Abstract
Despite that the availability of new therapeutic options has expanded the multiple sclerosis (MS) disease-modifying therapy arsenal, interferon β (IFN-β) remains an important therapy option in the current decision-making process. This review will summarize the present knowledge of IFN-β mechanism of action, the overall safety, and the short- and long-term efficacy of its use in relapsing remitting MS and clinically isolated syndromes. Data on secondary progressive MS is also provided, although no clear benefit was identified.
From the first report of intrathecal interferon (IFN) use by Jacobs et al. (1981) and the approval of IFN-β-1b (Betaseron) in 1993 (The IFNB Multiple Sclerosis Study Group 1993), IFN-β therapies became the first major therapeutic class of drugs developed for use in multiple sclerosis (MS) (Weinstock-Guttman 2013). Additional IFN-β-based products, including intramuscular (IM) IFN-β-1a (Avonex, Biogen), subcutaneous (SC) IFN-β-1a (Rebif, EMD Serono), and PEGylated IFN (PEG-IFN)-β-1a (Plegridy, Biogen) have expanded the repertoire of IFN-β Food and Drug Administration (FDA)-approved drugs. Even though IFNs only partially attenuate the relapse rate and slow the progression of the disease, they opened a new era of therapeutic interventions that has reshaped the natural history of the disease (Trojano et al. 2007; Cree et al. 2016). The complex and heterogeneous immunological milieu of the MS disease process may explain the partial effectiveness of IFN-β in a subset of patients. Therefore, a better understanding of the pathophysiology of the disease itself and of the IFN-β-mediated signaling pathways will ultimately lead to more effective and safer treatment decisions. This article summarizes the 25-year-old successful use of IFN-β treatments for MS. In particular, this article reviews the mechanism of action (MOA), clinical efficacy, safety profile, and adherence of IFN-β therapy in MS.
MECHANISM OF ACTION OF INTERFERON β IN MULTIPLE SCLEROSIS
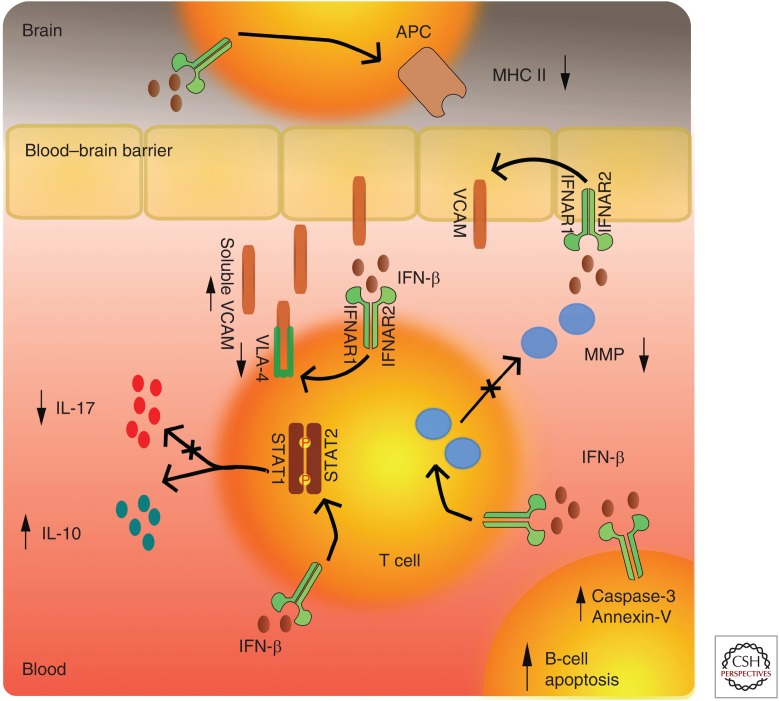
The initial interest in IFN as a potential therapeutic option in MS was motivated primarily by the known antiviral activities of IFNs. Subsequently, the immunomodulatory and antiproliferative properties of IFNs were discovered. The exact MOA of IFN-β therapies in MS is not fully understood. Their beneficial treatment effect is considered to be related to several overlapping mechanisms such as the down-regulation of the major histocompatibility complex (MHC) class II expression present on the antigen-presenting cells (i.e., dendritic cells, Langerhans cells, and B-cell lymphocytes), induction of T-cell production of interleukin 10 (IL-10), which shifts the balance toward the anti-inflammatory T helper (Th)-2 cells, and inhibition of T-cell migration as a result of blockade of metalloproteases and adhesion molecules (Dhib-Jalbut and Marks 2010) (a short synopsis of IFN MOA is shown in Fig. 1). These MOAs are mediated through transcriptional factors and subsequent gene regulation. The major route in which IFN-β produces its effect is by activating the Janus kinase (JAK)-signal transducers and activators of transcription (STAT) pathway. IFN-β binding to the type I IFN receptor causes phosphorylation of STAT1 and STAT2 and the formation of STAT1-STAT2 heterodimers, which translocate to the nucleus, bind the IFN-stimulated response element (ISRE), and modulate the expression of ISRE-regulated genes (Ransohoff 1998). The cellular response to IFNs is complex and results in changes in the expression of more than 500 genes representing ∼0.5% of the human genome (Weinstock-Guttman et al. 2003). More recently, up-regulation of mechanisms that require FAS-receptor/TACI (transmembrane activator and CAML interactor) signaling and production of apoptotic markers like Annexin-V and caspase-3 were shown as specific inducers of B-cell apoptosis. Because of the increasing evidence of the pivotal B-cell role in MS immunopathology, this IFN-β MOA becomes even more relevant (Rizzo et al. 2016).
Figure 1.
Molecular mechanisms of action of interferon β (IFN-β). Decrease of cellular expression of adhesion molecules (VLA-4) and cleavage of endothelial vascular cell adhesion protein (VCAM) results in decrease in cell sequestration through the blood–brain barrier. Additional decrease of matrix metalloproteinase (MMP) diminishes the ability for lymphocyte penetration. Activation of IFNAR1/2 receptors results in phosphorylation of signal transducers and activators of transcription (STAT)1-STAT2 factors that further results in secretion of anti-inflammatory cytokine profiles. Activation of IFNAR1/2 also results in decrease of major histocompatibility complex II (MHC II) expression and diminishes lymphocyte activation. IFN-β also increases apoptotic markers like Annexin-V and active caspase-3 via Fas-receptor/transmembrane activator and CAML interactor (TACI) signaling, resulting in specific depletion of memory B cells. IL, Interleukin.
There are two forms of recombinant IFN-β that are in use for treatment of MS. IFN-β-1b is produced in Escherichia coli, it is not glycosylated, and has subtle, but important amino acid differences from the naturally occurring IFN-β. These differences result in lower biological-specific activity, which requires larger doses and increases the risk of developing neutralizing antibodies. IFN-β-1b also shows strong and reversible binding to human serum albumin, which contributes to decrease the relative potency to 10% when compared to the equivalent IFN-β-1a formulation (Goodin 2005). IFN-β-1a is produced in the Chinese hamster ovary cell line and its amino acid sequence is identical to that of the native IFN.
CLINICAL EFFICACY OF INTERFERON-β IN RELAPSING REMITTING MULTIPLE SCLEROSIS (RRMS)
The IFN-β Multiple Sclerosis Study Group
The initial 2-year, double-blinded, placebo-controlled trial for IFN-β-1b that started enrollment in 1988 was the first study to report beneficial treatment effects in MS (The IFNB Multiple Sclerosis Study Group 1993). The study participants were randomized into placebo, low-dose IFN-β (1.6 million international units [MIU]), and high-dose IFN-β (8 MIU) administered subcutaneously every other day (QOD). The relapse rate was less in the 8 MIU IFN-β group compared to the 1.6 MIU group and placebo (0.84 vs. 1.17 vs. 1.27, respectively). The 3-year extension of this trial failed to show statistical significance, although it indicated a trend in decreasing disability compared to placebo (p = 0.161). Additionally, 52 patients enrolled at the British Columbia site underwent frequent magnetic resonance imaging (MRI) exams (every 6 weeks for 3 years) for MRI analysis (Paty and Li 1993). For the first time, MRI analysis was used as a surrogate measure for MS disease activity in a clinical trial supporting the clinical findings. The 8 MIU dose group had a median of 80% reduction in the number of active scans and a 23% reduction in the MRI-detected burden of the disease. The lesion load in the 8 MIU group decreased by 6.2%, whereas in the 1.6 MIU and the placebo group it increased by 1.1% and 17.1%, respectively. In the 16-year follow-up analysis of the original treatment groups, mortality rates significantly differed from 18.3% in the placebo group, 9.3% in the 1.6 MIU dose group, to the lowest 5.6% in the 8 MIU group (Ebers et al. 2009; Reder et al. 2010). Out of the 113 people that reached the expanded disability status scale (EDSS) of 6.0, 45.6% were originally assigned to placebo, 38.8% of those assigned to IFN-β-1b 1.6 MIU, and 45.8% in IFN-β-1b 8 MIU. However, this analysis did not consider death as part of the disability outcome, skewing the results against the treatment arm. Similarly, in the longest 21-year follow-up analysis, IFN-β-1b showed a 46.8% reduction in the long-term all-cause mortality when compared to placebo (Goodin et al. 2012a). During the 16-year follow-up, substantial technological changes in terms of MRI usage prevented direct comparison with the initial results. The new analysis showed that higher EDSS scores were associated with a higher T2 lesion load, T1 hypointense lesions (black holes), lower brain volumes, and smaller cervical cord volumes (Li et al. 2006). Additionally, the long-term assessment used multiple cognitive outcome measures like the symbol digit modality task (SDMT), paced auditory serial addition test (PASAT), and California verbal learning test II (CVLT-II), among others. Baseline EDSS scores, T2 lesion load, and premorbid IQ were calculated as independent predictors of poorer cognitive performance after the 16-year follow-up (Langdon et al. 2007). Even though both trial groups showed comparably stable neurocognitive performance, the placebo-treated patients had higher decline in both immediate and delayed verbal memory functioning when compared to the treatment group (p = 0.021 and p = 0.044, respectively) (Lacy et al. 2013).
Multiple Sclerosis Collaborative Research Group (MSCRG) Interferon β-1a
IFN-β-1a (Avonex) is an IM IFN-β-1a product that is administered once a week. This treatment was tested on 301 RRMS patients in a double-blind, placebo-controlled, randomized trial (Jacobs et al. 1996). In comparison to IFN-β-1b (Betaseron), the primary outcome of this trial was disability progression over the 104-week study period measured as a sustained increase of 1.0 EDSS unit from baseline and sustained for 6 months (confirmed disability progression [CDP]). The IFN-β-1a treatment group showed a 37% reduction in probability of CDP versus placebo. The study was discontinued early, as the primary outcome was already reached at 18 months (before all patients completed the 2-year follow-up). The annual relapse rate (ARR) in the treatment arm decreased to 0.67 when compared to 0.82 for the placebo patients (reduction of 18.3% and p = 0.04). Twofold more placebo patients had at least three exacerbations when compared to the IFN-β-1a-treated patients and the median time to initial exacerbation was delayed by 11.2 weeks. Even though the presence of gadolinium-enhancing lesions (GdELs) at study baseline strongly correlated with additional development of new lesions in both groups, a significant reduction in the number of new lesions (p = 0.006), enlarging lesions (p = 0.024), or a composite of both (p = 0.002) were seen in the IFN-β-1a-treated group when compared to placebo (Simon et al. 1998). With all subjects analyzed, the IFN-β-1a group had median T2 lesion volume increase of 628 mm3 versus 1410 mm3 seen in the placebo arm (not reaching significance, p = 0.22). However, the treatment effect was seen in patients with baseline GdEL+ at their baseline scan, indicating that the beneficial effect of IFN-β-1a is strongest in highly active inflammatory disease. Therefore, the placebo patients with a presence of MRI activity captured at baseline MRI scan had higher T2 lesion volume accumulation when compared to their IFN-β-1a GdEL+ counterparts (p = 0.022). Moreover, the treatment with IFN-β-1a resulted in a reduction of brain atrophy during the second year of the trial (mean % brain parenchymal fraction [BPF] change of −0.233 in the IFN-β-1a arm versus −0.521 in the placebo arm, p = 0.03) (Rudick et al. 1999). This MRI post hoc analysis revealed that brain atrophy progression worsened yearly without correlations with clinical manifestations. Unfortunately, the treatment effects seen at year 2 on preventing brain atrophy measured as a mean percent change in BPF was not significant at the 8-year follow-up (−2.5% vs. −3.0%, IFN-β-1a vs. initial placebo, respectively). Patients randomized into the treatment arm were significantly less likely to progress to EDSS of 4.0 or greater at the 8-year follow-up as compared to the initial placebo group. The sustained EDSS increase of 1.0 unit during the initial pivotal study has shown to be the strongest predictor of reaching clinically significant disability (EDSS of 4 and 6) after 8 years (Rudick et al. 2010). Other early predictors of limited response to IFN-β-1a therapy at year 8 were two or more clinical relapses during the study period and greater baseline T2 lesion volume. Furthermore, the Assessment of Drug Utilization, Early Treatment and Clinical Outcomes (ASSURANCE) 15-year long-term follow-up study showed that patients that remained on the IM IFN-β-1a had less disability and progression to meaningful EDSS milestones compared to placebo patients (Bermel et al. 2010). Breakthrough disease activity as measured with cumulative GdEL+ lesions found at year 1 and year 2 scans while on therapy strongly correlated with severe disability (the highest rate of GdEL+ was seen in the highest quartile group containing patients with Δ-EDSS of 4.50–8.50, p = 0.0047) after 15 years (Bermel et al. 2013).
Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis
As a result of the unresolved clinical benefits and dose dependency seen in the previously mentioned pivotal trials of IM and SC IFN-β-1b, an additional placebo-controlled SC IFN-β-1a given three times per week (TIW) (Rebif) with larger doses and more frequent treatment regimen was initiated. Prevention of relapses and disability by IFN-β-1a subcutaneously in MS (PRISMS) study recruited 560 RRMS patients and randomized them into three groups: 22 µg IFN-β-1a, 44 µg IFN-β-1 given TIW, and placebo (PRISMS Study Group 1998). The treatment groups showed a reduction of 27% and 33%, respectively, in ARR compared to placebo, as well as decrease in sustained disability progression (decrease of 22% and 30%, respectively, as compared to placebo). The MRI analysis showed a reduction in accumulation of T2 active lesion (67% and 78%, respectively, decrease as compared to placebo) that confirmed the effectiveness and the dose dependency of SC IFN-β-1a. The extension of this study (PRISMS-4) evaluated the long-term efficacy of SC IFN β-1a TIW (PRISMS Study Group and the University of British Columbia 2001). Although the initial placebo-assigned patients from the original PRISMS crossed over into one of the treatment arms, the efficacy measures remained favorable to the original IFN-β-1a 44 µg and IFN-β-1a 22 µg groups. This extension study, similar to the previous IFN extension studies, supported the importance of early treatment initiation, which will be more broadly discussed in the clinically isolated syndrome (CIS) section of this article. The prevention of disability conclusively shows the superiority of the 44 µg dose when compared to the IFN-β-1a 22 µg dose. Patients in the 44 µg dose displayed fewer confirmed EDSS changes over 4 years (0.17/patient/year) when compared to the crossover groups (0.24/patient/year) and to the 22 µg dose group (0.22/patient/year) (PRISMS Study Group and the University of British Columbia 2001). Three hundred eighty-two out of the original 560 enrolled patients continued into the long-term, open-label, follow-up study (Kappos et al. 2006b). When compared to the other groups, the original IFN-β-1a 44 µg group showed the longest time to reach confirmed progression and a clinically relevant progression EDSS of 4.0. Additionally, the medication effect that successfully decreased the inflammatory component translated into reduction of brain volume loss over the 8 years. The trend of better performance by the original IFN-β-1a 44 µg group was shown by the reduction in brain atrophy when compared to the low-dose 22 µg formulation. Additional post hoc analysis highlighted the long-term adherence as a factor associated with better clinical outcomes. The group labeled as “max dose, max time” had lower ARRs, lower secondary progressive MS (SPMS) conversion rates, and lower T2 burden of disease (Uitdehaag et al. 2011). A pilot placebo-controlled study of patients using 44 µg IFN-β-1a showed accelerated brain atrophy within the first 6 months, correlating with a decrease of T-cell expression and active inflammatory cytokine reduction (Dwyer et al. 2015). This supported the immunological basis of the pseudoatrophy seen after IFN treatment initiation (Dwyer et al. 2015). In the same study, increased magnetization transfer ratio (MTR) signal within the white matter suggested that the medication may provide active remyelination (Zivadinov et al. 2014).
Pegylated Interferon β-1a (ADVANCE Trial)
In the pivotal phase III trial of PEG-IFN-β-1a (Plegridy) 1512 patients were randomized into 1:1:1 ratio (placebo, PEG-IFN-β-1a 125 µm every 2 weeks, and PEG-IFN-β-1a 125 µm every 4 weeks, respectively) (Calabresi et al. 2014). At week 48, relapses were significantly lower in patients taking the active medication when compared to placebo. The ARR was 0.256 in the every-2-week group compared to 0.397 in the placebo group (p = 0.0007). Patients randomized to the 4-week interval dosing had an ARR of 0.288 (nonsignificant when compared to placebo, p = 0.0114). Additionally, multiple MRI-derived end points were statistically favoring the 2-week interval group. PEG-IFN-β-1a once every 2 weeks showed inflammatory suppression when compared to placebo as assessed by GdEL+ lesion number (−0.26 vs. 0.77), new T2 lesions (3.7 vs. 11.2), and T1 hypointense lesions (1.8 vs. 3.8). Although still significantly improved, the effect of PEG-IFN on the sustained disability progression was less apparent (0.62 hazard ratio [HR], p = 0.038). Advanced brain imaging like MTR also showed treatment improvement when compared to placebo, whereas whole brain atrophy failed to do so (supplementary data of Calabresi et al. 2014). Similarly to the PRISMS trial crossover design, after the initial first year of the trial, the placebo group was randomized into the active treatment arm, whereas the original PEG-IFN-β-1a groups remained on their preassigned doses (Kieseier et al. 2015). This extension study further provided evidence into the efficacy of PEG-IFN-β-1a, where patients originally assigned to the treatment arm outperformed those who were switched after year 1 (Kieseier et al. 2015). No evidence of disease activity (NEDA), a measure composite of no clinical relapse, disease progression, and MRI activity, was achieved in 33.9% of PEG-IFN-β-1a q2-week groups versus 15.1% and 21.5% for placebo and q4-week groups, respectively (Arnold et al. 2014). Patients continued participating in ATTAIN, a long-term, dose-frequency, blinded extension, which showed significant increase in NEDA over 3 years of treatment (30.5% vs. 19.8% 2-week vs. 4-week groups, respectively) (Arnold et al. 2015).
Dose Comparison Trials and IFN-β Outcomes
As the initial pivotal trials left certain doubts about the dose dependency and bioactivity of the different preparation, several randomized head-to-head trials were initiated. Both EVIDENCE (comparing SC IFN β-1a TIW vs. weekly IM IFN-β-1a) and INCOMIN (comparing SC IFN-β-1b QOD to IM IFN-β-1a) studies showed superiority of higher-dose and more frequent administration regiments (Durelli et al. 2002; Panitch et al. 2002). However, differences in efficacy seen in EVIDENCE only persisted in the first 24 weeks into the trial and was not maintained later on (Panitch et al. 2005). Similarly, INCOMIN trial used groups that were not well matched with regard to baseline clinical characteristics favoring the younger and healthier IFN-β-1b arm (Vartanian 2003). The inconsistency of the results was further complicated by negative results of several open-label dose-comparative studies (Clanet et al. 2002; Trojano et al. 2003; Minagar et al. 2008). The European IFN-β-1a comparison trial showed that there are neither clinical nor MRI differences between 30 µg and 60 µg IM doses. Even though it was not the primary purpose of this trial, the study provided invaluable information regarding the clinical efficacy as well. It showed that the nonlinear trajectory of brain atrophy seen in the first 4 months reflected the anti-edematous changes induced by the IFN treatment (Zivadinov et al. 2008). With an initial pretreatment relative annualized atrophy rate of −1.06%, baseline to month 4 rate of −1.49%, and −0.33% from month 4 to year 3 showed that IFN-β significantly reduces the atrophy rate, which corroborates the findings seen from initial Multiple Sclerosis Collaborative Research Group (MSCRG) trial (Hardmeier et al. 2005).
OBSERVATIONAL OPEN-LABEL IFN STUDIES
Assessing the slowly accumulating disability in MS can be a challenge, with an average 15-year gap in the natural history of progression from no disability to impaired walking (EDSS 6.0) (Weinshenker et al. 1989). In pooled analysis of 31 placebo-randomized trials, detection of clinical efficacy in short-term interventional trials by using the existing definitions of disease progression could not be validated (Ebers et al. 2008). In addition, considerable variations in disability and function within the first years of enrollment failed to translate into predicting future long-term disability (Goodin et al. 2012b). However, because of the high study costs and ethical concerns regarding placebo-controlled trials, they are conducted only up to 3 years (Table 1). Therefore, the most reliable long-term efficacy data for IFN-β comes from open-label extensions and observational studies both having unfortunately specific limitations (Freedman 2011; Signori et al. 2016). Real-world studies provide data on the effectiveness among different IFN-β formulations, while controlling for factors like comorbidities, other medication use, and adherence, which may play a substantial role in the varying disease outcomes. Meta-analysis on observational IFN-β-based studies included 32,026 patients and showed that there are no differences among the different IFN-β treatment effects (Einarson et al. 2017).
Table 1.
Clinical and magnetic resonance imaging (MRI) outcomes for the four pivotal relapse-remitting multiple sclerosis trials
| Clinical and MRI outcomes | Subgroups | Study name | ||||||
|---|---|---|---|---|---|---|---|---|
| IFN-β study group | MSCRGa | PRISMS | ADVANCEb | |||||
| 1.6 MIU | 8 MIU | 30 µg | 22 µg | 44 µg | q4 wk | q2 wk | ||
| ARR | IFN-β | 1.04 | 0.85 | 0.61 | 1.82 | 1.73 | 0.288 | 0.256 |
| Placebo | 1.18 | 0.90 | 2.56 | 0.397 | ||||
| p-value | 0.030c | 0.002 | <0.005c | 0.0007d | ||||
| Proportion of patients with disease progression | IFN-β | 47% | 35% | 21.20% | 18.5/23%e | 21.3/31%e | 6.05% | 6.05% |
| Placebo | 46% | 33.30% | 11.9e | 10% | ||||
| p-value | 0.096 | 0.02 | <0.05e | 0.038 | ||||
| GdEL+ lesion number | IFN-β | 1.8 ± 0.4 | 2.0 ± 0.7 | 0.8 ± 0.22 | 1.4 (0–6.7) | 1.3 (0–4) | 0.9 ± 0.15 | 0.2 ± 0.05 |
| Placebo | 4.9 ± 1.3 | 1.65 ± 0.48 | 8.0 (2.7–27) | 1.4 ± 0.17 | ||||
| p-value | 0.0089 | 0.05 | <0.0001 | <0.0001 | ||||
| T2-lesion volume change in % | IFN-β | 10.50% | −0.10% | 6.50% | −1.20% | −3.80% | 7.3 (35%)f | 3.7 (67%)f |
| Placebo | 20.00% | 13.20% | 10.90% | 11.2f | ||||
| p-value | <0.001 | 0.36 | <0.0001 | <0.0001f | ||||
| Whole brain volume change | IFN-β | N/A | N/A | −0.233 ± 0.74 | N/A | N/A | −0.67 ± 0.83 | −0.72 ± 0.75 |
| Placebo | N/A | −0.521 ± 0.8 | N/A | −0.62 ± 0.89 | ||||
| p-value | N/A | 0.03g | N/A | 0.084 | ||||
Interferon β (IFN-β) study group—Betaseron pivotal trial; MSCRG—Multiple Sclerosis Collaborative Research Group pivotal trial for Avonex; PRISMS—Prevention of Relapses and Disability by IFN-β-1a Subcutaneously in Multiple Sclerosis pivotal trial for Rebif; ADVANCE—pegylated interferon (PEG-IFN)-β-1a for relapsing remitting multiple sclerosis pivotal trial for Plegridy. Alpha level of 0.05 was considered as significant and is shown in bold.
MIU, Million international units; q4 wk, every 4 weeks; q2 wk, every 2 weeks; ARR, annualized relapse rate; GdEL, gadolinium-enhancing lesion; N/A, not available.
a104 weeks study period.
bResults at 48 weeks follow-up.
cRelapse rate.
dBased on negative binomial regression; adjusted for baseline expanded disability status scale (EDSS) (<4 vs. ≥4), baseline relapse rate, and age (<40 vs. ≥40).
eTime in months for EDSS progression <1/reduction in disease progression.
fAdjusted mean number of lesions (percentage reduction vs. placebo).
gMean % brain parenchyma fraction change post hoc analysis of Avonex trial.
Long-term follow-up data enabled the identification of early clinical and MRI-derived IFN-β efficacy markers, which led to the development of scores that can predict treatment outcomes. For example, the measurement of MRI-derived disease activity just after 1 year of IFN-β initiation was able to identify patients that will continue to accumulate disability over the following year (Rio et al. 2008). Additionally, an analysis within the large MRI in MS (MAGNISMS) network database showed that while on IFN-β therapy, substantial MRI activity (≥3 new T2 lesions) in the presence of at least one relapse is associated with a high risk of disability worsening over the following 2–3 years (Sormani et al. 2016). This can help clinicians in detecting short-term treatment failure and influence their decision on switching IFN-β as their first-line therapy. In an independent analysis of the PRISMS trial, the combination of 1-year MRI lesion activity and occurrence of relapses explained 100% of the treatment effect on disability progression over 2 years (Sormani et al. 2011). After adjusting for these scores, the differences between the treatment arms disappeared and no residual effect of IFN on EDSS was observed. Although persistent clinical activity during the early IFN-β treatment remained associated with poor long-term prognosis, some MRI activity failed to translate in long-term EDSS change (Rio et al. 2017). The emergence of new disease-modifying therapies (DMTs) offers clinicians new therapeutic options; however, they do not lack toxicity and are not risk-free treatments. Therefore, there is growing emphasis on the need for developing tools that will better capture patients at risk for long-term disability. Composite measure of clinical disability progression, relapses, and MRI activity termed NEDA or routine brain atrophy measurement may help contribute to the understanding of the unpredictability of long-term IFN-β treatment outcomes (Zivadinov et al. 2016; Uher et al. 2017).
BIOMARKERS FOR ASSESSMENT OF IFN CLINICAL EFFICACY
The effects of IFN-β activity can be assessed by measuring the up-regulation of IFN-β-induced messenger RNAs (mRNAs) such as human myxovirus resistance protein I (MxA), IL-10, β2-microglobulin, and tumor necrosis-related apoptosis inducing ligand (TRAIL) via the activation of the JAK-STAT pathway (Weinstock-Guttman et al. 2008). However, the timing of blood draw is a critical factor because the peak of MxA mRNA in the plasma occurs 6–12 h after the IFN-β injection (Pachner et al. 2005). Furthermore, the lack of significance seen between MxA expression and with the clinical and MRI phenotypes limited their clinical use and meaningfulness (Weinstock-Guttman et al. 2007). IFN-driven up-regulation of genes involved in the sterol synthesis pathways enabled use of differential cholesterol profiles as a potential biomarker for treatment efficacy. Early decreases in cholesterol markers, mainly high-density lipoprotein (HDL) cholesterol, were shown to be associated with short- to midterm brain atrophy outcomes (Uher et al. 2016). Similarly, the Dicer protein, which cleaves the double-stranded RNA and pre-microRNA, has been shown to be a potential marker of IFN-β response (Magner et al. 2016). Along with these examples, cluster analysis on levels of monocyte-derived IFNAR1, IFNAR2, and pSTAT1-2 were able to determine the phenotype associated with good IFN-β response (Hurtado-Guerrero et al. 2017). In a comparative study of three IFN-β formulations, IFN-β-1b showed higher bioactivity as measured by levels of MxA and vascular cell adhesion molecules when compared to the two IFN-β-1a preparations (Deisenhammer et al. 2000). One of the possible reasons for the heterogeneity of IFN-β activity is the heightened expression of circulating “interferon-inhibitory activity” (IIA) and free-soluble IFN-α/β receptors (sIFNRs) (Chadha et al. 2006). Elevated levels of IIA in patients resulted in partial response to the IFN treatment and were significantly linked with the T2 lesion volume. Although multiple biomarkers have tried to effectively measure the treatment response to IFN-β, not one has yet been identified as clinically meaningful.
Antibody formation as immunogenic response toward molecules with any genetically modified properties is expected. However, the mechanism of IFN-β antibody occurrence is not because of the classical immunological immune induction, but rather the slow and continuous breakdown of immunogenic tolerance (Schellekens 2002). Based on the antibody ability to inhibit the effect of IFN-β, both common binding (BAb) and rare neutralizing (NAb) antibodies have been characterized (Bendtzen 2003). The current consensus is that BAbs are used for initial screening, whereas NAbs are confirmatory tests of IFN-inhibiting antibodies (Sorensen et al. 2005a). Furthermore, BAbs titer larger than 1:2400 had 98.5% specificity and 74.7% sensitivity in predicting NAbs formation and strengthen the notion of use of BAbs as an antibody-screening tool (Hegen et al. 2014). The development of NAbs toward IFN-β largely depends on the specific preparation in terms of differences in molecular structure, application site, dosage, and frequency. The direct IFN-β comparison trial (EVIDENCE) showed substantially lower immunogenicity of IM IFN-β-1a over SC IFN-β-1a (2% vs. 25% in ≥20 neutralizing units/mL, respectively). Similarly, an open-label comparison corroborated the results showing 2-year NAb positivity in 42% patients in the 22 µg Rebif group, 34% for Betaferon 8 MIU, and finally, only 8% for 30 µg Avonex-treated patients (Sorensen et al. 2003). This immunogenicity difference was also confirmed in a large dataset of 42,555 samples from six European countries. Moreover, an observed prescribing switch toward the less immunogenic preparation of IM IFN-β-1a was noted (Link et al. 2017). Several studies have shown that after prolonged use of, especially, IFN-β-1b, there is noted reduction in the antibody presence. It has been hypothesized that disappearance of an immune reaction toward the medication could be because of reconstitution of the immune tolerance or epitope maturation (Sorensen et al. 2005b). Additionally, it has been shown that the persistency of the antibodies is a multifactorial process determined by the antibody titer, affinity maturation, and IgG subclass switching. Patients with transient appearance of IFN-β showed predominantly IgG1 and IgG3 subclass, whereas, the predominance of IgG2 and IgG4 contributed toward their persistence (Dujmovic et al. 2017). The inhibiting effects of NAbs has been shown in both original pivotal trials of Betaseron and Rebif, where NAbs attenuated the treatment effect, which returned the relapse rate, and the MRI response back to placebo levels (The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group 1996). In the PRISMS trial, it was shown that NAb+ patients had significantly higher ARR when compared to NAb patients (0.85 vs. 0.52, p < 0.05) (Francis et al. 2005). In addition to the effect seen on increasing relapse rates and MRI-derived activity, IFN-β NAb also directly correlated to the quantification of the biological response as measured by MxA assays (Santos et al. 2006). Both European Federation of Neurological Societies (EFNS) (see Table 3) and the Neutralizing Antibodies on Interferon β in Multiple Sclerosis (NABINMS) consortium have published guidelines and recommendations regarding the clinical use of IFN-β NAbs (Sorensen et al. 2005a; Polman et al. 2010). In their conclusion, apart from deducting clinical and practical NAbs cutoff titers, a consideration of switching patients with sustained high NAb+ titer and/or lack in MxA bioactivity to non-IFN-β therapy was suggested. The presence of NAbs remains the only biomarkers known to be associated with decrease in clinical benefit.
Table 3.
Guidelines on the use of anti-interferon β (IFN-β) antibody testing in multiple sclerosis
| Recommendations by the European Federation of Neurological Societies (EFNS) | Level of recommendation |
|---|---|
| BAb assays can be used for screening of IFN-β antibodies | Level A |
| NAb testing should be performed in specialized laboratories | Level A |
| Nab should be performed with validated CPE or MxA assays using serial dilution test | Level A |
| NAb should be tested within first 24 months of therapy | Level A |
| NAb measurement is not needed in NAb patients after 24 months of IFN therapy | Level B |
| Patients with NAb should be retested every 3–6 months | Level A |
| Therapy should be discontinued in patients with high titer NAbs and retested in 3–6 months | Level A |
Data modified from Sorensen et al. (2005a).
BAb, Binding antibodies; Nab, neutralizing antibodies; CPE, cytopathic effect; MxA, myxovirus resistance protein 1.
CLINICAL EFFICACY OF INTERFERON β IN CLINICALLY ISOLATED SYNDROME
CIS is a disease phenotype with first clinical neurological presentation and features that resemble inflammatory MS demyelination; however, it does not fully fulfill the McDonald criteria for dissemination in time. Based on three positive CIS trials, IFN-β today is routinely prescribed for this subset of patients. All studies (Betaferon/Betaseron in Newly Emerging MS for Initial Treatment [BENEFIT], Controlled High-Risk Subjects Avonex Multiple Sclerosis Prevention Study [CHAMPS], and Early Treatment of Multiple Sclerosis Study [ETOMS]; Table 2) testify to the importance of early initiation of therapy, even before the official diagnosis of clinically defined MS (CDMS). With the early IFN-β use, the rate of CDMS conversion has been reduced by 44%–50% when compared to placebo after long-term follow-up periods (Freedman et al. 2014).
Table 2.
Major interferon β (IFN-β) trials in clinically isolated syndromes
| CDMS | p-value | ||||
|---|---|---|---|---|---|
| Study | Therapy | Time from symptom onset | IFN-β | Placebo | |
| CHAMPS | IM IFN-β-1a | ≤1 month | 20% | 38% | <0.001 |
| ETOMS | SC IFN-β-1a | ≤3 months | 34% | 45% | 0.047 |
| BENEFIT | SC IFN-β-1b | ≤2 months | 28% | 45% | <0.001 |
| REFLEX | SC IFN-β-1a | ≤2 months | 21%/22%a | 38% | <0.001/0.002a |
Data modified from Freedman et al. (2014).
Clinically defined multiple sclerosis (CDMS) at 2 years follow-up (defined as two attacks with clinical evidence of two separate lesions, with at least one of the lesions producing objective central nervous system [CNS] dysfunction and with paraclinical evidence of demyelinating lesions by brain magnetic resonance imaging [MRI] examination).
IM, Intramuscular; SC, subcutaneous.
aREFLEX study had two treatment arms, IFN-β-1a 44 µg three times a week and IFN-β-1a 44 µg once weekly.
Controlled High-Risk Subjects Avonex Multiple Sclerosis Prevention Study
The first randomized placebo-controlled trial (CHAMPS) recruited 383 patients who had their first demyelinating event (Jacobs et al. 2000). After the initial corticosteroid therapy, they were randomized into two groups of IM IFN-β-1a and IM injections of placebo. At the 3-year follow-up, the cumulative probability of transition into CDMS was reduced by half when compared to placebo (rate ratio of 0.56, 95% CI 0.38–0.81). The IFN-β-1a group had a transition probability of 35%, whereas the placebo had a 50% chance of converting into CDMS. The beneficial effects were seen on the MRI analysis as well. The IFN-β-1a group had 42% fewer GdEL+ lesions at 6 months, 55% fewer at 12 months, and 67% fewer at 18 months. In addition, the IFN-β-1a group had a median increase of only 1% of lesion volume from baseline to 18 months when compared to a 16% increase seen in the placebo group. The trial was stopped after preplanned interim analysis and patients from the placebo arm were switched to IFN-β-1a. The median time of the late initiation of IFN-β-1a was 29 months after the CHAMPS placebo randomization. The immediate therapy group had lower probability of developing CDMS at 5-year follow-up when compared to the delayed therapy group (36% vs. 49%, HR of 0.65) (Kinkel et al. 2006). Similarly, at the 10-year follow-up, the immediate group had a lower CDMS conversion rate (HR of 0.65); however, early IFN-β-1a initiation did not improve disability measures when compared to late initiators (Kinkel et al. 2012). This highlighted the progressive nature of the later stages of the disease, despite the reduced relapse rates over 10 years of treatment. In the MRI post hoc analysis, 86% of CIS patients that had low T2 burden (two to eight lesions) at baseline showed additional activity, had fewer new brain lesions, lower brain volume loss, and less disability accumulation compared to the “high” T2 burden group (Simon et al. 2015).
Early Treatment of Multiple Sclerosis Study
In a similar study, patients after their initial demyelinating attack were randomized into 22 µg IFN-β-1b or placebo every week and were followed for 2 years (Comi et al. 1995). The follow-up analysis showed that 34% of 22 µg IFN-β-1b and 45% of placebo patients converted to CDMS (odds ratio [OR] 0.61, 95% CI 0.37–0.99) (Comi et al. 2001). Additionally, the second relapse was substantially delayed in the IFN-β-1b group (569 days for IFN-β-1b vs. 252 days for placebo, p = 0.023). Even though the treatment group had significantly more GdEL+ lesions at baseline, the follow-up MRI data showed fewer new T2 lesions compared to placebo (p < 0.001). Several explanations can be used to understand the lower efficacy seen in ETOMS when compared to CHAMPS. One explanation can be that to lessen the attack, mandatory high dose steroid was used in the CHAMPS study, whereas only 70% of CIS patients in ETOMS did receive variable doses of steroids (Beck et al. 1992). In addition, the long interval and the low dose of the weekly 22 µg IFN-β-1b used were probably subtherapeutic and not sufficient to create satisfactory disease suppression. Finally, the ETOMS study recruited CIS patients that had multifocal symptoms, whereas CHAMPS had only patients with a unifocal attack.
Because of the mentioned low-dose formulation used in ETOMS, an additional Rebif Flexible Dosing in Early MS (REFLEX) study was initiated (Comi et al. 2012). The patients were randomized into three groups of IFN-β-1b 44 µg TIW, IFN-β-1b once a week, and placebo. Both treatment regiments showed a 2-year cumulative lowering the probability for establishing the MS McDonald criteria and CDMS when compared to placebo, with rates of conversion of 20.6% versus 21.6% and 37.5% (IFN-β-1b 44 µg TIW, once a week, and placebo, respectively). Both treatment arms showed reduction of unique active lesions (81% for TIW and 63% once a week when compared to placebo) (De Stefano et al. 2014). REFLEXION, an open-label extension study of REFLEX, has already published its 3- and 5-year results (Comi et al. 2017). Patients that did not reach CDMS continued with their initial randomization regimen, whereas patients on placebo and CDMS patients switched to the IFN-β-1b 44 µg TIW protocol. Two major conclusions were reached from the extension study. Over the period of 5 years, early initiation of IFN-β-1b (within 2 months of the focal neurological attack) significantly delayed the time of conversion to CDMS up to 2 years. Second, based on MRI outcomes, the IFN-β-1b 44 µg TIW regimen did outperform the weekly dosage.
IFN-β-lb (Betaferon/Betaseron) in Newly Emerging MS for Initial Treatment
The IFN-β-lb (Betaferon/Betaseron) in Newly Emerging MS for Initial Treatment (BENEFIT) trial randomized 487 patients into a 2:1 ratio of IFN-β-1b 8 MIU or placebo accordingly (Kappos et al. 2006a). The study confirmed the two previous CIS trials showed its effectiveness as early as a few months into the trial. At the end of the 2-year scheduled period, the probability of not developing McDonald MS criteria was twice as high in the IFN-β-1b group (31%) when compared to placebo. Also, when CDMS criteria was used, only 28% of IFN-β-1b-treated CIS patients had a second neurological attack, whereas 45% of the placebo patients did. The risk reduction calculated through HR favored IFN-β-1b versus placebo, with a 50% decrease in probability (HR 0.50, 95% CI 0.36–0.70). Interestingly, when the Barkhof criteria for dissemination in space at baseline was used, the 3-year predictive value of conversion to CDMS was not affected by treatment intervention (Moraal et al. 2009). The patients enrolled in this trial were extensively followed in a longitudinal manner, which resulted in cross-sectional summaries at 3 years (Kappos et al. 2007), 5 years (Kappos et al. 2009), 8 years (Edan et al. 2014), and 11 years of follow-up (Kappos et al. 2016). All of them provide conclusive data that although the delay in treatment of only 2 years was relatively short, the early use of IFN-β-1b prolongs the time to reach CDMS. Even 11 years after the trial entry, the IFN-β-1b group had 33% reduced risk of conversion into CDMS. In terms of neuropsychological measures, the early treatment arm had better PASAT-3 scores when compared to the later switched placebo group (p = 0.007). Having long-term data, this study also reported important patient-related outcome measures like employment, ability to care for themselves, and new hospitalizations. Both groups had similar resource utilizations with a high rate of employment (73.4% when compared to 81.3% at baseline), 97.5% of the patients were still living independently, and 91.4% were not hospitalized because of MS-related reasons (Kappos et al. 2016).
CLINICAL EFFICACY OF INTERFERON β IN PROGRESSIVE MULTIPLE SCLEROSIS
In most cases, after a certain disease course of acute relapses followed by complete or partial recovery, patients continue to evolve into a phase of gradual progression called SPMS. Moreover, the greater extent of brain volume loss, formation of T1-hypointensities, and greater T2 lesion load display already developed pathology, which may prove as a potential limiting factor in IFN-β use. Even though SPMS features clinical and MRI differences when compared to RRMS, the immunological processes are mostly overlapping. Therefore, these similarities provided rationale for consideration of IFN trials for the SPMS population. The partially understood pathophysiology of the secondary progressive course and issues with the responsiveness of the available measurement scales contributed to discrepancies within the SPMS phase III trials. The initial positive results coming from the European Study Group on Interferon-β-1b in SPMS (EUSPMS) were not confirmed by later trials. Several meta-analyses showed the inability to prevent further disability progression, despite the treatment-related reduced risk of relapses (La Mantia et al. 2012). With recent new developments in the MS therapeutic field, further large IFN-β trials will most probably not take place.
European IFN-β Study in Secondary Progressive Multiple Sclerosis
The EUSPMS trial randomized 718 patients into eight MIU IFN-β-1b or placebo groups with 1:1 ratio (European Study Group on Interferon-β-1b in Secondary Progressive MS 1998). The eligibility for the study included baseline EDSS between 3.0 and 6.5, clinical progression defined as clinical deterioration (EDSS increase of ≥1.0 point for baseline EDSS of 3.0–6.0 or increase of ≥0.5 over baseline EDSS of 6.0–6.5) independent of relapses, and a record of two or more relapses with an increase of at least 1.0 in the EDSS within the last 2 years. The primary outcome of the trial was met, where the time to reach CDP was significantly delayed in favor of IFN-β-1b (p = 0.0008). Furthermore, 38.9% patients taking IFN-β-1b and 49.8% of placebo reached CDP (European Study Group on Interferon-β-1b in Secondary Progressive MS 1998). The treatment arm also showed significant reduction in the total T2 lesion volume (p < 0.0001). The sustained effect of IFN-β-1b on the inflammatory disease was shown by the differences in the longitudinal total lesion volume. Placebo-assigned patients had year-to-year lesion volume accumulation with mean increase of 16% by year 3 from baseline. On the other hand, the treatment arm showed reduction of 4% by year 1, 5% by year 2, and, finally, 2% reduction at year 3 (Miller et al. 1999). Additional analysis examining brain atrophy and T1 hypointense lesion load were acquired to assess neurodegenerative processes presumed to be seen in SPMS patients (Barkhof et al. 2001). The IFN-β-1b treated group showed a decrease in the black-hole volumes by 7.7% per year, whereas the placebo-treated patients accumulated additional black-hole lesion volume by 14% per year. This treatment significance was not seen in terms of reducing the brain atrophy loss. IFN-β patients had a mean 2.9% loss when compared to 3.9% in placebo (p = 0.34) (Molyneux et al. 2000). Further post hoc analysis concluded that 30% more IFN-β-1b patients remained both disability progression and relapse free. Additionally, patients that had substantially more active disease before entering the study, benefited with noticeably larger treatment effect (Kappos et al. 2001). Despite the initial short-term effect, the 10-year follow-up reevaluation of the patient cohort did not provide confirmation of favorable IFN-β outcomes (Kuhle et al. 2016).
North American IFN-β Study in Secondary Progressive Multiple Sclerosis
Similar to the EUSPMS study, an additional 3-year multicenter IFN-β-1b trial took place in 35 centers in both the United States and Canada (Panitch et al. 2004). Using inclusion criteria that matched the European predecessor, it recruited 939 SPMS patients that were randomized into placebo, IFN-β-1b 8 MIU (250 µg) or 5 MIU/m2 (160 µg/m2). Results did not show a dose-dependent effect; however, the group randomized to 5 MIU/m2 equalized to the mean dose of 9.6 MIU, which was not considerably different from the established 8 MIU. Even though the treatment arm had a significant decrease in ARRs, the primary outcome of time to CDP was not reached. Contrary to the EUSPMS that was terminated early because of futility, the NASPMS-planned interim analysis showed ineffectiveness and was concluded early as a negative trial. Because of the conflicting results, a pooled analysis of both trials was conducted (Kappos et al. 2004). The conclusion from this comparative investigation showed that the EUSPMS trial recruited younger patients with lower average disease duration, higher relapse rates, and higher inflammatory MRI activity, characteristics that still support an early inflammatory stage of the disease rather than the advanced progressive stage characterized by a different underlying pathobiology (Weiner 2009).
SPECTRIMS AND IMPACT TRIALS
Two additional SPMS trials were conducted in which SC IFN-β-1b (SPECTRIMS) and IM IFN-β-1a (IMPACT) were used (Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-β-1a in MSSG 2001; Cohen et al. 2002). The SPECTRIMS trial used the high 44 µg IFN-β-1b dose and showed lack of efficacy in prolonging the time to CDP, whereas the IMPACT study used high-dose 60 µg IFN-β-1a evaluated with the MS functional composite (MSFC) score that included a timed 25-foot walk (T25FW) test, 9-hole peg test (9HPT), and PASAT as primary outcomes (Cohen et al. 2001). Because of the difficulties in detecting meaningful change in the later stages of the disease using EDSS, this trial was the first to use MSFC as the primary outcome measure. As the study showed significant 40.4% reduction in MSFC z-scores between treatment groups (p = 0.033), EDSS failed to observe this benefit. In further composite deconstruction, the skewed noticeable benefit was driven by the 9HPT (p = 0.024) and PASAT (p = 0.061) versus the nonsignificant T25FW (p = 0.378). A common pattern of ARR reduction (p = 0.008), T2-hyperintense lesion, and GdEL+ lesion reduction (both p < 0.001) was detected. One of the explanations of this discrepancy is that the temporal order of different function deterioration is not simultaneous. As walking ability starts to worsen early in the secondary progressive stage, potentially higher disease burden would be needed to produce worsening of the hand and cognitive function (Giovannoni et al. 2017). In addition, the noticeable worsening in T25FW in both groups over 2 years failed to respond to IFN-β treatment.
COMPARATIVE HEAD-TO-HEAD STUDIES
Because of their established efficacy in reducing MS inflammation, IFN-β formulations played the role as an active comparator in many new pivotal trials. The additional data from the IM IFN-β-1a use in the fingolimod trial (Cohen et al. 2010) and from the SC IFN-β-1a TIW use in both the alemtuzumab and ocrelizumab trials (Cohen et al. 2012; Hauser et al. 2016) further testify to the beneficial effect of IFN-β in the management of MS. Even though in terms of efficacy the newly approved DMTs outperformed the β IFNs, and a higher rate of adverse effects was noted. Until further long-term safety data regarding the second-line DMTs emerges, the desirable risk–benefit ratio of IFN-β remains clinically relevant.
SAFETY AND ADHERENCE TO TREATMENT
Although all MS therapeutics carry inherited risk of potential adverse effects, IFN-β is usually well tolerated (Reder et al. 2014). Most common IFN-β reported side effects are “flu-like” symptoms of fever, chills and headache, injection-site reactions, myalgia, depression, and increase of liver enzymes. Most of these effects are a result of the transient increases in cytokines like IL-6 and tumor necrosis factor α (TNF-α); therefore, the symptoms appear a few hours after the administration and dissipate after 12 to 24 h (Langer-Gould et al. 2004). However, because of persistent “flu-like” side effects, a small number of patients (20%) do require continuous use of complementary anti-inflammatory therapy after the initial 2–3 months of treatment. The differences in application and formulation cause some discrepancies in site reactions reported. For example, in a direct comparison of both medications (EVIDENCE trial), the more frequent and higher dose of SC 44 µg IFN-β-1b noted 83% site reactions versus only 28% in IFN-β-1a (Panitch et al. 2002). All IFN-based medications have some intrinsic associated risk with hepatotoxicity and increase liver aminotransferases (Chan et al. 2011). In a retrospective analysis of the three IFN products, there were elevations of AST and ALT in IFN-β patients when compared to placebo (p < 0.005). The pattern of liver enzyme changes varied among formulations, where de novo abnormalities were seen in SC IFN-β-1a and SC IFN-β-1b, but not in IM IFN-β-1a. In addition, the temporal peak ranged within the first 6 months in SC IFN-β-1a and SC IFN-β-1b and between 6 and 12 months for IM IFN-β-1a (Tremlett et al. 2004). This increase of liver markers usually is asymptomatic and transient; however, threefold or greater increase calls for dose reduction or discontinuation of the medication. Therefore, heightened vigilance in terms of liver function monitoring, especially in the first year of treatment initiation is advised (Francis et al. 2003).
As MS is affecting women at their childbearing age, the choice of a safe DMT is crucial. Even though all DMTs currently used in MS do carry potential risk to pregnancy outcomes, recent studies indicate that IFN-β can be continued during the first trimester of the childbearing period (Thiel et al. 2016). Exposure of IFN-β within the first 4 weeks was shown not to be associated with increased risk of spontaneous abortion and significant fetal complications (Amato et al. 2010). Combined with the increased risk of disease breakthrough occurring immediately postpartum and the pregnancy-related acceleration of SPMS transition, personalized decision-making of IFN-β therapy continuation will be needed until conception is confirmed (Pozzilli et al. 2015; Alroughani et al. 2016).
Both adherence (percentage of doses taken as prescribed over a set period) and persistence (total number of days taking the medication) are crucial in acquiring better clinical outcomes. In a large retrospective analysis of 2446 patients, the adherent groups showed a decrease in clinical relapses, MS-related hospitalizations, and medical costs (Tan et al. 2011). Based on the data from all prospective studies, the adherence in different IFN-β agents ranged from 83.9% in IM IFN-β-1a to 72.0% in SC IFN-β-1a and to 64.7% in IFN-β-1b (Menzin et al. 2013). Addressing factors like adverse events, ability to self-inject, treatment complexity, and cognitive barriers will potentially increase the adherence and benefit for the MS-prescribed therapies (Mohr et al. 2001; Devonshire et al. 2011).
CONCLUSION
Numerous long-term data testify for the significant contribution of IFN-β in improving the care of MS patients. IFN-β still holds an important role in the treatment options for RRMS, CIS, and in SPMS patients that still experience highly active disease. Even though IFN-β produces only partial long-term responsiveness when compared to the newly developed DMTs, the well-known risk–benefit ratio offers certain reassurance in its everyday use. Better understanding of the drug MOA and developing specific IFN-β activity biomarkers will allow further stratification of patient responders.
Footnotes
Editors: Howard L. Weiner and Vijay K. Kuchroo
Additional Perspectives on Multiple Sclerosis available at www.perspectivesinmedicine.org
REFERENCES
- Alroughani R, Altintas A, Al Jumah M, Sahraian M, Alsharoqi I, AlTahan A, Daif A, Dahdaleh M, Deleu D, Fernandez O, et al. 2016. Pregnancy and the use of disease-modifying therapies in patients with multiple sclerosis: Benefits versus risks. Mult Scler Int 2016: 1034912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato MP, Portaccio E, Ghezzi A, Hakiki B, Zipoli V, Martinelli V, Moiola L, Patti F, La Mantia L, Mancardi GL, et al. 2010. Pregnancy and fetal outcomes after interferon-β exposure in multiple sclerosis. Neurology 75: 1794–1802. [DOI] [PubMed] [Google Scholar]
- Arnold DL, Calabresi PA, Kieseier BC, Sheikh SI, Deykin A, Zhu Y, Liu S, You X, Sperling B, Hung S. 2014. Effect of peginterferon β-1a on MRI measures and achieving no evidence of disease activity: Results from a randomized controlled trial in relapsing-remitting multiple sclerosis. BMC Neurol 14: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D, You X, Shang S, Sperling B, Evilevitch V. 2015. Long-term efficacy in MRI and no evidence of disease activity outcomes in patients with relapsing-remitting multiple sclerosis treated with peginterferon β-1a. Neurology 84 (Suppl P7.266). [Google Scholar]
- Barkhof F, van Waesberghe JH, Filippi M, Yousry T, Miller DH, Hahn D, Thompson AJ, Kappos L, Brex P, Pozzilli C, et al. 2001. T1 hypointense lesions in secondary progressive multiple sclerosis: Effect of interferon β-1b treatment. Brain 124: 1396–1402. [DOI] [PubMed] [Google Scholar]
- Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR, et al. 1992. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 326: 581–588. [DOI] [PubMed] [Google Scholar]
- Bendtzen K. 2003. Anti-IFN BAb and NAb antibodies: A minireview. Neurology 61: S6–S10. [DOI] [PubMed] [Google Scholar]
- Bermel RA, Weinstock-Guttman B, Bourdette D, Foulds P, You X, Rudick RA. 2010. Intramuscular interferon β-1a therapy in patients with relapsing-remitting multiple sclerosis: A 15-year follow-up study. Mult Scler 16: 588–596. [DOI] [PubMed] [Google Scholar]
- Bermel RA, You X, Foulds P, Hyde R, Simon JH, Fisher E, Rudick RA. 2013. Predictors of long-term outcome in multiple sclerosis patients treated with interferon β. Ann Neurol 73: 95–103. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, Liu S, Zhu Y, Seddighzadeh A, Hung S, et al. 2014. Pegylated interferon β-1a for relapsing-remitting multiple sclerosis (ADVANCE): A randomised, phase 3, double-blind study. Lancet Neurol 13: 657–665. [DOI] [PubMed] [Google Scholar]
- Chadha K, Weinstock-Guttman B, Zivadinov R, Bhasi K, Muhitch J, Feichter J, Tamano-Blanco M, Abdelrahman N, Ambrus J Sr, Munschauer F, et al. 2006. Interferon inhibitory activity in patients with multiple sclerosis. Arch Neurol 63: 1579–1584. [DOI] [PubMed] [Google Scholar]
- Chan S, Kingwell E, Oger J, Yoshida E, Tremlett H. 2011. High-dose frequency β -interferons increase the risk of liver test abnormalities in multiple sclerosis: A longitudinal study. Mult Scler 17: 361–367. [DOI] [PubMed] [Google Scholar]
- Clanet M, Radue EW, Kappos L, Hartung HP, Hohlfeld R, Sandberg-Wollheim M, Kooijmans-Coutinho MF, Tsao EC, Sandrock AW; European IFNβ-1a (Avonex) Dose-Comparison Study Investigators. 2002. A randomized, double-blind, dose-comparison study of weekly interferon β-1a in relapsing MS. Neurology 59: 1507–1517. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Jak AJ, Kniker JE, Kooijmans MF, Lull JM, Sandrock AW, et al. 2001. Use of the multiple sclerosis functional composite as an outcome measure in a phase 3 clinical trial. Arch Neurol 58: 961–967. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Kooijmans MF, Sandrock AW, Rudick RA, Simon JH, Simonian NA, et al. 2002. Benefit of interferon β-1a on MSFC progression in secondary progressive MS. Neurology 59: 679–687. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, et al. 2010. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362: 402–415. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, et al. 2012. Alemtuzumab versus interferon β 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 380: 1819–1828. [DOI] [PubMed] [Google Scholar]
- Comi G, Barkhof F, Durelli L, Edan G, Fernandez O, Filippi M, Hartung HP, Hommes OR, Seeldrayers P, Soelberg-Sorensen P. 1995. Early treatment of multiple sclerosis with Rebif (recombinant human interferon β): design of the study. Mult Scler 1: S24–S27. [PubMed] [Google Scholar]
- Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernandez O, Hartung H, Seeldrayers P, Sorensen PS, Rovaris M, et al. 2001. Effect of early interferon treatment on conversion to definite multiple sclerosis: A randomised study. Lancet 357: 1576–1582. [DOI] [PubMed] [Google Scholar]
- Comi G, De Stefano N, Freedman MS, Barkhof F, Polman CH, Uitdehaag BM, Casset-Semanaz F, Hennessy B, Moraga MS, Rocak S, et al. 2012. Comparison of two dosing frequencies of subcutaneous interferon β-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): A phase 3 randomised controlled trial. Lancet Neurol 11: 33–41. [DOI] [PubMed] [Google Scholar]
- Comi G, De Stefano N, Freedman MS, Barkhof F, Uitdehaag BM, de Vos M, Marhardt K, Chen L, Issard D, Kappos L. 2017. Subcutaneous interferon β-1a in the treatment of clinically isolated syndromes: 3-year and 5-year results of the phase III dosing frequency-blind multicentre REFLEXION study. J Neurol Neurosurg Psychiatry 88: 285–294. [DOI] [PubMed] [Google Scholar]
- Cree B, Gourraud PA, Oksenberg JR, Bevan C, Crabtree-Hartman E, Gelfand JM, Goodin DS, Graves J, Green AJ, Mowry E, et al. 2016. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 80: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhammer F, Mayringer I, Harvey J, Dilitz E, Gasse T, Stadlbauer D, Reindl M, Berger T. 2000. A comparative study of the relative bioavailability of different interferon β preparations. Neurology 54: 2055–2060. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Comi G, Kappos L, Freedman MS, Polman CH, Uitdehaag BM, Hennessy B, Casset-Semanaz F, Lehr L, Stubinski B, et al. 2014. Efficacy of subcutaneous interferon β-1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry 85: 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire V, Lapierre Y, Macdonell R, Ramo-Tello C, Patti F, Fontoura P, Suchet L, Hyde R, Balla I, Frohman EM, et al. 2011. The Global Adherence Project (GAP): A multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 18: 69–77. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S, Marks S. 2010. Interferon-β mechanisms of action in multiple sclerosis. Neurology 74: S17–S24. [DOI] [PubMed] [Google Scholar]
- Dujmovic I, Hegen H, Paz P, Croze E, Deisenhammer F. 2017. Persistency of neutralizing anti-interferon-β antibodies in patients with multiple sclerosis treated with subcutaneous interferon-β depends on antibody titers, IgG subclasses, and affinity maturation. J Interferon Cytokine Res 37: 317–324. [DOI] [PubMed] [Google Scholar]
- Durelli L, Verdun E, Barbero P, Bergui M, Versino E, Ghezzi A, Montanari E, Zaffaroni M; Independent Comparison of Interferon Trial Study Group. 2002. Every-other-day interferon β-1b versus once-weekly interferon β-1a for multiple sclerosis: Results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 359: 1453–1460. [DOI] [PubMed] [Google Scholar]
- Dwyer MG, Zivadinov R, Tao Y, Zhang X, Kennedy C, Bergsland N, Ramasamy DP, Durfee J, Hojnacki D, Weinstock-Guttman B, et al. 2015. Immunological and short-term brain volume changes in relapsing forms of multiple sclerosis treated with interferon β-1a subcutaneously three times weekly: An open-label two-arm trial. BMC Neurol 15: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebers GC, Heigenhauser L, Daumer M, Lederer C, Noseworthy JH. 2008. Disability as an outcome in MS clinical trials. Neurology 71: 624–631. [DOI] [PubMed] [Google Scholar]
- Ebers GC, Reder AT, Traboulsee A, Li D, Langdon D, Goodin DS, Wolf C, Beckmann K, Konieczny A; Investigators of the 16-Year Long-Term Follow-Up Study. 2009. Long-term follow-up of the original interferon-β-1b trial in multiple sclerosis: Design and lessons from a 16-year observational study. Clin Ther 31: 1724–1736. [DOI] [PubMed] [Google Scholar]
- Edan G, Kappos L, Montalban X, Polman CH, Freedman MS, Hartung HP, Miller D, Barkhof F, Herrmann J, Lanius V, et al. 2014. Long-term impact of interferon β-1b in patients with CIS: 8-year follow-up of BENEFIT. J Neurol Neurosurg Psychiatry 85: 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarson TR, Bereza BG, Machado M. 2017. Comparative effectiveness of interferons in relapsing-remitting multiple sclerosis: A meta-analysis of real-world studies. Curr Med Res Opin 33: 579–593. [DOI] [PubMed] [Google Scholar]
- European Study Group on Interferon β-1b in Secondary Progressive MS. 1998. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon β-1b in secondary progressive MS. Lancet 352: 1491–1497. [PubMed] [Google Scholar]
- Francis GS, Grumser Y, Alteri E, Micaleff A, O’Brien F, Alsop J, Stam Moraga M, Kaplowitz N. 2003. Hepatic reactions during treatment of multiple sclerosis with interferon-β-1a: Incidence and clinical significance. Drug Safety 26: 815–827. [DOI] [PubMed] [Google Scholar]
- Francis GS, Rice GP, Alsop JC, Group PS. 2005. Interferon β-1a in MS: Results following development of neutralizing antibodies in PRISMS. Neurology 65: 48–55. [DOI] [PubMed] [Google Scholar]
- Freedman MS. 2011. Long-term follow-up of clinical trials of multiple sclerosis therapies. Neurology 76: S26–S34. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Comi G, De Stefano N, Barkhof F, Polman CH, Uitdehaag BM, Lehr L, Stubinski B, Kappos L. 2014. Moving toward earlier treatment of multiple sclerosis: Findings from a decade of clinical trials and implications for clinical practice. Mult Scler Relat Disord 3: 147–155. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, Cutter G, Pia-Sormani M, Belachew S, Hyde R, Koendgen H, Knappertz V, Tomic D, Leppert D, Herndon R, et al. 2017. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord 12: 70–78. [DOI] [PubMed] [Google Scholar]
- Goodin DS. 2005. Treatment of multiple sclerosis with human β interferon. Int MS J 12: 96–108. [PubMed] [Google Scholar]
- Goodin DS, Reder AT, Ebers GC, Cutter G, Kremenchutzky M, Oger J, Langdon D, Rametta M, Beckmann K, DeSimone TM, et al. 2012a. Survival in MS: A randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology 78: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin DS, Traboulsee A, Knappertz V, Reder AT, Li D, Langdon D, Wolf C, Beckmann K, Konieczny A, Ebers GC, et al. 2012b. Relationship between early clinical characteristics and long term disability outcomes: 16 year cohort study (follow-up) of the pivotal interferon β-1b trial in multiple sclerosis. J Neurol Neurosurg Psychiatry 83: 282–287. [DOI] [PubMed] [Google Scholar]
- Hardmeier M, Wagenpfeil S, Freitag P, Fisher E, Rudick RA, Kooijmans M, Clanet M, Radue EW, Kappos L; European IFN-1a in Relapsing MS Dose Comparison Trial Study Group. 2005. Rate of brain atrophy in relapsing MS decreases during treatment with IFNβ-1a. Neurology 64: 236–240. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, et al. 2016. Ocrelizumab versus interferon β-1a in relapsing multiple sclerosis. N Engl J Med 376: 221–234. [DOI] [PubMed] [Google Scholar]
- Hegen H, Millonig A, Bertolotto A, Comabella M, Giovanonni G, Guger M, Hoelzl M, Khalil M, Killestein J, Lindberg R, et al. 2014. Early detection of neutralizing antibodies to interferon-β in multiple sclerosis patients: Binding antibodies predict neutralizing antibody development. Mult Scler 20: 577–587. [DOI] [PubMed] [Google Scholar]
- Hurtado-Guerrero I, Pinto-Medel MJ, Urbaneja P, Rodriguez-Bada JL, Leon A, Guerrero M, Fernandez O, Leyva L, Oliver-Martos B. 2017. Activation of the JAK-STAT signaling pathway after in vitro stimulation with IFNβ in multiple sclerosis patients according to the therapeutic response to IFNβ. PLoS ONE 12: e0170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L, O’Malley J, Freeman A, Ekes R. 1981. Intrathecal interferon reduces exacerbations of multiple sclerosis. Science 214: 1026–1028. [DOI] [PubMed] [Google Scholar]
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, Fischer JS, Goodkin DE, Granger CV, Simon JH, et al. 1996. Intramuscular interferon β-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39: 285–294. [DOI] [PubMed] [Google Scholar]
- Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, Simonian NA, Slasor PJ, Sandrock AW. 2000. Intramuscular interferon β-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 343: 898–904. [DOI] [PubMed] [Google Scholar]
- Kappos L, Polman C, Pozzilli C, Thompson A, Beckmann K, Dahlke F; European Study Group in Interferon β-1b in Secondary-Progressive MS. 2001. Final analysis of the European multicenter trial on IFNβ-1b in secondary-progressive MS. Neurology 57: 1969–1975. [DOI] [PubMed] [Google Scholar]
- Kappos L, Weinshenker B, Pozzilli C, Thompson AJ, Dahlke F, Beckmann K, Polman C, McFarland H; European (EUSPMS) Interferon β-1b in Secondary Progressive Multiple Sclerosis Trial Steering Committee and Independent Advisory Board, et al. 2004. Interferon β-1b in secondary progressive MS: A combined analysis of the two trials. Neurology 63: 1779–1787. [DOI] [PubMed] [Google Scholar]
- Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Bauer L, Jakobs P, et al. 2006a. Treatment with interferon β-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 67: 1242–1249. [DOI] [PubMed] [Google Scholar]
- Kappos L, Traboulsee A, Constantinescu C, Eralinna JP, Forrestal F, Jongen P, Pollard J, Sandberg-Wollheim M, Sindic C, Stubinski B, et al. 2006b. Long-term subcutaneous interferon β-1a therapy in patients with relapsing-remitting MS. Neurology 67: 944–953. [DOI] [PubMed] [Google Scholar]
- Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Radu EW, Bauer L, et al. 2007. Effect of early versus delayed interferon β-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: A 3-year follow-up analysis of the BENEFIT study. Lancet 370: 389–397. [DOI] [PubMed] [Google Scholar]
- Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Radu EW, Metzig C, et al. 2009. Long-term effect of early treatment with interferon β-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 8: 987–997. [DOI] [PubMed] [Google Scholar]
- Kappos L, Edan G, Freedman MS, Montalban X, Hartung HP, Hemmer B, Fox EJ, Barkhof F, Schippling S, Schulze A, et al. 2016. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology 87: 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseier BC, Arnold DL, Balcer LJ, Boyko AA, Pelletier J, Liu S, Zhu Y, Seddighzadeh A, Hung S, Deykin A, et al. 2015. Peginterferon β-1a in multiple sclerosis: 2-year results from ADVANCE. Mult Scler 21: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkel RP, Kollman C, O’Connor P, Murray TJ, Simon J, Arnold D, Bakshi R, Weinstock-Gutman B, Brod S, Cooper J, et al. 2006. IM interferon β-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology 66: 678–684. [DOI] [PubMed] [Google Scholar]
- Kinkel RP, Dontchev M, Kollman C, Skaramagas TT, O’Connor PW, Simon JH; Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. 2012. Association between immediate initiation of intramuscular interferon β -1a at the time of a clinically isolated syndrome and long-term outcomes: A 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Arch Neurol 69: 183–190. [DOI] [PubMed] [Google Scholar]
- Kuhle J, Hardmeier M, Disanto G, Gugleta K, Ecsedi M, Lienert C, Amato MP, Baum K, Buttmann M, Bayas A, et al. 2016. A 10-year follow-up of the European multicenter trial of interferon β-1b in secondary-progressive multiple sclerosis. Mult Scler 22: 533–543. [DOI] [PubMed] [Google Scholar]
- Lacy M, Hauser M, Pliskin N, Assuras S, Valentine MO, Reder A. 2013. The effects of long-term interferon-β-1b treatment on cognitive functioning in multiple sclerosis: A 16-year longitudinal study. Mult Scler 19: 1765–1772. [DOI] [PubMed] [Google Scholar]
- La Mantia L, Vacchi L, Di Pietrantonj C, Ebers G, Rovaris M, Fredrikson S, Filippini G. 2012. Interferon β for secondary progressive multiple sclerosis. Cochrane Database Syst Rev 1: CD005181. [DOI] [PubMed] [Google Scholar]
- Langdon D, Reder AT, Ebers G. 2007. EDSS and MRI burden of disease predict cognitive status at 16 years: Data from the long-term follow-up study. Mult Scler 13: S268–S269. [Google Scholar]
- Langer-Gould A, Moses HH, Murray TJ. 2004. Strategies for managing the side effects of treatments for multiple sclerosis. Neurology 63: S35–S41. [DOI] [PubMed] [Google Scholar]
- Li D, Ebers G, Traboulsee A, Tam R, Goodin D, Konieczny A,2006. Interferon β-1b 16-year long-term follow-up study: MRI outcomes. Mult Scler 12: S188–S189. [Google Scholar]
- Link J, Ramanujam R, Auer M, Ryner M, Hassler S, Bachelet D, Mbogning C, Warnke C, Buck D, Hyldgaard Jensen PE, et al. 2017. Clinical practice of analysis of anti-drug antibodies against interferon β and natalizumab in multiple sclerosis patients in Europe: A descriptive study of test results. PLoS ONE 12: e0170395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magner WJ, Weinstock-Guttman B, Rho M, Hojnacki D, Ghazi R, Ramanathan M, Tomasi TB. 2016. Dicer and microRNA expression in multiple sclerosis and response to interferon therapy. J Neuroimmunol 292: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzin J, Caon C, Nichols C, White LA, Friedman M, Pill MW. 2013. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm 19: S24–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DH, Molyneux PD, Barker GJ, MacManus DG, Moseley IF, Wagner K. 1999. Effect of interferon-β-1b on magnetic resonance imaging outcomes in secondary progressive multiple sclerosis: Results of a European multicenter, randomized, double-blind, placebo-controlled trial. European Study Group on Interferon-β-1b in Secondary Progressive Multiple Sclerosis. Ann Neurol 46: 850–859. [DOI] [PubMed] [Google Scholar]
- Minagar A, Murray TJ, Investigators PS. 2008. Efficacy and tolerability of intramuscular interferon β-1a compared with subcutaneous interferon β-1a in relapsing MS: Results from PROOF. Curr Med Res Opin 24: 1049–1055. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Boudewyn AC, Likosky W, Levine E, Goodkin DE. 2001. Injectable medication for the treatment of multiple sclerosis: The influence of self-efficacy expectations and injection anxiety on adherence and ability to self-inject. Ann Behav Med 23: 125–132. [DOI] [PubMed] [Google Scholar]
- Molyneux PD, Kappos L, Polman C, Pozzilli C, Barkhof F, Filippi M, Yousry T, Hahn D, Wagner K, Ghazi M, et al. 2000. The effect of interferon β-1b treatment on MRI measures of cerebral atrophy in secondary progressive multiple sclerosis. European Study Group on Interferon β-1b in Secondary Progressive Multiple Sclerosis. Brain 123: 2256–2263. [DOI] [PubMed] [Google Scholar]
- Moraal B, Pohl C, Uitdehaag BM, Polman CH, Edan G, Freedman MS, Hartung HP, Kappos L, Miller DH, Montalban X, et al. 2009. Magnetic resonance imaging predictors of conversion to multiple sclerosis in the BENEFIT study. Arch Neurol 66: 1345–1352. [DOI] [PubMed] [Google Scholar]
- Pachner AR, Dail D, Pak E, Narayan K. 2005. The importance of measuring IFNβ bioactivity: Monitoring in MS patients and the effect of anti-IFNβ antibodies. J Neuroimmunol 166: 180–188. [DOI] [PubMed] [Google Scholar]
- Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O’Connor P, Monaghan E, Li D, Weinshenker B. 2002. Randomized, comparative study of interferon β-1a treatment regimens in MS: The EVIDENCE Trial. Neurology 59: 1496–1506. [DOI] [PubMed] [Google Scholar]
- Panitch H, Miller A, Paty D, Weinshenker B; North American Study Group on Interferon β-1b in Secondary Progressive MS. 2004. Interferon β-1b in secondary progressive MS: Results from a 3-year controlled study. Neurology 63: 1788–1795. [DOI] [PubMed] [Google Scholar]
- Panitch H, Goodin D, Francis G, Chang P, Coyle P, O’Connor P, Li D, Weinshenker B; EVIDENCE (Evidence of Interferon Dose-Response: European North American Comparative Efficacy Study Group and EV the University of British Columbia MS/MRI Research Group. 2005. Benefits of high-dose, high-frequency interferon β-1a in relapsing-remitting multiple sclerosis are sustained to 16 months: Final comparative results of the EVIDENCE trial. J Neurol Sci 239: 67–74. [DOI] [PubMed] [Google Scholar]
- Paty DW, Li DK. 1993. Interferon β-1b is effective in relapsing-remitting multiple sclerosis. II: MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 43: 662–667. [DOI] [PubMed] [Google Scholar]
- Polman CH, Bertolotto A, Deisenhammer F, Giovannoni G, Hartung HP, Hemmer B, Killestein J, McFarland HF, Oger J, Pachner AR, et al. 2010. Recommendations for clinical use of data on neutralising antibodies to interferon-β therapy in multiple sclerosis. Lancet Neurol 9: 740–750. [DOI] [PubMed] [Google Scholar]
- Pozzilli C, Pugliatti M, Paradig MSG. 2015. An overview of pregnancy-related issues in patients with multiple sclerosis. Eur J Neurol 22: 34–39. [DOI] [PubMed] [Google Scholar]
- PRISMS Study Group. 1998. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 352: 1498–1504. [PubMed] [Google Scholar]
- PRISMS Study Group and the University of British Columbia. 2001. PRISMS-4: Long-term efficacy of interferon-β-1a in relapsing MS. Neurology 56: 1628–1636. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. 1998. Cellular responses to interferons and other cytokines: The JAK-STAT paradigm. N Engl J Med 338: 616–618. [DOI] [PubMed] [Google Scholar]
- Reder AT, Ebers GC, Traboulsee A, Li D, Langdon D, Goodin DS, Bogumil T, Beckmann K, Konieczny A; Investigators of the 16-Year Long-Term Follow-Up Study. 2010. Cross-sectional study assessing long-term safety of interferon-β-1b for relapsing-remitting MS. Neurology 74: 1877–1885. [DOI] [PubMed] [Google Scholar]
- Reder AT, Oger JF, Kappos L, O’Connor P, Rametta M. 2014. Short-term and long-term safety and tolerability of interferon β-1b in multiple sclerosis. Mult Scler Relat Disord 3: 294–302. [DOI] [PubMed] [Google Scholar]
- Rio J, Rovira A, Tintore M, Huerga E, Nos C, Tellez N, Tur C, Comabella M, Montalban X. 2008. Relationship between MRI lesion activity and response to IFN-β in relapsing-remitting multiple sclerosis patients. Mult Scler 14: 479–484. [DOI] [PubMed] [Google Scholar]
- Rio J, Rovira A, Tintore M, Otero-Romero S, Comabella M, Vidal-Jordana A, Galan I, Castillo J, Arrambide G, Nos C, et al. 2017. Disability progression markers over 6–12 years in interferon-β -treated multiple sclerosis patients. Mult Scler 10.1177/1352458517698052. [DOI] [PubMed]
- Rizzo F, Giacomini E, Mechelli R, Buscarinu MC, Salvetti M, Severa M, Coccia EM. 2016. Interferon-β therapy specifically reduces pathogenic memory B cells in multiple sclerosis patients by inducing a FAS-mediated apoptosis. Immunol Cell Biol 94: 886–894. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. 1999. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology 53: 1698–1704. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Lee JC, Cutter GR, Miller DM, Bourdette D, Weinstock-Guttman B, Hyde R, Zhang H, You X. 2010. Disability progression in a clinical trial of relapsing-remitting multiple sclerosis: Eight-year follow-up. Arch Neurol 67: 1329–1335. [DOI] [PubMed] [Google Scholar]
- Santos R, Weinstock-Guttman B, Tamano-Blanco M, Badgett D, Zivadinov R, Justinger T, Munschauer F III, Ramanathan M. 2006. Dynamics of interferon-β modulated mRNA biomarkers in multiple sclerosis patients with anti-interferon-β neutralizing antibodies. J Neuroimmunol 176: 125–133. [DOI] [PubMed] [Google Scholar]
- Schellekens H. 2002. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov 1: 457–462. [DOI] [PubMed] [Google Scholar]
- Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-β-1a in MSSG. 2001. Randomized controlled trial of interferon-β-1a in secondary progressive MS: Clinical results. Neurology 56: 1496–1504. [DOI] [PubMed] [Google Scholar]
- Signori A, Gallo F, Bovis F, Di Tullio N, Maietta I, Sormani MP. 2016. Long-term impact of interferon or Glatiramer acetate in multiple sclerosis: A systematic review and meta-analysis. Mult Scler Relat Disord 6: 57–63. [DOI] [PubMed] [Google Scholar]
- Simon JH, Jacobs LD, Campion M, Wende K, Simonian N, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. 1998. Magnetic resonance studies of intramuscular interferon β-1a for relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group. Ann Neurol 43: 79–87. [DOI] [PubMed] [Google Scholar]
- Simon JH, Kinkel RP, Kollman C, O’Connor P, Fisher E, You X, Hyde R, CHAMPIONS Investigators Group CI. 2015. Ten-year follow-up of the “minimal MRI lesion” subgroup from the original CHAMPS Multiple Sclerosis Prevention Trial. Mult Scler 21: 415–422. [DOI] [PubMed] [Google Scholar]
- Sorensen PS, Ross C, Clemmesen KM, Bendtzen K, Frederiksen JL, Jensen K, Kristensen O, Petersen T, Rasmussen S, Ravnborg M, et al. 2003. Clinical importance of neutralising antibodies against interferon β in patients with relapsing-remitting multiple sclerosis. Lancet 362: 1184–1191. [DOI] [PubMed] [Google Scholar]
- Sorensen PS, Deisenhammer F, Duda P, Hohlfeld R, Myhr KM, Palace J, Polman C, Pozzilli C, Ross C; EFNS Task Force on Anti-IFN-β Antibodies in Multiple Sclerosis. 2005a. Guidelines on use of anti-IFN-β antibody measurements in multiple sclerosis: Report of an EFNS task force on IFN-β antibodies in multiple sclerosis. Eur J Neurol 12: 817–827. [DOI] [PubMed] [Google Scholar]
- Sorensen PS, Koch-Henriksen N, Ross C, Clemmesen KM, Bendtzen K, Danish Multiple Sclerosis Study Group. 2005b. Appearance and disappearance of neutralizing antibodies during interferon-β therapy. Neurology 65: 33–39. [DOI] [PubMed] [Google Scholar]
- Sormani MP, Li DK, Bruzzi P, Stubinski B, Cornelisse P, Rocak S, De Stefano N. 2011. Combined MRI lesions and relapses as a surrogate for disability in multiple sclerosis. Neurology 77: 1684–1690. [DOI] [PubMed] [Google Scholar]
- Sormani MP, Gasperini C, Romeo M, Rio J, Calabrese M, Cocco E, Enzingher C, Fazekas F, Filippi M, Gallo A, et al. 2016. Assessing response to interferon-β in a multicenter dataset of patients with MS. Neurology 87: 134–140. [DOI] [PubMed] [Google Scholar]
- Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. 2011. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 28: 51–61. [DOI] [PubMed] [Google Scholar]
- The IFNB Multiple Sclerosis Study Group. 1993. Interferon β-1b is effective in relapsing-remitting multiple sclerosis. I: Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 43: 655–661. [DOI] [PubMed] [Google Scholar]
- The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. 1996. Neutralizing antibodies during treatment of multiple sclerosis with interferon β-1b: Experience during the first three years. Neurology 47: 889–894. [DOI] [PubMed] [Google Scholar]
- Thiel S, Langer-Gould A, Rockhoff M, Haghikia A, Queisser-Wahrendorf A, Gold R, Hellwig K. 2016. Interferon-β exposure during first trimester is safe in women with multiple sclerosis—A prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler 22: 801–809. [DOI] [PubMed] [Google Scholar]
- Tremlett HL, Yoshida EM, Oger J. 2004. Liver injury associated with the β-interferons for MS: A comparison between the three products. Neurology 62: 628–631. [DOI] [PubMed] [Google Scholar]
- Trojano M, Liguori M, Paolicelli D, Zimatore GB, De Robertis F, Avolio C, Giuliani F, Fuiani A, Livrea P; Southern Italy MS Group. 2003. Interferon β in relapsing-remitting multiple sclerosis: An independent postmarketing study in southern Italy. Mult Scler 9: 451–457. [DOI] [PubMed] [Google Scholar]
- Trojano M, Pellegrini F, Fuiani A, Paolicelli D, Zipoli V, Zimatore GB, Di Monte E, Portaccio E, Lepore V, Livrea P, et al. 2007. New natural history of interferon-β-treated relapsing multiple sclerosis. Ann Neurol 61: 300–306. [DOI] [PubMed] [Google Scholar]
- Uher T, Fellows K, Horakova D, Zivadinov R, Vaneckova M, Sobisek L, Tyblova M, Seidl Z, Krasensky J, Bergsland N, et al. 2016. Serum lipid profile changes predict neurodegeneration in interferon-β-1a treated multiple sclerosis patients. J Lipid Res 58: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher T, Havrdova E, Sobisek L, Krasensky J, Vaneckova M, Seidl Z, Tyblova M, Ramasamy D, Zivadinov R, Horakova D. 2017. Is no evidence of disease activity an achievable goal in MS patients on intramuscular interferon β-1a treatment over long-term follow-up? Mult Scler 23: 242–252. [DOI] [PubMed] [Google Scholar]
- Uitdehaag B, Constantinescu C, Cornelisse P, Jeffery D, Kappos L, Li D, Sandberg-Wollheim M, Traboulsee A, Verdun E, Rivera V. 2011. Impact of exposure to interferon β-1a on outcomes in patients with relapsing-remitting multiple sclerosis: Exploratory analyses from the PRISMS long-term follow-up study. Ther Adv Neurol Disord 4: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian T. 2003. An examination of the results of the EVIDENCE, INCOMIN, and phase III studies of interferon β products in the treatment of multiple sclerosis. Clin Ther 25: 105–118. [DOI] [PubMed] [Google Scholar]
- Weiner HL. 2009. The challenge of multiple sclerosis: How do we cure a chronic heterogeneous disease? Ann Neurol 65: 239–248. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC. 1989. The natural history of multiple sclerosis: A geographically based study. I: Clinical course and disability. Brain 112: 133–146. [DOI] [PubMed] [Google Scholar]
- Weinstock-Guttman B. 2013. An update on new and emerging therapies for relapsing-remitting multiple sclerosis. Am J Manag Care 19: s343–s354. [PubMed] [Google Scholar]
- Weinstock-Guttman B, Badgett D, Patrick K, Hartrich L, Santos R, Hall D, Baier M, Feichter J, Ramanathan M. 2003. Genomic effects of IFN-β in multiple sclerosis patients. J Immunol 171: 2694–2702. [DOI] [PubMed] [Google Scholar]
- Weinstock-Guttman B, Tamano-Blanco M, Bhasi K, Zivadinov R, Ramanathan M. 2007. Pharmacogenetics of MXA SNPs in interferon-β treated multiple sclerosis patients. J Neuroimmunol 182: 236–239. [DOI] [PubMed] [Google Scholar]
- Weinstock-Guttman B, Bhasi K, Badgett D, Tamano-Blanco M, Minhas M, Feichter J, Patrick K, Munschauer F, Bakshi R, Ramanathan M. 2008. Genomic effects of once-weekly, intramuscular interferon-β 1a treatment after the first dose and on chronic dosing: Relationships to 5-year clinical outcomes in multiple sclerosis patients. J Neuroimmunol 205: 113–125. [DOI] [PubMed] [Google Scholar]
- Zivadinov R, Reder AT, Filippi M, Minagar A, Stuve O, Lassmann H, Racke MK, Dwyer MG, Frohman EM, Khan O. 2008. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology 71: 136–144. [DOI] [PubMed] [Google Scholar]
- Zivadinov R, Dwyer MG, Markovic-Plese S, Kennedy C, Bergsland N, Ramasamy DP, Durfee J, Hojnacki D, Hayward B, Dangond F, et al. 2014. Effect of treatment with interferon β-1a on changes in voxel-wise magnetization transfer ratio in normal appearing brain tissue and lesions of patients with relapsing-remitting multiple sclerosis: A 24-week, controlled pilot study. PLoS ONE 9: e91098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Jakimovski D, Gandhi S, Ahmed R, Dwyer MG, Horakova D, Weinstock-Guttman B, Benedict RR, Vaneckova M, Barnett M, et al. 2016. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 16: 777–793. [DOI] [PubMed] [Google Scholar]