Abstract
A growing body of epigenetic research suggests that in-utero adaptations to environmental changes display important sex-specific variation. We tested this heterogeneous adaptation hypothesis using data from 900 children born at the University Hospital in São Paulo, Brazil, between October 2013 and April 2014. Crude and adjusting linear models were used to quantify the associations between prematurity, being small for gestational age, and children’s physical and mental development at 12 months of age. Prematurity was negatively associated with neuropsychological development in final models (z score difference, −0.42, 95% confidence intervals: −0.71, −0.14), but associations did not vary significantly by sex. For being small for gestational age, associations with height-for-age, weight-for-age, and neuropsychological development were also negative, but they were systematically larger for male than for female infants (P < 0.05 for all). These results suggest that male fetuses may be more vulnerable to intrauterine adversity than female fetuses. Further research will be needed to better understand the mechanisms underlying these sex-specific associations.
Keywords: child development, intrauterine growth restriction, prematurity, small for gestational age
According to the latest estimates, more than 250 million children under 5 years of age are not reaching their developmental potential (1), and more than one-third of 3- and 4-year-olds show delays in their cognitive or socioemotional development (2). Children in low- and middle-income countries continue to be exposed to a multitude of risk factors that can undermine their healthy development individually as well as jointly (1, 3, 4). Experiences in early life have lasting impacts, with often surprisingly large and long-term ramifications of very early intrauterine experiences (5, 6).
In 2010, an estimated 14.9 million infants were born preterm (7), and 32.4 million were born small for gestational age (SGA) (8). Both of these conditions are major risk factors for child mortality (9) and childhood stunting (10), and they pose a serious risk to healthy child development more generally (11–13). Children born SGA (below the 10th percentile of weight for gestational age) have been shown to lag behind in terms of their physical growth, neurodevelopmental performance, and cognition at preschool age (14), display learning difficulties in primary school (15), and demonstrate deficits in academic achievement as adolescents (16). A number of studies have also demonstrated associations between adverse birth outcomes, such as size at birth adjusted for gestational age, and behavioral problems, including hyperactivity/inattention and total behavioral difficulties (17–19). A separate, but closely related literature has examined the associations between prenatal stress and developmental outcomes of exposed children, and has identified relatively consistent links between various types of maternal stress and child hyperactivity, conduct and peer problems, and behavioral difficulties (20–25).
Within this larger literature, male sex has been increasingly recognized as a potential risk factor for adverse neurodevelopmental outcomes, particularly among low birth weight and preterm births (26, 27). Compared with female fetuses, male fetuses appear to face increased risks of stillbirth (28), prematurity (29), and infant mortality (30). Recent epigenetic research suggests that early-life DNA methylation varies substantially by sex and has raised the question of whether these sex-specific differences in methylation can at least partially explain the empirically observed differences in developmental outcomes (31).
Empirically, evidence on the interactions between infant sex and the developmental impact of prematurity and SGA remains scarce and somewhat mixed (32), with some studies showing increased developmental risk for male infants born preterm or SGA (27, 33, 34) and other studies finding no such sex differences (26).
In this study, we use a rich new data set from a child development cohort in São Paulo, Brazil, to further investigate the empirical relationships between sex, adverse birth outcomes, and child development.
METHODS
Study setting
The study was conducted in the Butantã-Jaguaré region, which is located in the Western Region of São Paulo, Brazil, municipality. The Butantã-Jaguaré region has an estimated population of 380,000. Infant mortality rates in the region vary between 4.4 deaths per 1,000 live births in Morumbi District and 10.3 deaths per 1,000 live births in Vila Sonia District (35, 36).
Study population
The study population was composed of 900 children forming part of the São Paulo Western Region Birth Cohort. The Western Region Birth Cohort comprises all infants born at the Hospital Universitario da Universidade de São Paulo (HU-USP) between 2012 and 2014. For this study, we used data from a subsample of children born between October 1, 2013, and March 31, 2014, who were assessed as part of the baseline survey for an ongoing intervention trial at 12 months of age (ClinicalTrials.gov NCT02704000). HU-USP is the main public general hospital of the Butantã-Jaguaré region, where 82% of the births by (the mostly poor) women covered in the public health sector (Sistema Único de Saúde, Brazil’s National Health System) and about 40% of all births in the region take place (37). The 900 children were randomly selected among all births occurring in HU-USP within the study period to mothers living in the Butantã-Jaguaré region for the aforementioned intervention study.
Hospital birth records
HU-USP maintains detailed digital records on gestational age at birth, size for gestational age, mode of delivery, date of delivery, and age of mother at delivery. Gestational duration is estimated using the modified Ballard scale (38). Weight and length are measured immediately after birth by delivering providers.
Outcome variables
The primary outcome of interest was children’s development at 12 months of age. For physical development, age- and sex-normalized height-for-age and weight-for-age z scores (HAZ and WAZ) were computed using the World Health Organization Anthro software (39). To account for premature deliveries, corrected ages of children were calculated as the number of months between the time of the assessment and the expected date of delivery. Children’s neuropsychological development was assessed using the Ages and Stages Questionnaire (ASQ), previously validated in Brazil (40). The ASQ is based on caregiver reports, which were collected as part of a home visit by trained interviewers. Raw ASQ scores were normalized within the sample to a mean of 0 and a standard deviation of 1.
Primary exposure variables
The 2 primary exposure variables of interest were prematurity and being born small for gestational age. Prematurity (delivery before 37 weeks of gestation) was based on provider-estimated gestational lengths. The Ballard scoring system used at HU-USP has been shown to have both specificity and sensitivity of >95% for prematurity in the Brazilian context (41). We also collected self-reported due dates from mothers after delivery; results did not change when this alternative measure was used to establish prematurity. As for gestation-adjusted weight, the hospital uses sex-specific reference norms derived for Brazil (42). To ensure that our results were not driven by these specific weight reference tables, we also used alternative SGA codes based on INTERGROWTH fetal growth standards (43) as well as Oken’s growth standards (44) in our sensitivity analyses.
Statistical analysis and empirical strategy
We first estimated multivariable linear regression models to quantify the associations between prematurity, SGA, and developmental outcomes. The basic model estimated can be described as follows:
| (1) |
where is the child development outcome of interest for child i in the cohort, SGA is an indicator for small for gestational age, preterm is an indicator for children born prior to 37 weeks of gestation, and X is a vector of maternal and child characteristics (displayed in Table 1). We fitted crude models and models with adjustments in the pooled (both sexes together) sample; we then fitted these models separately for male and female children. Finally, we tested whether the observed sex-specific differences were statistically significant in pooled linear models with interaction terms. We used robust standard errors to account for any heteroskedasticity in the data.
Table 1.
Descriptive Statistics, Children From the Western Region Cohort Who Were Born in Brazil Between October 1, 2013, and March 31, 2014
| Variable | Full Term | Preterm | Normal Weight for Gestation | SGA | All | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Proportion (SD) | Mean (SD) | Proportion (SD) | Mean (SD) | Proportion (SD) | Mean (SD) | Proportion (SD) | Mean (SD) | Proportion (SD) | |
| Height-for-age z score | −0.11 (1.4) | 0.18 (1.4) | −0.04 (1.4) | −0.71 (1.3) | −0.09 (1.4) | |||||
| Child is stunted (HAZ <−2) | 0.07 (0.3) | 0.05 (0.2) | 0.06 (0.2) | 0.12 (0.3) | 0.07 (0.3) | |||||
| Weight-for-age z score | 0.46 (1.3) | 0.61 (1.1) | 0.53 (1.3) | −0.23 (1.3) | 0.47 (1.3) | |||||
| ASQ z score | 0.03 (1.0) | −0.36 (1.1) | 0.01 (1.0) | −0.11 (1.0) | 0.00 (1.0) | |||||
| Adjusted age of child, months | 12.58 (1.8) | 11.33 (1.8) | 12.49 (1.8) | 12.23 (1.9) | 12.47 (1.8) | |||||
| Child is female | 0.56 (0.5) | 0.49 (0.5) | 0.55 (0.5) | 0.59 (0.5) | 0.55 (0.5) | |||||
| Biological mother is primary caregiver | 0.75 (0.4) | 0.69 (0.5) | 0.75 (0.4) | 0.71 (0.5) | 0.75 (0.4) | |||||
| Caregiver has secondary education | 0.41 (0.5) | 0.44 (0.5) | 0.42 (0.5) | 0.39 (0.5) | 0.41 (0.5) | |||||
| Caregiver has higher education | 0.16 (0.4) | 0.17 (0.4) | 0.17 (0.4) | 0.11 (0.3) | 0.16 (0.4) | |||||
| Home activity scorea | 4.79 (1.3) | 4.97 (1.2) | 4.82 (1.2) | 4.7 (1.5) | 4.81 (1.3) | |||||
| Assessor home environment scoreb | 7.90 (1.9) | 7.77 (1.9) | 7.88 (1.9) | 7.99 (2.3) | 7.89 (1.9) | |||||
| Asset quintile 2 | 0.27 (0.4) | 0.21 (0.4) | 0.26 (0.4) | 0.27 (0.4) | 0.26 (0.4) | |||||
| Asset quintile 3 | 0.14 (0.3) | 0.15 (0.4) | 0.14 (0.4) | 0.11 (0.3) | 0.14 (0.3) | |||||
| Asset quintile 4 | 0.20 (0.4) | 0.17 (0.4) | 0.20 (0.4) | 0.20 (0.4) | 0.20 (0.4) | |||||
| Asset quintile 5 | 0.19 (0.4) | 0.20 (0.4) | 0.20 (0.4) | 0.17 (0.4) | 0.19 (0.4) | |||||
Abbreviations: ASQ, Ages and Stages Questionnaire; HAZ, height-for-age z score; SD, standard deviation; SGA, small for gestational age.
a Caregivers were asked whether they had done any of the following activities with the child in the previous 3 days: read books; tell stories; name objects, count, or draw; play; sing songs; or take child outside. These questions are based on those in the Multiple Indicator Cluster Survey.
b At the end of the interview, assessors were asked to make a subjective assessment of the physical quality of the home overall on a scale for 1 (worst they have seen) to 10 (best they have seen).
All statistical analysis was performed using Stata, version 14 (StataCorp LP, College Station, Texas) (45).
Ethical clearance
Ethical clearance for the data collection was obtained from HU-USP’s institutional review board (protocol number 890.325).
RESULTS
Table 1 shows descriptive statistics by gestation and weight category. Fifty-five percent of children in the sample were female, 8% were born preterm, and 8% were born small for gestational age. On average, children in the sample were relatively close to the global reference population with respect to height, with 7% of children stunted (HAZ of <−2 standard deviations of the global growth standard median) and a mean HAZ of −0.09. Children were on average 12 months old at the time of the assessment. Mothers were the primary caregiver in 75% of cases. Forty-one percent of caregivers had at least a secondary education, and 16% had tertiary or higher-level education; virtually all caregivers had at least some primary education. On average, caregivers appeared to be very engaged in early childhood care, with caregivers reporting having engaged in an average of 4.8 different caregiver-child activities within 3 days of the interview.
Table 2 shows estimated adjusted and crude associations between HAZ, WAZ, and ASQ z scores as outcomes. In our final models (bottom panel), preterm birth was not associated with any of the physical measures, but it was associated with a 0.42-standard-deviation reduction in ASQ z scores (95% confidence intervals: 0.71, 0.14). SGA was not associated with ASQ z scores but showed negative associations with HAZ (standard deviation reduction of 0.73, 95% confidence intervals: 1.04, 0.42) and WAZ (standard deviation reduction of 0.78, 95% confidence intervals: 1.11, 0.45) in the pooled sample with final models.
Table 2.
Pooled Associations Between Prematurity, Being Small for Gestational Age, and Child Development Among Children From the Western Region Cohort Who Were Born in Brazil Between October 1, 2013, and March 31, 2014
| Outcome Variablea | ||||||
|---|---|---|---|---|---|---|
| HAZ (n = 894) | WAZ (n = 790) | ASQ z Score (n = 675) | ||||
| Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | |
| Crude estimates | ||||||
| SGA | −0.665b | −0.982, −0.347 | −0.762b | −1.093, −0.432 | −0.134 | −0.424, 0.156 |
| Preterm birth | 0.288c | −0.035, 0.612 | 0.140 | −0.140, 0.421 | −0.399d | −0.704, −0.093 |
| Adjusted estimates | ||||||
| SGA | −0.728b | −1.038, −0.418 | −0.783b | −1.114, −0.453 | −0.152 | −0.415, 0.112 |
| Preterm birth | 0.134 | −0.201, 0.469 | 0.170 | −0.117, 0.456 | −0.423b | −0.709, −0.138 |
Abbreviations: ASQ, Ages and Stages Questionnaire; CI, confidence interval; HAZ, height-for-age z score; SGA, small for gestational age; WAZ, weight-for-age z score.
a Each column represents the results of a separate linear regression model, with 95% confidence intervals based on heteroskedasticity-robust standard errors. Models adjusted for the full list of covariates displayed in Table 1, including age, sex, caregiver education, home activity scores, and socioeconomic variables.
bP < 0.01.
cP < 0.1.
dP < 0.05.
Table 3 shows the same (final) models stratified by sex. Similar to the results in Table 2, we found SGA to be predictive of physical growth outcomes only and preterm birth to be predictive of ASQ z scores as a measure of neuropsychological development in both the male and female subsamples. While the association between prematurity and ASQ appears to be larger for girls than for boys, the estimated coefficients are not statistically different from each other (P = 0.705). The same is not true for SGA, which showed consistently larger associations with growth outcomes for boys than for girls. The estimated coefficients for HAZ and WAZ were more than twice as large for boys as for girls, and the interaction term was significant at the 10% level (P = 0.063 and 0.059, respectively). Estimated sex differences with respect to SGA were largest for ASQ scores, which showed no evidence of associations with SGA for girls but large negative associations for boys (z score difference, −0.55, 95% confidence intervals: 1.11, 0.01; statistically significant at the 10% level). We rejected the null of equal associations with a P value of 0.043.
Table 3.
Sex-Specific Associations Between Prematurity, Being Small for Gestational Age, and Child Development Among Children From the Western Region Cohort Who Were Born in Brazil Between October 1, 2013, and March 31, 2014
| Outcome Variablea | ||||||
|---|---|---|---|---|---|---|
| HAZ | WAZ | ASQ z Score | ||||
| Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | |
| Girls | ||||||
| SGA | −0.485b | −0.839, −0.130 | −0.509b | −0.830, −0.188 | 0.094 | −0.180, 0.368 |
| Preterm birth | −0.043 | −0.444, 0.358 | 0.112 | −0.214, 0.438 | −0.507c | −1.001, −0.013 |
| Boys | ||||||
| SGA | −1.108b | −1.662, −0.553 | −1.177b | −1.794, −0.559 | −0.549d | −1.110, 0.013 |
| Preterm birth | 0.275 | −0.266, 0.816 | 0.212 | −0.257, 0.682 | −0.392c | −0.722, −0.062 |
| H0: No sex difference for SGA (P value) | 0.063d | 0.059d | 0.043c | |||
| H0: No sex difference for prematurity (P value) | 0.354 | 0.730 | 0.705 | |||
Abbreviations: ASQ, Ages and Stages Questionnaire; CI, confidence interval; HAZ, height-for-age z score; SGA, small for gestational age; WAZ, weight-for-age z score.
a All models adjusted for child sex, child age in months, caregiver education, Multiple Indicator Cluster Survey home activity score, assessor home score, and asset quintiles. The 95% CIs are based on heteroskedasticity-robust standard errors. P values are based on a pooled model with linear interaction terms.
bP < 0.01.
cP < 0.05.
dP < 0.1.
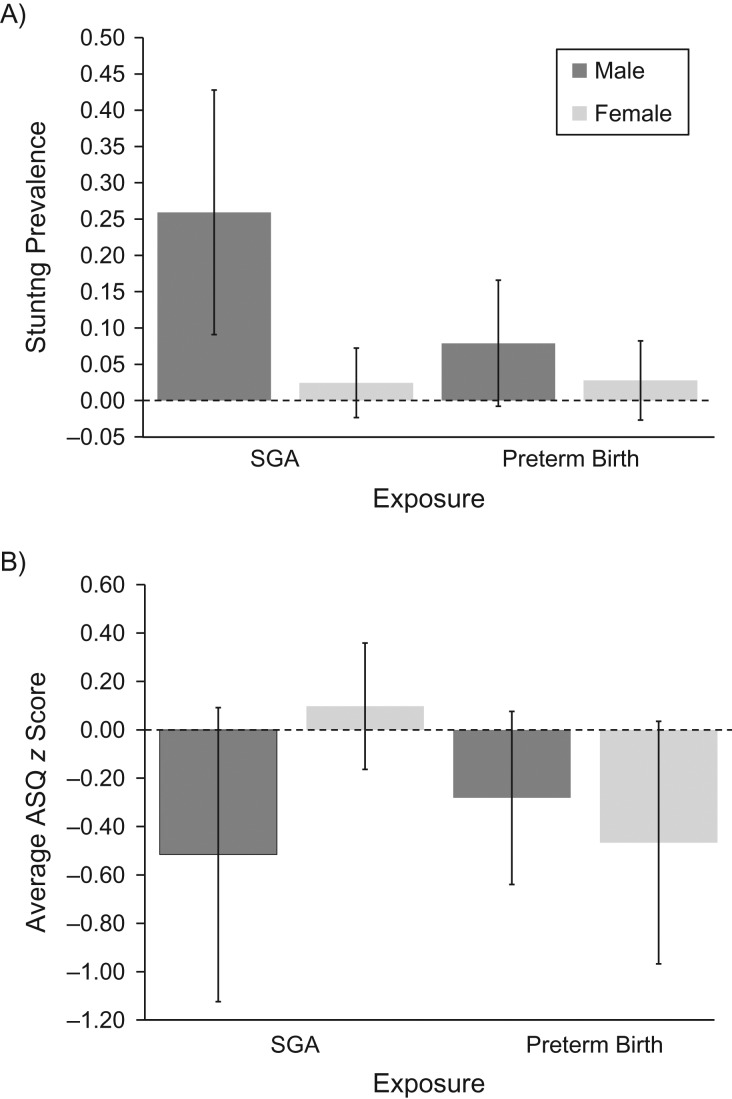
Figure 1 further illustrates these estimated sex differences with respect to SGA. Overall, 12% of SGA children were stunted at age 12 months (age corrected for gestational length) in the sample; this high stunting prevalence is, however, almost exclusively driven by boys in this subgroup, with an estimated stunting prevalence of 25.9% among boys born SGA compared with an estimated rate of 2.4% among girls born SGA. For ASQ, boys born SGA had an average score that was 0.5 standard deviations below the sample average, while girls scored slightly higher than the sample average.
Figure 1.
Stunting prevalence (A) and average Ages and Stages Questionnaire (ASQ) z score (B), stratified by sex, being small for gestational age (SGA), and preterm status, Brazil, 2013–2015. Children were part of the Western Region Cohort and were born between October 1, 2013, and March 31, 2014. ASQ z scores and stunting prevalence were assessed at 12 months of age.
DISCUSSION
In this study, we used data from São Paulo’s Western Region Birth Cohort to test the extent to which birth outcomes predict child development at age 1 year as well as to assess the extent to which associations between adverse pregnancy outcomes and child development vary according to child sex. Overall, we found strong negative associations between SGA and measures of physical development but no association with neuropsychological development. The opposite was true for prematurity, which was not predictive of physical development at age 1 year but highly predictive of neuropsychological development. While we found relatively minor sex differences for the associations between prematurity and neuropsychological development, we observed large sex-specific differences with respect to SGA. For boys, we found that being born SGA (compared with being born normal size for gestational age) was associated with an HAZ and WAZ decrease of more than 1 standard deviation, as well as substantial reduction in ASQ. For girls, SGA was associated with smaller reductions in HAZ and WAZ and was not associated with ASQ scores at all.
Our sex-specific results are consistent with other studies that found that female infants with extremely low birthweight or who were born extremely preterm had better cognitive outcomes than male infants with the same birth conditions (26, 32). Our study further adds to this literature by studying both preterm and SGA infants and by examining both neuropsychological and physical growth outcomes.
Even though our data do not allow us to directly identify the causal mechanisms underlying these differentials, the overall patterns observed suggest that male infants born SGA either have different in-utero experiences than female infants do or that male fetuses respond more strongly to such adversity in terms of programmed changes in their early life growth and development, as well as potentially in terms of their long-term metabolic systems (5, 6). Given that SGA rates were marginally higher among female infants than among male infants in our sample, one possibility is that male SGA births may represent a subset of infants with more severe adversity. Empirically, the differences in SGA prevalence appear to be minor, however. Using the hospital’s classification system, 7.2% of boys and 8.2% of girls in the sample were classified as SGA. Using the most recently published INTERGROWTH standards, the rates were 11.2% and 13.6% for boys and girls, respectively. In both cases, sex differences were present but relatively small, so that large differences in selection appear unlikely (and the sex-specific associations estimated do not change qualitatively when these alternative measures are used).
The findings presented in this study seem consistent with a growing body of evidence highlighting large sex differences in children’s general developmental trajectories. A recent review concluded that boys might be less resilient to early adversity but that the true extent of these differences is hard to measure due to higher perinatal mortality experienced by male infants (46). The results presented here definitely seem consistent with this finding. The large sex differentials observed also appear to align well with a broader set of research documenting sex-specific responses to adversity. Several recent studies have shown that girls experiencing trauma, physical abuse, or maternal distress during infancy show higher rates of depression, anxiety, and posttraumatic stress disorder compared with boys with the same experience (47, 48). In contrast, boys appear more vulnerable to developing schizophrenic, autism, and attention deficit hyperactivity disorder symptoms in response to perinatal and intrauterine stress (49–51).
Our findings also corroborate previous literature examining the general relationship between preterm and neuropsychological development (27, 52) and SGA and anthropometric measures (53). We did not find preterm birth to be associated with the anthropometric measures of HAZ and WAZ when we used gestation-adjusted ages, which suggests that infants born preterm can catch up physically to those born at full term. Preterm is, however, associated with lower ASQ scores, which suggests that neuropsychological development remains delayed even when adjusting for age, as we did in our analysis. While we found that SGA was associated with delayed physical growth, we did not find that SGA was associated with ASQ scores in this sample. This suggests that the causal determinants of SGA—which likely include both genetic and environmental factors—shape the medium- to long-run linear growth trajectories of children but that that these physical growth trajectories are not necessarily predictive of neurocognitive outcomes.
The analysis presented here has several limitations. First, despite the longitudinal nature of the study, our ability to interpret estimated associations causally is limited; it is possible that prematurity and SGA are correlated with other causal determinants of child development not included in our model, which may confound our results. To address this, we included in our model a large number of family characteristics collected through personal surveys with caregivers, which should reduce the risk of large confounding biases to a minimum. Confounding biases seem even less likely in the male-female comparisons given that there is no evidence of sex selection through abortion in the study setting, which means that the correlation between sex and potential unobserved parental confounders should be minimal. Another general concern with studies related to in-utero exposures is that the exact measurement of gestational length, as well as an exact classification of gestation-adjusted weight categories, is challenging. To address this, we used both maternal reports and provider estimates of gestational length for the classification of prematurity, and we explored 3 different standards for the classification of SGA. While the specific standard applied changes the estimated average SGA prevalence noticeably—from 7.8% to 12.6% if INTERGROWTH rather than Brazilian standards are applied, and to 8.0% if Oken’s US-based standard are applied—the overall associations found appear to be highly consistent across measures in our sensitivity analysis (results available in Web Tables 1 and 2, available at https://academic.oup.com/aje). One further limitation of the present study is that our core exposure variables (SGA and prematurity) do not allow for direct assessment of the nature or intensity of the causal determinants of these outcomes. Although SGA is sometimes used as a proxy measure for intrauterine growth restriction, some children are born small for genetic reasons rather than due to lack of nutrients or exposure to stress. The extent to which SGA is driven by in-utero lack of nutrients and oxygen needed for proper growth and development in our sample is unknown; larger associations seem likely if the empirical model could be restricted to those children who experienced such in-utero adversity. Finally, while the ASQ has been extensively used and validated (54–56), it relies on parental report of child development measures, which may be subject to social desirability bias (57, 58). However, we have no reason to believe that this bias is differential by sex or by SGA or preterm.
In conclusion, the results presented in this paper suggest that adverse birth outcomes may be more harmful to the development of male infants than to the development of female infants. Further research is needed to better understand the mechanisms underlying these sex-specific relationships as well as to remediate any effects.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Swiss Tropical and Public Health Institute, Basel, Switzerland (Günther Fink); University of Basel, Basel, Switzerland (Günther Fink); Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Kathryn G. Andrews); Department of Psychiatry, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil (Helena Brentani); Department of Pediatrics, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil (Sandra Grisi, Alexandra Brentani); and Child Institute—Instituto da Criança do Hospital das Clínicas da Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil (Ana Paula Scoleze Ferrer).
This project was funded by Grand Challenges Canada and Fundação Maria Cecília Souto Vidigal.
Conflict of interest: none declared.
Abbreviations
- ASQ
Ages and Stages Questionnaire
- HAZ
height-for-age z score
- HU-USP
Hospital Universitario da Universidade de São Paulo
- SGA
small for gestational age
- WAZ
weight-for-age z score
REFERENCES
- 1. Black MM, Walker SP, Fernald LCH, et al. Early childhood development coming of age: science through the life course. Lancet. 2017;389(10064):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCoy DC, Peet ED, Ezzati M, et al. Early childhood developmental status in low- and middle-income countries: national, regional, and global prevalence estimates using predictive modeling. PLoS Med. 2016;13(6):e1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engle PL, Black MM, Behrman JR, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369(9557):229–242. [DOI] [PubMed] [Google Scholar]
- 4. Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker DJ. Childhood causes of adult diseases. Arch Dis Child. 1988;63(7):867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barker DJ, Eriksson JG, Forsén T, et al. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–1239. [DOI] [PubMed] [Google Scholar]
- 7. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. [DOI] [PubMed] [Google Scholar]
- 8. Lee AC, Katz J, Blencowe H, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1(1):e26–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. [DOI] [PubMed] [Google Scholar]
- 10. Danaei G, Andrews KG, Sudfeld CR, et al. Risk factors for childhood stunting in 137 developing countries: a comparative risk assessment analysis at global, regional, and country levels. PLoS Med. 2016;13(11):e1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walker SP, Chang SM, Powell CA, et al. Early childhood stunting is associated with poor psychological functioning in late adolescence and effects are reduced by psychosocial stimulation. J Nutr. 2007;137(11):2464–2469. [DOI] [PubMed] [Google Scholar]
- 12. Patrianakos-Hoobler AI, Msall ME, Marks JD, et al. Risk factors affecting school readiness in premature infants with respiratory distress syndrome. Pediatrics. 2009;124(1):258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murray E, Fernandes M, Fazel M, et al. Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG. 2015;122(8):1062–1072. [DOI] [PubMed] [Google Scholar]
- 14. Leitner Y, Fattal-Valevski A, Geva R, et al. Six-year follow-up of children with intrauterine growth retardation: long-term, prospective study. J Child Neurol. 2000;15(12):781–786. [DOI] [PubMed] [Google Scholar]
- 15. Low JA, Handley-Derry MH, Burke SO, et al. Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am J Obstet Gynecol. 1992;167(6):1499–1505. [DOI] [PubMed] [Google Scholar]
- 16. Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six-year follow-up of the 1970 British Birth Cohort. JAMA. 2000;283(5):625–632. [DOI] [PubMed] [Google Scholar]
- 17. Wiles NJ, Peters TJ, Heron J, et al. Fetal growth and childhood behavioral problems: results from the ALSPAC cohort. Am J Epidemiol. 2006;163(9):829–837. [DOI] [PubMed] [Google Scholar]
- 18. Lahti J, Räikkönen K, Kajantie E, et al. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J Child Psychol Psychiatry. 2006;47(11):1167–1174. [DOI] [PubMed] [Google Scholar]
- 19. Schlotz W, Jones A, Godfrey KM, et al. Effortful control mediates associations of fetal growth with hyperactivity and behavioural problems in 7- to 9-year-old children. J Child Psychol Psychiatry. 2008;49(11):1228–1236. [PubMed] [Google Scholar]
- 20. Bergman K, Sarkar P, O’Connor TG, et al. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1454–1463. [DOI] [PubMed] [Google Scholar]
- 21. Gutteling BM, de Weerth C, Willemsen-Swinkels SH, et al. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry. 2005;14(1):41–51. [DOI] [PubMed] [Google Scholar]
- 22. O’Connor TG, Heron J, Golding J, et al. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. [DOI] [PubMed] [Google Scholar]
- 23. Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75(4):1085–1097. [DOI] [PubMed] [Google Scholar]
- 24. Van den Bergh BR, Mulder EJ, Mennes M, et al. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29(2):237–258. [DOI] [PubMed] [Google Scholar]
- 25. Markussen Linnet K, Obel C, Bonde E, et al. Cigarette smoking during pregnancy and hyperactive-distractible preschoolers: a follow-up study. Acta Paediatr. 2006;95(6):694–700. [DOI] [PubMed] [Google Scholar]
- 26. Hintz SR, Kendrick DE, Vohr BR, et al. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006;95(10):1239–1248. [DOI] [PubMed] [Google Scholar]
- 27. Morsing E, Asard M, Ley D, et al. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127(4):e874–e882. [DOI] [PubMed] [Google Scholar]
- 28. Mondal D, Galloway TS, Bailey TC, et al. Elevated risk of stillbirth in males: systematic review and meta-analysis of more than 30 million births. BMC Med. 2014;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peelen MJ, Kazemier BM, Ravelli AC, et al. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstet Gynecol Scand. 2016;95(9):1034–1041. [DOI] [PubMed] [Google Scholar]
- 30. Pongou R. Why is infant mortality higher in boys than in girls? A new hypothesis based on preconception environment and evidence from a large sample of twins. Demography. 2013;50(2):421–444. [DOI] [PubMed] [Google Scholar]
- 31. Maschietto M, Bastos LC, Tahira AC, et al. Sex differences in DNA methylation of the cord blood are related to sex-bias psychiatric diseases. Sci Rep. 2017;7:44547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hindmarsh GJ, O’Callaghan MJ, Mohay HA, et al. Gender differences in cognitive abilities at 2 years in ELBW infants. Extremely low birth weight. Early Hum Dev. 2000;60(2):115–122. [DOI] [PubMed] [Google Scholar]
- 33. Hoffman EL, Bennett FC. Birth weight less than 800 grams: changing outcomes and influences of gender and gestation number. Pediatrics. 1990;86(1):27–34. [PubMed] [Google Scholar]
- 34. Peacock JL, Marston L, Marlow N, et al. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71(3):305–310. [DOI] [PubMed] [Google Scholar]
- 35. Brazil Ministry of Health Portal da Saúde: sistema de informação sobre mortalidade. http://svs.aids.gov.br/cgiae/sim/. Accessed June 5, 2018.
- 36. Secretaria Municipal da Saúde Prefeitura da Cidade de São Paulo: mortalidade no municipio de Sao Paulo. http://www.prefeitura.sp.gov.br/cidade/secretarias/saude/tabnet/mortalidade/index.php?p=6529. Accessed June 5, 2018.
- 37. Brentani A, Grisi SJFE, Taniguchi MT, et al. Rollout of community-based family health strategy (programa de saude de familia) is associated with large reductions in neonatal mortality in São Paulo, Brazil. SSM Popul Health. 2016;2:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ballard JL, Khoury JC, Wedig K, et al. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119(3):417–423. [DOI] [PubMed] [Google Scholar]
- 39. World Health Organization WHO Anthro (version 3.2.2, January 2011) and macros. World Health Organization; http://www.who.int/childgrowth/software/en/. Accessed June 5, 2018. [Google Scholar]
- 40. Santana CMT, Filgueiras A, Landeira-Fernandez J. Ages & Stages Questionnaire-Brazil-2011: adjustments on an early childhood development screening measure. Glob Pediatr Health. 2015;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pereira AP, Dias MA, Bastos MH, et al. Determining gestational age for public health care users in Brazil: comparison of methods and algorithm creation. BMC Res Notes. 2013;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Segre CA, Colletto GM, Bertagnon JR. Intrauterine growth curves in a high-income population. J Pediatr (Rio J). 2001;77(3):169–174. [DOI] [PubMed] [Google Scholar]
- 43. Stirnemann J, Villar J, Salomon LJ, et al. International estimated fetal weight standards of the INTERGROWTH‐21st Project. Ultrasound Obstet Gynecol. 2017;49(4):478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oken E, Kleinman KP, Rich-Edwards J, et al. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. StataCorp Stata Statistical Software: Release 14 College Station, TX: StataCorp LP; 2015. https://www.stata.com/. [Google Scholar]
- 46. Radulescu L, Ferechide D, Popa F. The importance of fetal gender in intrauterine growth restriction. J Med Life. 2013;6(1):38–39. [PMC free article] [PubMed] [Google Scholar]
- 47. Baker A, Shalhoub-Kevorkian N. Effects of political and military traumas on children: the Palestinian case. Clin Psychol Rev. 1999;19(8):935–950. [DOI] [PubMed] [Google Scholar]
- 48. MacMillan HL, Fleming JE, Streiner DL, et al. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158(11):1878–1883. [DOI] [PubMed] [Google Scholar]
- 49. Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology (Berl). 2011;214(1):89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Class QA, Abel KM, Khashan AS, et al. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med. 2014;44(1):71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of the Netherlands. Br J Psychiatry. 1998;172:324–326. [DOI] [PubMed] [Google Scholar]
- 52. Sansavini A, Guarini A, Caselli MC. Preterm birth: neuropsychological profiles and atypical developmental pathways. Dev Disabil Res Rev. 2011;17(2):102–113. [DOI] [PubMed] [Google Scholar]
- 53. Christian P, Lee SE, Donahue Angel M, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42(5):1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schonhaut L, Armijo I, Schönstedt M, et al. Validity of the Ages and Stages Questionnaires in term and preterm infants. Pediatrics. 2013;131(5):e1468–e1474. [DOI] [PubMed] [Google Scholar]
- 55. Vameghi R, Sajedi F, Kraskian Mojembari A, et al. Cross-cultural adaptation, validation and standardization of Ages and Stages Questionnaire (ASQ) in Iranian children. Iran J Public Health. 2013;42(5):522–528. [PMC free article] [PubMed] [Google Scholar]
- 56. Kvestad I, Taneja S, Kumar T, et al. The assessment of developmental status using the Ages and Stages Questionnaire–3 in nutritional research in north indian young children. Nutr J. 2013;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bennetts SK, Mensah FK, Westrupp EM, et al. The agreement between parent-reported and directly measured child language and parenting behaviors. Front Psychol. 2016;7:1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.