Abstract
There are limited data on pregnancy outcomes in women with cirrhosis. To address this gap, we examined the records of singleton births from Sweden’s National Patient Register (NPR), Cause of Death Register (CDR), and Medical Birth Register (MBR) between 1997 and 2011 to assess exposure and pregnancy‐related and liver‐related outcomes of pregnant women with cirrhosis. Exposure status was defined as having an International Classification of Diseases (ICD) code for cirrhosis obtained prior to or during pregnancy. Poisson regression with cluster‐robust standard errors was used to estimate relative risks (RRs) adjusted for maternal age, smoking, and body mass index (BMI). We identified 103 pregnancies in women with cirrhosis and compared these to 1,361,566 pregnancies in women without cirrhosis. Pregnancies in women with cirrhosis were at increased risk of caesarean delivery (36% versus 16%, respectively; adjusted RR [aRR], 2.00; 95% confidence interval [CI], 1.47‐2.73), low birth weight (15% versus 3%; aRR, 3.87; 95% CI, 2.11‐7.06), and preterm delivery (19% versus 5%; aRR, 3.51; 95% CI, 2.16‐5.72). Rates of maternal mortality during pregnancy (no cases), gestational diabetes, preeclampsia, small for gestational age, congenital malformations, and stillbirth were not increased when compared to the pregnant women without cirrhosis. There were 12 hospitalizations during pregnancy due to liver‐related events, including one case with bleeding esophageal varices. Conclusion: Women with cirrhosis are at increased risk for adverse pregnancy outcomes. However, severe maternal and fetal adverse events were rare in our study, and most pregnancies in women with cirrhosis ended without complications.
Abbreviations
- aRR
adjusted relative risk
- BMI
body mass index
- CDR
Cause of Death Register
- CI
confidence interval
- ICD
International Classification of Diseases
- MBR
Medical Birth Register
- NPR
National Patient Register
- RR
relative risk
Chronic liver disease can lead to cirrhosis, which is known to affect a small proportion of women of childbearing age. Recently, chronic liver disease was ranked among the six most common causes of mortality in women between 15 and 44 years of age in the United States.1 Historically, women with cirrhosis have had reduced fertility due to hormonal and metabolic changes leading to anovulation.2, 3, 4 As care of patients with liver disease has improved, fertility has increased, leading to more pregnancies in women with cirrhosis.5
In cirrhosis, increased portal blood flow and intrahepatic resistance cause increased pressure in the portal vein, leading to portal hypertension and subsequent formation of esophageal varices. Pregnancy further causes a rise in intra‐abdominal pressure, and for this reason, women with cirrhosis are considered to have an increased risk of bleeding from esophageal varices, particularly during repeated Valsalva maneuvers at delivery.6 Previous data suggest that more than 1 in 20 pregnant women with cirrhosis in developed countries will die during pregnancy.5, 7 However, more recent studies have reported better outcomes, with mortality rates <2%;8, 9 it is therefore likely that clinicians today are less inclined to discourage pregnancy. Previous studies also report an increased risk of adverse pregnancy outcomes, such as preeclampsia and preterm birth,5 with increasing risk by severity of cirrhosis.8 Therefore, some experts advocate that conception in women with cirrhosis should be carefully considered by the affected women and their physicians.10
To date, the majority of studies of pregnancy in women with cirrhosis derive from tertiary hospital settings9, 11, 12; this results in a degree of selection bias. A register‐based study of 339 U.S. women with cirrhosis reported an increased risk of some maternal and fetal outcomes but was unable to capture several important outcomes, including bleeding from esophageal varices.5 Hence, population‐based studies without selection bias and with a low risk of misclassification bias are lacking. Here, we investigated the risk of pregnancy‐related and liver‐related adverse outcomes in women with cirrhosis in a population‐based cohort study of more than 1.3 million births.
Materials and Methods
Registries
The Swedish MBR was founded in 1973 and contains data from the first antenatal visit until delivery and the postnatal period. Our study is restricted to pregnancies in the ICD, 10th revision, era that were registered in the MBR between 1997 and 2011. In total, we obtained data on 1,361,669 singleton pregnancies in 840,573 women.
The personal identity number is a unique 10‐digit code provided to all Swedish residents.13 Through this number, we linked the MBR to the NPR14 and the CDR.15 The NPR contains data on hospital discharge diagnoses since 1964, with full national coverage since 1987 and hospital‐based outpatient data since 2001. The validity of hospital discharge diagnoses and contacts with specialized care obtained from the NPR varies between 85% and 95% for most chronic diseases.14 The CDR contains data regarding the causes of death of all Swedish citizens. It is mandatory for the responsible physician to report the underlying cause of death (e.g., hepatocellular carcinoma) and any disease that may have contributed to the death (e.g., liver cirrhosis).
Variables
Cirrhosis and Liver Disease
Cirrhosis was defined by two approaches using ICD codes registered in the NPR: an ICD code for cirrhosis before or during pregnancy or an ICD code for hepatic decompensation (i.e., esophageal varices or ascites) before pregnancy in conjunction with a code for any specific liver disease before, during, or after pregnancy. Specific liver diseases were added as a requirement to reduce false‐positive cases. All ICD codes used in this study are listed in Supporting Table S1.
Covariates
Data on maternal age, parity, early pregnancy BMI, smoking status, and delivery year were obtained from the MBR. Data on smoking status, obtained at the first antenatal visit, are self‐reported as nonsmoking, light smoking (one to nine cigarettes per day), and heavy smoking (≥10 cigarettes per day). Diabetes prior to the first visit in antenatal care was defined as an ICD code for type 1 or type 2 diabetes in the NPR.
Outcomes
Pregnancy‐related outcomes were obtained from the MBR and included gestational diabetes, preeclampsia, caesarean delivery, low Apgar score (<7) at 5 minutes after birth, low birth weight (<2,500 g), extremely low birth weight (<1,500 g), small for gestational age (birth weight >2 SDs below the sex‐specific mean birth weight for gestational age),16 congenital malformations as listed in the MBR, preterm birth (<37 weeks), very preterm birth (<32 weeks), stillbirth, and neonatal mortality (up to 28 days after delivery). In women with cirrhosis, liver‐related outcomes included overall and liver‐specific maternal mortality during pregnancy or during the first 6 months after delivery. Hospital admission during pregnancy with liver‐related complications was defined as a discharge code of cirrhosis, ascites, esophageal varices, or liver failure (Supporting Table S1). Specifically, we examined coding for incident bleeding esophageal varices and upper endoscopy with therapy of esophageal varices during pregnancy.
Statistical Analysis
To determine whether there is an increased risk of the different binary outcomes for pregnant women with cirrhosis, Poisson regression with cluster‐robust standard errors was used17 to estimate aRR. All models were adjusted for maternal age, BMI, and smoking. For gestational diabetes, only women without diabetes type 1 or 2 were included. For pregnancy‐related outcomes (excluding stillbirth), only live births were included. We did not calculate RRs for liver‐related outcomes as these would logically only occur in women with cirrhosis, and findings of liver‐related outcomes in the control population could be misclassified.
To examine whether a diagnosis of cirrhosis became more common in pregnant women during the study period, we compared data from 2002 to 2011. We did not include 1997‐2001 cases in this analysis as outpatient visits in specialized care (where most cases with cirrhosis are normally followed) was introduced to the NPR in 2001. A chi‐square test was used to examine whether cirrhosis became more prevalent in pregnant women when the 2002‐2006 and 2007‐2011 calendar periods were compared.
Ethical Considerations
The study was approved by the regional research ethics review board in Stockholm, Sweden (No. 2008/1182 31/4). Because this was a register‐based study and none of the study participants were contacted, informed consent was waived by the review board.18
Results
During the study period, there were 1,361,669 singleton pregnancies in 840,573 women. Baseline characteristics of women with and without cirrhosis are presented in Table 1. From the study population, we identified 103 pregnancies in 76 women with cirrhosis. Of the 85 pregnancies in the 2002‐2011 cohort, there were 31 pregnancies between 2002 and 2006 and 54 between 2007 and 2011 (P = 0.02; Fig. 1). Liver diseases diagnosed before or during pregnancy included viral hepatitis, n = 32; autoimmune hepatitis, n = 29; cryptogenic cirrhosis, n = 13; alcoholic liver disease, n = 11; primary sclerosing cholangitis, n = 9; primary biliary cholangitis, n = 6; Budd‐Chiari syndrome, n = 3; Wilson’s disease, n = 2; and hereditary hemochromatosis, n = 2. Due to low power, we did not perform any additional subanalyses on specific liver diseases.
Table 1.
Descriptive Baseline Characteristics of Women With and Without Cirrhosis at First Visit in Antenatal Care in Sweden, 1997‐2011
| Parameter* | No Cirrhosis (n = 1,361,566) | Cirrhosis (n = 103) |
|---|---|---|
| Age (years) | 30.1 (5.2) | 30.9 (5.5) |
| Calendar period | ||
| 1997‐2001 | 354,494 (26.0%) | 18 (17.5%) |
| 2002‐2006 | 478,852 (35.2%) | 31 (30.1%) |
| 2007‐2011 | 528,217 (38.8%) | 54 (52.4%) |
| BMI (kg/m2) | 24.5 (4.5) | 24.9 (4.3) |
| Smoking | ||
| Nonsmoker | 1,172,072 (86.1%) | 74 (71.8%) |
| 1‐10 cigarettes/day | 83,746 (6.2%) | 15 (14.6%) |
| >10 cigarettes/day | 31,264 (2.3%) | 3 (2.9%) |
| Missing data | 74,484 (5.5%) | 22 (10.7%) |
| Diabetes† | 6,871 (0.4%) | 8 (7.8%) |
| Parity | ||
| Nulliparous | 603,580 (44.3%) | 54 (52.4%) |
| Multiparous | 757,986 (65.7%) | 49 (47.6%) |
*Continuous parameters are presented as mean values with SDs. Categorical parameters are presented as total numbers and percentages. †Diabetes is defined as ICD coding in the NPR for type 1 or type 2 diabetes prior to the first visit in antenatal care.
Figure 1.
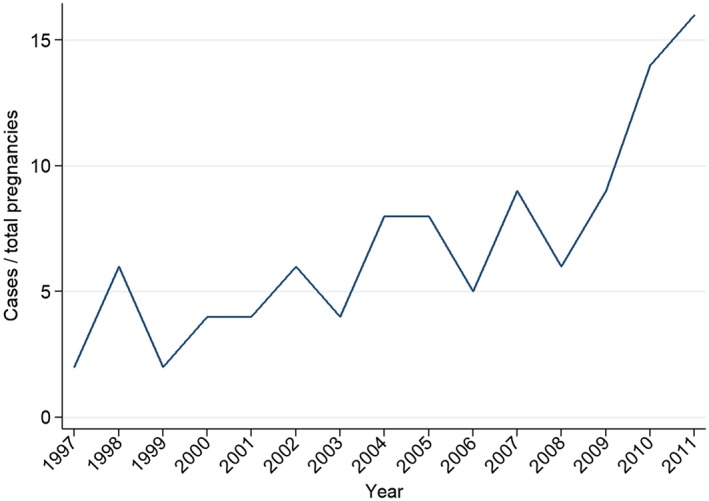
Line graph of cases of cirrhosis per total number of pregnancies in Sweden 1997‐2011. P = 0.02 when cases diagnosed in 2002‐2006 are compared with those in 2007‐2011.
Pregnancy‐Related Outcomes
Compared to women without cirrhosis, we found an increased risk for women with cirrhosis for caesarean delivery (36% versus 16%, respectively; aRR, 2.00; 95% CI, 1.47‐2.73), low birth weight (15% versus 3%; aRR, 3.87; 95% CI, 2.12‐7.06), very low birth weight (3% versus 0.6%; aRR, 7.01; 95% CI, 2.28‐21.49), preterm delivery (19% versus 5%; aRR, 3.51; 95% CI, 2.16‐5.72), and very preterm delivery (7% versus 0.9%; aRR ,7.27; 95% CI, 3.53‐14.98). There was no increase in the risk of preeclampsia (3.9% versus 2.8%; aRR, 1.39; 95% CI, 0.33‐5.80), small for gestational age (7.8% versus 2.6%; aRR, 0.90; 95% CI, 0.23‐3.57), gestational diabetes (three cases, 3% versus 1%; aRR, 2.41; 95% CI, 0.61‐9.60), stillbirth (one case, 1.0% versus 0.3%; aRR, 3.57; 95% CI, 0.52‐24.64), or malformations (two cases, 2% versus 3.5%; aRR, 0.70; 95% CI, 0.18‐2.71). There was one case of neonatal death in women with cirrhosis (1.0% versus 0.2%; aRR, 8.1; 95% CI, 1.14‐57.67), and no cases with low Apgar score at 5 minutes. Data on pregnancy outcomes for women with and without cirrhosis and RRs adjusted for maternal age, BMI, and smoking are presented in Table 2. [Corrections to results associated with stillbirth and neonatal death were made on 9 October, 2018 after initial online publication.]
Table 2.
Absolute Numbers, Percentages, and RRs of Pregnancy‐Related Outcomes in Women With Cirrhosis Compared to Women Without Cirrhosis in Sweden, 1997‐2011
| Parameter | No Cirrhosis (n = 1,361,566) | Cirrhosis (n = 103) | aRR* (95% CI) | P value |
|---|---|---|---|---|
| Stillbirth | 4,605 (0.3%) | 1 (1.0%) | 3.57 (0.51‐24.64) | 0.20 |
| Gestational diabetes | 13,301 (1.0%) | 3 (3.2%) | 2.41 (0.61‐9.60) | 0.21 |
| Preeclampsia | 38,128 (2.8%) | 4 (3.9%) | 1.39 (0.33‐5.80) | 0.65 |
| Caesarean section | 216,018 (15.9%) | 37 (35.9%) | 2.00 (1.47‐2.73) | <0.001 |
| Low Apgar score† | 14,765 (0.6%) | 0 (0%) | ‐ | ‐ |
| Low birth weight | 42,342 (3.1%) | 15 (14.7%) | 3.87 (2.12‐7.06) | <0.001 |
| Very low birth weight | 7,521 (0.6%) | 3 (2.9%) | 7.01 (2.28‐21.49) | 0.001 |
| Small for gestational age | 34,619 (2.6%) | 8 (7.8%) | 0.90 (0.23‐3.57) | 0.88 |
| Malformation | 47,574 (3.5%) | 2 (2.0%) | 0.70 (0.18‐2.71) | 0.61 |
| Preterm delivery | 67,497 (5.0%) | 19 (18.6%) | 3.51 (2.16‐5.72) | <0.001 |
| Very preterm delivery | 12,801 (0.9%) | 7 (6.9%) | 9.49 (4.56‐19.71) | <0.001 |
| Neonatal death | 2,231 (0.2%) | 1 (1.0%) | 8.10 (1.14‐57.67) | 0.037 |
| Maternal mortality | 130 (0.01%) | 0 (0%) | ‐ | ‐ |
*Adjusted for maternal age, smoking, and BMI using Poisson regression. †<7 after 5 minutes. See text for definitions of outcomes.
Liver‐Related Outcomes
There were no cases of maternal mortality during pregnancy or in the 6 months following delivery in women with cirrhosis. There were 12 (12%) hospitalizations during pregnancy due to liver‐related events, including one case with bleeding esophageal varices. In 17 of the pregnancies in women with cirrhosis (17%), upper endoscopy was performed during pregnancy; in five of these (5%), therapy of esophageal varices was performed. We identified one case with bleeding esophageal varices. In an additional five cases, therapy of esophageal varices had been performed prior to pregnancy.
Discussion
In this population‐based cohort study of more than 1.3 million births, we identified 103 pregnancies in women with cirrhosis during the study period. Although a rare event, a diagnosis of cirrhosis became more frequent during the study period, with 31 cases in the 2002‐2006 period compared to 54 cases in the 2007‐2011 period. We found an increased risk of caesarean delivery, low birth weight, and preterm birth in women with cirrhosis compared to population controls. Importantly, none of the women with cirrhosis died during pregnancy, although 12% were hospitalized during pregnancy due to liver disease. In five pregnancies, therapy for esophageal varices was required (one case had bleeding esophageal varices).
The main etiological causes of cirrhosis in this study were viral hepatitis and autoimmune liver diseases (autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis), which together accounted for 74% of all diagnoses. This differs from previous U.S. studies in which alcoholic liver disease was the main underlying cause of liver disease.5, 11 This likely reflects a difference in the prevalence of liver disease in these populations. Autoimmune liver diseases are common in Sweden compared to other countries and represent the principal indications for liver transplantation.19
Consistent with an earlier population‐based U.S. study,5 we found increased risks for preterm birth and caesarean delivery in women with cirrhosis. However, in contrast to that study, we found no cases of maternal death. This lack of mortality could be multifactorial, reflecting a true difference between the populations, and because the study periods differ (1993‐2005 versus 1997‐2011), there is also the possibility that improvement in the management and prognosis of pregnant women with cirrhosis contributes to this finding. On the other hand, this difference in mortality‐related outcomes could simply be explained by the relatively small number of cases with cirrhosis during pregnancy in our study. Importantly, our methodology allowed us to study postnatal complications, including neonatal death, where an increased risk was identified. Moreover, while the study by Shaheen and Myers5 was limited to hospital admissions for obstetric reasons, we had access to all hospital admissions.
Recently, Palatnik and Rinella11 published results from a single‐center study on 31 pregnancies in women with cirrhosis. As in our study, no woman with cirrhosis died during pregnancy but there was one case of stillbirth reported (3%). The prevalence of births in pregnancies in women with cirrhosis was much lower in our study (103 pregnancies in women with cirrhosis from 1.3 million pregnancies), which could be explained by the fact that the Palatnik and Rinella study included women from a single tertiary center representing a degree of ascertainment bias. Our findings are supported by a register‐based study from California that identified only 37 cases of cirrhosis in more than 2.2 million pregnancies, also without any report of maternal mortality.20 Therefore, it is reasonable to assume that improvements in both maternal and fetal care are contributing to decreased mortality in both instances in more recent publications. It is also noteworthy that in previous studies there was an increased risk for preeclampsia in women with cirrhosis, a finding not replicated in our cohort.
These results indicate that, while pregnancy in women with cirrhosis is a rare event, it is becoming more common, with growing recognition that severe adverse events during pregnancy are rare and perhaps decreasing in prevalence. Thus, it is likely that successful pregnancy is a realistic outcome for most women with cirrhosis. However, cirrhosis in itself is a risk factor for both maternal and fetal complications, and women with cirrhosis should be cared for by a multidisciplinary team of hepatologists and obstetricians. Because therapy for esophageal varices was required in around 5% (n = 5) of pregnancies in women with cirrhosis in this study, screening endoscopy for early detection of esophageal varices could be performed in pregnant women with known or suspected cirrhosis to prevent bleeding.
The major strength of our study is the population‐based cohort design, which enabled us to capture the clear majority of pregnant women in Sweden during the study period with very low loss to follow‐up. Previous studies were often performed in single institutions,9, 11, 12 and previous population‐based studies captured only obstetric hospitalizations, whereas we could ascertain all hospitalizations and from 2002 onward also identify visits for specialized outpatient care.
The primary limitation of our study is the low number of pregnant women with cirrhosis. However, point estimates of the risk for pregnancy‐related outcomes are likely robust as the CIs for these estimates were narrow. Furthermore, stillbirths in Sweden are only recorded from gestational week 22 (and until 2008 from week 28). Therefore, we cannot draw any conclusions about the risk of stillbirth from gestational weeks 22 to 28 for pregnant women with cirrhosis before 2008. In addition, the register‐based classification of the exposure and outcomes can be seen as a limitation. However, the validity of the NPR used to define the exposure of cirrhosis as well as outcomes unrelated to the pregnancy has been externally validated and found to have a positive predictive value between 85% and 95% for many chronic diseases.14 Furthermore, we did not have adequate statistical power to perform subgroup analyses based on the type of underlying liver disease, although given the positive outcomes it is likely that patients without Child class B or C cirrhosis were not represented. Another limitation is the lack of clinical data, including blood tests and the severity of cirrhosis, including calculation of the Model for End‐Stage Liver Disease score, which is known to affect outcomes of pregnancy in women with cirrhosis.8 We cannot know if the caesarean sections performed were due to complications of liver disease or if they were planned interventions.
The finding that cirrhosis during pregnancy seems to have increased in prevalence might be true, but the finding might also be a result of a higher rate of case finding in pregnant women. Another plausible explanation of this finding is that the study period started in 1997; women entered in the MBR at this time would have had little time prior to that to be diagnosed with cirrhosis. However, it is likely that pregnant women with known cirrhosis would have had contact with specialized care and therefore be captured by the registries.
In this population‐based cohort, cirrhosis was a rare but increasingly common diagnosis in pregnant Swedish women. Women with cirrhosis were at increased risk for hospitalization for liver‐related events and some adverse pregnancy outcomes. However, severe maternal and fetal adverse events were rare, leading to the conclusion that most women with cirrhosis will have a successful pregnancy.
Supporting information
Supported by a Stockholm County Council (SCC) clinical postdoctoral award (to H.H.), the Bengt Ihre Fellowship (to H.H.), and the Gastroenterology Fund (to H.H.).
Potential conflict of interest: Nothing to report.
References
- 1. Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H‐C. Deaths: preliminary data for 2009. NVSS 2011;59:1‐51. https://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdf. [PubMed] [Google Scholar]
- 2. Russell MA, Craigo SD. Cirrhosis and portal hypertension in pregnancy. Semin Perinatol 1998;22:156‐165. [DOI] [PubMed] [Google Scholar]
- 3. Joshi D, James A, Quaglia A, Westbrook RH, Heneghan MA. Liver disease in pregnancy. Lancet 2010;375:594‐605. [DOI] [PubMed] [Google Scholar]
- 4. Cundy TF, Butler J, Pope RM, Saggar‐Malik AK, Wheeler MJ, Williams R. Amenorrhoea in women with non‐alcoholic chronic liver disease. Gut 1991;32:202‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaheen AA, Myers RP. The outcomes of pregnancy in patients with cirrhosis: a population‐based study. Liver Int 2010;30:275‐283. [DOI] [PubMed] [Google Scholar]
- 6. Britton RC. Pregnancy and esophageal varices. Am J Surg 1982;143:421‐425. [DOI] [PubMed] [Google Scholar]
- 7. Steven MM. Pregnancy and liver disease. Gut 1981;22:592‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Westbrook RH, Yeoman AD, O’Grady JG, Harrison PM, Devlin J, Heneghan MA. Model for end‐stage liver disease score predicts outcome in cirrhotic patients during pregnancy. Clin Gastroenterol Hepatol 2011;9:694‐699. [DOI] [PubMed] [Google Scholar]
- 9. Westbrook RH, Yeoman AD, Kriese S, Heneghan MA. Outcomes of pregnancy in women with autoimmune hepatitis. J Autoimmun 2012;38:J239‐J244. [DOI] [PubMed] [Google Scholar]
- 10. Giard JM, Terrault NA. Women with cirrhosis: prevalence, natural history, and management. Gastroenterol Clin North Am 2016;45:345‐358. [DOI] [PubMed] [Google Scholar]
- 11. Palatnik A, Rinella ME. Medical and obstetric complications among pregnant women with liver cirrhosis. Obstet Gynecol 2017;129:1118‐1123. [DOI] [PubMed] [Google Scholar]
- 12. Rasheed SM, Abdel Monem AM, Abd Ellah AH, Abdel Fattah MS. Prognosis and determinants of pregnancy outcome among patients with post‐hepatitis liver cirrhosis. Int J Gynaecol Obstet 2013;121:247‐251. [DOI] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brooke HL, Talback M, Hornblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol 2017;32:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843‐848. [DOI] [PubMed] [Google Scholar]
- 17. Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013;22:661‐670. [DOI] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Haberg SE, Knudsen GP, Lafolie P, Zoega H, Sarkkola C, et al. Ethical aspects of registry‐based research in the Nordic countries. Clin Epidemiol 2015;7:491‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fosby B, Melum E, Bjoro K, Bennet W, Rasmussen A, Andersen IM, et al. Liver transplantation in the Nordic countries ‐ an intention to treat and post‐transplant analysis from The Nordic Liver Transplant Registry 1982–2013. Scand J Gastroenterol 2015;50:797‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puljic A, Salati J, Doss A, Caughey AB. Outcomes of pregnancies complicated by liver cirrhosis, portal hypertension, or esophageal varices. J Matern Fetal Neonatal Med 2016;29:506‐509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


