Abstract
Complex samples benefit from multidimensional measurements where higher resolution enables more complete characterization of biological and environmental systems. To address this challenge, we developed a drift tube-based ion mobility spectrometry-Orbitrap mass spectrometer (IMS-Orbitrap MS) platform. To circumvent the time scale disparity between the fast IMS separation and the much slower Orbitrap MS acquisition, we utilized a dual gate and pseudorandom sequences to multiplex the injection of ions and allow operation in signal averaging (SA), single multiplexing (SM), and double multiplexing (DM) IMS modes to optimize the signal-to-noise ratio of the measurements. For the SM measurements, a previously developed algorithm was used to reconstruct the IMS data. A new algorithm was developed for the DM analyses involving a two-step process that first recovers the SM data and then decodes the SM data. The algorithm also performs multiple refining procedures to minimize demultiplexing artifacts. The new IMSOrbitrap MS platform was demonstrated by the analysis of proteomic and petroleum samples, where the integration of IMS and high mass resolution proved essential for accurate assignment of molecular formulas.
Graphical Abstract
Interest in IMS analyses in areas such as biomolecule analysis and characterization, national security, petroleum, and environmental monitoring has greatly increased over the past decade.1–7 IMS separates ions on the basis of the balance between two forces that impact the ion movement, the electric field and the drag force from the collision with buffer gas molecules.8 For the vast majority of applications, the buffer gas is inert, but there are a few applications where a reactive buffer gas is also desired. Different variations of the application of electric field and stationary state of the buffer gas have given rise to multiple IMS-based platforms such as drift tube IMS (DTIMS), traveling wave IMS (TWIMS), trapped IMS (TIMS),9 overtone IMS (OIMS),10,11 differential IMS (DIMS),12 field asymmetric IMS (FAIMS),13–15 and transversal modulation IMS (TM-IMS).16 In the classical DTIMS, ions travel through the drift tube under the influence of a weak electric field while colliding with a stationary buffer gas. Ions with a small collisional cross section spend less time inside the drift tube, while ions of larger collision cross sections spend more time. The collision cross section depends on the ion mobility (which depends on ion–neutral interaction potential), effective temperature, charge, and reduced mass.17 Thus, IMS provides information on the shape of molecules not readily accessible from MS information alone. IMS also separates species on the basis of their charge state and their shape, which in turn depend on the chemical makeup and spatial structure of the molecules. The signal of ions that exit IMS can be acquired using a simple charge collector (Faraday plate) or using the more sophisticated MS.
While early IMS analyses focused on using the technique as a stand-alone device to study ion–neutral interactions and separate small molecules,8 the field has broadened dramatically with IMS integration with MS and soft ionization such as electrospray and matrix-assisted laser desorption (MALDI). While soft ionization broadened the range of molecules analyzed to include biomolecules, the IMS-MS analyses opened new areas of research where the two-dimensional separations provide new capabilities to characterize ions in the gas phase. However, to preserve the IMS duty cycle while also accurately profiling the IMS separation, the acquisition rate of the MS must be much faster than the IMS separation time. Because IMS normally distinguishes ions in a time scale of milliseconds, mass spectrometers such as time-of-flight (TOF) MS are a natural fit.18 A TOF MS samples ions on a microsecond time scale, allowing sampling of many points across an IMS peak forming and providing nested IMS-MS spectra. However, there are many benefits to combine IMS with much slower trapping-based instruments. In particular, FTICR and Orbitrap instruments can provide advantages in terms of the much higher mass resolution and accuracy, and have useful ancillary capabilities, for example, for performing MSn analyses.
Efforts to integrate IMS with slow detectors started in the early 1970s with the work of Karasek et al. using a dual grid gating technique.19–21 This approach was also adopted by many to enable mass and mobility selected ion activation as well as for fast screening.22–26 The dual grid gating approach relies on a first grid to inject ions into the drift cell (to initiate the IMS experiment), while a second grid at the end of the drift cell allows ions of a specific arrival time to be selected for transmission to the detector. At constant delay times between the two grids, this approach allows continuous monitoring of specific ions (i.e., single ion monitoring).27 Alternatively, scanning the delay time between the two grids allows the reconstruction of the whole IMS separation. This approach has the advantage of decoupling IMS speed from the acquisition speed of the detector or mass spectrometer but is slow due to the need to scan the entire mobility separation time and the long acquisition time of the mass spectrometer (especially ion traps) and suffers from overall low ion utilization efficiency. Traditionally, IMS measurements utilize a single grid configuration, which has a very low duty cycle as only a narrow pulse of ions is admitted into the drift cell. Adding a second gate (as in the dual grid gating approach) lowers the IMS duty cycle even further. An IMS separation time of 100 ms and a second gate window of 200 μs (to be transmitted to the mass spectrometer while discarding the rest of ions) result in a duty of cycle of 0.2% at the second gate. The IMS duty cycle can be improved by applying Hadamard-based multiplexing or frequency modulations schemes,28–30 which can provide duty cycles as high as 50%.26,28 Our laboratory has also introduced a modified pseudorandom sequence that incorporates a trapping stage before the IMS separation, and was shown to further improve the duty cycle for an IMS-TOF MS platform to beyond 50%.30,31
In this work, we show the application of the pseudorandom multiplexing scheme to the dual gates of the IMS-Orbitrap MS platform to maximize the sampling of ions into the Orbitrap and improve the signal-to-noise ratio of the measurements. We further demonstrate the advantages of this platform on the analysis of complex proteomic samples and petroleum substances to illustrate how the 2D separations provide higher peak capacity than each dimension alone.
EXPERIMENT SETUP
Instrument.
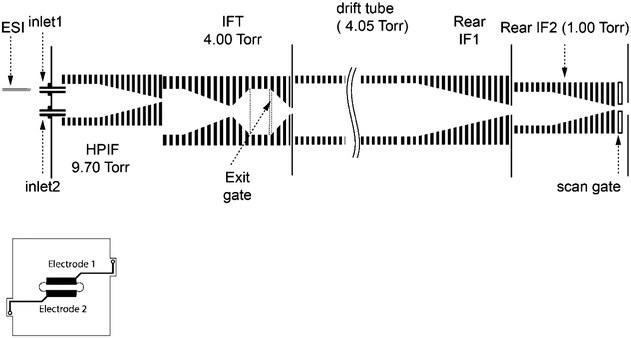
Experiments were performed using a home-built IMS drift tube (resolving power ~73)32 that was integrated with an Exactive Orbitrap MS (Thermo Fisher Scientific, San Jose, CA). Details of the IMS drift tube were published previously,32 and only information pertaining to the current configuration will be presented. Briefly, ions formed by nanoelectrospray ionization were transferred to the ion funnel trap (IFT)33 through a differentially pumped high-pressure ion funnel operating at 9.70 Torr. Ions were trapped at 4.00 Torr in the ion funnel trap for 4 ms and injected into the drift tube as 200–300 μs pulse. The diffused ions packets traveled through the 79 cm long and uniform field drift tube and were collected by a 15 cm long, 5 cm i.d. rear ion funnel (rear IF1) operating at the same pressure as the drift tube (4.05 Torr). Ions exited the rear IF1 through a 2.5 mm i.d. conductance limiting orifice into a second rear ion funnel IF2 that operated at a pressure of1.00 Torr. The rear IF2 was pumped using the same rough pump that backed the Exactive turbo pumps. To integrate IMS with the Exactive Orbitrap, the inlet capillary housing in the Exactive source interface was removed and replaced with the IF2 as shown in Figure 1. The IF2 is ~10 cm long with an acceptance diameter of 2.5 cm and an exit orifice of 2.5 mm. The rear IF2 was operated at an RF of 870 kHz and 100 Vp–p. A similar E/N (E is the field in V/cm and N is the number density) was maintained at ~4.5 V cm−1 Torr−1 (~14 Td) throughout the drift tube, rear IF1, and rear IF2. At the end of the ion funnel, a scan gate lens was used to modulate ion introduction to the Exactive interface. The scan gate (Figure 1) was fabricated using printed circuit board technology by depositing a thin gold layer (~65 μm) onto a 1.6 mm-thick nonconductive surface made from hydrocarbon ceramic (RO4000). The scan gate consisted of two electrodes separated by 4 mm gap and operates by applying different voltages to the two electrodes to block ion transmission and applying the same voltages to transmit ions. To shield the scan gate from the RF applied to the IF2 and downstream multipole, two DC-orifices of 3 mm i.d. separated the scan gate from the IF2 and first multipole of the Exactive interface.
Figure 1.
Top: Schematic diagram of the IMS-Exactive platform. Bottom: Schematic diagram of the scan gate electrodes.
Time Synchronization.
Because an Orbitrap is a trapping instrument while IMS is a pulsed technique, it is essential to synchronize the IMS timing with the curved linear trap (C-trap). In non-IMS mode (i.e., continuous ion beam), ions are initially injected into the C-trap for a time period corresponding to the automatic gain control (AGC) injection time. After the desired injection time is reached, the C-trap closes to incoming ions from the source, allowing ions to be trapped and then injected into the Orbitrap for detection. In IMS mode, ions arrive at the Orbitrap in packets that are temporally separated; therefore, it is crucial to time ions’ arrival to the scan gate and then to the C-trap, because otherwise ions are lost. Thus, the pulse from the C-trap corresponding to the injection into the Orbitrap was chosen to be the master trigger to initiate the IMS experiment. We also maximized the AGC injection time to increase the probability of the IMS ion packets arriving at the C-trap while being open.
Samples.
Tryptically digested bovine serum albumin and Enolase were purchased from Waters and were diluted in 50% methanol:50% water, which was acidified with 0.1% formic acid. Pierce LTQ ESI positive ion calibration solution was purchased from Thermo Scientific (Rockford, IL) and used without dilution. Petroleum substances of (i) heavy fuel oil and (ii) vacuum and hydrotreated gas oil were donated by an anonymous oil refining company and were diluted to 1 mg/mL in toluene/methanol buffer and acidified with formic acid. All samples were run in the positive electrospray mode.
Data Processing.
Data were collected using the Exactive software in Raw format and were converted to a Unified Ion Mobility Format (UIMF)34 file using a conversion tool written in C#, which utilized the MSFileReader from ThermoFisher.35 UIMF format allows easy data visualization as heat maps as well as integration with other bioinformatics data processing tools.
To recover the IMS data from the single multiplexed (SM) data, a previously developed algorithm was utilized.36 In this algorithm, the single multiplexed data are demultiplexed on the basis of the construction of the simplex matrix C, which is a square matrix of size n × n where n is the length of the pseudorandom sequence and the columns of the matrix are the elements of the pseudorandom sequence vector cyclically shifted. Following demultiplexing, a validation and artifact removal step is applied on the basis of the algorithm described previously.36 This two-stage algorithm first applies analytical tests to validate whether a real signal or an artifact exists in the data. The second stage of the algorithm uses a scoring mechanism to find each real signal and preserve it.
To decode the DM data, we developed a new algorithm that applies a two-step process. In the first step, the SM data that were used to construct the DM data are recovered. The second step involves decoding the single multiplexed data similar to the SM mode described above. To recover the SM data, the raw encoded DM data are subjected to a matrix transform. A matrix S of size m × n is generated from the encoding pseudorandom sequence, where n is the length of the pseudorandom sequence and m = 2 × n – 1. Note that S is a block diagonal matrix where each diagonal block contains the reverse pseudorandom sequence. The DM data acquired from the instrument are a matrix, A, of size k × l, where k is the m/z dimension and l is the drift time dimension in scan numbers. Each row of A is aligned by finding the maximum intensity scan number and subsequently shifting the row via modulus. The alignment permits peak correctness validation by shifting the DM data in such a way where the demultiplexed peak(s) will occupy the same scan numbers. For each row, the data are encoded by the pseudorandom sequence and an oversampling number, which determines the number of segments per row vector. The number of segments is obtained by dividing the row count by the length of the oversampling. Each row (vector) of matrix A is then resized into a matrix, R, that has a row count equal to the number of segments and a column count equal to the oversampling. The decoded matrix D is obtained by D = (STS)−1 × ST × R. The result is then resized to the size of the row count of A, and then validation is performed on the demultiplexed row. The output from validation is then shifted back to preserve accurate arrival times. Once the SM data D are recovered, it is then multiplied by an inverse matrix C−1 and treated as described previously.36
RESULTS
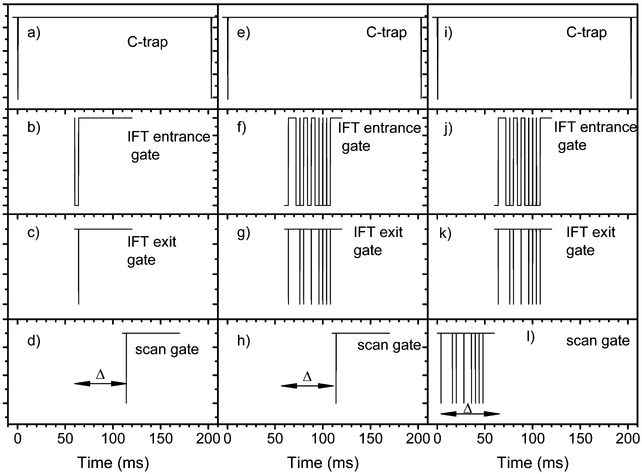
The IMS-Orbitrap MS platform can be operated in continuous as well as IMS modes. In the continuous mode, the IMS is disabled and the instrument is operated as an Orbitrap-only instrument. However, in the IMS mode, the platform can be operated in the signal averaging (SA), single multiplexing (SM), and double multiplexing (DM) IMS modes. The timing sequence for the three modes of operating the IMS-Orbitrap MS platform is shown in Figure 2. In the SA mode of operation (Figure 2a–d), ions are stored in the IFT by controlling the timing of the IFT entrance gate (Figure 2b). Following ion storage, the voltage on the exit grid is lowered for 200–300 μs (Figure 2c) to inject a single ion packet into the drift tube where the ions separate according to their mobilities. A voltage then is applied to one of the scan gate electrodes to either transmit or block ions from passing into the C-trap. The length of the scan gate pulse (200 μs) is the portion of the total IMS separation (e.g., 60 ms), which is sampled by the Orbitrap. By sequentially stepping the delay time between pulses applied to the scan gate and to the IFT exit grid, the entire IMS separation can be sampled. For example, 300 steps will be required to sample a 60 ms IMS separation time using a scan gate sweep rate of 200 μs/Orbitrap scan and a 200 μs wide scan gate pulse. Drift time can be calculated from the number of scan gate steps multiplied by the scan gate sweep rate. Figure 3 shows an example of the IMS separation for singly charged species from the Pierce calibration solution. As the delay time of the scan gate is stepped, the Orbitrap collects different mass spectra corresponding to different drift times. For instance, Figure 3b shows the mass spectrum collected at Orbitrap scan of 92 (drift time = 18.4 ms) that corresponds to the arrival time of 195 m/z. Figure 3c, on the other hand, shows ions of 1321.99 m/z collected at Orbitrap scan 178 (drift time = 35.6 ms). The total experiment time depends on the drift time range, scan gate width, sweep rate, and how fast the Orbitrap can acquire data, which in turn depend on the mass resolving power desired. The example shown in Figure 3 used a setting of 25 000 mass resolving power for the Orbitrap, and 300 scan gate steps were completed in 1 min. At 100 000 mass resolving power, the acquisition time can be as long as 5 min (for the Exactive MS), but future advancements in Orbitrap acquisition speed will hopefully reduce this time (e.g., QExactive MS has faster speed than Exactive). Also, reducing the number of scan gate steps by scanning over the useful IMS range will reduce the total time. For example, as shown in Figure 3, the useful IMS range extends only from 15 to 45 ms, which corresponds to a total acquisition time of 30 s (for 25 000 mass resolving power). Increasing the scan gate width and sweep rate to, for example, 400 μs can further reduce this time to 15 s, which is approaching the utilization time for liquid chromatography. Increasing the scan gate width, however, can reduce the number of points across an IMS peak especially for high mobility ions, which may lead to poorly defined IMS peaks.
Figure 2.
Timing sequence for the IMS-Orbitrap MS in (a–d) SA, (e–h) SM, and (i–l) DM IMS modes of operation. The delay time, Δt, between the scan gate and the IFT exit gate is stepped to collect an ion mobility spectrum.
Figure 3.
(a) The arrival time distribution of 1+ ions from Pierce calibration mixture utilizing SA mode (see text). The scan gate duration was 200 μs, while the scan gate sweep rate was 200 μs/Orbitrap scan. The m/z values of few ions are annotated. (b) Mass spectrum collected at Orbitrap scan number 92. (c) Mass spectrum collected at Orbitrap scan number 178.
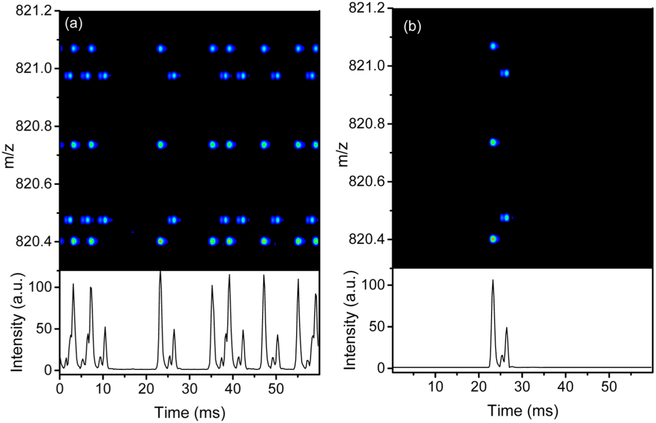
While the speed of the Orbitrap is fixed, the ion utilization efficiency can be improved. One way to maximize ion utilization is to multiplex ion packets’ introduction into the IMS drift tube. In the SM mode, a pseudorandom sequence is applied to the IFT exit gate to improve the IMS duty cycle by sequentially injecting multiple ion packets into the drift tube for 60 ms (the same time as a SA experiment). Ion packets of different m/z may overlap in the drift tube as their mobilities differ, but ion packets of the same mobility do not overlap and stay separated at the end of the drift tube. Utilizing mathematical transformation on the encoded data, the IMS peaks can be decoded with an improved signal-to-noise ratio. In the SM mode, the scan gate transmits ions only once per Orbitrap scan as shown in Figure 2e–h. In SM mode utilizing a 4-bit sequence, eight ion packets can be sampled by the scan gate. An example of the SM experiment is shown in Figure 4a for m/z region 820.3–821.2 from the tryptic digest of a BSA and Enolase mixture. A pseudorandom sequence of 100110101111000 was utilized where a 1 represents an event of ion packet release while 0 represents no ion packet release into the drift tube. Figure 4b show the demultiplexed (decoded) data with the correct IMS separation. In the SM scheme, the signal quality improves as compared to the SA scheme due to the larger number of ion packets being sampled as well as the reduction in random noise. SM also minimizes the IFT overfilling and undesirable space charge peak broadening effect37 by limiting the trap time to 4 ms for the 4-bit multiplexing.
Figure 4.
SM data for a tryptic digest of BSA and Enolase. (a) Multiplexed data for m/z region 820.3–821.2. (b) Demultiplexed data for the same m/z region as (a).
Despite the increased number of ion packets being injected into the drift tube, the scan gate samples each of these packets only once for every Orbitrap scan. Alternatively, the scan gate can be operated more than one time per Orbitrap scan. For instance, the same multiplexing sequence applied to the IFT can be also applied to the scan gate. The third IMS mode is the DM, where the same pseudorandom sequence is applied to the IFT and scan gate (Figure 2i–l). In this case, the scan gate transmits multiple ion packets for every Orbitrap scan. The resulting arrival time distribution then corresponds to the various combinations of multiplexing the IFT and the scan gate. To illustrate this operation, assume for simplicity, a 2-bit multiplexing sequence (represented as 101 sequence) where two ion packets are released from the IFT and injected into the drift tube. In the 101 sequence, the two released ion packets are separated by 0, that is, no ion packet release event. Applying the same sequence of 101 to the scan gate yields the results shown in Figure 5. Depending on the delay time between the IFT exit gate and scan gate, an ion packet can be blocked (0) or transmitted (1) to the Orbitrap. In the first acquisition cycle(i.e., time step 1), the first ion packet (i.e., status of 1) from the IFT arrived when the scan gate is in the second transmission event (i.e., status of 1); therefore, a signal of 1 can be detected in the first scan of the Orbitrap. In the second Orbitrap scan, the scan gate is stepped to time step 2, where the first packet has arrived, while the scan gate is closed followed by the scan gate in an open state (1) but with no ions arriving (0). The net result of time step 2 is no ions can be detected. The third Orbitrap scan corresponds to exact alignment between the two ion packets arriving at the scan gate while in the transmission status, resulting in two ion packets being transmitted to Orbitrap. The result is a packet of 2× ion intensity as compared to that resulting from step 1. The fourth and fifth time steps result in no signal (0) and one packet transmitted (1), respectively. The final chromatogram will have five peaks of intensities of 10201 corresponding to the five scan gate steps. Similarly, in the case of 4-bit multiplexing sequence(100110101111000) applied to the IFT and to the scan gate, the resulting distribution detected in Orbitrap scans will have intensity distributions that scale as 00011122333444844433322111000. The actual data are recovered by performing mathematical transformation on the double multiplexed data obtained from the experiment as discussed in Data Processing. To further illustrate this process, assume the example shown in Figure 5. For simplicity, assume one m/z bin (i.e., arrival time distribution for a single m/z value) and no oversampling. The spectrum (or the encoded data) from the double multiplexing process is shown in Figure 5b, which can be represented as a matrix Ak×l.
| (1) |
where k = 1 (i.e., one m/z bin).
| (2) |
assuming no oversampling.
Figure 5.
An example of the DM operation using a 101 multiplexing sequence at the IFT and scan gate. (a) The five time steps correspond to different combinations of the two ion packets’ arrival time and the voltage profile applied to the scan gate. (b) The resulting chromatogram from the application of the five time steps.
Because the data in Figure 5 are generated from a sequence of 101, then
| (3) |
where m = 2 × 3 − 1 = 5 and n = 3.
| (4) |
which is the single multiplexed data.
| (5) |
where C is the Simplex matrix of size 3 × 3.
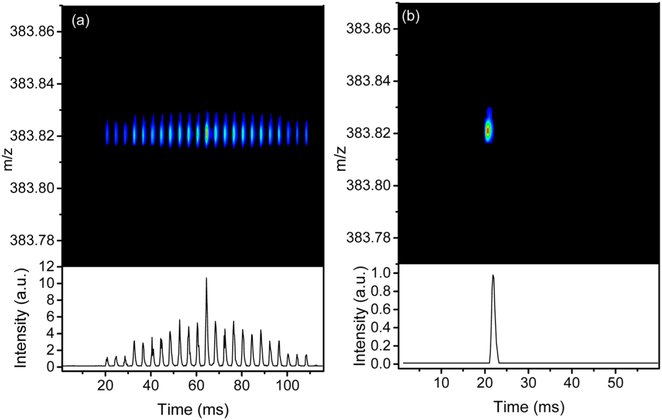
The results of the DM demultiplexing are shown in Figure 6. Figure 6a illustrates the encoded data for a peak from a sample of heavy gas oil, while Figure 6b is the corresponding demultiplexed data. The advantage of the DM mode is the increase in ions’ sampling into the Orbitrap, which can be (for a 4-bit sequence) 8 times higher than the SM mode and 64 times higher than the SA approach. The quality of the data from the DM mode depends on the minimization of demultiplexing artifacts and the reasons that contribute to them. For instance, signal variations due to ion source instability and insufficient ion statistics can lead to signal artifacts, which can affect the quality of the signal from DM data. Also, the ability of the demultiplexing algorithm to confidently distinguish real signal from artifacts is also key to the success of the DM approach. To demonstrate the advantage of the DM mode, we compared the three modes (SA, SM, and DM) for the analysis of a heavy gas oil sample.
Figure 6.
(a) DM encoded data for a selected peak of the heavy gas oil sample. (b) The demultiplexed data after applying the procedure outlined in the Data Processing.
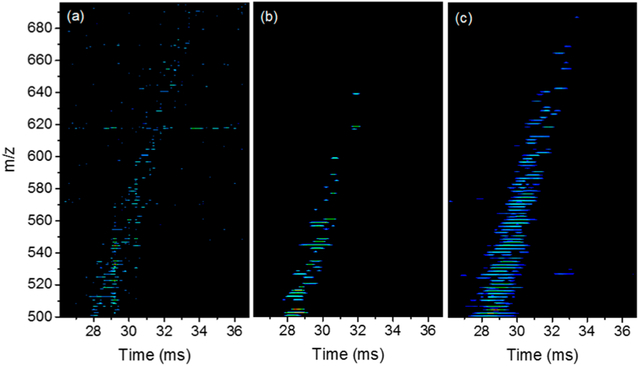
Figure 7 shows the comparison between the three modes for a selected m/z region of low abundance peaks to demonstrate the advantages of multiplexing. The full spectra for the three modes are included in the Figure S1. It is clear that DM has more peaks than SM, which in turn has more peaks than SA due to the increased sampling of ions by DM > SM > SA. Note the noise level in the demultiplexed spectra (SM and DM) is virtually eliminated as compared to the SA mode, which is key to high-quality features in the multiplexing modes. The advantage of the multiplexing is further illustrated in Figures S2 and S3. Figure S2 shows an expanded view of the data displayed in Figure 7, while Figure S3 shows the arrival time distribution for m/z of 558.5. As clearly indicated in Figure S2, the DM results in more peaks in the 2D display as compared to the SM and SA. Meanwhile, the SA has more background noise and fewer jagged peaks. A look into the arrival time distribution (Figure S3) of a low abundance ions of m/z 558.5 reveals that in the SA mode the peak suffers from low ion statistics that caused the peak to split, which is common to many low-abundance peaks in the SA mode. The peak in the SM, however, has better ion statistics but is inferior to the peak produced in the DM mode. Note that the peak intensity in the SM and DM modes is scaled to account for the increased number of packets as compared to the SA mode. The final peak intensity after the proper demultiplexing should be similar to that of the SA mode (see eqs 1–5). However, DM results in better data quality due to the increased sampling of ion packets.
Figure 7.
Selected m/z region for the heavy gas oil sample analyzed in the three IMS modes: (a) SA, (b) SM, and (c) DM.
Finally, to demonstrate the power of the IMS-Orbitrap platform for analyzing complex samples, several complex petroleum samples were analyzed because they have a very large number of constituents with subtle structure and mass differences. Challenges of the analytical characterization of petroleum substances have recently been addressed using mass spectrometers with ultrahigh resolving power such as FT-ICR and Orbitrap.38,39 These platforms allow formula assignments, which are then visualized on multiple graphs to quickly characterize the chemical classes in the samples. IMS-TOF MS has also been utilized for petroleum analysis and characterization as IMS profiles the data into trend lines.5,40,41 However, the TOF MS has lower mass resolving power than FT-based instruments and may not be adequate for correct formula assignments. The maximum mass resolving power of the Orbitrap utilized in this work is 100 000, and we believe its combination with IMS is beneficial for the analysis of complex environmental samples.
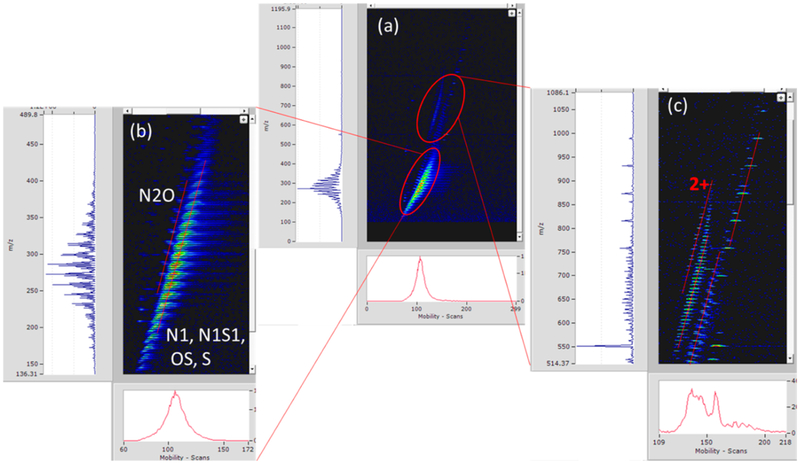
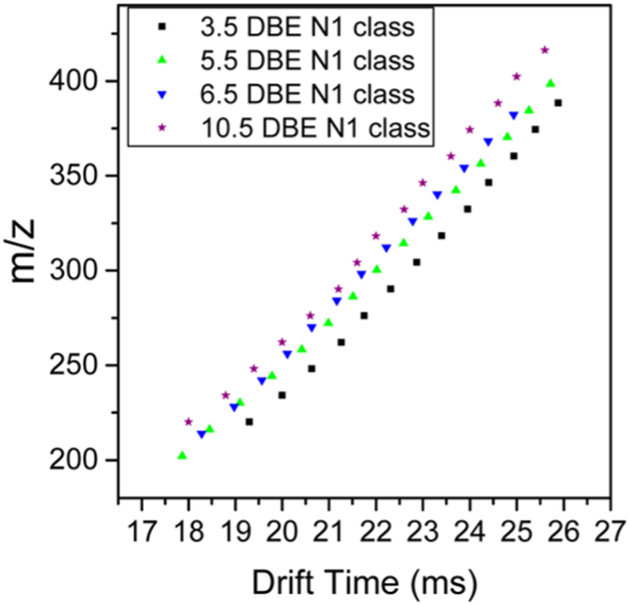
An example of a vacuum and hydrotreated gas oil sample is shown in Figure 8. There are distinct IMS-MS profiles for the different samples analyzed showing the utility of the IMS. Data in Figure 8 can be divided into two regions where each region is characterized by unique diagonal lines, or “trend lines”, on the IMS-MS heat map. These trend lines correspond to a different class of compounds that share core common structures or charge states. Focusing on the Gaussian mass distribution around ~280 m/z shows singly charged species that were identified as N, NS, OS, S, and N2O homologues series for the trend lines shown in Figure 8b. All identifications were made with a mass measurement accuracy of ≤1 ppm. Each of these trend lines consists of homologous series members that differ by CH2, and the trend line corresponds to structural growth of the homologous series for each additional CH2. Notice that the long trend lines contain also shorter subtrend lines with different slopes. Considering only the N series, these subtrend lines correspond to the change in double bond equivalence number (DBE). Figure 9 shows the multiple trend lines for a selected DBE. For ions of similar m/z, every additional double bond results in compactness of the carbon–carbon backbone. So ions of higher DBE are generally more compact and thus have higher mobilities. The trend lines can be used to correctly assign the molecular formulas to peaks by ensuring that these peaks fall into their correct trend lines. IMS also aids in resolving peaks of close m/z values.
Figure 8.
IMS-Orbitrap data for a vacuum and hydrotreated gas oil sample. Homologous series are noted on some of the trend lines.
Figure 9.
Trend lines for selected N1 homologous series members.
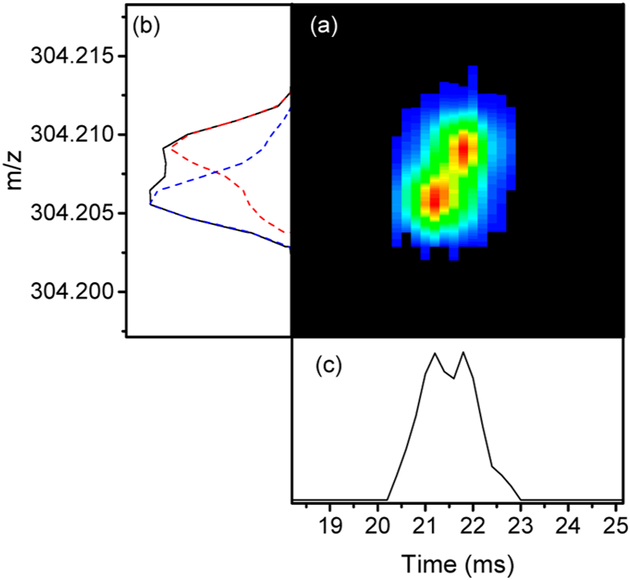
Figure 10 illustrates an example of two peaks that were partially resolved by the 100 000 mass resolving power of Orbitrap and IMS alone. However, both dimensions together are nicely separated as shown by selecting the mass spectra collected at arrival times of 20.8 and 22.4 ms. The two peaks were assigned to m/z 304.20584 and 304.20917 of molecular formula C22H26N and C19H30NS, respectively. The difference between the two peaks is only 3.33 mDa corresponding to the difference between SH4 and C3. This small difference is very common due to the many constituents in petroleum substance indicating the power of the IMS-Orbitrap MS platform for very complex samples.
Figure 10.
(a) 2D IMS-m/z plot of two ions that are partially resolved in (b) the m/z dimension (black solid line) and (c) the mobility dimension. The red and blue dashed lines in (b) represent the m/z peaks selected at drift times of 20.8 and 22.4 ms, respectively.
CONCLUSIONS
In this Article, we report on the development of a drift tube IMS-Orbitrap MS instrument operated in SA, SM, and DM IMS modes to understand the capabilities of each. The SM mode used previous algorithms for analysis, but a new algorithm was required for the DM scheme because it applies a pseudorandom sequence at the two gates before and after IMS separation. This algorithm resulted in minor to no artifacts in the data and the highest signal-to-noise ratio of all modes. In all three modes, the IMS-Orbitrap MS platform was able to acquire a 60 ms IMS separation in as little as 1 min for 25 000 mass resolving power and 5 min for the 100 000 mass resolving power with the duty cycle limited by the acquisition speed of the Orbitrap, desired mass resolution, and the sweep window and rate of the scan gate. Utilizing the useful separation time of IMS along with larger sweep rate, the acquisition time can be reduced to ~15 s, which is amenable to coupling with an LC. The performance of this platform can be further improved, especially for the highest mass resolving power (1 s/acquisition), through performing multiple IMS scans within the same 1 s. For instance, instead of one DM cycle per Orbitrap acquisition presented in this work, 8 DM cycles can be performed simultaneously within 1 s leading to the sampling of 512 ion packets into the Orbitrap (Figure S4). The new IMSOrbitrap MS platform was demonstrated for the analysis of proteomic and petroleum samples, where the integration of IMS and high mass resolution proved essential for accurate assignment of molecular formulas of overlapping peaks in the MS dimension for the complex samples. We believe that higher IMS resolving power from devices such as Structures for Lossless Ion Manipulations (SLIM) will provide even better peak capacity measurements and improved separations.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Drs. Satendra Prasad, Jean-Jacques Dunyach, and Alexander Makarov from Thermo Scientific for their help in the integration with the Exactive Orbitrap MS, and David Stranz from Sierra Analytics, Inc. for allowing us to utilize the Composer software. This research was partially supported by the Laboratory Directed Research and Development Program at Pacific Northwest National Laboratory, and by the U.S. Department of Energy Office of Biological and Environmental Research Genome Sciences Program under the Pan-omics Program, National Institutes of Health (NIH) NIGMS Proteomics Research Resource under grant P41 GM103493, and NIEHS (R01 ES022190). Work was performed at the W. R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a DOE national scientific user facility at the Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for the DOE under contract DEAC05–76RL0 1830.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:10.1021/acs.anal-chem.6b03027.
Figures S1–S4 (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Johnson PV; Beegle LW; Kim HI; Eiceman GA; Kanik I Int. J. Mass Spectrom 2007, 262, 1–15. [Google Scholar]
- (2).Kanu AB; Dwivedi P; Tam M; Matz L; Hill HH Jr. J. Mass Spectrom 2008, 43, 1–22. [DOI] [PubMed] [Google Scholar]
- (3).Karpas Z Food Res. Int 2013, 54, 1146–1151. [Google Scholar]
- (4).Fenn LS; McLean JA Anal. Bioanal. Chem 2008, 391, 905–909. [DOI] [PubMed] [Google Scholar]
- (5).Fernandez-Lima FA; Becker C; McKenna AM; Rodgers RP; Marshall AG; Russell DH Anal. Chem 2009, 81, 9941–9947. [DOI] [PubMed] [Google Scholar]
- (6).McLean JA; Ruotolo BT; Gillig KJ; Russell DH Int. J. Mass Spectrom 2005, 240, 301–315. [Google Scholar]
- (7).Buryakov IA J. Anal. Chem 2011, 66, 674–694. [Google Scholar]
- (8).Mason E; McDaniel E Transport Properites of Ions in Gases; Wiley: New York, 1988. [Google Scholar]
- (9).Michelmann K; Silveira JA; Ridgeway ME; Park MA J. Am. Soc. Mass Spectrom 2015, 26, 14–24. [DOI] [PubMed] [Google Scholar]
- (10).Ewing MA; Conant CRP; Zucker SM; Griffith KJ; Clemmer DE Anal. Chem 2015, 87, 5132–5138. [DOI] [PubMed] [Google Scholar]
- (11).Zucker SM; Ewing MA; Clemmer DE Anal. Chem 2013, 85, 10174–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Rus J; Moro D; Sillero JA; Royuela J; Casado A; Estevez-Molinero F; Fernandez de la Mora J Int. J. Mass Spectrom 2010, 298, 30–40. [Google Scholar]
- (13).Brown LJ; Creaser CS Curr. Anal. Chem 2013, 9, 192–198. [Google Scholar]
- (14).Guevremont RJ Chromatogr. A 2004, 1058, 3–19. [PubMed] [Google Scholar]
- (15).Kolakowski BM; Mester Z Analyst 2007, 132, 842–864. [DOI] [PubMed] [Google Scholar]
- (16).Vidal-de-Miguel G; Macía M; Cuevas J Anal. Chem 2012, 84, 7831–7837. [DOI] [PubMed] [Google Scholar]
- (17).Revercomb HE; Mason EA Anal. Chem 1975, 47, 970–983. [Google Scholar]
- (18).Hoaglund CS; Valentine SJ; Sporleder CR; Reilly JP; Clemmer DE Anal. Chem 1998, 70, 2236–2242. [DOI] [PubMed] [Google Scholar]
- (19).Karasek FW; Denney DW; DeDecker EH Anal. Chem 1974, 46, 970–973. [DOI] [PubMed] [Google Scholar]
- (20).Karasek FW; Kilpatrick WD; Cohen MJ Anal. Chem 1971, 43, 1441–1447. [Google Scholar]
- (21).Karasek FW; Tatone OS Anal. Chem 1972, 44, 1758–1763. [DOI] [PubMed] [Google Scholar]
- (22).Donohoe GC; Arndt JR; Valentine SJ Anal. Chem 2015, 87, 5247–5254. [DOI] [PubMed] [Google Scholar]
- (23).Donohoe GC; Maleki H; Arndt JR; Khakinejad M; Yi J; McBride C; Nurkiewicz TR; Valentine SJ Anal. Chem 2014, 86, 8121–8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sysoev A; Adamov A; Viidanoja J; Ketola RA; Kostiainen R; Kotiaho T Rapid Commun. Mass Spectrom 2004, 18, 3131–3139. [DOI] [PubMed] [Google Scholar]
- (25).Clowers BH; Hill HH Anal. Chem 2005, 77, 5877–5885. [DOI] [PubMed] [Google Scholar]
- (26).Morrison KA; Siems WF; Clowers BH Anal. Chem 2016, 88, 3121–3129. [DOI] [PubMed] [Google Scholar]
- (27).Hill HH; Siems WF; Louis RHS; McMinn DG Anal. Chem 1990, 62, 1201A–1209A. [DOI] [PubMed] [Google Scholar]
- (28).Clowers BH; Siems WF; Hill HH; Massick SM Anal. Chem 2006, 78, 44–51. [DOI] [PubMed] [Google Scholar]
- (29).Szumlas AW; Ray SJ; Hieftje GM Anal. Chem 2006, 78, 4474–4481. [DOI] [PubMed] [Google Scholar]
- (30).Belov ME; Buschbach MA; Prior DC; Tang K; Smith RD Anal. Chem 2007, 79, 2451–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Clowers BH; Belov ME; Prior DC; Danielson WF; Ibrahim Y; Smith RD Anal. Chem 2008, 80, 2464–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ibrahim YM; Baker ES; Danielson Iii WF; Norheim RV; Prior DC; Anderson GA; Belov ME; Smith RD Int. J. Mass Spectrom 2015, 377, 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ibrahim YM; Belov ME; Tolmachev AV; Prior DC; Smith RD Anal. Chem 2007, 79, 7845–7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Shah AR; Davidson J; Monroe ME; Mayampurath AM; Danielson WF; Shi Y; Robinson AC; Clowers BH; Belov ME; Anderson GA; Smith RD J. Am. Soc. Mass Spectrom 2010, 21, 1784–1788. [DOI] [PubMed] [Google Scholar]
- (35).RAW TO UIMF CONVERTER. This software can be requested from the corresponding author.
- (36).Prost SA; Crowell KL; Baker ES; Ibrahim YM; Clowers BH; Monroe ME; Anderson GA; Smith RD J. Am. Soc. Mass Spectrom 2014, 25, 2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Tolmachev AV; Clowers BH; Belov ME; Smith RD Anal. Chem 2009, 81, 4778–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Marshall AG; Rodgers RP Proc. Natl. Acad. Sci. U. S. A 2008, 105, 18090–18095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zhurov KO; Kozhinov AN; Tsybin YO Energy Fuels 2013, 27, 2974–2983. [Google Scholar]
- (40).Santos JM; Galaverna R. d. S.; Pudenzi MA; Schmidt EM; Sanders NL; Kurulugama RT; Mordehai A; Stafford GC; Wisniewski A; Eberlin MN Anal. Methods 2015, 7, 4450–4463. [Google Scholar]
- (41).Ponthus J; Riches E Int. J. Ion Mobility Spectrom 2013, 16, 95–103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.