Abstract
Background and aims
Effective strategies are needed to address dramatic increases in hepatitis C virus (HCV) infection among people who inject drugs (PWID) in rural settings of the United States (US). We determined the required scale-up of HCV treatment with or without scale-up of HCV prevention interventions to achieve a 90% reduction in HCV chronic prevalence or incidence by 2025 and 2030 in a rural US setting.
Design
An ordinary differential equation model of HCV transmission calibrated to HCV epidemiological data obtained primarily from a HIV-outbreak investigation in Indiana.
Setting
Scott County, Indiana (population 24,181), USA, a rural setting with negligible baseline interventions, increasing HCV epidemic since 2010, and 55.3% chronic HCV prevalence amongst PWID in 2015
Participants
PWID
Measurements
Required annual HCV treatments per 1000 PWID (and initial annual percentage of infections treated) to achieve a 90% reduction in HCV chronic prevalence or incidence by 2025/30, either with or without scaling-up syringe service programs (SSPs) and medication-assisted treatment (MAT) to 50% coverage. Sensitivity analyses considered whether this impact could be achieved without retreatment of reinfections, and whether greater intervention scale-up was required due to the increasing epidemic in this setting.
Findings
To achieve a 90% reduction in incidence and prevalence by 2030, without MAT and SSP scale-up, 159 per 1000 PWID (initially 25% of infected PWID) need to be HCV-treated annually. However, with MAT and SSP scaled-up, treatment rates are halved (89 per 1000 annually or 15%). To reach the same target by 2025 with MAT and SSP scaled-up, 121 per 1000 PWID (20%) need treatment annually. These treatment requirements are 3-fold higher than if the epidemic was stable, and the impact targets are unattainable without retreatment.
Conclusions
Combined scale-up of hepatitis C virus (HCV) treatment and prevention interventions is needed to decrease the increasing burden of HCV incidence and prevalence in rural Indiana, USA, by 90% by 2025/30.
Keywords: Hepatitis C, prescription opioid, people who inject drugs
Introduction
Globally, chronic hepatitis C virus (HCV) is a leading cause of liver disease and death.1,2 In the United States (U.S.), HCV has a high burden, with 3.4-6 million persons currently infected3 and annual deaths where HCV is an underlying cause now exceeding those from HIV.4 Injection drug use is the primary mode of HCV transmission.5 While incidence rates of HCV infection fell between 1989 and 2003, rates have increased over three-fold since 20076. Many of these infections have been amongst young people who inject drugs (PWID) in rural areas7. Reducing HCV transmission amongst this sub-group is critical for reducing associated morbidity8 and achieving elimination9.
Accumulating international evidence suggests syringe service programs (SSPs) and medication-assisted treatment (MAT, normally involving methadone or buprenorphine replacement therapy in the US) can reduce HCV transmission by 50-80%.10–14 Additionally, the availability of all-oral, highly effective direct-acting antiviral (DAA) HCV treatment has heralded a new era where HCV infection can be easily cured in 8-12 weeks.15,16 The robust evidence associated with these interventions suggests that treating current HCV infections while simultaneously preventing future infection could significantly reduce HCV transmission in the U.S. This is supported by mathematical modelling from non-U.S. settings,17–19 and advocated by the World Health Organisation (WHO) in their recent global strategy to eliminate viral hepatitis20.
In many U.S. regions, the number of PWID has increased markedly due to a growing prescription opioid and heroin epidemic,7 contributing to a marked increase in parenterally-acquired HCV infections.6 Recently, in Scott County, Indiana, a rural county of 24,000 persons, a recent HIV-outbreak occurred (192 cases) among persons injecting oxymorphone (Opana® ER), a prescription opioid.21 Contact tracing and targeted HIV and HCV testing revealed a sizeable network of PWID with considerable HCV infection (>70% HCV antibody positivity) and high rates of syringe sharing (68% report ever sharing needles/syringes) and re-use (mean: 12 times before disposal).22,23 Similar to other rural U.S. communities, programs for preventing HCV/HIV transmission and treating opioid addiction were extremely limited in this setting.
In response to the HIV-outbreak, the Scott County Health Department implemented an SSP in 2015 and MAT is reported to follow,22 with ongoing discussions on whether to scale-up HCV treatment. Due to the increasing HCV epidemic, and limited coverage of prevention and treatment interventions, Scott County provides an important setting for modelling the required scale-up of HCV treatment, MAT and SSP for reducing HCV transmission to low-levels. While numerous models24–27 have demonstrated that treating HCV-infected PWID, possibly in combination with scaling-up SSPs and MAT17, could reduce HCV infection transmission, none have considered the increased requirements in settings with increasing transmission risk, as currently occurring across many rural U.S. settings and other settings worldwide28–31.
This study aims to model the HCV-impact of scaling-up access to MAT, SSPs and HCV treatment in Scott County, to typify other rural U.S. settings with limited existing interventions and increasing HCV epidemics. Specifically, our aims are:
With or without scale-up of MAT and SSP services, project the required HCV treatment capacity needed to reduce HCV infection prevalence and incidence among PWID by 90% (as advocated by WHO’s elimination strategy20) by 2020/25/30.
Project what subsequent treatment rate is needed to maintain impact to 2040.
Estimate the degree to which the HCV treatment targets are heightened due to the increasing HCV epidemic occurring in this setting,
Determine whether the impact targets are still achievable without allowing treatment of re-infected PWID.
Methods
Mathematical model description
We developed a dynamic, deterministic, compartmental ordinary differential equation model of HCV transmission among PWID. The modelled PWID population was stratified by HCV (see Figure S1a) and intervention status (none, MAT or SSP only, and both, Figure S1b). PWID enter the modelled population through a time-varying rate that individuals initiate injecting, and leave through mortality (drug-related or other causes) or permanent cessation from injecting drug use. We did not include HIV because HIV mortality was not expected to be important over the time span of our projections due to HIV treatment being scaled-up. All new PWID are assumed susceptible to HCV, with no access to SSP or MAT.
The model is dynamic, in that it simulates HCV transmission at a per-capita transmission rate dependent on the current prevalence of chronic HCV infection. The baseline transmission rate for PWID not on MAT or SSP is decreased by fixed multiplicative cofactors (or rate ratios) for PWID on MAT, SSP or both. PWID mix randomly to form potential transmission contacts with other PWID (see supplementary materials for details).
Once infected, PWID either develop chronic infection (presence of viremia) or spontaneously clear their infection and become susceptible to re-infection32. Chronically infected PWID remain infected unless treated, whereupon they either achieve a sustained viral response (SVR -virologic cure)16, or fail treatment and remain chronically infected. Treated PWID who achieve SVR become susceptible to re-infection. Conservatively, we assume that re-infection will occur at the same rate as primary infection. We assume PWID who fail treatment or become re-infected can be retreated, but vary this assumption in the sensitivity analysis.
Further model details are in the supplementary materials.
Model parameterisation
Key model input parameters were estimated based on experiences in Scott County, Indiana, with most parameters estimated from state surveillance and contact-tracing data collected during the 2014-2015 HIV-outbreak. Data from intensive contact-tracing for the HIV-outbreak investigation in Indiana23 suggests a high prevalence of current PWID (436 individuals were identified that reported recently (in last 12 months) injecting in an estimated population of 24,18133) and high HCV chronic prevalence (55.3%, 95%CI 49.3-61.4% of PWID were RNA-positive). The model assumed a PWID population size 436-600, and chronic HCV prevalence of 45-65% in 2015 due to uncertainty in the representativeness of available data.
Based on state-level acute HCV case reports34,35 and data on new opioid dependent admissions to the drug treatment episode dataset (TEDs) for Indiana36, we assumed a steady PWID population and HCV epidemic up to 2008 which increased thereafter. Through an increased rate of individuals initiating injecting, we assumed a 2 to 3-fold increase in PWID population size over 2008-2013 based on increases in opioid dependent admissions (TEDs) over that period. Through increased HCV transmission risk, we also assumed a 4 to 7-fold increase in the annual number of incident HCV infections over 2010-2014 – as observed in the number of acute HCV case reports from Indiana, which increased from approximately 17 annually for 2004-2010 to 123 annually for 2011-2014. Although known to underestimate the real number of acute infections, the increasing trends in acute cases should still represent a real increase in the rate of new HCV infections as they are not thought to be due to changes in reporting or case definitions7. Importantly, the demographics of these recent acute HCV cases (2015) align closely with the HIV-outbreak cases from Scott County (98-99% white, median age 32-34 years and 50-58% male), suggesting the increase in acute HCV cases occurred amongst the same pool of PWID as the HIV-outbreak.
There is uncertainty in the overall duration that PWID inject drugs, and so we sampled from a wide range of 5-20 years reflecting the young age and short duration of current injecting in rural U.S. sites (23,37,38 and unpublished Scott County SSP data) and the long injection careers observed among PWID in U.S. cities.39 We assumed a drug-related mortality rate based on U.S. synthesised data40 and a non-drug related mortality rate for the U.S.41
Prior to 2015, there was no SSP or HCV treatment for PWID in this setting and negligible MAT. SSP opened in March 2015 and reached 200 PWID (33-46% of PWID) by the end of 2015, with the model assuming further scale-up to 50% of PWID by mid-2016. Estimates for the effectiveness of SSP and MAT in reducing an individual’s risk of HCV infection were taken from a recent Cochrane systematic review.13 The duration on MAT or using the SSP were based on U.S. data,42 after which PWID cease MAT or SSP but can return at existing recruitment rates. Because most PWID are likely to be young and recently infected, we assumed conservative SVR rates for DAA HCV treatment of 90% (varied 85-95%) with 12-week duration.43
The model parameters with uncertainty bounds are given in Table 1.
Table 1.
Parameter table including uncertainty intervals. TEDs denotes the drug treatment episode dataset.
| Parameter | Symbol | Units | Values (sampled range) | Reference | |
|---|---|---|---|---|---|
| PWID and HCV-related parameters | |||||
| PWID HCV chronic prevalence in 2015 | – | 45 – 65%, uniform distribution | Contact tracing data33 | ||
| Average infection rate |
|
per year | Varied to fit sampled HCV prevalence in 2015, and increased by factor after 2010 to achieve 4–7 fold increase in incident infections | Acute HCV surveillance data34,35 | |
| Average proportion of infections that spontaneously clear |
|
– | 0.22 – 0.29, uniform distribution | 32 | |
| PWID recruitment rate |
|
per year | Varied to fit total population of 436–600 PWID in 2015, and increased by factor after 2008 to fit 2–3 fold increase in PWID population between 2008–2013 | Contact tracing data33 and TEDs data36 | |
| Increase in PWID population size between 2008 and 2013 | p | 2 – 3, uniform distribution | TEDs data36 | ||
| Average duration of injecting until cessation |
|
years | 5 – 20, uniform distribution | Unpublished Scott county SSP data and other data23,37–39 | |
| Average drug-related mortality rate |
|
per year | 0.57% (0.41–0.73%), Poisson distribution | USA sites from40 | |
| Average non drug-related mortality rate |
|
per year | 0.14%, Poisson distribution | 41 for 35–39 year olds in US | |
| Treatment parameters | |||||
| SVR rate |
|
– | 85 – 95%, uniform distribution | 43 | |
| Duration of treatment |
|
weeks | 12 | ||
| Treatment number |
|
number per year | Varied to reduce HCV prevalence or incidence by 90% by 2020/25/30. | ||
| Harm reduction intervention parameters | |||||
| Relative risk of acquiring HCV while: | |||||
| On MAT |
|
– | 0.50 (0.40–0.63), log normal distribution | Cochrane Systematic review13 | |
| On SSP |
|
– | 0.44 (0.24–0.80), log normal distribution | ||
| On both MAT and SSP |
|
– | 0.29 (0.13–0.65), log normal distribution | ||
| Duration on MAT/SSP | 1/ | years | 0.99 (0.64–1.50), normal distribution | 42 | |
| Recruitment rate MAT |
|
per year | Varied to give 25% or 50% coverage by mid-2017 | ||
| Recruitment rate SSP |
|
per year | Varied to give 50% coverage by mid-2016 | ||
Model calibration and analyses
While incorporating parameter uncertainty (1000 random samples of parameter distributions in Table 1), the model was calibrated (using a least-squared solver in MATLAB) to a sampled estimate for the PWID population size in 2015, increase in PWID population over 2008-2013, chronic prevalence in 2015, and 4-7-fold increase in annual incident infections over 2010-2014. The supplementary materials include more details of the methods.
The model was then run from 2015 to consider the impact of scaling-up SSP from March 2015, and MAT and HCV treatment from mid-2016. We projected the impact of a few illustrative treatment scenarios, and then estimated the annual treatment rate needed to result in a 90% reduction in HCV infection prevalence or incidence from 2016 to 2020, 2025 or 2030. These projections considered the impact both with and without scaling-up both MAT and SSP to 50% coverage alongside increased HCV treatment, with the added assumption that no more than 80% of chronic infections can be treated annually. We then projected the required treatment rates needed to maintain the impact achieved by 2020/25/30 to 2040, and assessed whether the same impact targets for 2020/25/30 could be reached with no retreatment.
Lastly, we undertook a sensitivity analysis to determine the degree to which the increasing epidemic in Indiana may be heightening our required treatment rates for achieving a 90% reduction in incidence and prevalence. The model was recalibrated to the same HCV prevalence in 2015, but assuming no increase in transmission risk or injecting in recent years (see supplementary materials), and the treatment rates needed to achieve a 90% reduction in prevalence or incidence were re-estimated.
Uncertainty analysis
To determine which parameter uncertainties are important for driving the variability in our model projections, a linear regression analysis of covariance44 was performed on the projected number of HCV treatments needed to reduce HCV prevalence or incidence by 90% by 2030 when MAT and SSP are also scaled-up. The proportion of the model outcome’s sum-of-squares contributed by each parameter was calculated to estimate the importance of individual parameters to the overall uncertainty.
Results
Baseline epidemic projections and illustrative intervention scenarios
Figure 1 shows that between 2008 and 2010, the model projects that both HCV infection prevalence and incidence decreased due to the increased recruitment of new susceptible PWID initiating injecting. However, this trend reversed when HCV transmission risk increased in 2010. Chronic prevalence increased to 56.0% by 2015, agreeing with available data from the HIV-outbreak investigation, but is then projected to reach 83.2% by 2030, and HCV incidence is projected to increase from 44.9 to 66.8 per 100 person years from 2015 to 2030.
Figure 1.
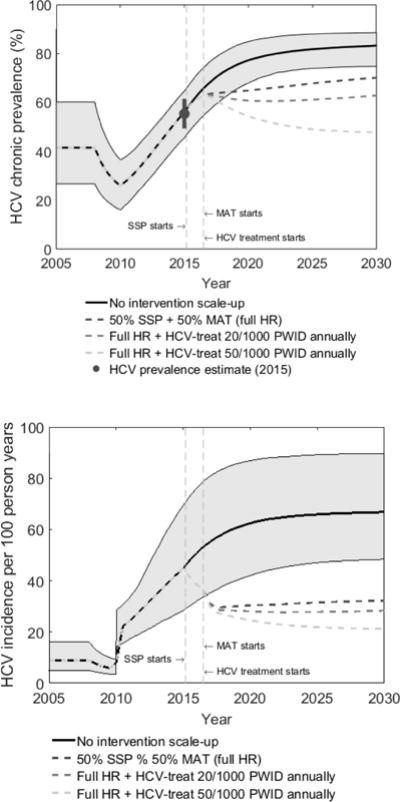
HCV chronic prevalence (a) and incidence (b) amongst PWID over time for different intervention scenarios.
Figure 1 shows median projections from 1000 model fits, with 95% credibility intervals only shown for the no intervention scale-up scenario. SSP denotes syringe service programs and MAT denotes medication-assisted treatment. Full HR denotes full harm reduction which is defined as 50% coverage of both SSP and MAT. HCV treatment started in mid-2016 with two scenarios being shown (20 or 50 per 1000 PWID being treated annually). Incidence is estimated amongst susceptible PWID. Figure 1a also shows chronic HCV prevalence estimate model was calibrated to.
Figure 1 also shows the impact of three intervention scenarios, illustrating that scaling-up both SSP and MAT to 50% coverage will be enough to decrease incidence by 2030, but that chronic prevalence will only decrease if this is combined with treating 50 PWID per 1000 annually (7.7% of infections treated in first year).
Treatment scale-up needed for reducing and maintaining HCV at low-levels
Projections in Figure 2 suggest that a 90% decrease in both HCV infection prevalence and incidence can be achieved by 2030. With no scale-up of SSP and MAT, 159 per 1000 PWID (24.9% of HCV infections treated in first year) need to receive HCV treatment annually to reach both these targets. Conversely, if SSP and MAT are both scaled-up to 50% coverage, then the yearly number needing treatment approximately halves to 89 per 1000 PWID (14.5% in first year).
Figure 2.
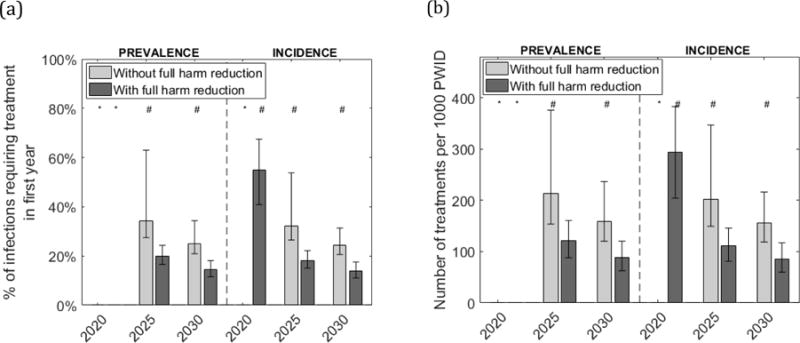
Required yearly HCV treatment rate needed to decrease HCV prevalence or incidence by 90% by 2020/25/30.
Figures show projected number per 1000 PWID needing to be HCV-treated each year (2b), and percentage of infections this translates to in the first year (2a), to result in a 90% reduction in chronic HCV prevalence or incidence by 2020, 2025 or 2030, with or without full harm reduction (50% coverage of both SSP and MAT). In both figures, bars show the median projections from a sample of 1000 model runs and whiskers show the 95% credibility intervals. *Less than 5% of parameter sets achieved the target. #Only a proportion of parameter sets achieved the target.
Projections also suggest it should be possible to achieve these same impact targets by 2025. When SSP and MAT are scaled-up, a 90% reduction in prevalence and incidence is possible if 121 per 1000 PWID (19.9% of HCV infections treated in first year) are treated annually. However, if SSP and MAT are not scaled-up then the yearly number needing HCV treatment doubles to 213 per 1000 PWID (34.1% in first year), although 17% of model simulations suggest it is not possible in this time-frame.
Lastly, it is not possible to achieve a 90% reduction in HCV prevalence by 2020, but a 90% decrease in incidence may be achievable (in 55% of simulations) if SSP and MAT are scaled-up and 294 per 1000 PWID (54.9% in first year) are treated in the first year, with this decreasing in subsequent years.
After achieving a 90% decrease in prevalence and/or incidence by 2030, Supplementary Figure S2 shows that prevalence and incidence would rebound quickly (increasing up to 10-fold by 2040) if treatment was not maintained, although the rebound would be smaller (about half) if MAT and SSP are scaled-up. To maintain the impact achieved on incidence from 2030 to 2040, 21 per 1000 PWID need to be treated annually if MAT and SSP are scaled-up, and 33 per 1000 PWID otherwise (Supplementary Table S1). Fewer treatments are needed to maintain the impact on prevalence.
Uncertainty and sensitivity analysis
There is considerable variability in the projected number of treatments needed to reach different impact targets (Figure 2). Analyses of covariance indicate that uncertainty in the chronic HCV infection prevalence in 2015 accounts for most variability in the required number of treatments (with SSP and MAT scaled-up) for achieving a 90% reduction in prevalence or incidence by 2030 (40-42% of variability – Figure 3). Other important parameters were the efficacy estimate for SSP (13% of variability), the PWID population size in 2015 (9-11% of variability), and the drug-related mortality rate (10-12%). The efficacy of SSP and MAT combined and the projected increase in incidence after 2010 also contributed 6-10% of variability each. Other parameters had a small effect.
Figure 3.
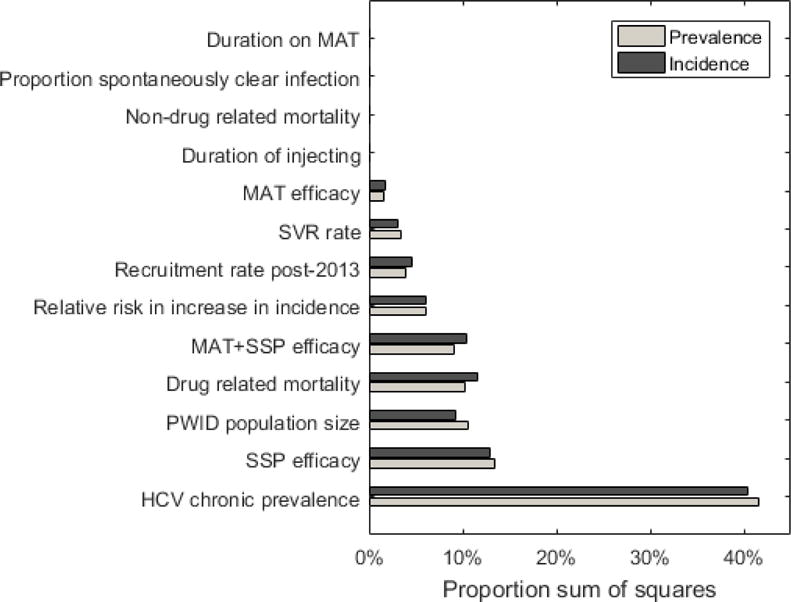
Contribution of each model parameter to the variability in the required number of HCV treatments to reduce incidence/prevalence by 90% by 2030.
Figures show the proportion of sum of squares each of the parameters contributes to the model outcome, indicating the importance of the parameters to the variation that is seen in the treatment number needed to reduce prevalence and incidence by 90% by 2030. The projections assume 50% coverage of SSP and MAT.
The baseline model assumed an increasing HCV epidemic and allowed re-treatment of re-infected PWID. If we assume a stable HCV-epidemic in Indiana, then two-thirds fewer treatments are needed to achieve a 90% decrease in prevalence/incidence (Supplementary Figure S3). Additionally, without retreatment it is not possible to decrease prevalence/incidence by 90% by 2030 (Supplementary Figure S4), with considerable re-treatment being needed (24-37% of all treatments) to achieve these targets.
Discussion
Since 2010, dramatic increases in HCV infections in the U.S. have occurred concurrently with the country’s growing opioid epidemic, linked to increasing injection drug use in rural settings.38 This increase in HCV transmissions was facilitated by limited HCV prevention services in rural settings, which contrasts with most U.S. urban areas which have established harm reduction programs, and have experienced long-term decreases in HIV transmission.45,46 The success of these urban efforts demonstrates the need to expand MAT and SSP in rural settings. However, because no modelling has so far considered similar settings experiencing increasing epidemics, the required scale-up remains unknown.
This modelling study helps to fill this knowledge-gap. It demonstrates that achievable scale-up of HCV treatment, when paired with expanded SSP and MAT, could dramatically reduce the burden of HCV among PWID in rural U.S. settings experiencing increasing HCV epidemics20. Our findings indicate that a 90% reduction in HCV incidence and prevalence are achievable by 2025 if 20% of currently HCV-infected PWID receive HCV treatment each year with no restriction on re-treatment, and half of the population are on MAT or SSP. Due to the small number of PWID in Scott County (estimated at 436-600), these targets should be achievable (<65 HCV-infected needing treatment annually), suggesting that such rural jurisdictions could be viable settings for conducting demonstration studies to test whether combining HCV treatment with MAT and SSP scale-up can control and eliminate HCV.
To reduce the HCV-burden among PWID, this study highlights the importance of scaling-up both HCV treatment and MAT and SSP capacity, with each playing a complementary role. HCV treatment is essential for rapidly reducing the infection burden to low-levels, which otherwise would take decades with just expanded MAT and SSP. Conversely, scaling-up MAT and SSP dramatically reduces (by half) the required levels of HCV treatment and underlying risk for new infections, which is key for maintaining a low infection burden.
Our projections also highlight the necessity of allowing treatment of re-infections, without which large reductions in HCV transmission are not possible, and the importance of maintaining levels of HCV treatment after the epidemic has reached low-levels, because otherwise prevalence and incidence quickly rebound to pre-intervention levels. Crucially, we also illustrate for the first time that much higher treatment rates (3-fold higher in our projections) will be needed to reach the WHO elimination targets in settings experiencing increasing HCV epidemics, such as Indiana, other rural U.S. settings, and numerous global settings experiencing increased injecting risk28–31. Further analyses need to confirm the generalisability of our findings to other similar settings, with these analyses emphasising the importance of accurately characterising a setting’s on-going epidemic when assessing required prevention and treatment needs for tackling HCV.
Limitations
Our analyses are subject to several potential limitations. First, there was limited epidemiological and behavioural data available to parameterise and calibrate the model. To address this, we used data from other U.S. settings7,37 when necessary and incorporated parameter uncertainty in our projections.
Second, we used acute HCV surveillance data34,35 and substance abuse treatment admission data36 to parameterise the likely increase in HCV infection and injecting drug use occurring in Indiana. Given these data sources do not capture all new HCV infections and many PWID do not access drug treatment, it is possible that the real-life increases in the HCV epidemic and levels of injection drug use may differ from what these datasets suggest. To counter this, uncertainty was incorporated in to these modelled trends and our projections were robust despite this.
Third, we did not incorporate network effects in to our model, which data suggests contributed to the HIV-outbreak in Indiana.23 This could be an important area of future modelling since previous modelling has suggested the network structure of PWID can affect the impact of HCV treatment interventions.48 Further, due to a lack of data, the model did not incorporate periods of temporary cessation of injecting, but instead assumed their effect was incorporated into the overall transmission risk of PWID, especially amongst those on MAT who are more likely to temporarily cease injecting.49 This model simplification should not have affected our model projections as illustrated by previous modelling.17,18
Lastly, we did not consider the mechanism by which the scale-up in treatment will be financed. Until recently, the high costs of all oral DAA drugs ($84,000-96,000 per treatment course in 2014), and restrictions within Medicaid programs43 (drug/alcohol abstinence and disease severity requirements and restrictions on treatment providers) presented serious obstacles to using HCV treatment as a prevention strategy in the U.S.50 However, the costs of HCV medications are now decreasing51,52 (through competition and negotiations), and Medicaid programs are easing restrictions on HCV treatment53, including amongst those with current drug/alcohol use (CDC, unpublished data). Economic modelling is now needed to help identify the required HCV drug prices to ensure HCV treatment-based prevention strategies are cost-effective. Economic modelling should also compare the costs of these HCV treatment-based prevention strategies to the costs of scaling-up MAT and SSP, whose yearly costs are much cheaper per PWID reached (estimated as ~$5,000 for MAT54 and ~$100 for SSP55 if 200 syringes are exchanged per year). However, this is not a simple comparison because HCV treatment exerts a one-off cost per infected PWID, while SSP and MAT exert yearly costs amongst both infected and susceptible PWID and have other economic benefits.
Comparison with other studies
This is the first modelling study to project the impact on HCV transmission of scaling-up combination prevention interventions (MAT and SSP) and HCV treatment for PWID in the U.S. Other analyses in non-U.S. settings have considered the HCV prevention impact of these combined interventions,17,18 but have not considered an increasing epidemic setting. Two other analyses have modelled the impact of scaling-up HCV treatment amongst prisoners27 or PWID in urban U.S.26 Our modelling builds on these analyses by considering a rural setting with increasing injection drug use and HCV transmission, which characterises the main expansion of HCV transmission in the U.S. Additionally, we incorporate the important benefits of scaling-up SSP and MAT,12,14,56,57 which generally have very low coverage in rural settings.
Conclusion
Scott County’s rapid HIV-outbreak occurred in a region that has witnessed increasing injection drug use and HCV transmission over recent years.7 Many other rural U.S. regions are witnessing similar problems.7 Like Scott County before the HIV-outbreak, many of these regions have insufficient infrastructure or resources to respond effectively, raising serious concerns that similar increases in HCV and HIV infections may occur elsewhere23,58. For areas of the U.S. and internationally experiencing similar epidemics and suffering analogous limitations in intervention coverage, our findings are of particular importance. They emphasise the need to scale-up HCV treatment, in combination with MAT and SSP, for reducing levels of HCV transmission. Importantly, this would also address current inequities in the provision of MAT and SSP, which will have other benefits.59,40,60–62 Demonstrating the effectiveness and potential costs and savings of our combined scale-up strategies in the field is an important next step.
Supplementary Material
Acknowledgments
Funding and disclosure: This study was supported by Contract No. 200-2013-M-53964B GS-10F-0097L from the Centers for Disease Control and Prevention (CDC) to RTI International and a subcontract from RTI International to the University of Bristol. The opinions expressed in this paper are solely those of the authors and do not necessarily represent the opinions of the CDC, RTI International, or the University of Bristol.
JZ was an employee of the Center for the Study of Human Health, Emory University, Atlanta, Georgia, USA, at the time the study began.
NKM, PV, and MH were additionally supported by the National Institute for Drug Abuse [grant number R01 DA037773-01A1], and NM was partially funded by the University of California San Diego Center for AIDS Research(CFAR), a National Institute of Health (NIH) funded program [grant number P30 AI036214]. PV and MH acknowledge support from the National Institute of Health Research Health Protection Research Unit in Evaluation of Interventions.
NKM and PV have received unrestricted research grants from Gilead unrelated to this work, and NKM has received honoraria from Merck, AbbVie, and Janssen.
List of abbreviations
- HCV
Hepatitis C virus
- PWID
People who inject drugs
- SSP
Syringe-service programs
- MAT
Medication-assisted treatment
- US
United States
- DAA
Direct-acting antivirals
- WHO
World Health Organization
- HIV
Human immunodeficiency virus
- TEDs
Drug treatment episode database
Footnotes
Conflict of interest: MH has received honoraria unrelated to this work from Merck, Abbvie and Gilead. JW, SH, HF, CV, AK, JZ and TH declare no conflict of interest
Contributions: JW, JZ and PV conceived of the study. PV and HF provided overall leadership for the study design, analysis and interpretation of the findings with input off the other co-authors. HF developed the model and performed all model analyses. JZ, CV, SH and JW collated and contributed data for the analysis. HF wrote the first draft with PV, JZ and TH. All authors have contributed to interpreting the results, and to writing the manuscript.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. The Lancet infectious diseases. 2005;5(9):558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L, et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C and hepatitis B: Results from the Global Burden of Disease GBD 2013 study. Lancet Infectious Disease. 2016 doi: 10.1016/S1473-3099(16)30325-5. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353–63. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of internal medicine. 2012;156(4):271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 5.Schoener EP, Hopper JA, Pierre JD. Injection drug use in North America. Infectious disease clinics of North America. 2002;16(3):535–51. doi: 10.1016/s0891-5520(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 6.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clinical Infectious Diseases. 2014:ciu643. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 7.Zibbell JE, Iqbal K, Patel R, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged≤ 30 years-kentucky, tennessee, virginia, and west virginia, 2006-2012. MMWR Morbidity and mortality weekly report. 2015;64(17):453–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Alter MJ, Moyer LA. The importance of preventing hepatitis C virus infection among injection drug users in the United States. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1998;18:S6–S10. doi: 10.1097/00042560-199802001-00003. [DOI] [PubMed] [Google Scholar]
- 9.Edlin BR, Winkelstein ER. Can hepatitis C be eradicated in the United States? Antiviral research. 2014;110:79–93. doi: 10.1016/j.antiviral.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Vickerman P, Page K, Maher L, Hickman M. Commentary on Nolan et al.(2014): Opiate substitution treatment and hepatitis C virus prevention: building an evidence base? Addiction. 2014;109(12):2060–1. doi: 10.1111/add.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmateer NE, Taylor A, Goldberg DJ, et al. Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PLoS One. 2014;9(8):e104515. doi: 10.1371/journal.pone.0104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106(11):1978–88. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 13.Platt L, Reed J, Minozzi S, et al. Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. The Cochrane Library. 2016 doi: 10.1002/14651858.CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolan S, Dias Lima V, Fairbairn N, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction. 2014;109(12):2053–9. doi: 10.1111/add.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir”. Clinical Infectious Diseases. 2015;60(12):1829–36. doi: 10.1093/cid/civ197. [DOI] [PubMed] [Google Scholar]
- 16.Yang HJ, Ryoo JY, Yoo BK. Meta-analysis of the efficacy and safety of sofosbuvir for the treatment of hepatitis C virus infection. International journal of clinical pharmacy. 2015;37(5):698–708. doi: 10.1007/s11096-015-0144-x. [DOI] [PubMed] [Google Scholar]
- 17.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(Suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vickerman P, Martin N, Turner K, Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction. 2012;107(11):1984–95. doi: 10.1111/j.1360-0443.2012.03932.x. [DOI] [PubMed] [Google Scholar]
- 19.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Draft global health sector strategies. Viral hepatitis, 2016–2021. 2016 [Google Scholar]
- 21.Conrad C, Bradley HM, Broz D, et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–4. [PMC free article] [PubMed] [Google Scholar]
- 22.Strathdee SA, Beyrer C. Threading the Needle—How to Stop the HIV Outbreak in Rural Indiana. New England Journal of Medicine. 2015;373(5):397–9. doi: 10.1056/NEJMp1507252. [DOI] [PubMed] [Google Scholar]
- 23.Peters PJ, Pontones P, Hoover KW, et al. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. New England Journal of Medicine. 2016;375(3):229–39. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- 24.Martin NK, Vickerman P, Miners A, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55(1):49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 25.Cousien A, Tran VC, Deuffic-Burban S, Jauffret-Roustide M, Dhersin JS, Yazdanpanah Y. Hepatitis C Treatment as Prevention of Viral Transmission and Liver-Related Morbidity in Persons Who Inject Drugs. Hepatology. 2016;63(4):1090–101. doi: 10.1002/hep.28227. [DOI] [PubMed] [Google Scholar]
- 26.Durham DP, Skrip LA, Bruce RD, et al. The impact of enhanced screening and treatment on hepatitis C in the United States. Clinical Infectious Diseases. 2016;62(3):298–304. doi: 10.1093/cid/civ894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He T, Li K, Roberts MS, et al. Prevention of hepatitis C by screening and treatment in US prisons. Annals of internal medicine. 2016;164(2):84–92. doi: 10.7326/M15-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paraskevis D, Nikolopoulos G, Fotiou A, et al. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS One. 2013;8(11):e78941. doi: 10.1371/journal.pone.0078941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Health Protection Scotland. HPS Weekly Report. 2016 [Google Scholar]
- 30.Des Jarlais DC, Kerr T, Carrieri P, Feelemyer J, Arasteh K. HIV infection among persons who inject drugs: ending old epidemics and addressing new outbreaks. Aids. 2016;30(6):815–26. doi: 10.1097/QAD.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarján A, Dudás M, Wiessing L, et al. HCV prevalence and risk behaviours among injectors of new psychoactive substances in a risk environment in Hungary—An expanding public health burden. International Journal of Drug Policy. 2017;41:1–7. doi: 10.1016/j.drugpo.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13(1):34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 33.Spiller MW, Shields J, Bradley HM, et al. Network Analysis of a Contact Network from an Investigation of a Community Outbreak of HIV Infection Linked to Injection Drug Use – Indiana. IAS; Vancouver, Canada: 2015. [Google Scholar]
- 34.Centers for Disease Control and Prevention. Surveillance Data for Acute Viral Hepatitis – United States, 2008. 2010 [Google Scholar]
- 35.Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States, 2013. 2015 [Google Scholar]
- 36.United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set - Admissions (TEDS-A) - Concatenated, 1992 to 2012 (ICPSR 25221) [Google Scholar]
- 37.Havens JR, Lofwall MR, Frost SD, Oser CB, Leukefeld CG, Crosby RA. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. American journal of public health. 2013;103(1):e44–e52. doi: 10.2105/AJPH.2012.300874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zibbell JE, Hart-Malloy R, Barry J, Fan L, Flanigan C. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. American journal of public health. 2014;104(11):2226–32. doi: 10.2105/AJPH.2014.302142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiller MW, Broz D, Wejnert C, Nerlander L, Paz-Bailey G. HIV Infection and HIV-Associated Behaviors Among Persons Who Inject Drugs-20 Cities, United States, 2012. MMWR Morbidity and mortality weekly report. 2015;64(10):270–5. [PMC free article] [PubMed] [Google Scholar]
- 40.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2013;91(2):102–23. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Detailed Tables for the National Vital Statistic Report (NVSR) “Deaths: Final Data for 2013”. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf (accessed 27th Jan 2016)
- 42.Bao YP, Liu ZM, Epstein DH, Du C, Shi J, Lu L. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35(1):28–33. doi: 10.1080/00952990802342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Association for the Study of Liver Diseases, America IDSo. Recommendations for Testing, Managing, and Treating Hepatitis C. 2016 [Google Scholar]
- 44.Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford; Oxford University Press; 2006. [Google Scholar]
- 45.Des Jarlais DC, Perlis T, Arasteh K, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. Aids. 2005;19:S20–S5. doi: 10.1097/01.aids.0000192066.86410.8c. [DOI] [PubMed] [Google Scholar]
- 46.Mehta SH, Astemborski J, Kirk GD, et al. Changes in blood-borne infection risk among injection drug users. J Infect Dis. 2011;203(5):587–94. doi: 10.1093/infdis/jiq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Indiana State Department of Health. Indiana State Deparment of Health investigates additional HIV cases tied to Southeastern Indiana Outbreak. 2015 [Google Scholar]
- 48.Hellard M, Rolls DA, Sacks-Davis R, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60(6):1861–70. doi: 10.1002/hep.27403. [DOI] [PubMed] [Google Scholar]
- 49.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3(3) doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Annals of internal medicine. 2015;163(3):226–8. doi: 10.7326/M15-0320. [DOI] [PubMed] [Google Scholar]
- 51.Young K, Rudowitz R, Garfield R, Musumeci M. Medicaid’s Most Costly Outpatient Drugs. The Henry J. Kaiser Family Foundation. 2016 [Google Scholar]
- 52.Silverman E. Washington State Told to Lift Restrctions on Hepatitis C Medicines. Stat News. 2016 https://www.statnews.com/pharmalot/2016/05/27/washington-state-hepatitis-drug-prices/
- 53.Center for Medicaid and CHIP Services. Assuring Medicaid Beneficiaries access to Hepatitis C (HCV) drugs. Medicaid Drug Rebate Prgram Notice. 2015 [Google Scholar]
- 54.Jackson H, Mandell K, Johnson K, Chatterjee D, Vanness DJ. Cost-effectiveness of injectable extended-release naltrexone compared with methadone maintenance and buprenorphine maintenance treatment for opioid dependence. Substance abuse. 2015;36(2):226–31. doi: 10.1080/08897077.2015.1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen TQ, Weir BW, Des Jarlais DC, Pinkerton SD, Holtgrave DR. Syringe exchange in the United States: a national level economic evaluation of hypothetical increases in investment. AIDS and Behavior. 2014;18(11):2144–55. doi: 10.1007/s10461-014-0789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med. 2014;174(12):1974–81. doi: 10.1001/jamainternmed.2014.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White B, Dore GJ, Lloyd AR, Rawlinson WD, Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med J Aust. 2014;201(6):326–9. doi: 10.5694/mja13.00153. [DOI] [PubMed] [Google Scholar]
- 58.Van Handel MM, Rose CE, Hallisey EJ, et al. County-level Vulnerability Assessment for Rapid Dissemination of HIV or HCV Infections among Persons who Inject Drugs, United States. JAIDS Journal of Acquired Immune Deficiency Syndromes 9000. doi: 10.1097/QAI.0000000000001098. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol. 2014;43(1):235–48. doi: 10.1093/ije/dyt243. [DOI] [PubMed] [Google Scholar]
- 60.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. Bmj. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Low AJ, Mburu G, Welton NJ, et al. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clinical Infectious Diseases. 2016;63(8):1094–104. doi: 10.1093/cid/ciw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. 2007 doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


