Abstract
Hepatocellular carcinoma (HCC) is one of the most common and lethal cancer types worldwide, especially in Asian countries. Genetic alterations, including hyperactivation of oncogenes and loss of expression of tumor suppressor genes, greatly contribute to the initiation and progression of HCC. Here we report that down‐regulation of trophoblast cell surface antigen 2 (TROP‐2) was frequently detected in HCC. Transcriptome sequencing of non‐tumor and HCC patient samples revealed down‐regulation of TROP‐2 in tumor tissues. Immunohistochemical staining showed nearly undetectable levels of TROP‐2 in HCC tissues but distinct and strong staining of TROP‐2 in adjacent non‐tumor tissues. The frequent down‐regulation of TROP‐2 expression was further confirmed in an in‐house cohort of 205 pairs of HCC patient samples and in the Cancer Genome Atlas (TCGA) databases. Furthermore, the down‐regulation of TROP‐2 was associated with poor overall survival of HCC patients, severe adjacent organ invasion, and poor differentiation of HCC. Using bisulfite genomic sequencing and methylation‐specific polymerase chain reaction analyses, we show that higher levels of promoter methylation were detected in the DNA samples of HCC tissues (low TROP‐2 expression) than that of the non‐tumor tissues (high TROP‐2 expression). Conclusion: Taken together, our data suggest that promoter hypermethylation contributes to the frequent down‐regulation of TROP‐2 in HCC, and that TROP‐2 down‐regulation predicts poor prognosis of HCC patients.
Abbreviations
- BGS
bisulfite genomic sequencing
- FPKM
fragments per kilobase of transcript per million mapped reads
- HCC
hepatocellular carcinoma
- mRNA
messenger RNA
- MSP
methylation‐specific PCR
- qRT‐PCR
quantitative reverse‐transcription polymerase chain reaction
- TCGA
the Cancer Genome Atlas
- TROP‐2
trophoblast cell surface antigen 2
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and has been rated the sixth most prevalent and the third most lethal cancer type.1 Effective HCC therapies appear to be very limited due to the complex mechanisms of pathogenesis and heterogeneity of HCC, the 5‐year survival rate of which has been reported to be lower than 20%.2 Therefore, early detection of this disease using biomarkers with high sensitivity and specificity is in urgent need.
Trophoblast cell surface antigen 2 (TROP‐2, also known as TACSTD2) is a cell surface antigen identified on trophoblast cells using monoclonal antibodies.3 The location of the TROP‐2 gene was first mapped to the human chromosome 1p32 by Calabrese et al. in 2001 through in situ hybridization.4 The TROP‐2 protein possesses a large extracellular domain of 244 amino acids, a single transmembrane region of 23 amino acids, and a 30‐amino acid intracellular tail with potential serine and tyrosine phosphorylation sites.5 The expression of the TROP‐2 protein was found in most human carcinomas.6 Following stimulation by monoclonal TROP‐2 antibodies, the intracellular calcium signals of OvCa‐432 ovarian cancer cells and MCF‐7 breast cancer cells were raised by up to 40% with mean latencies of 65 and 77 seconds, respectively. Therefore, TROP‐2 is regarded as a tumor‐associated calcium signal transducer.7
The clinical significance of TROP‐2 has been implicated in various types of human carcinomas. In colorectal cancer, complementary DNA microarray and quantitative reverse‐transcription polymerase chain reaction (qRT‐PCR) analyses showed significantly higher expression of TROP‐2 in tumor samples than in non‐tumor samples, and the high expression of TROP‐2 is associated with liver metastasis and predicts poor patient prognosis.8, 9 Similarly, the overexpression of TROP‐2 was linked to shorter overall survival, lymph node metastasis, and advanced tumor stage in pancreatic cancer patients.10 In addition, TROP‐2 protein expression has been detected to be significantly higher in invasive prostate cancer tissues and it was suggested that TROP‐2 can promote prostate cancer cell metastasis in vivo by regulating the relocalization and downstream signaling pathways of integrin α5β1.11, 12 Interestingly, in lung adenocarcinoma and head and neck squamous cell carcinoma, the expression of TROP‐2 was reported to be dow‐nregulated in tumor tissues when compared with non‐tumor tissues.13, 14 In HCC, genome‐wide methylation and microarray analyses showed that the TROP‐2 gene was significantly hypermethylated in tumor tissues, hinting a loss of expression of this gene in HCC.15 Therefore, whether the inactivation or overexpression of TROP‐2 predicts poor patient outcome could be organ and cancer type dependent.
In this study, we report that the expression of TROP‐2 is significantly down‐regulated in tumor samples of HCC as indicated by our in‐house cohort and the Cancer Genome Atlas (TCGA) databases. The depletion of TROP‐2 is associated with poor overall survival of patients, adjacent organ invasion, and poor differentiation of HCC. Results of bisulfite genomic sequencing (BGS) and methylation‐specific PCR (MSP) analyses suggested promoter hypermethylation of TROP‐2, providing a possible explanation for the down‐regulation of TROP‐2 in HCC. Our findings could pave the way of exploiting TROP‐2 as a diagnostic and prognostic marker for HCC patients.
Materials and Methods
Clinical HCC Tissue Samples
HCC patient samples of tumor and adjacent non‐tumor tissues were collected from the Sun Yat‐Sen University Cancer Center (Guangzhou, China). All HCC patients gave written consent on the use of clinical specimens for medical research. The collection of human tissues was approved by the Committees for Ethical Review of Research Involving Human Subjects at the Sun Yat‐Sen University Cancer Center.
Bisulfite Genomic Sequencing and Methylation‐Specific PCR
Genomic DNA was extracted from HCC patient tissue samples, followed by bisulfite DNA treatments with the EpiTECT Bisulfite Kit (Qiagen, Hilden, Germany). After the bisulfite treatment, unmethylated cytosines were converted to uracils, leaving methylated cytosines unchanged. For BGS, the CpG island in the TROP‐2 promoter region was predicted using an online software (MethPrimer v1.1 beta, Li Lab, Department of Urology, University of California San Francisco). The predicted CpG island was then amplified by PCR. The PCR products were subsequently cloned into pMD18‐T vector and transformed into DH5α competent cells. Sanger sequencing was performed on five randomly picked colonies from each sample. After obtaining data from BGS, primers were designed for MSP to test the methylation status of the CpG island on a larger amount of HCC patient DNA samples. Sequences of primers used in this study were listed in Supporting Table S1.
Immunohistochemistry
Paraffin‐sectioned clinical samples were first dewaxed at 70ºC for 1‐2 hours, followed by incubation with xylene and gradient concentrations of ethanol. Quenching of endogenous peroxidase was achieved by incubation of samples in 3% H2O2 for 5‐10 minutes. For antigen retrieval, samples were boiled for 10‐15 minutes in sodium citrate buffer with 0.05% Tween‐20 (pH 6.04) to remove the cross‐linking of formaldehyde.
SPlink Detection Kits (Biotin‐Streptavidin HRP Detection Systems; OriGene, Rockville, MD) were used for immunohistochemical staining and signal detection according to manufacturer's instructions. For signal development and visualization, 3,3'‐diaminobenzidine was added to the samples. Nuclear staining was carried out by hematoxylin (DAKO, Glostrup, Denmark) staining for 1‐2 minutes. Dried samples were mounted with DPX mounting media (Thermo Scientific) and analyzed under a light microscope.
Quantitative Reverse‐Transcription Polymerase Chain Reaction
After RNA extraction using RNAiso Plus reagent (Takara, Kyoto, Japan), complementary DNA was synthesized using PrimeScript RT Master Mix (Takara). The expression levels of genes of interest were detected using the SYBR Green PCR Kit (Roche, Basel, Switzerland) on an ABI Prism 7900 System (Applied Biosystems, Foster City, CA). Relative expressions were calculated using the 2‐ΔΔCt method. 18S ribosomal RNA was used as endogenous reference.
Statistical Analyses
Statistical analyses of clinical data were performed using SPSS version 20.0 (SPSS, Inc., Armonk, NY). The clinicopathological features of patients were analyzed using Pearson's χ2 test for categorical variables. Kaplan‐Meier plots and log‐rank test were used for overall survival analysis. Student t test was applied to compare the mean values of two groups using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA). Error bars represent standard deviation values. Statistical significance was defined as P < 0.05.
Results
TROP‐2 Is Frequently Down‐regulated in HCC
To search for novel genetic markers of HCC, transcriptome Solexa sequencing on three pairs of clinical HCC samples (non‐tumor and tumor) was performed. The sequencing data of the 3 HCC patients (numbered 488, 473, and 510) showed that TROP‐2 was consistently down‐regulated in tumor tissues when compared with adjacent non‐tumor tissues. In patient 488, the expression of TROP‐2 was down‐regulated by 39.32 folds in tumor tissue (non‐tumor: 7.1522 fragments per kilobase of transcript per million mapped reads [FPKM]; tumor: 0.1819 FPKM). In patient 473, the expression of TROP‐2 was down‐regulated by 32.36 folds in tumor tissue (non‐tumor: 2.3329 FPKM; tumor: 0.0721 FPKM). In patient 510, the expression of TROP‐2 was down‐regulated by 2.59 folds in tumor tissue (non‐tumor: 0.3724 FPKM; tumor: 0.1437 FPKM) (Fig. 1A). Representative images from immunohistochemical staining showed a nearly undetectable level of TROP‐2 in HCC tumor tissues and high expression of TROP‐2 in adjacent non‐tumor tissues (n = 5) (Fig. 1B). To verify these results, TROP‐2 messenger RNA (mRNA) expression was compared between non‐tumor and tumor tissues in our in‐house cohort consisting of 205 pairs of clinical HCC tissues and in the TCGA databases. qRT‐PCR analysis of our in‐house cohort confirmed the down‐regulation of TROP‐2 in HCC tumor samples (P < 0.05) (Fig. 1C). Among the 205 HCC patients, 56% of patients showed no less than 2‐fold TROP‐2 down‐regulation in tumor tissues, and 26% of patients showed no less than 10‐fold TROP‐2 down‐regulation in tumor tissues. Meanwhile, RNA sequencing data from the TCGA databases demonstrated that TROP‐2 mRNA expression was significantly down‐regulated in HCC tumor tissues (n = 368) when compared with non‐tumor tissues (n = 50; P < 0.0001) (Fig. 1D).
Figure 1.
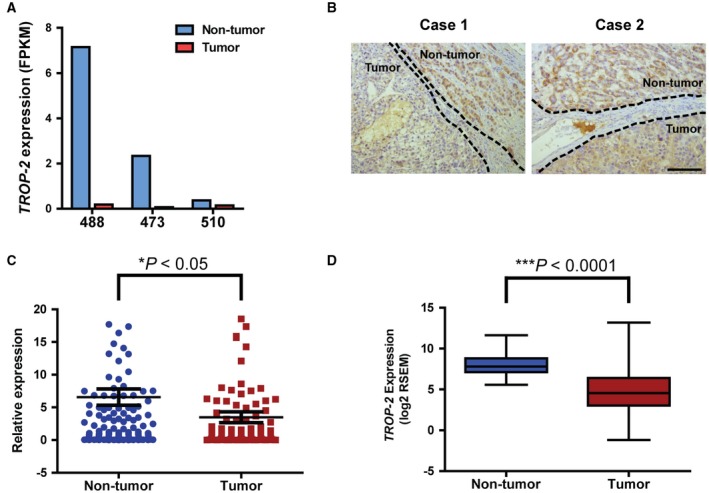
TROP‐2 is frequently down‐regulated in HCC. (A) Expression levels of TROP‐2 as detected by transcriptome sequencing of three pairs of HCC patient tissue samples. Results were expressed in FPKM. Numbers 488, 473, and 510 represent 3 HCC patients. (B) Representative images showing TROP‐2 protein expression in HCC patient tumor sections. Dotted lines were used to separate non‐tumor and tumor regions. Scale bar, 50 μm. (C) Comparison of TROP‐2 mRNA expression between non‐tumor and tumor tissues in 205 HCC patients as detected by qRT‐PCR. * P < 0.05, Student t test. (D) Comparison of TROP‐2 expression between non‐tumor and HCC tumor samples in TCGA databases. *** P < 0.0001, Student t test.
TROP‐2 Down‐regulation Predicts Poor Prognosis of HCC Patients
Following validation of the frequent down‐regulation of TROP‐2 in HCC, we further explored whether such down‐regulation has any clinical impacts on HCC patients. Using Kaplan‐Meier plots and log‐rank test, we found that the down‐regulation of TROP‐2 (at least 10‐fold down‐regulation in tumor tissue when compared with non‐tumor tissues as indicated by qRT‐PCR data) was tightly associated with poor overall survival of HCC patients (P < 0.05) (Fig. 2). In addition, clinicopathological analysis by Pearson's χ2 test revealed that TROP‐2 down‐regulation is associated with adjacent organ invasion (P < 0.05) and poor differentiation of HCC (P < 0.05) (Table 1).
Figure 2.
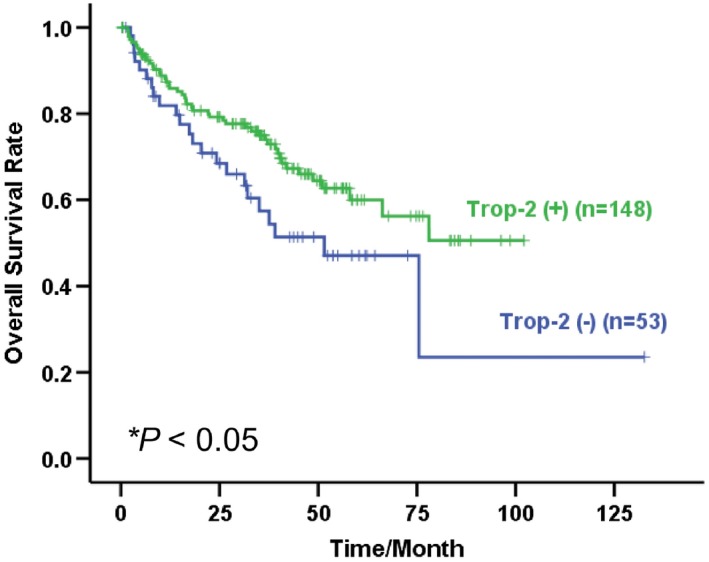
Kaplan‐Meier overall survival of HCC patients correlated with TROP‐2 expression. * P < 0.05, log‐rank test. Abbreviations: TROP‐2 (+), patients with TROP‐2 normal expression; TROP‐2 (‐), patients with TROP‐2 down‐regulation.
Table 1.
Association of TROP‐2 Downregulation With Clinicopathological Features in 205 HCC Patients
| Features | Total Samples | TROP‐2 Expression | P value | |||
|---|---|---|---|---|---|---|
| Down‐regulation | Normal Expression | |||||
| Sex | ||||||
| Male | 175 | 44 (25.1%) | 131 (74.9%) | 0.360 | ||
| Female | 30 | 9 (30%) | 21 (70%) | |||
| Age (years) | ||||||
| ≤ 60 | 167 | 42 (25.1%) | 125 (74.9%) | 0.384 | ||
| > 60 | 38 | 11 (28.9%) | 27 (71.1%) | |||
| Hepatitis B surface antigen | ||||||
| Negative | 39 | 8 (20.5%) | 31 (79.5%) | 0.251 | ||
| Positive | 164 | 45 (27.4%) | 119 (72.6%) | |||
| Serum α‐fetoprotein level (ng/mL) | ||||||
| < 400 | 116 | 32 (27.6%) | 84 (72.4%) | 0.349 | ||
| ≥ 400 | 87 | 21 (24.1%) | 66 (75.9%) | |||
| Cirrhosis | ||||||
| Absent | 88 | 22 (25%) | 66 (75%) | 0.264 | ||
| Present | 41 | 16 (39%) | 25 (61%) | |||
| Tumor stage | ||||||
| I | 142 | 39 (27.5%) | 103 (72.5%) | 0.384 | ||
| II + IIIa + IIIb | 58 | 14 (24.1%) | 44 (75.9%) | |||
| Tumor size | ||||||
| < 5 cm | 74 | 18 (24.3%) | 56 (75.7%) | 0.383 | ||
| ≥ 5 cm | 128 | 35 (27.3%) | 93 (72.7%) | |||
| Differentiation | ||||||
| Low grade | 123 | 27 (22%) | 96 (78%) | < 0.05* | ||
| High grade | 76 | 26 (34.2%) | 50 (65.8%) | |||
| Adjacent organ invasion | ||||||
| Absent | 160 | 36 (22.5%) | 124 (77.5%) | < 0.05* | ||
| Present | 44 | 16 (36.4%) | 28 (63.6%) | |||
| Recurrence | ||||||
| No | 79 | 18 (22.8%) | 61 (77.2%) | 0.244 | ||
| Yes | 124 | 35 (28.2%) | 89 (71.8%) | |||
Clinicopathological data of certain patients were not available. Statistical analysis was based on available data.
* P < 0.05, Pearson's χ2 test
Promoter Hypermethylation Results in TROP‐2 Downregulation in HCC
To examine whether promoter hypermethylation plays a role in TROP‐2 down‐regulation, BGS, and MSP were used to analyze the promoter methylation status of the TROP‐2 gene. The presence and location of the CpG island within the TROP‐2 promoter region were predicted using MethPrimer software, and such analysis identified a 749‐bp CpG island consisting of 79 CpG sites in the promoter region of TROP‐2 (Fig. 3A). Primers were subsequently designed to amplify and clone the genomic sequence of this CpG island from two non‐tumor and two tumor samples from HCC patients. The results from BGS indicated higher levels of promoter methylation in tumor samples with low TROP‐2 expression (samples 365T and 411T) than that in non‐tumor samples with high TROP‐2 expression (samples 389N and 411N), especially on CpG sites 21, 22, 23 and 38, 39, 40 (Fig. 3B). Thereafter, primers (methylated sequence‐ and unmethylated sequence‐specific) were designed to target these six CpG sites for MSP. Five pairs of HCC DNA samples underwent MSP analysis. PCR results showed stronger bands in tumor DNA samples than that in non‐tumor samples when detected with methylated sequence‐specific primers, whereas non‐tumor DNA samples showed stronger bands than tumor samples when detected with unmethylated sequence‐specific primers (Fig. 3C).
Figure 3.
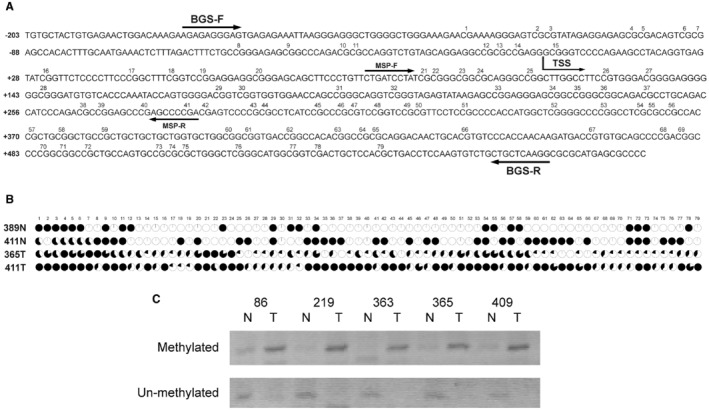
Promoter hypermethylation results in TROP‐2 down‐regulation. (A) Illustration of the CpG island sequence and primer designs in BGS and MSP experiments. The numbers indicate the order of CpG sites within this CpG island. Arrowheads indicate the locations and directions of BGS and MSP primers. (B) BGS results of non‐tumor and tumor tissue samples collected from HCC patients. The black‐filled areas indicate the percentages of clones with methylation at the specified CpG site. (C) MSP results in five pairs of HCC patient DNA samples. Numbers 86, 219, 363, 365 and 409 represent 5 HCC patients. Abbreviations: N, non‐tumor; T, tumor; TSS, transcription start site.
Discussion
Down‐regulation of expression in tumor tissue is an important indicator in the identification of candidate tumor suppressor genes. Much effort was made to search for candidate tumor suppressor genes that could serve as biomarkers for cancer diagnosis and prognosis. However, effective diagnostic markers for HCC are still lacking. We reported herein a potential tumor suppressor in HCC‐TROP‐2. The expression of TROP‐2 was significantly down‐regulated in HCC tumor tissues when compared with non‐tumor tissues, and promoter hypermethylation could potentially provide an explanation for such down‐regulation. The loss of TROP‐2 predicts poor overall patient survival, invasion, and poor differentiation of HCC. However, the molecular mechanism of the effects of TROP‐2 leading to such patient outcomes is still unclear at this moment.
Loss of expression of genes could be attributed to multiple factors. Genetic and epigenetic alterations greatly contribute to changes in gene expression. For epigenetic alterations, DNA methylation is one of the most commonly seen mechanisms in inactivation of gene expression in cancer.16 In fact, the hepatic methylation profile has been shown to be correlated with recurrence‐free survival of HCC patients after hepatectomy.17 As reported by Revill et al., TROP‐2 was found to be hypermethylated in HCC.15 Therefore, by analyzing HCC patient DNA samples, we sought to confirm the cause of TROP‐2 down‐regulation in HCC being promoter hypermethylation. Indeed, using BGS and MSP, we found that the TROP‐2 promoter methylation level is comparatively higher in HCC tumor tissues with low TROP‐2 expression than that in non‐tumor tissues with high TROP‐2 expression (Fig. 3). However, whether promoter hypermethylation is the sole mechanism causing the loss of TROP‐2 expression in HCC remains to be elucidated. It might be interesting to further explore whether genetic alterations such as chromosomal deletion also play a role in the loss of TROP‐2 in HCC.
During the three decades since TROP‐2 was initially identified in trophoblast cells, much attention has been given to study the tumorigenic effects and predictive significance in poor patient prognosis of this gene in cancer patients. This is partly due to the important functions of this gene in the highly invasive process of blastocyst implantation.18 Nevertheless, emerging evidence hints that there is more to it than that. Depending on the cancer types, TROP‐2 has exhibited distinct effects. In head and neck squamous cell carcinoma, the mesenchymal tumors showed decreased TROP‐2 expression. Depletion of the TROP‐2 protein in the cell surface stimulates the activation of epidermal growth factor receptor family member ErbB3 due to a lack of sequestering of neuregulin‐1 by TROP‐2.14 In lung cancer, TROP‐2 expression was inhibited by DNA hypermethylation, and the loss of TROP‐2 leads to hyperactivation of insulin‐like growth factor 1R and subsequent oncogenic effects.13 Thus, the effects of TROP‐2 in cancer development and determining patient outcome could be complex and cancer‐type dependent. In this study, we found that TROP‐2 was frequently down‐regulated in HCC, and the loss of TROP‐2 was associated with poor prognosis of HCC patients (Figs. 1 and 2, Table 1). This finding is encouraging and might provide support for using TROP‐2 as an effective biomarker in future diagnosis and prognosis predictions of HCC patients.
Conclusions
In conclusion, the results of RNA sequencing from TCGA databases and qRT‐PCR analysis of our in‐house cohort both indicated a frequent and significant down‐regulation of TROP‐2 in HCC tumor tissues. DNA methylation analysis linked the down‐regulation of TROP‐2 with promoter hypermethylation. Furthermore, the down‐regulation of TROP‐2 could predict poor patient prognosis of HCC. These findings indicate that TROP‐2 might be a potential biomarker in predicting prognosis and aiding early cancer diagnosis of HCC patients.
Author names in bold designate shared co‐first authorship.
Supporting information
View this article online at wileyonlinelibrary.com.
Funding
Supported by the General Research Fund (HKU/7668/11M, 767313), the Collaborative Research Fund (HKBU5/CRF/10, HKU3/CRF/11R), and the Theme‐Based Research Scheme Fund (T12‐403/11) of the Hong Kong Research Grants Council.
Potential conflict of interest: Nothing to report.
References
- 1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245‐1255. [DOI] [PubMed] [Google Scholar]
- 2. Burkhart RA, Ronnekleiv‐Kelly SM, Pawlik TM. Personalized therapy in hepatocellular carcinoma: molecular markers of prognosis and therapeutic response. Surg Oncol 2017;26:138‐145. [DOI] [PubMed] [Google Scholar]
- 3. Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell‐surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A 1981;78:5147‐5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calabrese G, Crescenzi C, Morizio E, Palka G, Guerra E, Alberti S. Assignment of TACSTD1 (alias TROP1, M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2 (alias TROP2, M1S1) to human chromosome 1p32 by in situ hybridization. Cytogenet Cell Genet 2001;92:164‐165. [DOI] [PubMed] [Google Scholar]
- 5. McDougall AR, Tolcos M, Hooper SB, Cole TJ, Wallace MJ. Trop2: from development to disease. Dev Dyn 2015;244:99‐109. [DOI] [PubMed] [Google Scholar]
- 6. Alberti S, Miotti S, Stella M, Klein CE, Fornaro M, Menard S, et al. Biochemical characterization of Trop‐2, a cell surface molecule expressed by human carcinomas: formal proof that the monoclonal antibodies T16 and MOv‐16 recognize Trop‐2. Hybridoma 1992;11:539‐545. [DOI] [PubMed] [Google Scholar]
- 7. Ripani E, Sacchetti A, Corda D, Alberti S. Human Trop‐2 is a tumor‐associated calcium signal transducer. Int J Cancer 1998;76:671‐676. [DOI] [PubMed] [Google Scholar]
- 8. Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res 2006;12:3057‐3063. [DOI] [PubMed] [Google Scholar]
- 9. Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, et al. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis 2009;24:875‐884. [DOI] [PubMed] [Google Scholar]
- 10. Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer 2008;99:1290‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trerotola M, Ganguly KK, Fazli L, Fedele C, Lu H, Dutta A, et al. Trop‐2 is up‐regulated in invasive prostate cancer and displaces FAK from focal contacts. Oncotarget 2015;6:14318‐14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trerotola M, Jernigan DL, Liu Q, Siddiqui J, Fatatis A, Languino LR. Trop‐2 promotes prostate cancer metastasis by modulating β(1) integrin functions. Cancer Res 2013;73:3155‐3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, Chang YL, et al. TROP2 is epigenetically inactivated and modulates IGF‐1R signalling in lung adenocarcinoma. EMBO Mol Med 2012;4:472‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang K, Jones L, Lim S, Maher CA, Adkins D, Lewis J, et al. Loss of Trop2 causes ErbB3 activation through a neuregulin‐1‐dependent mechanism in the mesenchymal subtype of HNSCC. Oncotarget 2014;5:9281‐9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Revill K, Wang T, Lachenmayer A, Kojima K, Harrington A, Li J, et al. Genome‐wide methylation analysis and epigenetic unmasking identify tumor suppressor genes in hepatocellular carcinoma. Gastroenterology 2013;145:1424‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daura‐Oller E, Cabre M, Montero MA, Paternain JL, Romeu A. Specific gene hypomethylation and cancer: new insights into coding region feature trends. Bioinformation 2009;3:340‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishida N, Kudo M. Alteration of epigenetic profile in human hepatocellular carcinoma and its clinical implications. Liver Cancer 2014;3:417‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer 2015;6:84‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


