Abstract
Arsenic in drinking water is known to cause cancer and noncancer diseases, but little is known about its association with age at exposure. Here, we investigated age at arsenic exposure and mortality in Antofagasta, Chile, 30–40 years after a distinct period of very high water arsenic concentrations (1958–1970). We calculated standardized mortality ratios (SMRs) comparing Antofagasta with the rest of Chile for 2001–2010 by sex and age at potential first exposure. A remarkable relationship with age at first exposure was found for bronchiectasis, with increased risk in adults 30–40 years after exposure being confined to those who were in utero (SMR = 11.7, 95% confidence interval (CI): 4.3, 25.4) or aged 1–10 years (SMR = 5.4, 95% CI: 1.1, 15.8) during the high-exposure period. Increased SMRs for lung, bladder, and laryngeal cancer were evident for exposures starting at all ages, but the highest SMRs were for exposures beginning at birth (for bladder cancer, SMR = 16.0 (95% CI: 10.3, 23.8); for laryngeal cancer, SMR = 6.8 (95% CI: 2.2, 15.8); for lung cancer, SMR = 3.8 (95% CI: 2.9, 4.9)). These findings suggest that interventions targeting early-life arsenic exposure could have major impacts in reducing long-term mortality due to arsenic 30–40 years after exposure ends.
Keywords: age at first exposure, arsenic, Chile, drinking water
Arsenic is a naturally occurring element in the earth’s crust and is frequently detected in drinking water. Millions of people around the world are exposed to arsenic through drinking water (1). Arsenic is classified by the International Agency for Research on Cancer as a human carcinogen, and there is sufficient evidence that it can cause cancer of the lung, urinary bladder, and skin (2). Many noncancer diseases have been found to be associated with arsenic exposure, including reproductive, cardiovascular, pulmonary, neurological, and dermal effects (3).
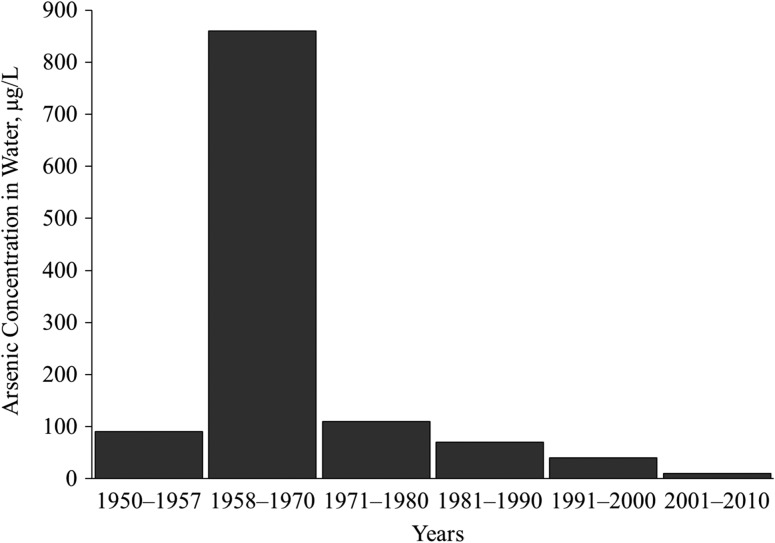
Northern Chile is the driest habitable place on earth, and almost all of the population there relies exclusively on public water systems, with no other significant water sources such as bottled water or private wells (4). Antofagasta (Figure 1) is the largest city in northern Chile (population 378,244 in 2015 (5)). Before 1958, the arsenic concentration in its water supply was 90 μg/L (6). In 1958, because of population growth, the city’s water source was changed to the Toconce and Hojalar rivers, which emanate as geothermal water (a type of groundwater) from the Andes mountains. This water contains high levels of arsenic, resulting in city water arsenic concentrations of approximately 860 μg/L (6–8). In 1970 a treatment plant was installed, and water arsenic levels decreased dramatically to about 100 μg/L. Current concentrations are less than 10 μg/L, following improvements to the arsenic removal plant.
Figure 1.
Map of Chile showing the locations of the study cities, Antofagasta and Mejillones, in region II.
Relatively few people in other studies have been exposed to arsenic concentrations over 500 μg/L. For example, in a study carried out in southwestern Taiwan, 8,251 participants were exposed to water arsenic levels over 600 μg/L (9). In a study in northeastern Taiwan, 698 people were exposed to water arsenic levels over 300 μg/L (10). In a study conducted in the South 24 Parganas district of West Bengal, India, 902 people drank well water with arsenic concentrations over 500 μg/L (11). In Matlab, a rural area in Bangladesh, 10,430 participants were exposed to arsenic levels over 300 μg/L from drinking water (12). However, in Antofagasta, approximately 125,000 residents were exposed to arsenic at concentrations of 860 μg/L from the city’s only water source until 1970, with over 10 times more people being exposed to high arsenic concentrations than in any other study. Since there was no other water source, all residents were exposed to the same high level of arsenic. This scenario, with its distinct high-exposure period (1958–1970), large population, good exposure records, and appropriate latency period, provided us with a unique opportunity to investigate the long-term consequences of arsenic exposure (13).
In previous studies, we found that mortality from lung, bladder, laryngeal, and kidney cancers and from noncancer diseases such as acute myocardial infarction had increased after the high arsenic exposure period of 1958–1970 in Chile’s region II (14–17). In several analyses, we examined mortality among adults who were in utero or were young children at the time of the high-exposure period in Antofagasta (1958–1970) and found especially high mortality ratios for lung, bladder, liver, and laryngeal cancers, chronic renal disease, and bronchiectasis in adulthood, even before age 50 years (13, 18–20). These previous early-life arsenic exposure mortality findings involved the years 1989–2000 (14–20), and in the present study, which involved mortality during the years 2001–2010, we were able to again assess the impact of early-life arsenic exposure with nonoverlapping mortality data. In addition, only limited information concerning the impact of age at first exposure has been published so far. Here we present evidence concerning age at potential first arsenic exposure and mortality from arsenic-related causes of death 30–40 years later in the largest population in the world with a distinct period of high exposure to arsenic in drinking water.
METHODS
Exposure data
Historical data on water arsenic levels in Chile have been provided in previous studies (4, 6) (Figure 2). After the water source change in the city of Antofagasta, the average arsenic concentration in the public water supply increased greatly, up to 860 μg/L, between 1958 and 1970, until a water treatment plant commenced operations in 1970. Now arsenic concentrations in Antofagasta are below 10 μg/L, the World Health Organization guideline for arsenic in drinking water (21). Antofagasta and Mejillones are neighboring cities that share the same water system, so in this article, “Antofagasta” refers to both cities together, accounting for more than 65% of the population of region II in Chile. Although the rest of region II also had arsenic in drinking water, the degree of contamination was less than that in Antofagasta. The “rest of Chile” in this paper is defined as any part of Chile outside of region II, which had low arsenic concentrations of generally less than 10 μg/L.
Figure 2.
Concentrations of drinking water arsenic in Antofagasta, Chile, 1950–2010. New water sources with high levels of arsenic were used from 1958 onward, and a water treatment plant for arsenic removal was installed in 1970, with improved removal efficiency thereafter. The current concentration of arsenic in drinking water in Antofagasta is below the World Health Organization standard of 10 μg/L (21).
Exposure data in this paper were based on the locations where subjects died, without detailed histories of residential location over the years. Since some people will have migrated into Antofagasta later in life, when we identify “early-life exposure” it means that there was potential for early-life exposure if the person was living in Antofagasta at that time. The impact of migration into Antofagasta from elsewhere in Chile would have the effect of biasing estimates of association towards the null, which is not of concern given the major increases in risk we report.
Mortality data
Mortality data were computerized and provided by the Chilean Ministry of Health for all regions of Chile for the years 2001–2010 (T.R., Ministry of Health of Chile, unpublished data, 2015). The causes of death were based on death certificates signed by physicians, and trained nosologists coded them according to the International Classification of Diseases, Tenth Revision. Nosologists were blinded to the place of death of subjects to prevent bias. We converted International Classification of Diseases, Tenth Revision, codes to International Classification of Diseases, Ninth Revision (ICD-9) codes for our analysis in order to compare results obtained over the years with previous findings. In this study, we focused on lung cancer (ICD-9 code 162), bladder cancer (ICD-9 code 188), kidney cancer (ICD-9 code 189), laryngeal cancer (ICD-9 code 161), and the noncancer diseases of bronchiectasis (ICD-9 code 494), acute myocardial infarction (ICD-9 code 410), and chronic renal failure (ICD-9 codes 582, 585, 586, and 587), all of which are reported to be associated with arsenic exposure. ICD-9 code 189 includes both renal cell carcinoma and transitional cell carcinoma, so those conditions could not be separated for this analysis. “Chronic renal disease” combines related diseases such as chronic renal failure, unspecified renal failure, chronic glomerulonephritis, and renal sclerosis (18).
Statistical analysis
We estimated standardized mortality ratios (SMRs) for deaths occurring over the age of 30 years in Antofagasta by dividing the observed number of deaths by the expected number of deaths for each age group at potential first exposure: birth, 1–10 years, 11–20 years, 21–30 years, 31–40 years, and ≥41 years. Age at potential first exposure was identified on the basis of birth cohort: births occurring during 1958–1970 (the high-exposure period in Antofagasta), 1948–1957, 1938–1947, 1928–1937, 1918–1927, and before 1918. The expected number of deaths in Antofagasta was calculated on the basis of the population of the rest of Chile using indirect age standardization. Population data by age (in 10-year age groups) and sex were obtained from Chilean census data for 1992, 2002, and 2012 (T.R., National Statistics Institute of Chile, unpublished data, 2015) and were interpolated linearly to estimate person-years for Antofagasta and the rest of Chile in 2001–2010. Exact 95% confidence intervals and P values for the SMRs were calculated on the basis of the Poisson distribution. Average percent changes for trends in SMRs by age group at first exposure were evaluated using the linear Poisson regression model (22, 23). One-sided statistical tests were used because there is a clear direction in the hypothesis that arsenic increases mortality from the selected diseases. All statistical analyses were conducted with SAS software (version 9.4; SAS Institute, Inc., Cary, North Carolina).
RESULTS
Table 1 shows the SMRs for lung, bladder, kidney, and laryngeal cancers by age at potential first exposure and sex for 2001–2010, comparing Antofagasta with the rest of Chile. The highest lung cancer SMR among males was 5.0 (95% confidence interval (CI): 4.3, 5.8) for persons likely to have been first exposed to arsenic at ages 1–10 years. In women, the SMR for lung cancer was 5.1 (95% CI: 3.4, 7.3) when they had probably been exposed to arsenic at birth, and the risk remained elevated over all age groups, even when they were first exposed at age 41 years or older (SMR = 2.7, 95% CI: 1.6, 4.2). For men and women combined, the lung cancer SMRs gradually decreased with likely age of exposure, with an 11% reduction for each 10-year increase in age (P for trend < 0.001) (Figure 3).
Table 1.
Observed and Expected Numbers of Deaths and Standardized Mortality Ratios for Cancer According to Age at Potential First Exposure to High Levels of Arsenic in Drinking Water, Antofagastaa, Chile, 2001–2010
| Disease and Age at First Exposure, years | Total | Men | Women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Exp | SMR | 95% CI | P Valueb | Obs | Exp | SMR | 95% CI | P Value | Obs | Exp | SMR | 95% CI | P Value | |
| Lung cancer | |||||||||||||||
| Birth | 57 | 15.1 | 3.8 | 2.9, 4.9 | <0.001 | 27 | 9.3 | 2.9 | 1.9, 4.2 | <0.001 | 30 | 5.9 | 5.1 | 3.4, 7.3 | <0.001 |
| 1–10 | 237 | 52.4 | 4.5 | 4.0, 5.1 | <0.001 | 178 | 35.8 | 5.0 | 4.3, 5.8 | <0.001 | 59 | 17.4 | 3.4 | 2.6, 4.4 | <0.001 |
| 11–20 | 417 | 101.6 | 4.1 | 3.7, 4.5 | <0.001 | 328 | 69.2 | 4.7 | 4.2, 5.3 | <0.001 | 89 | 32.9 | 2.7 | 2.2, 3.3 | <0.001 |
| 21–30 | 457 | 122.1 | 3.7 | 3.4, 4.1 | <0.001 | 322 | 77.7 | 4.1 | 3.7, 4.6 | <0.001 | 135 | 43.1 | 3.1 | 2.6, 3.7 | <0.001 |
| 31–40 | 176 | 62.6 | 2.8 | 2.4, 3.3 | <0.001 | 112 | 33.8 | 3.3 | 2.7, 4.0 | <0.001 | 64 | 27.6 | 2.3 | 1.8, 3.0 | <0.001 |
| ≥41 | 28 | 13.6 | 2.1 | 1.4, 3.0 | <0.001 | 9 | 5.6 | 1.6 | 0.7, 3.0 | 0.12 | 19 | 7.1 | 2.7 | 1.6, 4.2 | <0.001 |
| P for trendc | <0.001 | 0.001 | 0.006 | ||||||||||||
| Bladder cancer | |||||||||||||||
| Birth | 24 | 1.5 | 16.0 | 10.3, 23.8 | <0.001 | 17 | 1.0 | 16.8 | 9.8, 27.0 | <0.001 | 7 | 0.5 | 13.6 | 5.5, 27.9 | <0.001 |
| 1–10 | 35 | 4.8 | 7.4 | 5.1, 10.3 | <0.001 | 28 | 3.5 | 7.9 | 5.3, 11.5 | <0.001 | 7 | 1.3 | 5.3 | 2.2, 11.0 | <0.001 |
| 11–20 | 59 | 9.3 | 6.3 | 4.8, 8.1 | <0.001 | 32 | 6.7 | 4.8 | 3.3, 6.8 | <0.001 | 27 | 2.7 | 9.9 | 6.5, 14.5 | <0.001 |
| 21–30 | 112 | 16.6 | 6.7 | 5.5, 8.1 | <0.001 | 65 | 11.1 | 5.9 | 4.5, 7.5 | <0.001 | 47 | 5.3 | 8.8 | 6.5, 11.7 | <0.001 |
| 31–40 | 80 | 15.6 | 5.1 | 4.1, 6.4 | <0.001 | 39 | 8.8 | 4.4 | 3.1, 6.0 | <0.001 | 41 | 6.4 | 6.4 | 4.6, 8.7 | <0.001 |
| ≥41 | 22 | 5.3 | 4.2 | 2.6, 6.3 | <0.001 | 13 | 2.6 | 4.9 | 2.6, 8.4 | <0.001 | 9 | 2.5 | 3.6 | 1.6, 6.8 | 0.001 |
| P for trend | <0.001 | <0.001 | 0.01 | ||||||||||||
| Kidney cancer | |||||||||||||||
| Birth | 8 | 6.2 | 1.3 | 0.6, 2.5 | 0.28 | 4 | 4.5 | 0.9 | 0.2, 2.3 | 0.50 | 4 | 1.8 | 2.2 | 0.6, 5.6 | 0.11 |
| 1–10 | 25 | 14.4 | 1.7 | 1.1, 2.6 | 0.007 | 18 | 10.8 | 1.7 | 1.0, 2.6 | 0.03 | 7 | 3.9 | 1.8 | 0.7, 3.7 | 0.10 |
| 11–20 | 49 | 21.5 | 2.3 | 1.7, 3.0 | <0.001 | 32 | 15.3 | 2.1 | 1.4, 3.0 | <0.001 | 17 | 6.4 | 2.6 | 1.5, 4.2 | <0.001 |
| 21–30 | 46 | 26.1 | 1.8 | 1.3, 2.4 | <0.001 | 29 | 16.7 | 1.7 | 1.2, 2.5 | 0.009 | 17 | 9.0 | 1.9 | 1.1, 3.0 | 0.01 |
| 31–40 | 38 | 14.1 | 2.7 | 1.9, 3.7 | <0.001 | 16 | 7.4 | 2.2 | 1.2, 3.5 | 0.004 | 22 | 6.5 | 3.4 | 2.1, 5.1 | <0.001 |
| ≥41 | 7 | 3.4 | 2.0 | 0.8, 4.2 | 0.06 | 3 | 1.5 | 2.0 | 0.4, 5.9 | 0.19 | 4 | 1.9 | 2.1 | 0.6, 5.4 | 0.12 |
| P for trend | 0.12 | 0.24 | 0.39 | ||||||||||||
| Laryngeal cancer | |||||||||||||||
| Birth | 5 | 0.7 | 6.8 | 2.2, 15.8 | 0.001 | 3 | 0.6 | 4.8 | 1.0, 14.1 | 0.03 | 2 | 0.1 | 14.4 | 1.3, 51.9 | 0.009 |
| 1–10 | 6 | 2.6 | 2.3 | 0.8, 4.9 | 0.05 | 5 | 2.4 | 2.1 | 0.7, 4.8 | 0.10 | 1 | 0.3 | 3.3 | 0.1, 18.3 | 0.26 |
| 11–20 | 17 | 5.3 | 3.2 | 1.9, 5.1 | <0.001 | 15 | 4.8 | 3.1 | 1.7, 5.1 | <0.001 | 2 | 0.5 | 3.7 | 0.4, 13.2 | 0.10 |
| 21–30 | 12 | 7.6 | 1.6 | 0.8, 2.7 | 0.09 | 10 | 6.5 | 1.5 | 0.7, 2.8 | 0.12 | 2 | 1.0 | 2.1 | 0.3, 7.5 | 0.25 |
| 31–40 | 8 | 3.9 | 2.1 | 0.9, 4.1 | 0.04 | 6 | 3.0 | 2.0 | 0.7, 4.3 | 0.09 | 2 | 0.7 | 2.9 | 0.4, 10.6 | 0.15 |
| ≥41 | 1 | 0.8 | 1.3 | 0.1, 7.1 | 0.50 | 1 | 0.5 | 1.9 | 0.1, 10.3 | 0.42 | 0 | 0.2 | 0 | ||
| P for trend | 0.03 | 0.18 | 0.06 | ||||||||||||
| All cancers | |||||||||||||||
| Birth | 94 | 23.5 | 4.0 | 3.2, 4.9 | <0.001 | 51 | 15.5 | 3.3 | 2.4, 4.3 | <0.001 | 43 | 8.4 | 5.1 | 3.7, 6.9 | <0.001 |
| 1–10 | 303 | 74.2 | 4.1 | 3.6, 4.6 | <0.001 | 229 | 52.5 | 4.4 | 3.8, 5.0 | <0.001 | 74 | 22.9 | 3.2 | 2.5, 4.1 | <0.001 |
| 11–20 | 542 | 137.8 | 3.9 | 3.6, 4.3 | <0.001 | 407 | 95.9 | 4.2 | 3.8, 4.7 | <0.001 | 135 | 42.6 | 3.2 | 2.7, 3.8 | <0.001 |
| 21–30 | 627 | 172.5 | 3.6 | 3.4, 3.9 | <0.001 | 426 | 112.0 | 3.8 | 3.4, 4.2 | <0.001 | 201 | 58.4 | 3.4 | 3.0, 4.0 | <0.001 |
| 31–40 | 302 | 96.1 | 3.1 | 2.8, 3.5 | <0.001 | 173 | 53.1 | 3.3 | 2.8, 3.8 | <0.001 | 129 | 41.2 | 3.1 | 2.6, 3.7 | <0.001 |
| ≥41 | 58 | 23.0 | 2.5 | 1.9, 3.3 | <0.001 | 26 | 10.9 | 2.4 | 1.6, 3.5 | <0.001 | 32 | 11.7 | 2.7 | 1.9, 3.9 | <0.001 |
| P for trend | <0.001 | 0.002 | 0.06 | ||||||||||||
| All other cancers | |||||||||||||||
| Birth | 278 | 238.8 | 1.2 | 1.0, 1.3 | 0.007 | 133 | 97.3 | 1.4 | 1.1, 1.6 | <0.001 | 145 | 138.2 | 1.0 | 0.9, 1.2 | 0.29 |
| 1–10 | 454 | 389.2 | 1.2 | 1.1, 1.3 | <0.001 | 239 | 170.0 | 1.4 | 1.2, 1.6 | <0.001 | 215 | 216.9 | 1.0 | 0.9, 1.1 | 0.53 |
| 11–20 | 652 | 614.7 | 1.1 | 1.0, 1.1 | 0.07 | 353 | 308.8 | 1.1 | 1.0, 1.3 | 0.007 | 299 | 305.7 | 1.0 | 0.9, 1.1 | 0.64 |
| 21–30 | 833 | 803.2 | 1.0 | 1.0, 1.1 | 0.15 | 453 | 420.0 | 1.1 | 1.0, 1.2 | 0.06 | 380 | 378.5 | 1.0 | 0.9, 1.1 | 0.48 |
| 31–40 | 604 | 582.6 | 1.0 | 1.0, 1.1 | 0.19 | 305 | 274.9 | 1.1 | 1.0, 1.3 | 0.04 | 299 | 301.0 | 1.0 | 0.9, 1.1 | 0.53 |
| ≥41 | 166 | 189.5 | 0.9 | 0.7, 1.0 | 0.95 | 68 | 75.7 | 0.9 | 0.7, 1.1 | 98 | 112.3 | 0.9 | 0.7, 1.1 | 0.91 | |
| P for trend | 0.001 | <0.001 | 0.37 | ||||||||||||
Abbreviations: CI, confidence interval; Exp, expected number; Obs, observed number; SMR, standardized mortality ratio.
a All data presented for Antofagasta include neighboring Mejillones, which had the same water sources.
b One-sided P value for mortality being increased in Antofagasta as compared with the rest of Chile.
c Two-sided P value for linear trend in the SMRs for each 10-year increase in age at first exposure in Antofagasta.
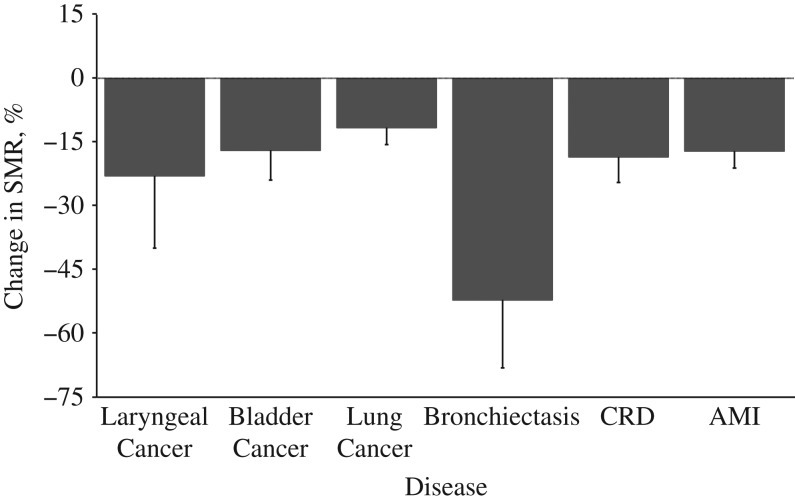
Figure 3.
Change in the standardized mortality ratio (SMR) for selected diseases per 10-year increase in age at first exposure to drinking water arsenic, Antofagasta, Chile, 2001–2010. Among cancers, the strongest relationship with age at first exposure was for cancer of the larynx, with a 23% reduction in the SMR for each 10-year increase in age (P for trend = 0.03). Among noncancer diseases, the strongest age relationship was for bronchiectasis, with a 49% reduction for each 10-year increase in age at first exposure (P for trend < 0.001). P values for trends are provided in Table 2. Bars, 95% confidence intervals. AMI, acute myocardial infarction; CRD, chronic renal disease.
Bladder cancer SMRs for men and women who were probably exposed very early in their lives were extremely high. For people exposed at birth, the SMR was 16.8 (95% CI: 9.8, 27.0) in men and 13.6 (95% CI: 5.5, 27.9) in women. For men and women combined, the SMRs dropped at a rate of 17% for each 10-year increase in age (P for trend < 0.001) (Figure 3) but remained elevated in all groups of age at first exposure, within a range of 4–10. Laryngeal cancer showed a similar trend as bladder cancer when subjects were probably exposed starting in early life. Among cancer causes of death, laryngeal cancer showed the strongest relationship with age at first exposure, with a 23% reduction in SMRs for each 10-year increase in age at exposure (P for trend = 0.03) (Figure 3). SMRs for all of the cancers above combined were increased in all age groups at first exposure (Table 1).
Table 2 presents the SMRs for arsenic-related noncancer deaths in Antofagasta from bronchiectasis, acute myocardial infarction, and chronic renal disease. The SMRs for all of these diseases were elevated only when subjects had been exposed starting at younger ages. For bronchiectasis, mortality was greatly increased in the group probably exposed to arsenic in early life (at birth and ages 1–10 years). In fact, the SMR for bronchiectasis was not elevated for persons who were probably exposed to arsenic only at age 11 years or more. Mortality from acute myocardial infarction was increased in those exposed to high levels of arsenic before age 21 years. The SMR for chronic renal disease was highest in those exposed to arsenic before age 31 years. The SMRs for acute myocardial infarction and chronic renal failure decreased by 17% and 18%, respectively, for each 10-year increase in age at first exposure (P for trend < 0.001) (Figure 3). Mortality from all of these noncancer diseases combined was increased with exposure starting early in life. Mortality from all other noncancer diseases not associated with arsenic did not differ between Antofagasta and the rest of Chile in any age group at first exposure (Table 2).
Table 2.
Observed and Expected Numbers of Deaths and Standardized Mortality Ratios for Noncancer Diseases According to Age at Potential First Exposure to High Levels of Arsenic in Drinking Water, Antofagastaa, Chile, 2001–2010
| Disease and Age at First Exposure, years | Total | Men | Women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Exp | SMR | 95% CI | P Valueb | Obs | Exp | SMR | 95% CI | P Value | Obs | Exp | SMR | 95% CI | P Value | |
| Bronchiectasis | |||||||||||||||
| Birth | 6 | 0.5 | 11.7 | 4.3, 25.4 | <0.001 | 4 | 0.2 | 24.9 | 6.8, 63.7 | <0.001 | 2 | 0.3 | 5.9 | 0.7, 21.3 | 0.05 |
| 1–10 | 3 | 0.6 | 5.4 | 1.1, 15.8 | 0.02 | 1 | 0.4 | 2.4 | 0.1, 13.5 | 0.34 | 2 | 0.2 | 13.2 | 1.6, 47.7 | 0.01 |
| 11–20 | 1 | 0.7 | 1.3 | 0.1, 7.5 | 0.50 | 1 | 0.4 | 2.6 | 0.1, 14.6 | 0.32 | 0 | 0.4 | 0 | ||
| 21–30 | 2 | 1.7 | 1.2 | 0.1, 4.3 | 0.50 | 0 | 0.9 | 0 | 2 | 0.8 | 2.4 | 0.3, 8.8 | 0.20 | ||
| 31–40 | 0 | 2.0 | 0 | 0 | 0.8 | 0 | 0 | 1.2 | 0 | ||||||
| ≥41 | 1 | 1.1 | 0.9 | 0.1, 5.1 | 0.50 | 1 | 0.4 | 2.6 | 0.1, 14.2 | 0.32 | 0 | 0.7 | 0 | ||
| P for trendc | <0.001 | 0.004 | 0.006 | ||||||||||||
| Myocardial infarction | |||||||||||||||
| Birth | 94 | 51.5 | 1.8 | 1.5, 2.2 | <0.001 | 74 | 44.4 | 1.7 | 1.3, 2.1 | <0.001 | 20 | 9.2 | 2.2 | 1.3, 3.4 | 0.001 |
| 1–10 | 171 | 107.3 | 1.6 | 1.4, 1.9 | <0.001 | 139 | 86.5 | 1.6 | 1.4, 1.9 | <0.001 | 32 | 23.3 | 1.4 | 0.9, 1.9 | 0.05 |
| 11–20 | 231 | 188.2 | 1.2 | 1.1, 1.4 | 0.001 | 162 | 137.7 | 1.2 | 1.0, 1.4 | 0.02 | 69 | 51.9 | 1.3 | 1.0, 1.7 | 0.01 |
| 21–30 | 275 | 260.3 | 1.1 | 0.9, 1.2 | 0.19 | 181 | 158.9 | 1.1 | 1.0, 1.3 | 0.05 | 94 | 98.9 | 1.0 | 0.8, 1.2 | 0.33 |
| 31–40 | 220 | 228.6 | 1.0 | 0.8, 1.1 | 0.70 | 113 | 108.5 | 1.0 | 0.9, 1.3 | 0.34 | 107 | 117.5 | 0.9 | 0.7, 1.1 | 0.82 |
| ≥41 | 69 | 112.1 | 0.6 | 0.5, 0.8 | 0.99 | 27 | 36.7 | 0.7 | 0.5, 1.1 | 0.94 | 42 | 75.6 | 0.6 | 0.4, 0.8 | 0.99 |
| P for trend | <0.001 | <0.001 | <0.001 | ||||||||||||
| Chronic renal disease | |||||||||||||||
| Birth | 23 | 9.4 | 2.5 | 1.6, 3.7 | <0.001 | 13 | 5.9 | 2.2 | 1.2, 3.8 | 0.008 | 10 | 3.6 | 2.8 | 1.3, 5.2 | 0.004 |
| 1–10 | 39 | 16.6 | 2.3 | 1.7, 3.2 | <0.001 | 19 | 10.1 | 1.9 | 1.1, 2.9 | 0.008 | 20 | 6.7 | 3.0 | 1.8, 4.6 | <0.001 |
| 11–20 | 60 | 34.1 | 1.8 | 1.3, 2.3 | <0.001 | 33 | 19.2 | 1.7 | 1.2, 2.4 | 0.003 | 27 | 15.0 | 1.8 | 1.2, 2.6 | 0.003 |
| 21–30 | 106 | 61.5 | 1.7 | 1.4, 2.1 | <0.001 | 54 | 32.2 | 1.7 | 1.3, 2.2 | <0.001 | 52 | 28.8 | 1.8 | 1.3, 2.4 | <0.001 |
| 31–40 | 77 | 61.0 | 1.3 | 1.0, 1.6 | 0.03 | 38 | 28.4 | 1.3 | 0.9, 1.8 | 0.05 | 39 | 31.9 | 1.2 | 0.9, 1.7 | 0.12 |
| ≥41 | 24 | 29.8 | 0.8 | 0.5, 1.2 | 0.83 | 10 | 10.9 | 0.9 | 0.4, 1.7 | 0.53 | 14 | 18.7 | 0.7 | 0.4, 1.3 | 0.84 |
| P for trend | <0.001 | 0.02 | <0.001 | ||||||||||||
| All noncancer diseases | |||||||||||||||
| Birth | 123 | 61.3 | 2.0 | 1.7, 2.4 | <0.001 | 91 | 50.5 | 1.8 | 1.5, 2.2 | <0.001 | 32 | 13.1 | 2.4 | 1.7, 3.4 | <0.001 |
| 1–10 | 213 | 124.5 | 1.7 | 1.5, 2.0 | <0.001 | 159 | 97.0 | 1.6 | 1.4, 1.9 | <0.001 | 54 | 30.2 | 1.8 | 1.3, 2.3 | <0.001 |
| 11–20 | 292 | 223.1 | 1.3 | 1.2, 1.5 | <0.001 | 196 | 157.2 | 1.2 | 1.1, 1.4 | 0.002 | 96 | 67.2 | 1.4 | 1.2, 1.7 | <0.001 |
| 21–30 | 383 | 323.4 | 1.2 | 1.1, 1.3 | <0.001 | 235 | 192.0 | 1.2 | 1.1, 1.4 | 0.001 | 148 | 128.5 | 1.2 | 1.0, 1.4 | 0.05 |
| 31–40 | 297 | 291.6 | 1.0 | 0.9, 1.1 | 0.38 | 151 | 137.7 | 1.1 | 0.9, 1.3 | 0.13 | 146 | 150.7 | 1.0 | 0.8, 1.1 | 0.63 |
| ≥41 | 94 | 143.0 | 0.7 | 0.5, 0.8 | 0.99 | 38 | 48.0 | 0.8 | 0.6, 1.1 | 0.92 | 56 | 95.0 | 0.6 | 0.4, 0.8 | 0.99 |
| P for trend | <0.001 | <0.001 | <0.001 | ||||||||||||
| All other noncancer diseases | |||||||||||||||
| Birth | 773 | 872.6 | 0.9 | 0.8, 1.0 | 0.99 | 546 | 710.1 | 0.8 | 0.7, 0.8 | 0.99 | 227 | 195.2 | 1.2 | 1.0, 1.3 | 0.01 |
| 1–10 | 957 | 911.6 | 1.0 | 1.0, 1.1 | 0.07 | 647 | 669.2 | 1.0 | 0.9, 1.0 | 0.80 | 320 | 260.0 | 1.2 | 1.1, 1.4 | <0.001 |
| 11–20 | 1,412 | 1,284.7 | 1.1 | 1.0, 1.2 | <0.001 | 888 | 839.9 | 1.1 | 1.0, 1.1 | 0.05 | 524 | 451.3 | 1.2 | 1.1, 1.3 | <0.001 |
| 21–30 | 2,306 | 1,978.5 | 1.2 | 1.1, 1.2 | <0.001 | 1,250 | 1,080.3 | 1.2 | 1.1, 1.2 | <0.001 | 1,056 | 885.5 | 1.2 | 1.1, 1.3 | <0.001 |
| 31–40 | 2,434 | 2,219.8 | 1.1 | 1.1, 1.1 | <0.001 | 1,053 | 947.7 | 1.1 | 1.0, 1.2 | <0.001 | 1,381 | 1,256.3 | 1.1 | 1.0, 1.2 | <0.001 |
| ≥41 | 1,289 | 1,399.7 | 0.9 | 0.9, 1.0 | 0.99 | 353 | 417.3 | 0.8 | 0.8, 0.9 | 0.99 | 936 | 989.6 | 0.9 | 0.9, 1.0 | 0.96 |
| P for trend | 0.44 | <0.001 | <0.001 | ||||||||||||
Abbreviations: CI, confidence interval; Exp, expected number; Obs, observed number; SMR, standardized mortality ratio.
a All data presented for Antofagasta include neighboring Mejillones, which had the same water sources.
b One-sided P value for mortality being increased in Antofagasta as compared with the rest of Chile.
c Two-sided P value for linear trend in the SMRs for each 10-year increase in age at first exposure in Antofagasta.
DISCUSSION
We found especially strong relationships with age at first exposure for bladder cancer, laryngeal cancer, bronchiectasis, myocardial infarction, and chronic renal disease. Persons born during the high-exposure period (1958–1970) had a nearly 12-fold increase in mortality from bronchiectasis in 2001–2010 (SMR = 11.7, 95% CI: 4.3, 25.4), 30–40 years after the high exposure ceased. We previously reported that in the years 1989–2000, this same birth cohort had a bronchiectasis SMR of 46.2 (95% CI: 21.1, 87.7; P < 0.001) (19). Thus, 2 nonoverlapping studies with nonoverlapping mortality data both demonstrated an astonishingly increased risk of death from bronchiectasis following early-life arsenic exposure. Persons first exposed at ages 1–10 years had a 5-fold increased risk of mortality, but if exposure to arsenic did not start until after age 10 years, there was no meaningful increase in mortality from bronchiectasis (although the numbers were small and confidence intervals wide). In our previous study, we did not assess children with first exposure after age 10 years, and we are not aware of any other studies with data on age at first exposure that separated out persons first exposed only after age 10 years. The findings we report here suggest that arsenic may affect the growing lung, making it particularly susceptible to bronchiectasis in later years.
Bladder cancer also had a remarkably strong relationship to early-life exposure (for persons exposed from birth, SMR = 16.0, 95% CI: 10.3, 23.8). This finding is also consistent with our previously published findings for bladder cancer mortality in 1989–2000 (SMR = 18.1, 95% CI: 11.3, 27.4) (18). However, unlike bronchiectasis, bladder cancer mortality continued to be increased for older ages at first exposure, even for first exposures after age 40 years (Table 1). The finding for laryngeal cancer was rather similar (for exposure from birth, SMR = 6.8, 95% CI: 2.2, 15.8), although increased risks were evident only if exposure started before age 41 years. Once again, we confirmed the earlier finding for mortality in 1989–2000 following exposure from birth (SMR = 8.1, 95% CI: 3.5, 16.0) (18).
Lung cancer SMRs were increased with all ages at first exposure, with a small trend downward by age, but kidney cancer showed an upward trend with age at first exposure (P for trend = 0.12). This finding could have been due to chance, but it was surprising since in 1989–2000 we reported a kidney cancer SMR of 3.5 (95% CI: 2.1, 5.4) for potential exposure from birth (18). Mortality from myocardial infarction and chronic renal disease both trended downward with increasing age. Increased numbers of myocardial infarction deaths were only evident if exposure started before age 21 years, but some increase in chronic renal disease persisted with exposure starting at older ages.
The observed trends by age group at first exposure for bladder cancer, laryngeal cancer, bronchiectasis, myocardial infarction, and chronic renal disease were very strong and were beyond what could be attributed to unaccounted potential bias.
Our findings on lung and bladder cancer are consistent with the results of a previous case-control study of incident cancer we conducted in the same area (13). In a case-control study of 221 lung cancer patients and 160 bladder cancer patients with 508 matched controls from northern Chile for the years 2007–2010, we observed that the risks of lung and bladder cancer were elevated with early-life arsenic exposure in a dose-response manner (13). The trends with age of exposure in the case-control study were similar to those we report for cancer mortality here. For lung cancer, high odds ratios for males and females combined were seen in the case-control study for arsenic exposures incurred at birth and up to approximately age 20 years, with a gradual decline in odds ratios for exposures incurred at age 21 years and beyond. In this paper, for males and females combined, the highest SMRs were seen for ages 1–20 years, also with a decline in relative risk for exposure after these ages. For bladder cancer, the highest odds ratios in the case-control study were seen for exposure at birth, with a marked decrease in relative risk for exposure at older ages. A similar pattern was seen for mortality here, with the highest SMRs by far being seen for likely exposure at birth and markedly lower SMRs being seen for first exposure at older ages.
In this study, we considered the subjects exposed if they died in Antofagasta or Mejillones during 2000–2010 and were of ages at which they would have been born or lived in Antofagasta during the high-exposure period (1958–1970). Because our study design was ecological, we considered the possibility that exposure misclassification might have affected our results. As we mentioned above, the region of Chile containing Antofagasta (region II) receives very little rainfall, and there are very few water sources in the area. Because of this, essentially everyone who lived in Antofagasta during the high-exposure period received their drinking water from the single public water source supplying the city. Detailed records of historical arsenic water concentrations are available, including those from the high-exposure period, and until recently bottled water use was minimal (4). In a case-control study we are currently conducting among 296 participants who were living in region II before 1970, only 1 person reported using bottled water while residing in region II (17).
Consequently, if a person resided in Antofagasta during the high-exposure period, we can be assured that he or she was highly exposed to arsenic. A few other areas in the rest of Chile, the site of our comparison population, had measurable arsenic levels in drinking water, but these areas were relatively few, and none of the arsenic levels approached anything near those seen in Antofagasta. Because of this unique situation, operation of the ecological fallacy in our study is virtually inconceivable.
Migration is another issue we considered. Because we did not have people’s full residential history, we could not account for people moving in and out of Antofagasta. However, both migration into and out of Antofagasta during our study period would most likely have biased our results towards the null, not towards the large SMRs we identified. In addition, the migration rate in Chile was low (only 0.6% per year) during the study period (18, 24).
Another potential concern is that the associations we identified might have been affected by confounding. For example, smoking is the main risk factor for bladder cancer, but it is not possible that our results for this cancer could have been due to confounding by smoking. In a meta-analysis based on 83 studies carried out in many different countries (25), the pooled relative risk of bladder cancer mortality for smokers versus nonsmokers was approximately 1.5. Given this, even if all subjects in Antofagasta were smokers and all subjects in the rest of Chile were nonsmokers (a highly improbable exaggerated scenario), the SMR due to confounding by smoking would only be around 1.5. However, our results showed that SMRs for bladder cancer were much higher (e.g., SMR = 16.0 in people potentially first exposed at birth). In fact, data from the Chile National Health Survey (26) and elsewhere show that smoking rates do not differ between region II and the rest of Chile (Table 3). In our previous paper, we also reported similar smoking patterns in region II and all of Chile, even showing lower smoking rates in region II in some years between 1990 and 2014 (17).
Table 3.
Distribution (%) of Demographic, Lifestyle-Related, and Medical Risk Factors for Mortality in Region IIa and All of Chile, 2003–2010
| Characteristicb | Region II | All of Chile |
|---|---|---|
| Demographic risk factors | ||
| Female sex | 48.1 | 51.1 |
| Urban residence | 97.7 | 91.6 |
| Higher education (university/professional) | 17.0 | 14.0 |
| Poverty-level SES | 11.4 | 18.8 |
| Lifestyle-related risk factors | ||
| Current smoking (yes/no) | 42.8 | 40.5 |
| Passive tobacco smoke exposure | 7.1 | 9.6 |
| Tobacco smoking, cigarettes/day | 7.7 | 10.4 |
| Alcohol consumption, g/day | 41.5 | 55.6 |
| Fruit/vegetable consumption, g/day | 174.0 | 186.0 |
| Salt consumption, g/day | 9.6 | 9.8 |
| Regular physical activityc | 13.8 | 10.6 |
| Medical risk factors | ||
| Average BMId | 27.2 | 27.4 |
| Obesity (BMI ≥30) | 24.7 | 25.1 |
| Hypertension (BP ≥140/90 mm Hg) | 21.1 | 26.9 |
| Diabetes mellitus | 9.3 | 9.4 |
Abbreviations: BMI, body mass index; BP, blood pressure; SES, socioeconomic status.
a Antofagasta is the second-largest city in Chile, and combined with Mejillones, its population accounts for more than 65% of the population of region II.
b Data were obtained from the Ministry of Health (2010) (26), the National Statistics Institute (2003) (30), the Ministry of Health (2003) (31), and the Ministry of Planning and Cooperation (2004) (32).
c Engaging in physical activity for at least 30 minutes 3 or more times per week.
d Weight (kg)/height (m)2.
Overall, given the very high SMRs we identified and the fact that no major differences in smoking rates were observed between Antofagasta and the rest of Chile, we can conclude that the associations we identified here were not due to confounding by smoking. Relatively small differences in other demographic and risk factors were seen between region II and the rest of Chile (Table 3). In addition, there is no notable occupational or environmental exposure that is sufficiently prevalent or sufficiently strongly related to the outcomes we assessed to cause the very high SMRs we identified. Our previous lung and bladder cancer case-control study in this same area showed that age, sex, smoking, diet, occupational exposure, socioeconomic status, and obesity had little influence on the arsenic-cancer associations identified (13).
Some outcomes appeared to show differences in SMRs by sex. For example, the SMRs for laryngeal cancer in men were lower than those for women. However, there was considerable overlap in the confidence intervals. Additionally, the relative risks for men were lower because they had a much higher background rate of laryngeal cancer (2.86 deaths per 100,000 in men and 0.41 deaths per 100,000 in women for the rest of Chile) due to smoking and alcohol drinking, which are major risk factors for laryngeal cancer.
In this study, for most outcomes we found higher relative risks for exposures occurring in early life, and there are several plausible mechanisms that may explain this finding. The fetus and young children may have undeveloped immune systems and may undergo metabolic, excretory, and other pharmacokinetic processes that decrease their ability to counteract early toxic effects (27). They have greater exposure per body weight for a given level of toxicant in water, and rapid organ growth also makes them more vulnerable to toxicants (27). In addition, molecular changes, including epigenetic modification, may result in increased risk of diseases in later life (28, 29). We found that age was an effect modifier in diseases associated with arsenic exposure 30–40 years after cessation. Cancer mortality remained elevated with exposure commencing at older ages, although mortality from most noncancer diseases was increased during this period only in people who had early-life arsenic exposure. For bronchiectasis, bladder cancer, and laryngeal cancer, young age at first exposure was associated with major increases in mortality in adults.
Overall, the findings we present here for the 2001–2010 period, combined with the very high SMRs associated with early-life exposure we previously published for the 1989–2000 period (14–20), provide strong evidence that the fetus and young children are particularly susceptible to arsenic exposure. Millions of people worldwide are exposed to high concentrations of arsenic in drinking water. Although attempts should be made to decrease these exposures in all population groups, our results suggest that reducing exposures in pregnant women and young children could have major impacts on reducing the long-term mortality risks from arsenic, even 30–40 years after exposure.
ACKNOWLEDGMENTS
Author affiliations: Arsenic Health Effects Research Group, School of Public Health, University of California, Berkeley, Berkeley, California (Taehyun Roh, Craig Steinmaus, Jane Liaw, Allan H. Smith); Departamento de Estadística, Facultad de Matemáticas, Pontificia Universidad Católica de Chile, Santiago, Chile (Guillermo Marshall); and Advanced Center for Chronic Diseases, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile (Catterina Ferreccio).
This work was funded by the National Institute of Environmental Health Sciences, US National Institutes of Health (grants R01 ES014032 and P42 ES04705).
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- ICD-9
International Classification of Diseases, Ninth Revision
- SMR
standardized mortality ratio
REFERENCES
- 1. Nordstrom KD Worldwide occurrences of arsenic in ground water. Science. 2022;296(5576):2143–2145. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer Arsenic, Metals, Fibres and Dusts (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, volume 100C). Lyon, France: International Agency for Research on Cancer; 2012. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100C.pdf. Accessed January 11, 2018. [PMC free article] [PubMed]
- 3. National Research Council, National Academy of Sciences Arsenic in Drinking Water. Washington, DC: National Research Council; 2001. [Google Scholar]
- 4. Steinmaus CM, Ferreccio C, Romo JA, et al. . Drinking water arsenic in northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev. 2013;22(4):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Instituto Nacional de Estadísticas, Gobierno de Chile Estadísticas Vitales Anuario 2015 Santiago, Chile: Instituto Nacional de Estadísticas; 2017. http://www.ine.cl/docs/default-source/publicaciones/2017/anuario-de-estadisticas-vitales-2015.pdf. Accessed January 11, 2018.
- 6. Ferreccio C, González C, Milosavjlevic V, et al. . Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11(6):673–679. [DOI] [PubMed] [Google Scholar]
- 7. Borgoño JM, Vicent P, Venturino H, et al. . Arsenic in the drinking water of the city of Antofagasta: epidemiological and clinical study before and after the installation of a treatment plant. Environ Health Perspect. 1977;19:103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smedley PL, Kinniburgh DG, Huq I, et al. . International perspective on naturally occurring arsenic problems in groundwater In: Chappell WR, Abernathy CO, Calderon RL, eds. Arsenic Exposure and Health Effects IV. Amsterdam, the Netherlands: Elsevier BV; 2001:9–26. [Google Scholar]
- 9. Tseng WP, Chu HM, How SW, et al. . Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J Natl Cancer Inst. 1968;40(3):453–463. [PubMed] [Google Scholar]
- 10. Chiou HY, Chiou ST, Hsu YH, et al. . Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am J Epidemiol. 2001;153(5):411–418. [DOI] [PubMed] [Google Scholar]
- 11. Guha Mazumder DN, Haque R, Ghosh N, et al. . Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int J Epidemiol. 1998;27(5):871–877. [DOI] [PubMed] [Google Scholar]
- 12. Sohel N, Persson LA, Rahman M, et al. . Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009;20(6):824–830. [DOI] [PubMed] [Google Scholar]
- 13. Steinmaus C, Ferreccio C, Acevedo J, et al. . Increased lung and bladder cancer incidence in adults after in utero and early life arsenic exposure. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan Y, Marshall G, Ferreccio C, et al. . Kidney cancer mortality: fifty-year latency patterns related to arsenic exposure. Epidemiology. 2010;21(1):103–108. [DOI] [PubMed] [Google Scholar]
- 15. Yuan Y, Marshall G, Ferreccio C, et al. . Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epidemiol. 2007;166(12):1381–1391. [DOI] [PubMed] [Google Scholar]
- 16. Smith AH, Goycolea M, Haque R, et al. . Marked increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147(7):660–669. [DOI] [PubMed] [Google Scholar]
- 17. Smith AH, Marshall G, Roh T, et al. . Lung, bladder, and kidney cancer mortality 40 years after arsenic exposure reduction. J Natl Cancer Inst. 2018;110(3):241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith AH, Marshall G, Liaw J, et al. . Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect. 2012;120(11):1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith AH, Marshall G, Yuan Y, et al. . Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114(8):1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith AH, Yunus M, Khan AF, et al. . Chronic respiratory symptoms in children following in utero and early life exposure to arsenic in drinking water in Bangladesh. Int J Epidemiol. 2013;42(4):1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization Arsenic in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality Geneva, Switzerland: World Health Organization; 2011. (Publication no. WHO/SDE/WSH/03.04/75/Rev/1). http://www.who.int/water_sanitation_health/dwq/chemicals/arsenic.pdf. Accessed January 11, 2018.
- 22. Boshuizen HC, Feskens EJ. Fitting additive Poisson models. Epidemiol Perspect Innov. 2010;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Hoek J, Roorda LD, Boshuizen HC, et al. . Trend in and predictors for cardiovascular mortality in patients with rheumatoid arthritis over a period of 15 years: a prospective cohort study. Clin Exp Rheumatol. 2016;34(5):813–819. [PubMed] [Google Scholar]
- 24. Soto R, Torche A. Spatial inequality, migration and economic growth in Chile. Cuad Econ. 2004;41(124): 401–424. [Google Scholar]
- 25. Cumberbatch MG, Rota M, Catto JW, et al. . The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70(3):458–466. [DOI] [PubMed] [Google Scholar]
- 26. Ministerio de Salud, Gobierno de Chile Encuesta Nacional de Salud ENS Chile, 2009–2010 Santiago, Chile: Ministerio de Salud; 2010. http://web.minsal.cl/portal/url/item/bcb03d7bc28b64dfe040010165012d23.pdf. Accessed December 7, 2017.
- 27. Miller MD, Marty MA, Arcus A, et al. . Differences between children and adults: implications for risk assessment at California EPA. Int J Toxicol. 2002;21(5):403–418. [DOI] [PubMed] [Google Scholar]
- 28. Bailey KA, Smith AH, Tokar EJ, et al. . Mechanisms underlying latent disease risk associated with early life arsenic exposure: current research trends and scientific gaps. Environ Health Perspect. 2016;124(2):170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas DJ. The die is cast: arsenic exposure in early life and disease susceptibility. Chem Res Toxicol. 2013;26(12):1778–1781. [DOI] [PubMed] [Google Scholar]
- 30. Instituto Nacional de Estadísticas, Gobierno de Chile Censo 2002 Resultados. Volumen I. Población País-Región Santiago, Chile: Instituto Nacional de Estadísticas; 2003. http://historico.ine.cl/canales/usuarios/cedoc_online/censos/pdf/censo_2002_volumen_I.pdf. Accessed December 7, 2017.
- 31. Ministerio de Salud, Gobierno de Chile Resultados I Encuesta de Salud, Chile 2003 Santiago, Chile: Ministerio de Salud; 2003. http://epi.minsal.cl/wp-content/uploads/2016/03/InformeFinalENS2003.vent_.pdf. Accessed December 7, 2017.
- 32. Ministerio de Planificacion y Cooperacion, Gobierno de Chile Volumen 1: Pobreza, Distribución del Ingreso e Impacto Distributivo del Gasto Social Santiago, Chile: Ministerio de Planificacion y Cooperacion; 2004. http://www.cooperativa.cl/noticias/site/artic/20040819/asocfile/20040819114630/ASOCFILE120040819114630.pdf. Accessed December 7, 2017.