Abstract
The term “neoantigen,” as applied to molecules newly expressed on tumor cells, has a long history. The groundbreaking discovery of a cancer causing virus in chickens by Rous over 100 years ago, followed by discoveries of other tumor-causing viruses in animals, suggested a viral etiology of human cancers. The search for other oncogenic viruses in the 1960s and 1970s resulted in the discoveries of Epstein–Barr virus (EBV), hepatitis B virus (HBV), and human papilloma virus (HPV), and continues until the present time. Contemporaneously, the budding field of immunology was posing the question can the immune system of animals or humans recognize a tumor that develops from one’s own tissues and what types of antigens would distinguish the tumor from normal cells. Molecules encoded by oncogenic viruses provided the most logical candidates and evidence was quickly gathered for both humoral and cellular recognition of viral antigens, referred to as neoantigens. Often, however, serologic responses to virus-bearing tumors revealed neoantigens unrelated to viral proteins and expressed on multiple tumor types, foreshadowing later findings of multiple changes in other genes in tumor cells creating nonviral neoantigens.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
Use the right word, not its second cousin.
—Mark Twain
Groundbreaking discoveries in the early 1970s, starting with the identification of the src oncogene, showed that cancer was a genetic disease and that abnormal expression (activation or suppression) of proto-oncogenes could cause malignant transformation. Products of mutated proto-oncogenes became candidate tumor neoantigens (Hellstrom and Hellstrom 1989; Urban and Schreiber 1992). Simultaneous development of molecular techniques and cellular assays to evaluate T-cell responses in vitro identified numerous mutated neoantigens, products of single-point mutations in genes encoding proteins directing various cellular functions newly expressed and unique to each individual mouse and human tumor (Sibille et al. 1990; Lennerz et al. 2005; Sensi and Anichini 2006; Coulie et al. 2014). These data recapitulated results from early work on mouse carcinogen-induced tumors that identified unique mutations generating neoantigens that elicited immunity and protected mice from challenge with the original tumor but no other tumors caused by the same carcinogen (Srivastava 2015).
All of these neoantigens (viral, oncogene-encoded, or randomly mutated) were the predicted targets of cancer immunosurveillance, a function of the immune system that was at that time very much in doubt and for which their identification provided an indisputable support (Dunn et al. 2004). What was not predicted when the search for tumor antigens began was the finding of tumor-specific humoral and cellular immune responses in mice and in cancer patients, which recognized nonmutated cellular proteins as specific antigens on tumor cells and not on normal cells (Sjogren 1967; Finn 1993; Henderson and Finn 1996). Because these were present on multiple tumors rather than being unique to each tumor, they were given a designation “shared tumor antigens” (Ting and Herberman 1974). Because they were tumor-associated versions of proteins expressed by normal cells and thus not theoretically “tumor-specific,” they were also named tumor-associated antigens (TAAs). Over the last two decades, many TAAs have been identified and the nature of tumor-specific changes in normal cellular proteins that created these neoantigens was elucidated. Shared neoantigens belong to one of several categories: oncofetal antigens (expressed in fetal but not adult tissues and reexpressed in tumors), cancer/testis (CT) antigens (present in germ cells that lack major histocompatibility complex [MHC] and not presented to the immune system except on tumor cells), differentiation antigens (specific to differentiated tissues and organs from which the tumor originated), overexpressed antigens and differentially processed antigens (tumor-specific changes in protein glycosylation [Vlad and Finn 2004; Vankemmelbeke et al. 2016], phosphorylation [Mohammed et al. 2008], and citrullination [Brentville et al. 2016]), among others.
In summary, over a period of more than half a century, a long list of neoantigens has been compiled, which regardless of their origin (viral, mutated, nonmutated) share the same characteristics: (1) they are newly and preferentially present on tumor cells; (2) recognized by antibodies and T cells; and (3) elicit spontaneous immunity in cancer patients and tumor-rejection immunity in animal models. It is thus unjustified that the term “neoantigens” has recently been usurped specifically for products of mutated gene segments uncovered by whole exome sequencing or by mass spectrometric analysis of MHC/human leukocyte antigen (HLA)-bound peptides (Bassani-Sternberg et al. 2015; Kalaora et al. 2016). This misuse of terminology hides the nature and immunogenic potential of other neoantigens mentioned above, which have already been shown to be recognized by the immune system, to induce tumor-rejection immunity in animal models, and to have clinical benefit in cancer patients either as targets of immunotherapy or as vaccine antigens (Cheever et al. 2009). To use the right word and not its second cousin, which is especially important in science communication, what are recently being referred to as “neoantigens” should be referred to as “mutated neoantigens” in deference to other members of a large and varied family of tumor neoantigens.
CANCER VACCINES BASED ON MUTATED NEOANTIGENS CAN BE DEVELOPED BUT DO NOT WARRANT SPECIAL ATTENTION
Because it is now relatively easy to sequence genes, a cottage industry has sprung up around identifying and cataloging hundreds to thousands of mutations in cancers, each potentially a candidate neoantigen for an individual tumor/individual patient-specific vaccine, or a target for immunotherapy (Boegel et al. 2014; Yadav et al. 2014). Those who are in the field of tumor immunology trying to better understand tumor immunity and immunosurveillance appreciate the challenges brought about by tumor-specific/patient-specific mutated epitopes (Lutz and Jaffee 2014; Gubin et al. 2015) and their use for immunotherapy or vaccines. The major challenges include (1) how to select from a large number of mutations those few that are made into proteins and processed and presented as antigenic peptides in MHC class I or class II antigens; (2) how to show convincingly that immune responses against mutated neoantigens are superior to nonmutated neoantigens; (3) how to deal with MHC/HLA restriction and further mutations that can generate antigen-escape variants; and (4) how to obtain evidence, other than through single-cell sequencing, that specific mutations are present in all tumor cells, thus not allowing immune escape (Verdegaal et al. 2016).
Some of these challenges are the same for viral and nonmutated neoantigen vaccines but others are not. For example, every tumor cell in a virally induced tumor expresses viral neoantigens required for continued oncogenic transformation and thus each tumor cell is a target of a vaccine based on these neoantigens (McAllister 1965; Javier and Butel 2008). Similarly, many nonmutated neoantigens, but especially those that have oncogenic functions (Cheever et al. 1995; Bright et al. 2014), such as Her-2neu (Disis et al. 1994), MUC1 (Cheever et al. 1995), hTERT (Vonderheide 2002), and p53 (Pedersen et al. 2011), are expressed in all tumor cells and on multiple tumor types, which makes them optimal candidates for vaccines and immunotherapy. Short of sequencing single cells from various sections of primary tumors and metastatic sites, the same cannot be assumed for mutated neoantigens. Furthermore, many nonmutated neoantigens trigger immune responses because of their overexpression in tumors compared to normal cells. Protein abundance and protein turnover are important factors for HLA presentation of antigens (Bassani-Sternberg et al. 2015). Identification of mutated peptides as potential neoantigens currently requires a combination of exome sequencing, messenger RNA (mRNA) microarrays, and epitope prediction algorithms, but also knowing the level of expression of the source protein, which determines whether it can reach the threshold required for its efficient processing and presentation in HLA.
MUTATED VERSUS NONMUTATED TUMOR PEPTIDES AS HLA LIGANDS
Rammensee and colleagues have characterized immunopeptidomes of a number of primary tumors and cells, including leukemias and solid tumors (Walz et al. 2015; Löffler et al. 2016), by eluting and sequencing peptides from purified HLA class I and class II molecules (Berlin et al. 2015). With the current sensitivity of the mass spectrometry methods, 5000 unique nonmutated peptides can be isolated from 1 g of tissue. Hundreds of nonmutated peptides identified from each sample appear to be tumor specific on the grounds that they are not found on any of the numerous normal tissues similarly analyzed. Many of these peptides are immunogenic, as tested by in vitro priming of T cells from healthy donors or by measuring recall T-cell responses from patients. In chronic lymphocytic leukemia (CLL) patients, such T-cell responses correlated with patients’ overall survival (Kowalewski et al. 2015a).
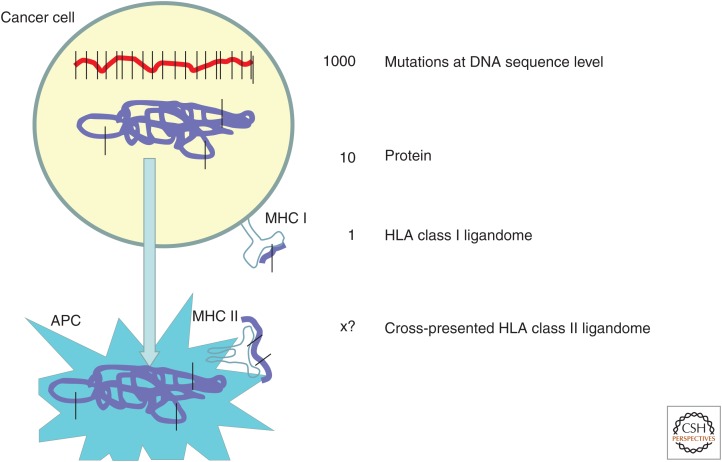
By combining exome sequencing, prediction of mutated peptides as HLA ligands, and their verification by mass spectrometry, a few mutated peptides were also identified; however, only a very small fraction of mutations at the DNA sequence level ended up as peptides in HLA class I. Based on their own mass spectrometry data and the published data on the expression of mutations in proteins and HLA ligands, Rammensee and colleagues (Löffler et al. 2016) estimate that from 1000 nonsynonymous DNA mutations, 10 manifest in mutated proteins and only one as a mutated HLA class I ligand (for illustration, see Fig. 1). Assuming also that the tumor proteome is cross-presented on HLA class II molecules on tumor-associated antigen-presenting cells, the frequency of mutated HLA class II ligands is estimated to be close to the number of mutated proteins, 10/1000. These numbers are roughly in accordance with clinical observations on mutation-specific T cells from patients treated with checkpoint blockade (Schumacher and Schreiber 2015).
Figure 1.
Frequency of mutated neoantigens presented in major histocompatibility complex (MHC) class I and cross-presented in MHC class II. APC, Antigen-presenting cell; HLA, human leukocyte antigen.
In contrast, the number of nonmutated but still highly tumor-specific peptides is much higher with several dozens of such peptides identified in a given tumor tissue, most of them present in one particular tumor and not in most other tumors or normal tissues. One hypothesis is that a mutation anywhere in a protein would lead to changes in its expression and likely expression of downstream proteins in the same functional pathway, thereby influencing their processing and consequently a difference in the tumor immunopeptidome. For example, a mutation in a member of the WNT-signaling pathway (MacDonald et al. 2009) increases levels of cyclin D1, which in turn increases the number of its HLA ligands and leads to their recognition as tumor-specific, nonmutated neoantigens. Peptides from nonmutated cyclin D1 have been shown to be immunogenic and are already being tested in cancer immunotherapy (Walter et al. 2012; Löffler et al. 2016). Another example is cyclin B1, a shared nonmutated tumor antigen that is overexpressed in human tumors where p53 is either deleted or mutated (Kao et al. 2001; Yu et al. 2002). The nonmutated but overexpressed cyclin B1 serves as a tumor-rejection antigen in mouse models (Vella et al. 2009) and elicits antibodies and T cells in cancer patients (Suzuki et al. 2005). Mutation-induced alterations can affect mRNA expression levels, RNA splicing, and other posttranscriptional or posttranslational modifications, resulting in many tumor-specific MHC ligands as nonmutated neoantigens (Kowalewski et al. 2015a,b).
IMPORTANCE OF CHOOSING THE RIGHT NEOANTIGEN(S) FOR EFFECTIVE CANCER VACCINES
A recent explosion in the number of mutated neoantigens and the enthusiasm for their potential as cancer vaccines should not distract from the fact that mutated neoantigen vaccines are not a new concept and have been made and tested before. This includes vaccines based on mutated H-ras (Gjertsen and Gaudernack 1998) and K-ras oncogenes (Carbone et al. 2005), mutated p53 (Carbone et al. 2005; Vermeij et al. 2011), and heat-shock proteins that bind mutated tumor peptides (Srivastava 1993). These vaccines have had as much or as little success as vaccines based on nonmutated overexpressed antigens, CT antigens, or differentiation antigens. The rationale for their development as vaccines was that they were tumor-specific and foreign to the immune system and should induce stronger immune responses than nonmutated neoantigens that might be subject to immune tolerance. This predicted difference between mutated and nonmutated neoantigens did not materialize, nor did the immunogenic superiority of mutated antigens. A good example is p53 where vaccines based on patient- and tumor-specific p53-mutated neoepitopes achieved the same results as vaccines based on nonmutated peptides derived from overexpressed wild-type p53 (Vermeij et al. 2011). It is clear now that the immunosuppressive tumor microenvironment (Palucka and Coussens 2016) can have a much greater effect on vaccine immunogenicity and efficacy than the nature of the vaccine antigen. Given past experience with mutated neoantigens, it is hard to justify the labor- and cost-intensive development of personalized vaccines based on these antigens. It is more than likely that to be more effective in the therapeutic setting, all cancer vaccines will need help from currently available or soon-to-be-developed immunotherapies directed at modulating the tumor microenvironment (Kourie et al. 2016). If these immunomodulators are successful, both nonmutated and mutated neoepitope vaccines will experience a renaissance.
NEOANTIGENS AS PROPHYLACTIC CANCER VACCINES?
In addition to combination therapies designed to enhance efficacy of therapeutic cancer vaccines, some cancer vaccines are already being tested for increased efficacy in individuals without cancer but at an increased risk for cancer. The hypothesis is that in the absence of tumor the immune system is not compromised and effective immunity and immune memory can be elicited to protect from or delay tumor development (Finn 2014). Initial trials are based on several well-known shared tumor antigens that have been extensively studied and thoroughly characterized for their expression on tumors (Finn and Beatty 2016). One of these is MUC1 that is overexpressed in its hypoglycosylated form on all human adenocarcinomas as well as on multiple myeloma and some leukemias and lymphomas. The expectation is that the MUC1 vaccine would elicit or boost strong immune responses and long-term immune memory to prevent cancer development. Finn and colleagues are vaccinating individuals diagnosed with advanced adenomas of the colon, precursors to colon cancer. The vaccine is given postadenoma removal and a booster is administered a year later. Very strong anti-MUC1 immune responses were induced in the initial study that greatly surpassed frequency and intensity of responses seen in cancer patients. Importantly, the vaccine induced immune memory with no evidence of toxicity (Kimura et al. 2013). Vaccine-elicited antibodies had a range of affinities and reacted only with MUC1 on tumors and not on normal tissues (Lohmueller et al. 2016). Inasmuch as the nonmalignant adenomas also overexpress hypoglycosylated MUC1, this vaccine is expected to prevent recurrence of premalignant lesions or their progression to cancer. This is being tested in an ongoing randomized trial.
The absence of cancer as a source of mutated neoantigens would appear to exclude the possibility of developing personalized prophylactic cancer vaccines. Whereas this is the case in the true prevention setting in the complete absence of disease, it does not apply to the setting of premalignant disease (Finn 2003). Most human adenocarcinomas start with readily diagnosed premalignant lesions that can be biopsied and subjected to exome sequencing to identify specific mutations and mutated neoantigens. Efforts are already underway to better understand the molecular events in cancer development from premalignant to malignant disease, with the goal of creating a Pre-Cancer Genome Atlas (PCGA) to parallel efforts on The Cancer Genome Atlas (TCGA) (Campbell et al. 2016). This effort is expected to uncover new targets for cancer prevention (Kensler et al. 2016). Importantly, it would also set the stage for routinely biopsying and sequencing premalignant lesions, which could support development of patient-specific mutated neoantigen vaccines for cancer prevention. The same issues and challenges that apply to the mutated neoantigens of therapeutic vaccines would apply to the prophylactic vaccines, including challenging the wisdom of their development.
CONCLUSION
Sophisticated high-throughput methodologies have been developed for identifying and cataloging genetic mutations in tumors, and these studies as applied to tumor immunity will provide new information about immunosurveillance of genome integrity and its targets (Fritsch et al. 2014; Tran et al. 2015; Gros et al. 2016). Although mutated neoantigens uncovered in this process already have their proponents for immediate clinical application as targets for immunotherapy or antigens in vaccines (Gros et al. 2016; Stronen et al. 2016), the labor-intensive and likely to be a very expensive approach to their identification and vaccine development should not replace or even compete with the development of more broadly applicable, off-the-shelf, cost-effective vaccines based on nonmutated, shared tumor neoantigens.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Bassani-Sternberg M, Pletscher-Frankild S, Jensen LJ, Mann M. 2015. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol Cell Proteomics 14: 658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C, Kowalewski DJ, Schuster H, Mirza N, Walz S, Handel M, Schmid-Horch B, Salih HR, Kanz L, Rammensee HG, et al. 2015. Mapping the HLA ligandome landscape of acute myeloid leukemia: A targeted approach toward peptide-based immunotherapy. Leukemia 29: 647–659. [DOI] [PubMed] [Google Scholar]
- Boegel S, Lower M, Bukur T, Sahin U, Castle JC. 2014. A catalog of HLA type, HLA expression, and neo-epitope candidates in human cancer cell lines. Oncoimmunology 3: e954893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentville VA, Metheringham RL, Gunn B, Symonds P, Daniels I, Gijon M, Cook K, Xue W, Durrant LG. 2016. Citrullinated vimentin presented on MHC-II in tumor cells is a target for CD4+ T-cell-mediated antitumor immunity. Cancer Res 76: 548–560. [DOI] [PubMed] [Google Scholar]
- Bright RK, Bright JD, Byrne JA. 2014. Overexpressed oncogenic tumor-self antigens. Hum Vaccin Immunother 10: 3297–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JD, Mazzilli SA, Reid ME, Dhillon SS, Platero S, Beane J, Spira AE. 2016. The case for a pre-cancer genome atlas (PCGA). Cancer Prev Res (Phila) 9: 119–124. [DOI] [PubMed] [Google Scholar]
- Carbone DP, Ciernik IF, Kelley MJ, Smith MC, Nadaf S, Kavanaugh D, Maher VE, Stipanov M, Contois D, Johnson BE, et al. 2005. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: Immune response and clinical outcome. J Clin Oncol 23: 5099–5107. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Disis ML, Bernhard H, Gralow JR, Hand SL, Huseby ES, Qin HL, Takahashi M, Chen W. 1995. Immunity to oncogenic proteins. Immunol Rev 145: 33–59. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. 2009. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15: 5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. 2014. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat Rev Cancer 14: 135–146. [DOI] [PubMed] [Google Scholar]
- Disis ML, Bernhard H, Gralow JR, Hand SL, Emery SR, Calenoff E, Cheever MA. 1994. Immunity to the HER-2/neu oncogenic protein. Ciba Found Symp 187: 198–207; discussion 207-111. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21: 137–148. [DOI] [PubMed] [Google Scholar]
- Finn OJ. 1993. Tumor-rejection antigens recognized by T lymphocytes. Curr Opin Immunol 5: 701–708. [DOI] [PubMed] [Google Scholar]
- Finn OJ. 2003. Premalignant lesions as targets for cancer vaccines. J Exp Med 198: 1623–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn OJ. 2014. Vaccines for cancer prevention: A practical and feasible approach to the cancer epidemic. Cancer Immunol Res 2: 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn OJ, Beatty PL. 2016. Cancer immunoprevention. Curr Opin Immunol 39: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch EF, Rajasagi M, Ott PA, Brusic V, Hacohen N, Wu CJ. 2014. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol Res 2: 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjertsen MK, Gaudernack G. 1998. Mutated Ras peptides as vaccines in immunotherapy of cancer. Vox Sang 74: 489–495. [DOI] [PubMed] [Google Scholar]
- Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, et al. 2016. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 22: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. 2015. Tumor neoantigens: Building a framework for personalized cancer immunotherapy. J Clin Invest 125: 3413–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom KE, Hellstrom I. 1989. Oncogene-associated tumor antigens as targets for immunotherapy. FASEB J 3: 1715–1722. [DOI] [PubMed] [Google Scholar]
- Henderson RA, Finn OJ. 1996. Human tumor antigens are ready to fly. Adv Immunol 62: 217–256. [DOI] [PubMed] [Google Scholar]
- Javier RT, Butel JS. 2008. The history of tumor virology. Cancer Res 68: 7693–7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaora S, Barnea E, Merhavi-Shoham E, Qutob N, Teer JK, Shimony N, Schachter J, Rosenberg SA, Besser MJ, Admon A, et al. 2016. Use of HLA peptidomics and whole exome sequencing to identify human immunogenic neo-antigens. Oncotarget 7: 5110–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H, Marto JA, Hoffmann TK, Shabanowitz J, Finkelstein SD, Whiteside TL, Hunt DF, Finn OJ. 2001. Identification of cyclin B1 as a shared human epithelial tumor-associated antigen recognized by T cells. J Exp Med 194: 1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Spira A, Garber JE, Szabo E, Lee JJ, Dong Z, Dannenberg AJ, Hait WN, Blackburn E, Davidson NE, et al. 2016. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev Res (Phila) 9: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ. 2013. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 6: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie HR, Awada G, Awada AH. 2016. Learning from the “tsunami” of immune checkpoint inhibitors in 2015. Crit Rev Oncol Hematol 101: 213–220. [DOI] [PubMed] [Google Scholar]
- Kowalewski DJ, Schuster H, Backert L, Berlin C, Kahn S, Kanz L, Salih HR, Rammensee HG, Stevanovic S, Stickel JS. 2015a. HLA ligandome analysis identifies the underlying specificities of spontaneous antileukemia immune responses in chronic lymphocytic leukemia (CLL). Proc Natl Acad Sci 112: E166–E175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewski DJ, Stevanovic S, Rammensee HG, Stickel JS. 2015b. Antileukemia T-cell responses in CLL—We don’t need no aberration. Oncoimmunology 4: e1011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerz V, Fatho M, Gentilini C, Frye RA, Lifke A, Ferel D, Wolfel C, Huber C, Wolfel T. 2005. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci 102: 16013–16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler MW, Chandran PA, Laske K, Schroeder C, Bonzheim I, Walzer M, Hilke FJ, Trautwein N, Kowalewski DJ, Schuster H, et al. 2016. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol 65: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmueller JJ, Sato S, Popova L, Chu IM, Tucker MA, Barberena R, Innocenti GM, Cudic M, Ham JD, Cheung WC, et al. 2016. Antibodies elicited by the first non-viral prophylactic cancer vaccine show tumor-specificity and immunotherapeutic potential. Sci Rep 6: 31740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz ER, Jaffee EM. 2014. Can we predict mutant neoepitopes in human cancers for patient-specific vaccine therapy? Cancer Immunol Res 2: 518–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. 2009. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev Cell 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister RM. 1965. Viruses and cancer. Calif Med 102: 344–352. [PMC free article] [PubMed] [Google Scholar]
- Mohammed F, Cobbold M, Zarling AL, Salim M, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Engelhard VH, Willcox BE. 2008. Phosphorylation-dependent interaction between antigenic peptides and MHC class I: A molecular basis for the presentation of transformed self. Nat Immunol 9: 1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, Coussens LM. 2016. The basis of oncoimmunology. Cell 164: 1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AE, Stryhn A, Justesen S, Harndahl M, Rasmussen S, Donskov F, Claesson MH, Pedersen JW, Wandall HH, Svane IM, et al. 2011. Wildtype p53-specific antibody and T-cell responses in cancer patients. J Immunother 34: 629–640. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. 2015. Neoantigens in cancer immunotherapy. Science 348: 69–74. [DOI] [PubMed] [Google Scholar]
- Sensi M, Anichini A. 2006. Unique tumor antigens: Evidence for immune control of genome integrity and immunogenic targets for T cell-mediated patient-specific immunotherapy. Clin Cancer Res 12: 5023–5032. [DOI] [PubMed] [Google Scholar]
- Sibille C, Chomez P, Wildmann C, Van Pel A, De Plaen E, Maryanski JL, de Bergeyck V, Boon T. 1990. Structure of the gene of tum- transplantation antigen P198: A point mutation generates a new antigenic peptide. J Exp Med 172: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren HO. 1967. Immunology of virus-induced tumours. Mod Trends Med Virol 1: 207–222. [PubMed] [Google Scholar]
- Srivastava PK. 1993. Peptide-binding heat shock proteins in the endoplasmic reticulum: Role in immune response to cancer and in antigen presentation. Adv Cancer Res 62: 153–177. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. 2015. Neoepitopes of cancers: Looking back, looking ahead. Cancer Immunol Res 3: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronen E, Toebes M, Kelderman S, van Buuren MM, Yang W, van Rooij N, Donia M, Boschen ML, Lund-Johansen F, Olweus J, et al. 2016. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 352: 1337–1341. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Graziano DF, McKolanis J, Finn OJ. 2005. T cell-dependent antibody responses against aberrantly expressed cyclin B1 protein in patients with cancer and premalignant disease. Clin Cancer Res 11: 1521–1526. [DOI] [PubMed] [Google Scholar]
- Ting CC, Herberman RB. 1974. Serological analysis of immune response to friend virus-induced leukemia. Cancer Res 34: 1676–1683. [PubMed] [Google Scholar]
- Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, Gartner JJ, Zheng Z, Li YF, Ray S, et al. 2015. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350: 1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JL, Schreiber H. 1992. Tumor antigens. Annu Rev Immunol 10: 617–644. [DOI] [PubMed] [Google Scholar]
- Vankemmelbeke M, Chua JX, Durrant LG. 2016. Cancer cell associated glycans as targets for immunotherapy. Oncoimmunology 5: e1061177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella LA, Yu M, Phillips AB, Finn OJ. 2009. Immunity against cyclin B1 tumor antigen delays development of spontaneous cyclin B1-positive tumors in p53–/– mice. Ann NY Acad Sci 1174: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne CE, Schotte R, Spits H, et al. 2016. Neoantigen landscape dynamics during human melanoma–T cell interactions. Nature 536: 91–95. [DOI] [PubMed] [Google Scholar]
- Vermeij R, Leffers N, van der Burg SH, Melief CJ, Daemen T, Nijman HW. 2011. Immunological and clinical effects of vaccines targeting p53-overexpressing malignancies. J Biomed Biotechnol 2011: 702146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad AM, Finn OJ. 2004. Glycoprotein tumor antigens for immunotherapy of breast cancer. Breast Dis 20: 73–79. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH. 2002. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene 21: 674–679. [DOI] [PubMed] [Google Scholar]
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. 2012. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 18: 1254–1261. [DOI] [PubMed] [Google Scholar]
- Walz S, Stickel JS, Kowalewski DJ, Schuster H, Weisel K, Backert L, Kahn S, Nelde A, Stroh T, Handel M, et al. 2015. The antigenic landscape of multiple myeloma: Mass spectrometry (re)defines targets for T-cell-based immunotherapy. Blood 126: 1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T, et al. 2014. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515: 572–576. [DOI] [PubMed] [Google Scholar]
- Yu M, Zhan Q, Finn OJ. 2002. Immune recognition of cyclin B1 as a tumor antigen is a result of its overexpression in human tumors that is caused by non-functional p53. Mol Immunol 38: 981–987. [DOI] [PubMed] [Google Scholar]