The prevalence of fungal infections has seen a rise in the past decades due to advances in modern medicine leading to an expanding population of device-associated and immunocompromised patients. Furthermore, the spectrum of pathogenic fungi has changed, with the emergence of multidrug-resistant strains such as C. auris. High mortality related to fungal infections points to major limitations of current antifungal therapy and an unmet need for new antifungal drugs. We screened a library of repurposed FDA-approved inhibitors to identify compounds with activities against a diverse range of fungi in varied phases of growth. The assays identified alexidine dihydrochloride (AXD) to have pronounced antifungal activity, including against preformed biofilms, at concentrations lower than mammalian cell toxicity. AXD potentiated the activity of fluconazole and amphotericin B against Candida biofilms in vitro and prevented biofilm growth in vivo. Thus, AXD has the potential to be developed as a pan-antifungal, antibiofilm drug.
KEYWORDS: Candida albicans, FDA, HTS, antifungal agents, biofilms, panfungal
ABSTRACT
Invasive fungal infections due to Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans constitute a substantial threat to hospitalized immunocompromised patients. Further, the presence of drug-recalcitrant biofilms on medical devices and emergence of drug-resistant fungi, such as Candida auris, introduce treatment challenges with current antifungal drugs. Worse, currently there is no approved drug capable of obviating preformed biofilms, which increase the chance of infection relapses. Here, we screened a small-molecule New Prestwick Chemical Library, consisting of 1,200 FDA-approved off-patent drugs against C. albicans, C. auris, and A. fumigatus, to identify those that inhibit growth of all three pathogens. Inhibitors were further prioritized for their potency against other fungal pathogens and their ability to kill preformed biofilms. Our studies identified the bis-biguanide alexidine dihydrochloride (AXD) as a drug with the highest antifungal and antibiofilm activity against a diverse range of fungal pathogens. Finally, AXD significantly potentiated the efficacy of fluconazole against biofilms, displayed low mammalian cell toxicity, and eradicated biofilms growing in mouse central venous catheters in vivo, highlighting its potential as a pan-antifungal drug.
IMPORTANCE The prevalence of fungal infections has seen a rise in the past decades due to advances in modern medicine leading to an expanding population of device-associated and immunocompromised patients. Furthermore, the spectrum of pathogenic fungi has changed, with the emergence of multidrug-resistant strains such as C. auris. High mortality related to fungal infections points to major limitations of current antifungal therapy and an unmet need for new antifungal drugs. We screened a library of repurposed FDA-approved inhibitors to identify compounds with activities against a diverse range of fungi in varied phases of growth. The assays identified alexidine dihydrochloride (AXD) to have pronounced antifungal activity, including against preformed biofilms, at concentrations lower than mammalian cell toxicity. AXD potentiated the activity of fluconazole and amphotericin B against Candida biofilms in vitro and prevented biofilm growth in vivo. Thus, AXD has the potential to be developed as a pan-antifungal, antibiofilm drug.
INTRODUCTION
Fungal pathogens responsible for invasive fungal infections (IFIs) are a leading cause of human mortality, killing approximately one and a half million people every year despite treatment with antifungal drugs (1). Of concern, the current incidence of fungal infection-related deaths is reported to be even higher than mortality due to tuberculosis or malaria (2). A vast majority of IFIs result from species belonging to Cryptococcus, Candida, or Aspergillus (3). However, fungi such as molds other than Aspergillus, and non-albicans Candida species, including the multidrug-resistant pathogen Candida auris, are becoming increasingly frequent and difficult to treat (4). Furthermore, other IFIs such as those due to Mucorales cause highly angioinvasive and tissue-destructive infections, which in many cases have mortality rates close to 100% (2).
The challenge in treatment of IFIs is directly linked to an ever-expanding population of immunocompromised patients requiring modern medical interventions and a paucity of currently approved antifungal agents (5, 6). Indwelling medical devices infected with fungi develop biofilms that are notoriously resistant to all classes of antifungal drugs and serve as a reservoir of infectious cells with direct access to the vasculature (7, 8). The current therapeutic armamentarium for IFIs is sparse, including only three classes of antifungal agents: polyenes, azoles, and echinocandins. These drugs have drawbacks, including significant limitations in spectrum of activity, human toxicity, and emergence of drug resistance, thereby underscoring a need for development of new antifungal agents (9, 10).
To fulfil this unmet need, we employed a high-throughput screening (HTS) assay to screen and characterize FDA (U.S. Food and Drug Administration)-approved, off-patent library drugs for their abilities to kill/inhibit three of the most invasive and drug-resistant human-pathogenic fungi, Candida albicans, C. auris, and Aspergillus fumigatus. This assay allowed us to identify core fungicidal molecules against all three pathogens. One of the leading compounds identified was a bis-biguanide dihydrochloride called alexidine dihydrochloride (AXD). AXD is an anticancer drug that targets a mitochondrial tyrosine phosphatase, PTPMT1, in mammalian cells and causes mitochondrial apoptosis (11). We found that AXD not only inhibited planktonic growth but also prevented biofilm formation as well as killing biofilms formed by a variety of drug-resistant and susceptible isolates of diverse fungal organisms. Further, when used in combination, AXD reduced the MIC of fluconazole and amphotericin B and rendered them efficacious against drug-resistant C. albicans biofilms. Finally, the antibiofilm property of AXD was also recapitulated in an in vivo mouse central venous catheter model of C. albicans biofilm formation. Overall, our studies warrant the further development of AXD as a panfungal antibiofilm drug, which could be used in combination therapeutics against diverse fungal pathogens.
RESULTS AND DISCUSSION
High-throughput screening (HTS) for identification of antifungal molecules.
We used an HTS assay to test the ability of a commercially available, small-molecule library containing 1,233 FDA-approved compounds (New Prestwick Chemical [NPW] Library). We reckoned that repositioning existing off-patent drugs with known human safety and bioavailability profiles can accelerate the antifungal drug-discovery process without undergoing the arduous FDA approval process. These compounds were screened to identify a core set of inhibitors and fungicidals against C. albicans, A. fumigatus, and C. auris. The former two fungi represent two of the top four fungal pathogens causing IFIs with 40 to 70% mortality rates (3). C. auris is a newly emerging fungus that represents a serious global health threat due to its multidrug-resistant properties (12). We used cell viability as a parameter for prioritizing the broad-spectrum FDA-approved molecules as lead drugs for developing panfungal therapeutics. The purpose was to first identify a core set of molecules that could inhibit a diverse collection of fungi spanning different genera and species, under planktonic growth conditions.
HTS was performed in a 384-well plate screening format where the NPW library was screened against planktonic yeast or spore suspensions of the three fungal organisms, at a single concentration of 10 µM. The spectrum of activity of these drugs was compared to clinically used azole drugs (fluconazole or voriconazole) at a concentration ranging from 0.03 to 32 µg/ml. MICs of drugs were determined in agreement with the CLSI M27-A3 (for yeast) and M38-A2 (for filamentous fungi) reference standards for antifungal susceptibility testing (13, 14). After 3 days of incubation at 37°C, turbidity of the wells (OD600) was measured and molecules displaying >50% reduction in turbidity compared to control non-drug-treated wells (MIC50) were considered primary “hits.” Z′ factor was calculated as a parameter of HTS quality, and an average Z′ factor of 0.75 was computed for our assays (a value of >0.5 represents an excellent quality of HTS) (15).
From this hit list, a core set of molecules that inhibited planktonic growth of all three fungi as identified by >50% growth inhibition measured by MIC were identified and shortlisted. C. albicans was sensitive to fluconazole at concentrations of <0.125 to 0.25 µg/ml, as has been reported previously (16), while, consistent with its drug-resistant nature, C. auris was resistant to fluconazole with MICs of >16 µg/ml (see Table 2). A. fumigatus succumbed to voriconazole at 0.25 µg/ml, similarly to previously reported antifungal drug susceptibility studies (16). Recently, Siles et al. investigated the ability of Prestwick Chemical Library to specifically inhibit C. albicans biofilms and revealed 38 pharmacologically active agents against the fungus (17). While our study also identified a number of molecules individually inhibiting the three fungi, respectively (Table 1), only the following six compounds were successful at inhibiting all three organisms: chloroxine, thimerosal, alexidine dihydrochloride, haloprogin, clioquinol, and butenafine hydrochloride (Table 1). NPW contains a number of antifungal drugs, such as imidazoles, triazoles, and the polyene class of drugs. C. auris was by far the most resistant fungus, inert against the azoles and polyenes in the library. The six molecules were further evaluated for their ability to curtail biofilm formation as well as kill preformed biofilms developed by the three fungi. Table 1 lists primary hits that were confirmed and narrowed after a repeat screen under similar planktonic conditions.
TABLE 1.
Hits obtained from replicate primary screening of the New Prestwick Chemical Library against planktonic cells of C. albicans, A. fumigatus, and C. auris
| Organism | Hits |
|---|---|
| C. albicans | Alexidine dihydrochloride, amphotericin B, antimycin A, butenafine hydrochloride, chloroxine, ciclopirox ethanolamine, clioquinol, clotrimazole, dequalinium dichloride, econazole nitrate, enilconazole, fluconazole, flucytosine, haloprogin, isoconazole, isoxsuprine hydrochloride, itraconazole, ketoconazole, methyl benzethonium chloride, pyrvinium pamoate, sertaconazole nitrate, sulconazole nitrate, terconazole, thimerosal, thonzonium bromide, tioconazole, voriconazole |
| A. fumigatus | Alexidine dihydrochloride, butenafine hydrochloride, isoxsuprine hydrochloride, clioquinol, thimerosal, dequalinium dichloride, pyrvinium pamoate, haloprogin, chlorhexidine, voriconazole, amphotericin B, antimycin A, econazole nitrate, enilconazole, methyl benzethonium chloride, tioconazole |
| C. auris | Alexidine dihydrochloride, butenafine hydrochloride, chloroxine, clioquinol, thimerosal, haloprogin |
Secondary assays for determination of antibiofilm activity.
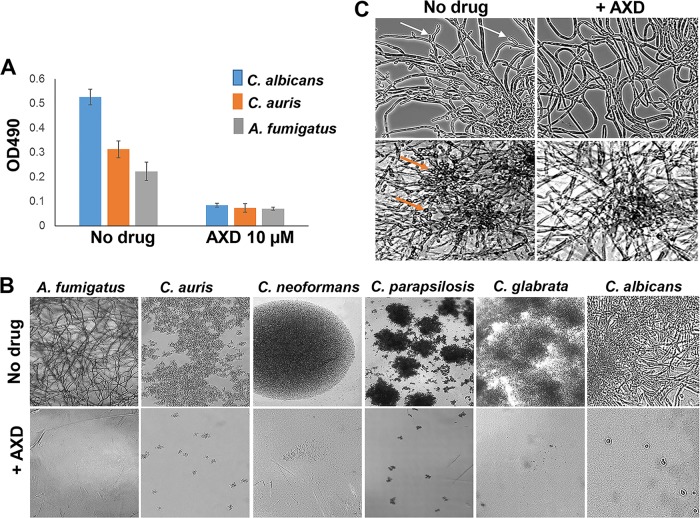
Wells with C. albicans, C. auris, and A. fumigatus were either treated with inhibitors at the time of yeast/spore inoculation (start of biofilm initiation) or allowed to grow without drugs for 48 h to allow biofilm development (mature biofilm). This assay was performed in a 96-well microtiter plate assay, as previously reported by us (18). For the effect on formation of biofilm, all six inhibitors could inhibit biofilm formation in the three fungal organisms, as adjudged by a significant decrease in turbidity of the media in the wells 48 h following incubation with the drugs (data not shown). However, only two drugs, alexidine dihydrochloride and thimerosal, could significantly kill 80% of mature biofilm community at the tested concentration of <10 µM (Fig. 1A; see also Fig. S1A in the supplemental material). We chose to focus our attention to studying alexidine dihydrochloride (AXD), since it was more attractive with respect to drug development, having indications for use as an antibacterial and antiplaque agent and with limited side effects (19–21).
FIG 1.
Inhibition of biofilm growth, C. albicans biofilm dispersal, and abrogation of planktonic growth in diverse fungi by alexidine dihydrochloride (AXD). (A) Fungal cells were allowed to form a biofilm for 48 h and treated for 24 h with 10 µM AXD. Biofilm inhibition was as determined by XTT reading (OD490). (B) Fungal yeast cells or spores were incubated with different concentrations of AXD under planktonic conditions. Inhibition of growth and filamentation of the fungi visualized by phase-contrast microscopy (20× magnification), at their respective AXD MIC80s. (C) C. albicans planktonic hyphae (top two panels) and biofilms (bottom two panels) were treated for 12 h with 150 ng/ml of AXD. AXD inhibited lateral yeast production from hyphal cells and hyphal layers of biofilms, as visualized microscopically. Arrows point to lateral yeasts.
Primary screening: MIC80 of alexidine dihydrochloride and thimerosal against preformed biofilms of three different fungi as determined by XTT reading (OD490). Both drugs kill biofilm cells at <10 µM concentrations. Download FIG S1, TIF file, 2.9 MB (3MB, tif) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Dose-response assays of AXD.
AXD was first evaluated in planktonic and biofilm dose-response assays and further tested for its ability to inhibit growth of other fungal pathogens, including drug-resistant clinical isolates, a number of non-albicans Candida spp., and members of the Mucorales family. The results for this study are described in Table 2, which lists the fungal strains used for evaluation of the efficacy of AXD, under three different growth conditions—planktonic, biofilm inhibition, and preformed biofilms, compared to the MIC of the control azole antifungal drugs. For example, AXD displayed activity against most Candida spp.; MIC values of ≤1.5 μg/ml were observed for all isolates tested under planktonic conditions, with the exception of Candida parapsilosis and Candida krusei. Interestingly, AXD also displayed striking activity against clinically relevant fluconazole-resistant Candida isolates: C. albicans (CA2, CA6, and CA10), C. glabrata (CG2 and CG5), C. parapsilosis (CP5), and C. auris (CAU-09 and CAU-03). Furthermore, the MIC values of AXD against C. neoformans were comparable to the MIC values for fluconazole.
TABLE 2.
MICs of AXD against clinical isolates of different fungal species versus fluconazole or voriconazolea
| Isolate | MIC (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| Planktonic azole | Planktonic AXD |
Biofilm-inhibitory AXD |
Mature biofilm AXD |
||||
| 50% | 80% | 50% | 80% | 50% | 80% | ||
| Fluconazole | |||||||
| CA:SC5314 | <0.5 | 0.79 | 0.73 | 0.73 | 0.73 | 3 | 12 |
| CA1 | <0.125 | 1.5 | 1.5 | 0.73 | 0.73 | 6 | 12 |
| *CA2 | 16 | 0.94 | 0.94 | 1.5 | 1.5 | 3 | 20 |
| CA4 | <0.125 | 1.5 | 1.5 | 0.73 | 0.73 | 3 | 12 |
| *CA6 | 16 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 6 |
| *CA10 | 32 | 1.5 | 1.5 | 0.73 | 1.5 | 3 | 6 |
| CG1 | 2 | 0.73 | 1.5 | 0.73 | 1.5 | 3 | 6 |
| CG2 | 32 | 0.73 | 1.5 | 0.15 | 1.5 | 3 | 6 |
| CG3 | 2 | 0.73 | 1.5 | 0.73 | 1.5 | 3 | 6 |
| CG4 | 4 | 1.10 | 1.1 | 1.5 | 3 | 3 | 6 |
| *CG5 | 256 | 1.14 | 1.14 | 1.5 | 3 | 3 | 12 |
| CP1 | 0.25 | 1.5 | 3 | 3 | 3 | 3 | 3 |
| CP2 | 0.25 | 3 | 6 | 3 | 3 | 3 | 6 |
| CP3 | 2 | 3 | 6 | 3 | 3 | 6 | >12 |
| CP4 | <0.12 | 1.4 | 1.4 | 2.5 | 3 | 3 | 3 |
| *CP5 | 64 | 2.29 | 3 | 4 | 6 | 6 | 6 |
| CK | NA | 2.21 | 3 | 2.5 | 6 | 6 | 6 |
| CT1 | 2 | 0.84 | 0.84 | 0.84 | 1.5 | 1.5 | 3 |
| *CT2 | >256 | 0.84 | 0.84 | 0.84 | 1.5 | 1.5 | 3 |
| CN1 | 1 | 0.73 | 0.73 | 1.5 | 6 | 3 | 6 |
| CN2 | 0.5 | 0.73 | 0.73 | 0.73 | 3 | 3 | 3 |
| CN3 | 1 | 1.5 | 1.5 | 1.5 | 3 | 1.5 | 1.5 |
| CAU-03 | 32 | 0.73 | 1.5 | 3 | 3 | 3 | 3 |
| CAU-09 | 16 | 1.5 | 1.5 | 6 | 6 | 3 | 6 |
| Posaconazole | |||||||
| R. delemar 99.880 | 0.25 | 1.5 | 1.5 | NT | NT | NT | NT |
| R. oryzae 99.892 | 0.25 | 1.5 | 3 | NT | NT | NT | NT |
| Voriconazole | |||||||
| M. circinelloides 131 | 8 | 0.73 | 3 | NT | NT | NT | NT |
| L. corymbifera 008049 | >32 | 3 | 6 | NT | NT | NT | NT |
| C. bertholletiae 182 | 8 | 3 | 6 | NT | NT | NT | NT |
| S. apiospermum DI16-478 | 8 | 1.5 | 1.5 | NT | NT | NT | NT |
| AF293 | 0.25 | 0.73 | 3 | 0.73 | 3 | 6 | 6 |
| AF1 | 1 | 0.73 | 3 | 0.73 | 3 | 6 | 6 |
| AF2 | 0.25 | 1.5 | 6 | 1.5 | 3 | 6 | 6 |
| AF3 | 0.25 | 1.5 | 3 | 1.5 | 3 | 6 | 6 |
CA, C. albicans; CAU, C. auris; CG, C. glabrata; CP, C. parapsilosis; CK, C. krusei; CN, C. neoformans; AF, A. fumigatus; NA, not available; NT, not tested. The asterisks signify drug-resistant clinical isolates.
In the case of filamentous fungi, low AXD MIC50 values of 1.5 to 3 μg/ml were observed for all filamentous fungi (Mucorales and Aspergillus spp., plates read at 48 h), including the molds L. corymbifera and S. apiospermum (read at 72 h), which have poor outcomes with current clinically available antifungal drugs (22). Inhibition of planktonic growth by AXD monitored microscopically revealed a complete inhibition of filamentation or proliferation of the imaged fungi (Fig. 1B). Of particular importance was the finding that AXD was able to decimate at low concentrations (1.5 to 6 μg/ml) mature biofilms of Candida, Cryptococcus, and Aspergillus spp. that are known to be resistant to almost all classes of antifungal drugs (Table 2; also see Fig. S2 for AXD activity on Candida spp.). In fact, at 10-fold-lower concentrations (150 ng/ml) of planktonic MICs, AXD could inhibit lateral yeast formation and biofilm dispersal in C. albicans (Fig. 1C). The dispersal of lateral yeast cells from a biofilm biomass is the link between contaminated catheters and disseminated candidiasis (8, 23). Inhibition of dispersal with just nanomolar levels of AXD can help seal the biofilm reservoir and curtail further proliferation and robustness of a biofilm.
Inhibitory effect of AXD against preformed biofilms of four different fluconazole-resistant Candida spp. AXD kills preformed biofilms between 3 and 6 µg. Download FIG S2, TIF file, 2.7 MB (2.7MB, tif) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Alexidine dihydrochloride is a bis-biguanide in which the common 2-ethylhexyl chain has been attached to each biguanide unit and the two units are linked by a 1,6-hexanediamine chain (Fig. S3A). This compound, initially identified for its antibacterial properties, is also found as an inducer of mitochondrial dysfunction and apoptosis (11, 24). AXD has been proposed in several studies as an antiplaque agent and mouthwash and with potential to be used in endodontic treatment to eliminate biofilms (19, 20, 25). These reports, along with our present findings, serve as precedents for the development of AXD as an antibiofilm agent. Another bis-biguanide with structural similarity to AXD, metformin, has recently been shown to have antifungal activity (and synergistic potentiation of clinically used antifungal drugs) against C. glabrata, albeit only at physiologically inapt high concentrations (26). AXD, on the other hand, is active even at levels as low as 0.75 µg/ml against an array of fungal species in our current study. In fact, AXD has been reported to have activity against the fungus C. neoformans, by targeting phospholipases (27). Whether specific inhibition of fungal phospholipases is the cause of AXD’s antifungal activity against a spectrum of pathogenic fungi is unknown and remains to be explored in future studies.
(A) Chemical structure of alexidine dihydrochloride. (B) Inhibition of early cellular division of HL60 cells by AXD. Download FIG S3, TIF file, 2.9 MB (2.9MB, tif) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mammalian cell cytotoxicity assays and synergy of AXD with fluconazole.
Considering that AXD displayed enhanced efficacy against fungal organisms, we evaluated the extent of its cell toxicity (CC50) to mammalian cells. The results showed that AXD resulted in 50% killing of HUVECs and lung epithelial cells, at concentrations 5- to 10-fold higher than the MIC required to kill planktonically growing fungal pathogens (CC50 of >7.37 μg/ml versus planktonic MIC50 of 0.73 to 1.5 μg/ml) (Fig. 2A and B). Previous studies have reported similar cytotoxicity levels of AXD against various other cell lines (19, 27, 28).
FIG 2.
Toxicity of AXD on host cells and on biofilm killing in combination with fluconazole. Different concentrations of AXD were incubated with HUVECs (A), lung A549 cells (B), or macrophages (C) for 24 h at 37°C, for testing the CC50 of the drug to the respective cell lines. (D) C. albicans biofilms were developed for 48 h and then treated with different concentrations of AXD and fluconazole in a checkerboard format. Metabolic activity of biofilm cells was measured by the XTT assay. Bright red represents growth above the MIC50, dull red represents growth at the MIC50, and black/dark red represents growth below the MIC50.
We further tested the toxicity of AXD for a human bone marrow-derived macrophage cell line to understand its effect on the immune cells. AXD displayed a slightly higher toxicity to the macrophages than the mammalian tissue cell lines, with a CC50 of over 5 μg/ml (Fig. 2C). A similar study was also done to test the impact of AXD toxicity on HL60 monocyte proliferation. HL60 cells stained with CFSE were treated with various concentrations of AXD, or the control PMA (phorbol 12-myristate 13-acetate) as a positive stimulant that induces cellular proliferation. Inhibition of cellular proliferation corresponds to toxicity, and the concentration of AXD that could prevent early cell division in HL60 cells was examined. As expected, cells stimulated by PMA showed proliferation, while those treated with the highest dose of AXD (10 µg/ml) did not divide. AXD at 5 µg/ml prevented cell division in the human bone marrow-derived HL60 cells (Fig. S3B). This level of toxicity matched the macrophage CC50 value. We note that concentrations detrimental to host cells are at least 3- to 4-fold higher than AXD levels required to inhibit planktonic cells of many different fungi, including C. albicans. These moderately low cytotoxicities of the FDA-approved drug pave the way to a potential repurposing of AXD as an antifungal agent and warrant its further development into a compound with higher efficacy and bioavailability and less toxicity.
This inhibitory potential was further highlighted in our studies evaluating synergistic action of AXD in combination with fluconazole against mature C. albicans biofilms. Fluconazole is completely inert against C. albicans biofilms, with an MIC50 of >250 μg/ml (this study and references 7, 29, and 30). When used together, AXD at 1.25 μg/ml strikingly reduced the MIC50 of fluconazole from >256 μg/ml to a clinically relevant 1 μg/ml (Fig. 2D), providing an FIC index of 0.42, which indicated a synergistic interaction (30). These results further emphasize AXD’s prospects as an antibiofilm agent, especially due to its ability to lower MICs of fluconazole, highlighting the possibility of bringing a biofilm-redundant drug back into clinical use.
Inhibition of biofilm in vivo by AXD.
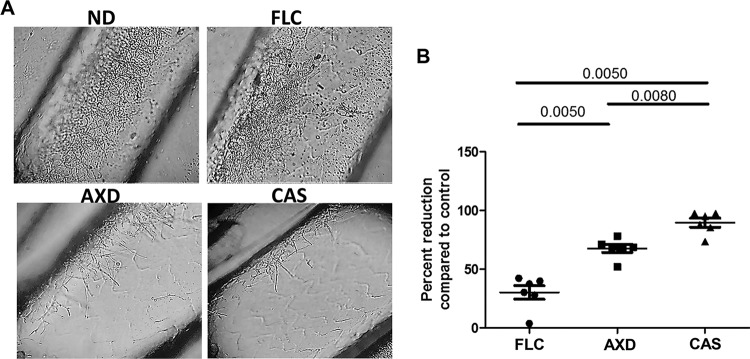
Our studies showed that AXD could arrest growth and kill biofilm cells formed by various Candida species, C. neoformans, and A. fumigatus in in vitro assays. We next examined the ability of AXD to decimate preformed biofilms in an in vivo model. For this study, we chose to focus on biofilm formation by C. albicans, since a murine biofilm model has been well established in this fungus and used for testing the effects of established and new antifungal agents (31). The effect of the drugs on the 24-h-old biofilms growing in the jugular vein catheters of mice was visualized microscopically, which revealed significantly lower density of the biofilms in catheters treated with AXD and caspofungin, versus the control untreated catheters (Fig. 3A). In fact, fungal CFU determination revealed that AXD inhibited 67% of fungal biofilm growth and viability, compared to the control untreated biofilms (Fig. 3B). As expected, caspofungin (an antifungal drug known to be hyperactive against C. albicans biofilms) decimated >90% of the biofilm community growing within the catheters. On the other hand, fluconazole (a drug with enhanced activity against planktonic fungi, but with limited activity against biofilm cells) was found to reduce biofilms by only 30% (P value = 0.028) (Fig. 3B). Overall, our data show that AXD can inhibit biofilm growth in vivo. A better understanding of the pharmacokinetics/pharmacodynamics of AXD could be invaluable in assessing its utility as a systemic antifungal drug, especially in a disseminated mouse model of fungemia.
FIG 3.
Impact of AXD, fluconazole (FLC), and caspofungin (CAS) as lock therapy against C. albicans biofilm cells in an in vivo catheter model. (A) Biofilms were grown for 24 h followed by intraluminal drug treatment for 24 h. Following compound exposure, the catheters were removed for microscopy and CFU enumeration. Each of the four panels represent a 40× magnification under phase-contrast microscope. Panel columns: no drug treatment (ND), control biofilm treated with saline; FLC, 125-μg/ml fluconazole exposure; AXD, catheters exposed to AXD at 3 μg/ml; CAS, catheters exposed to 0.25 μg/ml caspofungin. (B) After ND or drug treatment, catheters were cut into pieces, vortexed, and sonicated to release adhered cells in sterile PBS, and dilutions of the suspension were plated on solid medium for CFU enumeration. Results are presented as percent biofilm reduction in drug-treated catheters compared to the untreated catheter biofilms and analyzed statistically by using a nonparametric t test. A P value of <0.05 is significant.
In summary, our HTS identified alexidine dihydrochloride to have profound activity against various growth forms of fungi: planktonic, biofilm, and biofilm dispersal. AXD was fungicidal to a number of different pathogenic fungi, including common as well as emerging drug-resistant pathogens. The fact that AXD retains its activity against azole-resistant clinical isolates indicates its potential use in recalcitrant fungal infections. Importantly, AXD reduces the MIC of fluconazole, a clinically used first-line antifungal drug, ironically considered dispensable for biofilm treatment, thereby pointing to its extended utility as an antibiofilm combination drug. Perhaps the most intriguing activity of AXD was seen against Mucorales, including Rhizopus, a species that leads to devastating infections and very poor outcomes in patients, despite conventional antifungal treatment. Furthermore, the drug was also potent against those fungi that are therapeutically unmanageable in clinics with current antifungal agents such as L. corymbifera and S. apiospermum. Future studies will focus on the mechanism of action of AXD at a molecular level and evaluate its feasibility as a pan-antifungal drug to combat infections in different clinical settings.
MATERIALS AND METHODS
Strains, media, and culture conditions.
The following fungal strains were used in this study: C. albicans strain SC5314, which is a human clinical isolate recovered from a patient with generalized candidiasis (32), and several clinical isolates of Candida spp. received from the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio—fluconazole-sensitive C. albicans CA1 and CA4; fluconazole-resistant C. albicans CA6 and CA10; C. glabrata fluconazole-sensitive CG1 and CG3 and fluconazole-resistant CG2; C. parapsilosis CP1, CP2, and CP3; C. neoformans CN1, CN2, and CN3; and A. fumigatus AF1, AF2, and AF3. Some Candida strains were also obtained from the Division of Infectious Disease, Massachusetts General Hospital, Boston, MA: fluconazole-resistant strains of C. albicans CA2, C. parapsilosis CP4 and CP5, C. krusei CK, and C. tropicalis CT2. The two C. auris isolates CAU-03 and CAU-09 were a kind gift from Shawn Lockhart, Centers for Disease Control and Prevention (CDC), and the filamentous fungi, including Rhizopus delemar 99.880 and Rhizopus oryzae 99.892, L. corymbifera 008049, C. bertholletiae 182, M. circinelloides 131, and S. apiospermum DI16-478, were a part of the fungal bank at Division of Infectious Diseases, Los Angeles Biomedical Research Center. All cultures were maintained by subculture on yeast-peptone-dextrose medium (YPD) at 37°C, and stocks of these cultures were stored in 20% glycerol at −80°C.
HTS.
Screening was performed at the Molecular Screening Shared Resource facility at the University of California, Los Angeles. A total of 50 µl of 1 × 104 fungal yeast cells/ml (C. albicans and C. auris) or spores (Aspergillus) was suspended in RPMI 1640 supplemented with l-glutamine (Cellgro), buffered with 165 mM morpholinepropanesulfonic acid (MOPS), and plated into individual 384-well plates using an automated Multidrop 384 system (Thermo Labsystems). The New Prestwick Chemical Library consisting of 1,233 drugs was used to pin one compound per well at 10 µM final concentration, using a Biomek FX liquid handler. Forty-eight hours later the plates were scanned with a Flex Station II 384-well plate reader (Molecular Devices) to measure turbidity (OD600) of the wells. Molecules displaying >80% reduction in turbidity compared to control non-drug-treated wells (MIC80) were considered primary “hits.” Compounds commonly inhibiting all three fungal organisms were prioritized for planktonic dose-response assays and for their activity against biofilm growth.
Dose-response assays.
Dose-response assay of AXD against planktonically grown fungi was performed in agreement with the CLSI M27-A3 (for yeast) and M38-A2 (for filamentous fungi) reference standards for antifungal susceptibility testing (13, 14). Each drug was used in the concentration range of 0.19 µg/ml to 24 µg/ml, and the MIC of AXD was compared to the MIC of fluconazole, posaconazole, or voriconazole, as controls. All strains described in Table 2 were tested at the LA Biomedical Research Institute; however, several of the Candida strains were also verified for their susceptibility to AXD independently at Massachusetts General Hospital. Inhibition of planktonic growth or filamentation due to drug treatment was also visualized and imaged using bright-field microscopy. Microscopy was also used to directly visualize lateral yeast formation from planktonic C. albicans hyphae or lateral yeast cells formed on the surface of the biofilms (dispersal) using microtiter plates.
Biofilm growth and drug susceptibility testing.
Biofilms of Candida spp., C. neoformans, and A. fumigatus were developed in 96-well microtiter plates, and susceptibility of the biofilm cells to AXD or thimerosal was determined as described previously (33, 34). Biofilms were initiated in either the presence or absence of the drugs, or the drugs were tested on 48-h preformed biofilms, for efficacy evaluation. Inhibition of biofilm growth was measured by a standard colorimetric assay (XTT) that measures metabolic activity of the biofilm cells (18). Absorbance at 490 nm was measured using an automated plate reader. Biofilms formed by several other Candida spp. were further studied for their susceptibility to AXD.
Potential of AXD for synergistic use with fluconazole against C. albicans biofilms was investigated using a checkerboard assay, where dilutions of fluconazole (0.25 to 250 µg/ml) and AXD (0.3 to 2 µg/ml) were examined alone and in combination. Biofilm killing was measured by XTT assay. Drug concentration associated with 50% reduction in optical density compared to the no-drug control wells (EC50) was determined. The fractional inhibitory concentration (FIC) was then calculated as follows: [(EC50 of drug A in combination)/(EC50 of drug A alone)] + [(EC50 of drug B in combination)/(EC50 of drug B alone)]. Values of ≤0.5 revealed synergy, those of >0.5 but <2 indicated no interaction, and those of >2 were antagonistic (30).
Mammalian cell toxicity assays.
Primary human umbilical vascular endothelial cells (HUVECs) and human lung carcinoma-derived A549 epithelial cell lines were used to determine the cytotoxicity of AXD. HUVECs were isolated and propagated by the method of Jaffe et al. (35). The cells were grown in M-199 (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum, 10% defined bovine calf serum, and 2 nM l-glutamine, with penicillin and streptomycin. Second- or third-passage endothelial cells were grown on collagen matrix on 96-well microtiter plates. Treatment with AXD was conducted in M-199 medium.
A549 cells were purchased from the American Type Culture Collection and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. A549 cells (1.5 × 105/well) were used to seed 96-well plates and incubated at 37°C in a humidified atmosphere containing 5% CO2 for 24 h. The medium was then removed by aspiration, and the cells were washed twice with phosphate-buffered saline. Treatment with AXD was conducted with DMEM supplemented with 1% FBS.
Different concentrations of AXD in respective media were introduced into the cell lines and incubated for 24 h at 37°C in 5% CO2. The extent of cellular damage to both cell lines caused by AXD was quantified by a chromium release assay (36). Briefly, confluent mammalian cells were incubated overnight in respective media containing Na2 51CrO4 (6 µCi per well; ICN Biomedicals, Irvine, CA). The next day, the unincorporated tracer was aspirated and the wells were rinsed three times with warm HBSS. Two hundred microliters of media containing various concentrations of AXD (ranging from 0.12 to 59 µg/ml) was added to each well, and the plate was incubated for 24 h at 37°C in 5% CO2. At the end of the incubation, 100 µl of medium was gently aspirated from each well, after which the cells were lysed by the addition of 6 N NaOH. The lysed cells were aspirated, and the wells were rinsed twice with RadicWash (Atomic Products, Inc., Shirley, NY). These rinses were added to the lysed cells, and the 51Cr activity of the medium and the cell lysates was determined. Control wells containing no drug were processed in parallel to measure the spontaneous release of 51Cr. After corrections were made for the differences in the incorporation of 51Cr in each well, the specific release of 51Cr was calculated by the following formula: (2× experimental release – 2× spontaneous release)/(total incorporation – 2× spontaneous release).
Cytotoxicity to immune cells.
Wild-type C57BL/6 primary bone marrow-derived macrophages were cultured by plating bone marrow cells in 50 ng/ml of M-CSF (Peprotech, Rocky Hill, NJ) in complete RPMI (RPMI 1640 with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum, and 1% penicillin-streptomycin) for 7 days and then counted and seeded at 1 × 105 in 100 µl of complete RPMI overnight to allow for adhesion.
To examine cytotoxicity of AXD, bone marrow-derived macrophages were incubated in various concentrations of AXD for 24 h and stained with DAPI (Invitrogen, Carlsbad, CA) for viability assessment using an inverted epifluorescence microscope (Olympus IX70, Center Valley, PA) using 10× objective, with an X-cite 120 metal halide light source (EXFO, Mississauga, ON, Canada). Percent cell viability was determined using 1 – (DAPI-positive cells divided by total cells by phase contrast) × 100.
AXD was also examined for its capacity to block proliferation of a human promyelocytic cell line, HL-60. Cells were stained with 2 mM CFSE (carboxyfluorescein succinimidyl ester) for 5 min and washed with 1× RPMI medium three times. This dye is commonly used to measure cell proliferation; with each cell division the amount of CFSE is diluted in half, which can be observed via flow cytometry (37). After the staining, the cells were counted and adjusted at a cell density of 5 × 106 cells/ml and plated at 100 µl/well in a round-bottom 96-well plate. Twofold serially diluted AXD was added in wells containing cells. The final drug concentration obtained was between 0.004 and 10 µg/ml. Non-drug-treated and unstained cells in a number of wells were included as controls. Plates were incubated at 37°C for 48 h to allow the cell proliferation. After 48 h, the cells were collected and acquired in a flow cytometer. The unstained cells were used to gate the CFSE-positive HL60 cells. The shift in the peak of CFSE+ HL60 cells was considered proliferating cells.
In vivo biofilm drug susceptibility.
A mouse central venous catheter infection model was used for biofilm studies as previously described (31). These in vivo experiments were approved by the Los Angeles Biomedical Research Institute, Harbor-UCLA IACUC. Briefly, we used catheterized 8-week-old C57BL/6 male mice, purchased from Charles River Labs (Wilmington, MA), where the surgery was performed. The surgery involves insertion of a Silastic catheter into the jugular vein of the mice. Patency is tested, and the catheter is filled with heparin lock solution and plug-sealed. Following receipt of the jugular vein-catheterized mice, the catheters were instilled with 25 µl of C. albicans inoculum of 5 × 106 cells/ml (entire catheter volume) using a 23-gauge blunt-ended needle after removal of the plug and the lock solution (the plug was put back in place after inoculation). Cells were allowed to develop biofilms for 24 h, after which the catheters were treated with 3 µg/ml AXD for 48 h. Biofilms growing in replicate mouse catheters were also subjected to fluconazole (250 µg/ml) or caspofungin (0.125 µg/ml) treatment, as comparative controls. The catheters were cut laterally and imaged under a phase-contrast microscope to visualize the morphology of the cells growing within the catheters of the individual groups. Additionally, the distal 2 cm of the catheters was cut into small pieces, vortexed vigorously, and homogenized for plating on YPD plates for viability count measurements.
Statistical methods.
All in vitro secondary assays were done in triplicate and repeated once. Experiments were conducted in a randomized fashion and subjected to unpaired two-tailed t tests and/or ANOVA with Kruskal-Wallis posttest to determine significance of results (for P ≤ 0.05). For in vivo studies, differences in catheter fungal burden between the four groups (6 mice per group) were presented as percent reduction in CFU in the individual drug-treated groups compared to the control untreated mice. A two-tailed t test with a P value of <0.05 was considered significant.
Data availability.
Compounds identified after the first primary screening of the NPW library against planktonic cells of C. albicans, A. fumigatus, and C. auris are presented in Table S1 in the supplemental material. Any further data are available upon request from the corresponding author.
Hit list from primary screening of the New Prestwick Chemical library (>50% inhibition) for three fungal organisms under planktonic conditions. Download Table S1, XLSX file, 0.01 MB (11.8KB, xlsx) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We thank Nathan Wiederhold, Director, Fungus Testing Laboratory, University of Texas Health Sciences Center at San Antonio, for providing us with several clinical isolates of fungi used in this study.
These studies were funded by a grant from the National American Heart Association, #16SDG30830012, to P.U. A.S.I. is supported by a Public Health Service grant from the National Institutes of Allergy and Immunology, R01 AI063503.
REFERENCES
- 1.Vallabhaneni S, Mody RK, Walker T, Chiller T. 2016. The global burden of fungal diseases. Infect Dis Clin North Am 30:1–11. doi: 10.1016/j.idc.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 4.Lamoth F, Lockhart SR, Berkow EL, Calandra T. 2018. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother 73:i4–i13. doi: 10.1093/jac/dkx444. [DOI] [PubMed] [Google Scholar]
- 5.Brown GD, Denning DW, Levitz SM. 2012. Tackling human fungal infections. Science 336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- 6.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. 2010. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 9:719–727. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 7.Nobile CJ, Johnson AD. 2015. Candida albicans biofilms and human disease. Annu Rev Microbiol 69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Kohler JR, Kadosh D, Lopez-Ribot JL. 2010. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 6:e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odds FC, Brown AJ, Gow NA. 2003. Antifungal agents: mechanisms of action. Trends Microbiol 11:272–279. doi: 10.1016/S0966-842X(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 10.Vandeputte P, Ferrari S, Coste AT. 2012. Antifungal resistance and new strategies to control fungal infections. Int J Microbiol 2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doughty-Shenton D, Joseph JD, Zhang J, Pagliarini DJ, Kim Y, Lu D, Dixon JE, Casey PJ. 2010. Pharmacological targeting of the mitochondrial phosphatase PTPMT1. J Pharmacol Exp Ther 333:584–592. doi: 10.1124/jpet.109.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osei Sekyere J. 2018. Candida auris: a systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 7:e00578. doi: 10.1002/mbo3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Zhang JH, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 16.Wiederhold NP, Patterson TF, Srinivasan A, Chaturvedi AK, Fothergill AW, Wormley FL, Ramasubramanian AK, Lopez-Ribot JL. 2017. Repurposing auranofin as an antifungal: iIn vitro activity against a variety of medically important fungi. Virulence 8:138–142. doi: 10.1080/21505594.2016.1196301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siles SA, Srinivasan A, Pierce CG, Lopez-Ribot JL, Ramasubramanian AK. 2013. High-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother 57:3681–3687. doi: 10.1128/AAC.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS, Woo Chang S, Baek SH, Han SH, Lee Y, Zhu Q, Kum KY. 2013. Antimicrobial effect of alexidine and chlorhexidine against Enterococcus faecalis infection. Int J Oral Sci 5:26–31. doi: 10.1038/ijos.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Linares M, Aguado-Pérez B, Baca P, Arias-Moliz MT, Ferrer-Luque CM. 2017. Efficacy of antimicrobial solutions against polymicrobial root canal biofilm. Int Endod J 50:77–83. doi: 10.1111/iej.12598. [DOI] [PubMed] [Google Scholar]
- 21.Silveira LFM, Baca P, Arias-Moliz MT, Rodríguez-Archilla A, Ferrer-Luque CM. 2013. Antimicrobial activity of alexidine alone and associated with N-acetylcysteine against Enterococcus faecalis biofilm. Int J Oral Sci 5:146–149. doi: 10.1038/ijos.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiederhold NP, Lewis RE. 2009. Antifungal activity against Scedosporium species and novel assays to assess antifungal pharmacodynamics against filamentous fungi. Med Mycol 47:422–432. doi: 10.1080/13693780802510224. [DOI] [PubMed] [Google Scholar]
- 23.Uppuluri P, Lopez-Ribot JL. 2016. Go forth and colonize: dispersal from clinically important microbial biofilms. PLoS Pathog 12:e1005397. doi: 10.1371/journal.ppat.1005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yip KW, Ito E, Mao X, Au PY, Hedley DW, Mocanu JD, Bastianutto C, Schimmer A, Liu FF. 2006. Potential use of alexidine dihydrochloride as an apoptosis-promoting anticancer agent. Mol Cancer Ther 5:2234–2240. doi: 10.1158/1535-7163.MCT-06-0134. [DOI] [PubMed] [Google Scholar]
- 25.Barrios R, Ferrer-Luque CM, Arias-Moliz MT, Ruiz-Linares M, Bravo M, Baca P. 2013. Antimicrobial substantivity of alexidine and chlorhexidine in dentin. J Endod 39:1413–1415. doi: 10.1016/j.joen.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Xu S, Feliu M, Lord AK, Lukason DP, Negoro PE, Khan NS, Dagher Z, Feldman MB, Reedy JL, Steiger SN, Tam JM, Soukas AA, Sykes DB, Mansour MK. 2018. Biguanides enhance antifungal activity against Candida glabrata. Virulence 9:1150–1162. doi: 10.1080/21505594.2018.1475798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganendren R, Widmer F, Singhal V, Wilson C, Sorrell T, Wright L. 2004. In vitro antifungal activities of inhibitors of phospholipases from the fungal pathogen Cryptococcus neoformans. Antimicrob Agents Chemother 48:1561–1569. doi: 10.1128/AAC.48.5.1561-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varadan P, Ganesh A, Konindala R, Nagendrababu V, Ashok R, Deivanayagam K. 2017. Comparison of the antibacterial efficacy of alexidine and chlorhexidine against Enterococcus faecalis. An in vitro study. Cureus 9:e1805. doi: 10.7759/cureus.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas LJ. 2003. Candida biofilms and their role in infection. Trends Microbiol 11:30–36. doi: 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 30.Uppuluri P, Nett J, Heitman J, Andes D. 2008. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother 52:1127–1132. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazzell AL, Chaturvedi AK, Pierce CG, Prasad D, Uppuluri P, Lopez-Ribot JL. 2009. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J Antimicrob Chemother 64:567–570. doi: 10.1093/jac/dkp242. [DOI] [PubMed] [Google Scholar]
- 32.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 33.Uppuluri P, Chaturvedi AK, Lopez-Ribot JL. 2009. Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia 168:101–109. doi: 10.1007/s11046-009-9205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins N, Uppuluri P, Nett J, Rajendran R, Ramage G, Lopez-Ribot JL, Andes D, Cowen LE. 2011. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog 7:e1002257. doi: 10.1371/journal.ppat.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffe EA, Nachman RL, Becker CG, Minick CR. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim AS, Gebremariam T, Liu M, Chamilos G, Kontoyiannis D, Mink R, Kwon-Chung KJ, Fu Y, Skory CD, Edwards JE Jr, Spellberg B. 2008. Bacterial endosymbiosis is widely present among zygomycetes but does not contribute to the pathogenesis of mucormycosis. J Infect Dis 198:1083–1090. doi: 10.1086/591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung S, Kim SH, Seo Y, Kim SK, Lee JY. 2017. Quantitative analysis of cell proliferation by a dye dilution assay: application to cell lines and cocultures. Cytometry A 91:704–712. doi: 10.1002/cyto.a.23105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary screening: MIC80 of alexidine dihydrochloride and thimerosal against preformed biofilms of three different fungi as determined by XTT reading (OD490). Both drugs kill biofilm cells at <10 µM concentrations. Download FIG S1, TIF file, 2.9 MB (3MB, tif) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Inhibitory effect of AXD against preformed biofilms of four different fluconazole-resistant Candida spp. AXD kills preformed biofilms between 3 and 6 µg. Download FIG S2, TIF file, 2.7 MB (2.7MB, tif) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Chemical structure of alexidine dihydrochloride. (B) Inhibition of early cellular division of HL60 cells by AXD. Download FIG S3, TIF file, 2.9 MB (2.9MB, tif) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Hit list from primary screening of the New Prestwick Chemical library (>50% inhibition) for three fungal organisms under planktonic conditions. Download Table S1, XLSX file, 0.01 MB (11.8KB, xlsx) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Compounds identified after the first primary screening of the NPW library against planktonic cells of C. albicans, A. fumigatus, and C. auris are presented in Table S1 in the supplemental material. Any further data are available upon request from the corresponding author.
Hit list from primary screening of the New Prestwick Chemical library (>50% inhibition) for three fungal organisms under planktonic conditions. Download Table S1, XLSX file, 0.01 MB (11.8KB, xlsx) .
Copyright © 2018 Mamouei et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.