Abstract
Caspase-8 (Casp8)-mediated signaling triggers extrinsic apoptosis while suppressing receptor interacting protein kinase (RIPK)3-dependent necroptosis. Even though Casp8 is dispensable for the development of innate and adaptive immune compartments in mice, the importance of this proapoptotic protease in the orchestration of immune response to pathogens remains to be fully explored. Here, Casp8−/−Ripk3−/− C57BL/6 mice show robust innate and adaptive immune responses to the natural mouse pathogen, murine cytomegalovirus. When young, these mice lack lpr-like lymphoid hyperplasia and accumulation of either B220+CD3+ or B220−CD3+ CD4+ and CD8+ T cells with increased numbers of immature myeloid cells that are evident in older mice. Dendritic cell activation and cytokine production drive both NK and T cell responses to control viral infection in these mice, suggesting that Casp8 is dispensable to the generation of antiviral host defense. Curiously, NK and T cell expansion is amplified, with greater numbers observed by 7 days post infection compared to either Casp8+/−Ripk3−/− or wild type (Casp8+/+Ripk3+/+) littermate controls. Casp8 and RIPK3 are natural targets of virus-encoded cell death suppressors that prevent infected cell apoptosis and necroptosis, respectively. It is clear from the current studies that the initiation of innate immunity and the execution of cytotoxic lymphocyte functions are all preserved despite the absence of Casp8 in responding cells. Thus, Casp8 and RIPK3 signaling is completely dispensable to the generation of immunity against this natural herpesvirus infection even though the pathways driven by these initiators serve as a crucial first line for host defense within virus-infected cells.
Keywords: cell death, murine cytomegalovirus, herpesvirus, antiviral immunity
Introduction:
The self-activating protease caspase-8 (Casp8) is an important cell signaling protease that controls alternate apoptotic and necroptotic cell death pathways (1) in host defense (2, 3). Casp8 also influences the activation of cytokines and differentiation of myelomonocytic lineage cells (4, 5). Best characterized following TNF superfamily (TNFR1, TRAIL and Fas) death receptor (DR) activation (5, 6), soluble Casp8 has been shown to form a death-inducing signaling complex (also called complex II or ripoptosome) with Fas-associated death domain protein (FADD), the long form of FLIP (cFLIPL), receptor-interacting protein kinase (RIPK)1 and RIPK3 (5) in the cytosol. In addition to DR activation, this complex controls signaling following either the activation of TLR3, TLR4 (7–9) or TCR (10, 11), or the induction of genotoxic stress (12). Several different outcomes may occur following complex formation: (i) cytokine activation via NF-κB, JNK and p38 signaling pathways, (ii) extrinsic apoptosis following autocleavage activation of Casp8, and, (iii) alternate RIPK3-dependent, mixed-lineage kinase domain-like (MLKL)-mediated necroptosis when Casp8 is compromised (5). Taken together, Casp8 is now recognized for its importance in integrating divergent cell death and inflammatory signals.
Considerable experimental evidence supports physiological interplay between alternate Casp8-mediated apoptosis and RIPK3-mediated necroptosis in host defense against human (H) (13, 14) and mouse (M) cytomegalovirus (CMV) (15–20), vaccinia virus (21–23), Sendai virus (24) as well as human influenza A virus (25–28) and herpes simplex virus (HSV) (29, 30). MCMV, a natural mouse viral pathogen, has provided unambiguous evidence revealing the importance of Casp8 (31) and RIPK3 (17–19) interactions in anti-viral host defense (20). The MCMV M36 and M45 gene-encoded functions inhibit cell death signaling to sustain infection, dissemination, and persistence. The M36-encoded viral inhibitor of caspase 8 activation (vICA) blocks basal activity as well as auto-cleavage activation of this protease (15, 16). The M45-encoded viral inhibitor of RIP activation (vIRA) (18) is a RIP homotypic interaction motif (RHIM) competitor that naturally suppresses recruitment of RIPK3 by Z-form nucleic acid binding protein (ZBP)1 (also called DAI or DLM1) (19). vIRA blocks subsequent phosphorylation of MLKL and execution of necroptosis (7). ΔM36 and M45mutRHIM mutant viruses fail to sustain infection in the host due to premature death of infected cells through Casp8- and RIPK3-driven pathways (14, 18, 20), respectively. Importantly, the attenuation of ΔM36, M45mutRHIM or combined ΔM36/M45mutRHIM viruses in wild type (WT) mice is reversed in Casp8−/−Ripk3−/− (DKO) mice (20), reinforcing the importance of both pathways in restricting virus infection in vivo. HCMV and MCMV encode evolutionarily-conserved Casp8 inhibitors (15, 16), although these betaherpesviruses block different points in the necroptotic pathway (13, 29). The mechanism of cell death suppression by MCMV shows remarkable similarity to HSV, an alphaherpesvirus (30, 32). Overall, Casp8 and RIPK3 sustain potent cell-autonomous innate host defense to stop the spread of virus infection and so both pathways are targeted by virus-encoded cell death suppressors.
Despite the importance in host defense, Casp8 function appears dispensable in the activation of immune cells that control MCMV infection (33, 34). Initially, Casp8 was thought to be critical for the activation of NF-κB in immune cells given that lymphocytes from either mice or patients with a disruption in Casp8 display defective translocation of this transcription factor into the nucleus (35, 36). These observations were later clarified in mouse studies revealing the important role of Casp8 as a suppressor of RIPK3-mediated necroptosis following TCR activation as, once on a RIPK3-eliminated background, Casp8-deficient cells become activated and exhibit intact functionality (11). Cd4CreCasp8flox/flox and LckCreFADDdd mice with intact RIPK3-mediated necroptotic function exhibit compromised antiviral responses (11, 37) aligning with the observed immune defect in controlling herpesvirus infection in human patients carrying a destabilizing Casp8 mutation (Casp8C248T) (35). In contrast, the overall antiviral immunity is preserved in DKO and Ripk1−/−Casp8−/−Ripk3−/− mice despite the combined absence of Casp8 and RIPK3 (33, 34, 38). Further, Cd4CreCasp8flox/floxRipk3−/− (11) and LckCreFADDddRipk3−/− (37) mice that have T cell-specific genetic disruptions in Casp8 (or FADD) on a RIPK3-eliminated background mount effective antiviral immunity suggesting that pathways driven by these molecules are dispensable to T cell expansion, contraction and cytotoxicity. In fact, like the lpr mice that have a disruption in the DR Fas that is upstream of Casp8, patients with autoimmune lymphoproliferative syndrome (ALPS) are indeed immunocompetent (39). Neither lpr mice nor ALPS patients develop any opportunistic infection or other clinical signs of immunodeficiency (40) arguing strongly that anti-microbial immunity is intact when both Casp8 and RIPK3 signaling pathways are compromised. The combined evidence suggest that, in mice as well as humans, the pathways controlled by Casp8 are dispensable for the generation of antiviral immunity once RIPK3-mediated necroptosis is eliminated in parallel. Thus, DKO animals lack the confounding impact of unleashed necroptosis and therefore permit studies in a setting where Casp8 contribution to immune regulation may be assessed independent of necroptosis.
Even with the strong immune activation, DKO mice exhibit higher MCMV titers at 7 dpi compared to WT mice (33, 34), reminiscent of the increased viral titers seen in animals lacking both Fas and TNFR1 signaling (41). In contrast, Casp8+/+Ripk3−/− (Ripk3−/−) mice exhibit similar viral titers, infection-induced inflammation (18, 19) and antiviral T cell responses (34) compared to WT mice, suggesting that RIPK3 is dispensable for control of WT MCMV. Comparative assessment of Ripk3−/− and Casp8+/−Ripk3−/− (HET) mice did not reveal any additional impact on MCMV titers or antiviral immunity when one allele of Casp8 was deleted (unpublished data). Together, these studies suggest that increased WT MCMV titers in DKO mice (34) is attributable to the elimination of both Casp8 alleles and raise the question of whether DKO mice mount effective immune responses at different stages of MCMV infection.
In this manuscript, we take advantage of viable Casp8-deficient DKO mice along with HET and WT controls to investigate Casp8-mediated regulation of antiviral immunity. We focused on young (6- to 8-week old) mice to circumvent confounding contributions of the lymphoid hyperplasia and accumulating abnormal B220+CD3+ T cells that develop as DKO mice age (33, 42), first reported in Fas signaling-deficient lpr mice (43). We report that DKO mice develop antiviral immune responses sufficient to control acute MCMV infection. Our investigation reveals the capacity of these mice to generate elevated dendritic cell (DC), NK cell as well as T cell numbers against MCMV even though control of acute MCMV infection appears comparable to WT mice. Thus, despite the contribution of Casp8 and RIPK3-mediated pathways to host defense within virus-infected cells, these signaling components are completely dispensable for the generation of anti-viral immunity.
Materials and Methods:
Mice, viruses and experimental infection
DKO mice were derived as described previously (33) and backcrossed to >97% on the C57BL/6 background as indicated by single nucleotide polymorphism scanning (Jackson Laboratories). Casp8−/−Ripk3−/− (DKO), Casp8+/−Ripk3−/− (HET) and Casp8+/+Ripk3+/+ (WT) littermates were generated from mating Casp8+/−Ripk3+/− mice. C57BL/6 WT mice (Jackson Laboratories) used as controls for K181-BAC infection were bred and maintained in house. Mice were maintained at the Emory University Division of Animal Resources and experimental procedures were conducted in accordance with the National Institutes of Health and Emory University Institutional Animal Care and Use Committee guidelines. MCMV strain V70 (kindly provided by C. Biron, Brown University) (44) and was propagated in salivary glands (SG) as described (45). Briefly, BALB/c mice were inoculated intraperitoneally (i.p.) with 1 × 103 PFU V70 and SG were collected at 14 days post infection (dpi). Clarified supernatant (2700 × g at 4°C, 10 min) was collected from sonicated SG (10% weight/volume in Dulbecco’s Modified Eagle Medium; DMEM with 10% FBS/1% penicillin/streptomycin). Strain K181-BAC (46) and K181-BAC-derived Δm157 (kindly provided by W. Brune; Heinrich Pette Institute, Hamburg) (47) were propagated in NIH 3T3 murine fibroblasts (American Type Culture Collection CRL-1658) (18) and purified from clarified tissue culture medium. Aliquots of virus were stored at −80°C until use. Experimental mice were inoculated i.p. with either medium (mock control), 1 × 105 PFU V70, or 1 × 106 PFU tissue culture-propagated viruses.
Plaque assay
Tissues were homogenized in DMEM and overlaid onto 3T3 Swiss Albino murine fibroblasts (ATCC CCL-92). Virus titers were calculated at 4 days when cells were fixed with methanol and stained with Giemsa for plaque visualization (48).
Antibodies
For flow cytometry, Abs to CD16/CD32 (FcγRII/III; Clone 2.4G2), Ly6C (Clone AL-21), CD80 (Clone 16–10A1), IL-2 (Clone JES6–5H4), CD4 (Clone RM4–5), TNF (Clone MP6-XT22), CD62L (Clone MEL-14), NK1.1 (Clone PK136), B220 (Clone RA3–6B2), CD11b (Clone M1/70) and CD3ε (Clone 17A2) were purchased from BD PharMingen; IFNγ (Clone XMG1.2), CD49d (Clone R1–2), CD3ε (Clone 145–2C11), CD115, CD44 (Clone 1M7), CD86 (Clone GL-1) and I-A/I-E (Clone M5/114.15.2) were purchased from BioLegend; and CD11c (Clone N418), CD69 (Clone H1.2F3), CD3ε (Clone 145–2C11), Siglec H (Clone eBio440c), CD3ε (Clone 17A2), CD11b (Clone M1/70), Ly49H (Clone 3D10), Ly6C/6G (Gr-1; Clone RB6–8C5), CD11a (Clone M17/4) and CD28 (Clone 37.51) were purchased from eBioscience. The following Abs were purchased from Invitrogen: CD8α (Clone 5H10), F4/80 (Clone BM8) and CD45 (Clone 30-F11). For ELISA, purified mouse IgM, IgG1, IgG2b and IgG2c, and polyclonal nonconjugated and alkaline phosphatase (AP)-conjugated goat anti-mouse Ig isotypes were purchased from Southern Biotech.
ELISA
Total serum IgM and IgG isotypes were measured by ELISA (49). Well volumes were 50 μl, incubations were overnight at 4°C unless otherwise specified and plates were washed after each incubation. Polystyrene 96-well microtiter plates (Nunc ImmunoMaxisorp plates; Fisher Scientific) were coated with 10μg/ml goat anti-mouse Ig isotypes (Southern Biotech), blocked, then incubated 4 h at room temperature (RT) with three-fold serial dilutions of sera. Wells were then incubated with alkaline phosphatase -conjugated goat anti-mouse Ig isotypes. Binding was detected with p-nitrophenyl phosphate (Sigma) in diethanolamine buffer. Optical density readings were taken at 405 nm using a microplate reader (Biotek Synergy HT) and the associated Gen5 software. Concentrations were calculated from standard curves constructed with purified mouse Ig isotypes (Southern Biotech).
MCMV-specific IgGs were detected using a modified version of the protocol described above. Microtiter plates were incubated with 0.5 μg/ml virion protein purified on a sucrose gradient and endpoint titers for bound IgG2c and IgG1 were determined as the reciprocal of the highest serum dilution from infected mice with greater than twice the optical density of the corresponding dilution from naïve mice.
Serum dsDNA-specific Abs were assayed using a modified protocol described previously (34). Briefly, Immulon 1B plates (Fisher Scientific) were exposed to UV light overnight. Well volumes were 50 μl. Wells were incubated 1h at RT with 2 μg/ml calf-thymus DNA (Sigma), washed, blocked then incubated 4 h with three-fold serial dilutions of sera. Bound IgG was detected overnight at 4 °C using alkaline phosphatase-conjugated goat anti-mouse isotype-specific Ab and the assay was developed as above.
IL12(p70) production in mice was evaluated at 40 hpi with MCMV. For spleens, tissues were disrupted by sonification in 1 ml DMEM and clarified supernatants were assayed. Sera were prepared from blood collected by cardiac puncture. ELISA was conducted by using the Mouse IL-12 (p70) ELISA Set (BD OptEIA; BD Biosciences) following manufacturer’s instructions. Briefly, 96-well microtiter polystyrene plates (Costar) were coated overnight at 4 °C with capture Ab. Incubations were conducted at RT and followed by a washing step unless otherwise stated. Plates were blocked, incubated with samples and IL-12(p70) standard followed by the detection Ab-HRP-SAv mixture then developed using the 3,3′,5,5′-tetramethylbenzidine substrate reagent set. 1M H3PO4 was added directly wells to stop the enzymatic reaction and optical density readings were taken at 450 nm with λ correction 570 nm.
Flow cytometry
Spleens were harvested from euthanized mice, disrupted by mashing through a metal sieve, incubated with erythrocyte lysis buffer then passed through a filter (100 mm mesh). Viable cell counts were performed on a hemocytometer using trypan blue dye exclusion. Cell surface FcγRII/III were blocked prior to incubating with surface Ag-specific Abs for multiparametric flow cytometric analyses. CD45+ leukocytes were gated to identify precursor-like (pre-) CD3−CD11c+MHC-IIlo conventional(c) DCs and more mature CD3−CD11c+MHC-IIhi cDCs that were further separated into CD8α+ cDC1 and CD11b+ cDC2 subsets. Leukocytes were also identified as B220+Siglec-H+ plasmacytoid (p)DCs, CD115+CD11b+ Ly6C+Gr-1−F4/80+CD11c−CD62L– inflammatory monocytes (IMs), CD115+CD11b+Ly6C−Gr-1−F4/80+CD11c+CD62L+ patrolling monocytes (PMs) and CD11b+F4/80+CD11c− macrophages (Macs). CD45+ leukocytes were also gated to identify B220−CD3−NK1.1+ NK cells and the Ly49H+ subset, B220+CD3+ aberrant T cells, and conventional B220−CD3+CD8−CD4+ (CD4) and B220−CD3+CD4−CD8+ (CD8) T cell subsets. For intracellular cytokine staining (ICCS), cells were either evaluated directly ex vivo, as in the case of NK cells, or stimulated 5 h with anti-CD3/CD28 or with M45, M38 or IE3 peptides (50) in the presence of brefeldin A, as was done for T cell analyses, using the Cytofix/Cytoperm kit (BD Biosciences). Data were acquired by flow cytometry (BD LSRII cytometer and FACSDiva Software; BD Biosciences), analyzed with FlowJo (TreeStar), and graphed with Prism 7 (GraphPad).
T cell proliferation
B220–CD3+ T cells were isolated by EasySep Mouse T Cell Isolation Kit (Stem Cell), labeled with 0.5 μM CFSE in 0.1% BSA/PBS for 10 min at 37 °C then stimulated with plate-bound anti-CD3 and anti-CD28 for 3 days in a 37 °C incubator. Isolated T cells (2 × 105) were resuspended in 200 μl RPMI 1640 with 10% FBS/1% penicillin/streptomycin (complete RPMI) as well as 50μM 2-mercaptoethanol in 96-well plate. Cells were stained with 7-AAD for dead cell exclusion as well as Abs specific for surface markers. All samples were resuspended in an equal volume of FACS staining buffer and collected for the same amount of time on the flow cytometer (37).
In vivo CTL killing assay
CTL killing in spleens of MCMV-infected WT, HET and DKO mice was assessed at 7 dpi. To generate target cells, splenocytes from naïve C57BL/6 WT mice were pulsed with M45 peptide by one hour incubation at 37 °C in RPMI complete medium with 2-mercaptoethanol. The M45-pulsed/unpulsed cells were labeled with 0.5μM/5μM CFSE, respectively, at 2 × 107 cells/ml concentration in PBS supplemented with 0.1% BSA. The CFSEhi and CFSElo cells were mixed at a 1:1 ratio to obtain 2 × 107 cells in 250 μl medium, and injected into tail vein. Spleens were harvested at 4 or 18 hour post transfer from recipient mice and frequencies of CFSE+ splenocytes were analyzed by flow cytometry (51). The specific killing was defined as 100 x [1 – Ratio (naïve) / Ratio (infected)], with Ratio representing CFSEhi / CFSElo.
Statistical analysis
Statistical analysis was performed by unpaired one-way or two-way ANOVA with Tukey analysis, using GraphPad Prism 7. P ≤ 0.05 was considered significant.
Results:
Myeloid cell homeostasis in naïve DKO mice
Prior to evaluating the response to MCMV infection, we set out to determine whether viable Casp8-deficient mice develop myeloid and lymphoid compartments sufficient to support antiviral immunity. DKO mice showed the expected lymphadenopathy that developed with age (33, 42). The immune compartments of 8-week-old naïve DKO mice were characterized, because this was a time when lymphoid organs appeared more normal (52). We compared these younger mice to older (16-week-old) adult mice as well as to age-matched WT and HET controls.
Although young WT and DKO mouse spleens comprised similar frequencies (Fig. 1 A, top panels) and numbers (Fig. 1 B) of CD45+CD3−CD11c+MHC-IIlo pre-cDCs (53, 54), DKO cells expanded dramatically by 16 weeks of age to numbers six-fold greater than WT mice (Fig. 1 A, bottom panels and 1B). In contrast, the frequencies and numbers of more mature CD45+CD3−CD11c+MHC-IIhi cDCs in DKO mice were sustained at these ages; however, cell numbers were approximately twice that of WT controls regardless of age (Fig. 1C) due to increased numbers of CD8α+ cDC1 and CD11b+ cDC2 subsets (Fig. 1D – F). CD45+B220+SiglecH+ pDCs accumulated faster with age in DKO mice to levels greater than 2.5-fold that of WT controls (Fig. 1G). Curiously, in HET mice, numbers of both cDCs and pDCs decreased with age to levels lower than observed in 16-week-old WT mice (Fig. 1C - G), suggesting that RIPK3 impacts DC numbers when Casp8 is present. Given the higher levels of DCs in DKO mice compared to HET mice, elimination of Casp8 appeared to reverse the reduced DC numbers in RIPK3-deficient mice. Thus, our data show that DKO mice develop the more mature DC subsets known to contribute to the antiviral immune response in WT mice, even though the contribution of Casp8 to pre-cDCs and cDCs homeostasis appears similar to that shown in mice with CD11c-specific disruption of Casp8 (55, 56).
Figure 1. Age-dependent impact of Casp8 and RIPK3 deficiency on myeloid cell homeostasis.
(A - F) Analysis of developmentally (A - C) and functionally (D - F) distinct DCs in spleens of WT, HET and DKO mice at the indicated weeks of age. Splenic CD45+B220–CD3–leukocytes from 8 (top panels) and 16 (bottom panels) weeks-of-age were displayed to identify CD11c+MHCIIlo pre-cDCs and CD11c+MHC-IIhi cDCs. Representative flow cytometry plots comparing frequencies of pre-cDCs and cDCs (A), and graphs showing the total numbers of the subsets represented as the mean ± S.E.M. (B and C). cDCs were then separated to identify CD8α+ cDC1 and CD11b+ cDC2 subsets. Representative plots comparing frequencies of cDC1s and cDC2s (D), and graphs showing the total mean numbers ± S.E.M. of the subsets (E and F). (G – J) Graphs showing total numbers of myeloid cell subsets represented as the mean ± S.E.M. CD45+ leukocytes were gated to identify B220+Siglec-H+ pDCs (G), CD115+CD11b+ Ly6C+Gr-1−F4/80+CD11c−CD62L– IMs (H), CD115+CD11b+Ly6C− Gr-1− F4/80+CD11c+CD62L+ PMs (I) and CD11b+F4/80+CD11c−Macs (J). Combined data of n = 11 mice from up to three independent experiments are shown. Significant differences between DKO and WT (*) or HET (#) mice are indicated as * or #, p < 0.05; ** or ##, p < 0.01; *** or ###, p < 0.001.
Next, we investigated the impact of Casp8 and RIPK3 deficiency on the development of splenic monocyte subsets and CD45+CD11b+F4/80+CD11c− Macs. By 16 weeks of age, both CD45+CD115+CD11b+ Ly6C+Gr-1−F4/80+CD11c−CD62L– IMs (Fig. 1H) and CD45+CD115+CD11b+Ly6C− Gr-1− F4/80+CD11c+CD62L+ PMs (Fig. 1I) in DKO mice expanded to numbers that were 8-fold greater than WT controls. Importantly, this pattern was comparable to that observed with LysM-specific disruption of Casp8 or Fas (57–59), implicating the Fas-Casp8 axis in maintaining homeostasis of less mature myeloid cells. Mac numbers in younger DKO mice were greater than WT controls and expanded faster as animals aged (Fig. 1J). Interestingly, although Mac numbers were also greater in 8-week-old HET mice, the levels dropped over time to match the increase in WT animals by 16 weeks of age, indicating that RIPK3 impacts numbers of more mature myeloid cell. These data suggest that the myeloid compartment of DKO mice is relatively complete and may be expected to support MCMV infection and antiviral response even though Casp8 appears to contribute to the homeostatic equilibrium of less mature myeloid cells and Macs.
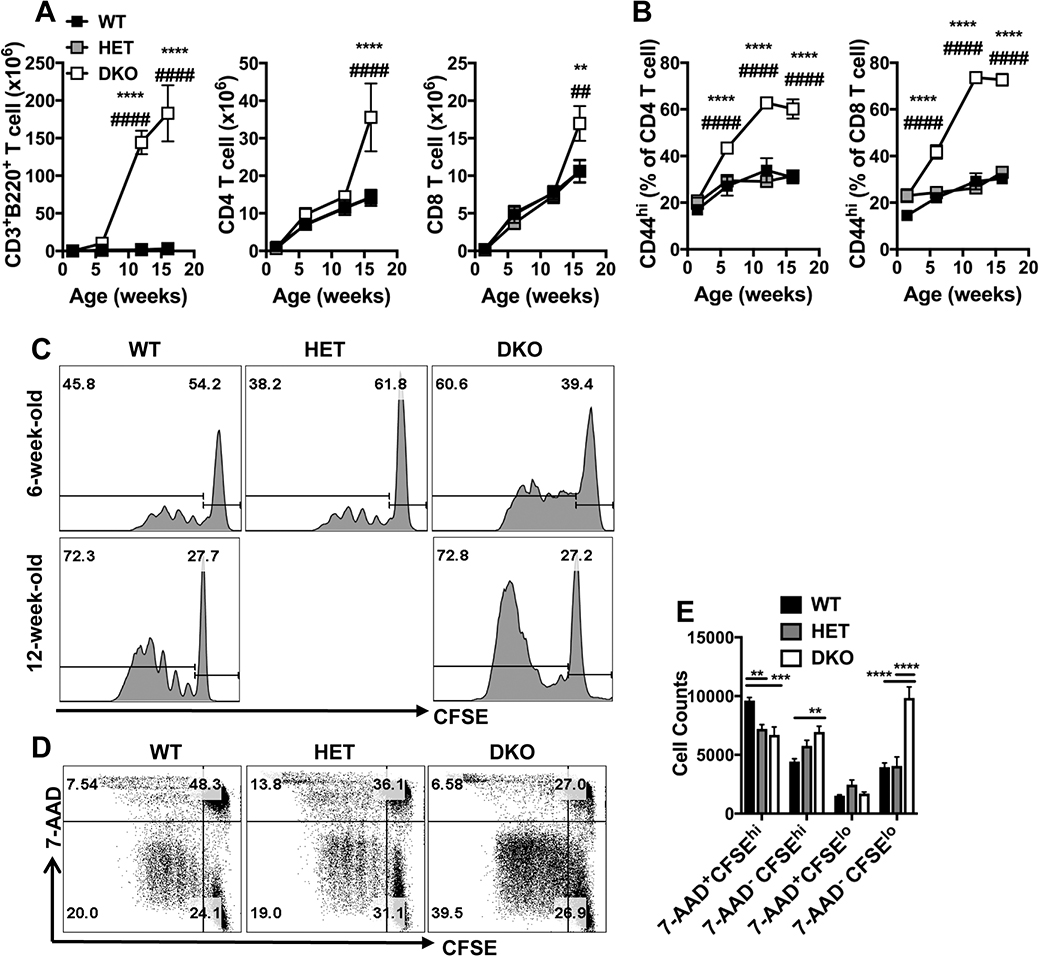
T cells in naïve DKO mice accumulate and acquire activation markers with age
Next, we sought to characterize T cells in naïve DKO mice. Consistent with previous observations (52), aberrant CD45+CD3+B220+ T cells are detectable by six weeks of age and continue to elevation in numbers over time (Fig. 2A). As expected (33), conventional CD45+B220–CD3+ CD4+ and CD8+ T cells accumulated to significantly greater numbers by 16 weeks of age in DKO mice compared to controls. T cells in lpr mice express high levels of CD44 ascribed to defective turnover in response to self-antigens or cytokines (43). Increasing proportions of T cells from DKO mice expressed CD44 over time such that frequencies of CD44hi CD4 and CD44hi CD8 T cells reached 60% and 70%, respectively, by 12 weeks of age compared to the 30% levels in WT and HET controls (Fig. 2B). Notably, the increase in frequencies in DKO mice was less dramatic at 6 weeks of age compared to controls. Altogether, these data confirm that Casp8 is dispensable for T cell development (33) but may contribute to the suppression of T cell accumulation and homeostatic activation state in aging mice (60).
Figure 2. Impact of Casp8 and RIPK3 deficiency on T cell homeostasis at different ages.
(A and B) Graphs showing levels of T cell subsets represented as the mean ± S.E.M. in spleens of WT, HET and DKO mice at the indicated weeks of ages. Splenic CD45+ leukocytes were gated to identify B220+CD3+ T cells, B220–CD3+CD8–CD4+ (CD4) and B220–CD3+CD4–CD8+ (CD8) T cells. Graphs showing the total numbers of the T cell subsets (A) and the frequencies of CD44hi CD4 and CD44hi CD8 T cells (B). Significant differences between DKO and WT (*) or HET (#) mice are indicated as * or #, respectively, * or #, p < 0.05; ** or ##, p < 0.01; *** or ###, p < 0.001; **** or ####, p < 0.0001.
(C - E) Flow cytometry plots showing CD8 T cell proliferation and death. CFSE dilution analyses of purified splenic T cells after stimulation with anti-CD3 and anti-CD28 for 72 hr. Representative histograms comparing CFSE distribution of 7-AAD– CD8 T cells from mice at the indicated ages (C). Representative plots showing CFSE vs. 7-AAD of cells from 6-week-old mice (D) and bar graphs showing the numbers of different cell population recovered in culture following stimulation, represented as the mean ± S.E.M. Combined data of n = 11 mice from up to three independent experiments are shown. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Given the observed increase in activation state during homeostasis, we sought to investigate the capacity of T cells from DKO mice to respond to TCR engagement. Purified splenic conventional T cells from 6 and 12-week-old mice were labeled with CFSE, stimulated with CD3 and CD28 Abs then analyzed at 72 h by flow cytometry for extent of proliferation. In contrast to observations made using young T cell-specific Cd4CreCasp8flox/floxRipk3–/– mice (11), proliferating CD8 T cells accumulated to greater levels in DKO mice compared to WT controls regardless of age (Fig. 2C), consistent with the high proportion of CD44hi cells in DKO mice (Fig. 2B).
To better understand how Casp8 and RIPK3 regulate cell death in CD8 T cells following TCR stimulation, we quantified 7-AAD+ events in cultures of CFSE-labelled cells purified from spleens of 6-week-old mice. Cells from WT mice had the highest proportion (Fig. 2D) and numbers (Fig. 2E) of dead, undivided (7-AAD+CFSEhi) CD8 T cells at 72 h post-stimulation whereas 20% of the population had remained alive and had divided (7-AAD−CFSElo). In contrast, the levels of dead, undivided cells in cultures from both HET and DKO mice were significantly reduced (Fig. 2D and E), suggesting a RIPK3-dependent but Casp8-independent contribution to cell death following TCR stimulation. of CD8 T cells. Upon entering the cell cycle, CFSElo DKO cells underwent extensive proliferation and, compared to controls, a smaller proportion of dead (7-AAD+CFSElo) cells was observed (Fig. 2D). Notably, similar numbers of these dead, divided 7-AAD+CFSElo cells were recovered from all cultures (Fig. 2E). Thus, notwithstanding the heightened activation state, DKO CD8 T cells respond to TCR stimulation in vitro and undergo more extensive proliferation with dampened putative Casp8-dependent activation-induced-cell-death (43).
Virus-induced DC activation is robust in DKO mice
Previously, DKO mice, similar to Cd4CreCasp8flox/floxRipk3–/– and Ripk1–/–Casp8–/–Ripk3–/–mice, were found to be immunocompetent and capable of controlling viral infections (11, 33, 34); however, detailed characterization of innate and adaptive immune response parameters have not been reported. We characterized the antiviral response to MCMV in young (6 to 8-week-old) DKO mice because they retained intact leukocyte populations without exhibiting the gross myeloid and lymphoid abnormalities that became apparent with age. Given their important role in activating NK cells and in being the predominant APCs responsible for priming adaptive immunity (61), we characterized the DC response in spleens of WT, HET and DKO mice at 1.5 dpi. Even though the cDC frequency was lower in infected DKO mice (Fig. 3A), the total numbers of cDC1 and cDC2 subsets were greater in these mice than in littermate controls (Fig. 3B). WT and HET mice exhibited comparable numbers of both subsets, revealing that RIPK3 is dispensable for the cDC response to infection despite an apparent contribution to homeostasis (Fig. 1C-F). Importantly, cDC maturation following infection was evident in all three genotypes where CD80 and CD86 were upregulated (Fig. 3C-D), indicating cDC activation in response to virus despite Casp8 and/or RIPK3 deficiency.
Figure 3. Impact of Casp8 and RIPK3 deficiency on DC activation following MCMV infection.
(A and B) Representative contour plots showing frequencies of mature cDCs (A) and bar graphs showing total mean numbers ± S.E.M. of cDC1s and cDC2s (B) in spleens of infected mice. (C and D) Representative histograms showing CD80 and CD86 expression on cDC1s (C) and cDC2s (D) from either mock or infected mice. (E and F) Bar graphs showing concentrations ± S.E.M. of IL-12(p70) in spleen homogenate (E) and serum (F) as quantified by ELISA. Dash lines indicate limit of detection. Eight-week-old WT, HET and DKO mice were inoculated intraperitoneally (i.p.) with 105 PFU MCMV (V70 strain) per mouse. Splenocytes were analyzed at 1.5 dpi by flow cytometry, as described in Figure 1. Spleen homogenates or sera were analyzed by ELISA. Combined data of n = 11 mice from up to three independent experiments are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
IL-12 produced by DCs early after MCMV infection is critical for promoting cytotoxic NK and CD8 T effector cell activation (44, 61). Given that DCs are the major source of IL-12 (62), we assessed concentrations in spleen and serum at 1.5 dpi to understand whether Casp8 and RIPK3 influenced the production of this cytokine. Infection increased IL-12 levels in spleens regardless of genotype; although, levels in DKO mice were notably lower than controls even following infection (Fig. 3E). Thus, Casp8 contributes to the optimal production of this cytokine in splenocytes. As expected (44), systemic IL-12 production was undetectable in mock-treated WT mice but increased at 1.5 dpi (Fig. 3F) in a pattern that was independent of Casp8 and RIPK3. Even though splenic levels were lower in DKO mice, serum IL-12 levels were similar to controls. Thus, despite the absence of Casp8 and RIPK3, higher numbers of cDC appeared to be accompanied by sufficient systemic IL-12 production in response to MCMV infection.
Antiviral NK cell response is robust in DKO mice
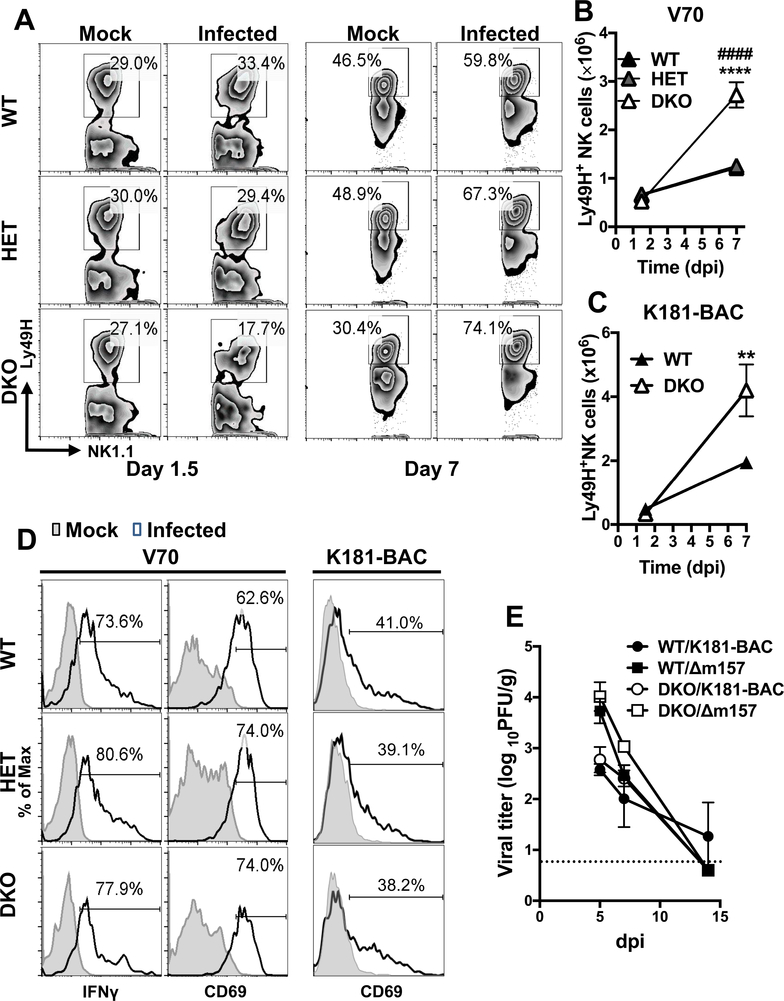
Activated DCs collaborate with Ly49H activation by viral m157 to promote NK cell proliferation and maturation (63, 64), contributing to early control of MCMV infection in C57BL/6 mice. Here, CD45+B220−CD3−NK1.1+Ly49H+ NK cells responded more robustly between 1.5 and 7 dpi in the absence of Casp8 and RIPK3 than in controls (Fig. 4A and B). The percentages of Ly49H+ NK cells at 1.5 dpi appeared substantially lower in DKO mice than in infected WT or HET mice or mock-treated controls (Fig. 4A). Nevertheless, all three genotypes of mice showed increased percentages over mock by 7 dpi, indicating Ly49H+ NK cells respond to MCMV independent of Casp8. Consistent with the proportional assessment, the numbers of Ly49H+ NK cells expanded between 1.5 and 7 dpi in all three genotypes, with a more dramatic expansion in DKO mice (Fig. 4B and C). Despite differences in expansion, Ly49H+ NK cells exhibited comparable elevation of IFNγ and CD69 at 1.5 dpi in all mice (Fig. 4D), indicating that Casp8 deficiency did not impair NK cell activation. Virulence of MCMV depends on the source as well as preparation of virus stock (45, 65). Importantly, NK cells in DKO mice exhibited both enhanced cell expansion (Fig. 4C) and robust activation (Fig. 4D) in response to either tissue culture-propagated strain K181-BAC or the more virulent SG-propagated strain V70. Thus, the patterns of NK cell response in DKO mice occurred independent of virus strain and preparation.
Figure 4. Impact of Casp8 and RIPK3 deficiency on NK cell response following MCMV infection.
(A – D) Graphs showing the levels and phenotype of Ly49H+ NK cells responding to MCMV infection. Splenic total CD45+CD3–B220–NK1.1+ (NK) cells gated to identify Ly49H+ NK cells from 8-week-old WT, HET and DKO mice infected for 1.5 or 7 days with MCMV as described in Figure 3. Representative flow cytometry contour plots comparing Ly49H+ NK cell frequencies (A) and graph showing mean total numbers ± S.E.M (B) in mock or MCMV V70-infected mice. Graph showing mean numbers ± S.E.M. of Ly49H+ NK cells in K181-BAC-infected mice (C). Representative histograms showing frequencies of Ly49H+ NK cells expressing IFNγ or CD69 at day 1.5 post infection with strain V70 or K181-BAC (D). (E) Graph showing viral titers in spleens. WT and DKO mice were i.p.-inoculated with 106 PFU MCMV K181-BAC or Δm157. Tissues were harvested on day 5, 7 and 14 post infection and homogenates were analyzed by plaque assay. Dash line indicates limit of detection. * and # denote the significantly differences of DKO cells relative to WT and HET cells, respectively. ** or ##, p < 0.01; *** or ###, p < 0.001. Data represent two to three independent experiments using n = 4 – 5 mice per group.
Next, to assess the antiviral function of NK cells, we compared K181-BAC to Δm157 mutant derivative that was unable to activate Ly49H+ NK cells (66). At 5 dpi, when NK cell-mediated antiviral immunity is dominant, WT and DKO mice exhibited similar 10-fold lower virus titers with K181-BAC compared to Δm157 (Fig 4E). Thus, competent NK cell function developed despite Casp8 and RIPK3 deficiency. The difference in titer was less pronounced at 7 dpi, a time point when CD8 T cells contribute to control of this virus (66). Thus, DKO mice mount a robust NK cell response that controls MCMV early during infection; however, the pattern suggests that Casp8 may normally dampen NK cell expansion without impacting overall antiviral function.
Antiviral T cell response is robust in DKO mice
We sought to characterize the T cell response against MCMV given its importance in the ultimate control of virus replication and maintenance of latency (67). We compared the splenic CD8 T cell response in infected WT, HET and DKO mice by evaluating the virus-specific response to M45, the dominant epitope during acute infection, as well as the response to inflationary epitope IE3 (50), employing stimulation with specific peptides. CD8 T cells responded robustly to infection independent of Casp8 and/or RIPK3 deficiency (Fig. 5A and B). The three genotypes of mice exhibited similar frequencies of M45-specific CD8 T cells at either 5 or 14 dpi (Fig. 5A); however, at 7 dpi when the acute response peaked, significantly greater frequencies of Ag-specific T cell populations were present in DKO mice. Thus, Casp8 appears to restrict initial T cell expansion without any apparent impact on contraction. Instead of an acute response, IE3-specific CD8 T cells are not detectable until at least three weeks post infection. Whereas WT and HET mice exhibited the expected low percentages of this subset by 14 dpi, DKO mice developed faster expansion of IE3-specific CD8 cells between 7 and 14 dpi (Fig. 5B). Similar patterns of CD8 T cell expansion were observed in DKO mice infected with MCMV K181-BAC (Fig. 5C and D). In summary, DKO mice mount a robust CD8 T cell response to MCMV infection, although the unexpected pattern suggests a role for Casp8 in restricting CD8 T cell expansion without affecting contraction.
Figure 5. Impact of Casp8 and RIPK3 deficiency on T cell antiviral response.
(A – D) Graphs comparing mean ± S.E.M. frequencies of MCMV-specific CD8 T cell subsets in spleens from 8-week-old WT, HET and DKO mice infected with MCMV. Frequencies of IFNγ+ CD8 T cells following ex vivo M45 (A) or IE3 (B) peptide stimulation at 5, 7 and 14 dpi with V70 as described in Figure 3. Frequencies of M45 (C) or IE3 (D) tetramer-positive CD8 T cells in K181-BAC-infected mice at 0, 7 or 14 dpi. Significant differences between DKO and WT (*) or HET (#) mice are indicated as * or #, p < 0.05; ** or ##, p < 0.01; *** or ###, p < 0.001; **** or ####, p < 0.0001. (E and F) Bar graphs comparing mean ± S.D. CD8 T cell-specific killing. Non-pulsed CFSElo and M45 peptide-pulsed CFSEhi splenocytes were adoptively transferred into naïve or infected recipients by V70 (E) or K181BAC strain (F) at 7 dpi, and spleens were harvested at 18 (E) or 4 (F) hour post transfer from recipient mice and frequencies of CFSE+ splenocytes were analyzed by flow cytometry. (G – M) Flow cytometric analyses of CD4 T cells in spleens at 14 dpi. Bar graphs showing the numbers of total (G) and CD11a+CD49d+ (H) CD4 T cells represented as the mean ± S.E.M.. Pie charts depicting mean percentages of indicated CD4 T cell subsets following stimulation of anti-CD3/CD28 for 5h (I). Bar graphs showing mean ± S.E.M. total numbers of CD4 T cells that were IFNγ+(J), TNF+ (K), IL-2+ (L), or IFNγ+TNF+IL-2+ (M). Data represent two to three independent experiments using n = 5 mice per group.
To evaluate CD8 T cell cytotoxic function, we injected 2 × 107 WT M45 peptide-pulsed and CFSE-labeled splenocytes into mice at 7 dpi. A comparable level of killing was observed over an 18 h time frame in all genotypes (Fig. 5E). Specific killing of Ag-pulsed cells was nearly complete in all mice regardless of whether infected with V70 or K181-BAC (Fig. 5F), indicating CD8 T cell cytotoxicity was preserved in DKO mice. The antiviral function of CD8 T cells in DKO mice was evaluated with Δm157 virus to circumvent the contribution of NK cells. DKO mice controlled MCMV infection by 14 dpi independent of NK cell activation by m157 (Fig. 4E). WT and DKO mice exhibited similar patterns of Δm157 virus clearance, demonstrating intact T cell function in DKO mice. In addition, the sharper drop in titer between 5 and 7 dpi in all Δm157 virus-infected mice is consistent with enhanced T cell effector function in the absence of activated NK cells (66, 68). Taken together, mice mount cytotoxic CD8 T cell responses with full antiviral capacity independent of Casp8.
Whereas acute MCMV infection is controlled via overlapping NK and CD8 T cell responses, CD4 T cells contribute to viral clearance from SGs, as well as the Ab response and establishment of latency (69). We evaluated the splenic CD4 T cell response at 14 dpi following expression of CD11a and CD49d (70). Total CD4 T cells showed modest elevation in WT and HET mice (Fig. 5G); whereas, this population of cells started out higher in mock-treated DKO mice and increased significantly in response to infection. Ag-experienced CD49d+CD11a+ CD4 T cell frequencies increased significantly in WT and HET mice by 14 dpi; whereas, in DKO mice, this population again started three-fold higher and increased during infection, although with high animal-to-animal variability (Fig. 5H). When evaluated further, these Ag-experienced CD4 T cells showed proportionally increased intracellular cytokine staining for IFNγ+ as well as for IFNγ+TNF+ and IFNγ+TNF+IL-2+ (Fig. 5I). Consistent with this assessment, DKO mice developed significantly greater numbers of CD4 T cells producing single (Fig. 5J - L) and multiple cytokines (Fig. 5M), indicating the presence of high numbers of terminally differentiated CD4 T cells. The elevated cytokine production may be a phenotype already present in naïve cells, given that CD4 T cells in mock-treated DKO mice displayed increased activation markers and cytokine production. Thus, Casp8 appears to dampen the expansion of Ag-experienced CD4 T cells but is dispensable for mounting a response to virus infection.
Ab response patterns in DKO mice
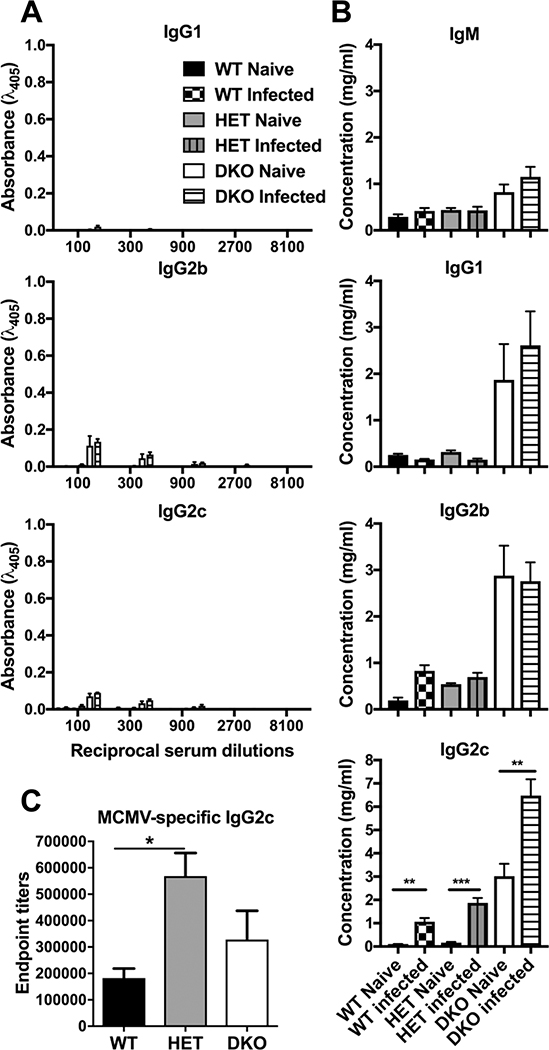
Although dispensable for limiting initial MCMV replication, Abs play a crucial role in preventing reinfection (71). We assessed the serum Ab response, comparing naïve to virus-infected mice at 14 dpi. Consistent with previous observations (52), naïve 8-week-old DKO mice had elevated anti-dsDNA Abs (Fig. 6A) compared to HET and WT mice; however, these levels did not significantly increase after infection in either DKO or control mice, regardless of Ab isotype examined. We also observed higher levels of total Ig (IgM, IgG1, IgG2b and IgG2c) in DKO mice compared to WT or HET controls (Fig. 6B), correlating with enhanced anti-dsDNA Ab levels. Despite these differences in homeostasis, all genotypes exhibited an increase in total IgG2c during infection. DKO and WT mice developed comparable MCMV-specific IgG2c titers (Fig. 6C); whereas HET mice had unexpectedly higher levels than WT controls. These data demonstrate that mice mount Ab in response to MCMV infection independent of Casp8 function.
Figure 6. Impact of Casp8 and RIPK3 deficiency on Ab levels following MCMV infection.
Mean ± S.E.M. concentrations of different Ig isotypes in sera from mice infected as described in Fig. 5H – N. Bars graph depict relative dsDNA-reactive IgG levels (A), total Ab concentrations (B) and MCMV-specific IgG2c endpoint titers (C) as derived by ELISA. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data represent one experiment using n = 5 mice per group.
Discussion:
Our study demonstrates that Casp8-deficient mice mount a robust and functional innate and adaptive immune response to natural virus infection so long as pro-necroptotic RIPK3 signaling has been eliminated. This is remarkable because both Casp8 and RIPK3 execute potent antiviral activities in the WT mouse that eliminate virus-infected cells if not blocked by virus-encoded cell death suppressors (2, 3). DKO mice survive MCMV infection and higher splenic titers are detected at 7 dpi (34) but are nevertheless controlled by 14 dpi (20), demonstrating that viral control is complete despite the absence of Casp8 and RIPK3 host defense pathways. Innate and adaptive immune control of this viral pathogen proceeds in even when both pathways are eliminated from the natural mammalian host. The cell-autonomous role of Casp8 and RIPK3 in extrinsic cell death and innate immune host defense (5), together with the contribution of these pathways to inflammatory consequences of infection (20) remain clear evidences supporting our long-standing hypothesis that Casp8 and RIPK3 evolved to eliminate pathogens rather to regulate the immune response (2, 3). It is not yet clear whether this hypothesis applies to infections with other viruses or bacteria, but such studies are clearly warranted (4, 5). Innate DC activation along with the crucial antiviral cytotoxic function of NK and T cells proceeds independent of Casp8 and RIPK3 signaling pathways, with a pattern of faster and enhanced NK and T cell expansion. Given the strong immune response in these animals, the slower viral clearance showed in the previous literature (34) may in fact be the result of a deficiency in components necessary to control virus infection in target cells when Casp8 is lacking.
Hyper-accumulation of virus-specific T cells in DKO mice was not observed in T cell-specific Cd4CreCasp8flox/floxRipk3−/− mice (11), suggesting that the influence of Casp8 is not T cell-autonomous. A previous study indicates that DCs from Cd11cCreCasp8flox/floxRipk3–/– mice exhibit improved ability to induce T cell proliferation in vitro (55). Separate studies demonstrate the resistance of Fas-deficient DCs to CTL killing in vivo, resulting in prolonged DC survival and enhanced antiviral T cell numbers (72, 73). These data suggest that disruption of the Fas-Casp8 signaling axis may extend DC lifespan and promote T cell expansion. Furthermore, increased proportion of T cells in unchallenged DKO mice upregulate CD44, CD11a and CD49d, and produce cytokines, indicating that cells acquire an activation phenotype and are more prone to proliferation when stimulated by Ag (74). It was shown that Casp8 deficiency in DCs or Macs results in T cell hyperactivation in naive mice (55, 57), a dysregulation that is reversed by eliminating Myd88 instead of RIPK3 in these myeloid cells. The data indicate a TLR-dependent dysregulation in APCs contributing to T cell hyperactivation when Casp8 is absent. Similarly, hyperaccumulation of DKO NK cells in response to viral infection may be due to their enhanced activation during homeostasis, though a marker to confirm this is yet to be identified. Taken together, Casp8 is predicted to control APC survival and innate immune signaling, thereby contributing to homeostatic T cell activation as well as cell expansion in response to viral infection.
DCs and Macs are among the most important host cell targets of MCMV infection, contributing to Ag presentation, virus dissemination and establishment of latency (61). It has become very clear that the success of infection in these virus-susceptible myeloid cell populations is dependent on virus-encoded Casp8 and RIPK3 inhibitors, vICA (15, 16, 75) and vIRA (17, 19), responsible for suppression of cell death pathways to prolong infection enough to support pathogenesis. In other words, MCMV-mediated suppression of cell death pathways in WT Macs and DCs reflects the virus-host relationship in DKO DCs where apoptosis and necroptosis are absent. Nevertheless, infected DCs constitute only a small portion of total splenic DCs in vivo and are functionally paralyzed by additional viral suppressors of Ag presentation as well as cytokine secretion (61). Indeed, activation of MCMV-specific CD8 T cells has been attributed to the role of uninfected DCs (>95% of total DCs in vivo) and Ag cross-presentation. Despite the Casp8 and RIPK3 deficiencies, both DC1s and DC2s undergo robust activation by 1.5 day after MCMV infection as indicated by the upregulation of costimulatory molecules that are critical for T cell activation (61, 76). Taken together, our data suggest that DCs retain their function in response to MCMV infection despite the absence of death-dependent and -independent functions of Casp8 and/or RIPK3.
Proinflammatory cytokines are produced through signal transduction downstream of innate pathogen sensors, playing a crucial role in DC maturation as well as cytotoxic effector cell activation. Cytokine production defects result in a compromised immune response that fails to protect hosts from pathogen invasion (77). The death-independent role of Casp8 contributes to innate immune signaling transduction directly downstream of TLR3, TLR4 and ZBP1, or indirectly through the engagement of DRs (5). Recently, this protease has been shown to regulate the priming process of inflammasome as well as to directly interact with the components in the complex (5, 78). Casp8-deficient mice exhibit low systematic cytokine levels in response to Citrobacter rodentium, Yersinia or influenza A infection (25, 28, 79–81), correlated with a defect in producing proinflammatory cytokines. Furthermore, a study of Yersinia reveals the consequence of low cytokine levels driving poor lymphocyte responses (81), especially Th1 cells that are necessary for ultimate control of the invading bacteria (82). The manifestation of immune hypoactivation to bacterial infection in Casp8-deficient mice is likely an indication of the unique role that CD4 T cells play in such settings. Surprisingly, Casp8-deficient mice exhibited normal systemic IL-12 production sufficient to promote NK and CD8 T cell responses necessary for controlling acute MCMV infection. Thus, the contributions of Casp8 to anti-microbial immune control may not be as clear cut as initially perceived as it is now evident that Casp8-dependent and independent functions in the host are likely dictated by the nature of the infecting pathogen.
The discrepancy between current natural herpesvirus pathogen and previous cross-species bacterial and influenza studies in mice may be attributed to species-specific regulation of Casp8 depending on the pathogen sensor(s) engaged. This hypothesis is supported by the defective cytokine secretion of DKO bone marrow-derived Macs in response to TLR3 and TLR4 stimulation but not to TLR2 stimulation or Sendai virus infection, which is TLR7-dependent (24, 81). Furthermore, multiple cell surface and cytosolic receptors sensing MCMV infection (83) coordinate a balanced outcome of cell survival, cell death and innate cytokine production to control infection. It is possible these sensors compensate for each other to overcome any signaling transduction defect that is a consequence of Casp8 deficiency, ensuring the induction of antiviral immunity that brings infection under control. Taken together, instead of a ubiquitous influence on innate immune signaling, Casp8 appears dispensable downstream of the innate sensors triggered by MCMV infection.
Our study shows that RIPK3-deficient mice mount robust antiviral immunity despite dysregulated homeostasis. A previous study reports that both Ripk3–/– and Ripk3K51A/K51A (38) mice retain intact antiviral immunity, questioning whether adaptor function or kinase activity of RIPK3 is generally important for the anti-MCMV immune response. By using WT and RIPK3-deficient HET mice as controls to compare with DKO mice, we have confirmed the requirement of Casp8 for suppressing the hyperaccumulation phenotype. However, the adaptor function of RIPK3 may certainly influence antiviral immunity in Casp8-deficient mice. Future studies comparing Casp8–/–Ripk3K51A/K51A or Casp8–/–MLKL–/– mice to Casp8–/–Ripk3–/– mice will help dissect the necroptosis-independent contribution of RIPK3 to Casp8-deficient animal during infection.
Although Casp8 function is critical for cell-autonomous host defense and innate signaling transduction, mice lacking Casp8 and/or RIPK3 trigger robust innate and adaptive immune response to MCMV infection. Importantly, the cytotoxic NK and CD8 T cells in DKO mice retain the full capacity to recognize and control virus, reinforcing long-standing principles in the immune control of herpesvirus infection. MCMV-infected mice develop enhanced NK and T cell expansion, possibly due to the Casp8-deficient APCs that elevate the activation state of these lymphocytes prior to the spread of infection.
Acknowledgements:
We thank Rafi Ahmed for reagents and discussion, Linda Roback for laboratory support, the National Institute of Health (NIH) Tetramer Core Facility for tetramers and the Emory Vaccine Center Flow Core for materials and operating assistance.
This work was supported by Public Health Service Grants R01 AI020211 and R01 AI118853 (to E.S.M.) and an ARCS Foundation Graduate Fellowship (to D.L.R).
Abbreviations:
- Casp
caspase
- DR
death recptor
- FADD
Fas-associated death domain protein
- cFLIPL
the long form of cellular FLICE inhibitory protein
- RIPK
receptor-interacting protein kinase
- MLKL
mixed-lineage kinase domain-like
- HCMV
human cytomegalovirus
- MCMV
murine cytomegalovirus
- HSV
herpes simplex virus
- vICA
viral inhibitor of caspase 8 activation
- vIRA
viral inhibitor of RIP activation
- ZBP, Z
nucleic acid binding protein
- RHIM, RIP
homotypic interaction motif
- WT
wild type
- DKO
Casp8−/−Ripk3−/−
- HET
Casp8+/−Ripk3−/−
- DC
dendritic cell
- ALPS
autoimmune lymphoproliferative syndrome
- SG
salivary gland
- i.p.
intraperitoneally
- RT
room temperature
- ICCS
intracellular cytokine staining
- pre
precursor-like
- cDC
conventional DC
- pDC
plasmacytoid DC
- Mac
macrophage
- IM
inflammatory monocyte
- PM
patrolling monocyte
References:
- 1.Chan FK, Luz NF, and Moriwaki K 2015. Programmed necrosis in the cross talk of cell death and inflammation. Annual review of immunology 33: 79–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocarski ES, Kaiser WJ, Livingston-Rosanoff D, Upton JW, and Daley-Bauer LP 2014. True grit: programmed necrosis in antiviral host defense, inflammation, and immunogenicity. Journal of immunology (Baltimore, Md. : 1950) 192: 2019–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mocarski ES, Guo H, and Kaiser WJ 2015. Necroptosis: The Trojan horse in cell autonomous antiviral host defense. Virology 479–480: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorgensen I, Rayamajhi M, and Miao EA 2017. Programmed cell death as a defence against infection. Nature reviews. Immunology 17: 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tummers B, and Green DR 2017. Caspase-8: regulating life and death. Immunological reviews 277: 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micheau O, and Tschopp J 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114: 181–190. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, and Mocarski ES 2013. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. The Journal of biological chemistry 288: 31268–31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, and Leverkus M 2011. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Molecular cell 43: 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He S, Liang Y, Shao F, and Wang X 2011. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proceedings of the National Academy of Sciences of the United States of America 108: 20054–20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ch’en IL, Beisner DR, Degterev A, Lynch C, Yuan J, Hoffmann A, and Hedrick SM 2008. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proceedings of the National Academy of Sciences of the United States of America 105: 17463–17468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, and Hedrick SM 2011. Mechanisms of necroptosis in T cells. The Journal of experimental medicine 208: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, and Meier P 2011. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Molecular cell 43: 432–448. [DOI] [PubMed] [Google Scholar]
- 13.Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, and Mocarski ES 2015. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. The Journal of biological chemistry 290: 11635–11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormick AL, and Mocarski ES 2013. Cell death pathways controlled by cytomegaloviruses In Cytomegaloviruses: From Molecular Pathogenesis to Intervention. Reddehase MJ, ed. Caister Scientific Press, Norfolk, United Kingdom: 263–276. [Google Scholar]
- 15.McCormick AL, Skaletskaya A, Barry PA, Mocarski ES, and Goldmacher VS 2003. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316: 221–233. [DOI] [PubMed] [Google Scholar]
- 16.Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, and Goldmacher VS 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proceedings of the National Academy of Sciences of the United States of America 98: 7829–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upton JW, Kaiser WJ, and Mocarski ES 2008. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. The Journal of biological chemistry 283: 16966–16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upton JW, Kaiser WJ, and Mocarski ES 2010. Virus inhibition of RIP3-dependent necrosis. Cell host & microbe 7: 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upton JW, Kaiser WJ, and Mocarski ES 2012. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell host & microbe 11: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daley-Bauer LP, Roback L, Crosby LN, McCormick AL, Feng Y, Kaiser WJ, and Mocarski ES 2017. Mouse cytomegalovirus M36 and M45 death suppressors cooperate to prevent inflammation resulting from antiviral programmed cell death pathways. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, and Beg AA 2000. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. Journal of virology 74: 7470–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, and Chan FK 2009. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137: 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koehler H, Cotsmire S, Langland J, Kibler KV, Kalman D, Upton JW, Mocarski ES, and Jacobs BL 2017. Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proceedings of the National Academy of Sciences of the United States of America 114: 11506–11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schock SN, Chandra NV, Sun Y, Irie T, Kitagawa Y, Gotoh B, Coscoy L, and Winoto A 2017. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway. Cell death and differentiation 24: 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH 3rd, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O, Verbist K, Gough PJ, Bertin J, Hartmann BM, Sealfon SC, Kaiser WJ, Mocarski ES, Lopez CB, Thomas PG, Oberst A, Green DR, and Balachandran S 2016. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell host & microbe 20: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, Sridharan H, Kosoff R, Shubina M, Landsteiner VJ, Andrake M, Vogel P, Sigal LJ, tenOever BR, Thomas PG, Upton JW, and Balachandran S 2016. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell host & microbe 20: 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann BM, Albrecht RA, Zaslavsky E, Nudelman G, Pincas H, Marjanovic N, Schotsaert M, Martinez-Romero C, Fenutria R, Ingram JP, Ramos I, Fernandez-Sesma A, Balachandran S, Garcia-Sastre A, and Sealfon SC 2017. Pandemic H1N1 influenza A viruses suppress immunogenic RIPK3-driven dendritic cell death. Nature communications 8: 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuriakose T, Man SM, Subbarao Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, and Kanneganti TD 2016. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Science immunology 1: aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo H, Kaiser WJ, and Mocarski ES 2015. Manipulation of apoptosis and necroptosis signaling by herpesviruses. Medical microbiology and immunology 204: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, and Mocarski ES 2015. Herpes simplex virus suppresses necroptosis in human cells. Cell host & microbe 17: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Čičin-Šain L, Ruzsics Z, Podlech J, Bubić I, Menard C, Jonjić S, Reddehase MJ, and Koszinowski UH 2008. Dominant-Negative FADD Rescues the In Vivo Fitness of a Cytomegalovirus Lacking an Antiapoptotic Viral Gene. Journal of virology 82: 2056–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo H, Gilley RP, Fisher A, Lane R, Landsteiner VJ, Ragan KB, Dovey CM, Carette JE, Upton JW, Mocarski ES, and Kaiser WJ 2018. Species-independent contribution of ZBP1/DAI/DLM-1-triggered necroptosis in host defense against HSV1. Cell death & disease 9: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, and Mocarski ES 2011. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, and Mocarski ES 2014. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proceedings of the National Academy of Sciences of the United States of America 111: 7753–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, and Lenardo MJ 2002. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419: 395–399. [DOI] [PubMed] [Google Scholar]
- 36.Misra RS, Russell JQ, Koenig A, Hinshaw-Makepeace JA, Wen R, Wang D, Huo H, Littman DR, Ferch U, Ruland J, Thome M, and Budd RC 2007. Caspase-8 and c-FLIPL associate in lipid rafts with NF-kappaB adaptors during T cell activation. The Journal of biological chemistry 282: 19365–19374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, Srinivas P, Bell BD, Luhrs KA, Lane TE, Salvesen GS, and Walsh CM 2011. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proceedings of the National Academy of Sciences of the United States of America 108: 15312–15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, and Kaiser WJ 2014. RIP3 induces apoptosis independent of pronecrotic kinase activity. Molecular cell 56: 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bidere N, Su HC, and Lenardo MJ 2006. Genetic disorders of programmed cell death in the immune system. Annual review of immunology 24: 321–352. [DOI] [PubMed] [Google Scholar]
- 40.Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, Fleisher TA, Lim MS, Jaffe ES, Puck JM, Lenardo MJ, and Straus SE 1997. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood 89: 1341–1348. [PubMed] [Google Scholar]
- 41.Fleck M, Kern ER, Zhou T, Podlech J, Wintersberger W, Edwards CK, and Mountz JD 1998. Apoptosis mediated by Fas but not tumor necrosis factor receptor 1 prevents chronic disease in mice infected with murine cytomegalovirus. The Journal of Clinical Investigation 102: 1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, and Green DR 2011. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471: 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng L, Li J, and Lenardo M 2017. Restimulation-induced cell death: new medical and research perspectives. Immunological reviews 277: 44–60. [DOI] [PubMed] [Google Scholar]
- 44.Orange JS, and Biron CA 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. Journal of immunology (Baltimore, Md. : 1950) 156: 1138–1142. [PubMed] [Google Scholar]
- 45.Livingston-Rosanoff D, Daley-Bauer LP, Garcia A, McCormick AL, Huang J, and Mocarski ES 2012. Antiviral T cell response triggers cytomegalovirus hepatitis in mice. Journal of virology 86: 12879–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redwood AJ, Messerle M, Harvey NL, Hardy CM, Koszinowski UH, Lawson MA, and Shellam GR 2005. Use of a murine cytomegalovirus K181-derived bacterial artificial chromosome as a vaccine vector for immunocontraception. Journal of virology 79: 2998–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Handke W, Luig C, Popovic B, Krmpotic A, Jonjic S, and Brune W 2013. Viral inhibition of BAK promotes murine cytomegalovirus dissemination to salivary glands. Journal of virology 87: 3592–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning WC, Stoddart CA, Lagenaur LA, Abenes GB, and Mocarski ES 1992. Cytomegalovirus determinant of replication in salivary glands. Journal of virology 66: 3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sangster MY, Topham DJ, D’Costa S, Cardin RD, Marion TN, Myers LK, and Doherty PC 2000. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. Journal of immunology (Baltimore, Md. : 1950) 164: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 50.Snyder CM, Cho KS, Bonnett EL, Allan JE, and Hill AB 2011. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS pathogens 7: e1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daley-Bauer LP, Wynn GM, and Mocarski ES 2012. Cytomegalovirus impairs antiviral CD8+ T cell immunity by recruiting inflammatory monocytes. Immunity 37: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvarez-Diaz S, Dillon CP, Lalaoui N, Tanzer MC, Rodriguez DA, Lin A, Lebois M, Hakem R, Josefsson EC, O’Reilly LA, Silke J, Alexander WS, Green DR, and Strasser A 2016. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity 45: 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durai V, and Murphy KM 2016. Functions of Murine Dendritic Cells. Immunity 45: 719–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schraml BU, and Reis e Sousa C 2015. Defining dendritic cells. Current opinion in immunology 32: 13–20. [DOI] [PubMed] [Google Scholar]
- 55.Cuda CM, Misharin AV, Gierut AK, Saber R, Haines GK 3rd, Hutcheson J, Hedrick SM, Mohan C, Budinger GS, Stehlik C, and Perlman H 2014. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. Journal of immunology (Baltimore, Md. : 1950) 192: 5548–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang TB, Yang SH, Toth B, Kovalenko A, and Wallach D 2013. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38: 27–40. [DOI] [PubMed] [Google Scholar]
- 57.Cuda CM, Misharin AV, Khare S, Saber R, Tsai F, Archer AM, Homan PJ, Haines GK 3rd, Hutcheson J, Dorfleutner A, Budinger GR, Stehlik C, and Perlman H 2015. Conditional deletion of caspase-8 in macrophages alters macrophage activation in a RIPK-dependent manner. Arthritis research & therapy 17: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuda CM, Agrawal H, Misharin AV, Haines GK 3rd, Hutcheson J, Weber E, Schoenfeldt JA, Mohan C, Pope RM, and Perlman H 2012. Requirement of myeloid cell-specific Fas expression for prevention of systemic autoimmunity in mice. Arthritis and rheumatism 64: 808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, Ramakrishnan P, Lapidot T, and Wallach D 2004. Caspase-8 serves both apoptotic and nonapoptotic roles. Journal of immunology (Baltimore, Md. : 1950) 173: 2976–2984. [DOI] [PubMed] [Google Scholar]
- 60.Katagiri T, Cohen PL, and Eisenberg RA 1988. The lpr gene causes an intrinsic T cell abnormality that is required for hyperproliferation. The Journal of experimental medicine 167: 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexandre YO, Cocita CD, Ghilas S, and Dalod M 2014. Deciphering the role of DC subsets in MCMV infection to better understand immune protection against viral infections. Frontiers in microbiology 5: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, and Biron CA 2002. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. The Journal of experimental medicine 195: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, and Yokoyama WM 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proceedings of the National Academy of Sciences of the United States of America 99: 8826–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, and Degli-Esposti MA 2003. Functional interactions between dendritic cells and NK cells during viral infection. Nature immunology 4: 175–181. [DOI] [PubMed] [Google Scholar]
- 65.Selgrade MK, Nedrud JG, Collier AM, and Gardner DE 1981. Effects of cell source, mouse strain, and immunosuppressive treatment on production of virulent and attenuated murine cytomegalovirus. Infection and immunity 33: 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitrovic M, Arapovic J, Jordan S, Fodil-Cornu N, Ebert S, Vidal SM, Krmpotic A, Reddehase MJ, and Jonjic S 2012. The NK cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8(+) T-cell response. Journal of virology 86: 2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jonjic S, Pavic I, Lucin P, Rukavina D, and Koszinowski UH 1990. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. Journal of virology 64: 5457–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, Scalzo AA, Smyth MJ, and Degli-Esposti MA 2010. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. The Journal of experimental medicine 207: 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walton S, Mandaric S, and Oxenius A 2013. CD4 T cell responses in latent and chronic viral infections. Frontiers in immunology 4: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verma S, Weiskopf D, Gupta A, McDonald B, Peters B, Sette A, and Benedict CA 2015. Cytomegalovirus-Specific CD4 T Cells Are Cytolytic and Mediate Vaccine Protection. Journal of virology 90: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, and Koszinowski UH 1994. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. The Journal of experimental medicine 179: 1713–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, and Chervonsky AV 2007. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26: 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Varanasi V, Khan AA, and Chervonsky AV 2014. Loss of the death receptor CD95 (Fas) expression by dendritic cells protects from a chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America 111: 8559–8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho BK, Rao VP, Ge Q, Eisen HN, and Chen J 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. The Journal of experimental medicine 192: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menard C, Wagner M, Ruzsics Z, Holak K, Brune W, Campbell AE, and Koszinowski UH 2003. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. Journal of virology 77: 5557–5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, and Biron CA 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. The Journal of experimental medicine 197: 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loewendorf A, and Benedict CA 2010. Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything. Journal of internal medicine 267: 483–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mandal P, Feng Y, Lyons JD, Berger SB, Otani S, DeLaney A, Tharp GK, Maner-Smith K, Burd EM, Schaeffer M, Hoffman S, Capriotti C, Roback L, Young CB, Liang Z, Ortlund EA, DiPaolo NC, Bosinger S, Bertin J, Gough PJ, Brodsky IE, Coopersmith CM, Shayakhmetov DM, and Mocarski ES 2018. Caspase-8 Collaborates with Caspase-11 to Drive Tissue Damage and Execution of Endotoxic Shock. Immunity 49: 42–55.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK, Kelliher MA, Harris PA, Bertin J, Gough PJ, Shayakhmetov DM, Goguen JD, Fitzgerald KA, Silverman N, and Lien E 2014. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proceedings of the National Academy of Sciences of the United States of America 111: 7391–7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, and Kanneganti TD 2014. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. Journal of immunology (Baltimore, Md. : 1950) 192: 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM, Shin S, Wei L, Parker M, Zhang J, Oberst A, Green DR, and Brodsky IE 2014. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proceedings of the National Academy of Sciences of the United States of America 111: 7385–7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Autenrieth IB, Tingle A, Reske-Kunz A, and Heesemann J 1992. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infection and immunity 60: 1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rathinam VA, and Fitzgerald KA 2011. Innate immune sensing of DNA viruses. Virology 411: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]