Abstract
Prevalence of diabetes mellitus (DM), a multifactorial disease often diagnosed with high blood glucose levels, is rapidly increasing in the world. Association of DM with multi-organ dysfunction including cardiomyopathy makes it a leading cause of morbidity and mortality. There are two major types of DM: type 1 DM (T1D) and type 2 DM (T2D). T1D is diagnosed by reduced levels of insulin and high levels of glucose in the blood. It is caused due to pancreatic beta cell destruction/loss, and mostly found in juveniles (juvenile DM). T2D is diagnosed by increased levels of insulin and glucose in the blood. It is caused due to insulin receptor dysfunction, and mostly found in the adults (adult DM). Both T1D and T2D impair cardiac muscle function, which is referred to as diabetic cardiomyopathy. We and others have reported that miRNAs, a novel class of tiny non-coding regulatory RNAs, are differentially expressed in the diabetic heart and they contribute to diabetic cardiomyopathy. Here, we elaborated the biogenesis of miRNA, how miRNA regulates a gene, cardioprotective roles of different miRNAs including miRNAs present in exosomes, underlying molecular mechanisms by which miRNA ameliorates diabetic cardiomyopathy, and the role of miRNA as a potential therapeutic target for juvenile and adult diabetic cardiomyopathy.
Keywords: Diabetic heart, T1D, T2D, miRNA, exosome
1. Introduction
Diabetes mellitus (DM) is a rapidly increasing menace throughout the world (1, 2). It is associated with multi-organ disorders (3, 4), including cardiovascular disease (5, 6). Despite current therapeutic strategies, prevalence of diabetes-mediated cardiomyopathy has markedly increased (7, 8). Considering the increasing trend for prevalence of DM in the world (9), this number is projected to be higher in future. Therefore, a novel therapeutic strategy is warranted to ameliorate diabetic cardiomyopathy. Recent studies revealed that microRNA (miRNA) is a novel class of non-coding RNA, which is endogenously biosynthesized and regulates gene expression (10). Empirical evidences demonstrate that miRNA has potential to mitigate/prevent pathological remodeling in various diseases (11–15), which makes it an attractive therapeutic target for various diseases. As per the Clinicaltrials.gov website, to date there are more than 300 clinical trials on miRNA for different diseases, including anti-miR-122 for Hepatitis C (clinicaltrails.gov # NCT01200420), and miR-34 mimic for primary liver cancer and solid tumors (clinicaltrials.gov #NCT01829971). MiRNA is also emerging as a novel therapeutic target for cardiovascular diseases (16, 17), and diabetic cardiomyopathy (18–20). It is reported that several miRNAs are differentially expressed in the diabetic heart (21–25). Restoring the levels of specific miRNA in the diabetic heart may have therapeutic benefits. For example, miR-133a is downregulated in the diabetic heart (21, 26), and miR-133a mimic treatment to the diabetic heart improves contractility and ameliorates diabetic cardiomyopathy (27). MiRNAs are also present in an exosome, a small vesicle formed by inward folding of cell membrane. Exosomes are secreted from cardiomyocytes (28) and cardiac progenitor cells (29), and play a crucial role in cardiac remodeling (30–33). This chapter embodies the role of miRNA in mitigating diabetic cardiomyopathy in juvenile and adult hearts.
2. Diabetes Mellitus
i). Background
DM is often diagnosed by an elevated blood glucose levels (fasting blood glucose level is higher than 120mg/dL). The normal fasting blood glucose level in humans is 80mg/dL. When the fasting blood glucose level ranges between >80 and <120 mg/dL, it is considered a pre-diabetic condition. In addition to increase in prevalence of DM population (1, 2), the number of pre-diabetic population is also rapidly increasing (34). DM is a complex disease with metabolic disorder and multiple etiology (4, 35). Despite insulin treatment to lower the blood glucose level especially in T1D, and metformin drug treatment to improve insulin sensitivity especially in T2D there are numerous incidence of morbidity and mortality in DM patients, which is corroborated in several clinical trials including Action to Control Cardiovascular Risk in Diabetes (ACCORD), Action in Diabetes and Vascular Disease (ADVANCE), and Veterans’ Administration Diabetes (VADT) (36). Therefore, novel therapeutic strategies are warranted to ameliorate diabetic cardiomyopathy.
ii). Types of Diabetes Mellitus
Based on insulin levels in the blood, DM is categorized into two major types: type 1 DM (T1D) and type 2 DM (T2D). In T1D the pancreatic beta cells, which biosynthesize and secretes insulin, are either less in number or non-functional that results in decreased insulin production and/ or secretion in the blood. Reduced insulin levels in the blood compromise glucose uptake and metabolism that result in elevated blood glucose level. T1D is mostly prevalent in young individual and that is why they are also called juvenile DM. In T2D the pancreatic beta cells are functional and release insulin in the blood but circulating insulin is unable to inter a cell due to impaired insulin receptor function or insulin insensitivity. It results in accumulation of insulin in the blood. Therefore, in T2D both insulin and glucose levels are high in the blood. T2D are mostly prevalent in the adults. Besides T1D and T2D there is a third type of DM, which is present during gestation/pregnancy and it is called gestational DM. This DM is present only during gestation and after delivery of the baby blood glucose level is normalized. Pre-diabetes can be categorized as the fourth type of DM, however, it may progress to either T1D/T2D or revert to normal blood glucose levels (37).
iii). Diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is a disease of heart muscle which leads to heart failure. It is defined as heart failure caused due to DM without any symptoms of hypertension, coronary artery disease, valvular disease, or ischemia (38). DCM is categorized into three types: early stage where the changes are mostly at molecular levels such as altered calcium homeostasis, depleted glucose transporters GLUT1 and GLUT4, middle stage where the changes are also observed at structural levels such as increased size of left ventricle and cardiac fibrosis, and functional levels such as diastolic dysfunction, and late stage where besides molecular and structural changes both diastolic and systolic functions are compromised (39). DM contributes to micro-, and macro-vascular complications (34, 40–42), and increases the chances of heart failure 2–4 folds as compared to age and gender matched non-diabetic individuals (43, 44). The molecular mechanisms underlying DCM and the predictors and prevention of DCM at different stages are elaborated in one of our recent review articles (39).
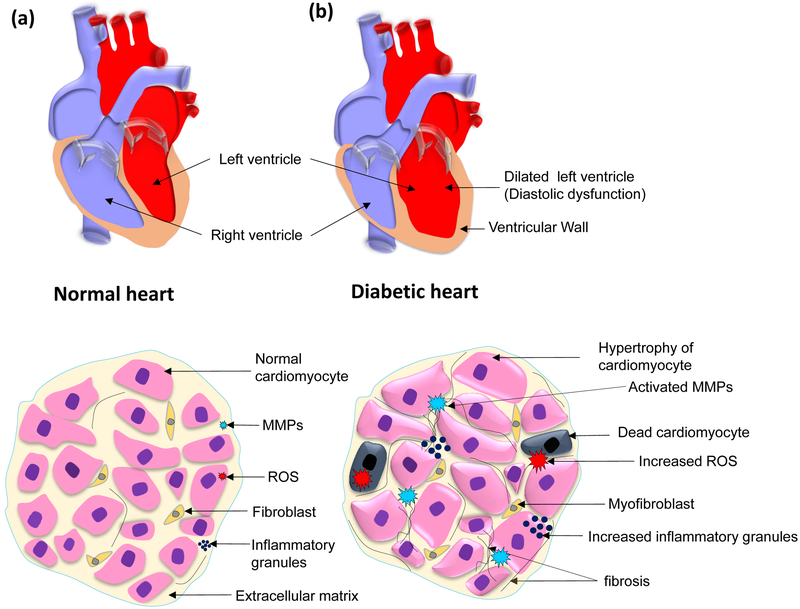
Hyperglycemia leads to several changes in diabetic hearts. There is an increased production of reactive oxygen species (ROS) due to mitochondrial damage (45–47). The ROS contributes to cardiac fibrosis by instigating matrix metalloproteinases (MMPs), especially MMP-2 and −9 which are collagenases that degrade extracellular matrix (48–52). Cardiac fibrosis compromises cardiomyocyte contractility and cellular signaling leading to apoptosis. The remaining cardiomyocytes have more workload for contractility of the heart. As an adaptation, cardiomyocytes increase in size and hypertrophied. Hyperglycemia also increases the accumulation of inflammatory granules. Pro-inflammatory cytokines are increased in the diabetic heart and accumulate near hypertrophic cardiomyocytes (21). Increased fibrosis of extracellular matrix compromises the compliance of the heart that leads to diastolic dysfunction. Further, the left ventricular wall becomes thin and the geometric shape of the heart changes and left ventricle become more round-shape (21, 51) (Figure 1).
Figure 1.
Schematic representation for anatomical and histological features of diabetic cardiomyopathy. Top, schematic diagram showing a) a normal heart and, b) a diabetic heart. The dialated left ventricle is highlighted in the diabetic heart, which is a hallmark for diastolic dysfunction. Bottom, schematic diagram showing distinct histological featues of a a) normal and b) diabetic hearts, such as cardiomyocytes in diabetic heart shows hypertrophic cardiomyocytes, increased cardiomyocyte death, presence of myofibroblasts that respond to inflammation, increased interstitial and perivascular fibrosis, and ROS production, inflammation, and MMP activation.
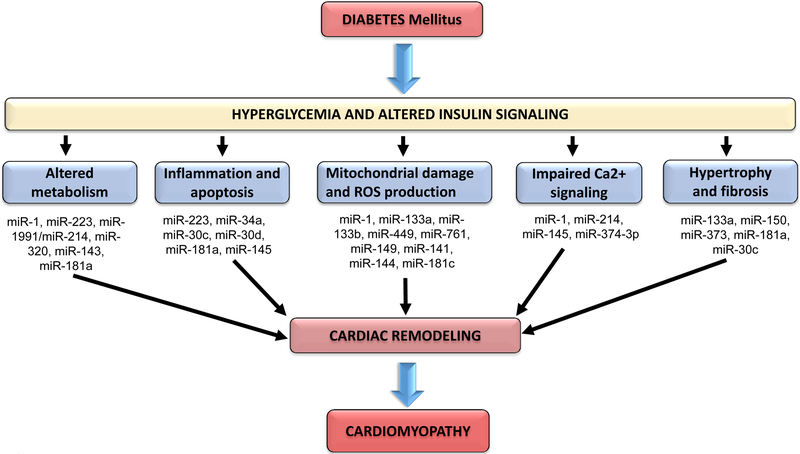
In DM hearts insulin signaling is compromised which causes altered metabolism, inflammation and apoptosis, mitochondrial damage and ROS generation, impaired calcium signaling, and cardiac hypertrophy and fibrosis (39, 41, 53). Several miRNAs are involved in the regulation of these changes in the DM heart, and differential expression of these miRNAs lead to pathological cardiac remodeling and cardiomyopathy (20, 27, 54, 55) (Figure 2).
Figure 2.
Altered cellular pathways in diabetic hearts. MiRNAs associated with regulation of signaling genes in each pathways that lead to cardiac remodeling and diabetic cardiomyopathy.
3. MicroRNA
i). Background
MicroRNAs (miRNAs) are a novel class of non-coding RNA that modulate gene expression either by mRNA degradation or translational repression (10). They are an evolutionary conserved regulatory molecule which control almost all genes in biological processes. After the discovery of the first miRNA Lin-4 in 1993, miRNA field has progressed enormously within a decade and miRNA is emerging as a potential therapeutic target for several diseases (56). There are more than two thousand miRNAs in humans (57), and each miRNA regulate more than one genes. Even one gene may be regulated by several miRNAs, which provides a layer of regulatory network for genes. Several members of the same family of miRNA may regulate a signaling cascade of a biological pathway or even one miRNA may regulate more than one genes in the same biological pathway (58). This complex regulatory network is not completely understood, however, there is consensus that miRNAs are a crucial regulator for a gene and/or several genes in a signaling pathway. In the human heart, there are nearly 18 miRNA families that contribute to nearly 90% of the total miRNAs present in the heart, and several dozens of them are differentially expressed in the failing heart (59). Overexpressing a downregulated miRNA or inhibiting an upregulated miRNA are novel approaches for mitigating cardiac remodeling and improving cardiac function in the diabetic heart.
ii). MiRNA biogenesis and function
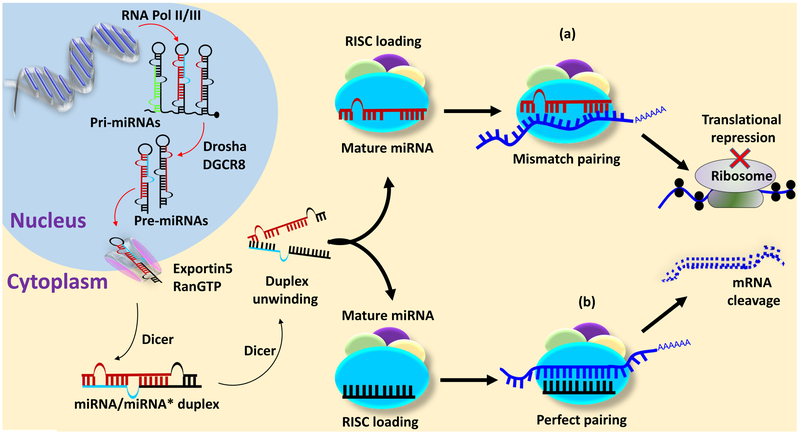
MiRNA is transcribed as a primary miRNA (pri-miRNA) from intronic or intergenic region by RNA polymerase II/III as a single transcript or a polycistronic transcript (16). The pri-miRNA is approximately 200 nucleotides long with a hairpin loop and contains 5´ cap and 3´ poly A tail. The Pri-miRNA is processed by Drosha and DiGeorge syndrome critical region gene 8 (DGRC8) to form a precursor miRNA (pre-miRNA), which is approximately 70 nucleotides long double stranded structure. The pre-miRNA assembles in exportin5 and RanGTP to make a complex, which is exported into cytoplasm. In the cytoplasm pre-miRNA is processed by dicer, a RNase III endonuclease, into a mature miRNA which is immediately loaded into RNA induced silencing complex (RISC). The two strands of pre-miRNA may form one or two mature miRNAs depending on degradation of passenger strand of miRNA (miRNA* or miR-5p) (19, 60) (Figure 3).
Figure 3.
MiRNA biogenesis and functions. Transcription of miRNA in the nucleus from non-coding DNA, their processing in the nucleus into primary miRNA (pri-miRNA) and precursor miRNA (pre-miRNA). Transport of pre-miRNA into cytoplasm and its processing by dicer into a mature miRNA, which is loaded into a RNA induced silencing complex (RISC). Mature miRNA can degrade mRNA when the seed sequence of miRNA perfectly match with the untranslated region of mRNA or inhibits translation of protein when the seed sequence imperfectly match with the untranslated region of mRNA.
Mature miRNA has a seed sequence which is 2–8 nucleotide from the 5´end. The seed sequence of miRNA if binds perfectly to the 3´untranslated region (UTR) of mRNA of a gene then it will degrade the mRNA but if the seed sequence match imperfectly with the 3´UTR of mRNA then it will block the translational machinery and impair protein synthesis (16, 19) (Figure 3).
iii). MiRNAs in cardiac regeneration
MiRNAs are documented to regulate cardiac stem cell differentiation, which is pivotal for cardiac regeneration (61–64). The extracellular matrix stiffness contributes to stem cell self-renewal (65), and survival and differentiation of cardiac stem cell (66, 67). MiR-1 and miR-133a promotes differentiation of embryonic stem cell into cardiac lineage by targeting histone deacetylase-4 and serum response factor, respectively (68). MiR-499 is also involved in regulation of embryonic stem cell into cardiomyocyte lineage (69).
MiRNAs also regulate stem cell homing, differentiation and maturation, which is crucial for cardiac regeneration (70, 71). Several recent reviews elaborate the potential roles of miRNA in cardiac regeneration (31, 72–78).
iv). MiRNAs in exosome improve cardiac functions of diabetic hearts
MiRNAs are released into circulation after encapsulation into a membrane-bound vesicle called exosome (31). These exosomes are also secreted from stem cell (79) and other cell types including cardiomyocytes (29), and plays a pivotal role in cardiac regeneration and regulation of cardiac functions (80–85). In diabetic heart, cardiomyocytes-derived exosomes have elevated levels of miR-320, which is detrimental to the heart (86). Therefore, exosome secretion inhibitor such as GW4869 could be a potential therapeutic strategy to mitigate exosome-mediated cardiac dysfunction in diabetic hearts (86–88).
4. MicroRNA as therapeutic target for diabetic cardiomyopathy
The expression of miRNA changes in the diabetic heart (21). It is not necessary that all miRNAs showing altered expression in diabetic heart may have a crucial role in diabetic cardiomyopathy, however, empirical evidences based on loss-, and gain-of function studies on miRNA revealed several miRNAs that regulate diabetic cardiomyopathy (89). For example, silencing of miR-195 (90) or upregulation of miR-30 (91) mitigates diabetic cardiomyopathy. MiR-141 is upregulated in diabetic hearts and it decreases ATP production by suppressing ATP synthase activity (92). Therefore, suppression of miR-141 can be a potential strategy to improve ATP production in the diabetic heart. MiR-133a is downregulated in the diabetic heart (21, 55, 93), and overexpression of miR-133a by a mimic reduces cardiac hypertrophy (55), fibrosis (94), and cardiac contractility (27). Therefore, targeting a particular miRNA involved in a specific signaling pathway in the diabetic heart may provide a therapeutic effect to ameliorate diabetic cardiomyopathy.
5. Conclusions
The two major types of diabetes, T1D and T2D, may have different pathophysiological adaptations due to different levels of insulin in the blood (95). The miRNA profiling in diabetic hearts revealed several crucial miRNAs that contribute to diabetic cardiomyopathy. These miRNAs can be either present in the heart or are released into an exosome. Empirical evidences demonstrate that modulating the expression of miRNA by either a mimic or an inhibitor has potential to ameliorate diabetic cardiomyopathy. Although these are encouraging results for developing miRNA-based therapeutic strategy for diabetic cardiomyopathy, further investigations are required to understand the regulatory mechanisms by which miRNAs cross-talk to optimize the gene expression in diabetic hearts, considering the fact that one miRNA may have more than one target and one gene can be targeted by more than one miRNA. From therapeutic point of view, it is also important to investigate how miRNA should be delivered and which route of delivery is better. Overall, there is consensus that miRNA is emerging as a therapeutic target for diabetic cardiomyopathy and future studies will determine its clinical application.
Ackowledgement:
This work was supported in part by American Heart Association Postdoctoral fellowship award 16POST30180003 to S.S.N., and the National Institutes of Health grants HL-113281 and HL-116205 to P.K.M.
References:
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–31. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. [DOI] [PubMed] [Google Scholar]
- 3.Shah S, Win Z, Al-Nahhas A. Multiorgan dysfunctions in diabetic patients: the role of functional imaging. Minerva Endocrinol. 2009;34(3):223–36. [PubMed] [Google Scholar]
- 4.Gluhovschi GH, Gluhovschi C, Vlad A, Timar R, Bob F, Velciov S, et al. Diabetic nephropathy and multiorgan protection. Part I. Rom J Intern Med. 2011;49(3):163–77. [PubMed] [Google Scholar]
- 5.Sardu C, Barbieri M, Rizzo MR, Paolisso P, Paolisso G, Marfella R. Cardiac Resynchronization Therapy Outcomes in Type 2 Diabetic Patients: Role of MicroRNA Changes. J Diabetes Res. 2016;2016:7292564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113(Pt A):600–9. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadizar F, Fazeli Farsani S, Souverein PC, van der Vorst MM, de Boer A, Maitland-van der Zee AH. Cardiovascular medication use and cardiovascular disease in children and adolescents with type 1 diabetes: a population-based cohort study. Pediatr Diabetes. 2016;17(6):433–40. [DOI] [PubMed] [Google Scholar]
- 8.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaacks LM, Siegel KR, Gujral UP, Narayan KM. Type 2 diabetes: A 21st century epidemic. Best Pract Res Clin Endocrinol Metab. 2016;30(3):331–43. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 11.Berber P, Grassmann F, Kiel C, Weber BH. An Eye on Age-Related Macular Degeneration: The Role of MicroRNAs in Disease Pathology. Mol Diagn Ther. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abente EJ, Subramanian M, Ramachandran V, Najafi-Shoushtari SH. MicroRNAs in obesity-associated disorders. Arch Biochem Biophys. 2016;589:108–19. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg MW, Moore KJ. MicroRNA Regulation of Atherosclerosis. Circ Res. 2016;118(4):703–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Sarkar FH. MicroRNA Targeted Therapeutic Approach for Pancreatic Cancer. Int J Biol Sci. 2016;12(3):326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson K, Wonnacott A, Fraser DJ, Bowen T. MicroRNAs in Diabetic Nephropathy: From Biomarkers to Therapy. Curr Diab Rep. 2016;16(3):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular diseases. J Cell Mol Med. 2009;13(4):778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Chen Q, Lew KS, Richards AM, Wang P. Discovery of Potential Therapeutic miRNA Targets in Cardiac Ischemia-Reperfusion Injury. J Cardiovasc Pharmacol Ther. 2016;21(3):296–309. [DOI] [PubMed] [Google Scholar]
- 18.Greco S, Fasanaro P, Castelvecchio S, D’Alessandra Y, Arcelli D, Di Donato M, et al. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes. 2012;61(6):1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyagi AC, Sen U, Mishra PK. Synergy of microRNA and stem cell: a novel therapeutic approach for diabetes mellitus and cardiovascular diseases. Curr Diabetes Rev. 2011;7(6):367–76. [DOI] [PubMed] [Google Scholar]
- 20.Caporali A, Miscianinov V, Saif J, Emanueli C. MicroRNA transport in cardiovascular complication of diabetes. Biochim Biophys Acta. 2016. [DOI] [PubMed] [Google Scholar]
- 21.Chavali V, Tyagi SC, Mishra PK. Differential expression of dicer, miRNAs, and inflammatory markers in diabetic Ins2+/− Akita hearts. Cell Biochem Biophys. 2014;68(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costantino S, Paneni F, Luscher TF, Cosentino F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J. 2016;37(6):572–6. [DOI] [PubMed] [Google Scholar]
- 23.Dangwal S, Stratmann B, Bang C, Lorenzen JM, Kumarswamy R, Fiedler J, et al. Impairment of Wound Healing in Patients With Type 2 Diabetes Mellitus Influences Circulating MicroRNA Patterns via Inflammatory Cytokines. Arterioscler Thromb Vasc Biol. 2015;35(6):1480–8. [DOI] [PubMed] [Google Scholar]
- 24.Yildirim SS, Akman D, Catalucci D, Turan B. Relationship between downregulation of miRNAs and increase of oxidative stress in the development of diabetic cardiac dysfunction: junctin as a target protein of miR-1. Cell Biochem Biophys. 2013;67(3):1397–408. [DOI] [PubMed] [Google Scholar]
- 25.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810–7. [DOI] [PubMed] [Google Scholar]
- 26.Nandi SS, Duryee MJ, Shahshahan HR, Thiele GM, Anderson DR, Mishra PK. Induction of autophagy markers is associated with attenuation of miR-133a in diabetic heart failure patients undergoing mechanical unloading. Am J Transl Res. 2015;7(4):683–96. [PMC free article] [PubMed] [Google Scholar]
- 27.Nandi SS, Zheng H, Sharma NM, Shahshahan HR, Patel KP, Mishra PK. Lack of miR-133a Decreases Contractility of Diabetic Hearts: A Role for Novel Cross Talk Between Tyrosine Aminotransferase and Tyrosine Hydroxylase. Diabetes. 2016;65(10):3075–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, et al. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol. 2013;304(7):H954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14(5):1064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol. 2015;24(4):199–206. [DOI] [PubMed] [Google Scholar]
- 31.Prathipati P, Nandi SS, Mishra PK. Stem Cell-Derived Exosomes, Autophagy, Extracellular Matrix Turnover, and miRNAs in Cardiac Regeneration during Stem Cell Therapy. Stem Cell Rev. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldenstrom A, Ronquist G. Role of exosomes in myocardial remodeling. Circ Res. 2014;114(2):315–24. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol. 2015;178:239–46. [DOI] [PubMed] [Google Scholar]
- 34.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karalliedde J, Gnudi L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol Dial Transplant. 2016;31(2):206–13. [DOI] [PubMed] [Google Scholar]
- 36.Bloomgarden ZT. Glycemic control in diabetes: a tale of three studies. Diabetes Care. 2008;31(9):1913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra PK, Singh SR, Joshua IG, Tyagi SC. Stem cells as a therapeutic target for diabetes. Front Biosci (Landmark Ed). 2010;15:461–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602. [DOI] [PubMed] [Google Scholar]
- 39.Chavali V, Tyagi SC, Mishra PK. Predictors and prevention of diabetic cardiomyopathy. Diabetes Metab Syndr Obes. 2013;6:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos C Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae. Postgrad Med. 2012;124(6):90–7. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol (Lausanne). 2012;3:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab. 2007;9(6):767–80. [DOI] [PubMed] [Google Scholar]
- 43.Mathew V, Gersh BJ, Williams BA, Laskey WK, Willerson JT, Tilbury RT, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2004;109(4):476–80. [DOI] [PubMed] [Google Scholar]
- 44.Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33(6):1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ni R, Cao T, Xiong S, Ma J, Fan GC, Lacefield JC, et al. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med. 2016;90:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma SK, Garikipati VN, Kishore R. Mitochondrial dysfunction and its impact on diabetic heart. Biochim Biophys Acta. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, et al. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008;57(11):2924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taye A, Abouzied MM, Mohafez OM. Tempol ameliorates cardiac fibrosis in streptozotocin-induced diabetic rats: role of oxidative stress in diabetic cardiomyopathy. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(12):1071–80. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Mo H, Guo R, You Q, Huang R, Wu K. Inhibition of the leptin-induced activation of the p38 MAPK pathway contributes to the protective effects of naringin against high glucose-induced injury in H9c2 cardiac cells. Int J Mol Med. 2014;33(3):605–12. [DOI] [PubMed] [Google Scholar]
- 50.Mishra PK, Tyagi N, Kundu S, Tyagi SC. MicroRNAs are involved in homocysteine-induced cardiac remodeling. Cell Biochem Biophys. 2009;55(3):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra PK, Tyagi N, Sen U, Joshua IG, Tyagi SC. Synergism in hyperhomocysteinemia and diabetes: role of PPAR gamma and tempol. Cardiovasc Diabetol. 2010;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prathipati P, Metreveli N, Nandi SS, Tyagi SC, Mishra PK. Ablation of Matrix Metalloproteinase-9 Prevents Cardiomyocytes Contractile Dysfunction in Diabetics. Front Physiol. 2016;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raut SK, Singh GB, Rastogi B, Saikia UN, Mittal A, Dogra N, et al. miR-30c and miR-181a synergistically modulate p53-p21 pathway in diabetes induced cardiac hypertrophy. Mol Cell Biochem. 2016;417(1–2):191–203. [DOI] [PubMed] [Google Scholar]
- 54.Beltrami C, Angelini TG, Emanueli C. Noncoding RNAs in diabetes vascular complications. J Mol Cell Cardiol. 2015;89(Pt A):42–50. [DOI] [PubMed] [Google Scholar]
- 55.Feng B, Chen S, George B, Feng Q, Chakrabarti S. miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes Metab Res Rev. 2010;26(1):40–9. [DOI] [PubMed] [Google Scholar]
- 56.Almeida MI, Reis RM, Calin GA. MicroRNA history: discovery, recent applications, and next frontiers. Mutat Res. 2011;717(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- 57.Friedlander MR, Lizano E, Houben AJ, Bezdan D, Banez-Coronel M, Kudla G, et al. Evidence for the biogenesis of more than 1,000 novel human microRNAs. Genome Biol. 2014;15(4):R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashimoto Y, Akiyama Y, Yuasa Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS One. 2013;8(5):e62589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumarswamy R, Thum T. Non-coding RNAs in cardiac remodeling and heart failure. Circ Res. 2013;113(6):676–89. [DOI] [PubMed] [Google Scholar]
- 60.Okamura K, Chung WJ, Lai EC. The long and short of inverted repeat genes in animals: microRNAs, mirtrons and hairpin RNAs. Cell Cycle. 2008;7(18):2840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogorek B, et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123(12):1287–96. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Foshay KM, Gallicano GI. miR-17 family miRNAs are expressed during early mammalian development and regulate stem cell differentiation. Dev Biol. 2009;326(2):431–43. [DOI] [PubMed] [Google Scholar]
- 63.Kuppusamy KT, Sperber H, Ruohola-Baker H. MicroRNA regulation and role in stem cell maintenance, cardiac differentiation and hypertrophy. Curr Mol Med. 2013;13(5):757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sartipy P, Olsson B, Hyllner J, Synnergren J. Regulation of ‘stemness’ and stem cell differentiation by microRNAs. IDrugs. 2009;12(8):492–6. [PubMed] [Google Scholar]
- 65.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mishra PK, Chavali V, Metreveli N, Tyagi SC. Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Can J Physiol Pharmacol. 2012;90(3):353–60. [DOI] [PubMed] [Google Scholar]
- 67.Mishra PK, Givvimani S, Chavali V, Tyagi SC. Cardiac matrix: a clue for future therapy. Biochim Biophys Acta. 2013;1832(12):2271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2(3):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson KD, Hu S, Venkatasubrahmanyam S, Fu JD, Sun N, Abilez OJ, et al. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499. Circ Cardiovasc Genet. 2010;3(5):426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seeger FH, Zeiher AM, Dimmeler S. MicroRNAs in stem cell function and regenerative therapy of the heart. Arterioscler Thromb Vasc Biol. 2013;33(8):1739–46. [DOI] [PubMed] [Google Scholar]
- 71.Bras-Rosario L, Matsuda A, Pinheiro AI, Gardner R, Lopes T, Amaral A, et al. Expression profile of microRNAs regulating proliferation and differentiation in mouse adult cardiac stem cells. PLoS One. 2013;8(5):e63041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12(3):135–42. [DOI] [PubMed] [Google Scholar]
- 73.Crippa S, Cassano M, Sampaolesi M. Role of miRNAs in muscle stem cell biology: proliferation, differentiation and death. Curr Pharm Des. 2012;18(13):1718–29. [DOI] [PubMed] [Google Scholar]
- 74.Hodgkinson CP, Kang MH, Dal-Pra S, Mirotsou M, Dzau VJ. MicroRNAs and Cardiac Regeneration. Circ Res. 2015;116(10):1700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamps JA, Krenning G. Micromanaging cardiac regeneration: Targeted delivery of microRNAs for cardiac repair and regeneration. World J Cardiol. 2016;8(2):163–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lei Z, Sluijter JP, van Mil A. MicroRNA Therapeutics for Cardiac Regeneration. Mini Rev Med Chem. 2015;15(6):441–51. [DOI] [PubMed] [Google Scholar]
- 77.Peng B, Chen Y, Leong KW. MicroRNA delivery for regenerative medicine. Adv Drug Deliv Rev. 2015;88:108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu K, Liu D, Lai H, Li J, Wang C. Developing miRNA therapeutics for cardiac repair in ischemic heart disease. J Thorac Dis. 2016;8(9):E918–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22. [DOI] [PubMed] [Google Scholar]
- 80.Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016;2016:7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J Stem Cells. 2016;8(9):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iaconetti C, Sorrentino S, De Rosa S, Indolfi C. Exosomal miRNAs in Heart Disease. Physiology (Bethesda). 2016;31(1):16–24. [DOI] [PubMed] [Google Scholar]
- 84.Kishore R, Khan M. More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ Res. 2016;118(2):330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang B, Yeo RW, Tan KH, Lim SK. Focus on Extracellular Vesicles: Therapeutic Potential of Stem Cell-Derived Extracellular Vesicles. Int J Mol Sci. 2016;17(2):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sahoo S, Emanueli C. Exosomes in Diabetic Cardiomyopathy: The Next-Generation Therapeutic Targets? Diabetes. 2016;65(10):2829–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, et al. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes. 2016;65(10):3111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, et al. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McClelland AD, Kantharidis P. microRNA in the development of diabetic complications. Clin Sci (Lond). 2014;126(2):95–110. [DOI] [PubMed] [Google Scholar]
- 90.Zheng D, Ma J, Yu Y, Li M, Ni R, Wang G, et al. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia. 2015;58(8):1949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raut SK, Kumar A, Singh GB, Nahar U, Sharma V, Mittal A, et al. miR-30c Mediates Upregulation of Cdc42 and Pak1 in Diabetic Cardiomyopathy. Cardiovasc Ther. 2015;33(3):89–97. [DOI] [PubMed] [Google Scholar]
- 92.Baseler WA, Thapa D, Jagannathan R, Dabkowski ER, Croston TL, Hollander JM. miR-141 as a regulator of the mitochondrial phosphate carrier (Slc25a3) in the type 1 diabetic heart. Am J Physiol Cell Physiol. 2012;303(12):C1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chavali V, Tyagi SC, Mishra PK. MicroRNA-133a regulates DNA methylation in diabetic cardiomyocytes. Biochem Biophys Res Commun. 2012;425(3):668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen S, Puthanveetil P, Feng B, Matkovich SJ, Dorn GW, 2nd, Chakrabarti S. Cardiac miR-133a overexpression prevents early cardiac fibrosis in diabetes. J Cell Mol Med. 2014;18(3):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanamori H, Takemura G, Goto K, Tsujimoto A, Mikami A, Ogino A, et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy. 2015;11(7):1146–60. [DOI] [PMC free article] [PubMed] [Google Scholar]