Abstract
Background.
Progressive physical frailty in older adults is associated with increased risk of falls, disability, institutionalization and mortality. Although associations between diabetes and frailty have been observed, the impact of diabetes on frailty in older Hispanics is largely unexplored. We examine the association of diabetes on the odds of frailty among older Mexican Americans.
Subjects and Methods.
Using data from the Hispanic Established Population for the Epidemiological Study of the Elderly (HEPESE) from 1995 until 2012, frailty was assessed by slow gait, weak hand grip strength, exhaustion, and unexplained weight loss (n=1327).
Results.
Logistic regression showed a large magnitude of effect of diabetes on the odds of frailty (OR 1.47, 95% CI 1.14–1.90). Other contributors to frailty included arthritis, heart attack, and hip fracture. Positive and negative affect had significant and opposing associations. Ordinal logit models assessed the odds of frail compared to non-frail and pre-frail. In these models, diabetes was associated with a 32% increase in the odds of a higher level of frailty.
Conclusions.
Diabetes is a significant contributor to increased frailty in older Mexican Americans. Interventions to reduce frailty rates should focus on mitigating the effects of diabetes and shifting away from negative and towards positive affect.
Keywords: diabetes, frail elderly, Mexican Americans
Introduction
Progressive physical frailty in older adults is associated with increased risk of falls, disability, institutionalization and mortality [1–4]. Frailty is conceptualized as an accumulation of deficits across multiple physical systems which reduce reserve capacity and render older adults vulnerable to acute events and adverse outcomes [1, 2, 4]. Due to the complex interaction of systems involved, frailty states vary over time.
Most frailty research has focused on non-Hispanic White populations. Research on frailty among Hispanics is limited. However, similar to existing reports on non-Hispanic Whites, research has shown a strong relationship between age and frailty among Hispanics [5, 6]. Other factors associated with increased frailty among Hispanics include adverse life events, low social support, acculturation, and neighborhoods with low proportions of Hispanics [7–9]. Importantly, frailty is associated with decreased quality of life and increased risk of mortality, disability, and cognitive decline among Hispanics [6, 10–13].
Diabetes affects nearly 11 million adults age 65 and older in the US, approximately 26% of the population of older adults [14]. Age-adjusted prevalence among non-Hispanic Whites is 7.6%, while the rate among Mexican-origin Hispanics is nearly double at 13.9%[14]. Diabetes is associated with numerous health conditions including hypertension, hyperlipidemia, heart disease and stroke, retinopathy, kidney disease, peripheral vascular disease and amputation, as well as premature mortality. These complications are particularly problematic among populations such as Hispanics for whom low socioeconomic attainment may lead to poor diet, limited exercise, and lack of preventive health care[15].
Research examining the association of diabetes with frailty in older populations has been sparse, especially with long-term follow up. Using data from the Cardiovascular Health Study (CHS) which included non-Hispanic Whites and Blacks in the US, Walston and colleagues found that a third of frail subjects were diabetic, nearly twice the rate of non-frail subjects [16]. In addition, elevated levels of fasting glucose and insulin were associated with increased odds of being frail compared to not frail [16]. Similarly, researchers in Spain found that elevated glycated hemoglobin (HbA1c) was associated with an increased odds of frailty (OR 1.48, 95% CI 1.20–1.81 per 1% increase) [17]. Researchers in Mexico found substantially higher rates of self-reported diabetes among frail subjects (23.7%) compared to non-frail subjects (9.9%)[18], while others found that diabetes was associated with a more than two-fold increase in the odds of a high frailty index score [19]. Prior studies of Hispanics in the US have also found associations between diabetes and frailty, but have been limited in their scope and methodology (e.g., limited follow-up time, no measure of frailty over time).
In order to examine the relationship between diabetes and frailty among US Hispanics, we examined data from the Hispanic Established Population for the Epidemiological Study of the Elderly (HEPESE). We hypothesized that diabetes would be associated with increased odds of frailty over time.
Materials and Methods
Sample
We used data from the Hispanic Established Populations for the Epidemiologic Study of the Elderly (H-EPESE), an on-going population-based study of 3,050 non-institutionalized Mexican Americans aged 65 and older at baseline (1993–94) from five Southwestern US states (Texas, California, New Mexico, Colorado, and Arizona). Eight waves of data have been collected (1993–94 n = 3,050; 1995–96 n = 2,438; 1998–99 n = 1,981; 2000–01 n = 1,682; 2004–05 n = 1,167; 2006–07 n = 921; 2010–11 n = 659; 2012–13 n=444). Respondents were interviewed in Spanish or English. Details regarding the methods have been described elsewhere [20]. Our study sample includes respondents beginning in Wave 2 (here-after referred to as baseline), the first wave where an important component of our frailty measure (weight loss) could be assessed. Subjects were then followed over the 6 subsequent waves of data collection resulting in nearly 18 years of follow-up time. Respondents were included if they were able to complete the frailty assessment and had complete data for included variables at baseline (N=1327). All research protocols and informed consents were approved by the University’s Institutional Review Board.
Dependent variable
Frailty status was determined based on a modification of the frailty phenotype described by Fried and colleagues [1]. The original phenotype measures included weight loss, exhaustion, slow gait, low grip strength, and low physical activity. An assessment of physical activity was not available in all waves and, thus, was excluded from our frailty measure. The 4-item measure has been used in previous studies and shown to have similar properties to the 5-item measure [5, 9, 13, 21–23]. Weight loss was defined as a 10 pound difference in weight from prior observation. Exhaustion was assessed using responses to 2 items on the Center for Epidemiological Studies Depression (CES-D) scale: “I felt everything was an effort” and “I could not get going”. Responses were scored as 0 for less than daily or 1–2 days and scored 1 for feelings occurring 3 or more days. Exhaustion was operationally defined as a positive response to either of the questions.
Gait speed was assessed using a timed performance of an 8 foot walk test. Subjects who were unable to perform the task or were in the bottom 20% adjusted for height and sex were coded as 1 (slow gait). Grip strength was assessed with a hand-held dynamometer. Subjects unable to perform the test or whose grip strength was in the bottom 20% (adjusted for weight and sex) were coded “weak grip strength”. Each of the dichotomous indicators of the frailty phenotype were then aggregated and categorized to form a frailty rating (range 0–4). Because participants could refuse to perform assessment activities, we allowed for the range to still be reported if a single item was skipped. Thus, our aggregate may underestimate the presence of frailty based on the measure developed by Fried and colleagues [1].
Covariates
Health Conditions.
We included major health conditions associated with functional decline [24, 25]. Health conditions were ascertained through self-report. Respondents were asked if they had “ever been told by a doctor that you had…” hypertension, diabetes, arthritis, cancer, heart attack, stroke, or hip fracture. Type of diabetes (insulin and non-insulin compared to not diabetic) and duration of diabetes in years (0–9, 10–19 and 20 or more compared to not diabetic) were also included. Pain was assessed with the question “In the past month, have you experienced pain or discomfort when you stood or walked?” Answers were coded as yes or no at each wave. Cognitive impairment was defined as a score less than 21 on the Mini-Mental State Examination (MMSE)[13, 24, 26–28]. Positive and negative affect was assessed summing the responses to subsets of questions from the CESD (4 items range 0–12 and 5 items range 0–15 respectively; see appendix Table i for details).
Other Covariates.
Other variables included age, sex (female), low education (≤5 years based on the sample mean), US born, over-weight or obese (25 ≤ BMI < 30 and BMI ≥ 30 respectively; BMI<25 as control), current smoker, and currently married. Financial strain was assessed as “yes” if the respondent expressed either (1) difficulty paying bills or (2) not having enough money left at the end of the month.
Statistical analysis
Demographic characteristics were examined for the selected study subjects and comparisons by diabetes status were made. We then assessed the odds of frailty (having 2 or more frailty measures) during follow-up using logistic regression accounting for repeated measurements of individuals. This analysis was chosen rather than a time to event approach because frailty is not an absorbing state but is rather fluid.
In addition, an ordered logit model was used to assess the odds of being frail compared to non-frail or pre-frail. The Brant test was used to determine if any covariates violated the proportional odds assumption. Low education (<5 years) and positive affect were both shown to violate the assumption, thus, a generalized ordered logit model was used. This generalized approach employs a partial proportional odds model that allows some of the coefficients to be the same for all values of the outcome, while other coefficients can differ across levels of the outcome. Possible collinearity between covariates was examined by the Variance Inflation Factor which showed all VIF values to be below 2.0, well below the threshold of concern (10.0). All analyses were performed using Stata 14mp (Stata Corp, College Station TX).
Results
The characteristics of the diabetics and non-diabetics are described in Table 1. The groups of respondents were similar in proportion female, US born, education, and marital status. Diabetics had higher rates of self-reported hypertension and arthritis, and were more likely to be obese. Non-diabetics were significantly older (73 compared to 71 years, p≤0.005).
Table 1:
Characteristics of sample by diabetes status, HEPESE Wave 2
| Non-diabetic | Diabetic | ||
|---|---|---|---|
| N | 1026 | 301 | |
| Age | 72.67 (5.51) | 71.38 (4.57) | * |
| Female | 59.84 | 60.47 | |
| US Born | 56.73 | 59.14 | |
| School ≤ 5 | 60.43 | 61.46 | |
| Overweight | 36.45 | 33.22 | |
| Obese | 27.19 | 39.87 | *** |
| Married | 57.02 | 58.14 | |
| Hypertension | 39.57 | 57.48 | *** |
| Arthritis | 42.4 | 53.16 | *** |
| Frail count† | 0.53 (0.71) | 0.69 (0.85) | ** |
| DM Medications | |||
| Any | 88.7 | ||
| Pills | 73.78 | ||
| Injections | 23.78 | ||
| DM Duration | 11.98 (9.69) |
p<0.05
p<0.01
p<0.001
count of positive frailty measures
DM - Diabetes; HEPESE - Hispanic Established Population for the Epidemiologic Study of the Elderly
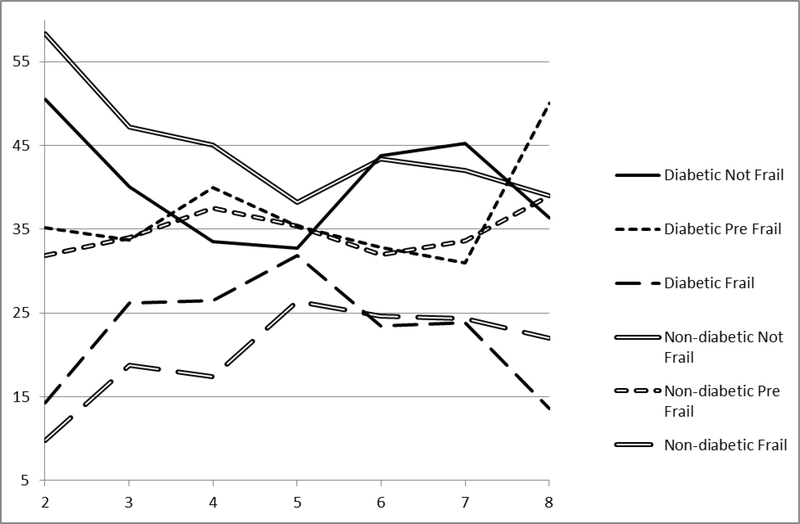
Figure 1 displays the proportion of frail states by diabetes status across waves. A higher proportion of non-diabetics were not frail at each assessment until wave 6 beyond which the proportions were similar between groups. Also, fewer non-diabetics were frail at each assessment until Wave 6, at which the rates were similar. The proportions of pre-frail (having a single frailty measure) were similar between diabetics and non-diabetic across follow-up until the last assessment.
Figure 1.
Proportion of frail states by diabetes status (solid and dash lines), HEPESE wave 2 to wave 8.
The results of logistic regression models assessing the impact of covariates on the odds of frailty are presented in Table 2. Diabetes was associated with a nearly 50% increase in the odds of frailty over time. Diabetics using insulin had two-fold increase in the odds of frailty compared to non-diabetics. Diabetics regardless of duration of disease had nearly 50% higher odds of frailty. Low education (<5 years) was also associated with a nearly 50% increase in the odds of frailty as was cognitive impairment (MMSE<21). Arthritis and heart attack were both associated with increased odds of frailty (43% and 58% respectively), and hip fracture was associated with a three-fold increase in odds of frailty over time. Overweight and obese were both associated with reduced odds of frailty. Positive affect was associated with a 6% reduction in the odds of frailty while negative affect was associated with a 17 % increase in odds of frailty.
Table 2.
Logistic regression predicting odds of being frail (2 or more frailty measures, none missing) from the HEPESE wave 2 to wave 8 (n = 1327)
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
|---|---|---|---|---|---|---|
| Time (years) | 1.07 | (1.05 – 1.09) | 1.07 | (1.05 – 1.09) | 1.07 | (1.05 – 1.09) |
| 70–74 | 1.18 | (0.93 – 1.50) | 1.18 | (0.93 – 1.50) | 1.18 | (0.93 – 1.50) |
| 75–80 | 2.01 | (1.51 – 2.67) | 2.00 | (1.50 – 2.65) | 2.00 | (1.51 – 2.66) |
| 80+ | 2.78 | (1.90 – 4.05) | 2.81 | (1.92 – 4.10) | 2.78 | (1.90 – 4.06) |
| Female | 0.81 | (0.65 – 1.01) | 0.81 | (0.65 – 1.02) | 0.81 | (0.65 – 1.02) |
| School ≤ 5 | 1.49 | (1.20 – 1.85) | 1.49 | (1.20 – 1.85) | 1.50 | (1.21 – 1.87) |
| Overweight | 0.63 | (0.50 – 0.78) | 0.62 | (0.50 – 0.78) | 0.63 | (0.50 – 0.78) |
| Obese | 0.71 | (0.56 – 0.92) | 0.71 | (0.55 – 0.91) | 0.72 | (0.56 – 0.92) |
| Arthritis | 1.43 | (1.16 – 1.76) | 1.43 | (1.16 – 1.76) | 1.42 | (1.16 – 1.75) |
| Hypertension | 1.12 | (0.91 – 1.37) | 1.10 | (0.90 – 1.36) | 1.12 | (0.91 – 1.38) |
| MMSE < 22 | 1.52 | (1.25 – 1.86) | 1.52 | (1.24 – 1.85) | 1.52 | (1.25 – 1.85) |
| Heart attack | 1.58 | (1.15 – 2.17) | 1.57 | (1.14 – 2.16) | 1.58 | (1.15 – 2.17) |
| Stroke | 1.25 | (0.83 – 1.90) | 1.25 | (0.82 – 1.89) | 1.25 | (0.83 – 1.90) |
| Hip | 3.06 | (1.66 – 5.62) | 3.05 | (1.66 – 5.62) | 3.05 | (1.66 – 5.60) |
| Married | 1.13 | (0.92 – 1.40) | 1.14 | (0.92 – 1.40) | 1.14 | (0.92 – 1.40) |
| Positive Affect | 0.94 | (0.92 – 0.96) | 0.94 | (0.92 – 0.96) | 0.94 | (0.92 – 0.96) |
| Negative Affect | 1.17 | (1.14 – 1.21) | 1.17 | (1.14 – 1.21) | 1.17 | (1.14 – 1.21) |
| Diabetes | 1.47 | (1.14 – 1.90) | ||||
| DM status (non-DM ref): | ||||||
| Non-insulin | 1.35 | (1.03 – 1.77) | ||||
| Insulin | 2.03 | (1.17 – 3.50) | ||||
| DM duration (non-DM ref): | ||||||
| 1–9 | 1.54 | (1.07 – 2.21) | ||||
| 10+ | 1.48 | (1.05 – 2.08) | ||||
Abbreviations: DM - Diabetes Mellitus; HEPESE - Hispanic Population for the Epidemiologic Study of the Elderly; MMSE - Mini Mental State Examination
Table 3 presents the results of the generalized ordered logit model (full model). The odds ratios represent the odds of being in a higher category compared to a lower category. That is, the column labeled “not frail” displays the odds ratios of being either pre-frail or frail compared to the not frail category. Similar to the logit model, diabetes was associated with increased odds of both pre-frail and frail compared to non-frail. Arthritis, heart attack and hip fracture were also associated with increased odds of pre-frail and frail compared to non-frail, as was cognitive impairment and negative affect. The effects of low education varied across levels of frailty: comparing non-frail to pre-frail or frail there is no effect of education; however, comparing non-frail and pre-frail to frail, low education was associated with a 41% increased odds of being frail. Positive affect also varied across levels of frailty but was associated with reduced odds of increased levels of frailty.
Table 3.
Generalized ordered logit predicting odds of frail level, HEPESE wave 2 to wave 8 (n = 1327)
| Non-frail | Pre-frail | |||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| Time (years) | 1.04 | (1.03 – 1.05) | 1.04 | (1.03 – 1.05) |
| 70–74 | 1.14 | (0.99 – 1.32) | 1.14 | (0.99 – 1.32) |
| 75–80 | 1.62 | (1.35 – 1.96) | 1.62 | (1.35 – 1.96) |
| 80+ | 2.08 | (1.59 – 2.71) | 2.08 | (1.59 – 2.71) |
| Female | 0.91 | (0.79 – 1.05) | 0.91 | (0.79 – 1.05) |
| School ≤ 5 | 1.09 | (0.95 – 1.25) | 1.41 | (1.18 – 1.68) |
| Overweight | 0.74 | (0.64 – 0.85) | 0.74 | (0.64 – 0.85) |
| Obese | 0.76 | (0.65 – 0.89) | 0.76 | (0.65 – 0.89) |
| Diabetes | 1.32 | (1.12 – 1.55) | 1.32 | (1.12 – 1.55) |
| Arthritis | 1.28 | (1.12 – 1.46) | 1.28 | (1.12 – 1.46) |
| Hypertension | 1.09 | (0.96 – 1.25) | 1.09 | (0.96 – 1.25) |
| MMSE < 22 | 1.37 | (1.20 – 1.57) | 1.37 | (1.20 – 1.57) |
| Heart attack | 1.43 | (1.11 – 1.84) | 1.43 | (1.11 – 1.84) |
| Stroke | 1.34 | (1.00 – 1.78) | 1.34 | (1.00 – 1.78) |
| Hip | 2.83 | (1.73 – 4.61) | 2.83 | (1.73 – 4.61) |
| Married | 1.11 | (0.97 – 1.27) | 1.11 | (0.97 – 1.27) |
| Positive Affect | 0.97 | (0.96 – 0.99) | 0.94 | (0.93 – 0.96) |
| Negative Affect | 1.14 | (1.12 – 1.17) | 1.14 | (1.12 – 1.17) |
Note: The non-frail column represents the odds of being pre-frail or frail compared to non-frail. The pre-frail column represents the odds of being frail compared to non-frail or pre-frail. Where the proportional odds assumption is met the odds are the same in both columns. Where the proportional odds assumption is violated, the odds differ (e.g., School<5).
Discussion
In order to assess the odds of frailty associated with diabetes, we analyzed longitudinal data from the HEPESE with approximately 18 years of follow-up. We observed a strong and consistent increase in the odds of frailty associated with diabetes controlling for numerous demographic, health and social factors. These results were also largely consistent when comparing non-frail to pre-frail and frail, and comparing no-frail and pre-frail to frail.
Other studies have shown that diabetic adults age 65 and older are more likely to be frail compared to non-diabetic peers, and frail diabetics have higher rates of mortality than the non-frail [6, 29, 30]. This association may be partially explained by glucose dysregulation and changes in micro-vascularization leading to impaired muscle function [31]. The resulting weakness manifests as reduced grip strength and slower gait among older diabetics compared to non-diabetics. Similarly, arthritis is associated with increased risk of mortality [32], as well as increased risk of functional decline [33] and frailty [6] through joint damage, weakness and fatigue associated with inflammatory processes.
The association of longer duration of diabetes and increased odds of frailty was not surprising. While not examining frailty directly, other studies have found associations with duration of diabetes and potential contributors to frailty. Young and colleagues found that the presence of neuropathy increased from 21% of diabetics with disease duration of less than 5 years to nearly 37% of diabetics with duration of 10 years or more [34]. In their small study of diabetics and matched controls, Hatef and colleagues found that diabetics with duration of less than 10 year and those with greater than 10 year duration both had decreased knee flexor and extensor strength compared to non-diabetic controls [35]. Longer duration coincides with decreased glycemic control, contributing to changes in micro- vascularization and impaired function [31].
Most prior studies of the association of diabetes and frailty have focused on the presence or absence of the disease or insulin resistance. We were able to extend this prior work by also examining both non-insulin and insulin dependent diabetics. Insulin is usually started when the patient is no longer achieving desired levels of glycemic control with oral medications, thus an indicator of disease progression or severity. It is possible that prolonged dysregulation leads to increased advanced glycation end products that have been linked to peripheral neuropathy [36], slow walking speed [37], and weak grip strength [38]. The increased odds of being frail associated with insulin dependence in our results (twice the odds of non-diabetics) further supports the idea of glycemic dysregulation playing a role in becoming frail.
The association of arthritis with increased frailty is consistent with other reports [39–41]. Persistent pain when standing or walking provides negative feedback encouraging sedentary behavior. Such immobility further exacerbates muscle loss and weakness in older adults resulting in more rapid onset of frailty symptoms. Arthritis co-occurs among an estimated 50% of diabetics, and inactivity in diabetics with arthritis is higher than those with diabetes alone (30% and 21% respectively) [42]. It is likely that this additional barrier compounds the metabolic effects of diabetes resulting in increased frailty.
The association of frailty and cognitive impairment in our data was expected. Links between cognitive decline and physical frailty have been described in other research [5, 13, 26, 43]. Potential explanations for this association include Alzheimer’s disease related plaque development, chronic inflammatory disease, nutritional imbalance and cardiovascular disease. It is plausible that cognitive decline and physical frailty share a common underlying pathology [44]. Two common models of frailty, the phenotype proposed by Fried et al [1] and the index proposed by Rockwood et al [4], diverge on the inclusion of cognition. While others propose a separate measure of cognitive frailty [45]. Indeed, there is considerable disagreement whether or not to include cognitive impairment in measures of frailty leading to difficulty in cross-study evaluations [46].
While diabetes, arthritis, and cognitive impairment can independently lead to reduced physical activity and subsequently increased risk of frailty, it is important to note that increases in physical exercise may diminish those effects [47, 48]. Such findings underscore the importance of maintaining or increasing strength and mobility in older adults as well as support for community programs aimed at increasing levels of physical exercise. This may be of particular importance for older Mexican origin adults who report high rates of diabetes and low rates of physical activity [49].
The opposing influences of positive and negative affect also warrant noting. While the effects were modest (6% reduction and 17% increase in the odds of frailty respectively), the impact of shifting away from negative toward positive could be substantial. In their recent meta-analysis, Boumparis and colleagues found moderate effects of interventions aimed at enhancing positive and reducing negative affect [50]. In small studies, positive affect interventions have showed promise in promoting physical activity following coronary procedures among non-Hispanic Whites, non-Hispanic Blacks and Hispanics [51] as well as improved medication adherence in hypertensive African Americans [52]. While no work has focused specifically on Mexican Americans and frailty, such interventions could lead to reductions in frailty through increased motivation to remain active and/or increase activity levels, seek appropriate preventive health care, and encourage other health promoting behaviors.
While some previous studies have found significant association of gender (female) with frailty in the general population and to some extent among Hispanics [1, 13], other work using group based models found little or no effect among older Mexican Americans [9]. The lack of evidence for an effect of nativity, marital status or financial strain among our sample of Mexican Americans also echoes prior work [9]. In regards to health conditions, it is possible that the lack of effect of hypertension and stroke in our analyses was due to severe events resulting in early mortality rather than frailty.
Our study has several important limitations. Our study sample was drawn from a representative sample of Mexican origin older adults from 5 southwestern US states. Thus, our findings cannot be compared to the broader US population or Hispanic groups. Also, we used a modified measure of frailty that did not incorporate physical activity. Although our findings are not directly comparable to those where the complete phenotype is used [1], the modified measure has been widely used and shown to have similar properties to the 5-item measure [5, 9, 13, 21–23]. Those excluded from the study had higher rates of cancer, hip fracture, heart attack and stroke which may have resulted in an underestimation of frailty.
Conclusion.
A large magnitude of effect of diabetes on odds of frailty over time was evident in our sample of older Mexican Americans. This effect persisted despite controlling for demographics, and health and social factors. Programs aimed at decreasing the effects of diabetes, arthritis and combating negative affect in older adults may have potential to slow the frailty process.
Acknowledgments
Funding: This work was supported in part by the National Institute on Aging (R01 MD01035501; AG10939)
Sponsor’s Role: The sponsorship by the National Institute on Aging had no active role in the design, methods, subject recruitment, data collections, analysis or preparation of the manuscript.
Abbreviations:
- HEPESE
Hispanic Established Population for the Epidemiological Study of the Elderly
Appendix Table i.
CESD subscale items for Positive and Negative Affect
| Positive Affect: |
| I felt that I was just as good as other people |
| I felt hopeful about the future |
| I was happy |
| I enjoyed life |
| Negative Affect: |
| I felt that I could not shake off the blues even with help from my family and friends |
| I felt depressed |
| I felt lonely |
| I had crying spells |
| I felt sad |
CESD - Center for Epidemiologic Studies Depression Scale
Footnotes
Conflict of Interest: The authors have no conflict of interest to report.
References cited
- 1.Fried LP, et al. , Frailty in older adults evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2001. 56(3): p. M146–M157. [DOI] [PubMed] [Google Scholar]
- 2.Bergman H, et al. , Frailty: an emerging research and clinical paradigm—issues and controversies. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2007. 62(7): p. 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitnitski A, et al. , Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. Journal of the American Geriatrics Society, 2005. 53(12): p. 2184–2189. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Hogan DB, and MacKnight C, Conceptualisation and measurement of frailty in elderly people. Drugs & Aging, 2000. 17(4): p. 295–302. [DOI] [PubMed] [Google Scholar]
- 5.Ottenbacher KJ, et al. , Frailty in Older Mexican Americans. Journal of the American Geriatrics Society, 2005. 53(9): p. 1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ottenbacher KJ, et al. , Becoming Frail: Findings from the Hispanic Established Populations Epidemiologic Study of the Elderly. American Journal of Public Health, 2009. 99(4): p. 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aranda MP, et al. , The Protective Effect of Neighborhood Composition on Increasing Frailty Among Older Mexican Americans A Barrio Advantage? Journal of Aging and Health, 2011. 23(7): p. 1189–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masel MC, Howrey B, and Peek MK, The effect of acculturation on frailty among older Mexican Americans. Journal of Aging and Health, 2011. 23(4): p. 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peek MK, et al. , Social Support, Stressors, and Frailty Among Older Mexican American Adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 2012. 67(6): p. 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masel MC, Ostir GV, and Ottenbacher KJ, Frailty, Mortality, and Health Related Quality of Life in Older Mexican Americans. Journal of the American Geriatrics Society, 2010. 58(11): p. 2149–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham JE, et al. , Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology, 2009. 55(6): p. 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Snih S, et al. , Frailty and incidence of activities of daily living disability among older Mexican Americans. Journal of Rehabilitation medicine, 2009. 41(11): p. 892–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raji MA, et al. , Cognitive status and future risk of frailty in older Mexican Americans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2010. 65(11): p. 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention, National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014, 2014, US Department of Health and Human Services: Atlanta, GA. [Google Scholar]
- 15.Escarce JJ and Kapur K, Access to and Quality of Health Care, in Hispanics and the Future of America, Tienda M and Mitchell F, Editors. 2006, National Academies Press: Washington DC. [PubMed] [Google Scholar]
- 16.Walston J, et al. , Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the cardiovascular health study. Archives of Internal Medicine, 2002. 162(20): p. 2333–2341. [DOI] [PubMed] [Google Scholar]
- 17.García-Esquinas E, et al. , Diabetes and Risk of Frailty and Its Potential Mechanisms: A Prospective Cohort Study of Older Adults. Journal of the American Medical Directors Association, 2015. 16(9): p. 748–754. [DOI] [PubMed] [Google Scholar]
- 18.Aguilar-Navarro SG, et al. , Frailty among Mexican community-dwelling elderly: a story told 11 years later. The Mexican Health and Aging Study. Salud Pública de México, 2015. 57: p. s62–s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castrejón-Pérez RC, et al. , Frailty, diabetes, and the convergence of chronic disease in an age-related condition: a population-based nationwide cross-sectional analysis of the Mexican nutrition and health survey. Aging Clin Exp Res, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Markides KS, et al. , Health Status of Hispanic Elderly, in Racial and Ethnic Differences in the Health of Older Americans, Martin L and Soldo B, Editors. 1997, National Academies Press: Washington DC: p. 285–300. [PubMed] [Google Scholar]
- 21.Cano C, et al. , Frailty and cognitive impairment as predictors of mortality in older Mexican Americans. The Journal of Nutrition, Health & Aging, 2012. 16(2): p. 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez‐García S, et al. , Frailty among community dwelling elderly Mexican people: Prevalence and association with sociodemographic characteristics, health state and the use of health services. Geriatrics & Gerontology International, 2014. 14(2): p. 395–402. [DOI] [PubMed] [Google Scholar]
- 23.Akin S, et al. , The prevalence of frailty and related factors in community-dwelling Turkish elderly according to modified Fried Frailty Index and FRAIL scales. Aging Clinical and Experimental Research, 2015. 27(5): p. 703–9. [DOI] [PubMed] [Google Scholar]
- 24.Raji MA, et al. , Cognitive status and incident disability in older Mexican Americans: findings from the Hispanic established population for the epidemiological study of the elderly. Ethnicity and Disease, 2004. 14(1): p. 26–31. [PubMed] [Google Scholar]
- 25.Stuck AE, et al. , Risk factors for functional status decline in community-living elderly people: a systematic literature review. Social Science & Medicine, 1999. 48(4): p. 445–469. [DOI] [PubMed] [Google Scholar]
- 26.Samper‐Ternent R, et al. , Relationship between frailty and cognitive decline in older Mexican Americans. Journal of the American Geriatrics Society, 2008. 56(10): p. 1845–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raji MA, et al. , The interaction of cognitive and emotional status on subsequent physical functioning in older Mexican Americans: findings from the Hispanic established population for the epidemiologic study of the elderly. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2002. 57(10): p. M678–M682. [DOI] [PubMed] [Google Scholar]
- 28.Leveille SG, et al. , Black/white differences in the relationship between MMSE scores and disability: the Women’s Health and Aging Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 1998. 53(3): p. P201–P208. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard RE, et al. , Comparison of the prognostic importance of diagnosed diabetes, co-morbidity and frailty in older people. Diabetic Medicine, 2010. 27(5): p. 603–606. [DOI] [PubMed] [Google Scholar]
- 30.Cacciatore F, et al. , Clinical frailty and long-term mortality in elderly subjects with diabetes. Acta Diabetologica, 2013. 50(2): p. 251–260. [DOI] [PubMed] [Google Scholar]
- 31.Evans WJ, et al. , Frailty and muscle metabolism dysregulation in the elderly. Biogerontology, 2010. 11(5): p. 527–536. [DOI] [PubMed] [Google Scholar]
- 32.Dadoun S, et al. , Mortality in rheumatoid arthritis over the last fifty years: Systematic review and meta-analysis. Joint Bone Spine, 2013. 80(1): p. 29–33. [DOI] [PubMed] [Google Scholar]
- 33.Howrey BT, et al. , Stability and Change in Activities of Daily Living Among Older Mexican Americans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2016. 71(6): p. 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young MJ, et al. , A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia, 1993. 36(2): p. 150–154. [DOI] [PubMed] [Google Scholar]
- 35.Hatef B, Bahrpeyma F, and Mohajeri Tehrani MR, The comparison of muscle strength and short-term endurance in the different periods of type 2 diabetes. Journal of Diabetes & Metabolic Disorders, 2014. 13(1): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreassen C, et al. , Expression of neurotrophic factors in diabetic muscle—relation to neuropathy and muscle strength. Brain, 2009. 132(10): p. 2724–2733. [DOI] [PubMed] [Google Scholar]
- 37.Semba RD, et al. , Relationship of an advanced glycation end product, plasma carboxymethyl-lysine, with slow walking speed in older adults: the InCHIANTI study. European Journal of Applied Physiology, 2009. 108(1): p. 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalal M, et al. , Elevated Serum Advanced Glycation End Products and Poor Grip Strength in Older Community-Dwelling Women. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2009. 64A(1): p. 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shega JW, et al. , Persistent Pain and Frailty: A Case for Homeostenosis. Journal of the American Geriatrics Society, 2012. 60(1): p. 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blyth FM, et al. , Pain, frailty and comorbidity on older men: The CHAMP study. PAIN, 2008. 140(1): p. 224–230. [DOI] [PubMed] [Google Scholar]
- 41.Patel KV, et al. , Prevalence and impact of pain among older adults in the United States: Findings from the 2011 National Health and Aging Trends Study. PAIN®, 2013. 154(12): p. 2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention, Arthritis as a Potential Barrier to Physical Activity Among Adults with Diabetes --- United States, 2005 and 2007. MMWR. Morbidity and Mortality Weekly Report, 2008. 57(18): p. 486–9. [PubMed] [Google Scholar]
- 43.Mitnitski A, et al. , Transitions in cognitive status in relation to frailty in older adults: A Comparison of three frailty measures. The Journal of Nutrition, Health & Aging, 2011. 15(10): p. 863–867. [DOI] [PubMed] [Google Scholar]
- 44.Buchman AS, et al. , Brain Pathology Contributes to Simultaneous Change in Physical Frailty and Cognition in Old Age. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelaiditi E, et al. , Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. The Journal of Nutrition, Health & Aging, 2013. 17(9): p. 726–734. [DOI] [PubMed] [Google Scholar]
- 46.Sternberg SA, et al. , The Identification of Frailty: A Systematic Literature Review. Journal of the American Geriatrics Society, 2011. 59(11): p. 2129–2138. [DOI] [PubMed] [Google Scholar]
- 47.Heyn P, Abreu BC, and Ottenbacher KJ, The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis1. Archives of Physical Medicine and Rehabilitation, 2004. 85(10): p. 1694–1704. [DOI] [PubMed] [Google Scholar]
- 48.Peterson MJ, et al. , Physical Activity as a Preventative Factor for Frailty: The Health, Aging, and Body Composition Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2009. 64A(1): p. 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neighbors CJ, Marquez DX, and Marcus BH, Leisure-time physical activity disparities among Hispanic subgroups in the United States. American Journal of Public Health, 2008. 98(8): p. 1460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boumparis N, et al. , The effect of psychotherapeutic interventions on positive and negative affect in depression: A systematic review and meta-analysis. Journal of Affective Disorders, 2016. 202: p. 153–162. [DOI] [PubMed] [Google Scholar]
- 51.Peterson JC, et al. , A randomized controlled trial of positive-affect induction to promote physical activity after percutaneous coronary intervention. Archives of Internal Medicine, 2012. 172(4): p. 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogedegbe GO, et al. , A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive african americans. Archives of Internal Medicine, 2012. 172(4): p. 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]