Abstract
A prognostic system for acute liver failure (ALF) with a higher predictive value is urgently needed. The role of extracellular matrix (ECM) remodeling in ALF has not been fully elucidated. We hypothesized that serologic fibrosis markers, which reflect ECM remodeling, are predictive of ALF outcome at first presentation. This observational study included 110 patients with acute liver dysfunction, of which 73 had non‐acetaminophen‐associated ALF (NAA‐ALF). We evaluated serum levels of hyaluronic acid, 7S domain of type IV collagen (4COL7S), and Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein at first presentation to a tertiary center. Serologic fibrosis markers were significantly higher in NAA‐ALF compared with acute hepatitis. Elevated hyaluronic acid and 4COL7S levels at first presentation correlated significantly with worse clinical outcomes. 4COL7S, along with age, ammonia, and the Model for End‐Stage Liver Disease (MELD) score, was a significant prognostic factor in multivariate analysis; 4COL7S correlated significantly with coagulopathy, decreased hepatic synthetic functions, advanced hepatic encephalopathy, and liver atrophy and also predicted 180‐day transplant‐free survival. Cox regression models incorporating 4COL7S with the MELD system had profoundly improved predictive values that significantly surpassed the MELD system alone. Conclusion: Elevation of serologic fibrosis markers reflecting ECM remodeling in NAA‐ALF predicted a worse clinical outcome. Incorporation of 4COL7S at first presentation to a transplant center improves the specificity while retaining the sensitivity of the MELD system. External validation of a fibrosis marker as part of a clinical prediction tool in ALF warrants further investigation.
Abbreviations
- 4COL7S
7S domain of type IV collagen
- AH
acute hepatitis
- AIH
autoimmune hepatitis
- ALF
acute liver failure
- ALT
alanine aminotransferase
- AT‐III
anti‐thrombin‐III
- AUROC
area under receiver operating characteristic
- ECM
extracellular matrix
- HA
hyaluronic acid
- HSC
hepatic stellate cell
- KCC
King’s College Hospital Criteria
- ln
logarithmic transformation
- LSEC
liver sinusoidal endothelial cell
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- NAA‐ALF
non‐acetaminophen‐associated acute liver failure
- PT‐INR
prothrombin time–international normalized ratio
- T/D
transplant or deceased
- TFS
transplant‐free survival
- WFA+‐M2BP
Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein
Introduction
Acute liver failure (ALF) is a rare and life‐threatening clinical syndrome caused by severe impairment of liver function due to massive or submassive liver necrosis. It occurs most often in patients who do not have preexisting liver diseases.( 1 ) Due to transplant organ insufficiency( 2, 3 ) or comorbidities, such as uncontrolled active infection, social background, and severe cerebral edema, many patients with ALF do not benefit from emergent liver transplantation (LT).( 4, 5, 6 ) The ALF survival rate without LT varies from approximately 20% to 60%( 4, 7, 8 ) and remains far from satisfactory. Additionally, as many as 20% of patients with ALF may have undergone unnecessary transplants.( 9 ) Therefore, patients who may definitely benefit from LT should be properly evaluated. The two best studied prognostic systems are the King’s College Hospital Criteria (KCC) and the Model for End‐Stage Liver Disease (MELD) score. However, in a meta‐analysis of studies conducted after 2005, McPhail et al.( 10 ) showed that the sensitivity of the KCC to predict the outcome of non‐acetaminophen‐associated ALF (NAA‐ALF) was reduced to approximately 58%. Several researchers have suggested that modifications might be needed to improve the prognostic value of the MELD system.( 11, 12 )
Although patients with ALF may have similar clinical manifestations, the initial symptoms are usually obscure. Furthermore, the time from liver insult to the presence of ALF varies greatly.( 1, 4, 7, 13 ) Acetaminophen is the leading cause (around 44%) of ALF in Europe and the United States( 7 ); however, non‐acetaminophen‐associated causes account for over half of ALF cases in the Western world and nearly all ALF cases in Japan.( 14 ) The duration from the time of onset to hepatic encephalopathy has prognostic value and is incorporated into the scoring systems for outcome prediction in the KCC and the criteria for ALF in Japan,( 15 ) but chronological evolution is usually not clear‐cut. With regard to ALF, the heterogeneity of its etiology, the chronology of its evolution, its predisposing background, its severity, and its rarity have limited evidence‐based guides for care. Possible biomarkers that can cope with these complexities are warranted.
ALF is histopathologically characterized by not only massive hepatocyte necrosis but also variable inflammation, periportal ductular reaction, and a background comprising an admixture of extracellular matrix (ECM).( 16 ) Cellular components of liver sinusoids and the surrounding ECM can be described as a multidirectional interaction complex and have been emphasized in various models of liver injury, fibrosis, and regeneration.( 17, 18, 19 ) Hepatic ECM remodeling, featuring multiple serologic markers, such as hyaluronic acid (HA), 7S domain of type IV collagen (4COL7S), and Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein (WFA+‐M2BP), has been studied extensively in chronic liver diseases. The elevation of serologic markers for liver fibrosis( 20 ) and liver stiffness( 21, 22 ) along with evidence of hepatic stellate cell (HSC) activation( 20, 21 ) have been reported in ALF; however, the clinical implications of serologic fibrosis markers in ALF have not been fully elucidated.
In order to know whether serologic fibrosis markers that reflect ECM remodeling associate with clinical presentations in ALF and whether they help predict prognosis, we conducted this observational study in our tertiary medical center (a transplant center) and assessed the potential use of HA, 4COL7S, and WFA+‐M2BP as prognostic biomarkers in NAA‐ALF.
Patients and Methods
Study Subjects
The Institutional Review Board of Keio University School of Medicine approved this observational case‐control study (No. 20120395 and No. 20160453) according to the guidelines of the 1975 Declaration of Helsinki (2008 revision). The study subjects were prospectively recruited, and each provided prior written informed consent to blood sampling, study participation, and analysis of clinical data. All the study subjects received standard care and treatment according to their clinical presentations. The analysis was conducted retrospectively. We recruited 110 consecutive adult patients who had been admitted to our tertiary center, a transplant center in metropolitan Tokyo, with acute liver dysfunction between June 2007 and February 2018. No donor organs were obtained from executed prisoners or other institutionalized persons. Seventy‐three patients were diagnosed with established ALF; 24 patients with acute hepatitis and 13 patients with acute exacerbation of previously established chronic liver diseases were also included.
The study scheme is shown in Supporting Fig. S1A. Clinical and biochemical parameters were collected at first presentation (admission) to our center. Outcomes, including transplant‐free survival (TFS), transplant, and death, were defined as status within 180 days of the onset of symptoms. TFS was defined as survival with improved liver function by standard medical management without LT. (See Supporting Methods for more information about diagnosis, standard medical care, and indications for LT.)
Measurement of Serologic Fibrosis Markers
Serum levels of 4COL7S, HA, and WFA+‐M2BP were determined at first presentation (at admission to our center) as described.( 23, 24, 25 ) These measurements are all clinically approved and available with commercial kits in Japan. In brief, 4COL7S levels were measured using the radioimmunoassay two‐antibodies method (reference level for normal adult, below 6.0 ng/mL); HA levels were measured using the latex agglutination method (reference level for normal adult, below 50 ng/mL); and WFA+‐M2BP levels were measured using the chemiluminescent enzyme immunoassay method. Quantitative evaluation of WFA+‐M2BP was expressed as a cut‐off index (reference level for normal adult, below 1.0).
Statistics
The data were analyzed using JMP12 (SAS Institute, Inc. Cary, NC) and are expressed as medians with interquartile ranges or averages ± SD, as appropriate. Graphs and linear correlations were constructed using Prism 7.0 (GraphPad Software, Inc. San Diego, CA). Nonparametric Kruskal–Wallis tests were used to assess differences between groups. Categorical variables were analyzed using chi‐square analysis. Spearman correlation was used for correlation analysis. Area under receiver operating characteristic (AUROC) analysis was performed to confirm the usefulness of various parameters for predicting outcome and generating optimal cutoffs based on the Youden index. The DeLong method( 26 ) was used to compare the differences between AUROC curves. Kaplan–Meier analysis determined the cumulative percentage of survival, and the differences between groups were compared using log‐rank tests. Cox regression analysis was used to build a model stratified by outcome. The Shapiro–Wilk test was used to confirm normality of the distribution of various parameters. Natural logarithmic transformation (ln) of a non‐normally distributed parameter was used. Competing risk estimates of cumulative incidence function for death (with transplantation as a competing risk) were determined by Gray’s test. R software (version 3.3.3) was used for internal validation performed by bootstrapping analysis and competing risk analysis. The results were considered significant when P < 0.05.
Results
Background Characteristics, Outcomes, and Histology Supporting ECM Remodeling in NAA‐ALF
Baseline characteristics of the enrolled 73 patients with NAA‐ALF and their clinical parameters (collected at the first presentation to our tertiary center but not at the onset of any signs or symptoms) are presented in Table 1. No patient had acetaminophen‐induced liver injury. Forty‐three patients (61%) survived without LT (the TFS group); 20 patients (27%) died without undergoing LT, and 7 patients (10%) died who underwent LT (the transplant or deceased T/D group). All transplant recipients with ALF survived for the duration of the study period. The detailed outcomes regarding LT determination at admission and their enforcement are shown in Supporting Fig. S1B
Table 1.
Background Characteristics, Clinical Parameters, and Serologic Fibrosis Markers of Patients With NAA‐ALF at First Presentation
| Parameter | All patients | TFS | T/D | P * | P † | Multivariate OR |
|---|---|---|---|---|---|---|
| (95% CI) | ||||||
| Number (%) | 73 | 43 (61) | 27‡ (39) | – | ||
| Etiologies, n (%) | ||||||
| Viral infection | 30 (41) | 18 (39) | 12 (44) | 0.33 | – | |
| HAV | 8 (12) | 7 (16) | 1 (4) | – | ||
| HBV | 16 (24) | 9 (21) | 7 (28) | – | ||
| Others§ | 6 (9) | 2 (5) | 4 (16) | – | ||
| Autoimmune hepatitis | 22 (30) | 14 (30) | 8 (30) | – | ||
| DILI | 6 (8) | 4 (9) | 2 (7) | – | ||
| Indeterminate | 13 (18) | 10 (22) | 3 (11) | – | ||
| Others|| | 2 (3) | 0 (0) | 2 (7) | – | ||
| Sex (M/F)/ n (%) | 37 (51)/ 36 (49) | 20 (43)/ 26 (57) | 17 (63)/ 10 (37) | 0.15 | ||
| Age in years | 50 [23] | 40.5 [25.5] | 56 [13] | 0.0014 | 0.014 | 9.1 (1.8–71) |
| Clinical parameters | ||||||
| PT‐INR | 2.21 ± 0.75 | 2.06 ± 0.64 | 2.48 ± 0.85 | 0.02 | 0.56 | – |
| Creatinine, mg/dL | 1.23 ± 1.55 | 0.98 ± 1.07 | 1.65 ± 2.10 | 0.08 | ||
| T‐ Bil, mg/dL | 14.7 ± 10.0 | 13.5 ± 9.9 | 16.7 ± 10.0 | 0.19 | ||
| AST, IU/L | 1,719 ± 2,817 | 1,880 ± 2,866 | 1,444 ± 2,764 | 0.53 | ||
| ALT, IU/L | 1,625 ± 2,176 | 1,775 ± 2,122 | 1,372 ± 2,283 | 0.45 | ||
| NH3, μg/dL | 68 ± 39 | 56 ± 28 | 87 ± 47 | 0.0006 | 0.006 | 47 (4.5–1440) |
| MELD score | 26 [5.5] | 24 [5.4] | 29 [7.5] | <0.0001 | 0.002 | 65 (7.4–1770) |
| Fibrosis markers | ||||||
| HA¶, ng/mL | 4,766 ± 7,641 | 2,437 ± 3,204 | 8,649 ± 10,833 | 0.0012 | 0.98 | – |
| 4COL7S#, ng/mL | 16.8 ± 6.5 | 14.8 ± 6.0 | 19.9 ± 6.1 | 0.012 | 0.017 | 7.4 (1.6–46) |
| WFA+‐M2BP**, COI | 10.7 ± 5.1 | 10.4 ± 5.0 | 11.2 ± 5.4 | 0.56 | – |
Data were collected at first presentation to a tertiary center. Data are shown as mean ± SD or median with interquartile range in brackets, as appropriate. *Significance for univariate analyses; †significance for multivariate analyses; ‡liver transplantation in seven cases; §including three cases of HEV, two cases of EBV, and one case of VZV; ||acute Budd–Chiari syndrome. Statistics were analyzed from ¶64 cases, #66 cases, and **60 cases. P < 0.05 was significant.
Abbreviations: AFP, alpha‐fetoprotein; AST, aspartate aminotransferase; CI, confidence interval; COI, cut‐off index; DILI, drug‐induced liver injury; EBV, Epstein–Barr virus; F, female; HAV, hepatitis A virus; HBV, hepatitis B virus; HEV, hepatitis E virus; M, male; OR, odds ratio; T‐Bil, total bilirubin; VZV, varicella zoster virus.
Because of a bleeding tendency and the lack of evidence to support definite diagnostic benefit in patients with ALF, routine liver biopsies are discouraged by the American Gastroenterological Association guidelines.( 27 ) However, in the present study, liver histology information was obtained by biopsy in the recovery phase in several patients or from liver explants during LT. The histology of liver biopsies in two representative cases in their recovery phases along with two liver explants from representative patients who underwent LT are shown in Fig. 1. In all four cases, Mallory’s azan staining revealed increased density of the blue‐stained fibrous tissue that reflects the loss of hepatocytes and pale blue‐gray young fibrous tissue surrounding the sites of ductular reaction, which is characteristic of hepatocyte regeneration in the periportal regions (Fig. 1; see Supporting Table S1 for clinical details). ECM remodeling might be a universal histologic phenomenon in NAA‐ALF.
Figure 1.
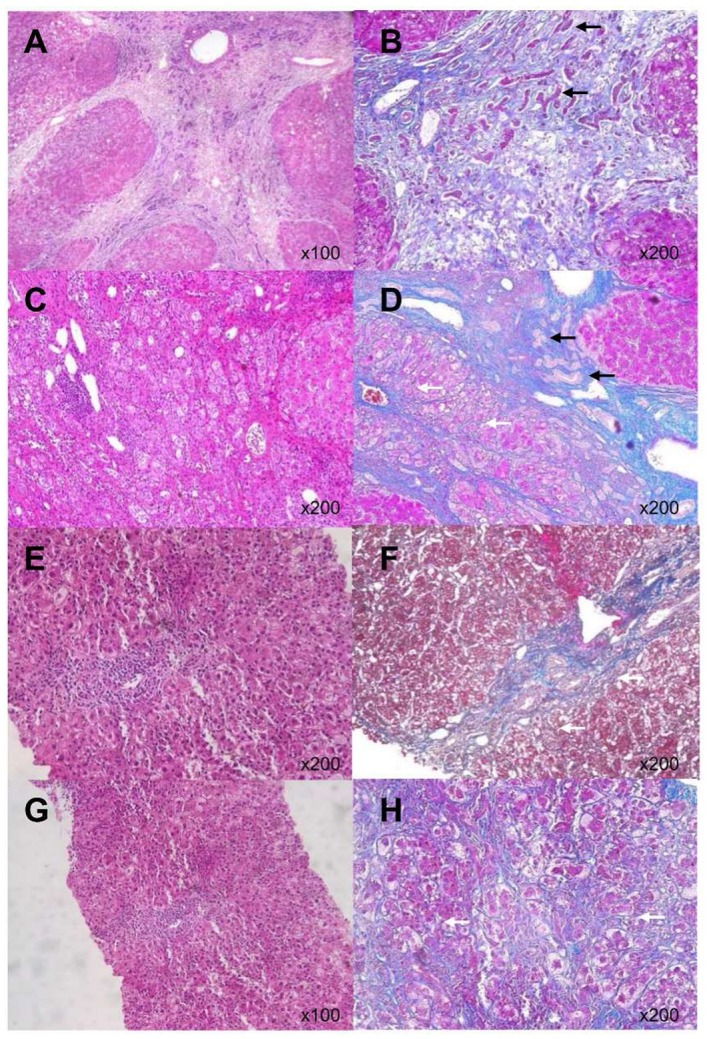
Histology of NAA‐ALF tissue samples. (A,B) Case 1. (C,D) Case 2. (E,F) Case 3. (G,H) Case 4. Histopathologic examination, including hematoxylin and eosin staining (A,C,E,G) and Mallory’s azan staining (B,D,F,H) revealed increased density of total fibrous tissue due to hepatocyte loss and robust ECM remodeling around ductular reactions (black arrows), suggestive of liver regeneration. There were also variable degrees of hepatocyte necrosis, inflammatory cell infiltration, and perisinusoidal fibrosis (white arrows). (See Supporting Table S1 for clinical details.)
Elevation of Serologic Fibrosis Markers was Observed in NAA‐ALF, and the Levels of 4COL7S Correlated to Clinical Outcomes
We compared serologic fibrosis markers (HA, 4COL7S, and WFA+‐M2BP) at admission in patients with NAA‐ALF and other acute liver injuries, such as acute hepatitis (AH) and acute exacerbation (Fig. 2A). The levels of 4COL7S differed significantly among all three groups (ALF, AH, and acute exacerbation). Of the 66 patients with NAA‐ALF whose 4COL7S levels were available at admission, over 98% (65 cases) had 4COL7S levels that were higher than the upper limit of normal reference range (6 ng/mL). Levels of HA and WFA+‐M2BP also differed significantly between ALF and AH (Fig. 2A). We confirmed that the serologic markers of liver fibrosis were elevated at first presentation to a tertiary center in the patients with NAA‐ALF.
Figure 2.
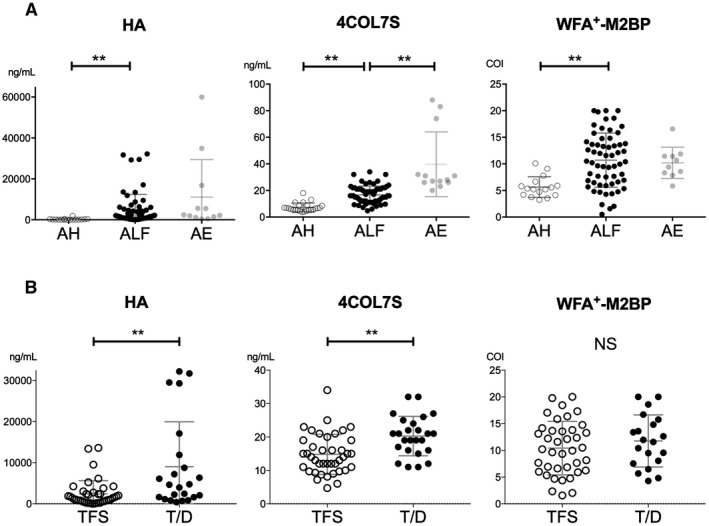
Serologic fibrosis markers in acute liver dysfunction and their correlations with clinical outcomes in NAA‐ALF. (A) Serologic fibrosis markers, i.e., HA, 4COL7S, and WFA+‐M2BP, were evaluated at first presentation and were compared among groups of AH, ALF, and acute exacerbation of previously known chronic hepatitis. (B) Those makers were compared between groups of TFS and T/D patients with NAA‐ALF. Statistics are shown as average ± SD. **P < 0.01. Abbreviations: AE, acute exacerbation; NS, not significant.
Levels of 4COL7S and HA were significantly higher in the T/D group than in the TFS group (Table 1; Fig. 2B); levels of WFA+‐M2BP did not differ significantly. In univariate analysis, older age of onset, more prolonged prothrombin time–international normalized ratios (PT‐INRs), higher serum ammonia levels, and higher MELD scores at first presentation were also significant factors favoring worse outcomes. In multivariate analysis, levels of 4COL7S at admission along with age of onset, ammonia, and MELD score were significantly higher in the T/D group than in the TFS group (Table 1).
Serologic Fibrosis Markers Correlated to Demographic Characteristics and Clinical/Biochemical Derangements in ALF
Levels of HA and 4COL7S did not differ significantly between sex, age, and etiology of NAA‐ALF (Table 2). WFA+‐M2BP was significantly higher in patients with NAA‐ALF due to autoimmune hepatitis (AIH). The MELD score at a cutoff of 24, which is based on the Youden index in ROC analysis for outcome prediction, significantly differentiated all three serologic fibrosis markers. Statistical significance to KCC for NAA‐ALF( 28 ) (assessed at admission) was noticed only with 4COL7S. Notably, the presence of subacute onset of hepatic encephalopathy over grade 2 differentiated the levels of 4COL7S. In contrast, such differentiation was not observed with WFA+‐M2BP levels. Furthermore, the presence of liver atrophy differentiated the levels of 4COL7S but not HA and WFA+‐M2BP (Table 2).
Table 2.
Distribution of Serologic Fibrosis Markers Depending on Demographic and Clinical Characteristics of Patients With NAA‐ALF
| Parameters | HA* | 4COL7S† | WFA+‐M2BP‡ | |
|---|---|---|---|---|
| (ng/mL) | (ng/mL) | (COI) | ||
| Sex | Male, n = 37 | 5,988 ± 8,973 | 17.9 ± 6.5 | 9.5 ± 4.8 |
| Female, n = 36 | 3,381 ± 5,612 | 15.6 ± 6.3 | 11.9 ± 5.2 | |
| Age of onset | ≥54, n = 27 | 6,462 ± 9,821 | 17.1 ± 6.1 | 11.2 ± 4.6 |
| <54, n = 46 | 3,815 ± 628 | 16.7 ± 6.8 | 10.4 ± 5.4 | |
| Etiology of ALF | Viral, n = 30 | 6,352 ± 9,578 | 15.7 ± 6.6 | 11.1 ± 4.6 |
| Nonviral, n = 43 | 3,680 ± 5,870 | 17.6 ± 6.4 | 10.5 ± 5.5 | |
| Etiology of ALF | Autoimmune, n = 22 | 3,317 ± 6,604 | 19.1 ± 5.7 | 12.5 ± 5.0|| |
| Nonautoimmune, n = 51 | 5,378 ± 8,030 | 15.9 ± 6.6 | 9.7 ± 4.9|| (0.04) | |
| KCC§ | Positive, n = 21 | 6,556 ± 9,072 | 20.9 ± 5.8¶ | 12.9 ± 4.9 |
| Negative, n = 52 | 3,853 ± 6,857 | 15.1 ± 6.0¶ (0.0005) | 10.0 ± 5.0 | |
| MELD | ≥24, n = 49 | 6,237 ± 8,749|| | 18.0 ± 6.7|| | 11.6 ± 5.3|| |
| <24, n = 24 | 1,530 ± 2,081|| (0.02) | 14.2 ± 5.3|| (0.03) | 8.9 ± 4.3|| (0.049) | |
| HE ≥grade 2 | None, n = 40 | 2,773 ± 5,476¶ | 14.5 ± 5.6¶ | 10.1 ± 5.1 |
| Acute, n = 12 | 7,711 ± 8,989¶ (0.006) | 18.1 ± 8.1 | 10.4 ± 3.8 | |
| Subacute, n = 21 | 6,628 ± 9,383 | 20.0 ± 5.5¶ (0.002) | 12.1 ± 5.8 | |
| Liver atrophy | None, n = 36 | 4,525 ± 8,335 | 14.3 ± 6.3¶ | 9.6 ± 5.5 |
| Atrophied, n = 37 | 4,979 ± 7,094 | 19.0 ± 5.8¶ (0.002) | 11.9 ± 4.5 |
Number of study subjects included: *64 cases; †66 cases; ‡60 cases. §KCC was assessed at admission. Data with statistical significance assessed by Kruskal–Wallis tests with significance in parentheses, || P < 0.05; ¶ P < 0.01.
Abbreviations: COI, cut‐off index; HE, hepatic encephalopathy.
Serologic fibrosis markers correlated with various biochemical derangements in the patients with NAA‐ALF (Table 3). HA, 4COL7S, and WFA+‐M2BP all correlated significantly with PT‐INR but not with total bilirubin or creatinine levels. HA correlated significantly with levels of aspartate transaminase, alanine transaminase (ALT), fibrin degradation products, and ammonia; 4COL7S correlated significantly with albumin, fibrinogen, anti‐thrombin III (AT‐III) activities, and ammonia; and WFA+‐M2BP correlated significantly with choline‐esterase, fibrinogen, and AT‐III activities.
Table 3.
Correlations Between Various Serologic Fibrosis Markers and Biochemical Parameters of Patients With NAA‐ALF at First Presentation
| Significance; P | HA* | 4COL7S† | WFA+‐M2BP‡ |
|---|---|---|---|
| Correlation Coefficient (R) | |||
| AST, IU/L | 0.0012 (0.40) | 0.31 | 0.15 |
| ALT, IU/L | 0.0078 (0.33) | 0.07 | 0.17 |
| Albumin, g/dL | 0.26 | 0.0006 (–0.42) | 0.15 |
| Ch‐E, IU/L | 0.51 | 0.13 | <0.0001 (–0.49) |
| T‐Bil, mg/dL | 0.66 | 0.24 | 0.11 |
| Creatinine, mg/dL | 0.57 | 0.46 | 0.94 |
| PT‐INR | <0.0001 (0.48) | 0.02 (0.28) | 0.03 (0.28) |
| Fibrinogen, mg/dL | 0.06 | 0.0005 (–0.42) | 0.0059 (–0.35) |
| FDP, mg/dL | 0.0001 (0.46) | 0.15 | 0.60 |
| AT‐III activity, % | 0.47 | 0.0025 (–0.38) | 0.0033 (–0.39) |
| NH3, mg/dL | 0.04 (0.24) | 0.009 (0.32) | 0.73 |
Spearman correlation analysis was used for each single analysis, and each result was collectively incorporated. Correlation coefficients (R) are shown in parentheses in cases with statistical significance. Number of study subjects included: *64 cases; †66 cases; ‡60 cases.
Abbreviations: AST, aspartate aminotransferase; Ch‐E, choline‐esterase; FDP, fibrin degradation products; T‐Bil, total bilirubin.
Collectively, compared with the HA and WFA+‐M2BP levels, elevated levels of 4COL7S significantly predicted outcomes in patients with NAA‐ALF (Table 1). Levels of 4COL7S were more coherent with the KCC for NAA‐ALF at admission and exhibited a closer association with decreased hepatic protein synthesis, dysfunctional ammonia fixation, profound coagulopathy, encephalopathy, and liver atrophy. We further specified the analysis on 4COL7S for its predictive value of outcome.
Modification of the Meld System with 4COL7S Improved the Prediction of Outcomes in NAA‐ALF
ROC analysis and the Youden index were used to determine the optimal cut‐off values for predicting T/D with factors including age, 4COL7S, ammonia (as ln[NH3]), PT‐INR, total bilirubin, and MELD score in the 66 patients who had available 4COL7S values at first presentation. The details are shown in Supporting Table S2. In short, the extensively applied MELD system, along with age, 4COL7S, and ammonia, was found to have a prognostic value with statistical significance. With regard to the prediction of the T/D state, the MELD system had a sensitivity of 92%; however, it had a relatively low specificity of 55% at the cut‐off value of 24 (AUROC, 0.77). The MELD scores falsely categorized 20 cases, i.e., the diagnostic accuracy was 0.70 in this analysis. At the same time, the MELD system alone did not show superior prognostic value over age of onset, 4COL7S, or ammonia (Supporting Fig. S2).
By using Cox regression analysis, we tried to modify the MELD system with age, ammonia (as ln[NH3]), or 4COL7S or with the combination of some of the three (Table 4; details of Cox regression models are shown in Supporting Table S3). The combination of two or more factors with the MELD system significantly surpassed the MELD system in their prognostic values (Table 4). Notably, the inclusion of 4COL7S significantly improved (MELD + Age and MELD + ln[NH3]; P = 0.0497 and 0.0118, respectively) or tended to improve (MELD + Age + ln[NH3]; P = 0.0855) the prognostic values more than exclusion of 4COL7 did (Fig. 3; Table 4). Specificity (from 55% to 90%), positive predictive values (from 57% to 85%), and diagnostic accuracy (from 70% to 89%) were also profoundly improved, while the sensitivity of the MELD system was relatively retained (from 92% to 88%). In contrast, incorporation of age to the MELD system without 4COL7S also improved specificity but decreased the sensitivity of the MELD system (Table 4). The correlation matrices showed that 4COL7S did not strongly confound with MELD score, ammonia, or age (Supporting Table S3). Internal validation was done using 1,000 bootstrapped samples. Performances of the odds ratio of each parameter and its diagnostic values presented as AUROC in models with or without 4COL7S were relatively consistent with original multivariable models (Supporting Table S4).
Table 4.
Comparison Between AUROC Curve of MELD and Other Cox Regression Models for Differentiation Between TFS Versus T/D
| Parameters | AUROC | 95% CI | Sensitivity | Specificity | PPV | NPV | Accuracy | P | P *,†,‡,§ | P || | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | (%) | (%) | (%) | (%) | (%) | |||||
| MELD* | 0.77 | 0.63 | 0.87 | 92 | 55 | 57 | 92 | 70 | 0.0009 | ‐ | ‐ |
| 4COL7S | 0.73 | 0.59 | 0.84 | 77 | 63 | 57 | 81 | 68 | 0.0012 | ‐ | 0.6553 |
| MELD + 4COL7S*, ¶ | 0.83 | 0.71 | 0.90 | 69 | 85 | 75 | 81 | 79 | <0.0001 | 0.3419* | 0.2419 |
| MELD + Age†, ¶ | 0.84 | 0.73 | 0.91 | 58 | 95 | 88 | 78 | 80 | <0.0001 | ‐ | 0.1576 |
| MELD + Age + 4COL7S†, ¶ | 0.89 | 0.76 | 0.95 | 88 | 83 | 77 | 92 | 85 | <0.0001 | 0.0497† | 0.0135 |
| MELD + ln[NH3]‡, ¶ | 0.83 | 0.73 | 0.90 | 92 | 73 | 69 | 95 | 80 | <0.0001 | ‐ | 0.1537 |
| MELD + ln[NH3] + 4COL7S‡, ¶ | 0.90 | 0.79 | 0.95 | 92 | 73 | 69 | 95 | 80 | <0.0001 | 0.0118‡ | 0.0083 |
| MELD + Age + ln[NH3]§, ¶ | 0.90 | 0.79 | 0.95 | 96 | 70 | 68 | 97 | 80 | <0.0001 | ‐ | 0.0032 |
| MELD + Age + ln[NH3] + 4COL7S§, ¶ | 0.93 | 0.82 | 0.97 | 88 | 90 | 85 | 92 | 89 | <0.0001 | 0.0855§ | 0.0008 |
Statistics were evaluated in 66 non‐acetaminophen‐associated ALF cases with available 4COL7S levels. *,†,‡,§Significance with comparison between corresponding Cox regression models inclusive or exclusive of 4COL7S (by DeLong method); ||significance with comparison to MELD (by DeLong method); ¶details of each Cox regression model in Supporting Table S3. P < 0.05 was significant.
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; T‐Bil, total bilirubin.
Figure 3.
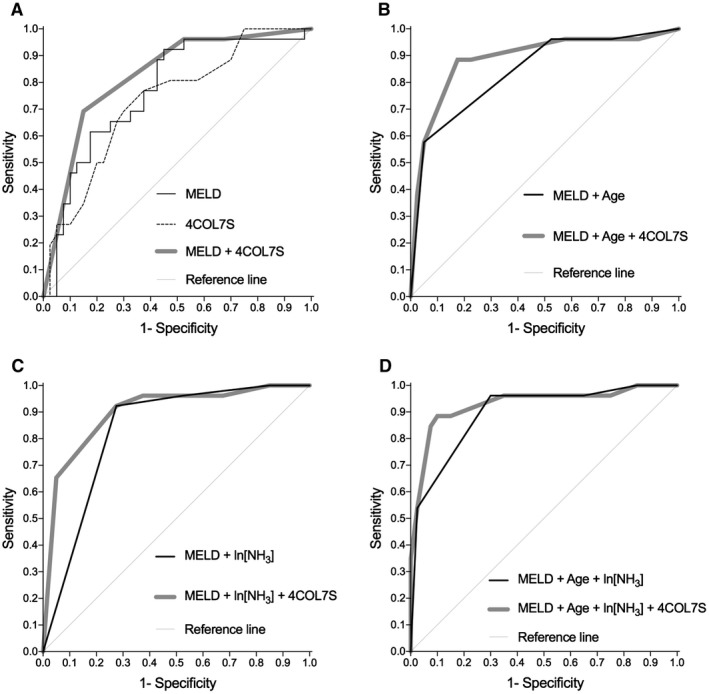
ROC curves for predicting TFS in patients with NAA‐ALF. We compared the prognostic value of various parameters for discrimination between TFS and T/D cases in 66 NAA‐ALF cases with available 4COL7S levels. The ROC curves for MELD, other single parameters, and Cox regression models comprising two to four factors, including MELD score, age, ln[NH3], or 4COL7S, are shown. Detailed statistics, including statistical comparison of the ROC curves by the DeLong method, are presented in Table 4 and Supporting Tables S2 and S3.
In Kaplan–Meier analysis, the 180‐day survival curves beginning at symptom onset showed that there were significant differences in the TFS group (Fig. 4A) and survival in patients without a transplant (total 59 patients, excluding 7 patients who underwent LT; Fig. 4B), in terms of 4COL7S at the optimal cutoff (16 ng/mL). Because the MELD system already had a good negative predictive value of 92% at the cutoff of 24, we analyzed whether 4COL7S helped differentiate survival in patients with MELD scores over 24. We found that 4COL7S (at the cutoff of 19 ng/mL, which was determined by the Youden index) helped differentiate TFS and T/D with statistical significance in patients with MELD scores over 24 (Fig. 4C). Finally, competing risk estimates of cumulative incidence function for death (with transplantation as a competing risk) by Gray’s test showed that the Cox regression model incorporating 4COL7S, MELD, age, and ammonia significantly discriminated TFS and death and tended to significantly discriminate transplantation and death (P = 0.052) (Fig. 4D; detailed statistics in Supporting Table S5).
Figure 4.
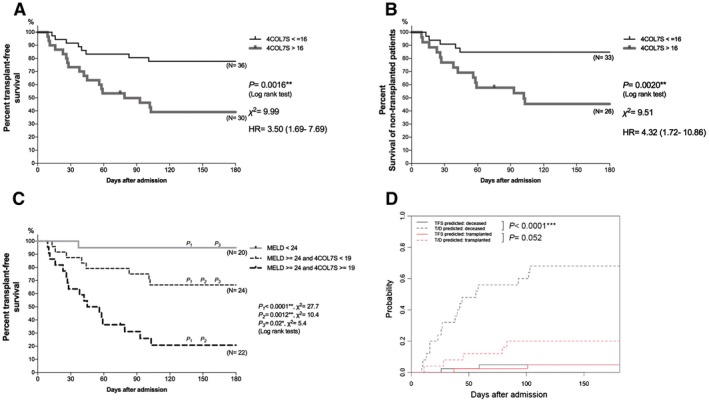
Kaplan–Meier analysis of 180‐day survival. In 66 NAA‐ALF cases with available 4COL7S levels, survival from the onset of symptoms up to 180 days was compared. Patients are stratified by 4COL7S at the optimal cut‐off value generated by ROC curve analyses. (A) Kaplan–Meier analysis was performed for 180‐day TFS, with 8 versus 18 events. (B) Kaplan–Meier analysis for survival of patients without a transplant, with 5 versus 14 events. (C) TFS of three groups stratified by MELD score <24, MELD score >24 + 4COL7S <19 ng/mL, and MELD score >24 + 4COL7S >19 ng/mL, with 1 versus 8 versus 17 events. (D) Competing risk estimates of cumulative incidence function for death (with transplantation as a competing risk) by Gray’s test of a Cox regression model incorporating 4COL7S, MELD, age, and NH3. P values from log‐rank tests are shown. *P < 0.05; **P < 0.01; ***P < 0.0001. Abbreviation: HR, hazard ratio.
A Significant Reduction in 4COL7S Levels was Observed in Transplant‐free Survivors of NAA‐ALF
In a subgroup of spontaneous survivors of NAA‐ALF (detailed clinical parameters in Supporting Table S6) whose serum 4COL7S levels were determined after approximately 4 weeks, all cases showed significantly reduced 4COL7S levels compared with those at admission; this was in accordance with the functional recovery from NAA‐ALF (Fig. 5). Interestingly, although decreased levels of 4COL7S were observed in patients with NAA‐ALF who recovered without transplantation, the levels of 4COL7S determined after approximately 4 weeks were still higher than the reference upper limit (6 ng/mL) of the normal population. This suggests that ECM remodeling continues for at least several weeks, even after the clinical signs of ALF subside.
Figure 5.
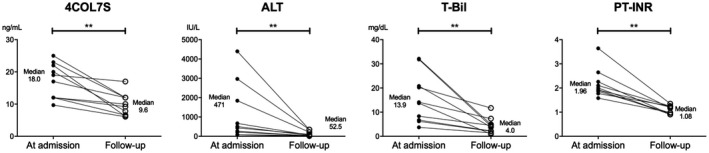
4COL7S significantly decreased in accordance with functional recovery from NAA‐ALF. 4COL7S, total bilirubin, and PT‐INR were evaluated at admission and at follow‐up approximately 4 weeks after admission in 10 transplant‐free survivors of NAA‐ALF. (See Supporting Table S6 for the clinical details of these study subjects.) **P < 0.01. Abbreviation: T‐Bil, total bilirubin.
Discussion
An ideal prognostic system for ALF would have optimal sensitivity and specificity.( 6, 7 ) Liver biopsies are usually contraindicated in patients with ALF due to the risk of bleeding and the lack of sufficient benefits( 27 ); therefore, serologic information derived from a “fluid biopsy” may be important. In the present study, we demonstrated that a Japanese cohort of patients with NAA‐ALF at first presentation had a prominent elevation of serologic fibrosis markers associated with the biochemical and clinical features of NAA‐ALF that could be used to predict outcomes. Serologic fibrosis markers could help improve the specificity of preexisting prognostic systems while retaining their sensitivity (Table 4). To the best of our knowledge, the potential usefulness of serologic fibrosis markers for predicting ALF has not been reported.
It is thought that transplantation may be unnecessary in as many as 20% of patients with ALF,( 9 ) and it has been suggested that because LTs usually equate to death in outcome analyses, positive predictive values might have been falsely elevated in some population‐based studies.( 29 ) Because of the extreme shortage of cadaveric donors and the consequent longer wait times for grafts in Japan,( 30 ) the Japanese cohort in the present study may have provided some useful insights for the assessment of “true” survival, which is important given the paucity of suitable liver graft donors.
The usefulness of elevated serologic fibrosis markers for prognosis in ALF should be discussed from two different perspectives. First is whether ECM remodeling occurs after an acute liver insult; second is whether there are premorbid or etiology‐associated factors that may influence ECM remodeling individually.
Young ECM protein deposition is one of the histologic features of ALF.( 16 ) Type IV collagen deposition has been emphasized in “sinusoidal capillarization,” the initial process of liver sinusoidal endothelial cell (LSEC) activation and subsequent angiogenesis in liver fibrosis.( 31 ) Suou et al.( 32 ) reported that serum 4COL7S levels rose abruptly following single transcatheter arterial embolization (used as a model of acute liver injury). In an investigation of immunohistochemistry, Inuzuka et al.( 33 ) showed that type IV collagen accumulates in HSCs and nearby hepatocytes and in the LSECs that form capillaries in acute viral hepatitis. In an observational study, Dechene et al.( 21 ) showed that serum levels of tissue inhibitor of metalloproteinase 1, a profibrogenic marker of HSC activation, and those of fibrinolytic matrix metalloproteinases were elevated in a cohort of 29 patients with ALF. He et al.( 20 ) showed that serum levels of HA, procollagen III, and type IV collagen were elevated in patients with hepatitis B virus‐related ALF compared with those with chronic hepatitis B or cirrhosis. ECM proteins are needed for liver regeneration during the physiologic wound‐healing process known as “liver fibrosis.”( 34 ) However, overactivation of HSCs, the main producers of hepatic ECM, may intensify liver injury and hinder liver regeneration.( 35 ) HSCs relay inflammatory signals( 36 ); they have also been shown to participate in inflammatory macrophage differentiation and local proliferation in a murine concanavalin A acute liver injury model.( 37 ) Using a murine tetrachloride liver fibrosis model, Issa et al.( 38 ) showed that a mutation in collagen‐I that confers resistance to collagenase degradation resulted in diminished stellate cell apoptosis and impaired hepatocyte regeneration. Activated HSCs participate in the termination of hepatocyte regeneration, probably through the elevated expression of transforming growth factor β, a well‐known hepatocyte antiproliferative factor, as demonstrated in various murine models of liver fibrosis.( 39 ) In the present study, the elevation of serologic fibrosis markers in ALF may imply the collective dysregulation of overexaggerated profibrogenesis with compensatory fibrinolysis and consequently delayed liver regeneration, which leads to poor clinical outcomes. Further evidence from human studies is needed to clarify the underlying mechanism.
ALF often occurs in patients who do not have preexisting liver diseases. Some clinical practice guides, such as that provided by the Acute Liver Failure Study Group Germany, more clearly defines differentiating between acute liver insults from acute‐on‐chronic or acute‐on‐cirrhosis liver injuries( 40 ); however, premorbid information might be lacking and clarification may not always be possible. Previous epidemiologic studies have shown that a predisposing background of subclinical steatosis due to obesity, unestablished alcohol intake, or asymptomatic hepatitis B virus infection might modify the course or severity of acute liver insults.( 41, 42, 43 ) In some countries, such as the United States, it is estimated that the prevalence of nonalcoholic fatty liver disease in the general population could be approximately 20% in adults( 44 ) and the prevalence of unhealthy alcohol intake has increased over the past decade.( 45 ) Because people with nonalcoholic fatty liver disease and those that drink excessive quantities of alcohol may also suffer from acute liver insults, the assessment of serologic fibrosis markers at first presentation to hospital in patients with ALF may help evaluate background hepatic status more precisely, thereby improving predictive accuracy (Fig. 3; Table 4). Moreover, cumulative demographic, laboratory, and outcome data on the etiology of ALF in the Acute Liver Failure Study Group Germany Adult Registry show that the presentation and progress of ALF usually depend on its etiology.( 7 ) AIH‐related ALF is usually characterized by less prominent ALT elevation and relatively higher serum bilirubin levels at first presentation. Serologic fibrosis markers at first presentation to hospital may collectively reflect these factors in patients with NAA‐ALF.
The reason that different serologic fibrosis markers exhibit different behaviors in NAA‐ALF is unclear. In our study cohort, the level of correlation between the serum levels of HA, 4COL7S, and WFA+‐M2BP was slight to moderate (Supporting Fig. S2). All three markers generally correlated with PT‐INR and several other factors associated with intravascular coagulation and fibrinolysis (Table 3). HA is mostly secreted by HSCs and degraded by LSECs, and the process is maintained by competent Kupffer cells.( 46 ) HA is also important for cell proliferation and migration in the steady state.( 19 ) The correlation of HA and ALT in ALF, as shown in Table 3, might imply destruction of the normal liver structure caused by massive necrosis. Basement membranes comprising type IV collagen appear around liver sinusoids after liver insults.( 31 ) The serum level of 4COL7S, which is the amino‐terminal triple‐helix domain of type IV collagen, has demonstrated better sensitivity and specificity for the detection of cirrhosis than type IV collagen itself.( 24 ) In this current study, 4COL7S was most closely associated with the various clinical features of ALF and might imply an unfavorable environment for liver regeneration. WFA+‐M2BP, a newly developed biomarker for liver fibrosis,( 47 ) is secreted by HSCs and induces Mac2 expression in Kupffer cells, which in turn activates HSCs during fibrogenesis.( 48 ) WFA+‐M2BP levels were prominently elevated in AIH‐related ALF (Table 2). Administration of corticosteroids for hypertransaminasemia might modify prognosis in AIH‐related ALF, making WFA+‐M2BP less useful for predicting prognosis than 4COL7S (Fig. 3A).
A major limitation of this study was its observational design, which was exploratory in nature. The small subject number of the study and its single‐center‐based design limit the power of the explanation of results. Because ALF is a rare clinical syndrome, subsequent prospective multicenter studies with larger subject numbers will be needed to externally validate the results and verify the associations described in this study. Acetaminophen‐associated ALF is rare in Asia (including Japan) and was absent from the present study. Therefore, it is not known whether serologic fibrosis markers predict prognosis in acetaminophen‐associated ALF; this warrants further investigation.
In conclusion, the elevation of certain serologic fibrosis markers reflects ECM remodeling in ALF and predicts poor clinical outcomes in patients with NAA‐ALF at first presentation. Inclusion of a serologic marker that reflects ECM remodeling in patients with ALF could lead to a better understanding of the pathophysiology underlying ALF, which could improve clinical decision making.
Potential Conflict of Interest
Nothing to report.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1233/full.
Acknowledgment
We thank Mr. Yuzo Koda for his technical and intellectual assistance when preparing this manuscript.
Funding
Supported in part by grants‐in‐aid from the Keio Gijuku Academic Development Funds (to P.‐S. C.).
Contributor Information
Po‐sung Chu, Email: pschu0928@iCloud.com.
Takanori Kanai, Email: takagast@z2.keio.jp.
References
- 1. Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013;369:2525‐2534. [DOI] [PubMed] [Google Scholar]
- 2. Chan G, Taqi A, Marotta P, Levstik M, McAlister V, Wall W, et al. Long‐term outcomes of emergency liver transplantation for acute liver failure. LiverTranspl 2009;15:1696‐1702. [DOI] [PubMed] [Google Scholar]
- 3. Yamashiki N, Sugawara Y, Tamura S, Nakayama N, Oketani M, Umeshita K, et al. Outcomes after living donor liver transplantation for acute liver failure in Japan: results of a nationwide survey. LiverTranspl 2012;18:1069‐1077. [DOI] [PubMed] [Google Scholar]
- 4. Bernal W, Cross TJ, Auzinger G, Sizer E, Heneghan MA, Bowles M, et al. Outcome after wait‐listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol 2009;50:306‐313. [DOI] [PubMed] [Google Scholar]
- 5. Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: summary of a workshop. Hepatology 2008;47:1401‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology 2012;55:965‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernal W, Lee WM, Wendon J, Larsen FS, Williams R. Acute liver failure: a curable disease by 2024? J Hepatol 2015;62(Suppl.):S112‐S120. [DOI] [PubMed] [Google Scholar]
- 8. Oketani M, Ido A, Nakayama N, Takikawa Y, Naiki T, Yamagishi Y, et al. Intractable Hepato‐Biliary Diseases Study Group of Japan: etiology and prognosis of fulminant hepatitis and late‐onset hepatic failure in Japan: summary of the annual nationwide survey between 2004 and 2009. Hepatol Res 2013;43:97‐105. [DOI] [PubMed] [Google Scholar]
- 9. Lake JR, Sussman NL. Determining prognosis in patients with fulminant hepatic failure: when you absolutely, positively have to know the answer. Hepatology 1995;21:879‐882. [PubMed] [Google Scholar]
- 10. McPhail MJ, Wendon JA, Bernal W. Meta‐analysis of performance of Kings's College Hospital Criteria in prediction of outcome in non‐paracetamol‐induced acute liver failure. J Hepatol 2010;53:492‐499. [DOI] [PubMed] [Google Scholar]
- 11. Bechmann LP, Jochum C, Kocabayoglu P, Sowa JP, Kassalik M, Gieseler RK, et al. Cytokeratin 18‐based modification of the MELD score improves prediction of spontaneous survival after acute liver injury. J Hepatol 2010;53:639‐647. [DOI] [PubMed] [Google Scholar]
- 12. Anastasiou OE, Kalsch J, Hakmouni M, Kucukoglu O, Heider D, Korth J, et al. Low transferrin and high ferritin concentrations are associated with worse outcome in acute liver failure. LiverInt 2017;37:1032‐1041. [DOI] [PubMed] [Google Scholar]
- 13. Reddy KR, Ellerbe C, Schilsky M, Stravitz RT, Fontana RJ, Durkalski V, et al. Acute Liver Failure Study Group. Determinants of outcome among patients with acute liver failure listed for liver transplantation in the United States. LiverTranspl 2016;22:505‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol 2012;47:849‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naiki T, Nakayama N, Mochida S, Oketani M, Takikawa Y, Suzuki K, et al. Intractable Hepato‐Biliary Disease Study Group supported by the Ministry of Health, Labor and Welfare of Japan. Novel scoring system as a useful model to predict the outcome of patients with acute liver failure: application to indication criteria for liver transplantation. Hepatol Res 2012;42:68‐75. [DOI] [PubMed] [Google Scholar]
- 16. Lefkowitch JH. The pathology of acute liver failure. Adv Anat Pathol 2016;23:144‐158. [DOI] [PubMed] [Google Scholar]
- 17. Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 2010;468:310‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, et al. Endothelial cell‐derived angiopoietin‐2 controls liver regeneration as a spatiotemporal rheostat. Science 2014;343:416‐419. [DOI] [PubMed] [Google Scholar]
- 19. Marrone G, Shah VH, Gracia‐Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol 2016;65:608‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He Y, Jin L, Wang J, Yan Z, Chen T, Zhao Y. Mechanisms of fibrosis in acute liver failure. LiverInt 2015;35:1877‐1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dechene A, Sowa JP, Gieseler RK, Jochum C, Bechmann LP, El Fouly A, et al. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology 2010;52:1008‐1016. [DOI] [PubMed] [Google Scholar]
- 22. Kuroda H, Kakisaka K, Oikawa T, Onodera M, Miyamoto Y, Sawara K, et al. Liver stiffness measured by acoustic radiation force impulse elastography reflects the severity of liver damage and prognosis in patients with acute liver failure. Hepatol Res 2015;45:571‐577. [DOI] [PubMed] [Google Scholar]
- 23. Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum "sweet‐doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013;3:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murawaki Y, Ikuta Y, Koda M, Yamada S, Kawasaki H. Comparison of serum 7S fragment of type IV collagen and serum central triple‐helix of type IV collagen for assessment of liver fibrosis in patients with chronic viral liver disease. J Hepatol 1996;24:148‐154. [DOI] [PubMed] [Google Scholar]
- 25. Wong VS, Hughes V, Trull A, Wight DG, Petrik J, Alexander GJ. Serum hyaluronic acid is a useful marker of liver fibrosis in chronic hepatitis C virus infection. J Viral Hepat 1998;5:187‐192. [DOI] [PubMed] [Google Scholar]
- 26. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837‐845. [PubMed] [Google Scholar]
- 27. Flamm SL, Yang Y‐X, Singh S, Falck‐Ytter YT, Flamm SL, Lim JK, et al. AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guidelines for the diagnosis and management of acute liver failure. Gastroenterology 2017;152:644‐647. [DOI] [PubMed] [Google Scholar]
- 28. O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989;97:439‐445. [DOI] [PubMed] [Google Scholar]
- 29. Craig DG, Ford AC, Hayes PC, Simpson KJ. Systematic review: prognostic tests of paracetamol‐induced acute liver failure. Aliment Pharmacol Ther 2010;31:1064‐1076. [DOI] [PubMed] [Google Scholar]
- 30. Akamatsu N, Sugawara Y, Kokudo N. Acute liver failure and liver transplantation. Intractable Rare Dis Res 2013;2:77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedman SL. Liver fibrosis – from bench to bedside. J Hepatol 2003;38(Suppl. 1):S38‐S53. [DOI] [PubMed] [Google Scholar]
- 32. Suou T, Yamada S, Kobayashi J, Hoshino U, Nishimuki E, Kawasaki H. Changes of serum 7S fragment of type IV collagen and N‐terminal propeptide of type III procollagen after transcatheter arterial embolization as a model of acute liver injury. Hepatology 1993;18:809‐815. [DOI] [PubMed] [Google Scholar]
- 33. Inuzuka S, Ueno T, Torimura T, Sata M, Abe H, Tanikawa K. Immunohistochemistry of the hepatic extracellular matrix in acute viral hepatitis. Hepatology 1990;12:249‐256. [DOI] [PubMed] [Google Scholar]
- 34. Klaas M, Kangur T, Viil J, Maemets‐Allas K, Minajeva A, Vadi K, et al. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci Rep 2016;6:27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest 2013;123:1902‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fujita T, Soontrapa K, Ito Y, Iwaisako K, Moniaga CS, Asagiri M, et al. Hepatic stellate cells relay inflammation signaling from sinusoids to parenchyma in mouse models of immune‐mediated hepatitis. Hepatology 2016;63:1325‐1339. [DOI] [PubMed] [Google Scholar]
- 37. Amiya T, Nakamoto N, Chu PS, Teratani T, Nakajima H, Fukuchi Y, et al. Bone marrow‐derived macrophages distinct from tissue‐resident macrophages play a pivotal role in Concanavalin A‐induced murine liver injury via CCR37 axis. Sci Rep 2016;6:35146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Issa R, Zhou X, Trim N, Millward‐Sadler H, Krane S, Benyon C, et al. Mutation in collagen‐1 that confers resistance to the action of collagenase results in failure of recovery from CCl4‐induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J 2003;17:47‐49. [DOI] [PubMed] [Google Scholar]
- 39. Ebrahimkhani MR, Oakley F, Murphy LB, Mann J, Moles A, Perugorria MJ, et al. Stimulating healthy tissue regeneration by targeting the 5‐HT(2)B receptor in chronic liver disease. Nat Med 2011;17:1668‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Canbay A, Tacke F, Hadem J, Trautwein C, Gerken G, Manns MP. Acute liver failure: a life‐threatening disease. Dtsch Arztebl Int 2011;108:714‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chu CM, Yeh CT, Liaw YF. Fulminant hepatic failure in acute hepatitis C: increased risk in chronic carriers of hepatitis B virus. Gut 1999;45:613‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Debes JD, Ashhab A. Acute liver failure and dengue: alcohol matters. Am J Trop Med Hyg 2017;96:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michaut A, Moreau C, Robin MA, Fromenty B. Acetaminophen‐induced liver injury in obesity and nonalcoholic fatty liver disease. LiverInt 2014;34:e171‐e179. [DOI] [PubMed] [Google Scholar]
- 44. Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988‐1994. Am J Epidemiol 2013;178:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han BH, Moore AA, Sherman S, Keyes KM, Palamar JJ. Demographic trends of binge alcohol use and alcohol use disorders among older adults in the United States, 2005‐2014. Drug Alcohol Depend 2017;170:198‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deaciuc IV, Bagby GJ, Lang CH, Spitzer JJ. Hyaluronic acid uptake by the isolated, perfused rat liver: an index of hepatic sinusoidal endothelial cell function. Hepatology 1993;17:266‐272. [PubMed] [Google Scholar]
- 47. Ito K, Murotani K, Nakade Y, Inoue T, Nakao H, Sumida Y, et al. Serum Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein levels and liver fibrosis: a meta‐analysis. J Gastroenterol Hepatol 2017;32:1922‐1930. [DOI] [PubMed] [Google Scholar]
- 48. Bekki Y, Yoshizumi T, Shimoda S, Itoh S, Harimoto N, Ikegami T, et al. Hepatic stellate cells secreting WFA+ ‐M2BP: its role in biological interactions with Kupffer cells. J Gastroenterol Hepatol 2017;32:1387‐1393. Author names in bold designate shared co‐first authorship. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1233/full.


