R.K.J.), NFCR (R.K.J.), Harvard Ludwig Cancer Center (R.K.J.), and NINDS (NS028478 to A.A.B.; NS088114 to A.I.P.; R01CA221969 and R01NS091620 to W.A.W.; NS081117 to J.J.P.; NS079697 to M.K.A.; NS088648 to S.M.; NS083513 to D.H.R.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures, five tables, and three videos and can be found with this article online at https://doi.org/10.1016/j.ccell.2018.03.020.
DECLARATION OF INTERESTS
The authors declare no disclosures relevant to these studies. A.A.-B. is co-founder and on the SAB of Neurona Therapeutics. T.T.B. received consultant fees or funding from Merck, NXDC, Amgen, Genomicare, Jiahui Health, Inc., and Pfizer. R.K.J. received consultant fees from Merck, Ophthotech, Pfizer, SPARC, SynDevRx, and XTuit; owns equity in Enlight, Ophthotech, SynDevRx, XTuit; serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Life Sciences Investors, Healthcare Opportunities Fund, and World Healthcare Fund. No reagents or funding from these organizations were used in this study.
REFERENCES
- Amankulor NM, Kim Y, Arora S, Kargl J, Szulzewsky F, Hanke M, Margineantu DH, Rao A, Bolouri H, Delrow J, et al. (2017). Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 31, 774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, et al. (2002). Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1, 269–277. [DOI] [PubMed] [Google Scholar]
- Bardehle S, Rafalski VA, and Akassoglou K (2015). Breaking boundaries-coagulation and fibrinolysis at the neurovascular interface. Front. Cell. Neurosci 9, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, et al. (2016). New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA 113, E1738–E1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigner SH, Bullard DE, Pegram CN, Wikstrand CJ, and Bigner DD (1981). Relationship of in vitro morphologic and growth characteristics of established human glioma-derived cell lines to their tumorigenicity in athymic nude mice. J. Neuropathol. Exp. Neurol 40, 390–409. [DOI] [PubMed] [Google Scholar]
- Boer JC, Walenkamp AM, and den Dunnen WF (2014). Recruitment of bone marrow derived cells during anti-angiogenic therapy in GBM: the potential of combination strategies. Crit. Rev. Oncol. Hematol 92, 38–48. [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, and Speed TP (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193. [DOI] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al. (2013). The somatic genomic landscape of glioblastoma. Cell 155, 462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, and Jain RK (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Liao S, Kit Wong H, Lacorre DA, di Tomaso E, Au P, Fukumura D, Jain RK, and Munn LL (2011). Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood 118, 4740–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, et al. (2014). Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med 370, 709–722. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, and Barres BA (2009). Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 106, 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon MJ, Imoto S, Nolan J, and Miyano S (2004). Open source clustering software. Bioinformatics 20, 1453–1454. [DOI] [PubMed] [Google Scholar]
- di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK, Fazlollahi L, Andronesi OC, Frosch MP, Wen PY, Plotkin SR, et al. (2011). Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 71, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emblem KE, and Jain RK (2016). Improving treatment of liver metastases by targeting nonangiogenic mechanisms. Nat. Med 22, 1209–1210. [DOI] [PubMed] [Google Scholar]
- Erdem-Eraslan L, van den Bent MJ, Hoogstrate Y, Naz-Khan H, Stubbs A, van der Spek P, Bottcher R, Gao Y, de Wit M, Taal W, et al. (2016). Identification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: a report from the BELOB trial. Cancer Res. 76, 525–534. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, et al. (2011). Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci 14, 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, Nathan MR, Wotherspoon A, Gao ZH, Shi Y, et al. (2016). Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med 22, 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, and Deneen B (2014). Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83, 283–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, et al. (2014). A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med 370, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow SM, Zhu W, Stolt CC, Huang TW, Chen F, LoTurco JJ, Neul JL, Wegner M, Mohila C, and Deneen B (2014). Mutual antagonism between Sox10 and NFIA regulates diversification of glial lineages and glioma subtypes. Nat. Neurosci 17, 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, and Kriegstein AR (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561. [DOI] [PubMed] [Google Scholar]
- Hu B, Wang Q, Wang YA, Hua S, Sauve CG, Ong D, Lan ZD, Chang Q, Ho YW, Monasterio MM, et al. (2016). Epigenetic activation of WNT5A Drives glioblastoma stem cell differentiation and invasive growth. Cell 167, 1281–1295.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangiri A, De Lay M, Miller LM, Carbonell WS, Hu YL, Lu K, Tom MW, Paquette J, Tokuyasu TA, Tsao S, et al. (2013). Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin. Cancer Res 19, 1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK (2014). Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, and Rabinovic A (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127. [DOI] [PubMed] [Google Scholar]
- Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, et al. (2011). Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl. Acad. Sci. USA 108, 3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, Dalvie N, Amelung RL, Datta M, Song JW, et al. (2016). Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc. Natl. Acad. Sci. USA 113, 4476–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski C, and Weimer RM (2012). An automated method to quantify microglia morphology and application to monitor activation state longitudinally in vivo. PLoS One 7, e31814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P, and Horvath S (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, and Amit I (2014). Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, and Rowitch DH (2004). The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J. Neuropathol. Exp. Neurol 63, 499–509. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, Bachoo RM, Kane M, Louis DN, Depinho RA, et al. (2007). Olig2-regulated line-age-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron 53, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, and Zong H (2011). Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al. (2013). Targeting wnt-driven cancer through the inhibition of porcupine by LGK974. Proc. Natl. Acad. Sci. USA 110, 20224–20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu-Emerson C, Duda DG, Emblem KE, Taylor JW, Gerstner ER, Loeffler JS, Batchelor TT, and Jain RK (2015). Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J. Clin. Oncol 33, 1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, et al. (2012). VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, and Rowitch DH (2002). Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86. [DOI] [PubMed] [Google Scholar]
- Mateo JL, van den Berg DL, Haeussler M, Drechsel D, Gaber ZB, Castro DS, Robson P, Crawford GE, Flicek P, Ettwiller L, et al. (2015). Characterization of the neural stem cell gene regulatory network identifies OLIG2 as a multifunctional regulator of self-renewal. Genome Res. 25, 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Huillard E, Kesari S, Maire CL, Golebiowski D, Harrington EP, Alberta JA, Kane MF, Theisen M, Ligon KL, et al. (2011). The central nervous system-restricted transcription factor Olig2 opposes p53 responses to genotoxic damage in neural progenitors and malignant glioma. Cancer Cell 19, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer DH, Sun Y, Liu T, Kane MF, Alberta JA, Adelmant G, Kupp R, Marto JA, Rowitch DH, Nakatani Y, et al. (2014). An amino terminal phosphorylation motif regulates intranuclear compartmentalization of Olig2 in neural progenitor cells. J. Neurosci 34, 8507–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olar A, and Aldape KD (2014). Using the molecular classification of glioblastoma to inform personalized treatment. J. Pathol 232, 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham MC, Langfelder P, and Horvath S (2012). Network methods for describing sample relationships in genomic datasets: application to Huntington’s disease. BMC Syst. Biol 6, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, et al. (2015). Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, and Barnholtz-Sloan JS (2015). CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 17 (Suppl 4), iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, et al. (2014). Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AI, Petritsch C, Swartling FJ, Itsara M, Sim FJ, Auvergne R, Goldenberg DD, Vandenberg SR, Nguyen KN, Yakovenko S, et al. (2010). Non-stem cell origin for oligodendroglioma. Cancer Cell 18, 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MA, Ryu JK, Chang KJ, Etxeberria A, Bardehle S, Mendiola AS, Kamau-Devers W, Fancy SPJ, Thor A, Bushong EA, et al. (2017). Fibrinogen activates BMP signaling in oligodendrocyte progenitor cells and inhibits remyelination after vascular damage. Neuron 96, 1003–1012.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, Datta M, Amoozgar Z, Seano G, Jung K, et al. (2016). Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl. Acad. Sci. USA 113, 4470–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzella F, and Gatter K (2015). Non-angiogenic tumours unveil a new chapter in cancer biology. J. Pathol 235, 381–383. [DOI] [PubMed] [Google Scholar]
- Phoenix TN, Patmore DM, Boop S, Boulos N, Jacus MO, Patel YT, Roussel MF, Finkelstein D, Goumnerova L, Perreault S, et al. (2016). Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 29, 508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posokhova E, Shukla A, Seaman S, Volate S, Hilton MB, Wu B, Morris H, Swing DA, Zhou M, Zudaire E, et al. (2015). GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep. 10, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados MD, Byron SA, Tran NL, Phillips JJ, Molinaro AM, Ligon KL, Wen PY, Kuhn JG, Mellinghoff IK, de Groot JF, et al. (2015). Toward precision medicine in glioblastoma: the promise and the challenges. Neuro Oncol. 17, 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, Heck R, Thom S, Macas J, Bockamp E, et al. (2012). Endothelial Wnt/beta-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J. Exp. Med 209, 1611–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, and Shuman MA (2000). Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ (2004). Java Treeview–extensible visualization of microarray data. Bioinformatics 20, 3246–3248. [DOI] [PubMed] [Google Scholar]
- Sandmann T, Bourgon R, Garcia J, Li C, Cloughesy T, Chinot OL, Wick W, Nishikawa R, Mason W, Henriksson R, et al. (2015). Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J. Clin. Oncol 33, 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Wilson MD, Spyrou C, Brown GD, Hadfield J, and Odom DT (2009). ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods 48, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, and McMahon AP (2008). Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Sun Y, Meijer DH, Alberta JA, Mehta S, Kane MF, Tien AC, Fu H, Petryniak MA, Potter GB, Liu Z, et al. (2011). Phosphorylation state of Olig2 regulates proliferation of neural progenitors. Neuron 69, 906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, et al. (2014). Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell 157, 580–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Boekhorst V, and Friedl P (2016). Plasticity of cancer cell invasion-mechanisms and implications for therapy. Adv. Cancer Res 132, 209–264. [DOI] [PubMed] [Google Scholar]
- Tirosh I, Venteicher AS, Hebert C, Escalante LE, Patel AP, Yizhak K, Fisher JM, Rodman C, Mount C, Filbin MG, et al. (2016). Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature 539, 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli C, Morelli MJ, Bianchi S, Rotta L, Capra T, Sabo A, Campaner S, and Amati B (2015). Genome-wide analysis of p53 transcriptional programs in B cells upon exposure to genotoxic stress in vivo. Oncotarget 6, 24611–24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Gothert JR, Malik AB, Valyi-Nagy T, and Zhao YY (2016). Endothelial beta-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation 133, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al. (2012). Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, and Fancy SP (2016). Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 351, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. (2004). In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848. [DOI] [PubMed] [Google Scholar]
- Venteicher AS, Tirosh I, Hebert C, Yizhak K, Neftel C, Filbin MG, Hovestadt V, Escalante LE, Shaw ML, Rodman C, et al. (2017). Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 355, 10.1126/science.aai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto H, Mohapatra G, Kanai R, Curry WT Jr., Yip S, Nitta M, Patel AP, Barnard ZR, Stemmer-Rachamimov AO, Louis DN, et al. (2012). Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 14, 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al. (2017). Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, and Sontheimer H (2014). Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun 5, 4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, et al. (2017). Lomustine and bevacizumab in progressive glioblastoma. N. Engl. J. Med 377, 1954–1963. [DOI] [PubMed] [Google Scholar]
- Wickstrom M, Dyberg C, Milosevic J, Einvik C, Calero R, Sveinbjornsson B, Sanden E, Darabi A, Siesjo P, Kool M, et al. (2015). Wnt/beta-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat. Commun 6, 8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO, and Tannous BA (2008). A secreted luciferase for ex vivo monitoring of in vivo processes. Nat. Methods 5, 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chen Y, Kim B, Wang H, Zhao C, He X, Liu L, Liu W, Wu LM, Mao M, et al. (2013). Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152, 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E, and Rowitch DH (2014). Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, and Zlokovic BV (2015). Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Tien AC, Alberta JA, Ficarro SB, Griveau A, Sun Y, Deshpande JS, Card JD, Morgan-Smith M, Michowski W, et al. (2017). A sequentially priming phosphorylation cascade activates the gliomagenic transcription factor Olig2. Cell Rep. 18, 3167–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, and Nathans J (2014). Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev. Cell 31, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.