Abstract
Purpose
Spinal interbody fusion cages are designed to provide immediate stabilization for adjoining vertebrae and ideally enable bony ingrowth to achieve successful integration. For such an implant, cells must be able to attach, move, grow, and differentiate on its surface. These cellular interactions are dependent on how the implant surface enables the coating and binding of blood and tissue fluid proteins that support cell adhesion. The purpose of this study was to evaluate the in vitro and in vivo osteoblast cell–implant surface interactions that result in osseointegration onto a surface composed of plasma-sprayed titanium on a polyetheretherketone (PEEK) substrate or titanium-coated PEEK (Ti-PEEK) (PlasmaporeXP®) as compared to uncoated PEEK implants.
Materials and methods
The influence of the Ti-PEEK surface modification on the biochemical, biomechanical, and histological properties at the bone–implant interface is demonstrated both in vitro using simulated bone-forming cell culture experiments and in vivo using a 12- and 24-week ovine implant model.
Results
Osteoblast-like cells attached to the Ti-PEEK surface upregulated early bone-forming activity as measured by an increase in transcription and translation of ALP and BMP-2 when compared to cells on PEEK. Similarly, a significant increase in new bone formation, bony apposition, and pullout strength was demonstrated on Ti-PEEK implants when compared to PEEK implants at 12 and 24 weeks in an ovine implant in vivo model.
Conclusion
The study shows that the Ti-PEEK surface demonstrated enhanced osseointegrative properties compared to PEEK both in vitro and in vivo.
Keywords: porous titanium, implant coating, osteoblast differentiation, osseointegration, pullout test, sheep
Introduction
In a spinal fusion, the role of the interbody implant is to provide primary stabilization to the degenerated spinal segment until complete arthrodesis occurs, which can take several months. At first, the implant bears the majority of the load; however, over time, the role of the device becomes obsolete as the vertebrae undergo arthrodesis. The interbody implant must support mechanical loading while at the same time prevent stress shielding of the adjoining vertebrae until the spinal segment is fully fused.1
Most interbody implants are currently either manufactured from titanium or polyetheretherketone (PEEK). PEEK has become the prevailing material for spinal interbodies not only due to the polymer’s radiolucency and proven biocompatibility but also because PEEK possesses an elastic modulus of 4.0 GPa, which is considerably less than titanium (105 GPa) and more similar to that of cortical bone (4.89 GPa). Although PEEK has several advantageous properties, the inert nature of the polymer restricts close contact between the vertebral end plate and the surface of the implant, which can result in pseudoarthrosis.2,3
Interbody fusion cages manufactured out of titanium alloy exhibit a more favorable biological response that promotes bone formation in vitro while possessing the necessary biomechanical strength.4,5 At the same time, however, the material’s X-ray opacity and greater stiffness make it less suitable for use as a spinal implant. Recent technological advances have resulted in the development of titanium-coated PEEK (Ti-PEEK) composite materials that bring together the mechanical advantages of PEEK with the desired bio-compatible characteristics of titanium. To create a composite material with X-ray translucency and a modulus of elasticity less prone to stress shielding, rough, porous titanium is bound to the surface of a PEEK substrate material through a plasma spray application process. This process results in a rough surface coating that can potentially inhibit implant migration while providing a porous texture that is optimal for osseointegration.5–8
It is well documented that the implant surface composition and topography at the macro-, micro-, and nanoscale have a direct effect on the osteogenic, osteoinductive, and osteoconductive activity of progenitor and mature osteoblast cells.8–11 Surface chemistry and texture have been shown to influence osseointegration at the implant surface to stabilize the implant as a contiguous fusion mass forms. For osseointegration to occur, bone-forming cells must be able to attach, grow, and differentiate on the surface of an implant. These cellular interactions are dependent on the coating and binding of blood and tissue fluid cell adhesive proteins to the implant surface that then serve as the substrate for specific cell receptor engagement and signaling.12,13 The signaling results in the spatial and temporal expression of growth factors, including the bone morphogenetic proteins (BMPs), members of the TGF-β superfamily of proteins. The expression of BMPs induce a biological cascade of cellular events, including chemo-taxis, proliferation, and differentiation that culminate in the formation of new bone.14–16
In the current study, the cell–implant surface interactions that result in osseointegration are evaluated on an implant surface composed of plasma-sprayed titanium on a PEEK substrate or Ti-PEEK. The influence of this surface modification on the biochemical, biomechanical, and histological properties at the bone–implant interface is demonstrated and compared to PEEK both in vitro using simulated bone- forming cell culture experiments and in vivo using an ovine implant model.
Materials and methods
Surface samples, preparation, and characterization
Surgical grade, 15 mm diameter PEEK (PEEK-Optima™; Invibio, Lancashire, UK), and Ti-PEEK (PlasmaporeXP®; Aesculap AG, Tuttlingen, Germany) disks were used for the in vitro portion of this study. Tissue culture plastic (TCP) served as the control growth surface. The Ti-PEEK surface is the result of a two-stage coating process applied to the surface of the PEEK substrate. It consists of a surface activation followed by an intermediate pure titanium layer. The combination of the surface activation of the PEEK material and the vacuum plasma spray (VPS) coating on the sandwiched pure titanium layer generates an adhesive composition on the PEEK substrate. The resulting coating has a very high static tensile, static shear, and shear fatigue strength as well as abrasion resistance when tested according to American Society for Testing and Materials F 1147-05, 1044-05, 1160-05, and 1978-00, respectively, exceeding all the related US Food and Drug Administration (FDA) requirements (data not shown).
Disks were machined and surface finished or coated, and if applicable (Ti-PEEK) washed in an acid bath to remove inorganic contaminants. All disks were ultrasonically cleaned, sonicated in ultra pure water, and sterilized with gamma irradiation. For transcriptional (reverse transcription quantitative PCR, RT-qPCR) and translational (ELISA) analyses, disk samples were ultrasonically cleaned, sonicated in pure water, and sterilized by autoclave (121°C, 20 minutes).
The surface of the Ti-PEEK material was characterized for morphology using scanning electron microscopy (SEM) with a Zeiss (Leo) 1550 field-emission scanning electron microscope (Carl Zeiss Meditec AG, Jena, Germany) in a secondary electron mode. Samples were evaluated before and after insulating with a thin layer of iridium metal to delineate the effect of titanium oxide charging on the surface.
Six Ti-PEEK samples were used to measure the coating thickness. The samples were prepared using standard mate-rialographic procedures (vacuum embedding, grinding, and polishing). Five pictures were taken for each sample (Leica DMRX at a magnification of 50:1), and the picture was divided using a superimposed grid with 20 crossing lines so that 20 measurements of the coating thickness could be made at random points. The same images were also used to determine the coating porosity. The measurement method used was a data-processing-assisted technique which identified the percentage of different levels of gray in the image (software: Dietermann & Heuser Solution, Greifenstein-Beilstein, version 13, Masterstand 5). The evaluation of the coating porosity was performed in the field of the average coating thickness determined on each image.
In addition, the Ti-PEEK was examined by atomic force microscopy (AFM) using an NT-MDT Solver NEXT system (NT-MDT Spectrum Instruments, Moscow, Russia). Silicon probes from NT-MDT (NSG10) had a nominal tip radius of 10 nm when unused. AFM analyses were performed using a semi-contact (tapping) mode, and data analysis was performed using the software program Gwyddion. Scans of square regions were performed with side lengths from 0.1 to 10.0 µm with scan rates between 0.5 and 1.0 Hz and 256×256 pixels.
Coating roughness for the Ti-PEEK was evaluated according to EN ISO 3274, EN ISO 4287, and EN ISO 4288, and the surface area of the Ti-PEEK was determined using a VK-X200 3D laser scanning microscope.
The PEEK machined surface was examined by confocal microscopy using µsurf (Nanofocus, Oberhausen, Germany), and roughness was measured according to DIN ISO 4287 and 4288.
In vitro cell culture
Human MG-63 osteosarcoma cell line (CRL-1427; American Type Culture Collection [ATCC], Manassas, VA, USA) and osteoblast-like cells were cultured on disk surfaces in 24-well TCP dishes (BD Falcon, Tewksbury, MA, USA). Cells were seeded at 10,000 cells/cm2 on each surface in Eagle’s Minimal Essential Medium (EMEM) supplemented with 10% heat-inactivated FBS (Lonza, Walkersville, MD, USA). Cells were cultured until reaching confluence on TCP, and full volume media exchanges were done 24 hours post seeding, then every 48 hours until reaching confluence on TCP. Once cells reached confluence on TCP, a final media exchange was done. Cells were harvested 24 hours later using trypsin–versene 0.25% in two exchanges to ensure maximum recovery. Cells were counted and characterized on a CASY-TTC cell counter (Omni Life Science, Bremen, Germany). Total cell number and viability per surface were compared for cell proliferative activity. Cell samples were pooled and pelleted from PCR plates, and spent media were pooled and frozen at –80°C for testing.
Cell adhesion assay
Cell adhesion to each surface was measured using the Wst1 assay (Omni Life Science). Implants were seeded at an initial density of 4E+04 cells and then incubated (37°C/5% CO2) for 3 hours. Implants were washed twice in 1× PBS to remove nonadherent cells. Fresh media containing Wst1 reagent were added according to the manufacturer’s recommendation, and cells were incubated for an additional hour (37°C/5% CO2). Spent media containing reagent were transferred to a 96-well plate. Absorbance at OD 450 nm was read, subtracting a background control without cells, and interpolated onto a serially diluted cell standard curve on a TCP control substrate.
ALP assay
Total cell yields from each sample were lysed by freeze thawing in the presence of 0.2% Triton X-100. Total protein concentration was determined using a bicinchoninic acid (BCA) protein assay, measuring absorbance at 562 nm (Pierce Biotechnology, Rockford, IL, USA). Samples were quantitated against a standard of known BSA concentration. ALP levels were determined in each sample using the AnaSpec SensoLyte pNPP ALP assay kit (AnaSpec, Inc., Fremont, CA, USA). Cell lysates were cleared of cellular debris by centrifugation at 2,500× g for 10 minutes at 4°C and were assayed for end point analysis after incubation for 30 minutes at room temperature. Absorbance was measured at 405 nm at the end of the incubation period, and the samples were quantitated against an ALP standard.
Gene expression by RT-qPCR
Relative gene expression of target mRNA was analyzed for BMP-2, BMP-4, BMP-7, ALP, and BGLAP. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene was used to normalize expression levels. Messenger RNA gene expression levels for each disk surface were compared to each other with TCP as the control. RNA was isolated from the total yield of cells from three combined surface samples using an RNAqueous Micro kit (Thermo Fisher Scientific, Waltham, MA, USA) with subsequent reverse transcription done using Quantitect RT kit (Qiagen NV, Venlo, the Netherlands). Taqman primer and probe cocktails for each target were added to Taqman Fast Master Mix and 50 ng of cDNA template. All qPCR assays were run on 7500 Fast PCR System (Thermo Fisher Scientific).
BMP ELISAs
Conditioned media collected at cell harvest were pooled from three surface samples (0.5 mL each), aliquoted, and stored at –80°C until being analyzed for secreted BMP-2 using DuoSet antibodies (R&D Systems, Inc., Minneapolis, MN, USA) in ELISA as per the manufacturer’s recommendations. Results were read on a microplate reader for luminescence at 425 nm. Data were interpolated on a standard curve of known BMP-2, BMP-4, and BMP-7 proteins and were normalized to cell number. A 1:10 dilution of the sample was used based on improved spike recovery (94.8%).
In vivo implants: surgical procedure and specimen preparation
Cylindrical dowels (8 mm × 30 mm) of either PEEK or Ti-PEEK were used in the in vivo portion of this study.
All surgeries were conducted at United States Department of Agriculture (USDA)-licensed Animal Research Facility Thomas D Morris, Inc. (TDMI, Reisterstown, MD, USA) following approval by the Institutional Animal Care and Use Committee (approved protocol no. 13-002). TDMI’s research activities followed the animal welfare guidelines laid out in the “Guide for the Care and use of Laboratory Animals” eighth edition (2011), as used by USDA and the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) as a reference standard and compliance tool.
After being assessed for general health, ten skeletally mature adult sheep (2–4 years old) were randomly assigned to a 12- or 24-week survival group. Each sheep received three cylindrical implants, which were placed in a triangular pattern in the lateral epicondyle region of the hind leg. The level of the lateral collateral ligament was used to determine the site of the most distal implant, and the two subsequent implants were spaced apart by at least 12 mm. To ensure good bone contact around the entire periphery of the implant, the drill bits used to prepare the femur were 0.05–0.15 mm smaller than the diameter of the PEEK or Ti-PEEK cylindrical implants. The holes were drilled to a depth of just over 30 mm, allowing each cylinder to be implanted in cancellous bone, parallel to each other and perpendicular to the condyle surface. Saline was used to irrigate the drilled implant sites, removing any issue fragments before the dowels were implanted with a light press fit into the femur. Porous Ti-PEEK cylinders were implanted into each animal’s left hind leg, while the uncoated PEEK cylinders were implanted into the animal’s right hind leg. All animals were returned to recovery pens and given food and water. At necropsy for each time point (12 and 24 weeks), the bone with the three cylindrical implants was sectioned from the limb. The implanted dowels were separated from each other along with ample surrounding bone so as not to affect pullout testing. Samples were preserved by covering gauze soaked in saline, then placed in bags with identification labels, and stored at −20°C.
In addition, six coated and six uncoated samples were inserted by the same procedure into bony segments obtained from sheep in the 24-week group at necropsy. This group was used to evaluate changes in pullout strength caused by the distinct surface roughness of each type of implant at a 0-week baseline.
Biomechanical pullout testing
An MTS Mini Bionix II system (MTS, Eden Prairie, MN, USA) was used to determine the pullout strength for two of each of the three specimens from each leg. For the test, the samples were positioned in a prefabricated test fixture referencing the front face of the dowel to ensure the cylindrical implant was always pulled out along its central axis. Ten coated and ten uncoated cylindrical implants were evaluated from each time point (12 and 24 weeks), along with six coated and six uncoated dowels that were immediately harvested from the baseline group (0 week). To assess maximum pullout force, the dowel was removed at a rate of 1 mm/min. Once peak load was acquired, the test speed was increased to a 10 mm/min rate. During the testing, load and displacement data were gathered at a rate of 20 Hz.
Histology
To evaluate bone formation and apposition, histological samples were obtained from both the coated and uncoated PEEK dowels at 12 and 24 weeks. Specimens were placed into appropriately labeled containers filled with a tenfold volume of 10% neutral buffered formalin and were shipped to an outside laboratory for undecalcified processing and analysis. Five samples from each group were stained with H&E and then evaluated by histomorphometry for inflammation, new bone formation, bone marrow adipose tissue, and fibrosis by measuring each as a percentage of the defect area. Bony apposition, defined as any apposition <0.1 mm away from the surface of the dowel, was measured as a percentage of implant circumference.
Statistical analyses
SD values for surface roughness measurements were determined using Minitab. For in vitro experiments, six independent cultures for each surface were completed for cell adhesion, yield, viability, and ALP enzyme activity. These cultures were repeated in duplicate for a total of 12 independent cultures. For RT-qPCR testing and BMP ELISA experiment, each surface was cultured in triplicate and repeated four times, combining cell pellets to ensure RNA yields for four data points. Spent media were pooled from each experiment as well as for four data points for BMP ELISA. Data for cell adhesion, proliferation, ALPL enzyme activity, and BMP ELISA experiments were analyzed for variances and normality, and a Student’s t-test was used for determining statistically significant differences; a P-value of <0.05 was considered significant.
The effect of time and surface coating on bony apposition, new bone formation, and mechanical pullout strength was evaluated using a two-way ANOVA with Bonferroni correct post hoc tests for pairwise comparisons. SPSS (IBM Corporation, Armonk, NY, USA) was used for all statistical analyses.
Results
Surface characterization
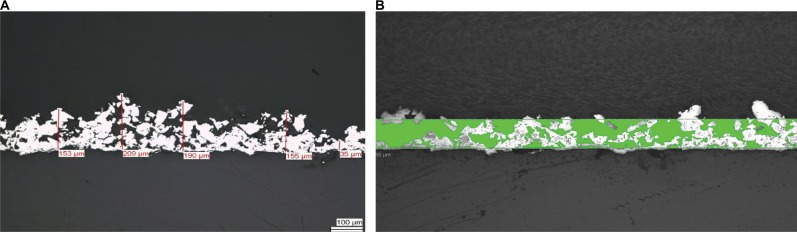
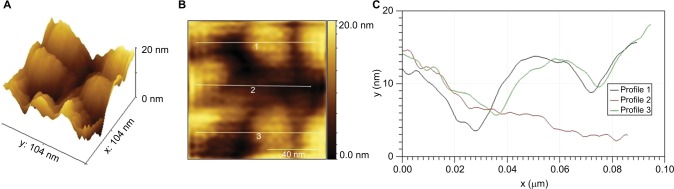
The Ti-PEEK coating has a thickness typically ranging from 60 to 150 µm and a highly microporous structure with an open porosity of between 35% and 60% and a pore diameter range of 25–150 µm (Figure 1A and B). The coating is extremely rough with a mean Ra value of 22.94 µm (SD of 0.98 µm) and a mean Rz value of 136.49 µm (SD of 6.25 µm). It has an isoelastic structure which prevents coating spalling, and the pure titanium spongy coating is completely randomized with random textural geometry visible at the macro-, micro-, and nanoscale in two dimensions under SEM (Figure 2) and in three dimensions under AFM (Figure 3). In particular, very small (<100 nm) particles or solidified droplets are apparent under high magnification (60,000×; Figures 2C and 3). These nanoscale features are visible in regions with and without iridium coating under SEM as well as under AFM confirming that they do not represent sample preparation artifacts. The Ti-PEEK coating has an average surface area 5.5 times larger (SD 0.1, 95% CI 0.1) than an uncoated theoretically smooth surface with comparable planar geometry. The machined PEEK surface by comparison is very flat and smooth, with an Ra value of 1.4 µm (SD =0.07 µm) and an Rz value of 5.4 µm (SD =0.42 µm).
Figure 1.
Exemplary sections through Ti-PEEK surface coating.
Notes: Coating thickness measurements show at selected points (A). Porosity measurement is green shaded area within the average coating thickness (B). Magnification 50×.
Abbreviation: Ti-PEEK, titanium-coated polyetheretherketone.
Figure 2.
Macro-, micro-, and nano-structure of Ti-PEEK.
Notes: SEM analysis of Ti-PEEK surface. Original magnification: ×150 (A), ×1,000 (B), and ×60,000 (C).
Abbreviations: PEEK, polyetheretherketone; SEM, scanning electron microscopy; Ti-PEEK, titanium-coated PEEK.
Figure 3.
Three-dimensional 100 × 100 nm2 AFM representation of PlasmaporeXP surface (A), planar view representation of AFM data (B), vertical profiles along lines represent in B (C).
Abbreviation: AFM, atomic force microscopy.
Cell adhesion and proliferation
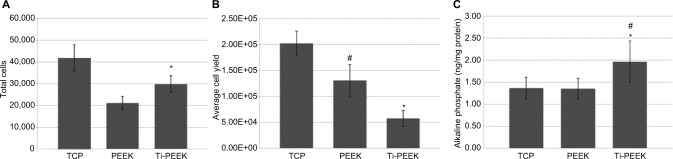
Cell adhesion activity of MG-63 human osteoblast-like cells after 3 hours of culture is 28% higher on the Ti-PEEK surface when compared to the PEEK surface (P≤0.05). Both Ti-PEEK and PEEK surfaces are less efficient at supporting cell adhesion when compared to the TCP control (Figure 4A). Similarly, differences are observed in average cell yield following 7–10 days of culture on the Ti-PEEK and PEEK surfaces. Cell growth and yield on both Ti-PEEK and PEEK are significantly lower compared to the control TCP surface (71% and 35%, respectively, P<0.05) with Ti-PEEK showing a significant difference compared to the PEEK surface (Figure 4B). Consistent with lower cell growth and yield, a significant increase in bone cell differentiation as measured by ALP levels (early bone cell differentiation marker) is observed on Ti-PEEK when compared to PEEK or TCP surfaces (46% increase, P<0.05). No difference in enzyme levels is observed between PEEK and the TCP control (Figure 4C). Viability for all surfaces remained above 80% following all culture conditions and time frames (data not shown).
Figure 4.
Osteoblast-like cell adhesion (A), proliferation (B), and differentiation (C) on Ti-PEEK compared to PEEK and TCP surfaces. ALP concentration (ng/mg protein) in cell lysates.
Notes: *P<0.05 vs PEEK; #P<0.05 vs TCP.
Abbreviations: PEEK, polyetheretherketone; Ti-PEEK, titanium-coated PEEK; TCP, tissue culture plastic.
Gene expression and BMP levels
Transcriptional analysis of osteogenic markers expressed by MG-63 human osteoblast-like cells cultured on TCP, PEEK, and Ti-PEEK surfaces demonstrates an increase in the expression of early (ALP, BMP-2) bone marker genes compared to expression levels on the TCP control surface. Gene expression levels of the other BMP proteins, BMP-4 and BMP-7, and BGLAP are not different between Ti-PEEK and PEEK surfaces (Figure 5).
Figure 5.
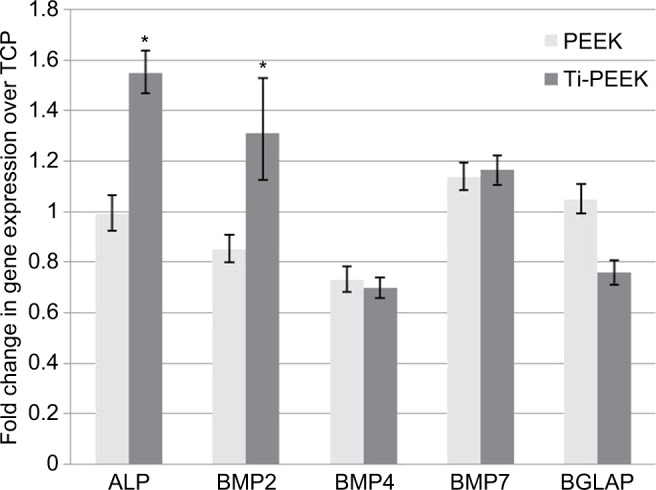
Fold change in gene expression of bone formation markers (normalized to GAPDH) over TCP control (calibrator =1) in osteoblast-like cells cultured on Ti-PEEK and PEEK surfaces.
Notes: *95% CIs of mean fold change values of Ti-PEEK and PEEK do not overlap.
Abbreviations: BMP, bone morphogenetic protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PEEK, polyetheretherketone; Ti-PEEK, titanium-coated PEEK; TCP, tissue culture plastic.
Translational analysis of BMP protein levels expressed by MG-63 human osteoblast-like cells cultured on TCP, PEEK, and Ti-PEEK surfaces demonstrates a significant increase in the expression of secreted BMP-2 protein compared to expression levels on the TCP control surface. Levels of BMP-2 protein secreted from cells on Ti-PEEK are also significantly higher than that observed from cells on PEEK (P≤0.05; Figure 6).
Figure 6.
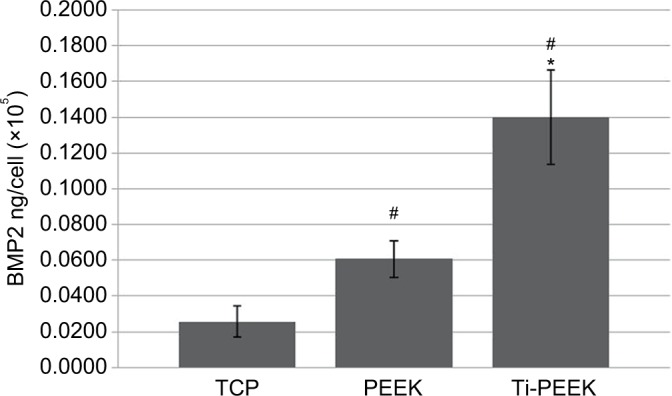
BMP-2 protein levels secreted by osteoblast-like cells cultured on Ti-PEEK, PEEK, and TCP surfaces.
Notes: *P<0.05 vs PEEK; #P<0.05 vs TCP.
Abbreviations: BMP, bone morphogenetic protein; PEEK, polyetheretherketone; Ti-PEEK, titanium-coated PEEK; TCP, tissue culture plastic.
In vivo analysis
Surgery and postoperative period Surgery and anesthesia were uneventful with no signs of lameness, deep or superficial infections, or other discomfort in the sheep. All animals showed normal food and water intake following recovery from surgery. All implants could be placed according to the intended technique and distribution. Despite the press fit, no delamination of the implant coating was visible during surgery. The light press fit of the implants resulted in a primary stability of all implants and prevented clinical mobility.
Biomechanical pullout strength
For the 0-week time point, no significant difference (P=0.661), in pullout force, was observed between the PEEK or Ti-PEEK specimens (Figure 7). A significant effect on implant anchorage strength was however detected over time for the Ti-PEEK group while for the PEEK group the differences were not significant. Looking at the Ti-PEEK dowels at the various time points, there was a significant difference in the pullout force at 12 weeks when compared to 0 week (P<0.001) and then again comparing 24 weeks to 12 weeks (P=0.007). At both 12 and 24 weeks, the difference in pullout force measured was also significantly higher (P<0.001) for the Ti-PEEK group compared to the PEEK group.
Figure 7.
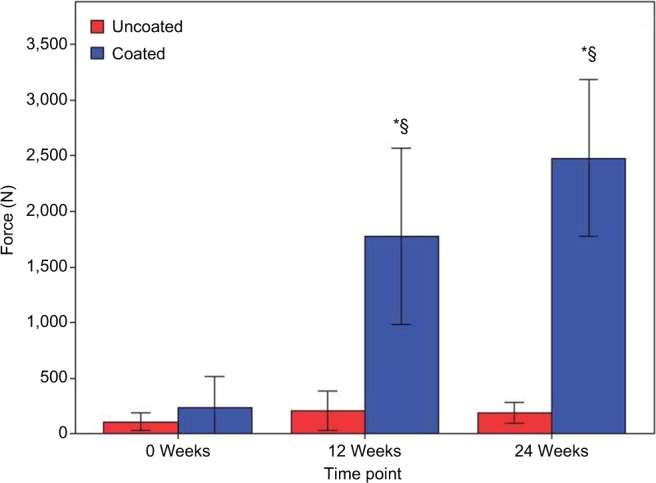
Average peak pullout force of Ti-PEEK and PEEK specimens over time.
Notes: *A significant difference from PEEK samples. §A significant difference from 0-week samples.
Abbreviations: PEEK, polyetheretherketone; Ti-PEEK, titanium-coated PEEK.
Histology
In both the Ti-PEEK and PEEK groups, there is a significant increase in new bone formation (P<0.001) and bony apposition (P=0.003) from 12 to 24 weeks (Figures 8 and 9). Figure 8 shows the mean and SD of new bone formation. Figure 9 shows the mean and SD of bony apposition. Greater new bone formation and bony apposition are found around the Ti-PEEK group when compared to the PEEK group at both 12-week and 24-week time points (P=0.004 and P=0.002, respectively). At the 12-week time point, bony apposition was significantly greater in the Ti-PEEK group (P=0.002). New bone formation was numerically greater in the Ti-PEEK group at this time point, but not to a degree of statistical significance (P=0.055). Conversely, new bone formation was significantly greater in the Ti-PEEK group at the 24-week time point (P=0.015), whereas the difference in bony apposition between the Ti-PEEK and PEEK group was not statistically significant at 24 weeks (P=0.141).
Figure 8.
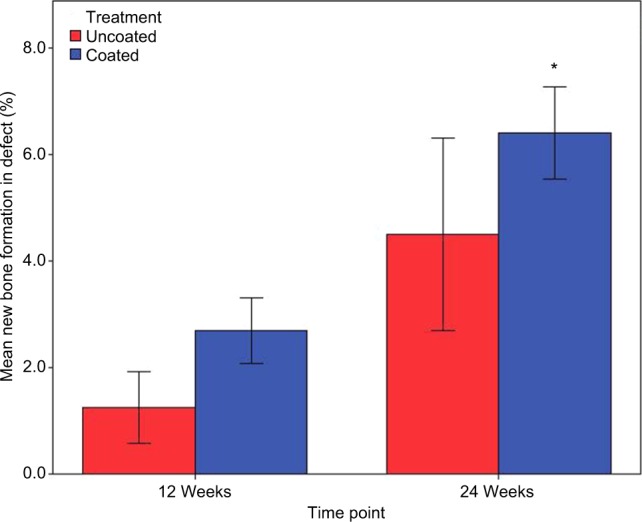
New bone formation (%) by the treatment group and time point (*significant difference compared to the PEEK 24-week group).
Abbreviation: PEEK, polyetheretherketone.
Figure 9.
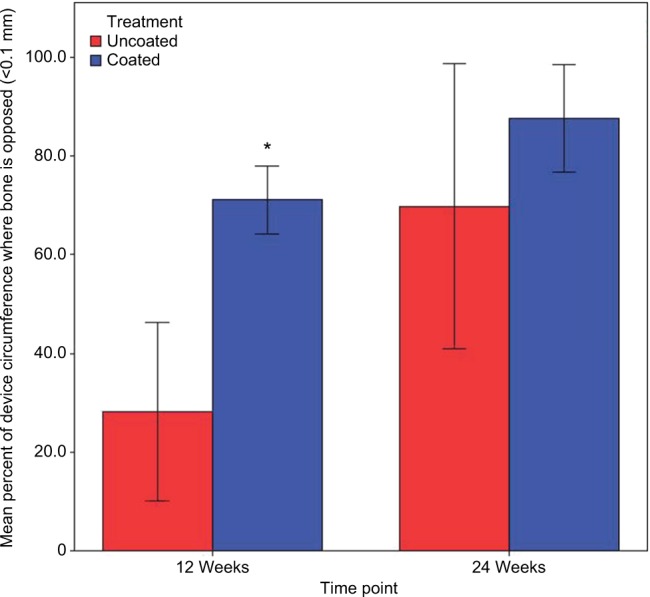
Bony apposition (%) by the treatment group and time point (*significant difference compared to the PEEK 12-week group).
Abbreviation: PEEK, polyetheretherketone.
In both the Ti-PEEK and PEEK treatment groups, there was no significant difference in the formation of bone marrow adipose tissue over time. However, the PEEK group did demonstrate significantly more inflammation and fibrosis irrespective of time points when compared to the Ti-PEEK group as a whole (P=0.022). However, when evaluating inflammation and fibrosis within the 12- and 24-week time points, there were no statistically significant differences noted between the two groups (P=0.123 and P=0.066, respectively).
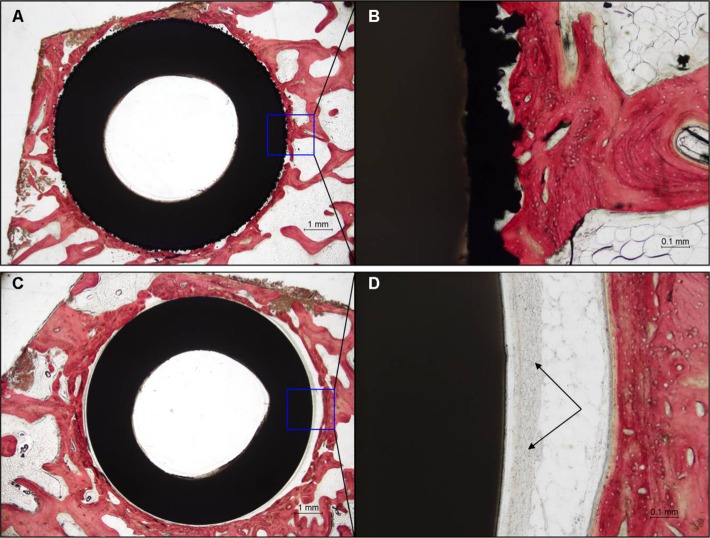
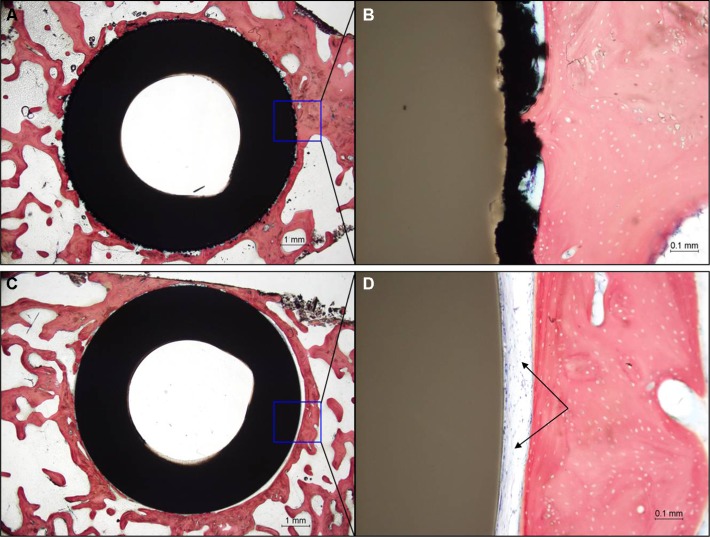
Histological examination of the Ti-PEEK group shows bony apposition immediately adjacent to the Ti-PEEK surface at the 12- and 24-week time points (Figures 10 and 11). The PEEK dowels on the other hand do not demonstrate the same extent of bony apposition. Compared to the PEEK group, the Ti-PEEK treatment group exhibited increased bone formation and apposition as well as a decrease in fibrous connective tissue found around the dowels. In the PEEK group, the fibrous connective tissue resulted in a smaller percentage of the circumference of the dowel being directly opposed to bone.
Figure 10.
Histology results at 12 weeks for a representative Ti-PEEK implant (A, B) compared to an uncoated PEEK implant (C, D).
Notes: Fibrous connective tissue (black arrows). H&E stain. Bar =1 mm (A, C), 0.1 mm (B, D).
Abbreviations: PEEK, polyetheretherketone; Ti-PEEK, titanium-coated PEEK.
Figure 11.
Histology results at 24 weeks for a representative Ti-PEEK implant (A, B) compared to an uncoated PEEK implant (C, D).
Notes: Fibrous connective tissue (black arrows). H&E stain. Bar =1 mm (A, C), 0.1 mm (B, D).
Abbreviations: PEEK, polyetheretherketon; Ti-PEEK, titanium-coated PEEK.
Overall, for the Ti-PEEK implant at the 12-week time point, there is good evidence of bone ingrowth and little evidence of fibrous connective tissue. Fibrous connective tissue is the only evident in the histological specimen taken from the PEEK group. As shown in Figure 9, by 24 weeks, the histology outcomes do not however indicate a significant difference in bony apposition between the Ti-PEEK and PEEK treatment groups.
Discussion
Regardless of implant design, the primary goal of a spinal fusion procedure is to eliminate pain caused by the unnatural movement of a degenerated spinal segment by restoring disk height and stability. Short-term stability is equally as important as advanced, long-term stability, both of which can be positively or negatively influenced by the mechanical and biological properties of the chosen implant. The rough surface of the Ti-PEEK implant supports the primary stability of the implant when compared to smoother implants. The demonstrated favorable in vitro results support osseointegration, and the in vivo demonstration of increased bone pullout strength indicates that the Ti-PEEK implant also encourages long-term, secondary stability of the segment.
In vitro bone-forming activity at the implant surface
Surface and mechanical properties of implants have been documented as an important determinant of implant device success for many years in the dental and orthopedic implant field and more recently in spine implants.8–11,17–23 The ability of a spinal implant surface to promote osseointegration quickly and uniformly can be evaluated through an analysis of cell attachment, migration, and growth properties as well as molecularly through osteogenesis pathway analysis. The in vitro studies presented in this study show that osteoblast-like cells exhibit a more differentiated phenotype on a Ti-PEEK surface compared to PEEK characterized by enhanced cell attachment, reduced cell growth, and increased transcriptional and translational expression of osteogenic genes and proteins.
The high level of cell attachment on Ti-PEEK compared to PEEK (Figure 4A) is consistent with a higher 3D surface area available on the rough, microporous Ti-PEEK surface (Figures 1–3). Cellular attachment is a prerequisite for implant osseo-integration, as an appropriate binding surface allows mobile bone-forming cells from the surrounding tissues to attach, proliferate, and establish the extracellular matrix required for bone formation.24,25 Cellular differentiation activity is generally associated with a decrease and eventual cessation of cell proliferation, consistent with the lower cell yield and significantly higher ALP levels observed after 7–10 days of culture on the Ti-PEEK surface (Figure 4B and C). The presence of ALP activity, one of the earliest markers of the osteoblast phenotype, provides high concentrations of inorganic phosphate by hydrolyzing pyrophosphate at sites of mineralization. ALP is one of the first enzymes activated in the process of calcification, and activity is induced immediately after the osteoblast proliferative phase and has been shown to increase more than tenfold after proliferation is downregulated.24–26,28,29
Transcriptional analyses of additional osteogenic pathway markers showed a similar increase in early bone differentiation markers chosen based on their temporal expression profiles. While all markers analyzed on both Ti-PEEK and PEEK surfaces were expressed at varying degrees relative to the TCP control, the expression of ALP and BMP-2 genes, activity, and protein levels was higher on Ti-PEEK (Figures 5 and 6). As indicated in previous research, gene expression patterns define a bone tissue developmental sequence which has three distinct and important components, including proliferation, extracellular matrix maturation, and mineralization.24,25,27 Each of these steps needs to occur, driven by gene expression patterns, for full tissue remodeling. Previous studies have shown the earliest osteoblast markers involved in cellular differentiation and maturation include BMP-2 and ALP.16,24,25 In a murine fracture-healing study, it was reported that BMP-2 expression was maximal at day 1 postfracture and was maintained out to 21 days postfracture. BMP-2 expression triggers and regulates the expression of downstream proteins during this time, including BMP-4 and BMP-7, which make significant contributions to bone healing at the point of calcified cartilage resorption.14,16 Similar results have been documented in spine fusion models.30–32 The in vitro results presented in this study indicate that Ti-PEEK promotes early osteogenesis pathway induction by a strong expression of the inductive regulator BMP-2 and the biosynthesis regulator ALP. These in vitro properties translate directly to enhanced osseointegration activity in an in vivo ovine model.
In vivo osseointegrative activity at the implant surface
The results of the histological portion of the in vivo study show that the Ti-PEEK implants demonstrate a significantly greater amount of bone ingrowth at 12 weeks when compared to the uncoated PEEK implants (Figure 9). In addition, at 24 weeks, a statistically greater amount of bony apposition around the implants is visible. Both of these observations are an indication of the potential for an improved healing process for patients (Figure 8). The area surrounding the Ti-PEEK implants quickly fills up with new bone that stabilizes the implant and progressively improves with time out to the 24-week time point. This is evidenced in the significant increase in biomechanical pullout strength at both 12 and 24 weeks (Figure 7).The decrease in fibrous connective tissue surrounding the Ti-PEEK compared to PEEK is consistent with previous work demonstrating the production of an inflammatory peri-implant environment surrounding PEEK implants in contrast to the bone-forming environment around titanium implants (Figures 10 and 11).22
Prior studies have been undertaken in vivo using animal models which examine and compare the bone ingrowth onto sample implant plugs or dowels. Pullout loads for the implanted plugs after a specified number of weeks postimplantation provide an indication of the rate of bony ingrowth. In addition, histological examination reveals the bone apposition onto the implant surface and the proximity of any fibrous tissue. One such study was performed by Aebli et al.8 In this study, an ovine model was used to compare the osseointegration of VPS titanium- and highly crystalline hydroxyapatite (HA)-coated implants with similar roughness. Each coating showed a significant increase in pullout strength after 2 or 4 weeks, but there was no significant difference between the coatings. Quantitative analysis using bone histomorphometry revealed that more implant/bone contact was visible after the 2-week time period for the HA-coated implants than for the titanium-coated implants after both 2 and 4 weeks. Another in vivo study performed by Schwarz et al compared four different implant surface treatments on titanium cylindrical implant dowels 1) glass pearl blasting, 2) sandblasting, 3) titanium plasma spray, and 4) titanium plasma spray with calcium phosphate coating. The results showed that increasing surface roughness led to a statistically higher bone pullout force at the 12-week time point. The addition of a calcium phosphate coating did not statistically improve the bone pullout strength. Histological bone implant contact was found to improve as the roughness increased as well as with the calcium phosphate coating.
Whereas all of these prior studies have evaluated the biomechanical and/or histological properties of the implant materials, this current study has investigated both the in vivo biomechanical and histological effects of a Ti-PEEK substrate and an in vitro molecular analysis of the microenvironment responsible for the in vivo response. This connection between biomechanical analysis, histological observation, and molecular data serves to provide a mechanism underlying the differences observed between PEEK and Ti-PEEK.
Limitations of the study include the use of an osteosarcoma cell line for the in vitro study and the lack of complex biomechanical loading in the adult ovine implant model. Human MG-63 osteosarcoma cells are well-accepted surrogates for normal human osteoblasts, particularly for studying response to titanium surfaces.20,21 The adult ovine model is a well-established model for direct comparison of implant bone ingrowth properties under ideal conditions, but it does not represent the more complex biomechanical and biological environment of the cervical and lumbar spine.6
Conclusion
The Ti-PEEK surface significantly increases early bone formation activity, as shown through the increase in gene transcripts for early bone formation markers such as ALP and BMP-2 as well as protein production of cell signaling cytokine BMP-2 and matrix maturation enzyme ALP. The increase in gene expression, enzyme activity, and protein production for these markers creates a favorable environment for increased rate and production of bony inclusions into Ti-PEEK implant areas. This increase in bone formation yields more stable spinal fusion after implantation. The clinical efficacy of spine implant devices such as interbody cages correlates directly with the ability of the implant to provide primary stability that can support a secondary, long-term stability through the formation of a contiguous, mature bony fusion mass. The Ti-PEEK surface technology meets this requirement based on the results described in this study, in particular when compared to non-coated PEEK implants.
Acknowledgments
This study was funded by Aesculap Implant Systems, LLC, and Aesculap Biologics, LLC. The authors wish to thank Dr Harold M Aberman for assistance in the in vivo study design and surgical procedure, Professor Himanshu Jain and Professor Mathias Falk of Lehigh University for performing the SEM analysis, and Stefan Schmiedberg for assistance with the statistical analysis.
Footnotes
Disclosure
RCS, CAW, and NW are paid employees of Aesculap Implant Systems, LLC, and Aesculap Biologics, LLC. BCC is a paid consultant of Aesculap Implant Systems, LLC. The other authors report no conflicts of interest in this work.
References
- 1.McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81(6):859–880. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Toth JM, Wang M, Estes BT, Scifert JL, Seim HB, 3rd, Turner AS. Poly-etheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324–334. doi: 10.1016/j.biomaterials.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12(3):265–272. doi: 10.1016/j.spinee.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6(2):81–89. doi: 10.1111/os.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh WR, Bertollo N, Christou C, Schaffner D, Mobbs RJ. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. 2015;15(5):1041–1049. doi: 10.1016/j.spinee.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Meers CM, Verleye GB, Smeets D, et al. Fine grained osseointegrative coating improves biocompatibility of PEEK in heterotopic sheep model. Int J Spine Surg. 2015;9:35. doi: 10.14444/2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aebli N, Krebs J, Stich H, et al. In vivo comparison of the osseointegration of vacuum plasma sprayed titanium- and hydroxyapatite-coated implants. J Biomed Mater Res A. 2003;66(2):356–363. doi: 10.1002/jbm.a.10508. [DOI] [PubMed] [Google Scholar]
- 9.Hunter A, Archer CW, Walker PS, Blunn GW. Attachment and proliferation of osteoblasts and fibroblasts on biomaterials for orthopaedic use. Biomaterials. 1995;16(4):287–295. doi: 10.1016/0142-9612(95)93256-d. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen HQ, Deporter DA, Pilliar RM, Valiquette N, Yakubovich R. The effect of sol-gel-formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implants. Biomaterials. 2004;25(5):865–876. doi: 10.1016/s0142-9612(03)00607-0. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz ML, Kowarsch M, Rose S, Becker K, Lenz T, Jani L. Effect of surface roughness, porosity, and a resorbable calcium phosphate coating on osseointegration of titanium in a minipig model. J Biomed Mater Res A. 2009;89:667–678. doi: 10.1002/jbm.a.32000. [DOI] [PubMed] [Google Scholar]
- 12.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51(3):475–483. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;67(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 14.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17(3):513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 15.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(80):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Harris MA, Rossini G, et al. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60(3):283–290. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- 17.Cook SD, Rust-Dawicki AM. Preliminary evaluation of titanium-coated PEEK dental implants. J Oral Implantol. 1995;21(3):176–181. [PubMed] [Google Scholar]
- 18.Engh C, Bobyn J, Glassman AH. Porous-coated hip replacement. J Bone Joint Surg Br. 1987;69(1):45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 19.Kienapfel H, Sprey C, Wilke A, Griss P. Implant fixation by bone ingrowth. J Arthroplasty. 1999;14(3):355–368. doi: 10.1016/s0883-5403(99)90063-3. [DOI] [PubMed] [Google Scholar]
- 20.Olivares-Navarrete R, Hyzy SL, Berg ME, et al. Osteoblast lineage cells can discriminate microscale topographic features on titanium-aluminum-vanadium surfaces. Ann Biomed Eng. 2014;42(12):2551–2561. doi: 10.1007/s10439-014-1108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olivares-Navarrete R, Hyzy SL, Gittens RA, 1st, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013;13(11):1563–1570. doi: 10.1016/j.spinee.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivares-Navarrete R, Hyzy SL, Slosar PJ, Schneider JM, Schwartz Z, Boyan BD. Implant Materials Generate Different Peri-implant Inflammatory Factors. Spine (Phila Pa 1976) 2015;40(6):399–404. doi: 10.1097/BRS.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivares-Navarrete R, Lee EM, Smith K, et al. Substrate stiffness controls osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS One. 2017;12(1):e0170312. doi: 10.1371/journal.pone.0170312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein GS, Lian JB, Gerstenfeld LG, et al. The onset and progression of osteoblast differentiation is functionally related to cellular proliferation. Connect Tissue Res. 1989;20(1–4):3–13. doi: 10.3109/03008208909023869. [DOI] [PubMed] [Google Scholar]
- 25.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4(13):3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 26.Golub EE, Harrison G, Taylor AG, Camper S, Shapiro IM. The role of alkaline phosphatase in cartilage mineralization. Bone Miner. 1992;17(2):273–278. doi: 10.1016/0169-6009(92)90750-8. [DOI] [PubMed] [Google Scholar]
- 27.Murshed M, Harmey D, Millán JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19(9):1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19(8):458–466. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 29.Rucci N. Molecular biology of bone remodelling. Clin Cases Miner Bone Metab. 2008;5(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- 30.Morone MA, Boden SD, Hair G, et al. The Marshall R. Urist Young Investigator Award. Gene expression during autograft lumbar spine fusion and the effect of bone morphogenetic protein 2. Clin Orthop Relat Res. 1998;351:252–265. [PubMed] [Google Scholar]
- 31.Suzuki H, Takahashi K, Yamagata M, Shimizu S, Moriya H, Yamazaki M. Spatial and temporal collagen gene expression in lumbar inter-transverse fusion in the rabbit. J Bone Joint Surg Br. 2001;83(5):760–766. doi: 10.1302/0301-620x.83b5.10792. [DOI] [PubMed] [Google Scholar]
- 32.Tang Y, Ye X, Klineberg EO, Curtiss S, Maitra S, Gupta MC. Temporal and Spatial Expression of BMPs and BMP Antagonists During Postero-lateral Lumbar Fusion. Spine (Phila Pa 1976) 2011;36(4):E237–E244. doi: 10.1097/BRS.0b013e3181d73541. [DOI] [PubMed] [Google Scholar]